Abstract
The autonomic nervous system consisting of two limbs, the sympathetic and parasympathetic nervous system, plays a key role in the modulation of various cardiac arrhythmias. Neurotransmitters, including catecholamines and acetylcholine, as well as other co-transmitters mediate supraventricular and ventricular arrhythmogenesis through distinctive electrophysiologic mechanisms. The intricate autonomic interplay depicts that most arrhythmias arise from sympathetic overactivity and parasympathetic attenuation, with the sympathetic nervous system mitigating the effects of antiarrhythmic drugs. While this remains true for adrenergic-driven arrhythmias such as atrial fibrillation, post-infarct ventricular tachycardia, and some long QT syndromes 1 and 2, parasympathetic nervous overactivity may act as a trigger for vagotonic arrhythmias including Brugada syndrome, long QT syndrome 3, and idiopathic ventricular fibrillation. Knowledge of the neuro-cardiac hierarchies and awareness of different methods for assessment of autonomic tone, combined with promising results from animal studies, has established a clear rationale for clinical trials of various autonomic neuromodulatory interventions, which have had varying success for defined arrhythmias. Whereas cardiac sympathetic denervation imparts clear therapeutic benefits in patients with long QT syndrome 1 and polymorphic catecholaminergic ventricular tachycardia, the impact of vagus nerve stimulation in heart failure patients is less defined. Sustainable and relatable therapeutic outcomes from future clinical trials of neuromodulatory interventions dictate conscientious patient selection, optimized study protocols, and the use of reproducible biomarkers. Novel tools such as optogenetics present an exciting direction in the future for noninvasive neuromodulatory interventions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Autonomic neuromodulatory interventions
- Arrhythmogenesis
- Autonomic neurotransmitters
- Neuro-cardiac axis
1 Introduction
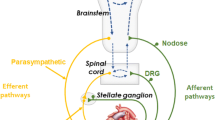
The autonomic nervous system (ANS) is composed of two limbs, the sympathetic (SNS) and parasympathetic nervous system (PNS), each exerting opposing effects in modulating arrhythmogenesis [1]. The ANS contributes to various supraventricular and ventricular arrhythmias through distinctive pro- and antiarrhythmic mechanisms. Intricate interaction occurs between the SNS and PNS at each anatomical hierarchy, from the central nervous system (brain and spinal cord) and intrathoracic extracardiac ganglia (stellate ganglia, dorsal root ganglia) to intrinsic cardiac nerves and ganglia, thereby enabling the many possibilities for autonomic interventions to modulate arrhythmogenesis in both atria and ventricles. The effect of arrhythmic triggers by the SNS and PNS are unique to each type of arrhythmia. At the atrial level, atrial fibrillation (AF) can be triggered by both the SNS and PNS [2]. At the ventricular level, SNS overactivation is the hallmark of most arrhythmias that occur with myocardial ischemia and myocardial infarction (MI), long QT syndrome (LQTS), and catecholaminergic polymorphic ventricular tachycardia (CPVT), including benign ventricular premature beats, whereas the PNS confers protective effects against such arrhythmias [3]. On the other hand, the PNS contributes to ventricular arrhythmias in certain cardiac channelopathies (Table 9.1).
In this chapter, the pathophysiological properties of SNS and PNS neurotransmitters will be discussed, linking their effects with various supraventricular and ventricular arrhythmias through distinctive mechanisms. The chapter proceeds to describe each of these arrhythmias, followed by discussion of up-to-date evidence-driven autonomic interventions applicable to the relevant arrhythmias. This is followed by outlining the current tools available for assessment of autonomic tone. And finally challenges of current autonomic interventions and potential future directions will be considered.
2 Autonomic Neurotransmitters and Their Electrophysiological Properties
2.1 Role of Autonomic Neurotransmitters in Arrhythmogenesis
The ANS exerts its effect on inducing and maintaining arrhythmias through the now well-known neurotransmitters, catecholamines (SNS) and acetylcholine (PNS). Briefly, SNS and PNS exert opposing effects on heart rate (chronotropy) and conduction through the atrioventricular node (dromotropy). While SNS stimulation leads to positive chronotropy and dromotropy, PNS stimulation results in negative chronotropy and dromotropy (i.e., slowing of sinus rate and atrioventricular (AV) node conduction). Sympatho-vagal interaction studies, however, revealed a phenomenon known as accentuated antagonism characterized by vagal dominance in both chronotropy and dromotropy [4]. In contrast, this phenomenon was absent at the ventricular level in terms of ventricular repolarization and ventricular fibrillation (VF) threshold during direct sympatho-vagal stimulation [5].
Recently SNS co-transmitters, notably neuropeptide Y (NPY) and galanin, were identified in association with certain arrhythmic states and play an important role in attenuating the chronotropic effect of the PNS. Both NPY and galanin reduce acetylcholine release, thereby attenuating vagal bradycardia through their corresponding receptors (NPY2, GalR1) located on the postganglionic vagal neurons projecting to the sinus node [6, 7]. At the ventricular level, NPY1 receptors found on cardiomyocytes exert pro-arrhythmic effects through the putative mechanism of increasing calcium transient amplitude and diastolic calcium sparks [7]. Indeed, in a study comprising 78 patients who underwent percutaneous coronary interventions, 8% experienced ventricular arrhythmias within 48 h, all of whom had noticeably elevated levels of NPY [8]. In an experimental ischemic and reperfusion model, NPY reduced VF threshold, leading to an increased incidence of ventricular tachycardia (VT, 60%) and VF (10%), the effect of which was nullified by an NPY1-receptor antagonist. Elsewhere, NPY receptors were also found in coronary vessels, mediating a vasoconstrictor effect via NPY5 receptors.
2.2 SNS-Mediated Arrhythmic Triggers
SNS and its circulating catecholamines (i.e., epinephrine and norepinephrine) account for the induction and triggering of many arrhythmias (Table 9.1) via different electrophysiological mechanisms (Table 9.2). SNS overactivity is accountable for most ventricular arrhythmias, notably LQTS, CPVT, and those associated with myocardial ischemia, mediated through beta-adrenoreceptors and cyclic-AMP/protein-kinase-dependent signaling pathways. On one hand, circulating catecholamines increase the rate of spontaneous depolarization, resulting in autonomic tachyarrhythmias. On the other hand, the heterogenous spatial distribution of sympathetic nerve endings may result in non-uniform repolarization, promoting reentrant tachycardias. Under certain conditions such as ischemia, hypoxia, or electrolyte imbalance, cardiomyocytes become more susceptible to the pro-fibrillatory effect of catecholamines [9]. It has been postulated that occurrence of VF in early ischemia is driven by the local metabolic effect of norepinephrine as opposed to a centrally induced effect [9]. In this respect, pharmacological agents with a beta-blockade effect are effective in antagonizing some of these arrhythmias precipitated by SNS overactivity. In certain refractory cases, cervical sympathetic denervation (CSD) presents an effective therapeutic option [10]. Table 9.3 outlines other physiological states, medical conditions, and drugs associated with SNS overactivity.
2.2.1 Physical Stress/Exercise
The health-enhancing effect of regular moderate exercise is well established in both normal individuals and those with cardiovascular diseases. A strategy of gradual increments in exercise intensity may enhance the predominance of the PNS, conferring cardioprotection. Conversely, SNS overactivity occurs during intense and strenuous exercise, conferring potentially adverse or even detrimental effects in provocation and exacerbation of potentially malignant arrhythmias in individuals with evident or occult underlying cardiovascular abnormalities. Given the pro-arrhythmic effects of the acute surge of SNS activity and its circulating catecholamines, acute bouts of vigorous exercise should be avoided in these individuals. When high-intensity exercise is entertained in these cases, appropriate screening is recommended beforehand to avoid unnecessary risks of adverse cardiovascular and arrhythmic events [11].
2.2.2 Emotional Stress
SNS overactivity is an autonomic profile typified in acute and chronic psychological or mental stress among individuals with cardiovascular diseases, including various cardiac arrhythmias. It is associated with increased frequency and malignancy of ventricular arrhythmias. Anger, anxiety, sadness, and stress have been found to be precipitants for ventricular arrhythmias and AF in patients with implantable cardioverter-defibrillators (ICDs). In contrast, happiness appears to be protective against such arrhythmias [12]. It remains inconclusive whether psychological interventions, such as in the form of cognitive-behavioral therapy, may alleviate stress-induced arrhythmias in this context [13, 14].
2.2.3 Sleep Disorders
An optimal sleep duration of ~7 h/night may contribute to a balanced ANS profile. Disturbed sleep in the form of reduced sleep duration, low sleep efficiency, sleep-disordered breathing, and insomnia are associated with attenuated PNS tone and/or elevated SNS tone, thereby increasing susceptibility to arrhythmias [15,16,17]. Sleep disruptions have been associated with both AF and ventricular arrhythmias [17]. While acute sleep deprivation modifies ANS activity by increased SNS, chronic sleep deprivation produces a constant hyperadrenergic state associated with pro-arrhythmic consequences. In individuals with sleep-disordered breathing, sympathetic overactivity may be one of the pathological drivers for increased incidence of arrhythmias [15, 16].
2.3 PNS-Mediated Arrhythmic Triggers
Parasympathetic nerve fibers form synapses with ganglionic plexuses (GPs) on the epicardial surface of the heart, facilitating adaptive changes in neuronal activity, i.e., neuroplasticity to fine-tune the neuro-cardiac status [18]. However, maladaptive changes of PNS overactivity or attenuation are a source of arrhythmogenesis [19]. One classical example is vagally induced AF due to spatially heterogeneous shortening of atrial refractoriness (Table 9.2), forming the rationale for certain ablation strategies that constitute a degree of autonomic intervention [10]. Furthermore, Brugada syndrome and LQT3 have been attributed to vagotonia (Table 9.1). Yet, vagus nerve stimulation (VNS) was shown to reduce VT/VF inducibility in the setting of myocardial ischemia and infarct.
The contribution to arrhythmias by ANS goes beyond the balance between SNS and PNS activity. On its own, VNS exerts differential effects on the heart based on its tonic (resting) and phasic activation pattern [18, 20]. For example, resting sinus bradycardia is common and thought to be normal among the athletes due to high vagal tone. Whereas in individuals suffering from neurocardiogenic syncope (NCS), the vagus nerve activation pattern is phasic, provoking cardioinhibitory and/or vasodepressor response under certain conditions [21]. AF in athletes, however, may not be solely due to vagus nerve overactivity as other factors such as atrial stretch, inflammation, and fibrosis have been implicated [11]. Finally, the different hierarchy of SNS and PNS at the neuro-cardiac axis provides a recipe for spatial heterogeneity in the interaction between the two arms of ANS with the temporal variation in this interaction providing additional mechanistic intricacies in precipitating different arrhythmias [22, 23].
2.3.1 Neurocardiogenic Syncope (NCS)
NCS is the most common form of syncope manifesting as profound slowing of heart rate (cardioinhibitory component, Type II) and/or hypotension from vasodilation (vasodepressor component, Type III) [24]. In spite of potential manifestations as isolated asystole/bradycardia vs. hypotension, most NCS is in fact a mixed response (i.e., Type I). Type II NCS is precipitated by cardio-inhibition by increased vagal input to the sinus and/or AV node, contributing to profound bradycardia (<40 bpm for >10 s) or even asystole (Type IIb). Type III NCS characterized by a vasodepressor response is attributed to a decrease in sympathetically mediated peripheral vasoconstriction. NCS have several well-identified triggers including emotional stress and upright posture, as well as specific activity triggers such as cough, micturition, deglutition, and pain (i.e., situational syncope). Post-exercise rebound vagotonia has been described in healthy individuals whereby exercise-induced sympathetic activation is swiftly followed by a neuro-cardiac interaction of sympathetic withdrawal coupled with excessive parasympathetic overdrive at the end of the exercise [25].
Cardio-neuroablation aiming to eliminate postganglionic parasympathetic neurons in atrial wall and GPs has been advocated as an alternative to pacemaker intervention for patients with medically refractory NCS with dominant cardioinhibitory component [26].
2.3.2 Bradyarrhythmias with Long-Short Sequence
A resting sinus bradycardia is generally a reflection of a favorable autonomic profile with a dominant parasympathetic state, as observed in athletes. However, excessive parasympathetic activity has been implicated in debilitating arrhythmias, including extreme sinus bradycardia, sinus exit blocks, sinus and AV blocks, and cardioinhibitory NCS, all of which can lead to pre-syncopal and syncopal episodes with a risk of head injury [24].
Vagotonia-induced bradycardia has, in addition, been associated with certain extrasystole-induced arrhythmias through the mechanism of long-short sequence. These have been found in patients with paroxysmal AF, LQTS, and VT/VF [27]. The underlying arrhythmogenic mechanism of long-short sequence is functional conduction block and/or slowed conduction, resulting in functional reentry when there is differential transmural repolarization, typically with a greater degree of refractoriness at midmyocardial and endocardial sites compared to epicardial sites [27]. This phenomenon has been observed in patients with cardiac implantable electronic devices (CIEDs) when an overzealous lowering of the pacing rate leads to bradycardia, which facilitates pause-dependent VT/VF in addition to polymorphic VT in the context of Torsades-de-pointes (TdP) [28]. This insight forms the basis of a higher programmed pacing rate in the first few weeks following AV node ablation for rate control in drug-refractory fast AF. In patients presenting with complete heart block, the possibility of polymorphic VT sometimes necessitates insertion of a temporary pacemaker for overdrive pacing until a definitive permanent pacemaker is inserted [29]. Similarly, overdrive ventricular pacing also effectively prevents arrhythmia recurrences in patients with LQTS by abolishing VT/VF induced by long-short sequence. On the other hand, continuous atrial pacing at 75–80 bpm anecdotally prevents recurrence of AF in patients with CIEDs [30].
As such, parasympathetic-modulated bradycardias are not all benign, especially in view of potential bradycardia/pause-dependent VT/VFs. Although pacing interventions may circumvent these bradyarrhythmias, ANS modulatory interventions may avoid the use of pacemakers [26, 31]. It should be emphasized that bradycardia/pause-dependent arrhythmias are not exclusive to ANS dysfunction but can occur independently [28, 29].
3 Tachyarrhythmogenic States Associated with Autonomic Imbalance
The two limbs of ANS modulates various supraventricular and ventricular arrhythmias (Table 9.1) either through the dominant effect of one of the limbs or the convergent effect of autonomic interaction via different electrophysiologic effects (Table 9.2).
3.1 Supraventricular Arrhythmias
Supraventricular tachycardia (SVT) associated with SNS overactivity can be induced by abnormal automaticity or triggered activity, typically under physical and/or emotional stress (atrial tachycardia, AF), upright postures (postural orthostatic tachycardia syndrome), or in some cases without any obvious precipitant (inappropriate sinus tachycardia) [32]. Other SVTs (atrioventricular nodal reentrant tachycardia, atrioventricular reentrant tachycardia, atrial tachycardia), especially those involving the ANS-innervated AV node, rely on a reentrant mechanism when catecholamines expose differential conduction velocities in the critical part of the reentrant circuit [33].
3.1.1 Inappropriate Sinus Tachycardia (IAST) and Postural Orthostatic Tachycardia Syndrome (POTS)
IAST is a clinical condition characterized by nonparoxysmal palpitation manifesting as a sinus rate of >100 bpm at rest, and/or a mean 24-h heart rate of >95 bpm without any obvious cause, out of proportion to physiological need [21]. This condition is generally regarded as a reflection of a hypersympathetic state, leading to the concept of beta-blockade as the mainstay of treatment to achieve sympatholysis. In some cases, ivabradine, a selective HCN channel (If current) blocker—largely considered to be without any other cardiovascular effect apart from chronotropy—is an effective adjunct or alternative [34]. Other more invasive therapeutic interventions involving sinus-node modification through catheter ablation of atrial GPs or sympathetic denervation are not recommended for treatment of IAST [21].
POTS is a clinical syndrome manifesting with a myriad of symptoms including light-headedness, palpitations, tremor, fatigue, general weakness, exercise intolerance, and visual blurring when sinus rate increases by ≥30 bpm upon standing (≥40 bpm in individuals aged 12–19 years) [21]. The underpinning mechanisms of POTS are complex and have been postulated to include peripheral autonomic denervation, hyperadrenergic stimulation, deconditioning, hypovolemia, and/or hypervigilance. Management of POTS overlaps with that of IAST encompassing lifestyle modifications (increased fluid and salt intake, avoidance of certain drugs and dehydration, compression stockings, structured exercise) and pharmacological approaches (fludrocortisone, midodrine, propranolol, ivabradine, central sympatholytic agents) [21].
3.1.2 Atrial Tachycardia
ANS imbalance with hyperadrenergic state contributes to neural triggers of atrial tachycardia. This is supported by the observation that paroxysmal atrial tachycardia can be abolished with ablation of extrinsic sympatho-vagal nerves akin to the effect of abolishing paroxysmal AF with the same method [23]. Focal atrial tachycardias are catecholamine-sensitive, occurring as a result of increased automaticity and/or triggered activity as opposed to reentrant mechanism in certain atrial tachycardia/flutter [32]. As such, focal atrial tachycardias tend to manifest in catecholamine-driven states, e.g., during exercise and/or stressful conditions. A further corroboration arises from the induction of these atrial tachycardias by infusion of isoproterenol during an electrophysiology study [32].
3.1.3 Atrial Fibrillation
The pulmonary veins (PV) are anatomically linked to the left atrium (LA) through myocardial sleeves, the latter being extensions of atrial myocardium over the PVs. These myocardial sleeves are typically more prominent around the superior than the inferior PVs, containing not only cardiac myocytes but also rich innervation of autonomic nerves [35]. Indeed, co-located sympathetic and vagal nerves are at their highest densities within 5 mm of the PV-LA junction [36]. While there is emerging evidence suggesting that histological remodeling at the PV-LA junction contributes to the development of AF, the exact role of neural remodeling in AF initiation remains uncertain [20].
The atria are richly innervated by both intrinsic cardiac nerves and extracardiac autonomic nerves/ganglia, with remodeling potential under various conditions [36, 37]. In particular, the PVs and posterior LA wall demonstrate unique depolarization and repolarization patterns associated with heterogenous vagal activity during autonomic manipulation, contributing to both the triggers and substrate for AF [37]. In a canine model of sustained AF induced by prolonged right atrial pacing, significant nerve sprouting and sympathetic hyperinnervation were observed [38]. As such, selective stimulation or inhibition of these extracardiac autonomic neural structures may form attractive therapeutic targets for autonomic interventions of AF.
While abnormal neuro-cardiac innervation at the PV-LA junction and other parts of LA provide the trigger and substrate for AF, the role of autonomic imbalance in the modulation of AF is far more complex as AF itself may result in autonomic disturbance, thus explaining the maxim “AF begets AF” [22]. Vagotonia is regarded as a classical trigger for AF as seen in young, healthy individuals with lone AF, yet stressful situations like acute mental stress have been shown to induce a transiently adverse LA electrophysiological state and hence provide the perfect substrate for AF [39]. Furthermore, it is thought that the initiation of AF does not rely exclusively on vagal or adrenergic activation, but rather the complex interaction between SNS and PNS to achieve a certain level of autonomic imbalance [1]. It has been postulated that while PNS overactivity is the culprit of spontaneous AF initiation, circulating catecholamines provide the appropriate adrenergic tone to modulate the initiation and maintenance of vagally triggered AF [2].
