Abstract
Several pathologies of the anterior skull base may involve the optic canal, the optic strut, and the optic nerve, requiring surgeon to understand such complex anatomical spaces and their proximity to the critical neurovascular structures, including the internal carotid artery and the cavernous sinus. The identification of definite anatomical landmarks, coupled with the knowledge of the most common anatomical variants, is essential for guiding the surgery, allowing surgeons to mentally visualize these relationships as one plan which approach will be applied. This is particularly true in the current surgical era, which is characterized by the continuous development of open and endoscopic approaches to select different regions of the optic canal. This chapter delineates the surgical anatomy of the optic canal, optic strut, and optic nerve, with the purpose to assist the surgical planning of optimal approaches tailored to each different lesion involving these regions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Aneurysm clipping
- Clinoidectomy
- Endoscopic surgery
- Optic canal
- Optic nerve decompression
- Skull base
- Surgical anatomy
1 Introduction
Detailed knowledge and understanding of the surgical anatomy of the optic canal, optic nerve, and optic strut is of particular importance in skull base surgery. The proximity of these structures with critical neurovascular structures (i.e., the internal carotid artery and cavernous sinus) requires the surgeon to mentally visualize these relationships as one plan which approach will be applied [1]. This is particularly true in the current surgical era, which is characterized by the continuous development of open and endoscopic approaches to select different regions of the optic canal [2].
This chapter describes the detailed surgical anatomy of the optic canal, optic strut, and optic nerve to further improve understanding and visualization of their relationship with adjacent critical structures. Ultimately, the purpose is to assist skull base surgeons in planning optimal approaches tailored to each different lesion involving this region. In addition, embryological development and common anatomical variants are summarized with their important surgical implications.
2 Embryological Development
Lang [3] identified 10 ossification sites in the sphenoid bone and stated that the complexity of the ossification process contributes to normal anatomical variants. The embryological development of the optic canal starts at the third gestation month and can be divided into three stages: (1) cartilaginous formation of the optic foramen, (2) ossification of the cartilaginous optic foramen, and (3) formation of the optic canal. The cartilaginous optic foramen is formed from the cartilaginous lesser wing of the sphenoid, with progressive chondrification from posterolateral to superomedial [4]. The ossification of the cartilaginous optic foramen occurs around the 12th–17th gestational week from the sphenoid’s lesser wing and presphenoid ossification centers, which outline the superolateral and medial margins [5]. The bony optic foramen takes a “keyhole” appearance with the inferolateral border formed by bony bridge joining the center of the lesser sphenoid wing to the postsphenoid center, delineating the anteroinferior segment of the optic strut. The transformation of the bony optic foramen into the bony optic canal begins at the fifth gestation month, with the intracranial ophthalmic artery initially located more inferiorly [5]. The posterosuperior segment of the optic strut develops as single bony ridge from the center of the lesser sphenoid wing to the presphenoid center or as two bony ridges originating separately from the two centers and growing toward one another. This optic strut segment transforms the optic foramen into the bony optic canal with the cranial opening of horizontal-oval shape and the orbital opening of vertical-oval shape [6]. The intracranial ophthalmic artery relocates occupying a more superior position. The two segments of the optic strut are initially separated by a non-functional foramen, which obliterates during the last two fetal months leading to the physiological fusion of the two segments and the formation of a single optic strut. Regarding the optic nerve, it appears around the fourth to seventh gestational week from the retinal nerve fibers of the optic stalk, which is the embryological structure connecting the optic cup to the brain [7].
3 Surgical Technique
Several skull base pathologies, including traumatic, neoplastic, and vascular, often involve the optic canal, optic strut, and optic nerve, requiring surgeons to gain a keen understanding of such complex spaces to avoid potentially devastating complications. The identification of definite anatomical landmarks is essential for guiding the surgery. Also, anatomical variants need to be considered and investigated during pre-operative imaging planning, to familiarize with the individual anatomy of each patient during surgery. A wide range of intracranial and endoscopic surgical windows have been investigated to safely expose the optic nerve, ophthalmic artery, and internal carotid artery [2]. The selection of an optimal approach should be modified on a case-by-case basis, governed by the extension of the pathology, the surgical goal, and the surgeon’s preference, including the expected risks of complications.
