Abstract
Cancer is considered to be one of the deadliest diseases ever due to its high rate of deaths and unpredictable initial stages. There are a number of techniques to diagnose and treatment of cancer therapies but cancer still remains untreatable is many cases. Traditional methods are not much advanced to kill tumours in the therapies without side effects. Tumours are most sensitive for the immune system to recognise, and tumour antigens are less immunogenic molecules. These have originated as a result of the transformation of natural and health cells. The immune response is the biggest ability of the body when body encounters with any antigen. Antigen should be enough capable to accelerate immune response as soon as possible but in the case of cancer tumours are very low immunogen and they are not capable of accelerating the immune response like T cells, Antigen presentation and many other releases of components in immune response. Food and drug administrations had already approved cancer vaccines over the past decade, but little research has being carried out to date. The vaccines are usually specific in nature in which toxic-virulent or toxic bacterial genes/proteins are suppressed in an attempt to activate the immune system of an individual. Nanoparticles are coated with specific cell membranes and proteins to enhance the immune response, to target dual molecules at a time and to lower the impact of immunosuppression.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
The immune system of homo sapiens is organized as a complex network comprising of various cells, organs, proteins, and chemical modulators along with anatomical and physical barriers which work collectively to resist many pathogens and antigen-causing infections. It can be also explained as the capability of the body to vanish foreign molecules in the body reduce risks for severe infections, but it has observed if it has not strength enough can lead to numerous diseases. Sometimes even an extremely active immune system can lead to autoimmunity and some inflammatory responses to the production of healthy tissues (Rosenblum et al., 2015; Wang et al., 2015). The immune system not only functions against external antigens with which our body encounters the clinical illness in the form of exo-antigens, but also can respond to numerous endo-antigens such as intracellular pathogenic viruses as well as certain tumour antigens. The natural immune system always tries to eliminate foreign pathogens, including cancer cells. The proper functioning of the immune system is a very important aspect of the treatment of cancer patients as either cancer itself can weaken the individual immune system or various cancer therapies might also weaken the immune system instead of fighting against the cancer.
5.2 Cancer and Treatments May Weaken Immunity
Cancer generally targets human immunity and generally undermines the functions of the immune system, especially when it causes the metastasis of bone marrow which makes diverse blood cells that will be the sole part of resistance to fight against infection. This will be most often observed in the case of certain types of cancers such as lymphoma or leukaemia in addition to other cancers. Cancer therapy inhibits bone marrow from being effective hemopoiesis that can make so many blood cells. Certain cancer treatments, such as radiation and chemotherapy, will target cell division and cannot have selective toxicity by which only the cancer cells will be destroyed and equally toxic to cancer cells as well as normal dividing cells which will temporarily weaken the immune system. This is because of the drop in the number of blood leukocytes which are generated in the bone marrow. Among the cancer treatments that are most likely to weaken the immune system are chemotherapy, radiotherapy, and high doses of steroids.
5.3 Cancer Treatments That Use the Immune System
Some of the cells of our immune system can recognise a wide range of different cancer cells as abnormal cells and effectively kill them through the identification of tumour markers on their surfaces. But this mechanism may not be sufficient to dispose of a cancer altogether. Hence some treatment strategies will aim to induce the individual immune system to fight against cancer, either with the inbuilt immune protection or with the protection methods developed after exposure to certain disease-causing agents by acquired immunity. Some advanced types of cancer treatments are currently used to boost the immune system to fight effectively against cancer, such as immunotherapy. This is one of the best treatment options for some types of cancer. It induces the immune system to precisely find the cancer cells, target them and kill cancer cells. They are much more helpful in cancer treatment since the cancer cells are metabolically and immunologically different from the normal cells of our body. Hence the targeted immune therapy helps the immune system to differentiate and kill the abnormal cancer cells. There are different types of chemicals that are part of the immune response with immunotherapy, such as monoclonal antibodies (MABs), which exclusively recognise and attack certain protein markers generally known as targeted antigens on the surface of cancer cells.
But if we can block the cellular transformation
-
Vaccines to help the immune system to recognise and attack cancer,
-
Cytokines to help to boost the immune system,
-
CAR T-cell therapy (also called adoptive cell transfer) to change the genes in a person’s white blood cells.