Neuromodulation via low-level VNS (LLVNS) at voltages below the bradycardia threshold achieved both antiadrenergic and anticholinergic effects [40, 41]. In experimental models of AF [41] and postoperative AF patients [42], LLVNS has been shown to reduce AF inducibility and duration. Chronic LLVNS has been shown to be associated with reduced stellate ganglia activity, leading to improved rate control in persistent AF [40].
Endurance athletes undergoing extreme exercise may have maladaptation of their autonomic tone in addition to development of an arrhythmogenic atrial substrate (i.e., atrial fibrosis), driving the high prevalence of AF in this group of individuals [11]. Indeed, an arrhythmogenic right ventricular cardiomyopathy (ARVC)-like phenotype has been described in these individuals although the role of chronic SNS overactivity in this phenotype remains unestablished [11].
3.1.4 Atrioventricular Nodal Reentrant Tachycardia (AVNRT)
The AV node forms a critical part of the reentrant circuit in patients with AVNRT, and is a structure richly innervated by autonomic nerves exerting positive (SNS) or negative (PNS) dromotropic effect. The inducibility of AVNRT by catecholamines is attested by triggering of tachycardia in some patients during exercise and/or emotional stress, in addition to the occasional requirement for isoproterenol infusion during electrophysiology studies and ablation procedures to initiate AVNRT [43]. Furthermore, the reversal effect of antiarrhythmic drugs by adrenergic agents (e.g., isoproterenol) in patients with SVT suggests a key role for adjunctive use of beta-blockers [44].
Interestingly, transient inappropriate sinus tachycardia (IAST) has been noted following ablation of the AV node fast- or slow-pathway due to thermal injury to the parasympathetic fibers at the right septal region. This phenomenon has been postulated to be a consequence of radiofrequency ablation at the anterior, mid, and posterior aspects of the interatrial septum disrupting preganglionic or postganglionic parasympathetic fibers innervating the sinus node, thus producing parasympathetic denervation that accounts for the IAST [45].
3.1.5 Atrioventricular Reentrant Tachycardia (AVRT)
In patients with pre-excitation syndromes, including those who are asymptomatic, malignant arrhythmias can occur during states of increased sympathetic activity. Exercise and/or administration of exogeneous catecholamines may unmask high-risk features in patients with Wolff–Parkinson–White (WPW) syndrome (i.e., patients with manifest/antegrade accessory pathway conduction) [46, 47]. Thus, appropriate risk assessment should be performed in these patients including those with purportedly low-risk features at baseline (i.e., intermittent pre-excitation) with isoproterenol/epinephrine administration or an electrophysiology study. Indeed, the use of exercise traditionally as a noninvasive risk assessment tool has been called into question in one study [48]. In extreme cases, increased sympathetic activity such as exercise/emotional stress can accelerate antegrade conduction over the accessory pathway facilitating degeneration of pre-excited atrial tachyarrhythmias (atrial flutter/AF) into VF causing sudden cardiac death (SCD) [49].
Administration of exogeneous catecholamines has also been shown to partially or completely reverse the therapeutic effect of certain antiarrhythmic drugs in patients with pre-excitation syndromes already established on antiarrhythmic drugs, further cementing the requirement for adjunctive use of a beta-blocker in such patients [44, 50].
3.2 Ventricular Arrhythmias
ANS dysfunction plays a key role in modulating the initiation and maintenance of ventricular arrhythmias. Sympathetic overdrive and parasympathetic attenuation are the hallmark of increased risk of sudden arrhythmic death in the setting of channelopathies, ARVC, and structural heart diseases (SHD) such as MI and heart failure [1]. SNS overactivity provides the trigger (increased automaticity and triggered activity) and the substrate (reduced action potential duration and refractoriness, reduced VF threshold) necessary for initiation and maintenance of VT/VF. In conjunction, PNS attenuation exacerbates pro-fibrillatory effect through reduced refractoriness, augmentation of dispersion of repolarization, and reduced VF threshold. However, in certain arrhythmic syndromes such as the Brugada syndrome and LQT3, increased PNS is conversely pro-arrhythmic [51].
3.2.1 Channelopathies
The underlying arrhythmogenesis of congenital LQT1 and LQT2 is prolongation of the QT interval by sympathetic activation. In these patients, heightened sympathetic activity under certain circumstances leads to increased malignant ventricular arrhythmias with arrhythmogenic events among LQT1 occurring during exercise or stress, while those among LQT2 occur during emotional stress, such as provoked by auditory stimuli (alarm clock, telephone ring, abrupt noises), especially at rest. In comparison, the arrhythmic events among LQT3 patients occur during states of high parasympathetic tone, i.e., at rest or during sleep. CPVT is another channelopathy relying on sympathetic triggers, characterized by the onset of bidirectional VT during exercise [1]. As such, patients with LQT1 and CPVT should be steered away from physical or emotional exertion to avoid potential ventricular arrhythmias.
Similar to LQT3, ventricular arrhythmias in Brugada syndrome tend to manifest during states of high parasympathetic tone, typically during rest or sleep. The vagotonic trigger in combination with an underlying electrophysiologic substrate (e.g., at the epicardial surface of the right ventricular outflow tract [RVOT]) mediates and perpetuates the ventricular arrhythmias [1, 51]. An abrupt surge in PNS activity characteristically occurs before episodes of VF in Brugada syndrome, further supported by the observation of adverse ECG changes enhanced by parasympathomimetic agents and mitigated by sympathomimetic agents [1].
In spite of autonomic imbalance being strongly characterized in these channelopathies, the underlying molecular pathology remains the culprit for the manifestation of malignant ventricular arrhythmias. As such, autonomic interventions remain limited in these conditions with the most success observed in LQT1.
3.2.2 Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC)
ARVC is an inherited arrhythmogenic syndrome characterized by abnormal fibrofatty infiltration of the right and/or left ventricular myocardium, progressing from epicardial to endocardial (i.e., out-to-in) aspects. A high incidence of ventricular arrhythmias with potential SCD events tend to occur at times of heighted sympathetic state, e.g., during sports and athletic activities [52]. Both exercise and isoproterenol infusion can lead to an arrhythmogenic response in patients with ARVC [53]. In summary, the role of SNS in ARVC-related arrhythmogenesis is supported by the occurrence of exercise-induced or catecholamine-sensitive VT, as well as the therapeutic effect of beta-blockade. However, the presence of fibrofatty replacement of ventricular myocardium providing the arrhythmogenic substrate should not be neglected. Like most channelopathies, the impact of autonomic modulation is reflected by a lack of concrete autonomic intervention to treat ventricular arrhythmias in ARVC, although patients with ARVC are advised against competitive sports [54].
3.2.3 Idiopathic Premature Ventricular Complexes (PVCs), VT, and VF
The relationship between ANS activity and premature ventricular complexes (PVCs) is complex. Although PVCs commonly occur with increased SNS activity, these can occur in the setting of increased PNS activity as previously described [3, 11]. Most of the idiopathic focal VTs (otherwise known as the normal heart VTs) arise from outflow tracts (70% RVOT), but can also arise from other locations (e.g., papillary muscles, left ventricular fascicles, mitral annulus, aortic cusps, and RV moderator band) [55]. The involvement of SNS in arrhythmogenesis is implicated by the inducibility of such tachyarrhythmias during physical and/or mental stress, as well as by catecholamine infusion. Furthermore, the origin of RVOT PVCs and VT has been found to have dense sympathetic innervation, suggesting the impact of sympathetic hyperactivity in the trigger and perpetuation of RVOT PVCs and VT [56]. Prognosis of patients with these idiopathic VTs is generally benign, but life-threating arrhythmic events have been reported [57].
Idiopathic VF is a rare arrhythmic conundrum, comprising 1.2% of out-of-hospital cardiac arrests with a shockable rhythm. However, given its predilection for young individuals and its malignant presentation, it has garnered considerable focus [58]. In particular, idiopathic VF among pediatric patients was noted to occur in situations associated with high adrenergic tone [59]. An implantable cardioverter-defibrillator (ICD) implantation is mandated in all survivors of idiopathic VF. Successful ablation of PVC triggers has been reported in selected cases of idiopathic VF [60]. Interestingly some of these patients were found to have elevated vagal tone, in association with an adverse ECG phenotype of J point elevation, with some similarities seen in Brugada syndrome [61].
3.2.4 VT/VF in Structural Heart Disease (SHD)
The left stellate ganglion, an integral component of the cardiac SNS, modulates ventricular electrophysiology in patients with SHD, thereby contributing to initiation and perpetuation of VT/VF. During myocardial ischemia, the myocardium becomes sensitive to the catecholamines from increased sympathetic activity, increasing its susceptibility to VF [9]. Indeed, left stellate ganglionectomy (more commonly known as left cardiac sympathetic denervation, LCSD) has been shown to prevent post-ischemic VF in experimental models [62]. Correction of autonomic imbalance during myocardial ischemia by pharmacologic stimulation of PNS with a muscarinic agonist has also been shown to protect against malignant ventricular arrhythmias [63].
A more elaborate and complex neural phenomenon occurs during MI. Sympathetic denervation occurring at the infarct zone undergoes neural remodeling characterized by nerve sprouting, heterogeneous sympathetic hyperinnervation with subsequent supersensitivity, and increased propensity for ventricular arrhythmogenesis [64]. Upstream autonomic intervention by LCSD, either with surgical or percutaneous approach, has been effective in treating patients with post-infarct ventricular tachyarrhythmias and electrical storm [65].
4 Autonomic Neuromodulatory Interventions (ANIs)
Interventions for autonomic neuromodulation have been introduced in neurological practices for over two decades, treating conditions such as epilepsy and depression [66]. As the knowledge of different ANS hierarchy at the neuro-cardiac axis expands [67], there has been a growing interest to develop autonomic neuromodulatory interventions (ANIs) aiming at different targets of the ANS hierarchy to treat a variety of cardiovascular diseases, in particular heart failure and arrhythmias [68]. ANIs may include lifestyle practices, pharmacological agents, and percutaneous and surgical interventions. Lifestyle practices such as habitual moderate aerobic exercise have been shown to improve ANS balance while mind-and-body practices including yoga and meditation may also exert a restorative effect on ANS imbalance [11, 20]. In this section, the antiarrhythmic impact of ANIs in clinical neurocardiology, from drug therapy to invasive approaches, will be discussed, focusing on the different modalities available for supraventricular and ventricular arrhythmias, respectively. Prospective clinical trials of ANIs are summarized in Tables 9.4 and 9.5 for corresponding supraventricular and ventricular arrhythmias.
4.1 Fundamental Principles of Autonomic Neuromodulatory Interventions
ANIs, similar to other neuro-stimulatory interventions, exhibit memory, allowing short interventional duration to provide long-lasting therapeutic effects [90, 91]. The physiological phenomenon of long-term potentiation or long-term depression, i.e., stimulatory or inhibitory effects of neuro-stimulation extending beyond the interventional period, is preserved in ANIs [92]. Indeed, cardiac GP has been shown to exhibit synaptic plasticity, a property that allows the neurons to strengthen synaptic communication to achieve long-term potentiation or long-term depression [93].
Different approaches of ANIs acting at either limb of the ANS at various hierarchies have been developed over the years, although the underlying therapeutic mechanisms remain to be fully elucidated. Several mechanisms have been proposed, including restoration of ANS neurotransmitter balance, potentiation of nitric oxide pathways, and inhibition of downstream processes including inflammation, fibrosis, and apoptosis [94,95,96,97,98,99].
4.2 Autonomic Modulation for Supraventricular Arrhythmias
4.2.1 Beta-Blocker
Beta-blocker therapy is an established pharmacological agent for all catecholamine-driven and/or exercise-induced arrhythmias. They act as a competitive antagonist for adrenoreceptors, specifically β1-receptors, which constitute 80% of the adrenoreceptors in the heart [100]. By inhibiting the effect of epinephrine and norepinephrine, they effectively counteract the arrhythmogenic effect of SNS activity. Unlike other antiarrhythmic drugs, they are virtually free from pro-arrhythmic effect and therefore present a safe and effective therapeutic option.
In patients with AVNRT or AVRT, which classically are triggered by SNS activity, beta-blocker therapy is a useful adjunctive therapy [44, 50]. In AF, beta-blockers are used primarily for control of ventricular rate, but in a subset of patients with adrenergic AF triggered by anger or stress, beta-blockers have a role in rhythm control, attenuating the SNS response [101]. The rhythm control property of beta-blockers has also been demonstrated in persistent AF following electrical cardioversion [102].
4.2.2 Vagus Nerve Stimulation (VNS)
Increased vagal activity with the resultant prolongation of atrial effective refractory period (ERP) is a well-known trigger for atrial fibrillation [103]. As such, the use of VNS in suppressing AF appears counterintuitive. While high-level VNS achieving >60% sinus rate slowing promotes AF inducibility, mild to moderate levels of VNS producing <40% sinus rate slowing do not have an impact on the inducibility of AF [104]. Studies using LLVNS, i.e., at levels 10 or 50% below the bradycardia threshold, in fact exhibit a strong antiarrhythmic effect [40, 41, 105, 106]. The targets of LLVNS appear to be the efferent nerve endings and the GPs. The lack of antiarrhythmic effect on sectioned sympatho-vagal trunks supports the notion that LLVNS mediates its AF suppressive effect at the efferent nerve endings [107]. In one study, LLVNS suppressed the bradycardic response from anterior right GP stimulation as well as markedly reduced both frequency and amplitude of the neural activity recorded from the anterior right or superior left GPs [105]. In another study, LLVNS of the right vagus nerve alone exerted a profound antiarrhythmic effect sufficient to suppress the neural activity of both adrenergic and cholinergic components of the intrinsic cardiac nerve [41].
In an experimental model of ambulatory dogs, LLVNS of the left vagus nerve suppressed left stellate ganglion neural activity which occurs in the morning as well as decreased tyrosine-hydroxylase positive cells in the left stellate ganglion 1-week post-LLVNS [40]. Importantly, AF inducibility by rapid atrial pacing in these dogs was prevented by left-sided LLVNS [40]. In a follow-up study by the same group of investigators, chronic LLVNS was shown to produce left stellate ganglion damage, resulting in attenuated ganglionic activity [108].
Application of LLVNS in clinical studies is currently limited to its demonstrated effect in the treatment of postoperative AF. One study randomized 54 patients undergoing cardiac surgery to active or sham LLVNS [42]. LLVNS was achieved by suturing a bipolar wire to the preganglionic vagus nerve fibers along the lateral aspect of the superior vena cava, and by delivering a mean 61 h of VNS (20 Hz, 50% bradycardia threshold). At 1-month follow-up, the incidence of postoperative AF was significantly lower in the LLVNS group compared to the sham group.
The electrophysiologic mechanism underlying LLVNS in AF suppression may involve prolongation of atrial and PV ERPs, but the induced structural changes during LLVNS should be highlighted: upregulation of atrial gap junctions by preventing loss of atrial connexins and phenotype-switching between adrenergic and cholinergic nerve fibers [22, 40, 41]. In support of the potential anti-inflammatory role of VNS beyond AF suppression through an antiadrenergic effect, there was a significant effect in peptide neuromodulation and reduction of inflammatory cytokines associated with VNS [109].
4.2.3 Tragus Stimulation
In view of the antiarrhythmic effect of VNS, transcutaneous stimulation of the tragus of the ear, where the auricular branch of the vagus nerve is located, has been developed as a method of noninvasive VNS, affirmed by observation of evoked potentials in the brainstem in humans [110].
In a canine model of AF induced by rapid atrial pacing, low-level tragus stimulation (LLTS) at 80% below the threshold of sinus node or AV node conduction slowing suppressed AF inducibility through attenuation of pacing-induced atrial ERP shortening [111]. In addition, the amplitude and frequency of GP firing, evident from direct neural recordings from the anterior right GP, were reduced. When the vagus nerves were bisected distal to the site of stimulation, the beneficial electrophysiologic effects of LLTS on atrial ERP and AF inducibility were abolished, suggesting that the LLTS effect was mediated through efferent nerve fibers [111].
In humans, a study reported LLTS in 40 patients with paroxysmal AF (20 LLTS, 20 sham) [69]. In the LLTS group, the patients were subjected to 1 h of LLTS (50% lower than voltage to achieve bradycardia threshold) in the right ear using a flat metal clip attached onto the tragus. Under general anesthesia, AF was induced at baseline and after 1 h of either LLTS or sham stimulation. In the LLTS group, pacing-induced AF duration was shorter and atrial ERP was longer compared to baseline, while in the sham group, there was no significant difference. In the same study, LLTS is associated with reduced serum levels of anti-inflammatory cytokines, namely tumor necrosis factor (TNF-α), compared to the sham control. Hence, akin to LLVNS, LLTS exerted its antiarrhythmic effect potentially through both antiadrenergic and anti-inflammatory pathways.
The feasibility and efficacy of LLTS in ambulatory settings has been demonstrated in the TREAT-AF (Transcutaneous Electrical Vagus Nerve Stimulation to Suppress Atrial Fibrillation) study [70]. In this study enrolling 53 patients with paroxysmal AF randomized to LLTS or sham group, LLTS was achieved using ear clip applied to the earlobe (a site devoid of vagal innervation) for 1 h daily over a 6-month study period. At 6 months, AF burden measured by a 14-day ECG performed at baseline, 3 months, and 6 months was 85% lower in the LLTS group compared with sham. Furthermore, the LLTS group also demonstrated a concomitant 23% reduction in TNF-α level. This study has several implications in therapeutic application of tragus stimulation. First, a self-administered noninvasive treatment with little or no risk can have a significant impact on both AF burden and systemic inflammatory response. Second, tragus stimulation preferentially activates afferent vagal nerve fibers with therapeutic potential [112]. Third, tragus stimulation has been shown to activate central vagal projections in humans with resultant decreased sympathetic output [113]. Fourth, tragus stimulation avoids the possibility of concomitant stimulation of sympathetic fibers collocated with vagal fibers in the cervical vagus nerve when compared to the cervical VNS in humans [114].
4.2.4 Renal Denervation (RDN)
Renal denervation (RDN) presents an attractive treatment for hypertension, as the proximal renal arteries are richly innervated by a network of sympathetic ganglia, as well as afferent and efferent nerve fibers linking the central nervous system, the kidney, and the cardiovascular system [115]. Although RDN was first proposed as an innovative tool for treatment of resistant hypertension with subsequent disappointing results from the SIMPLICITY HTN-3 trial (A Controlled Trial of Renal Denervation for Resistant Hypertension), several preclinical and clinical studies have demonstrated a beneficial effect of RDN on AF. In particular, the antiarrhythmic effect was suggested to be independent of an improvement in blood pressure in preclinical studies [116, 117]. In a goat model of pacing-induced AF, RDN resulted in reduced atrial sympathetic nerve sprouting and AF complexity [117], whereas in a porcine model of obstructive sleep apnea, RDN attenuated atrial ERP shortening and AF inducibility [116]. The beneficial effect of RDN on AF inducibility is purported to be mediated through favorable electrical (attenuation of atrial ERP shortening) and structural (reduced afferent sympathetic input to the central nervous system, with a resultant decrease in efferent sympathetic output to the heart) remodeling [115].