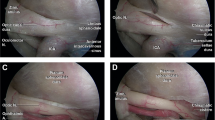
The orbital apex defines the region that connects the orbit with the intracranial space, housing the optic canal, the optic strut, and the superior and inferior orbital fissures. Most commonly, the optic canal constitutes a single bony opening in the sphenoid bone containing the optic nerve, ophthalmic artery, and postganglionic sympathetic nerves. In rare cases, the optic canal cranial opening may be duplicated, with the lower canal containing the ophthalmic artery and the upper canal containing the optic nerve [8]. Moving from posterior (intracranial) to anterior (intraorbital), Slavin et al. [9] divided the optic canal into three functional portions. The first, horizontal-oval portion opens into the middle cranial fossa, bounded superiorly by the falciform ligament and laterally by the anterior clinoid process. The cavernous sinus lies inferiorly with the cavernous (C4) segment of the internal carotid artery and cranial nerves IV, V1, V2, and VI, and the clinoid (C5) carotid segment turns superolaterally into the ophthalmic (C6) carotid segment that gives origin to the ophthalmic artery, which enters the optic canal below the optic nerve [10]. Similarly, in cases where the ophthalmic artery originates from the A1 segment of anterior cerebral artery, it also enters the optic canal below the optic nerve [11]. The ophthalmic artery may also originate from the middle meningeal artery [12] and then enter the orbit through the superior orbital fissure [13] or by penetrating the optic strut [14]. The second, round portion of the optic canal includes the canal itself, walled by two roots of the lesser wing of the sphenoid, from supero-medial-posterior to infero-lateral-anterior, at a 30° angle. Absence of the medial wall of the optic canal leads to a direct connection between the ethmoid sinus and the optic nerve, favoring the extension of infective agents [15]. The third, vertical-oval portion of the optic canal, known as optic foramen, involves the intraorbital opening, which can be identified immediately posterior to the optic tubercle creating a transorbital view. The optic canal is covered internally by two fused dural layers that continue from the intracranial dural lining and contain the optic nerve and ophthalmic artery [16]. While the dural covering of the optic canal’s floor continues to the anterior margin of the distal dural ring, the dural lining of the optic canal’s roof forms the falciform ligament from the base of the anterior clinoid process to the dura of the planum sphenoidale, and the dural layer of the optic canal’s medial wall fuses with the diaphragm sellae.
The optic strut defines the bony bridge of the lesser wing of the sphenoid located between the anterior clinoid process and the body of the sphenoid, forming the inferolateral wall of the optic canal, and separating it from the superior orbital fissure [17]. The optic strut has a triangular-shaped or wing-shaped appearance, delineating the anterior limit of the clinoid (C5) segment of the internal carotid artery [18] and the dorsal extent of the distal dural ring [19]. The base of the optic strut may be identified via the transsphenoidal endonasal endoscopic view as the “lateral optic-carotid recess,” which is a bony depression located between the optic nerve and internal carotid protuberances on the lateral wall of the sphenoid sinus [2]. Kerr et al. [20] identified four anatomical variants of the optic strut position in relation to the prechiasmatic sulcus: (1) sulcal or adjacent to the prechiasmatic sulcus; (2) postsulcal, when located posteriorly to the prechiasmatic sulcus; (3) asymmetric; and (4) presulcal, when located anteriorly to the prechiasmatic sulcus. The optic strut may be duplicated when the posterosuperior segment develops above the ophthalmic artery, forming two separate optic strut cranial openings with the optic nerve coursing through superior opening [5]. The optic strut may also assume a “keyhole” radiological appearance when its posterosuperior segment is absent or present only in a rudimentary form. In rare cases, the optic strut may be totally absent, with the optic canal and superior orbital fissure forming a single cranial opening [21].