5.4 Cellular Genomic Changes and Cancer
All cancers begin within a single cell. Our body are made up of more than a hundred million million cells. Generally, the cancer starts with genomic changes within one cell or a small group of cells. The cells will have cellular machinery to create signals to control the cellular growth and cell division. If any of these regulatory signals are faulty or missing, these cells attain the capacity to start to grow and multiply excessively, thereby leading to uncontrollable proliferation to form a lump called a primary tumour. A primary tumour of the body is generally brought about by genomic instability and escape from the natural defence of our immune system, making it the site where the cancer starts. Some types of cancers, such as like leukaemia, are associated with blood cells; in some circumstances, abnormal immature blood cells will be accumulated, and routine functions will not be held. They will not form solid tumours. Instead, the cancer cells build up either in the blood or in the bone marrow. For a cancer to start, certain cellular changes take place within the genes of a cell or a group of cells. Diverse types of cells in our body perform different functions, even though they are basically similar. But each of the cells have a unique genetic makeup to engage typical functionalities by expressing diverse proteins via RNA (ribonucleic acid). Genes will make sure that cells grow and reproduce in an orderly and controlled way to keep the body normal and healthy. Sometimes, a sudden change may happen because of either intrinsic or extrinsic factors in the genes when a cell divide is known as mutation. These mutations are also time to time restored by DNA damage repair, a natural phenomenon that occurs in all cells. If these mutations accumulated more and irreversibly damages the DNA, then this may lead to the genomic instability, which is a hallmark of cancer. These mutations can happen by chance when a cell is dividing. Some mutations insensitive to the cell no longer recognises cellular instructions. It can twitch to grow out of control. There must be about 6 diverse mutations before a normal cell transform into a cancer cell. Mutations results in a cell cause the expression of many proteins that trigger cell division; with mutation, a cell may stop protein expression that normally causes a cell to stop dividing. Alternatively, an abnormal proteins expression may produce a cell that works in an abnormal manner.
5.5 How Mutations Will Happen in the Cells
Mutations can happen by chance when a cell is in the process of division at the DNA level. This may occur either by the improper processes of metabolism inside the cell such as oxidative stress or by carcinogens and mutagens from outside the body, such as the chemicals in processed food, tobacco smoke, chronic alcoholism etc. In some cases, people will inherit activated oncogenes, in particular, the genes that make them more likely to prone to varieties of cancer. Some genes in the cells get damaged every day and cells are very good at repairing them. Over time, however, the damages that may accumulate may lead to the cells to start growing too fast; as a process, they are much prone to pick up additional mutations and less likely to be able to repair of these damaged genes.
5.6 What Are Cancer and Cancer Immunotherapy?
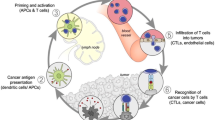
Cancer is usually defined as uncontrolled and mutated cells that aggregate at a point and are responsible for relentless cell proliferation. Tumours of cancers grows continuously and over time to escape from the recognise and in always make themselves to get a relief in continues battle with immune system (Kim et al., 2007). The biggest challenge to the modern technology is that the random behaviour of mutations in cells of cancers can even lead to high malignancy within the cells of the tumours. Due to this higher malignancy and the unstable mechanisms of immune response and against mutant cells still unknown and undetected (de Titta et al., 2013; Dagogo-Jack & Shaw, 2018). Therefore, a mechanism to target different cancer mutants and escape the behaviour of the immune system are as yet undefined Law, 1991). Therefore, cancer cells belong to the normal cells and normal cells releases a number of waste and unwanted products. These waste products also carry tumour-specific antigens and resist the parent tumour cells in recognition of cytotoxic T cells (Fig. 5.1).
Figure has been taken from the World Health Organization observatory of year 2020, collective research of worldwide cancer cases (Fig. 5.2).
Anticancer vaccines have been developed which allow the immune system to identify the tumours and enhance the immune response for a particular antigen such as tumours. This therapy is also known as immune therapy which has its main focus of training the immune system with releasing many components at a time (Rosenberg et al., 2004). These immunotherapies are antigen specific in their characteristics (Fig. 5.3).
Figure shown number of deaths by cancer in both females and males over the time/year (Siegel et al., 2021)
Vaccines were made to exercise control over the spreading of diseases all over the world. These vaccines work upon specific infections to accelerate immunity in that person. To date, vaccines have achieved benchmarks in health sciences, leading even in many cases to the complete eradications of some communicated diseases (Rosenberg et al., 2004; Guo et al., 2013) (Fig. 5.4).
