Abstract
Cardiac output is controlled by the autonomic nervous system to meet continually changing demands for the perfusion of systemic vascular beds. Dysfunction of autonomic control can contribute to a range of cardiopathies; conversely, robust autonomic function can help maintain a failing myocardium as heart diseases progress. Understanding the structure and operation of the intracardiac nervous system is thus essential to guide the formation of novel neuronally-directed cardiac therapies. Neural control of the heart operates through a hierarchy of interconnected reflex loops at the levels of the intracardiac neural network, the extracardiac intrathoracic ganglia and medullary and spinal autonomic nuclei. Within this hierarchy, the intracardiac nervous system represents the final common pathway for local cardiac control, capable of modulating chronotropy, dromotropy and inotropy on a fast, beat-by-beat basis. Intracardiac neurons constitute a series of interconnected ganglionated plexi distributed throughout the atrial walls and around the atrioventricular border; plexus nerves innervate all regions of the heart. This chapter reviews the position of the intracardiac nervous system in the autonomic control hierarchy and summarizes current knowledge of the neuroanatomy, physiology and potential roles of neuronal populations in cardiac control. Opportunities for future research to address remaining gaps in knowledge of this system are discussed in terms of the application of new tissue imaging technologies, genetic manipulations and novel experimental models.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Autonomic innervation of the heart
- Cardiac ganglia
- Postganglionic neurons
- Parasympathetic and sympathetic efferent neurons
- Neuroanatomy
- Cardiac neurochemical transmitters
- Neuronal heart rate control
1 Introduction and Scope
This chapter considers the role of the autonomic nervous system in controlling cardiac output to maintain cardiovascular homeostasis, a role that has been recognized at least since the middle of the nineteenth century. Modern concepts of the anatomy and organization of the autonomic nervous system were crystallized by Gaskell [1], Langley [2], and Cannon [3], whose works carried forward earlier ideas of the innervation and control of the viscera by the cranial and spinal nerves. These concepts were developed principally from the seventeenth century on (see [4] for a historical review). In the last four decades, the relationship between the nervous system and the heart has become a fertile area of research in which the interests of basic scientists and clinicians have converged. This convergence has led to the formation of the discipline of neurocardiology. A core tenet of this discipline is that in healthy individuals, normal control of the heart involves autonomic reflexes that drive rapid variations in cardiac output to meet changing demands for vascular perfusion under different physiological conditions such as exercise, arousal and sleep. Furthermore, in a wide variety of cardiovascular disease states, dysfunction of the neurocardiac axis can parallel or even precede the deterioration of the pumping capability of the heart. Therefore changes in neural and myocardial components must be considered together in developing many cardiac diseases [5]. It is thus essential to establish a clear understanding of the fundamental principles of the structure and operation of the autonomic network controlling the heart to guide the evolution of effective, neurally based therapies for managing cardiovascular diseases.
Progress in neurocardiology has been comprehensively represented in a series of review articles, book chapters and books covering both clinical and basic aspects of this field. In a landmark volume on neurocardiology edited by J. A. Armour and J. L. Ardell [6], the opening chapter by Dr W. Randall succinctly describes its genesis [5]. Dr Randall, with whom both of these investigators had worked, was a pioneer in the field and his article continues to reward the reader with valuable insights into major issues still driving research in neurocardiology. A second, expanded edition of that book [7] updated progress made in the decade after the publication of the first edition; both of those volumes remain essential reading. In a 2008 review by Armour, summarizing more than two decades of work from his and others’ laboratories, codified the proposition that the portion of the autonomic nervous system lying within the heart, the intracardiac nervous system (ICNS), comprised a “little brain on the heart”, that provides fast, local processing of responses to intracardiac events [8]. In 2016, special issues on neurocardiology were published in the Journal of Physiology [9] and in Autonomic Neuroscience [10]. These issues included articles that comprehensively summarized work on basic, translational and clinical aspects of autonomic control of the heart. Osteraas and Lee [11], Durães Campos et al. [12], Wake and Brack [13] and others have also recently reviewed aspects of neurocardiology. Together these reviews give a wide-ranging perspective of the state of this field at both central and peripheral levels of organization.
At the level of the mammalian central nervous system, nuclei involved in determining autonomic output to the heart and other viscera extend along the neuraxis from the insular cortex through the basal ganglia, hypothalamus, midbrain, medulla and spinal cord (see for example [14, 15]). These nuclei process inputs from generalized and specific visceral afferents conveying information to the central nervous system about changes in cardiorespiratory status. Cardiovascular-specific afferent information projecting to the central nervous system arises from two general populations of receptors, those with their somata in the petrosal and nodose ganglia of the vagus nerve, targeting secondary neurons in the nucleus of the tractus solitarius [16, 17] and receptors with their somata in the dorsal root ganglia associated with the lower cervical and upper thoracic segments of the spinal cord, targeting the dorsal horn of the cord [16, 18]. These inputs drive a wide range of reflexes generated by central autonomic nuclei that modify the heart to maintain cardiovascular homeostasis. The outputs of these reflexes are conveyed to the heart by the parasympathetic and sympathetic limbs of the autonomic nervous system through populations of peripheral neurons located in intrathoracic and intracardiac ganglia, connected in networked circuitry that modulates the activity of effectors within the heart to modify cardiac output.