The optic nerve is contained within the optic canal and borders inferiorly the optic strut, coursing from the optic chiasm to the eye bulb. Engin et al. [8] divided the optic nerve into four segments from posterior to anterior: (1) intracranial, (2) intracanalicular, (3) intraorbital, and (4) intraocular. The intracranial optic nerve segment lies inferior to the gyrus rectus and olfactory tract, coursing through the subarachnoid space intersecting the anterior cerebral artery and anterior communicating artery complex in the cistern of the lamina terminalis. While the anterior clinoid process is located laterally, proximal to the two dural rings that mark the transition from the cavernous (C4) carotid segment to the clinoidal (C5) segment, the tuberculum sellae and the middle clinoid process are located medially. The optic nerve enters the optic canal below the falciform ligament, forming the intracanalicular optic nerve segment that courses in an anterolateral direction, superomedially to the optic strut. The inferior surface of the intracanalicular ON segment forms a protuberance on the upper part of the sphenoid sinus, located anterolaterally to the sellar floor [10]. After endoscopic endonasal sphenoidotomy, the ON protuberance can be easily identified superiorly to ipsilateral carotid protuberance, which is located laterally to the sellar floor and corresponds to the cavernous (C4) internal carotid segment [10]. The lateral optic-carotid recess is sited between these two bony protuberances and can be used to detect the intracranial optic canal opening superomedially, the clinoid internal carotid segment inferomedially, the superior orbital fissure inferolaterally, and the orbital apex laterally [2]. From an endoscopic view, the intracanalicular optic nerve segment may be also identified using a vertical line touching the medial border of the anterior ascending cavernous (C4) internal carotid artery segment [22]. After entering the muscle cone within the orbital apex, the intraorbital optic nerve segment extends in the globe with a S-shape to allow the mobility of the globe within the orbit [15]. Both the intracanalicular and intraorbital segments are surrounded by the optic nerve sheath.
4 Operative Nuances
Surgical exposure of the optic canal is required to resect tumors extending into the optic canal from the orbital or intracranial compartments and to relieve compression of the edematous optic nerve in case of craniofacial trauma or pseudotumor cerebri [23]. Historically, ophthalmologists approached orbital apex lesions from a lateral orbitotomy corridor, which was introduced by Krönlein in 1889 [24]. In 1922, Dandy was the first to use the transcranial approach to operate on a patient with bilateral optic canal meningiomas, concluding to have obtained better optic canal exposure compared to the traditional lateral access [25]. From that point in time, multiple surgical windows, first open and then endoscopic, have been investigated to access the optic canal region [2]. Open surgical corridors, including the pterional, frontotemporal-orbital, supraorbital, and subfrontal, offer maximal exposure of the superior and lateral surfaces of the intracanalicular optic nerve segment. Transcranial routes to approach the optic canal can be extradural or intradural, with the use of either route depending on the surgeon’s preference and expertise [26]. Based on our experience, we prefer to access the optic canal extradurally by removing the anterior clinoid process, sectioning the falciform ligament, and unroofing the optic canal with optic strut removal to increase the decompression of the optic nerve. Such step, coupled with the distal dural ring excision particularly in cases of aneurysms, allow for the mobilization of the optic nerve and internal carotid artery creating an additional corridor in the optic-carotid space. This favors proximal control of the internal carotid artery, avoiding excessive traction of the optic nerve responsible for irreversible injury and offering safe dissection of the tumor from the optic nerve [27]. All these maneuvers reduce the risk of injuring the optic nerve. However, in cases of meningiomas, the surrounding dura should not be resected since its coagulation may compromise the optic nerve’s blood flow and impairing visual function by causing optic neuropathy [27]. An anterior clinoidectomy may also be performed with an intradural technique, but it may increase the risks of injuring the surrounding vasculature. We have recently used the ultrasonic bone curette (SONOPET®, Stryker, Kalamazoo, MI, USA) to reduce the risks of optic nerve and vascular injury, which may occur during bone drilling [28]. Any potential risk of thermal injury to the optic nerve associated with the use of ultrasonic bone curettes may be avoided by operating the SONOPET® at low power settings. In some cases, the anterior clinoid process may be pneumatized, or it can be fused with the middle clinoid process, forming the “caroticoclinoid foramen”, or with the posterior clinoid process, forming the “interclinoid bridge” [8, 29, 30]. These anatomical variations should be carefully evaluated at pre-operative CT scans, and, if present, an intradural clinoidectomy is recommended to avoid any injury to the internal carotid or ophthalmic artery. The main limitation of open transcranial approaches consists in the difficulty to safely expose the inferior and medial surfaces of the optic nerve without risking injury of the internal carotid artery and/or the cavernous sinus. Some authors suggest the use of contralateral transcranial approaches to access the optic canal and treat medially projecting aneurysms of the ophthalmic (C6) internal carotid artery segment [31].