Figure showing the number of deaths increasing in males and females up to the year 2018 (Siegel et al., 2021)
To date, vaccine research has achieved high degree of success in terms of both antiviral and antibacterial vaccines. Researchers have been continuously trying to make anticancer vaccines, but the biggest challenge for researchers has been the low immunogenicity of tumours. The main problem with tumours is that they arise from the healthy cells, and they are unable to accelerate immune responses. By 2010, the USA has already approved vaccines for cancer therapy with success rates of cancer therapies. Nanoparticles are highly advanced tools to cure diseases such as cancer. As traditional methods are not much enough effective upon tumours because of their low immunogenicity (Fang et al., 2015) (Figs. 5.5 and 5.6).
Figure describing heterogeneity in cancer in different patients (Kemp & Kwon, 2021)
Figure shown detection methods of cancers (Kemp & Kwon, 2021)
Therefore, the mechanism to treat tumours by immune response is non-specific. To attain specific target therapies, genetic engineering is the most favourable approach in cancer treatment therapies (Fesnak et al., 2016; Brudno & Kochenderfer, 2018). Chimeric antigen receptors isolated from donor or patient of leukapheresis (Rosenberg et al., 2008). In these techniques, the modifications can be done in the cells to identify tumour-associated antigens. These tumour-associated antigens are capable of recognition and deletion of cancerous cells.
5.6.1 The Current Status of Vaccines and Cancers
Cancer vaccines are made to reduce the tumour formation and their negative impacts over the immune system. If we are comparing traditional therapies with modern technology such as nanovaccines, they are specialized to work upon a specific target, unlike traditional therapies. The Food and Drug Administration of United States has already given approval to the anticancer vaccines in therapies in April 2010. These approvals were involved in the treatment of prostate cancer with sipuleucel-T (Cheever & Higano, 2011) (Fig. 5.7).
Figure showing how nanovaccines differ from traditional methods (Kemp & Kwon, 2021)
These kind of therapies involved prostate cancers as described earlier, involving the treatment of dendritic cells were isolated and exposed with the prosthetic acid phosphatase. This acid phosphatase was significantly observed in a number of patients of prostate cancer (Graddis et al., 2011).
The isolated cells from prostate cancer patients were injected again to the patients after exposing with granulocytes with macrophages. It was observed in the patient that the survival time was up to 4.1 months than other untreated cases. Therefore, the government had to approve this method of treatment (Cheever & Higano, 2011) (Fig. 5.8).
Even now, there are more than 200 active trials for cancer therapies. One of the most recent examples of these nanovaccines is glioblastoma or oncolytic ovarian cancer and also the recurrence of breast cancers with dendritic cell therapies.
5.6.2 Nanovaccines and Their Advantages
Nanotechnology has various applications in different fields such as food, waste treatment and environment etc., but medical sciences also need these advanced and emerging techniques to interlink different pathways that are unknown. Nanotechnologies are opening doors for many opportunities in cancer research. Unfortunately, the very low efficacy rate of traditional technologies in cancer therapies, nanotechnology can boost various research platforms (Fischer et al., 2013) (Fig. 5.9).
Figure showing conventional therapies, their side effects and the urgency of cancer nanovaccines (Kemp & Kwon, 2021)
The presence of flexibilities in nanotechnology can stimulate various components in cells such as proteins, polysaccharides, hormones, antibodies, adjuvants and many more. These nanoparticles are encapsulated particularly in biological components such as membranes, cells and proteins for targeting the specific site in the cell. There are some special chemicals that are involved in coating nanoparticles such as calcium phosphate etc. (Tam et al., 2016).
Therefore, nanocarriers are available to carry both antigen and adjuvants at same time (Tam et al., 2016). Nanoparticle-based vaccines are extremely nanosized in nature, which is why they can better design to enhance immune response, decrease the level of immunosuppression and target specificity. As they have narrow-sized characteristics so they can flow in lymph nodes and can achieve drug delivery in immune cells (Bachmann & Jennings, 2010; Shannahan et al., 2015; Reddy et al., 2007).
Nanoformulation can be undertaken in order to maximise biological activity to the payloads and increase direct penetration of cell membranes, Toll -ike receptors and surface molecule by coated with nanoparticles (Fig. 5.10).
Selection criteria based upon surface chemistry for formulations of nanovaccines in cancer therapies (Kemp & Kwon, 2021)
5.6.3 Nanoparticles Incorporated with Cancer Vaccines
5.6.3.1 Lack of Specificity
There are many immunomodulators based upon nanoparticles; these are the so-called non-specific because they can boost the immune system but are not target-specific in nature. These vaccines are not considered as vaccines due to their non-specificity and only rely upon the immune system of the patient, including tumour recognition and antigen presentation. Thus, the process can be attained by manipulations in immune response through a reduction in immunosuppression and an enhancement of the potential against cancer cells (Wei et al., 2018).