Small clinical studies in humans involving pulmonary vein isolation (PVI) with adjuvant RDN during AF ablation procedures appear to be supportive of the preclinical findings [71, 72]. In these studies, an effective RDN response manifested as abolishment of a sudden expected hypertensive response during high-frequency stimulation (HFS) of aortico-renal ganglion. The efficacy of RDN combined with standard PVI was assessed in the large multicenter ERADICATE-AF trial (Effect of Renal Denervation and Catheter Ablation vs. Catheter Ablation Alone on Atrial Fibrillation Recurrence Among Patients with Paroxysmal Atrial Fibrillation and Hypertension) [73]. In total, 302 patients with paroxysmal AF and hypertension on ≥1 antihypertensive drugs were recruited. At 12-month follow-up, the RDN+PVI group enjoyed greater freedom from AF off antiarrhythmic drugs compared to standalone PVI (71.4% vs. 57.8%; hazard ratio: 0.61; 95% confidence interval: 0.41–0.90; p = 0.011). Complication rates were comparable between the two groups. Blood pressure control was noted to be better in the RDN+PVI group. Interestingly, only 57% of patients with RDN demonstrated conclusive HFS response. As such, this calls into question whether the beneficial RDN effect on AF recurrence is related to favorable autonomic modulation, improved blood pressure control, or a combination of both mechanisms.
4.2.5 Ganglionic Plexi (GP) Ablation
In contrast to the extracardiac location of postganglionic sympathetic neurons at the paravertebral ganglia, the parasympathetic postganglionic neurons are located in vagal GPs within the epicardial fat pads and the ligament of Marshal [118, 119]. The short axons of these vagal neurons lend themselves to be feasible radiofrequency ablation targets from the endocardial surface. Variable measures have been used to define the GPs location, ranging from HFS associated with a positive vagal response defined as AV block >2 s to spectral analysis through fast Fourier transform analysis or anatomical guidance [118].
Given the parasympathetic contribution to initiation and maintenance of AF through electrophysiologic and structural mechanisms as discussed before, GP ablation has been proposed as an adjunct to PVI during AF ablation procedures [118]. A meta-analysis of four studies (n = 718, mean LA size 45.7 mm, mean LV EF 54.8%) comparing the strategy of GP ablation+PVI (n = 358) vs. standalone PVI (n = 360) demonstrated that adjunctive GP ablation was associated with significantly higher freedom from AF among patients with paroxysmal AF (75.8% vs. 60%; odds ratio [OR] 2.22, p = 0.001) [74]. For patients with persistent AF, the antiarrhythmic effect of GP ablation was more modest, with a non-significant trend toward higher rates of freedom from AF (54.7% vs. 43.3%; OR 1.55, p = 0.08). This meta-analysis concluded that GP ablation+PVI led to better AF freedom in patients with PAF and without significant SHD.
Ablation of complex fractionated atrial electrograms (CFAE) was employed as an adjunctive ablation strategy in addition to PVI, although no real benefit was demonstratable [120]. Part of the rationale for this approach was attributable to vagal atrial denervation, with these electrograms commonly encountered in areas juxtaposed to GPs [121, 122]. However, a meta-analysis of 14 studies (n = 1613 patients) revealed no benefit with adjunctive CFAE ablation compared to adjunctive GP ablation in both short- (OR 1.72; p = 0.003) and long-term (OR 2.0, p = 0.0006) AF freedom [123].
GP ablation remains a challenging approach to achieve sustainable vagal denervation due to the variable, inconsistent techniques of GP detection (electrical vs functional vs anatomical), assessment of complete GP ablation, and prevention of reinnervation [124]. Of note, denervation of postganglionic vagal neurons at the atrial level has been shown to be associated with increased susceptibility to ventricular arrhythmias [125, 126].
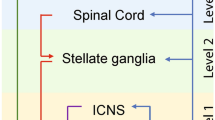
4.2.6 Spinal Cord Stimulation (SCS)
SCS is currently used to treat patients with refractory, chronic severe pain or angina pectoris by delivering electrical stimuli to the segments of spinal cord through implantable electrodes [127, 128]. Of relevance, SCS can exert electrophysiologic changes through modulation of afferent and efferent connections between the heart and the intrinsic cardiac autonomic nerves [129]. In a canine model with chronic AF induced by rapid atrial pacing, SCS prolonged atrial ERP as well as reduced AF burden and inducibility [130]. Another postulated mechanism includes direct suppression of neural activity in atrial GP and stellate ganglia [131, 132]. At the molecular level, SCS modulates relevant cytokine levels, reducing the expression of c-fos and nerve growth factor in addition to increasing the expression of the small conductance calcium-activated potassium channels type 2 within the stellate ganglia neurons [132]. However, adoption of SCS beyond pain management into the clinical realm of AF treatment is curtailed by its invasiveness.
4.2.7 Baroreceptor Receptor Activation Therapy (BAT)
Baroreceptors in the carotid sinus are an integral component of the neural feedback loop in modulating blood pressure. Elevation in blood pressure activates these baroreceptors, enhancing neural signals to the brainstem and ultimately reducing sympathetic output [133, 134]. Over the last few decades, several medical devices for baroreceptor receptor activation therapy (BAT) have been designed for the treatment of drug-refractory hypertension [135, 136]. In principle, BAT modulates ANS by sympathetic withdrawal and vagal activation [137, 138]. Low-level BAT (80% of threshold for blood pressure reduction) in a canine model invoked atrial electrophysiologic changes in the form of progressive prolongation of atrial ERP, reduction in AF inducibility, and attenuation of GP function, with the latter effect suggesting potential AF suppression through inhibition of atrial GP [139]. Moreover, low-level BAT was noted to attenuate atrial electrical remodeling induced by rapid atrial pacing in another canine AF model [140]. In spite of the promising experimental data hinting at prominent antiarrhythmic potential of BAT in the treatment of AF, the widespread applicability of BAT in clinical practice will be limited by its invasive nature.
4.3 Autonomic Modulation for Ventricular Arrhythmias
4.3.1 Beta-Blockers
Beta-blockers with their antiadrenergic properties are considered the mainstay therapy in managing patients with inherited channelopathies such as LQTS and CPVT. In patients with SHD, especially those with myocardial ischemia, post-myocardial infarct, or heart failure with reduced left ventricular ejection fraction including ischemic and non-ischemic cardiomyopathies, beta-blockers remain a steadfast therapy in reducing ventricular arrhythmias and sudden arrhythmic death [141].
Although selective β1 adrenoreceptor antagonists (metoprolol, bisoprolol) have been the beta-blockers of choice in a majority of the indicated cardiovascular conditions, non-selective beta-blockers (nadolol, propranolol) are the preferred agents for channelopathies (LQTS, CPVT) [142]. Indeed, propranolol achieved a better QT-shortening effect in LQTS compared to metoprolol and nadolol. While nadolol and propranolol are equally effective, metoprolol trails behind in its cardioprotective effect and should not be used for symptomatic LQTS individuals [142]. In contrast, selective β1-blockers (metoprolol, bisoprolol, carvedilol, nebivolol) are effective in patients with both ischemic and non-ischemic cardiomyopathies, especially in the setting of heart failure with reduced ejection fraction [141].
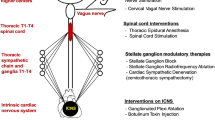
4.3.2 Cardiac Sympathetic Denervation (CSD)
Stellate ganglia serve not only as a key relay station for cardiac sympathetic afferents but also contain the cell bodies of postganglionic sympathetic efferent neurons with direct connections to the myocardium [143]. Importantly, they present an accessible target for ANIs through CSD, the latter aiming to interrupt the sympathetic input to the heart by removal or blockade of the extrinsic sympathetic nerves. In general, CSD involves bisecting the stellate ganglion with removal of the lower half of this ganglion along with a few ganglia below it, thus creating an adequate sympathetic block to the heart. This makes mechanistic sense as the arrhythmogenic sympathetic input has been shown to be more prominent from the caudal spinal segments and on the left [143]. The boundary of bisection is important as to prevent Horner’s syndrome when denervation occurs above the level intended. Bilateral or left CSD (LCSD) is regarded as an effective antiadrenergic therapy, disrupting both afferent and efferent sympathetic fibers [144]. The technique of LCSD has matured over the years, progressing from an open surgical approach via thoracotomy or supraclavicular access to a minimally invasive technique of video-assisted thoracic surgery [145, 146]. A percutaneous approach has been described, achieved either through radiofrequency ablation or use of an anesthetic agent for stellate ganglion blockade (SGB) [147].
The efficacy of CSD extends beyond a local antiadrenergic effect, with anti-fibrillatory impact on patients with LQTS, CPVT, and SHD [144, 148]. LCSD was first pioneered by Schwartz et al. for treatment of patients with LQTS refractory to beta-blockers [149]. Although LCSD led to significant reduction in the incidence of aborted sudden death and syncope in high-risk LQTS cohort compared to pre-LCSD period, the risk of sudden cardiac death persisted in the long-term follow-up [150]. As such, LCSD is currently a recommended adjunctive therapy to beta-blockers and ICD in LQTS patients experiencing breakthrough events [151]. Furthermore, the therapeutic effectiveness of LCSD extends to patients with CPVT [152]. In patients with either LQTS or CPVT, improvement in procedural technique enables LCSD to be performed via video-assisted thoracic surgeries [145].
In patients with SHD and refractory ventricular arrhythmias, CSD has proved to be a valuable anti-fibrillatory intervention. A multicenter CSD study involving 121 patients (26% female, mean age 55 ± 13 years, LVEF 30 ± 13%) with SHD and refractory VT/VT storm demonstrated a 1-year composite freedom from sustained VT, ICD shock, transplant, and death of 58% for LCSD and 50% for bilateral CSD [153]. In this study, 120 patients were on antiarrhythmic drugs before CSD with 39 (32%) no longer requiring antiarrhythmic drugs at follow-up. Interestingly, bilateral CSD appears to be more effective than LCSD in this group of patients as those undergoing bilateral CSD were found to have longer VT or ICD shock- and transplant-free survival.
The overall efficacy and safety of CSD was attested by a systematic review of 13 studies comprising 183 patients (70% male, mean age 54.6 years, 28% ischemic cardiomyopathy, 82% bilateral CSD) [148]. Freedom from arrhythmic events was noted to be 58–100% while most complications were transient with no procedural death reported. Complication rate was reported at 28% including transient hypotension (9%), pneumothorax (5%), neuropathic pain (4%), Horner’s syndrome (3%), abnormal perspiration pattern (3%), and hemothorax (2%).
Recently, percutaneous SGB has been advocated as a less invasive approach to achieve CSD in patients with refractory ventricular arrhythmias. A systematic review of 23 studies comprising 38 patients (71% male, mean age 52 ± 19.1 years, 45% ischemic cardiomyopathy, mean LVEF 31 ± 10%) presenting with an electrical storm (15 patients with acute MI; 7 patients with QT prolongation) who were refractory to antiarrhythmic drugs and subsequently underwent SGB concluded that SGB with the use of bupivacaine (0.25–0.5%) resulted in a significantly lower burden of ventricular arrhythmias (p < 0.001) and number of external and ICD shocks (p < 0.01) [65]. More importantly, the survival rate was 80.6% at discharge.
4.3.3 Vagus Nerve Stimulation
Ventricular electrophysiology depicts a cardioprotective effect exerted by parasympathetic dominance. Conversely, reduced vagal activity following MI is associated with heightened risks of ventricular arrhythmias [154, 155]. In experimental models, VNS and exogeneous cholinergic agonists prolong the ventricular ERP [156, 157]. Of note, VNS reduced the vulnerability to VF by increasing VF threshold, flattening the restitution curve (i.e., reducing the maximum slope of action potential duration restitution), attenuation of electrical alternans, and increasing ventricular ERP in an in vitro experimental model [158]. In a separate study, VNS was found to mediate these favorable ventricular electrophysiologic effects through a nitric-oxide-dependent pathway [159].
In addition to inducing a protective electrophysiologic profile, VNS in multiple animal models is shown to prevent adverse cardiac remodeling and ventricular arrhythmias [98, 160]. In a rat model of acute MI, VNS prevented the loss of phosphorylated connexin-43 [94]. In a canine model of healed MI, VNS prevented sudden arrhythmic death [161]. In a canine model of pacing-induced heart failure, chronic VNS improved cardiac autonomic control, promoted anti-inflammatory effects, and attenuated heart failure development [97]. Notably, VNS modulates all these beneficial effects independent of a reduction in heart rate [79, 162].
The promising findings in preclinical studies have led to the design of clinical trials of VNS in humans. An initial open-label, non-randomized trial in patients with heart failure demonstrated that VNS produced significant improvement in a multitude of outcomes including New York Heart Association (NYHA) class, quality of life, 6-min walk test, and LV end-systolic volume, without any major side effects [79]. However, three subsequent randomized trials of VNS in heart failure showed inconsistent results, with two trials reporting neutral results [82, 83] and one reporting modest benefits [80]. At present, ANTHEM-HFrEF (Autonomic Regulation Therapy to Enhance Myocardial Function and Reduce Progression of Heart Failure with Reduced Ejection Fraction) pivotal study (NCT03425422) is an ongoing multicenter randomized clinical trial for VNS in heart failure [163]. Its open-label feasibility study comprising 60 patients (ANTHEM-HF) has shown improvement in heart failure parameters at 6 months [80], with results sustained to a 12-month follow-up period (ENCORE [Extension Study of Neural Regulation Therapy on Myocardial Infarction with Heart Failure]) [81].
4.3.4 Tragus Stimulation
All clinical trials of VNS in heart failure populations involve an invasive approach of surgical placement of the electrodes around the cervical vagus nerve in the neck, which is not without procedural risk, with 1 death from implantation-related embolic stroke 3 days following the procedure in the ANTHEM-HF trial [80]. Furthermore, there is long-term implication for potential device-related complications, including infections, electrode fracture/displacement/malfunction, and battery depletion [164]. Direct stimulation of the cervical vagus nerve may be associated with such side effects as tinnitus, dysphonia, cough, and nausea [164, 165]. As such, tragus stimulation involving transcutaneous stimulation of the auricular branch of the vagus nerve presents an attractive noninvasive option [110].
Animal studies provided evidence for the effects of tragus stimulation in preventing adverse cardiac remodeling and ventricular arrhythmias. In a canine model of chronic MI, chronic intermittent LLTS 2 h daily for 2 months (80% below the bradycardia threshold) induced neuropeptide modulatory changes including suppression of left stellate ganglion activity, attenuation of cardiac sympathetic nerve sprouting, downregulation of nerve growth factor, and upregulation of small conductance calcium-activated potassium channel type 2. Notably favorable electrophysiological remodeling occurred with suppression of ventricular arrhythmias and a flattening of restitution slope [166]. Chronic intermittent LLTS also attenuated adverse structural remodeling, fibrosis, and inflammation [160]. In a proof-of-concept study, LLTS for 2 hours in humans undergoing primary coronary intervention for ST-segment elevation MI led to reduced ventricular arrhythmias, preserved cardiac function, and reduced biomarkers of myocardial injury/inflammation, suggesting a potential opportunity for noninvasive ANIs as an adjunctive nonpharmacological treatment in ST-segment elevation MI [84].
4.3.5 Renal Denervation
Early preclinical studies with RDN achieved using low-energy radiofrequency ablation along the renal arteries demonstrated reduced angiotensin receptor density and improved cardiac output [167]. In the SYMPLICITY HF study, a single-arm feasibility study enrolling 39 patients with mild-moderate symptoms, EF <40%, and renal impairment, RDN resulted in a reduction in NT-pro-brain natriuretic peptide 12 months after treatment without perceived deterioration in both cardiac and renal function [85]. In patients with cardiomyopathy and refractory VT, RDN has been shown to be an effective adjunctive therapy to catheter ablation of VT [168]. In patients with cardiomyopathy and recurrent VT following catheter ablation, RDN was used as an adjunct to CSD, leading to reduced ICD therapies in patients who underwent CSD and RDN as staged procedures [169].
4.3.6 Spinal Cord Stimulation (SCS)
In canine post-infarct heart failure models, SCS was consistently associated with reduced ventricular arrhythmias and recovery of left ventricular ejection fraction [170,171,172]. The improved electrical and structural remodeling mediating the antiarrhythmic effects of SCS was attributable to sympathetic withdrawal and vagal enhancement [171]. Initial case series of two patients with high ventricular arrhythmia burden depicted the same antiarrhythmic effect from SCS [173]. However, the two clinical trials investigating SCS in patients with heart failure, including the randomized controlled trial DEFEAT-HF (Determining the Feasibility of Spinal Cord Neuromodulation for the Treatment of Chronic Systolic Heart Failure), have yielded conflicting results [86, 87]. The conflicting outcomes of the two trials could be accounted for by variation in the duty cycle, intensity of stimulation, and design and position of the electrode, highlighting the need to optimize a systematic stimulation protocol to allow for meaningful translational research from animal studies to large clinical trials.
4.3.7 Baroreceptor Activation Therapy (BAT)
BAT has been shown to modulate ventricular electrophysiology through prolongation of ventricular ERP and flattening of the action potential duration restitution slope in canines [174]. At 80% below the voltage threshold for blood pressure lowering, low-level BAT was found to suppress premature ventricular complexes VT and VF in a canine model with acute ischemia induced by occlusion of the left anterior descending artery [175]. Additionally, BAT reversed adverse structural remodeling in canine models of heart failure with improvement in interstitial fibrosis, myocyte hypertrophy, LV end-diastolic pressure, and survival [176, 177].
In the clinical arena, a proof-of-concept, single-center, open-label study demonstrated the safety and efficacy of carotid BAT in improving quality of life and exercise capacity [178]. A small randomized clinical trial of BAT showed improvement in various heart failure markers at 6 months, including 6-min walk test, quality of life, NYHA class, and N-terminal pro-B-type natriuretic peptide level [88]. A large randomized clinical trial (BeAT-HF [Baroreflex Activation Therapy for Heart Failure]) comprising 245 patients (from a pool of 408 randomized patients) with heart failure and reduced ejection fraction (8% female, mean age 62 ± 11 years; LV EF 27 ± 6%) concluded that BAT is not only safe but also efficacious, resulting in significant improvement in quality of life, 6 min walk, exercise capacity, and functional status, as well as significantly lower N-terminal pro-B-type natriuretic peptide [89]. The impact of BAT on hard clinical endpoints is currently pending from this trial.
5 Assessment of Autonomic Tone
Distinctive methods have been developed over the years for assessment of specific aspects of sympathetic and parasympathetic nervous systems. These noninvasive and invasive methods can complement one another, contributing to a global picture of autonomic status. While these methods provided key insights into the abnormal autonomic characteristics in arrhythmias and heart failure, they remain rooted in the research arena, each with their unique strength and limitations.