The endoscopic transsphenoidal route provides an optimal surgical corridor for accessing lesions originating from the sphenoid wing, cavernous sinus, or tuberculum sellae and displacing the optic nerve superiorly and laterally [2]. Using the lateral optic-carotid recess as a landmark to identify the optic nerve, internal carotid artery, and ophthalmic artery, the bone overlying the tuberculum and planum sellae can be removed to access the optic chiasms and the inferomedial quadrant of the intracanalicular optic nerve segment. Navigation and micro-doppler may assist in identifying the internal carotid artery, especially in cases with tortuous “kissing ICA” where the ophthalmic internal carotid artery segment needs to be mobilized laterally or inferiorly to expose the inferior quadrant of the intracranial optic nerve segment. Bone removal may also be extended laterally to the tuberculum sellae (i.e., trans-medial optic-carotid recess) to access the distal intracranial optic nerve segment and the superomedial surface of the clinoid (C5) and ophthalmic (C6) carotid segments [2]. Abhinav et al. [32] reported the feasibility to decompress up to 270° of the optic canal using the endonasal endoscopic approach, but this technique requires extensive intracranial extension with delicate manipulation of the optic nerve and leads to high risks of causing cerebrospinal fluid leakage. Although the endonasal endoscopic corridor requires no brain retraction, the main limitation is the inability to expose the superior surface of the intracanalicular optic nerve segment and transection of the falciform ligament to free the optic nerve without opening the subarachnoid space and causing cerebrospinal fluid leakage. In addition, pneumatization of the sphenoid sinus plays a big role in determining the surgical access through the transsphenoidal route, expecting unfavorable outcomes in patients with poorly pneumatized sinuses, which require prolonged drilling and thermal injury to the optic nerve [33].
Although several studies have demonstrated that the transcranial approaches provide greater circumferential decompression and exposure of the optic nerve with minimal risk of cerebrospinal fluid leakage, the selection of the best surgical approach should be tailored on each patient’s anatomy, pathology, and clinical scenario [2, 22]. The pterional approach may be preferred to achieve a wide optic canal decompression or to access the superior and lateral surfaces of the intracranial optic nerve segment. The frontotemporal-orbital approach is indicated in treating lesions that extend into the lateral orbit. The subfrontal approach offers better inferomedial exposure of the distal intracranial optic nerve segment. The endoscopic endonasal transsphenoidal route provides the best access to the inferior and medial quadrants of the intracanalicular optic nerve, allowing expanded exposure of the intraorbital and intracranial segments using the transplanum trans-tuberculum, trans-medial optic-carotid recess, and transcanalicular approaches. The transcranial and endoscopic corridors should be considered as complementary options for accessing the optic canal, optic strut, and optic nerve regions, and not as mutually exclusive.
5 Conclusions
Knowledge of the surgical anatomy of the optic canal, optic strut, and optic nerve is necessary for skull base surgeons involved in the treatment of traumatic, oncological, and vascular pathologies to minimize the surgical morbidity and achieve optimal outcomes. Although some anatomical variants and abnormalities may be detected in several cases, the overall persistency of definite anatomical landmarks allows effective surgical planning. Open transcranial and endoscopic endonasal surgical approaches should be tailored on a case-by-case basis with relation to the individual patient’s anatomy, pathology, and clinical scenario.