5.6.3.2 Enhancement of Physical Proximity of Immune Cells
The main motive in enhancing the immune system of the patient and the elimination of the tumour cells from his or her body through specific targeting . In this technique the nanoparticles are used to design and in association of two antibodies take place. These two antibodies are being directed; one is to target the immune response to accelerate and other is to target tumour cells. These phenomena not only enhance immune system but are also helpful in releasing tumour antigens and antigen presentation. For example, nanoparticles coated with biodegradable components such as poly lactic to load antibodies which works against CD40+ markers that are co-stimulators of dendritic cells markers and HER2. HER2 are human epidermal growth factors that are commonly present in its overexpression in breast cancer patients (Dominguez & Lustgarten, 2010; Ashwini & Kumar, 2011).
5.6.3.3 Immunosuppression Reduction
We discussed earlier that immunosuppression is quite necessary in cases like autoimmune diseases. In some cases, it can be defined as being like the early stages of melanoma cancer vaccines are extremely effective in melanoma-specific antibodies. The specificity does not lead to effective treatment in the later stages of melanoma. This specificity is not retained in the later stages because of the increased level of immunosuppression of cytokines such as tumour factors TGF (Xu et al., 2013).
To overcome this condition, nanoparticles are coated with lysosome protamine-hyaluronic acid. In such cases, drug delivery are made to reduce TFGβ tumour effects with siRNA (Xu et al., 2013). But these nano-based drug deliveries are not so simple; it has many side effects to reduce immunosuppression such as inflammation and the blockages of different pathways such as PD1 (Mullard, 2013). One study suggests that these nano-based drug deliveries are also effective in antibodies that work against death ligand known as PDL1 (Zhu et al., 2014).
5.6.3.4 Activation of Immune System
Pathogen-associated molecular patterns are those molecules which are responsible for boosting the immune system of the patient. There are some other factors, such as co-stimulatory markers, signalling proteins and cytokines which are used to enhance the mechanisms in immune responses. It has been found that extremely nonspecific and strong modulator called adjuvants are used in the treatment of cancer. PAMs are mainly involved lipopolysaccharides, double-stranded RNA and single-stranded DNA and helpful in the activation of immune responses in cancer. Polysaccharides are involved in the mechanism of suppressing the effect of inflammation by the immune system and are recognised by toll-like receptors. These PAMs are recognised by TLR9 and patterns that are being recognized are CpG oligonucleotides by endosomal TLR9 (de Titta et al., 2013; Chinnathambi et al., 2012).
5.6.3.5 Combination of Immunosuppressive Intervention and Immune Activation
There are a number of different ways to enhance the immune system and its activation but combining immunosuppressive intervention with it can yield highly favourable results. It can be explained with an example for visualizing the efficacy of the immune response in a balanced for with Th1 and Th2 cytokines by mimicking the pathogenic behaviour of nanoparticles when combined with CpG ODN and si RNA for IL10 (Pradhan et al., 2014). There is an another example to explain it encapsulation of T cells in nanoparticles for targeting PDI (T cell expressing cells) for activation of R848 (Schmid et al., 2017). This technique can further explain the enhancement of the immune system as well as immunosuppressive intervention by achieving dual targeting nanoparticles which carry both antagonistic and agonistic antibodies on a same surface (Kosmides et al., 2017).
5.6.3.6 Nanoparticles Combining with Traditional Methods of Cancer Treatment
Some studies suggest that traditional therapies rely completely upon the tumour activation of the immune system in such cases as combine traditional methods with nanoparticles and relying of them onto tumour cells can give an accelerated response to new technology. In terms of decreasing the immune activation, nanoparticles have advantages over tumours. For example, potato virus X clone can be used to deactivate the growth of B16-F10 cancer cells (Lee et al., 2017). So-called sticky nanoparticles are being designed to recognise and capture antigens that are available for phagocytosis for immune cells (Min et al., 2017) (Fig. 5.11).
Figure shown mechanism of cancer nanovaccines as immunomodulators and targeting tumours (Kemp & Kwon, 2021)
5.6.4 Need for Vaccines with Specificity
The main purpose in combining nanoparticles with cancer therapies is to achieve the target of designing and applicable cancer vaccines with specificity. The already present vaccines are not so sufficient to downstream the immunosuppression and low impact on tumours. The ultimate goal of the nanoparticle-based vaccines for cancer must be specific for the tumours, and contains targeted antibodies and adjuvants. These cancer vaccines are found to be more effective; they have cross-representation and their dual targeting nature has an enhanced ability in terms of immune response. The characteristics of nano-based vaccines in cancer treatment are detailed below:
-
I.