5.1 Baroreflex Sensitivity (BRS)
Baroreflex sensitivity (BRS) describes the acuity of arterial baroreceptor control of heart rate in response to blood pressure changes. The heart rate changes in response to acute perturbation in blood pressure are thought to be an indication of reflex vagal response due to the preferential influence of cardiac cycles on the release of acetylcholine as opposed to norepinephrine. The strength of this reflex vagal response can be assessed by three techniques: (1) analysis of bradycardic response following the increase of blood pressure with vasoconstrictors such as phenylephrine; (2) assessment of reflex sympathetic-induced tachycardic response following the reduction of blood pressure by vasodilators such as nitroprusside or nitroglycerin; and (3) direct stimulation of carotid baroreceptors with neck suction or unloading by neck pressure, allowing these neck maneuvers to elicit counter-regulatory responses from aortic arch baroreceptors. These techniques are not without their limitations. Prolonged infusion of vasoactive agents can simulate a steady state, leading to competing sympathetic responses. Additionally, these vasoactive agents may exert a confounding effect on sino-atrial node discharges, baroreceptor nerve endings, and atrial and pulmonary mechanoreceptors [179, 180]. To circumvent these limitations, algorithms have been developed with the ability of identifying spontaneous concordant fluctuations in blood pressure and heart rate within the brief time window from continuous noninvasive or invasive recordings [181].
Central to all these techniques is the utilization of a regression equation correlating changes in pulse interval to changes in systolic blood pressure during the immediately preceding cardiac cycles. The gradient of the resulting graph describes the gain of the arterial baroreflex regulation on heart rate. The final results therefore indicate the sensitivity of vagal response toward stress response in blood pressure changes, allowing this method to be used as a bedside test comparable to the BRS derived from drug-induced changes in blood pressure. This method lacks the ability to identify the cause of autoimmune impairment. Nonetheless, low BRS has been shown to correlate with poor prognostic outcome. Canine models demonstrating reduced BRS after MI were more susceptible to VF [182]. These findings were extrapolated to post-MI human studies, demonstrating that a depressed BRS of <3 ms/mmHg was associated with a high incidence of arrhythmic deaths [183,184,185]. In heart failure patients receiving optimal medical therapy, the prognostication of BRS has recently been challenged [186].
5.2 Heart Rate Variability (HRV)
Beat-to-beat heart rate varies stochastically due to the influence of tonic vagal control of the sinus node, with direct or indirect influence of circadian rhythms, temperature regulations, and changes in autonomic nerve activity [187,188,189]. The positive chronotropy (increase in sinus rate) induced by catecholamines (epinephrine and norepinephrine) released by the SNS and the negative chronotropy (decrease in sinus rate) by acetylcholine released by the PNS are responsible for the subtle degree of variability in the intervals between consecutive beats [190,191,192]. Heart rate variability (HRV) is therefore a noninvasive surrogate marker of autonomic balance of the heart, with its analysis being performed in time, frequency, or nonlinear domains.
5.2.1 Time-Domain Measures
Time-domain measurements represent the most widely adopted technique to evaluate HRV due to its relatively straightforward computation of parameters derived from the time series of RR intervals. Long-term HRV is deduced from the standard deviation of RR intervals usually computed from 24-h Holter recordings, whereas short-term HRV is reflected by root mean square of successive differences of the RR intervals. Additionally, short-term HRV can also be assessed as the percentage of consecutive RR intervals with >50 ms difference. All these time-domain measures have been verified to be age-dependent, gender-sensitive, and decreased with aging, whereas preservation of HRV is associated with healthy longevity [193, 194].
5.2.2 Frequency-Domain Measures
Using fast Fourier transform and wavelet analysis quantifying the spectral and time-frequency content of HRV, the frequency-domain measures serve to complement the time-domain measurements of HRV. High frequencies (0.15–0.5 Hz) are thought to be a representation of the parasympathetic component of the ANS with vagal blockade abolishing oscillations in heart rate within this frequency band [195]. Low frequencies (0.05–0.15 Hz) are mediated by both the PNS and SNS and are affected by BRS. This is supported by observations that the low-frequency spectral power is increased by maneuvers that stimulate central sympathetic output, such as standing up, tilting, and exercising. Conversely the spectral power in this frequency band of 0.05–0.15 Hz is decreased by beta-blockade, clonidine, and during sleep. Very-low frequencies (<0.05 Hz) are under the influence of many in vivo factors including the renin-angiotensin system and thermoregulation [195]. As a rough guide, the ratio of low- to high-frequency power is therefore considered a measure of autonomic balance [189].
In heart failure, the spectral power appears to be markedly reduced and concentrated within the very-low- and low-frequency ranges [196]. Similar to BRS, HRV provides insights into sympathetic and parasympathetic contributions to heart rate modulation and contributes to estimation of prognostic outlook, despite the lack of specificity to the degree of regional sympathetic output. Historically, a depressed HRV demonstrated a high sensitivity and specificity in predicting susceptibility to VF in MI canine models [197]. This is further corroborated by the finding of HRV recovery in low-risk post-MI dogs compared to high-risk dogs with persistent depressed HRV parameters independent of beta-blockade [198]. These preclinical findings were echoed by subsequent human studies involving post-MI patients. In these trials, HRV was a significant predictor of SCD after adjusting for clinical and demographic factors, including ejection fraction [185, 199, 200]. Furthermore HRV improvement over time following MI coincided with decreasing risk of arrhythmic death [201]. In one study, a HRV of <5 ms conferred a hazard relative risk of 5.3 in SCD, compared with low-risk patients with a HRV of >10 ms during a follow-up period of 31 months [200]. Notably a depressed HRV has also been observed in patients with idiopathic dilated cardiomyopathy with a history of SCD compared to those without fatal ventricular arrhythmias [202, 203]. Other conditions in which frequency-domain measures have been utilized to evaluate autonomic balance include sleep apnea and hypertension [204, 205].
The concept of low- to high-frequency ratio as a reflection of autonomic balance has been questioned due to the fact that, in contrast to the previously conceived notion that low-frequency content comprises exclusively SNS activity, low-frequency content is now regarded to be a reflection of combined SNS and PNS inputs [192, 206].
5.2.3 Nonlinear Domain Measures
Nonlinear measures have been developed over the past decade to address complex nonlinear autonomic interactions [207,208,209]. Measurements such as SD1/SD2, Poincare maps, entropy, and detrended fluctuation analysis attempt to quantify the degree of information, disorder, or complexity of HRV.
SD1 and SD2 are measures of short- and long-term HRV, respectively, both based on RR intervals and derived from Poincare maps. SD1 measures immediate beat-to-beat variability, signifying parasympathetic tone, whereas SD2 reflects combined SNS and PNS influences on the heart [209]. The ratio SD1/SD2 therefore serves to indicate underlying autonomic tone, as well as short- and long-term variability in RR intervals. Indeed, intermittent VNS has been demonstrated to increase SD1/SD2 ratio in heart failure patients, suggesting a restorative role of VNS in autonomic balance [208]. In patients with dilated cardiomyopathy, Poincare plot analysis of HRV has been utilized for risk stratification [207]. Furthermore, approximate entropy has been shown to be raised in patients with heart failure compared with healthy subjects, indicating loss of autonomic control in association with more erratic heart rate [210]. Other measures such as sample entropy and detrended fluctuation analysis were used to demonstrate changes in autonomic drive and to assess the neural effects of HRV in both healthy individuals and those with spinal cord injury [211].
5.3 Neural Tracer Imaging
Over the last few decades, neural imaging techniques have been developed and employed using several different tracers that allow for direct visualization of sympathetic nervous innervation in the heart. These tracers target molecular landmarks at the presynaptic and postsynaptic side as well as second messenger systems of the sympathetic nervous system and are thereby capable of portraying the overall picture of sympathetic signal transduction. The majority of these radiotracers mimic the structure of norepinephrine and other catecholamines to enable them to target the endogenous reuptake pathway of sympathetic neurons. These tracers are exemplified by labeled neurotransmitters ([18F]-dopamine, [11C]-epinephrine), “false neurotransmitters” serving as substrate analogues ([123I]-MIBG, [11C]-mHED, [18F]-LMI1195, [11C]-phenylephrine, [11C]-phenethylguanidines), and uptake-1 inhibitors ([11C]=methylreboxetine, [11C]-desipramine). Each tracer possesses unique uptake and retention characteristics, contributing information on various aspects of neuronal reuptake, uptake-1 density, vesicular packaging, vesicular release, and norepinephrine metabolism. In heart failure, a chronic state of sympathetic overdrive fuels a decrease in neuronal reuptake, downregulation of uptake-1, and increased synaptic norepinephrine content and regional spillover.
The two most commonly utilized tracers in clinical practice are [123iodine]-metaiodobenzylguanidine ([123I]-MIBG) and [11C]-meta-hydroxyephedrine ([11C]-mHED). The former was first developed to image neuroendocrine tumors, with early studies identifying [123I]-MIBG uptake in myocardium to be in inverse correlation with plasma and urinary catecholamines [212] due to its high affinity to neuronal uptake-1 and extra-neuronal uptake-2 [213, 214]. Using semiquantitative analyses in single-photon emission computed tomography (SPECT), an estimation of sympathetic tone can be inferred from the rate of [123I]-MIBG washout, as well as early and late heart-to-mediastinum ratios [215]. Reduced late heart-to-mediastinum or raised [123I]-MIBG washout in semiquantitative myocardial [123I]-MIBG measurements has been shown to be a poor prognostic marker in a systematic meta-analysis [216]. Conversely beta-blockade and renin–angiotensin–aldosterone inhibition are characterized by an increase in [123I]-MIBG uptake and a reduced myocardial washout. Pertinently large-scale clinical trials have recognized [123I]-MIBG imaging as an independent prognostic tool in the identification of heart failure patients at the greatest risk of disease progression [217,218,219] and sudden cardiac death independent of left ventricular ejection fraction and B-type natriuretic peptide [216, 220,221,222,223]. In patients with ventricular tachycardia without any coronary artery disease, the uptake of [123I]-MIBG was noted to be reduced [224]. Notably, a reduced [123I]-MIBG predicted ventricular arrhythmias requiring ICD therapy in patients with heart failure [218]. Recently, [123I]-MIBG imaging was shown to accurately identify atrial GP locations as verified by HFS [225].
In positron emission tomography (PET), [11C]-mHED is the most widely used radiotracer for sympathetic neuronal imaging. As it is devoid of postsynaptic activity, the retention of [11C]-mHED reflects solely presynaptic function of sympathetic neurons due to its selectivity for uptake-1 over other reuptake transporters [226]. [11C]-mHED has a predilection for tissue retention in organs with complex adrenergic networks including the heart, adrenal glands, and spleen, with gradual accumulation in the liver. This is supported by the findings of selective competitive inhibitors of uptake-1, including true or false neurotransmitters, attenuating myocardial accumulation of [11C]-mHED, with consequent increase in hepatic activity due to the accumulation of metabolites [213, 227,228,229,230,231,232]. High-speed liquid chromatography further confirmed plasma accumulation of [11C]-mHED metabolites in guinea pigs and rats within 30 min after injection [229, 230, 232], and in human subjects within 10–20 min [233].
In spite of the attractiveness of these imaging modalities to measure autonomic tone noninvasively, complete assessment of autonomic tone is currently confounded by the lack of parasympathetic tracers.
5.4 Direct Measurement of Sympathetic Nerve Activity
While neural tracer imaging provides a means of direct visualization of sympathetic innervation, microneurography allows direct multi-fiber or single-fiber microelectrode measurements of postganglionic sympathetic nerve activity, thereby serving as a real-time recording of the dynamic sympathetic nerve and reflex controls [234, 235]. Sympathetic nerve activity is recorded from intraneural microelectrodes inserted percutaneously in a peripheral nerve, typically at the level of the peroneal nerve [236, 237]. Skin (SKNA) and muscle sympathetic nerve activities (MSNA) manifest as distinctive discharge patterns, the former preferentially responding to external acoustic, tactile, or temperature stimuli independent of cardiac cycles while the latter being heavily entrained by input from cardiopulmonary and arterial mechanoreceptors.
MSNA possesses unique pulse synchronicity, firing discharges 1.1–1.3 seconds after the preceding R wave of the electrocardiogram. At rest, MSNA is found to correlate with both cardiac and renal norepinephrine spillover, whereas during isometric exercises there is a concordant increase of sympathetic nerve activity between MSNA and cardiac norepinephrine spillover [238, 239]. This phenomenon is absent in heart failure [240]. Indeed, in heart failure, evidence of sympathetic hyperactivity manifested as increased firing probability and frequency with possible recruitment of otherwise silent sympathetic nerve fibers [235]. Importantly, increased MSNA predicts mortality in patients with heart failure in addition to its association with non-responders of cardiac resynchronization therapy [237, 241].
In view of the invasiveness and time-consuming nature of microneurography, MSNA is not routinely used despite its reproducibility and reliability in assessment of autonomic tone [236]. In contrast, SKNA activity can be measured noninvasively from ECG recordings [242]. SKNA is found to increase in subjects undergoing Valsalva maneuver as well as water pressor test due to increased sympathetic drive [242]. In ambulatory dogs, SKNA correlated well with stellate ganglion activity [243]. In humans, increased SKNA demonstrated a temporal relationship with paroxysmal atrial tachyarrhythmias, preceding the onset and termination of paroxysmal atrial tachycardia and AF [244]. In another study, sustained level of increased SKNA is noted to associate with temporal clustering of AF as well as spontaneous VT and VF in humans [245].
5.5 Alternans
Alternans in ventricular repolarization, measured as T-wave alternans (TWA), has been shown to surge in magnitude during elevated sympathetic activity in humans as well as in an end-stage heart failure animal model [246,247,248]. These surges in TWA amplitude are attenuated by beta-blockers, supporting the concept that sympathetic activity modulates TWA [249, 250]. It is postulated that beta-blockade-mediated attenuation of TWA amplitude occurs by (1) blunting of the exercise-related chronotropic response, thereby preventing patients from reaching the specific heart rate threshold necessary for TWA development, and (2) direct reduction of TWA magnitude [250]. Conversely, VNS was shown in an isolated rabbit heart model to reduce TWA amplitude and to increase the cycle length for VF induction [158].
P-wave alternans (PWA) has been proposed as a novel atrial substrate of electrical instability, predisposing to the development of AF [251]. Using a customized algorithm for PWA estimation [252, 253], LLTS for 1 h/day resulted in lower AF burden and diminished PWA voltage in a single patient compared to baseline and to another patient on a sham protocol [254]. AF and PWA burden for the patient with active LLTS was not increased over 6 months when compared to the sham patient.
6 Challenges and Limitations of Autonomic Neuromodulatory Interventions
The conflicting outcome among contemporary clinical trials of VNS in heart failure epitomizes the challenges and limitations of ANIs. In spite of a robust rationale to restore autonomic balance in heart failure supported by favorable preclinical data, the anticipated promising outcome of VNS in clinical populations of heart failure has failed to materialize [255]. The discrepancy between clinical trials is reflected by inconsistency in patient selection, VNS dosing, and stimulation parameters in terms of frequency and intensity. Future studies designed after homogenization of these challenges are imperative to showcase the efficacy of ANIs in various arrhythmias particularly in the heart failure population.
Use of a biomarker to identify appropriate candidates for ANIs is of paramount importance given that heterogeneous characteristics in subjects with heart failure may account for the conflicting results of the VNS clinical trials. This concept is in fact well recognized, aiding appropriate patient selection for AF ablation [251] and for cardiac resynchronization therapy. Furthermore, biomarkers capable of predicting long-term response to ANIs are currently lacking. Although MSNA [256], HRV [256, 257], TNF-α [69], global longitudinal strain [257], and possibly PWA have been shown to change accordingly following acute LLTS in humans, the predictive value of these biomarkers for response to chronic treatment remains elusive. A novel biomarker known as neurotrophic protein S100B, released from cardiac glial cells, appears to correlate with acute cardiac autonomic nerve damage in patients undergoing AF ablation [258]. Importantly, following AF ablation, higher levels of S100B predicted sinus rhythm maintenance during follow-up [258, 259]. Further studies are required to investigate the role of S100B in predicting response of ANIs.
7 Future Directions
7.1 Optogenetics
Optogenetics refers to the capability of specific cell types for optical imaging and genetic targeting [260]. In principle, illumination of genetically targeted brain circuits can regulate excitable tissue via the expression of opsins (light-sensitive microbial proteins) in the target cells [260]. The various functions of opsins as ion channels, ion pumps, or signaling receptors enable light-sensitive, temporally precise, focused low-energy control at cellular, tissue, and organ levels (see also Chap. 17). Application of optogenetics in cardiovascular research involves the use of viruses and exogenous “donor” cells expressing opsins as a means to create optically sensitive cardiac cells and syncytia [261]. Using this technique, two categories of opsins can be created: (1) excitatory opsins (e.g., channelrhodopsin2), which can provide currents sufficient for triggering action potentials, and (2) inhibitory opsins (e.g., archaerhodopsin, halorhodopsin) which suppresses action potentials [262]. The use of optogenetics at the neural-cardiac axis will allow precise dissection of neuro-cardiac interaction, as well as focused dynamic manipulation of cell signaling, neural feedback loop, and specific gene control through light transmission [263].
In an experimental model, left stellate ganglion was optogenetically silenced by using adenovirus vector to deliver archaerhodopsin T (ArchT, an inhibitory opsin) to the neurons within the left stellate ganglion of 20 male beagles [264]. All dogs successfully expressed ArchT, the latter activated by transient light-emitting diode illumination, resulting in significantly suppressed left stellate ganglion function and neural activity, depressed sympathetic indices of HRV, and prolongation of left ventricular ERP and action potential duration. These effects were enhanced by up to 30-min duration of illumination. Crucially, ischemia-induced ventricular arrhythmias were greatly reduced throughout the duration of illumination. All these observations returned to baseline within 2 h after illumination was turned off.
7.2 Transcutaneous Magnetic Stimulation (TCMS)
Although optogenetics presents a noninvasive revenue for autonomic neuromodulation, manipulation of left stellate ganglion activity using this method to reduce sympathetic input to the heart and to suppress ventricular arrhythmias is very much restricted in the preclinical realm [264]. In comparison, transcutaneous magnetic stimulation (TCMS) presents itself as a suitable noninvasive and nondestructive method of neuromodulation [265, 266]. Animal studies demonstrated the feasibility of magnetic stimulation to achieve antiarrhythmic effect in both AF and post-infarct ventricular arrhythmia by targeting cardiac sympathetic activation [267, 268].
Recently the first-in-human TCMS study was performed in five patients with drug-refractory VT storm [269]. In this study, a figure-of-8 TCMS coil was attached to a magnetic stimulation system which was positioned lateral to the C7 spinous process in approximation of the left stellate ganglion. TCMS was conducted at 80% left trapezius motor threshold (0.9 Hz for 60 min). Compared to baseline 24 h preceding TCMS, there was a lower burden of sustained VT in the 48 h ensuing TCMS. Additionally, there were no further external shocks during this period in contrast to an aggregate of 41 shocks prior to TCMS. One patient required subsequent catheter ablation 36 h after enrollment as a result of aborted TCMS from coil overheating. Importantly, no adverse event was reported. Given the promising findings of this case series, a randomized, sham-controlled trial evaluating the safety and efficacy of TCMS in patients with VT storm is currently ongoing (NCT04043312).