References
Demartini Z, Zanine SC. Microanatomic study of the optic canal. World Neurosurg. 2021;155:e792–6. https://doi.org/10.1016/j.wneu.2021.08.144.
Caporlingua A, Prior A, Cavagnaro MJ, et al. The intracranial and intracanalicular optic nerve as seen through different surgical windows: endoscopic versus transcranial. World Neurosurg. 2019;124:522–38. https://doi.org/10.1016/j.wneu.2019.01.122.
Lang J. Surgery of the cranial base tumors. In: Clinical anatomy of the head: neurocranium, orbit, craniocervical regions. Berlin: Springer-Verlag; 1983. p. 99–121.
Fawcett. Notes on the development of the human sphenoid. J Anat Physiol. 1910;44(Pt 3):207–22.
Kier EL. Embryology of the normal optic canal and its anomalies an anatomic and roentgenographic study. Investig Radiol. 1966;1(5):346–62. https://doi.org/10.1097/00004424-196609000-00023.
Duke-Elder S, Wybar KC. The anatomy of the visual system. In: System of ophthalmology, volume 2. London: H. Kimpton; 1958.
Lee AG, Morgan ML, Palau AEB, et al. Anatomy of the optic nerve and visual pathway. In: Nerves and nerve injuries. Amsterdam: Elsevier; 2015. p. 277–303. https://doi.org/10.1016/B978-0-12-410390-0.00020-2.
Engin Ö, Adriaensen GFJPM, Hoefnagels FWA, Saeed P. A systematic review of the surgical anatomy of the orbital apex. Surg Radiol Anat. 2021;43(2):169–78. https://doi.org/10.1007/s00276-020-02573-w.
Slavin KV, Dujovny M, Soeira G, Ausman JI. Optic canal: microanatomic study. Skull Base. 1994;4(3):136–44. https://doi.org/10.1055/s-2008-1058965.
Bouthillier A, van Loveren HR, Keller JT. Segments of the internal carotid artery: a new classification. Neurosurgery. 1996;38(3):425–33. https://doi.org/10.1097/00006123-199603000-00001.
Hassler W, Zentner J, Voigt K. Abnormal origin of the ophthalmic artery from the anterior cerebral artery: neuroradiological and intraoperative findings. Neuroradiology. 1989;31(1):85–7. https://doi.org/10.1007/BF00342037.
Hayreh SS, Dass R. The ophthalmic artery: I. Origin and intra-cranial and intra-canalicular course. Br J Ophthalmol. 1962;46(2):65–98. https://doi.org/10.1136/bjo.46.2.65.
Regoli M, Bertelli E. The revised anatomy of the canals connecting the orbit with the cranial cavity. Orbit. 2017;36(2):110–7. https://doi.org/10.1080/01676830.2017.1279662.
Hokama M, Hongo K, Gibo H, Kyoshima K, Kobayashi S. Microsurgical anatomy of the ophthalmic artery and the distal dural ring for the juxta–dural ring aneurysms via the pterional approach. Neurol Res. 2001;23(4):331–5. https://doi.org/10.1179/016164101101198703.
Selhorst J, Chen Y. The optic nerve. Semin Neurol. 2009;29(1):29–35. https://doi.org/10.1055/s-0028-1124020.
Meyer F. Zur Anatomie der Orbitalarterien. Morphol Jahr. 1887;12:414–58.
Curragh DS, Valentine R, Selva D. Optic strut terminology. Ophthalmic Plast Reconstr Surg. 2019;35(4):407–8. https://doi.org/10.1097/IOP.0000000000001394.
Seoane E, Rhoton AL, de Oliveira E. Microsurgical anatomy of the dural collar (carotid collar) and rings around the clinoid segment of the internal carotid artery. Neurosurgery. 1998;42(4):869–84. https://doi.org/10.1097/00006123-199804000-00108.
Beretta F, Sepahi AN, Zuccarello M, Tomsick TA, Keller JT. Radiographic imaging of the distal dural ring for determining the intradural or extradural location of aneurysms. Skull Base. 2005;15(4):253–62. https://doi.org/10.1055/s-2005-918886.