Entry in cytosol.
-
II.
Targeting immune cells.
-
III.
Dual deliver/co-delivery of adjuvants and antibodies.
- IV.
(a) and (b) representing boron dipyrromethene nanoparticles (c) and (d) representing phytotoxicity and immune efficacy of tumour cells (Kemp & Kwon, 2021)
(a) Various types of adjuvants, (b) nanovaccines for representing APCs. (c) drug delivery by nanovaccines (d) entry of nanovaccines in cystosol (Wei et al., 2018)
5.6.5 Types of Nanovaccines for Cancer Therapy (Fig. 5.14)
Types of nanoparticles used in nanovaccines for cancer therapies (Kemp & Kwon, 2021)
5.6.5.1 The Background of Nanovaccines for Anticancer Therapies
The treatment of diseases such as cancer offer never-ending challenges to medical researchers. New technologies are always being developed, but they are often slow to establish a secure footing. Nanovaccines are an advanced technology that can have offer many more applications in the field of cancer treatment if they are combined with an appropriate target molecule. Some of the previous studies have shown that both non-specificity and immunosuppression can play a huge part in cancer therapies. Upgrading treatment from specific to non-specific and reduction of immunosuppression has indeed demand of the immune system. Enhancing the immune response with target-based cell membranes of nanoparticles offer an advance in technology and highly acceptable in future perspectives. For example, RBC-coated cells are used with nanoparticles for an enhancement of immune clearance (Hu et al., 2011) (Fig. 5.15).
Usage of RBCs for antibacterial nanovaccines (Wei et al., 2018)
The cell-mimicking abilities of nanoparticles are used to transfer proteins of cell membranes onto the other membranes on the same surface (Hu et al., 2013; Angsantikul et al., 2018). Another example is to be found in the cases of nanoparticles coated with platelets to reduce bacterial vasculatures (Wei et al., 2018; Angsantikul et al., 2018; Plotkin, 2014). In another example WBCs are coated with nanoparticles to release toxins and remove infections such as sepsis (Gao et al., 2015; Thamphiwatana et al., 2017; Angsantikul et al., 2015).
5.6.5.1.1 Nanoparticles Coated with Cell Membrane as Antibacterial Vaccine
Vaccines can be defined as the world’s most effective and efficient way to resist infection and enhance the capabilities of the immune system. With the help of vaccines it has now become a simple task to combat and overcome many infectious diseases (Angsantikul et al., 2018). There is an effective form of vaccines which is known as toxoids vaccines. These vaccines are available in the health sector to kill infections such as tetanus and diphtheria (Mendoza et al., 2009). In the strategies of making antibacterial vaccines are meant for reduction of virulent protein present in the bacteria that is not capable of causing infection but able to generate immune system. These vaccines are made by reducing harmful virulent proteins, either by treating with harsh chemicals or through the heat treatment of proteins. When nanoparticles are mixed with bacterial proteins of RBC, which have the ability to neutralise bacterial toxins called nanosponges (Angsantikul et al., 2018; Gao et al., 2015).
Staphylococcus aureus is also used in making vaccines against bacteria MRSA infection. In this strategy, bacterial toxins are reduced through the use of heat treatment to denature the toxins of the proteins and make them nanotoxoids (Rosenberg et al., 2008; Plotkin, 2014). Outer membrane vesicles (OMVs) are also used in antibacterial vaccines, for example, sometimes E. coli is coated with nanoparticles of gold, which makes it appropriate for further use (Wei et al., 2018).
5.6.5.2 Anticancer Vaccines with Nanoparticles Coated by Cell Membrane
As we have seen, antibacterial vaccines has achieved a great success all across the world. In the case of cancer, the same scenario hadn’t predicted and not even more successful targets attained. IN recent studies, RBC have become a source of anticancer vaccines and came to the attention of researchers. Accordingly, anticancer vaccines loaded with nanoparticles are becoming the major area of research nowadays (Wang et al., 2015; Xu et al., 2013; Angsantikul et al., 2018). This platform of nanoparticles can be further modified with mannose to allow the surface targeting of dendritic cells. This surface target can enhance the immune response as well as the draining of lymph nodes. In the case of cancer cell membranes of autologous tumours contain plethora, which can be used as the nanoparticles coated cell membrane in anticancer activity. Initially, it was observed in B16-F10 melanoma. These melanoma membranes coated with nanoparticles are significant in enhancing the stimulation of T cells for antigen presentation and are also helpful in maturing dendritic cells from bone marrow (Gao et al., 2015) (Fig. 5.16).