The operating principles of those peripheral circuits are beginning to emerge from studies of the properties of their individual elements, the neurons in the intrathoracic and intracardiac ganglia. In this system, the ICNS represents the final common pathway for cardiac control. In the present chapter, I consider current knowledge of the physiological properties, patterns of synaptic connectivity, neurotransmitter phenotypes, receptor complements and cardio-modulatory influences of neurons in the ICNS and summarize the evidence for the operation of the circuitry in which they are embedded. Ultimately, clarifying the properties and functions of these neurons will have profound implications for the development of evidence-based therapies for cardiovascular diseases that target the intracardiac component of the autonomic nervous system. Clinical aspects of neurocardiology are considered elsewhere in this volume (see Chap. 9).
2 Hierarchical and Distributed: An Overview of Autonomic Innervation of the Heart
Autonomic neurons within the thoracic and intracardiac ganglia form a network coordinating the activities of myocardial effector cell populations that collectively determine cardiac output. This network innervates pacemaker cells in the sinoatrial and atrioventricular nodes, myocytes in the atrial and ventricular walls, the ventricular conducting system and the coronary vasculature. In the normal heart, the ongoing activity of effector cells is modulated by tonic influences and phasic fluctuations of inputs from this neural network to precisely match cardiac output with vascular perfusion demands.
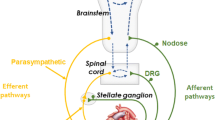
A major theme running through many of the reviews of neurocardiology cited in Sect. 8.1 is that the organization of autonomic neurons in the network controlling the heart is hierarchical, comprising several nested levels. This theme is embodied in a series of schematic diagrams that have evolved over time to encompass more and more elements as investigators became increasingly aware of the complexity of the system and as additional information became available about the details at each level in the hierarchy. One of the earliest of these schematics is shown in Fig. 8.1 [19]. A more recent version of Fig. 8.1 is shown in Fig. 8.2 [20] to illustrate the development of this concept. Broad acceptance of the principle of hierarchical organization and its usefulness as a conceptual framework for thinking about cardiac control has provided a focus for the rapid expansion of the field of neurocardiology in recent decades. This framework will be used here to structure the discussion of cardiac-associated neurons and how they may be organized into networks for control of the heart.
Schematic representation of a proposed hierarchical organization for populations of autonomic neurons involved in control of the heart and their interconnections. Three levels of hierarchy are shown: intracardiac neurons (“cardiac ganglia”); neurons within the thorax (“intrathoracic ganglia”) and central neurons (“medulla” and “spinal cord”) ([19] used by permission)
The “classical” concept of efferent cardiac innervation consists of a parallel series of simple, two-neuron pathways from the central nervous system to the target organ, in accord with the general plan of dual sympathetic and parasympathetic efferent autonomic outflow to the viscera proposed by Langley [2] and Cannon [3]. In this scheme, sympathetic cardiac preganglionic neurons are located in the intermediolateral cell column of the spinal cord in the caudal cervical and cranial thoracic segments [14, 21]. Axons arising from these neurons exit the cord via the white rami to synapse on somata of postganglionic neurons located predominantly in intrathoracic ganglia (middle cervical and stellate ganglia) of the sympathetic paravertebral ganglion chain. Neurons in these ganglia in turn project axons via cardiac nerves [22] to target virtually all regions of the heart [23], acting to accelerate pacemaker cell discharge, increase contractility of atrial and ventricular myocytes, enhance activity in conducting tissues and induce changes in radius of the cardiac vasculature. Parasympathetic preganglionic neurons, located in the nucleus ambiguus, the dorsal vagal motor nucleus and areas between these in the medulla, give rise to axons coursing peripherally via the vagosympathetic trunks and cardiac vagal branches to target postganglionic neurons in intracardiac ganglia distributed in subepicardial tissue. In turn, axons from intracardiac parasympathetic neurons innervate the same groups of cardiac effector cells as do sympathetic postganglionic axons [19], influencing the activity of these cells in the opposite direction to the actions of sympathetic innervation. Thus, in this “classical” view of autonomic cardiac control, sympathetic and parasympathetic drive is conveyed from the brain to the heart by pre- to postganglionic synapses functioning as basic 1:1 relays. In this view, it was also considered that these autonomic divisions acted antagonistically on the heart such that when sympathetic drive was elevated, parasympathetic activity was withdrawn and vice versa [24]. The elements of this simple innervation scheme can be readily identified in the schematics in Figs. 8.1 and 8.2.