Kerr R, Tobler W, Leach J, et al. Anatomic variation of the optic strut: classification schema, radiologic evaluation, and surgical relevance. J Neurol Surg B Skull Base. 2012;73(6):424–9. https://doi.org/10.1055/s-0032-1329626.
Le Double A. Traite Des Variations Des Os Du Crane de l’Homme, vol. 1. Paris: Vigot Freres; 1903.
Gogela SL, Zimmer LA, Keller JT, Andaluz N. Refining operative strategies for optic nerve decompression: a morphometric analysis of transcranial and endoscopic endonasal techniques using clinical parameters. Oper Neurosurg. 2018;14(3):295–302. https://doi.org/10.1093/ons/opx093.
Maurer J, Hinni M, Mann W, Pfeiffer N. Optic nerve decompression in trauma and tumor patients. Eur Arch Otorhinolaryngol. 1999;256(7):341–5. https://doi.org/10.1007/s004050050160.
Krönlein R. Pathologie and operativen Behandlung der Dermoidcysten der Orbita. Beitr z Klin Chir Tubing. 1889;4:149–63.
Dandy WE. Prechiasmal intracranial tumors of the optic nerves. Am J Ophthalmol. 1922;5(3):169–88. https://doi.org/10.1016/S0002-9394(22)90261-2.
Hassler W, Eggert HR. Extradural and intradural microsurgical approaches to lesions of the optic canal and the superior orbital fissure. Acta Neurochir. 1985;74(3–4):87–93. https://doi.org/10.1007/BF01418794.
Andaluz N, Beretta F, Bernucci C, Keller JT, Zuccarello M. Evidence for the improved exposure of the ophthalmic segment of the internal carotid artery after anterior clinoidectomy: morphometric analysis. Acta Neurochir. 2006;148(9):971–6. https://doi.org/10.1007/s00701-006-0862-x.
Spektor S, Dotan S, Mizrahi CJ. Safety of drilling for clinoidectomy and optic canal unroofing in anterior skull base surgery. Acta Neurochir. 2013;155(6):1017–24. https://doi.org/10.1007/s00701-013-1704-2.
Mikami T, Minamida Y, Koyanagi I, Baba T, Houkin K. Anatomical variations in pneumatization of the anterior clinoid process. J Neurosurg. 2007;106(1):170–4. https://doi.org/10.3171/jns.2007.106.1.170.
Kim JM, Romano A, Sanan A, van Loveren HR, Keller JT. Microsurgical anatomic features and nomenclature of the paraclinoid region. Neurosurgery. 2000;46(3):670–82. https://doi.org/10.1097/00006123-200003000-00029.
Andrade-Barazarte H, Kivelev J, Goehre F, et al. Contralateral approach to internal carotid artery ophthalmic segment aneurysms. Neurosurgery. 2015;77(1):104–12. https://doi.org/10.1227/NEU.0000000000000742.
Abhinav K, Acosta Y, Wang WH, et al. Endoscopic endonasal approach to the optic canal. Oper Neurosurg. 2015;11(3):431–46. https://doi.org/10.1227/NEU.0000000000000900.
Di Somma A, Cavallo LM, de Notaris M, et al. Endoscopic endonasal medial-to-lateral and transorbital lateral-to-medial optic nerve decompression: an anatomical study with surgical implications. J Neurosurg. 2017;127(1):199–208. https://doi.org/10.3171/2016.8.JNS16566.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Palmisciano, P., AlFawares, Y., Andaluz, N., Keller, J.T., Zuccarello, M. (2023). Optic Canal, Optic Strut, and Optic Nerve. In: Bonavolontà, G., Maiuri, F., Mariniello, G. (eds) Cranio-Orbital Mass Lesions. Springer, Cham. https://doi.org/10.1007/978-3-031-35771-8_2
Download citation
DOI: https://doi.org/10.1007/978-3-031-35771-8_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-35770-1
Online ISBN: 978-3-031-35771-8
eBook Packages: MedicineMedicine (R0)