It can also be defined as both an anticancer response, as in the case of antibacterial vaccines and targeting ligand, may work together and led to the artificial stimulation of the immune response. These cell membrane-coated nanoparticles work in a manner like the artificial generation of the immune response with target specificity (Zhu et al., 2014; Kang et al., 2018; Rosenberg, 2014; Rabinovich et al., 2007).
5.7 Conclusion
In this review the authors tried to explain different applications of the nanoparticles in treatment of diseases like cancer. Also, in this review authors tried to summarise all the factors and challenges that can affect the pathways and delivery system of cancer therapies. Therefore, it is extremely difficult to achieve a target drug delivery system and lower the immunogenicity with respect to time. Tumours can generate an immune response but can also lead to immunosuppression because tumours are arising from the same cells of the body. Nanovaccines can lead to a revolution in the field of cancer research with highly specific target attacking and the generation of an immune response. Nanovaccines have already achieved huge success in antibacterial vaccines in a similar manner, nanoparticle-coated cell membranes are principally effective with regard to tumours and T cells.
Furthermore, nanoparticles are becoming a boon to the medical science through their specific ability to recognise tumours and dual target abilities and antibodies-adjuvants loading characteristics. In this review the authors tried to explain the need for nanovaccines to be used in cancer treatment. By comparing nanovaccines with traditional medicines, nanoparticles are not non-specific, highly effective, and mainly work at dual targeting at a time. It is therefore clear that future studies will be required to developed the use of these nanovaccines as anti-cancer vaccines in an innovative fashion.
References
Angsantikul, P., Thamphiwatana, S., Gao, W., & Zhang, L. (2015). Cell membrane-coated nanoparticles as an emerging antibacterial vaccine platform. Vaccine, 3(4), 814–828. https://doi.org/10.3390/vaccines3040814
Angsantikul, P., Fang, R. H., & Zhang, L. (2018). Toxoid vaccination against bacterial infection using cell membrane-coated nanoparticles. Bioconjugate Chemistry, 29(3), 604–612. https://doi.org/10.1021/acs.bioconjchem.7b00692
Ashwini, K., Kumar, G., & Bhaskara Rao, K. V. (2011). Optimization, production and partial purification of extracellular α-amylase from Bacillus sp. marini. Archives of Applied Science Research, 3(1), 33–42. [Online]. Available: www.scholarsresearchlibrary.com
Bachmann, M. F., & Jennings, G. T. (2010). Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nature Reviews. Immunology, 10(11), 787–796. https://doi.org/10.1038/nri2868
Brudno, J. N., & Kochenderfer, J. N. (2018). Chimeric antigen receptor T-cell therapies for lymphoma. Nature Reviews. Clinical Oncology, 15(1), 31–46. https://doi.org/10.1038/nrclinonc.2017.128
Cheever, M. A., & Higano, C. S. (2011). PROVENGE (Sipuleucel-T) in prostate cancer: The first FDA-approved therapeutic cancer vaccine. Clinical Cancer Research, 17(11), 3520–3526. https://doi.org/10.1158/1078-0432.CCR-10-3126
Chinnathambi, S., Chen, S., Ganesan, S., & Hanagata, N. (2012). Binding mode of CpG oligodeoxynucleotides to nanoparticles regulates bifurcated cytokine induction via toll-like receptor 9. Scientific Reports, 2, 534. https://doi.org/10.1038/srep00534
Dagogo-Jack, I., & Shaw, A. T. (2018). Tumour heterogeneity and resistance to cancer therapies. Nature Reviews. Clinical Oncology, 15(2), 81–94. https://doi.org/10.1038/nrclinonc.2017.166
de Titta, A., et al. (2013). Nanoparticle conjugation of CpG enhances adjuvancy for cellular immunity and memory recall at low dose. Proceedings of the National Academy of Sciences of the United States of America, 110(49), 19902–19907. https://doi.org/10.1073/pnas.1313152110