This simplistic view of cardiac innervation has been belied by the results of studies in the last four decades using a variety of experimental models including open-chest, anesthetized mammalian preparations in which the heart was accessed in situ. The outcomes of these studies have driven the continued revision of our concepts of neural control of the heart, as demonstrated by (1) the addition of components representing populations of local-circuit neurons (LCN) and (2) afferent somata and their interconnections with the intrathoracic and intracardiac levels of the hierarchy, as shown in the schematic of Fig. 8.1. As more information became available about the operation of these components, the schematic was modified to reflect the increasing complexity of the system (Fig. 8.2).
According to the “classical” view of operation of the neurocardiac axis, all neuronal activity within intrathoracic and intracardiac ganglia should result from the activation of efferent postganglionic neurons by inputs from the central nervous system. However, recordings of neuronal activity in these ganglia during electrical stimulation of preganglionic axons showed that only a proportion of neurons were activated at the short latencies consistent with a monosynaptic pathway. Other neurons responded indirectly (presumably through multisynaptic pathways with varying degrees of latency) to such stimulations, or had activity patterns not correlated with the stimuli (summarized by Armour [19, 25]). These observations led to the concept of a population of neurons in peripheral cardiac ganglia that did not receive direct preganglionic inputs but were instead involved in processing information originating either from within the ganglia or, more distally, from cardiac afferents [8, 19]. This concept was expressed by the incorporation of LCN at the levels of the intrathoracic and intracardiac ganglia in schematic diagrams (Figs. 8.1 and 8.2).
A critical question in determining the origin of spontaneous neuronal activity within extra- and intracardiac ganglia was whether this activity could have arisen from events in the periphery rather than originating from the central nervous system. This question was investigated by disconnecting these peripheral ganglia from the central nervous system by surgical means aimed at severing preganglionic inputs (decentralization), leaving all other connections intact. In a landmark paper, Armour, Randall and coworkers [26] established that spontaneous activity in intracardiac ganglia, much of which was phase-related to events in the cardiac cycle, continued after decentralization, although the overall frequency of this activity was reduced. Likewise, spontaneous neuronal activity in decentralized stellate and middle cervical ganglia also survived decentralization [27, 28]. These observations led to the proposal that afferent neurons, transducing chemical or mechanical events in the heart, provided local inputs to LCN or directly to efferent postganglionic neurons, thus engendering cardio-cardiac reflexes that operated independently of the central nervous system. These inputs may have arisen as intraganglionic collaterals of cardiac sensory axons with their somata in the dorsal root [16, 18, 29] or vagal nodose [30, 31] ganglia or from somata of afferent neurons located within the intracardiac or intrathoracic ganglia themselves [8]. This concept was illustrated by representing afferent neurons at the levels of the intrathoracic and intracardiac ganglia in the cardiac control hierarchy (Figs. 8.1 and 8.2).
The presence within intracardiac ganglia of afferent neuronal somata (or collateral branching within these ganglia from axons of nodose or dorsal root cardiac afferents), along with LCN, together provide the necessary components for local, short-latency (on the order of <20–40 ms) cardio-cardiac reflexes that operate within the ICNS. Reflexes of slightly longer latency (100–200 ms) would involve projections through the intrathoracic ganglia [8]. Such reflexes are presumably capable of operating well within the duration of systole of a single cardiac cycle. Cardio-cardiac reflexes with the longest latencies (up to 350 ms [8]) would involve feedback from the heart originating from mechano- or chemosensitive afferents with their receptors in the atria or ventricles and their somata in the nodose ganglion or dorsal root ganglia. These would project to cardiomotor circuits in the central nervous system, indicated in the schematic diagrams at the “central” level of the network hierarchy (Figs. 8.1 and 8.2).
3 Elements of Intracardiac Neuronal Circuitry
To date, the vast majority of data on the organization and principles of control of the heart by the intrathoracic and intracardiac neural networks has resulted from studies recording spontaneous or evoked axonal or ganglion cell activity along with the behavior of cardiac functional indices (e.g., heart rate, atrial and ventricular chamber pressures, regional chamber wall tension). This work has been done in a variety of experimental models in which the beating heart (in vivo, in situ or in vitro) and components of the ICNS and extracardiac nerves are accessible for manipulation. These studies have provided major advances in our understanding of the principles of cardiac control at the systems level and are the subject of a number of recent reviews to which the reader is referred (e.g., [11, 12, 32,33,34]). While many of the issues addressed in the present chapter arise from aspects of this work, this material will not be covered in depth here.