8 Conclusions
The two divisions of the cardiac ANS, the SNS and the PNS, play a critical role in supraventricular and ventricular arrhythmogenesis. These arrhythmias arise from distinctive electrophysiologic mechanisms, being mediated by SNS and PNS neurotransmitters as well as co-transmitters interacting at various hierarchies of the neuro-cardiac axis. Knowledge of the intricate autonomic interplay derived from preclinical studies gives rise to exciting clinical application of ANIs in suppression of troublesome atrial arrhythmias and potentially fatal ventricular arrhythmias, albeit with conflicting results, especially in the clinical trials comprising HF patients. Novel methods such as optogenetics and TCMS offer noninvasive ANI tools to modulate arrhythmias. However, clinical trials of various ANIs demonstrating sustainable and meaningful therapeutic outcome in the suppression of various arrhythmias would require conscientious patient selection, optimization of study protocols, as well as utilization of reproducible biomarkers.
References
Shen MJ, Zipes DP. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res. 2014;114(6):1004–21. https://doi.org/10.1161/circresaha.113.302549.
Sharifov OF, Fedorov VV, Beloshapko GG, Glukhov AV, Yushmanova AV, Rosenshtraukh LV. Roles of adrenergic and cholinergic stimulation in spontaneous atrial fibrillation in dogs. J Am Coll Cardiol. 2004;43(3):483–90. https://doi.org/10.1016/j.jacc.2003.09.030.
Frigy A, Csiki E, Caraşca C, Szabó IA, Moga VD. Autonomic influences related to frequent ventricular premature beats in patients without structural heart disease. Medicine (Baltimore). 2018;97(28):e11489. https://doi.org/10.1097/md.0000000000011489.
Levy MN, Martin P. Parasympathetic control of the heart. In: Randall WC, editor. Nervous control of the cardiovascular function. New York: Oxford University Press; 1984. p. 68–94.
Chin SH, Allen E, Brack KE, Ng GA. Effects of sympatho-vagal interaction on ventricular electrophysiology and their modulation during beta-blockade. J Mol Cell Cardiol. 2020;139:201–12. https://doi.org/10.1016/j.yjmcc.2020.01.011.
Herring N, Cranley J, Lokale MN, Li D, Shanks J, Alston EN, et al. The cardiac sympathetic co-transmitter galanin reduces acetylcholine release and vagal bradycardia: implications for neural control of cardiac excitability. J Mol Cell Cardiol. 2012;52(3):667–76. https://doi.org/10.1016/j.yjmcc.2011.11.016.
Tan CMJ, Green P, Tapoulal N, Lewandowski AJ, Leeson P, Herring N. The role of neuropeptide Y in cardiovascular health and disease. Front Physiol. 2018;9:1281. https://doi.org/10.3389/fphys.2018.01281.
Kalla M, Hao G, Tapoulal N, Tomek J, Liu K, Woodward L, et al. The cardiac sympathetic co-transmitter neuropeptide Y is pro-arrhythmic following ST-elevation myocardial infarction despite beta-blockade. Eur Heart J. 2020;41(23):2168–79. https://doi.org/10.1093/eurheartj/ehz852.
Schömig A, Richardt G. The role of catecholamines in ischemia. J Cardiovasc Pharmacol. 1990;16(Suppl 5):S105–12.
Zhu C, Hanna P, Rajendran PS, Shivkumar K. Neuromodulation for ventricular tachycardia and atrial fibrillation: A clinical scenario-based review. J Am Coll Card Clin Electrophysiol. 2019;5(8):881–96. https://doi.org/10.1016/j.jacep.2019.06.009.
Manolis AS, Manolis AA. Exercise and arrhythmias: A double-edged sword. Pacing Clin Electrophysiol. 2016;39(7):748–62. https://doi.org/10.1111/pace.12879.
Buckley U, Shivkumar K. Stress-induced cardiac arrhythmias: The heart-brain interaction. Trends Cardiovasc Med. 2016;26(1):78–80. https://doi.org/10.1016/j.tcm.2015.05.001.
Irvine J, Firestone J, Ong L, Cribbie R, Dorian P, Harris L, et al. A randomized controlled trial of cognitive behavior therapy tailored to psychological adaptation to an implantable cardioverter defibrillator. Psychosom Med. 2011;73(3):226–33. https://doi.org/10.1097/PSY.0b013e31820afc63.
Chevalier P, Cottraux J, Mollard E, Sai N, Brun S, Burri H, et al. Prevention of implantable defibrillator shocks by cognitive behavioral therapy: a pilot trial. Am Heart J. 2006;151(1):191. https://doi.org/10.1016/j.ahj.2005.10.007.
Yamada S, Suzuki H, Kamioka M, Suzuki S, Kamiyama Y, Yoshihisa A, et al. Sleep-disordered breathing increases risk for fatal ventricular arrhythmias in patients with chronic heart failure. Circ J. 2013;77(6):1466–73. https://doi.org/10.1253/circj.cj-12-0836.
Padeletti M, Zacà V, Mondillo S, Jelic S. Sleep-disordered breathing increases the risk of arrhythmias. J Cardiovasc Med (Hagerstown). 2014;15(5):411–6. https://doi.org/10.2459/jcm.0000000000000019.
Manolis TA, Manolis AA, Apostolopoulos E, Melita H, Manolis AS. Cardiovascular complications of sleep disorders: A better night’s sleep for a healthier heart/from bench to bedside. Curr Vasc Pharmacol. 2020;19(2):210–32. https://doi.org/10.2174/1570161118666200325102411.
Armour JA. Potential clinical relevance of the ‘little brain’ on the mammalian heart. Exp Physiol. 2008;93(2):165–76. https://doi.org/10.1113/expphysiol.2007.041178.
Beaumont E, Salavatian S, Southerland EM, Vinet A, Jacquemet V, Armour JA, et al. Network interactions within the canine intrinsic cardiac nervous system: implications for reflex control of regional cardiac function. J Physiol. 2013;591(18):4515–33. https://doi.org/10.1113/jphysiol.2013.259382.
Shivkumar K, Ajijola OA, Anand I, Armour JA, Chen PS, Esler M, et al. Clinical neurocardiology defining the value of neuroscience-based cardiovascular therapeutics. J Physiol. 2016;594(14):3911–54. https://doi.org/10.1113/jp271870.
Sheldon RS, Grubb BP 2nd, Olshansky B, Shen WK, Calkins H, Brignole M, et al. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. 2015;12(6):e41–63. https://doi.org/10.1016/j.hrthm.2015.03.029.
Scridon A, Şerban RC, Chevalier P. Atrial fibrillation: Neurogenic or myogenic? Arch Cardiovasc Dis. 2018;111(1):59–69. https://doi.org/10.1016/j.acvd.2017.11.001.
Tan AY, Zhou S, Ogawa M, Song J, Chu M, Li H, et al. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation. 2008;118(9):916–25. https://doi.org/10.1161/circulationaha.108.776203.
Manolis AS, Linzer M, Salem D, Estes NA 3rd. Syncope: current diagnostic evaluation and management. Ann Intern Med. 1990;112(11):850–63. https://doi.org/10.7326/0003-4819-112-11-850.
van den Berg MP, Crijns HJ, Bouwmeester TR, Smit AJ, Lie KI. Cardiac asystole post-exercise: a report of two cases. Int J Cardiol. 1995;51(3):296–300. https://doi.org/10.1016/0167-5273(95)02432-v.
Hu F, Zheng L, Liang E, Ding L, Wu L, Chen G, et al. Right anterior ganglionated plexus: The primary target of cardioneuroablation? Heart Rhythm. 2019;16(10):1545–51. https://doi.org/10.1016/j.hrthm.2019.07.018.
El-Sherif N, Caref EB, Chinushi M, Restivo M. Mechanism of arrhythmogenicity of the short-long cardiac sequence that precedes ventricular tachyarrhythmias in the long QT syndrome. J Am Coll Cardiol. 1999;33(5):1415–23. https://doi.org/10.1016/s0735-1097(98)00700-1.
Sweeney MO, Ruetz LL, Belk P, Mullen TJ, Johnson JW, Sheldon T. Bradycardia pacing-induced short-long-short sequences at the onset of ventricular tachyarrhythmias: a possible mechanism of proarrhythmia? J Am Coll Cardiol. 2007;50(7):614–22. https://doi.org/10.1016/j.jacc.2007.02.077.
Kawasaki R, Machado C, Reinoehl J, Fromm B, Baga JJ, Steinman RT, et al. Increased propensity of women to develop torsades de pointes during complete heart block. J Cardiovasc Electrophysiol. 1995;6(11):1032–8. https://doi.org/10.1111/j.1540-8167.1995.tb00380.x.
Manolis AA, Manolis TA, Apostolopoulos EJ, Apostolaki NE, Melita H, Manolis AS. The role of the autonomic nervous system in cardiac arrhythmias: The neuro-cardiac axis, more foe than friend? Trends Cardiovasc Med. 2020;31(5):290–302. https://doi.org/10.1016/j.tcm.2020.04.011.
Qin M, Zhang Y, Liu X, Jiang WF, Wu SH, Po S. Atrial ganglionated plexus modification: A novel approach to treat symptomatic sinus bradycardia. J Am Coll Card Clin Electrophysiol. 2017;3(9):950–9. https://doi.org/10.1016/j.jacep.2017.01.022.
Brembilla-Perrot B, Terrier de la Chaise A, Pichené M, Aliot E, Cherrier F, Pernot C. Isoprenaline as an aid to the induction of catecholamine dependent supraventricular tachycardias during programmed stimulation. Br Heart J. 1989;61(4):348–55. https://doi.org/10.1136/hrt.61.4.348.
Manolis AS, Estes NA 3rd. Supraventricular tachycardia. Mechanisms and therapy. Arch Intern Med. 1987;147(10):1706–16.
Cappato R, Castelvecchio S, Ricci C, Bianco E, Vitali-Serdoz L, Gnecchi-Ruscone T, et al. Clinical efficacy of ivabradine in patients with inappropriate sinus tachycardia. A prospective, randomized, placebo-controlled, double-blind, crossover evaluation. J Am Coll Cardiol. 2012;60(15):1323–9. https://doi.org/10.1016/j.jacc.2012.06.031.
Ho SY, Cabrera JA, Tran VH, Farré J, Anderson RH, Sánchez-Quintana D. Architecture of the pulmonary veins: relevance to radiofrequency ablation. Heart. 2001;86(3):265–70. https://doi.org/10.1136/heart.86.3.265.
Tan AY, Li H, Wachsmann-Hogiu S, Chen LS, Chen PS, Fishbein MC. Autonomic innervation and segmental muscular disconnections at the human pulmonary vein-atrial junction: implications for catheter ablation of atrial-pulmonary vein junction. J Am Coll Cardiol. 2006;48(1):132–43. https://doi.org/10.1016/j.jacc.2006.02.054.
Arora R, Ng J, Ulphani J, Mylonas I, Subacius H, Shade G, et al. Unique autonomic profile of the pulmonary veins and posterior left atrium. J Am Coll Cardiol. 2007;49(12):1340–8. https://doi.org/10.1016/j.jacc.2006.10.075.
Chang CM, Wu TJ, Zhou S, Doshi RN, Lee MH, Ohara T, et al. Nerve sprouting and sympathetic hyperinnervation in a canine model of atrial fibrillation produced by prolonged right atrial pacing. Circulation. 2001;103(1):22–5. https://doi.org/10.1161/01.cir.103.1.22.
O’Neal WT, Hammadah M, Sandesara PB, Almuwaqqat Z, Samman-Tahhan A, Gafeer MM, et al. The association between acute mental stress and abnormal left atrial electrophysiology. J Cardiovasc Electrophysiol. 2017;28(10):1151–7. https://doi.org/10.1111/jce.13295.
Shen MJ, Shinohara T, Park HW, Frick K, Ice DS, Choi EK, et al. Continuous low-level vagus nerve stimulation reduces stellate ganglion nerve activity and paroxysmal atrial tachyarrhythmias in ambulatory canines. Circulation. 2011;123(20):2204–12. https://doi.org/10.1161/circulationaha.111.018028.
Sha Y, Scherlag BJ, Yu L, Sheng X, Jackman WM, Lazzara R, et al. Low-level right vagal stimulation: anticholinergic and antiadrenergic effects. J Cardiovasc Electrophysiol. 2011;22(10):1147–53. https://doi.org/10.1111/j.1540-8167.2011.02070.x.
Stavrakis S, Humphrey MB, Scherlag B, Iftikhar O, Parwani P, Abbas M, et al. Low-level vagus nerve stimulation suppresses post-operative atrial fibrillation and inflammation: A randomized study. J Am Coll Card Clin Electrophysiol. 2017;3(9):929–38. https://doi.org/10.1016/j.jacep.2017.02.019.
Yu WC, Chen SA, Chiang CE, Tai CT, Lee SH, Chiou CW, et al. Effects of isoproterenol in facilitating induction of slow-fast atrioventricular nodal reentrant tachycardia. Am J Cardiol. 1996;78(11):1299–302. https://doi.org/10.1016/s0002-9149(96)00607-8.
Manolis AS, Katsaros C, Cokkinos DV. Electrophysiological and electropharmacological studies in pre-excitation syndromes: results with propafenone therapy and isoproterenol infusion testing. Eur Heart J. 1992;13(11):1489–95. https://doi.org/10.1093/oxfordjournals.eurheartj.a060091.
Kocovic DZ, Harada T, Shea JB, Soroff D, Friedman PL. Alterations of heart rate and of heart rate variability after radiofrequency catheter ablation of supraventricular tachycardia. Delineation of parasympathetic pathways in the human heart. Circulation. 1993;88(4 Pt 1):1671–81. https://doi.org/10.1161/01.cir.88.4.1671.
Kubuš P, Vít P, Gebauer RA, Materna O, Janoušek J. Electrophysiologic profile and results of invasive risk stratification in asymptomatic children and adolescents with the Wolff-Parkinson-White electrocardiographic pattern. Circ Arrhythm Electrophysiol. 2014;7(2):218–23. https://doi.org/10.1161/circep.113.000930.
Aleong RG, Singh SM, Levinson JR, Milan DJ. Catecholamine challenge unmasking high-risk features in the Wolff-Parkinson-White syndrome. Europace. 2009;11(10):1396–8. https://doi.org/10.1093/europace/eup211.
Escudero CA, Ceresnak SR, Collins KK, Pass RH, Aziz PF, Blaufox AD, et al. Loss of ventricular preexcitation during noninvasive testing does not exclude high-risk accessory pathways: A multicenter study of WPW in children. Heart Rhythm. 2020;17(10):1729–37. https://doi.org/10.1016/j.hrthm.2020.05.035.
Timmermans C, Smeets JL, Rodriguez LM, Vrouchos G, van den Dool A, Wellens HJ. Aborted sudden death in the Wolff-Parkinson-White syndrome. Am J Cardiol. 1995;76(7):492–4. https://doi.org/10.1016/s0002-9149(99)80136-2.
Manolis AS, Estes NA 3rd. Reversal of electrophysiologic effects of flecainide on the accessory pathway by isoproterenol in the Wolff-Parkinson-White syndrome. Am J Cardiol. 1989;64(3):194–8. https://doi.org/10.1016/0002-9149(89)90456-6.
Polovina MM, Vukicevic M, Banko B, Lip GYH, Potpara TS. Brugada syndrome: A general cardiologist’s perspective. Eur J Intern Med. 2017;44:19–27. https://doi.org/10.1016/j.ejim.2017.06.019.
Thiene G, Nava A, Corrado D, Rossi L, Pennelli N. Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med. 1988;318(3):129–33. https://doi.org/10.1056/nejm198801213180301.
Denis A, Sacher F, Derval N, Martin R, Lim HS, Pambrun T, et al. Arrhythmogenic response to isoproterenol testing vs. exercise testing in arrhythmogenic right ventricular cardiomyopathy patients. Europace. 2018;20(Fi1):f30–f6. https://doi.org/10.1093/europace/euy007.
Ruwald A-C, Marcus F, Estes NAM III, Link M, McNitt S, Polonsky B, et al. Association of competitive and recreational sport participation with cardiac events in patients with arrhythmogenic right ventricular cardiomyopathy: results from the North American multidisciplinary study of arrhythmogenic right ventricular cardiomyopathy. Eur Heart J. 2015;36(27):1735–43. https://doi.org/10.1093/eurheartj/ehv110.
Arya A, Piorkowski C, Sommer P, Gerds-Li JH, Kottkamp H, Hindricks G. Idiopathic outflow tract tachycardias: current perspectives. Herz. 2007;32(3):218–25. https://doi.org/10.1007/s00059-007-2980-5.
Chang HY, Lo LW, Chen YR, Chou YH, Lin WL, Lin YJ, et al. The autonomic neural mechanism of right ventricular outflow tract tachycardia. Auton Neurosci. 2018;212:10–6. https://doi.org/10.1016/j.autneu.2018.03.006.
Chiladakis JA, Vassilikos V, Maounis T, Cokkinos DV, Manolis AS. Unusual features of right and left idiopathic ventricular tachycardia abolished by radiofrequency catheter ablation. Pacing Clin Electrophysiol. 1998;21(9):1831–4. https://doi.org/10.1111/j.1540-8159.1998.tb00288.x.
Conte G, Caputo ML, Regoli F, Marcon S, Klersy C, Adjibodou B, et al. True idiopathic ventricular fibrillation in out-of-hospital cardiac arrest survivors in the Swiss Canton Ticino: prevalence, clinical features, and long-term follow-up. Europace. 2017;19(2):259–66. https://doi.org/10.1093/europace/euv447.
Frontera A, Vlachos K, Kitamura T, Mahida S, Pillois X, Fahy G, et al. Long-term follow-up of idiopathic ventricular fibrillation in a pediatric population: Clinical characteristics, management, and complications. J Am Heart Assoc. 2019;8(9):e011172. https://doi.org/10.1161/jaha.118.011172.
Haïssaguerre M, Shoda M, Jaïs P, Nogami A, Shah DC, Kautzner J, et al. Mapping and ablation of idiopathic ventricular fibrillation. Circulation. 2002;106(8):962–7. https://doi.org/10.1161/01.cir.0000027564.55739.b1.
Kasanuki H, Ohnishi S, Ohtuka M, Matsuda N, Nirei T, Isogai R, et al. Idiopathic ventricular fibrillation induced with vagal activity in patients without obvious heart disease. Circulation. 1997;95(9):2277–85. https://doi.org/10.1161/01.cir.95.9.2277.
Puddu PE, Jouve R, Langlet F, Guillen JC, Lanti M, Reale A. Prevention of postischemic ventricular fibrillation late after right or left stellate ganglionectomy in dogs. Circulation. 1988;77(4):935–46. https://doi.org/10.1161/01.cir.77.4.935.
De Ferrari GM, Vanoli E, Curcuruto P, Tommasini G, Schwartz PJ. Prevention of life-threatening arrhythmias by pharmacologic stimulation of the muscarinic receptors with oxotremorine. Am Heart J. 1992;124(4):883–90. https://doi.org/10.1016/0002-8703(92)90968-2.
Chen PS, Chen LS, Cao JM, Sharifi B, Karagueuzian HS, Fishbein MC. Sympathetic nerve sprouting, electrical remodeling and the mechanisms of sudden cardiac death. Cardiovasc Res. 2001;50(2):409–16. https://doi.org/10.1016/s0008-6363(00)00308-4.
Meng L, Tseng CH, Shivkumar K, Ajijola O. Efficacy of stellate ganglion blockade in managing electrical storm: a systematic review. J Am Coll Card Clin Electrophysiol. 2017;3(9):942–9. https://doi.org/10.1016/j.jacep.2017.06.006.