Dominguez, A. L., & Lustgarten, J. (2010). Targeting the tumor microenvironment with anti-neu/anti-CD40 conjugated nanoparticles for the induction of antitumor immune responses. Vaccine, 28(5), 1383–1390. https://doi.org/10.1016/j.vaccine.2009.10.153
Fang, R. H., Kroll, A. V., & Zhang, L. (2015). Nanoparticle-based manipulation of antigen-presenting cells for cancer immunotherapy. Small, 11(41), 5483–5496. https://doi.org/10.1002/smll.201501284
Fesnak, A. D., June, C. H., & Levine, B. L. (2016). Engineered T cells: The promise and challenges of cancer immunotherapy. Nature Reviews. Cancer, 16(9), 566–581. https://doi.org/10.1038/nrc.2016.97
Fischer, N. O., Rasley, A., Corzett, M., Hwang, M. H., Hoeprich, P. D., & Blanchette, C. D. (2013). Colocalized delivery of adjuvant and antigen using nanolipoprotein particles enhances the immune response to recombinant antigens. Journal of the American Chemical Society, 135(6), 2044–2047. https://doi.org/10.1021/ja3063293
Gao, W., et al. (2015). Modulating antibacterial immunity via bacterial membrane-coated nanoparticles. Nano Letters, 15(2), 1403–1409. https://doi.org/10.1021/nl504798g
Graddis, T. J., McMahan, C. J., Tamman, J., Page, K. J., & Trager, J. B. (2011). Prostatic acid phosphatase expression in human tissues. International Journal of Clinical and Experimental Pathology, 4(3), 295–306.
Guo, C., Manjili, M. H., Subjeck, J. R., Sarkar, D., Fisher, P. B., & Wang, X.-Y. (2013). Therapeutic cancer vaccines: Past, present, and future. Advances in Cancer Research, 119, 421–475. https://doi.org/10.1016/B978-0-12-407190-2.00007-1
Hu, C.-M. J., Zhang, L., Aryal, S., Cheung, C., Fang, R. H., & Zhang, L. (2011). Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proceedings of the National Academy of Sciences of the United States of America, 108(27), 10980–10985. https://doi.org/10.1073/pnas.1106634108
Hu, C.-M. J., Fang, R. H., Luk, B. T., & Zhang, L. (2013). Nanoparticle-detained toxins for safe and effective vaccination. Nature Nanotechnology, 8(12), 933–938. https://doi.org/10.1038/nnano.2013.254
Kang, T., et al. (2018). Necroptotic cancer cells-mimicry nanovaccine boosts anti-tumor immunity with tailored immune-stimulatory modality. Biomaterials, 164, 80–97. https://doi.org/10.1016/j.biomaterials.2018.02.033
Kemp, J. A., & Kwon, Y. J. (2021). Cancer nanotechnology: Current status and perspectives. Nano Convergence, 8(1), 34. https://doi.org/10.1186/s40580-021-00282-7
Kim, R., Emi, M., & Tanabe, K. (2007). Cancer immunoediting from immune surveillance to immune escape. Immunology, 121(1), 1–14. https://doi.org/10.1111/j.1365-2567.2007.02587.x
Kosmides, A. K., Sidhom, J.-W., Fraser, A., Bessell, C. A., & Schneck, J. P. (2017). Dual targeting nanoparticle stimulates the immune system to inhibit tumor growth. ACS Nano, 11(6), 5417–5429. https://doi.org/10.1021/acsnano.6b08152
Law, S. K. (1991). Antigen shedding and metastasis of tumour cells. Clinical and Experimental Immunology, 85(1), 1–2. https://doi.org/10.1111/j.1365-2249.1991.tb05672.x
Lee, K. L., et al. (2017). Combination of plant virus nanoparticle-based in situ vaccination with chemotherapy potentiates antitumor response. Nano Letters, 17(7), 4019–4028. https://doi.org/10.1021/acs.nanolett.7b00107
Mendoza, N., Ravanfar, P., Satyaprakash, A., Pillai, S., & Creed, R. (2009). Existing antibacterial vaccines. Dermatologic Therapy, 22(2), 129–142. https://doi.org/10.1111/j.1529-8019.2009.01225.x
Min, Y., et al. (2017). Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nature Nanotechnology, 12(9), 877–882. https://doi.org/10.1038/nnano.2017.113
Mullard, A. (2013). New checkpoint inhibitors ride the immunotherapy tsunami. Nature Reviews Drug discovery, 12(7), 489–492. https://doi.org/10.1038/nrd4066
Plotkin, S. (2014). History of vaccination. Proceedings of the National Academy of Sciences of the United States of America, 111(34), 12283–12287. https://doi.org/10.1073/pnas.1400472111
Pradhan, P., et al. (2014). The effect of combined IL10 siRNA and CpG ODN as pathogen-mimicking microparticles on Th1/Th2 cytokine balance in dendritic cells and protective immunity against B cell lymphoma. Biomaterials, 35(21), 5491–5504. https://doi.org/10.1016/j.biomaterials.2014.03.039
Rabinovich, G. A., Gabrilovich, D., & Sotomayor, E. M. (2007). Immunosuppressive strategies that are mediated by tumor cells. Annual Review of Immunology, 25, 267–296. https://doi.org/10.1146/annurev.immunol.25.022106.141609
Reddy, S. T., et al. (2007). Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nature Biotechnology, 25(10), 1159–1164. https://doi.org/10.1038/nbt1332
Rosenberg, S. A. (2014). IL-2: The first effective immunotherapy for human cancer. Journal of Immunology, 192(12), 5451–5458. https://doi.org/10.4049/jimmunol.1490019
Rosenberg, S. A., Yang, J. C., & Restifo, N. P. (2004). Cancer immunotherapy: Moving beyond current vaccines. Nature Medicine, 10(9), 909–915. https://doi.org/10.1038/nm1100
Rosenberg, S. A., Restifo, N. P., Yang, J. C., Morgan, R. A., & Dudley, M. E. (2008). Adoptive cell transfer: A clinical path to effective cancer immunotherapy. Nature Reviews. Cancer, 8(4), 299–308. https://doi.org/10.1038/nrc2355
Rosenblum, M. D., Remedios, K. A., & Abbas, A. K. (2015). Mechanisms of human autoimmunity. The Journal of Clinical Investigation, 125(6), 2228–2233. https://doi.org/10.1172/JCI78088
Schmid, D., et al. (2017). T cell-targeting nanoparticles focus delivery of immunotherapy to improve antitumor immunity. Nature Communications, 8(1), 1747. https://doi.org/10.1038/s41467-017-01830-8
Shannahan, J. H., Bai, W., & Brown, J. M. (2015). Implications of scavenger receptors in the safe development of nanotherapeutics. Receptors & Clinical Investigation, 2(3), e811. https://doi.org/10.14800/rci.811
Siegel, R. L., Miller, K. D., Fuchs, H. E., & Jemal, A. (2021). Cancer statistics, 2021. CA: a Cancer Journal for Clinicians, 71(1), 7–33. https://doi.org/10.3322/caac.21654
Tam, H. H., et al. (2016). Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination. Proceedings of the National Academy of Sciences of the United States of America, 113(43), E6639–E6648. https://doi.org/10.1073/pnas.1606050113
Thamphiwatana, S., et al. (2017). Macrophage-like nanoparticles concurrently absorbing endotoxins and proinflammatory cytokines for sepsis management. Proceedings of the National Academy of Sciences of the United States of America, 114(43), 11488–11493. https://doi.org/10.1073/pnas.1714267114
Wang, L., Wang, F.-S., & Gershwin, M. E. (2015). Human autoimmune diseases: a comprehensive update. Journal of Internal Medicine, 278(4), 369–395. https://doi.org/10.1111/joim.12395
Wei, X., et al. (2018). Nanoparticle functionalization with platelet membrane enables multifactored biological targeting and detection of atherosclerosis. ACS Nano, 12(1), 109–116. https://doi.org/10.1021/acsnano.7b07720
Xu, Z., Ramishetti, S., Tseng, Y.-C., Guo, S., Wang, Y., & Huang, L. (2013). Multifunctional nanoparticles co-delivering Trp2 peptide and CpG adjuvant induce potent cytotoxic T-lymphocyte response against melanoma and its lung metastasis. Journal of Controlled Release, 172(1), 259–265. https://doi.org/10.1016/j.jconrel.2013.08.021
Zhu, M., Wang, R., & Nie, G. (2014). Applications of nanomaterials as vaccine adjuvants. Human Vaccines & Immunotherapeutics, 10(9), 2761–2774. https://doi.org/10.4161/hv.29589
Weblink
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jagadeesh Chandra Bose, K., Sarwan, J. (2023). Emerging Vaccine for the Treatment of Cancer Via Nanotechnology. In: Pal, K. (eds) Nanovaccinology. Springer, Cham. https://doi.org/10.1007/978-3-031-35395-6_5
Download citation
DOI: https://doi.org/10.1007/978-3-031-35395-6_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-35394-9
Online ISBN: 978-3-031-35395-6
eBook Packages: MedicineMedicine (R0)