Ben-Menachem E. Vagus-nerve stimulation for the treatment of epilepsy. Lancet Neurol. 2002;1(8):477–82. https://doi.org/10.1016/s1474-4422(02)00220-x.
Armour JA. Cardiac neuronal hierarchy in health and disease. Am J Physiol Regul Integr Comp Physiol. 2004;287(2):R262–71. https://doi.org/10.1152/ajpregu.00183.2004.
Florea VG, Cohn JN. The autonomic nervous system and heart failure. Circ Res. 2014;114(11):1815–26. https://doi.org/10.1161/circresaha.114.302589.
Stavrakis S, Humphrey MB, Scherlag BJ, Hu Y, Jackman WM, Nakagawa H, et al. Low-level transcutaneous electrical vagus nerve stimulation suppresses atrial fibrillation. J Am Coll Cardiol. 2015;65(9):867–75. https://doi.org/10.1016/j.jacc.2014.12.026.
Stavrakis S, Stoner JA, Humphrey MB, Morris L, Filiberti A, Reynolds JC, et al. TREAT AF (transcutaneous electrical vagus nerve stimulation to suppress atrial fibrillation): A randomized clinical trial. J Am Coll Card Clin Electrophysiol. 2020;6(3):282–91. https://doi.org/10.1016/j.jacep.2019.11.008.
Pokushalov E, Romanov A, Corbucci G, Artyomenko S, Baranova V, Turov A, et al. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol. 2012;60(13):1163–70. https://doi.org/10.1016/j.jacc.2012.05.036.
Feyz L, Theuns DA, Bhagwandien R, Strachinaru M, Kardys I, Van Mieghem NM, et al. Atrial fibrillation reduction by renal sympathetic denervation: 12 months’ results of the AFFORD study. Clin Res Cardiol. 2019;108(6):634–42. https://doi.org/10.1007/s00392-018-1391-3.
Steinberg JS, Shabanov V, Ponomarev D, Losik D, Ivanickiy E, Kropotkin E, et al. Effect of renal denervation and catheter ablation vs catheter ablation alone on atrial fibrillation recurrence among patients with paroxysmal atrial fibrillation and hypertension: The ERADICATE-AF randomized clinical trial. J Am Med Ass. 2020;323(3):248–55. https://doi.org/10.1001/jama.2019.21187.
Kampaktsis PN, Oikonomou EK, Choi DY, Cheung JW. Efficacy of ganglionated plexi ablation in addition to pulmonary vein isolation for paroxysmal versus persistent atrial fibrillation: a meta-analysis of randomized controlled clinical trials. J Interv Card Electrophysiol. 2017;50(3):253–60. https://doi.org/10.1007/s10840-017-0285-z.
Driessen AHG, Berger WR, Krul SPJ, van den Berg NWE, Neefs J, Piersma FR, et al. Ganglion plexus ablation in advanced atrial fibrillation: The AFACT study. J Am Coll Card. 2016;68(11):1155–65. https://doi.org/10.1016/j.jacc.2016.06.036.
Pokushalov E, Romanov A, Katritsis DG, Artyomenko S, Shirokova N, Karaskov A, et al. Ganglionated plexus ablation vs linear ablation in patients undergoing pulmonary vein isolation for persistent/long-standing persistent atrial fibrillation: a randomized comparison. Heart Rhythm. 2013;10(9):1280–6. https://doi.org/10.1016/j.hrthm.2013.04.016.
Katritsis DG, Giazitzoglou E, Zografos T, Pokushalov E, Po SS, Camm AJ. Rapid pulmonary vein isolation combined with autonomic ganglia modification: a randomized study. Heart Rhythm. 2011;8(5):672–8. https://doi.org/10.1016/j.hrthm.2010.12.047.
Katritsis DG, Pokushalov E, Romanov A, Giazitzoglou E, Siontis GC, Po SS, et al. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: a randomized clinical trial. J Am Coll Card. 2013;62(24):2318–25. https://doi.org/10.1016/j.jacc.2013.06.053.
De Ferrari GM, Crijns HJ, Borggrefe M, Milasinovic G, Smid J, Zabel M, et al. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J. 2011;32(7):847–55. https://doi.org/10.1093/eurheartj/ehq391.
Premchand RK, Sharma K, Mittal S, Monteiro R, Dixit S, Libbus I, et al. Autonomic regulation therapy via left or right cervical vagus nerve stimulation in patients with chronic heart failure: results of the ANTHEM-HF trial. J Card Fail. 2014;20(11):808–16. https://doi.org/10.1016/j.cardfail.2014.08.009.
Premchand RK, Sharma K, Mittal S, Monteiro R, Dixit S, Libbus I, et al. Extended follow-up of patients with heart failure receiving autonomic regulation therapy in the ANTHEM-HF study. J Card Fail. 2016;22(8):639–42. https://doi.org/10.1016/j.cardfail.2015.11.002.
Gold MR, Van Veldhuisen DJ, Hauptman PJ, Borggrefe M, Kubo SH, Lieberman RA, et al. Vagus nerve stimulation for the treatment of heart failure: The INOVATE-HF trial. J Am Coll Cardiol. 2016;68(2):149–58. https://doi.org/10.1016/j.jacc.2016.03.525.
Zannad F, De Ferrari GM, Tuinenburg AE, Wright D, Brugada J, Butter C, et al. Chronic vagal stimulation for the treatment of low ejection fraction heart failure: results of the NEural Cardiac TherApy foR Heart Failure (NECTAR-HF) randomized controlled trial. Eur Heart J. 2015;36(7):425–33. https://doi.org/10.1093/eurheartj/ehu345.
Yu L, Huang B, Po SS, Tan T, Wang M, Zhou L, et al. Low-level tragus stimulation for the treatment of ischemia and reperfusion injury in patients with ST-segment elevation myocardial infarction: A proof-of-concept study. J Am Coll Card Cardiovasc Interv. 2017;10(15):1511–20. https://doi.org/10.1016/j.jcin.2017.04.036.
Hopper I, Gronda E, Hoppe UC, Rundqvist B, Marwick TH, Shetty S, et al. Sympathetic response and outcomes following renal denervation in patients with chronic heart failure: 12-Month outcomes from the symplicity HF feasibility study. J Card Fail. 2017;23(9):702–7. https://doi.org/10.1016/j.cardfail.2017.06.004.
Tse HF, Turner S, Sanders P, Okuyama Y, Fujiu K, Cheung CW, et al. Thoracic spinal cord stimulation for heart failure as a restorative treatment (SCS HEART study): first-in-man experience. Heart Rhythm. 2015;12(3):588–95. https://doi.org/10.1016/j.hrthm.2014.12.014.
Zipes DP, Neuzil P, Theres H, Caraway D, Mann DL, Mannheimer C, et al. Determining the feasibility of spinal cord neuromodulation for the treatment of chronic systolic heart failure: The DEFEAT-HF study. J Am Coll Card Heart Fail. 2016;4(2):129–36. https://doi.org/10.1016/j.jchf.2015.10.006.
Abraham WT, Zile MR, Weaver FA, Butter C, Ducharme A, Halbach M, et al. Baroreflex activation therapy for the treatment of heart failure with a reduced ejection fraction. J Am Coll Card Heart Fail. 2015;3(6):487–96. https://doi.org/10.1016/j.jchf.2015.02.006.
Zile MR, Lindenfeld J, Weaver FA, Zannad F, Galle E, Rogers T, et al. Baroreflex activation therapy in patients with heart failure with reduced ejection fraction. J Am Coll Card. 2020;76(1):1–13. https://doi.org/10.1016/j.jacc.2020.05.015.
Salavatian S, Beaumont E, Longpré JP, Armour JA, Vinet A, Jacquemet V, et al. Vagal stimulation targets select populations of intrinsic cardiac neurons to control neurally induced atrial fibrillation. Am J Physiol Heart Circ Physiol. 2016;311(5):H1311–20. https://doi.org/10.1152/ajpheart.00443.2016.
Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci USA. 2016;113(29):8284–9. https://doi.org/10.1073/pnas.1605635113.
Gomes-Osman J, Indahlastari A, Fried PJ, Cabral DLF, Rice J, Nissim NR, et al. Non-invasive brain stimulation: Probing intracortical circuits and improving cognition in the aging brain. Front Aging Neurosci. 2018;10:177. https://doi.org/10.3389/fnagi.2018.00177.
Ashton JL, Burton RAB, Bub G, Smaill BH, Montgomery JM. Synaptic plasticity in cardiac innervation and its potential role in atrial fibrillation. Front Physiol. 2018;9:240. https://doi.org/10.3389/fphys.2018.00240.
Ando M, Katare RG, Kakinuma Y, Zhang D, Yamasaki F, Muramoto K, et al. Efferent vagal nerve stimulation protects heart against ischemia-induced arrhythmias by preserving connexin43 protein. Circulation. 2005;112(2):164–70. https://doi.org/10.1161/circulationaha.104.525493.
Stavrakis S, Scherlag BJ, Fan Y, Liu Y, Mao J, Varma V, et al. Inhibition of atrial fibrillation by low-level vagus nerve stimulation: the role of the nitric oxide signaling pathway. J Interv Card Electrophysiol. 2013;36(3):199–208. https://doi.org/10.1007/s10840-012-9752-8.
Zhou L, Filiberti A, Humphrey MB, Fleming CD, Scherlag BJ, Po SS, et al. Low-level transcutaneous vagus nerve stimulation attenuates cardiac remodelling in a rat model of heart failure with preserved ejection fraction. Exp Physiol. 2019;104(1):28–38. https://doi.org/10.1113/ep087351.
Zhang Y, Popovic ZB, Bibevski S, Fakhry I, Sica DA, Van Wagoner DR, et al. Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circ Heart Fail. 2009;2(6):692–9. https://doi.org/10.1161/circheartfailure.109.873968.
Beaumont E, Southerland EM, Hardwick JC, Wright GL, Ryan S, Li Y, et al. Vagus nerve stimulation mitigates intrinsic cardiac neuronal and adverse myocyte remodeling postmyocardial infarction. Am J Physiol Heart Circ Physiol. 2015;309(7):H1198–206. https://doi.org/10.1152/ajpheart.00393.2015.
Ardell JL, Foreman RD, Armour JA, Shivkumar K. Cardiac sympathectomy and spinal cord stimulation attenuate reflex-mediated norepinephrine release during ischemia preventing ventricular fibrillation. JCI Insight. 2019;4(23):e131648. https://doi.org/10.1172/jci.insight.131648.
Martínez-Milla J, Raposeiras-Roubín S, Pascual-Figal DA, Ibáñez B. Role of beta-blockers in cardiovascular disease in 2019. Rev Esp Cardiol (Engl Ed). 2019;72(10):844–52. https://doi.org/10.1016/j.rec.2019.04.014.
Lampert R, Burg MM, Jamner LD, Dziura J, Brandt C, Li F, et al. Effect of β-blockers on triggering of symptomatic atrial fibrillation by anger or stress. Heart Rhythm. 2019;16(8):1167–73. https://doi.org/10.1016/j.hrthm.2019.03.004.
Plewan A, Lehmann G, Ndrepepa G, Schreieck J, Alt EU, Schömig A, et al. Maintenance of sinus rhythm after electrical cardioversion of persistent atrial fibrillation; sotalol vs bisoprolol. Eur Heart J. 2001;22(16):1504–10. https://doi.org/10.1053/euhj.2000.2546.
Liu L, Nattel S. Differing sympathetic and vagal effects on atrial fibrillation in dogs: role of refractoriness heterogeneity. Am J Physiol. 1997;273(2 Pt 2):H805–16. https://doi.org/10.1152/ajpheart.1997.273.2.H805.
Zhang Y, Ilsar I, Sabbah HN, Ben David T, Mazgalev TN. Relationship between right cervical vagus nerve stimulation and atrial fibrillation inducibility: therapeutic intensities do not increase arrhythmogenesis. Heart Rhythm. 2009;6(2):244–50. https://doi.org/10.1016/j.hrthm.2008.10.043.
Yu L, Scherlag BJ, Li S, Sheng X, Lu Z, Nakagawa H, et al. Low-level vagosympathetic nerve stimulation inhibits atrial fibrillation inducibility: direct evidence by neural recordings from intrinsic cardiac ganglia. J Cardiovasc Electrophysiol. 2011;22(4):455–63. https://doi.org/10.1111/j.1540-8167.2010.01908.x.
Sheng X, Scherlag BJ, Yu L, Li S, Ali R, Zhang Y, et al. Prevention and reversal of atrial fibrillation inducibility and autonomic remodeling by low-level vagosympathetic nerve stimulation. J Am Coll Cardiol. 2011;57(5):563–71. https://doi.org/10.1016/j.jacc.2010.09.034.
Li S, Scherlag BJ, Yu L, Sheng X, Zhang Y, Ali R, et al. Low-level vagosympathetic stimulation: a paradox and potential new modality for the treatment of focal atrial fibrillation. Circ Arrhythm Electrophysiol. 2009;2(6):645–51. https://doi.org/10.1161/circep.109.868331.
Chinda K, Tsai WC, Chan YH, Lin AY, Patel J, Zhao Y, et al. Intermittent left cervical vagal nerve stimulation damages the stellate ganglia and reduces the ventricular rate during sustained atrial fibrillation in ambulatory dogs. Heart Rhythm. 2016;13(3):771–80. https://doi.org/10.1016/j.hrthm.2015.11.031.
Pavlov VA, Chavan SS, Tracey KJ. Molecular and functional neuroscience in immunity. Annu Rev Immunol. 2018;36:783–812. https://doi.org/10.1146/annurev-immunol-042617-053158.
Fallgatter AJ, Neuhauser B, Herrmann MJ, Ehlis AC, Wagener A, Scheuerpflug P, et al. Far field potentials from the brain stem after transcutaneous vagus nerve stimulation. J Neural Transm (Vienna). 2003;110(12):1437–43. https://doi.org/10.1007/s00702-003-0087-6.
Yu L, Scherlag BJ, Li S, Fan Y, Dyer J, Male S, et al. Low-level transcutaneous electrical stimulation of the auricular branch of the vagus nerve: a noninvasive approach to treat the initial phase of atrial fibrillation. Heart Rhythm. 2013;10(3):428–35. https://doi.org/10.1016/j.hrthm.2012.11.019.
Deuchars SA, Lall VK, Clancy J, Mahadi M, Murray A, Peers L, et al. Mechanisms underpinning sympathetic nervous activity and its modulation using transcutaneous vagus nerve stimulation. Exp Physiol. 2018;103(3):326–31. https://doi.org/10.1113/ep086433.
Frangos E, Ellrich J, Komisaruk BR. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimul. 2015;8(3):624–36. https://doi.org/10.1016/j.brs.2014.11.018.
Verlinden TJ, Rijkers K, Hoogland G, Herrler A. Morphology of the human cervical vagus nerve: implications for vagus nerve stimulation treatment. Acta Neurol Scand. 2016;133(3):173–82. https://doi.org/10.1111/ane.12462.
Linz D, Ukena C, Mahfoud F, Neuberger HR, Böhm M. Atrial autonomic innervation: a target for interventional antiarrhythmic therapy? J Am Coll Cardiol. 2014;63(3):215–24. https://doi.org/10.1016/j.jacc.2013.09.020.
Linz D, Mahfoud F, Schotten U, Ukena C, Neuberger HR, Wirth K, et al. Renal sympathetic denervation suppresses postapneic blood pressure rises and atrial fibrillation in a model for sleep apnea. Hypertension. 2012;60(1):172–8. https://doi.org/10.1161/hypertensionaha.112.191965.
Linz D, van Hunnik A, Hohl M, Mahfoud F, Wolf M, Neuberger HR, et al. Catheter-based renal denervation reduces atrial nerve sprouting and complexity of atrial fibrillation in goats. Circ Arrhythm Electrophysiol. 2015;8(2):466–74. https://doi.org/10.1161/circep.114.002453.
Aksu T, Güler TE, Mutluer FO, Oto MA. Vagal denervation in atrial fibrillation ablation: A comprehensive review. Anatol J Cardiol. 2017;18(2):142–8. https://doi.org/10.14744/AnatolJCardiol.2017.7788.
Chiou CW, Eble JN, Zipes DP. Efferent vagal innervation of the canine atria and sinus and atrioventricular nodes. The third fat pad. Circulation. 1997;95(11):2573–84. https://doi.org/10.1161/01.cir.95.11.2573.
Fadahunsi O, Talabi T, Olowoyeye A, Iluyomade A, Shogbesan O, Donato A. Ablation of complex fractionated atrial electrograms for atrial fibrillation rhythm control: A systematic review and meta-analysis. Can J Cardiol. 2016;32(6):791–802. https://doi.org/10.1016/j.cjca.2015.07.008.
You DJ, Chang D, Zhang SL, Yang DH, Gao LJ, Yin XM, et al. Substrate of complex fractionated atrial electrograms: evidence by pathologic analysis. Chin Med J (Engl). 2012;125(24):4393–7.
Lin J, Scherlag BJ, Zhou J, Lu Z, Patterson E, Jackman WM, et al. Autonomic mechanism to explain complex fractionated atrial electrograms (CFAE). J Cardiovasc Electrophysiol. 2007;18(11):1197–205. https://doi.org/10.1111/j.1540-8167.2007.00976.x.
Qin M, Liu X, Wu SH, Zhang XD. Atrial substrate modification in atrial fibrillation: Targeting GP or CFAE? Evidence from meta-analysis of clinical trials. PLoS One. 2016;11(10):e0164989. https://doi.org/10.1371/journal.pone.0164989.
Sakamoto S, Schuessler RB, Lee AM, Aziz A, Lall SC, Damiano RJ Jr. Vagal denervation and reinnervation after ablation of ganglionated plexi. J Thorac Cardiovasc Surg. 2010;139(2):444–52. https://doi.org/10.1016/j.jtcvs.2009.04.056.
Jungen C, Scherschel K, Eickholt C, Kuklik P, Klatt N, Bork N, et al. Disruption of cardiac cholinergic neurons enhances susceptibility to ventricular arrhythmias. Nat Commun. 2017;8:14155. https://doi.org/10.1038/ncomms14155.
Osman F, Kundu S, Tuan J, Jeilan M, Stafford PJ, Ng GA. Ganglionic plexus ablation during pulmonary vein isolation—predisposing to ventricular arrhythmias? Indian Pacing Electrophysiol J. 2010;10(2):104–7.
Kemler MA, de Vet HC, Barendse GA, van den Wildenberg FA, van Kleef M. Spinal cord stimulation for chronic reflex sympathetic dystrophy—five-year follow-up. N Engl J Med. 2006;354(22):2394–6. https://doi.org/10.1056/NEJMc055504.
Brodison A, Chauhan A. Spinal-cord stimulation in management of angina. Lancet. 1999;354(9192):1748–9. https://doi.org/10.1016/s0140-6736(99)00296-2.
Ardell JL. Heart failure: Mechanisms of spinal cord neuromodulation for heart disease. Nat Rev Cardiol. 2016;13(3):127–8. https://doi.org/10.1038/nrcardio.2016.8.
Bernstein SA, Wong B, Vasquez C, Rosenberg SP, Rooke R, Kuznekoff LM, et al. Spinal cord stimulation protects against atrial fibrillation induced by tachypacing. Heart Rhythm. 2012;9(9):1426–33.e3. https://doi.org/10.1016/j.hrthm.2012.04.038.
Yu L, Huang B, He W, Wang S, Liao K, Zhou X, et al. Spinal cord stimulation suppresses focal rapid firing-induced atrial fibrillation by inhibiting atrial ganglionated plexus activity. J Cardiovasc Pharmacol. 2014;64(6):554–9. https://doi.org/10.1097/fjc.0000000000000154.
Wang S, Zhou X, Huang B, Wang Z, Zhou L, Chen M, et al. Spinal cord stimulation suppresses atrial fibrillation by inhibiting autonomic remodeling. Heart Rhythm. 2016;13(1):274–81. https://doi.org/10.1016/j.hrthm.2015.08.018.
Scheffers IJ, Kroon AA, de Leeuw PW. Carotid baroreflex activation: past, present, and future. Curr Hypertens Rep. 2010;12(2):61–6. https://doi.org/10.1007/s11906-009-0087-5.
Carlsten A, Folkow B, Grimby G, Hamberger CA, Thulesius O. Cardiovascular effects of direct stimulation of the carotid sinus nerve in man. Acta Physiol Scand. 1958;44(2):138–45. https://doi.org/10.1111/j.1748-1716.1958.tb01615.x.
Schwartz SI, Griffith LS, Neistadt A, Hagfors N. Chronic carotid sinus nerve stimulation in the treatment of essential hypertension. Am J Surg. 1967;114(1):5–15. https://doi.org/10.1016/0002-9610(67)90034-7.
Mohaupt MG, Schmidli J, Luft FC. Management of uncontrollable hypertension with a carotid sinus stimulation device. Hypertension. 2007;50(5):825–8. https://doi.org/10.1161/hypertensionaha.107.099416.
Linz D, Mahfoud F, Schotten U, Ukena C, Neuberger HR, Wirth K, et al. Effects of electrical stimulation of carotid baroreflex and renal denervation on atrial electrophysiology. J Cardiovasc Electrophysiol. 2013;24(9):1028–33. https://doi.org/10.1111/jce.12171.
Heusser K, Tank J, Engeli S, Diedrich A, Menne J, Eckert S, et al. Carotid baroreceptor stimulation, sympathetic activity, baroreflex function, and blood pressure in hypertensive patients. Hypertension. 2010;55(3):619–26. https://doi.org/10.1161/hypertensionaha.109.140665.
Liao K, Yu L, Zhou X, Saren G, Wang S, Wang Z, et al. Low-level baroreceptor stimulation suppresses atrial fibrillation by inhibiting ganglionated plexus activity. Can J Cardiol. 2015;31(6):767–74. https://doi.org/10.1016/j.cjca.2015.01.007.
Dai M, Bao M, Liao J, Yu L, Tang Y, Huang H, et al. Effects of low-level carotid baroreflex stimulation on atrial electrophysiology. J Interv Card Electrophysiol. 2015;43(2):111–9. https://doi.org/10.1007/s10840-015-9976-5.
Al-Gobari M, El Khatib C, Pillon F, Gueyffier F. β-Blockers for the prevention of sudden cardiac death in heart failure patients: a meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. 2013;13:52. https://doi.org/10.1186/1471-2261-13-52.
Ackerman MJ, Priori SG, Dubin AM, Kowey P, Linker NJ, Slotwiner D, et al. Beta-blocker therapy for long QT syndrome and catecholaminergic polymorphic ventricular tachycardia: Are all beta-blockers equivalent? Heart Rhythm. 2017;14(1):e41–e4. https://doi.org/10.1016/j.hrthm.2016.09.012.
Herring N, Kalla M, Paterson DJ. The autonomic nervous system and cardiac arrhythmias: current concepts and emerging therapies. Nat Rev Cardiol. 2019;16(12):707–26. https://doi.org/10.1038/s41569-019-0221-2.
Olde Nordkamp LR, Driessen AH, Odero A, Blom NA, Koolbergen DR, Schwartz PJ et al. Left cardiac sympathetic denervation in the Netherlands for the treatment of inherited arrhythmia syndromes. Neth Heart J. 2014;22(4):160-166. doi:https://doi.org/10.1007/s12471-014-0523-2.
Collura CA, Johnson JN, Moir C, Ackerman MJ. Left cardiac sympathetic denervation for the treatment of long QT syndrome and catecholaminergic polymorphic ventricular tachycardia using video-assisted thoracic surgery. Heart Rhythm. 2009;6(6):752–9. https://doi.org/10.1016/j.hrthm.2009.03.024.
Odero A, Bozzani A, De Ferrari GM, Schwartz PJ. Left cardiac sympathetic denervation for the prevention of life-threatening arrhythmias: the surgical supraclavicular approach to cervicothoracic sympathectomy. Heart Rhythm. 2010;7(8):1161–5. https://doi.org/10.1016/j.hrthm.2010.03.046.
Hayase J, Vampola S, Ahadian F, Narayan SM, Krummen DE. Comparative efficacy of stellate ganglion block with bupivacaine vs pulsed radiofrequency in a patient with refractory ventricular arrhythmias. J Clin Anesth. 2016;31:162–5. https://doi.org/10.1016/j.jclinane.2016.01.026.
Shah R, Assis F, Alugubelli N, Okada DR, Cardoso R, Shivkumar K, et al. Cardiac sympathetic denervation for refractory ventricular arrhythmias in patients with structural heart disease: A systematic review. Heart Rhythm. 2019;16(10):1499–505. https://doi.org/10.1016/j.hrthm.2019.06.018.
Schwartz PJ, Locati EH, Moss AJ, Crampton RS, Trazzi R, Ruberti U. Left cardiac sympathetic denervation in the therapy of congenital long QT syndrome. A worldwide report. Circulation. 1991;84(2):503–11. https://doi.org/10.1161/01.cir.84.2.503.
Schwartz PJ, Priori SG, Cerrone M, Spazzolini C, Odero A, Napolitano C, et al. Left cardiac sympathetic denervation in the management of high-risk patients affected by the long-QT syndrome. Circulation. 2004;109(15):1826–33. https://doi.org/10.1161/01.Cir.0000125523.14403.1e.
Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10(12):1932–63. https://doi.org/10.1016/j.hrthm.2013.05.014.
Wilde AA, Bhuiyan ZA, Crotti L, Facchini M, De Ferrari GM, Paul T, et al. Left cardiac sympathetic denervation for catecholaminergic polymorphic ventricular tachycardia. N Engl J Med. 2008;358(19):2024–9. https://doi.org/10.1056/NEJMoa0708006.
Vaseghi M, Barwad P, Malavassi Corrales FJ, Tandri H, Mathuria N, Shah R, et al. Cardiac sympathetic denervation for refractory ventricular arrhythmias. J Am Coll Cardiol. 2017;69(25):3070–80. https://doi.org/10.1016/j.jacc.2017.04.035.
Zipes DP, Rubart M. Neural modulation of cardiac arrhythmias and sudden cardiac death. Heart Rhythm. 2006;3(1):108–13. https://doi.org/10.1016/j.hrthm.2005.09.021.
Schwartz PJ, Billman GE, Stone HL. Autonomic mechanisms in ventricular fibrillation induced by myocardial ischemia during exercise in dogs with healed myocardial infarction. An experimental preparation for sudden cardiac death. Circulation. 1984;69(4):790–800. https://doi.org/10.1161/01.cir.69.4.790.
Martins JB, Zipes DP, Lund DD. Distribution of local repolarization changes produced by efferent vagal stimulation in the canine ventricles. J Am Coll Cardiol. 1983;2(6):1191–9. https://doi.org/10.1016/s0735-1097(83)80350-7.
Litovsky SH, Antzelevitch C. Differences in the electrophysiological response of canine ventricular subendocardium and subepicardium to acetylcholine and isoproterenol. A direct effect of acetylcholine in ventricular myocardium. Circ Res. 1990;67(3):615–27. https://doi.org/10.1161/01.res.67.3.615.
Ng GA, Brack KE, Patel VH, Coote JH. Autonomic modulation of electrical restitution, alternans and ventricular fibrillation initiation in the isolated heart. Cardiovasc Res. 2007;73(4):750–60. https://doi.org/10.1016/j.cardiores.2006.12.001.
Brack KE, Patel VH, Mantravardi R, Coote JH, Ng GA. Direct evidence of nitric oxide release from neuronal nitric oxide synthase activation in the left ventricle as a result of cervical vagus nerve stimulation. J Physiol. 2009;587(Pt 12):3045–54. https://doi.org/10.1113/jphysiol.2009.169417.
Wang Z, Yu L, Wang S, Huang B, Liao K, Saren G, et al. Chronic intermittent low-level transcutaneous electrical stimulation of auricular branch of vagus nerve improves left ventricular remodeling in conscious dogs with healed myocardial infarction. Circ Heart Fail. 2014;7(6):1014–21. https://doi.org/10.1161/circheartfailure.114.001564.
Vanoli E, De Ferrari GM, Stramba-Badiale M, Hull SS Jr, Foreman RD, Schwartz PJ. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res. 1991;68(5):1471–81. https://doi.org/10.1161/01.res.68.5.1471.
Ardell JL, Nier H, Hammer M, Southerland EM, Ardell CL, Beaumont E, et al. Defining the neural fulcrum for chronic vagus nerve stimulation: implications for integrated cardiac control. J Physiol. 2017;595(22):6887–903. https://doi.org/10.1113/jp274678.
Konstam MA, Udelson JE, Butler J, Klein HU, Parker JD, Teerlink JR, et al. Impact of autonomic regulation therapy in patients with heart failure: ANTHEM-HFrEF pivotal study design. Circ Heart Fail. 2019;12(11):e005879. https://doi.org/10.1161/circheartfailure.119.005879.
Ben-Menachem E. Vagus nerve stimulation, side effects, and long-term safety. J Clin Neurophysiol. 2001;18(5):415–8. https://doi.org/10.1097/00004691-200109000-00005.
Spuck S, Tronnier V, Orosz I, Schönweiler R, Sepehrnia A, Nowak G, et al. Operative and technical complications of vagus nerve stimulator implantation. Neurosurgery. 2010;67(2 suppl operative):489–94. https://doi.org/10.1227/NEU.0b013e3181f88867.
Yu L, Wang S, Zhou X, Wang Z, Huang B, Liao K, et al. Chronic intermittent low-level stimulation of tragus reduces cardiac autonomic remodeling and ventricular arrhythmia inducibility in a post-infarction canine model. J Am Coll Card Clin Electrophysiol. 2016;2(3):330–9. https://doi.org/10.1016/j.jacep.2015.11.006.
Böhm M, Ewen S, Linz D, Reil JC, Schirmer SH, Ukena C, et al. Therapeutic potential of renal sympathetic denervation in patients with chronic heart failure. EuroIntervention. 2013;9 Suppl R:R122–6. https://doi.org/10.4244/eijv9sra21.
Remo BF, Preminger M, Bradfield J, Mittal S, Boyle N, Gupta A, et al. Safety and efficacy of renal denervation as a novel treatment of ventricular tachycardia storm in patients with cardiomyopathy. Heart Rhythm. 2014;11(4):541–6. https://doi.org/10.1016/j.hrthm.2013.12.038.
Bradfield JS, Hayase J, Liu K, Moriarty J, Kee ST, Do D, et al. Renal denervation as adjunctive therapy to cardiac sympathetic denervation for ablation refractory ventricular tachycardia. Heart Rhythm. 2020;17(2):220–7. https://doi.org/10.1016/j.hrthm.2019.09.016.
Southerland EM, Milhorn DM, Foreman RD, Linderoth B, DeJongste MJ, Armour JA, et al. Preemptive, but not reactive, spinal cord stimulation mitigates transient ischemia-induced myocardial infarction via cardiac adrenergic neurons. Am J Physiol Heart Circ Physiol. 2007;292(1):H311–7. https://doi.org/10.1152/ajpheart.00087.2006.
Issa ZF, Zhou X, Ujhelyi MR, Rosenberger J, Bhakta D, Groh WJ, et al. Thoracic spinal cord stimulation reduces the risk of ischemic ventricular arrhythmias in a postinfarction heart failure canine model. Circulation. 2005;111(24):3217–20. https://doi.org/10.1161/circulationaha.104.507897.
Lopshire JC, Zhou X, Dusa C, Ueyama T, Rosenberger J, Courtney N, et al. Spinal cord stimulation improves ventricular function and reduces ventricular arrhythmias in a canine postinfarction heart failure model. Circulation. 2009;120(4):286–94. https://doi.org/10.1161/circulationaha.108.812412.
Grimaldi R, de Luca A, Kornet L, Castagno D, Gaita F. Can spinal cord stimulation reduce ventricular arrhythmias? Heart Rhythm. 2012;9(11):1884–7. https://doi.org/10.1016/j.hrthm.2012.08.007.
Liao K, Yu L, He B, Huang B, Yang K, Saren G, et al. Carotid baroreceptor stimulation prevents arrhythmias induced by acute myocardial infarction through autonomic modulation. J Cardiovasc Pharmacol. 2014;64(5):431–7. https://doi.org/10.1097/fjc.0000000000000135.
Liao K, Yu L, Yang K, Saren G, Wang S, Huang B, et al. Low-level carotid baroreceptor stimulation suppresses ventricular arrhythmias during acute ischemia. PLoS One. 2014;9(10):e109313. https://doi.org/10.1371/journal.pone.0109313.
Sabbah HN, Gupta RC, Imai M, Irwin ED, Rastogi S, Rossing MA, et al. Chronic electrical stimulation of the carotid sinus baroreflex improves left ventricular function and promotes reversal of ventricular remodeling in dogs with advanced heart failure. Circ Heart Fail. 2011;4(1):65–70. https://doi.org/10.1161/circheartfailure.110.955013.
Zucker IH, Hackley JF, Cornish KG, Hiser BA, Anderson NR, Kieval R, et al. Chronic baroreceptor activation enhances survival in dogs with pacing-induced heart failure. Hypertension. 2007;50(5):904–10. https://doi.org/10.1161/hypertensionaha.107.095216.
Gronda E, Seravalle G, Brambilla G, Costantino G, Casini A, Alsheraei A, et al. Chronic baroreflex activation effects on sympathetic nerve traffic, baroreflex function, and cardiac haemodynamics in heart failure: a proof-of-concept study. Eur J Heart Fail. 2014;16(9):977–83. https://doi.org/10.1002/ejhf.138.
Musialek P, Casadei B. Nitrovasodilators and heart rate: more than the arterial baroreflex. Cardiovasc. Res. 2000;47(2):404–5.
Casadei B, Paterson DJ. Should we still use nitrovasodilators to test baroreflex sensitivity? J Hyperten. 2000;18(1):3–6.
Parati G, Di Rienzo M, Mancia G. How to measure baroreflex sensitivity: from the cardiovascular laboratory to daily life. J Hyperten. 2000;18(1):7–19.
Schwartz PJ, Vanoli E, Stramba-Badiale M, De Ferrari GM, Billman GE, Foreman RD. Autonomic mechanisms and sudden death. New insights from analysis of baroreceptor reflexes in conscious dogs with and without a myocardial infarction. Circulation. 1988;78(4):969–79.
Taggart P, Sutton P, Chalabi Z, Boyett MR, Simon R, Elliott D, et al. Effect of adrenergic stimulation on action potential duration restitution in humans. Circulation. 2003;107(2):285–9.
Schwartz PJ, Zaza A, Pala M, Locati E, Beria G, Zanchetti A. Baroreflex sensitivity and its evolution during the first year after myocardial infarction. J Am Coll Card. 1988;12(3):629–36.
La Rovere MT, Bigger JT Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351(9101):478–84.
Paleczny B, Olesińska-Mader M, Siennicka A, Niewiński P, Nowak K, Buldańczyk A, et al. Assessment of baroreflex sensitivity has no prognostic value in contemporary, optimally managed patients with mild-to-moderate heart failure with reduced ejection fraction: a retrospective analysis of 5-year survival. Eur J Heart Fail. 2019;21(1):50–8. https://doi.org/10.1002/ejhf.1306.
Schwab JO, Eichner G, Schmitt H, Weber S, Coch M, Waldecker B. The relative contribution of the sinus and AV node to heart rate variability. Heart. 2003;89(3):337–8. https://doi.org/10.1136/heart.89.3.337.
Stauss HM. Heart rate variability. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):R927–31. https://doi.org/10.1152/ajpregu.00452.2003.
Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043–65.
Koizumi K, Terui N, Kollai M. Effect of cardiac vagal and sympathetic nerve activity on heart rate in rhythmic fluctuations. J Auton Nerv Syst. 1985;12(2-3):251–9. https://doi.org/10.1016/0165-1838(85)90065-7.
Kleiger RE, Stein PK, Bigger JT Jr. Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiol. 2005;10(1):88–101. https://doi.org/10.1111/j.1542-474X.2005.10101.x.
Shaffer F, McCraty R, Zerr CL. A healthy heart is not a metronome: an integrative review of the heart’s anatomy and heart rate variability. Front Psychol. 2014;5:1040. https://doi.org/10.3389/fpsyg.2014.01040.
Zulfiqar U, Jurivich DA, Gao W, Singer DH. Relation of high heart rate variability to healthy longevity. Am J Cardiol. 2010;105(8):1181–5. https://doi.org/10.1016/j.amjcard.2009.12.022.
Umetani K, Singer DH, McCraty R, Atkinson M. Twenty-four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J Am Coll Cardiol. 1998;31(3):593–601. https://doi.org/10.1016/s0735-1097(97)00554-8.
Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213(4504):220–2. https://doi.org/10.1126/science.6166045.
Butler GC, Ando S, Floras JS. Fractal component of variability of heart rate and systolic blood pressure in congestive heart failure. Clinical science. 1997;92(6):543–50.
Hull SS Jr, Evans AR, Vanoli E, Adamson PB, Stramba-Badiale M, Albert DE, et al. Heart rate variability before and after myocardial infarction in conscious dogs at high and low risk of sudden death. J Am Coll Card. 1990;16(4):978–85.
Adamson PB, Huang MH, Vanoli E, Foreman RD, Schwartz PJ, Hull SS Jr. Unexpected interaction between beta-adrenergic blockade and heart rate variability before and after myocardial infarction. A longitudinal study in dogs at high and low risk for sudden death. Circulation. 1994;90(2):976–82.
Farrell TG, Bashir Y, Cripps T, Malik M, Poloniecki J, Bennett ED, et al. Risk stratification for arrhythmic events in postinfarction patients based on heart rate variability, ambulatory electrocardiographic variables and the signal-averaged electrocardiogram. J Am Coll Card. 1991;18(3):687–97.
Kleiger RE, Miller JP, Bigger JT Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Card. 1987;59(4):256–62.
Odemuyiwa O, Poloniecki J, Malik M, Farrell T, Xia R, Staunton A, et al. Temporal influences on the prediction of postinfarction mortality by heart rate variability: a comparison with the left ventricular ejection fraction. Br Heart J. 1994;71(6):521–7.
Lanza GA, Bendini MG, Intini A, De Martino G, Galeazzi M, Guido V, et al. Prognostic role of heart rate variability in patients with idiopathic dilated cardiomyopathy. Ital Heart J. 2000;1(1):56–63.
Hoffmann J, Grimm W, Menz V, Knop U, Maisch B. Heart rate variability and major arrhythmic events in patients with idiopathic dilated cardiomyopathy. Pacing Clin Electrophysiol PACE. 1996;19(11 Pt 2):1841–4.
Pagani M, Lombardi F, Guzzetti S, Sandrone G, Rimoldi O, Malfatto G, et al. Power spectral density of heart rate variability as an index of sympatho-vagal interaction in normal and hypertensive subjects. J Hypertens Suppl. 1984;2(3):S383–5.
Vanninen E, Tuunainen A, Kansanen M, Uusitupa M, Länsimies E. Cardiac sympathovagal balance during sleep apnea episodes. Clin Physiol. 1996;16(3):209–16. https://doi.org/10.1111/j.1475-097x.1996.tb00569.x.
Billman GE. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front Physiol. 2013;4:26. https://doi.org/10.3389/fphys.2013.00026.
Voss A, Fischer C, Schroeder R, Figulla HR, Goernig M. Segmented Poincaré plot analysis for risk stratification in patients with dilated cardiomyopathy. Methods Inf Med. 2010;49(5):511–5. https://doi.org/10.3414/me09-02-0050.
Libbus I, Nearing BD, Amurthur B, KenKnight BH, Verrier RL. Quantitative evaluation of heartbeat interval time series using Poincaré analysis reveals distinct patterns of heart rate dynamics during cycles of vagus nerve stimulation in patients with heart failure. J Electrocardiol. 2017;50(6):898–903. https://doi.org/10.1016/j.jelectrocard.2017.06.007.
Brennan M, Palaniswami M, Kamen P. Do existing measures of Poincaré plot geometry reflect nonlinear features of heart rate variability? IEEE Trans Biomed Eng. 2001;48(11):1342–7. https://doi.org/10.1109/10.959330.
Beckers F, Ramaekers D, Aubert AE. Approximate entropy of heart rate variability: validation of methods and application in heart failure. Cardiovasc Eng. 2001;1(4):177–82. https://doi.org/10.1023/A:1015212328405.
Merati G, Di Rienzo M, Parati G, Veicsteinas A, Castiglioni P. Assessment of the autonomic control of heart rate variability in healthy and spinal-cord injured subjects: contribution of different complexity-based estimators. IEEE Trans Biomed Eng. 2006;53(1):43–52. https://doi.org/10.1109/tbme.2005.859786.
Nakajo M, Shapiro B, Glowniak J, Sisson JC, Beierwaltes WH. Inverse relationship between cardiac accumulation of meta-[131I]iodobenzylguanidine (I-131 MIBG) and circulating catecholamines in suspected pheochromocytoma. J Nucl Med. 1983;24(12):1127–34.
Degrado TR, Zalutsky MR, Vaidyanathan G. Uptake mechanisms of meta-[123I]iodobenzylguanidine in isolated rat heart. Nucl Med Biol. 1995;22(1):1–12.
Dae MW, O’Connell JW, Botvinick EH, Chin MC. Acute and chronic effects of transient myocardial ischemia on sympathetic nerve activity, density, and norepinephrine content. Cardiovasc Res. 1995;30(2):270–80.
Narula J, Sarkar K. A conceptual paradox of MIBG uptake in heart failure: retention with incontinence! J Nucl Card. 2003;10(6):700–4. https://doi.org/10.1016/j.nuclcard.2003.09.006.
Verberne HJ, Brewster LM, Somsen GA, van Eck-Smit BL. Prognostic value of myocardial 123I-metaiodobenzylguanidine (MIBG) parameters in patients with heart failure: a systematic review. Eur Heart J. 2008;29(9):1147–59. https://doi.org/10.1093/eurheartj/ehn113.
Kasama S, Toyama T, Sumino H, Kumakura H, Takayama Y, Minami K, et al. Prognostic value of cardiac sympathetic nerve activity evaluated by [123I]m-iodobenzylguanidine imaging in patients with ST-segment elevation myocardial infarction. Heart. 2011;97(1):20–6. https://doi.org/10.1136/hrt.2010.204149.
Boogers MJ, Borleffs CJ, Henneman MM, van Bommel RJ, van Ramshorst J, Boersma E, et al. Cardiac sympathetic denervation assessed with 123-iodine metaiodobenzylguanidine imaging predicts ventricular arrhythmias in implantable cardioverter-defibrillator patients. J Am Coll Card. 2010;55(24):2769–77. https://doi.org/10.1016/j.jacc.2009.12.066.
Bax JJ, Kraft O, Buxton AE, Fjeld JG, Parizek P, Agostini D, et al. 123 I-mIBG scintigraphy to predict inducibility of ventricular arrhythmias on cardiac electrophysiology testing: a prospective multicenter pilot study. Circ Cardiovasc Imag. 2008;1(2):131–40. https://doi.org/10.1161/CIRCIMAGING.108.782433.
Tamaki S, Yamada T, Okuyama Y, Morita T, Sanada S, Tsukamoto Y, et al. Cardiac iodine-123 metaiodobenzylguanidine imaging predicts sudden cardiac death independently of left ventricular ejection fraction in patients with chronic heart failure and left ventricular systolic dysfunction: results from a comparative study with signal-averaged electrocardiogram, heart rate variability, and QT dispersion. JACC. 2009;53(5):426–35. https://doi.org/10.1016/j.jacc.2008.10.025.
Kuramoto Y, Yamada T, Tamaki S, Okuyama Y, Morita T, Furukawa Y, et al. Usefulness of cardiac iodine-123 meta-iodobenzylguanidine imaging to improve prognostic power of Seattle heart failure model in patients with chronic heart failure. J Am Coll Card. 2011;107(8):1185–90. https://doi.org/10.1016/j.amjcard.2010.12.019.
Kioka H, Yamada T, Mine T, Morita T, Tsukamoto Y, Tamaki S, et al. Prediction of sudden death in patients with mild-to-moderate chronic heart failure by using cardiac iodine-123 metaiodobenzylguanidine imaging. Heart. 2007;93(10):1213–8. https://doi.org/10.1136/hrt.2006.094524.
Jacobson AF, Lombard J, Banerjee G, Camici PG. 123I-mIBG scintigraphy to predict risk for adverse cardiac outcomes in heart failure patients: design of two prospective multicenter international trials. J Nucl Card. 2009;16(1):113–21. https://doi.org/10.1007/s12350-008-9008-2.
Mitrani RD, Klein LS, Miles WM, Hackett FK, Burt RW, Wellman HN, et al. Regional cardiac sympathetic denervation in patients with ventricular tachycardia in the absence of coronary artery disease. J Am Coll Cardiol. 1993;22(5):1344–53. https://doi.org/10.1016/0735-1097(93)90541-8.
Stirrup J, Gregg S, Baavour R, Roth N, Breault C, Agostini D, et al. Hybrid solid-state SPECT/CT left atrial innervation imaging for identification of left atrial ganglionated plexi: Technique and validation in patients with atrial fibrillation. J Nucl Cardiol. 2020;27(6):1939–50. https://doi.org/10.1007/s12350-018-01535-5.
Foley KF, Van Dort ME, Sievert MK, Ruoho AE, Cozzi NV. Stereospecific inhibition of monoamine uptake transporters by meta-hydroxyephedrine isomers. J Neural Trans. 2002;109(10):1229–40. https://doi.org/10.1007/s00702-002-0695-6.
Tipre DN, Fox JJ, Holt DP, Green G, Yu J, Pomper M, et al. In vivo PET imaging of cardiac presynaptic sympathoneuronal mechanisms in the rat. J Nucl Med. 2008;49(7):1189–95. https://doi.org/10.2967/jnumed.107.050252.
Thackeray JT, Renaud JM, Kordos M, Klein R, Dekemp RA, Beanlands RS, et al. Test-retest repeatability of quantitative cardiac 11C-meta-hydroxyephedrine measurements in rats by small animal positron emission tomography. Nucl Med Biol. 2013;40(5):676–81. https://doi.org/10.1016/j.nucmedbio.2013.03.007.
Thackeray JT, Beanlands RS, Dasilva JN. Presence of specific 11C-meta-Hydroxyephedrine retention in heart, lung, pancreas, and brown adipose tissue. J Nucl Med. 2007;48(10):1733–40. https://doi.org/10.2967/jnumed.107.043570.
Rosenspire KC, Haka MS, Van Dort ME, Jewett DM, Gildersleeve DL, Schwaiger M, et al. Synthesis and preliminary evaluation of carbon-11-meta-hydroxyephedrine: a false transmitter agent for heart neuronal imaging. J Nucl Med. 1990;31(8):1328–34.
Law MP, Schafers K, Kopka K, Wagner S, Schober O, Schafers M. Molecular imaging of cardiac sympathetic innervation by 11C-mHED and PET: from man to mouse? J Nucl Med. 2010;51(8):1269–76. https://doi.org/10.2967/jnumed.110.074997.
Law MP, Osman S, Davenport RJ, Cunningham VJ, Pike VW, Camici PG. Biodistribution and metabolism of [N-methyl-11C]m-hydroxyephedrine in the rat. Nucl Med Biol. 1997;24(5):417–24.
Link JM, Synovec RE, Krohn KA, Caldwell JH. High speed liquid chromatography of phenylethanolamines for the kinetic analysis of [11C]-meta-hydroxyephedrine and metabolites in plasma. J Chromat B, Biomed Sc App. 1997;693(1):31–41.
Wallin BG, Fagius J. Peripheral sympathetic neural activity in conscious humans. Ann Rev Physiol. 1988;50:565–76. https://doi.org/10.1146/annurev.ph.50.030188.003025.
Elam M, Macefield V. Multiple firing of single muscle vasoconstrictor neurons during cardiac dysrhythmias in human heart failure. J Appl Physiol. 2001;91(2):717–24.
Bernardi L, Spallone V, Stevens M, Hilsted J, Frontoni S, Pop-Busui R, et al. Methods of investigation for cardiac autonomic dysfunction in human research studies. Diabetes Metab Res Rev. 2011;27(7):654–64. https://doi.org/10.1002/dmrr.1224.
Goldberger JJ, Arora R, Buckley U, Shivkumar K. Autonomic nervous system dysfunction: JACC focus seminar. J Am Coll Card. 2019;73(10):1189–206. https://doi.org/10.1016/j.jacc.2018.12.064.
Wallin BG, Thompson JM, Jennings GL, Esler MD. Renal noradrenaline spillover correlates with muscle sympathetic activity in humans. J Physiol. 1996;491(Pt 3):881–7.
Wallin BG, Esler M, Dorward P, Eisenhofer G, Ferrier C, Westerman R, et al. Simultaneous measurements of cardiac noradrenaline spillover and sympathetic outflow to skeletal muscle in humans. J Physiol. 1992;453:45–58.
Rundqvist B, Elam M, Bergmann-Sverrisdottir Y, Eisenhofer G, Friberg P. Increased cardiac adrenergic drive precedes generalized sympathetic activation in human heart failure. Circulation. 1997;95(1):169–75.
Najem B, Unger P, Preumont N, Jansens JL, Houssière A, Pathak A, et al. Sympathetic control after cardiac resynchronization therapy: responders versus nonresponders. Am J Physiol Heart Circ Physiol. 2006;291(6):H2647–52. https://doi.org/10.1152/ajpheart.00373.2006.
Doytchinova A, Hassel JL, Yuan Y, Lin H, Yin D, Adams D, et al. Simultaneous noninvasive recording of skin sympathetic nerve activity and electrocardiogram. Heart Rhythm. 2017;14(1):25–33. https://doi.org/10.1016/j.hrthm.2016.09.019.
Jiang Z, Zhao Y, Doytchinova A, Kamp NJ, Tsai WC, Yuan Y, et al. Using skin sympathetic nerve activity to estimate stellate ganglion nerve activity in dogs. Heart Rhythm. 2015;12(6):1324–32. https://doi.org/10.1016/j.hrthm.2015.02.012.
Uradu A, Wan J, Doytchinova A, Wright KC, Lin AYT, Chen LS, et al. Skin sympathetic nerve activity precedes the onset and termination of paroxysmal atrial tachycardia and fibrillation. Heart Rhythm. 2017;14(7):964–71. https://doi.org/10.1016/j.hrthm.2017.03.030.
Kusayama T, Wan J, Doytchinova A, Wong J, Kabir RA, Mitscher G, et al. Skin sympathetic nerve activity and the temporal clustering of cardiac arrhythmias. JCI Insight. 2019;4(4):e125853. https://doi.org/10.1172/jci.insight.125853.
Shusterman V, McTiernan CF, Goldberg A, Saba S, Salama G, London B. Adrenergic stimulation promotes T-wave alternans and arrhythmia inducibility in a TNF-alpha genetic mouse model of congestive heart failure. Am J Physiol Heart Circ Physiol. 2010;298(2):H440–50. https://doi.org/10.1152/ajpheart.01024.2008.
Verrier RL, Antzelevitch C. Autonomic aspects of arrhythmogenesis: the enduring and the new. Curr Opin Cardiol. 2004;19(1):2–11. https://doi.org/10.1097/00001573-200401000-00003.
Lampert R, Shusterman V, Burg MM, Lee FA, Earley C, Goldberg A, et al. Effects of psychologic stress on repolarization and relationship to autonomic and hemodynamic factors. J Cardiovasc Electrophysiol. 2005;16(4):372–7. https://doi.org/10.1046/j.1540-8167.2005.40580.x.
Rashba EJ, Cooklin M, MacMurdy K, Kavesh N, Kirk M, Sarang S, et al. Effects of selective autonomic blockade on T-wave alternans in humans. Circulation. 2002;105(7):837–42. https://doi.org/10.1161/hc0702.104127.
Klingenheben T, Grönefeld G, Li YG, Hohnloser SH. Effect of metoprolol and d,l-sotalol on microvolt-level T-wave alternans. Results of a prospective, double-blind, randomized study. J Am Coll Card. 2001;38(7):2013–9. https://doi.org/10.1016/s0735-1097(01)01661-8.
Katritsis D, Merchant FM, Mela T, Singh JP, Heist EK, Armoundas AA. Catheter ablation of atrial fibrillation the search for substrate-driven end points. J Am Coll Card. 2010;55(21):2293–8. https://doi.org/10.1016/j.jacc.2010.03.016.
Smith JM, Rosenbaum DS, Cohen RJ. Variability in surface ECG morphology: signal or noise? Comput Cardiol. 1988;14:257–60.
Rosenbaum DS, Jackson LE, Smith JM, Garan H, Ruskin JN, Cohen RJ. Electrical alternans and vulnerability to ventricular arrhythmias. N Engl J Med. 1994;330(4):235–41. https://doi.org/10.1056/nejm199401273300402.
Stavrakis S, Kulkarni K, Singh JP, Katritsis DG, Armoundas AA. Autonomic modulation of cardiac arrhythmias: methods to assess treatment and outcomes. J Am Coll Card Clin Electrophysiol. 2020;6(5):467–83. https://doi.org/10.1016/j.jacep.2020.02.014.
Byku M, Mann DL. Neuromodulation of the failing heart: lost in translation? J Am Coll Card Basic Transl Sci. 2016;1(3):95–106. https://doi.org/10.1016/j.jacbts.2016.03.004.
Clancy JA, Mary DA, Witte KK, Greenwood JP, Deuchars SA, Deuchars J. Non-invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity. Brain Stimul. 2014;7(6):871–7. https://doi.org/10.1016/j.brs.2014.07.031.
Tran N, Asad Z, Elkholey K, Scherlag BJ, Po SS, Stavrakis S. Autonomic neuromodulation acutely ameliorates left ventricular strain in humans. J Cardiovasc Transl Res. 2019;12(3):221–30. https://doi.org/10.1007/s12265-018-9853-6.
Scherschel K, Hedenus K, Jungen C, Lemoine MD, Rübsamen N, Veldkamp MW, et al. Cardiac glial cells release neurotrophic S100B upon catheter-based treatment of atrial fibrillation. Sci Transl Med. 2019;11(493):eaav7770. https://doi.org/10.1126/scitranslmed.aav7770.
Scherschel K, Hedenus K, Jungen C, Münkler P, Willems S, Anwar O, et al. Impact of the ablation technique on release of the neuronal injury marker S100B during pulmonary vein isolation. Europace. 2020;22(10):1502–8. https://doi.org/10.1093/europace/euaa159.
Deisseroth K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat Neurosci. 2015;18(9):1213–25. https://doi.org/10.1038/nn.4091.
Ambrosi CM, Klimas A, Yu J, Entcheva E. Cardiac applications of optogenetics. Prog Biophys Mol Biol. 2014;115(2-3):294–304. https://doi.org/10.1016/j.pbiomolbio.2014.07.001.
Chow BY, Han X, Dobry AS, Qian X, Chuong AS, Li M, et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463(7277):98–102. https://doi.org/10.1038/nature08652.
Konermann S, Brigham MD, Trevino A, Hsu PD, Heidenreich M, Cong L, et al. Optical control of mammalian endogenous transcription and epigenetic states. Nature. 2013;500(7463):472–6. https://doi.org/10.1038/nature12466.
Yu L, Zhou L, Cao G, Po SS, Huang B, Zhou X, et al. Optogenetic modulation of cardiac sympathetic nerve activity to prevent ventricular arrhythmias. J Am Coll Card. 2017;70(22):2778–90. https://doi.org/10.1016/j.jacc.2017.09.1107.
McClintock SM, Reti IM, Carpenter LL, McDonald WM, Dubin M, Taylor SF, et al. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J Clin Psychiatry. 2018;79(1):16cs10905. https://doi.org/10.4088/JCP.16cs10905.
Repetitive transcranial magnetic stimulation for treatment-resistant depression: A systematic review and meta-analysis of randomized controlled trials. Ont Health Technol Assess Ser. 2016;16(5):1–66.
Yu L, Dyer JW, Scherlag BJ, Stavrakis S, Sha Y, Sheng X, et al. The use of low-level electromagnetic fields to suppress atrial fibrillation. Heart Rhythm. 2015;12(4):809–17. https://doi.org/10.1016/j.hrthm.2014.12.022.
Wang S, Zhou X, Huang B, Wang Z, Zhou L, Wang M, et al. Noninvasive low-frequency electromagnetic stimulation of the left stellate ganglion reduces myocardial infarction-induced ventricular arrhythmia. Sci Rep. 2016;6:30783. https://doi.org/10.1038/srep30783.
Markman TM, Hamilton RH, Marchlinski FE, Nazarian S. Case series of transcutaneous magnetic stimulation for ventricular Tachycardia Storm. J Am Med Ass. 2020;323(21):2200–2. https://doi.org/10.1001/jama.2020.3833.
Acknowledgments
Some of the work discussed here was supported by a British Heart Foundation Clinical Research fellowship (FS/12/52/29629 to S.H.C.) and a British Heart Foundation Programme Grant (RG/17/3/32774 to G.A.N.).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Chin, S.H., Ng, G.A. (2023). The Neuro-cardiac Axis in Arrhythmogenesis: Role and Impact of Autonomic Modulation. In: Tripathi, O.N., Quinn, T.A., Ravens, U. (eds) Heart Rate and Rhythm. Springer, Cham. https://doi.org/10.1007/978-3-031-33588-4_9
Download citation
DOI: https://doi.org/10.1007/978-3-031-33588-4_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-33587-7
Online ISBN: 978-3-031-33588-4
eBook Packages: MedicineMedicine (R0)