Extracellular recordings of action potential discharge in the peripheral cardiac nervous system (particularly when multiple recording electrodes are used to increase the regional scope of data recovery; see, e.g., [35]) and the changes in neuronal activity evoked by electrical, mechanical and chemical stimulation in this system are effective tools for enabling analysis of the behavior of populations of neurons. In that type of study, it may, however, be problematic to discern the ongoing activity pattern of a particular neuron from others recorded simultaneously at the same electrode site. Furthermore, the connectivity and physiological type of neuron from which such extracellular recordings are made may only be deduced indirectly from circumstantial evidence. In contrast, intracellular recordings or whole-cell patch recordings provide direct access to membrane properties, firing behavior and postsynaptic responses of single neurons, usually in reduced preparations of isolated tissues. Such recordings leave no doubt about the physiological type and the nature of synaptic inputs for each cell sampled. However, in those reduced preparations, the connections of the sampled neurons with extracardiac and distant intracardiac neurons have been disrupted. It is thus difficult to determine, based on single-cell recordings, what the roles and contributions of the sampled neurons might have been in the system in which they were originally embedded. Yet the determination of functional properties and input patterns of individual autonomic neurons in the intrathoracic and intracardiac ganglia is essential in understanding how neuronal networks are built from these basic elements.
A major principle of autonomic control of the viscera is that the sympathetic and parasympathetic limbs of this system are organized into “function-specific pathways” that precisely target visceral organs and specific tissues performing particular functions (hence “function-specific”) within those organs [36,37,38,39,40]. Understanding the properties, characteristics and connections of individual neurons in the networks controlling the heart enables a fundamental step to be taken in unraveling the integrative capabilities of this network. Neurons subserving various functions (motor neurons, afferents and those that integrate and process information locally) form the basic elements of a variety of local reflex arcs, the organization and operating principles of which will emerge from investigations of the properties and connectivity of these elements. In the following sections, I focus on the characteristics of intracardiac neurons and what is known of their connections to see how these neurons might work in combination to create function-specific pathways within the heart.
3.1 Overview of the Intracardiac Nervous System
Among the vertebrates autonomic innervation of the heart is phylogenetically ancient, with sympathetic and parasympathetic pathways employing the same basic operating principles in all extant orders from Teleostei [41] to mammals [42,43,44]. Interest in the gross innervation of the heart has been strong for centuries (see [4] for historical aspects). In the last century, works by Nonidez [45], Mizeres [22] and others have clarified the anatomical pathways of cardiac nerves into the heart in a number of mammalian species. It was recognized from the late 1800s that the ICNS consisted of collections of ganglia, associated with the epicardium, that were connected together into ganglionated plexi [46], but the details of this organization have only become clear relatively recently. The neuroanatomy of the ganglia and interganglionic plexi has now been well-established in both small and large mammals commonly used as experimental models in cardiovascular research. Among small mammals, the ICNS has been characterized in the mouse (estimated ~1000–1800 intracardiac neurons [47, 48]), rat (~4000 [49,50,51]) and guinea pig (~1500 [52,53,54]). Comparative studies of the neuroanatomy of the ICNS have also been done between rat and guinea pig [55, 56]. In larger mammals, the neuroanatomy of the ICNS has been analyzed in the pig (~3000 [57]), sheep (700–800 [58]) and dog (~20,000 [59]). Several studies have examined the innervation of the human heart and patterns of occurrence of ganglionated plexi (~40,000 [56, 60, 61]). Pauza et al. [56] presented a quantitative, comparative study of neurons in ganglionated plexi in rat, guinea pig, dog and human specimens, finding that the morphology of neuronal somata was similar across species and that the numbers of ganglia and the total numbers of neurons per heart scaled positively with body and heart size.
In mammalian hearts, the ganglionated plexi are associated with the external walls of both atria, the region adjacent to the atrioventricular border and within the interatrial septum. The occurrence of ganglionated plexi in the human heart is illustrated in Fig. 8.3 [34], where major plexi have been named for their proximate cardiac regions. This example clearly shows the distributed nature of the ICNS: ganglionated plexi are located close to the pacemaker loci in the right atrium (sinoatrial and atrioventricular nodes), the ostia of the inferior and superior vena cavae, adjacent to the pulmonary vein-atrial wall junctions and near the roots of the pulmonary artery and aorta. These ganglionated plexi are connected together into a network by numerous interganglionic nerves, so that there is communication among populations of neurons located in all regions of the heart [62].
Illustrations of distribution of major ganglionated plexi (dots) in the mammalian heart (left panel, dorsal view; right panel, superior view with dorsal aspect of heart toward the bottom of panel) ([34] used by permission)
Once details of the neuroanatomy of the ICNS became available, these were used to design a variety of physiological studies to electrically and chemically stimulate specific loci within the system to map the relationships between local neurons and cardiac effectors. As a general organizing principle for the ICNS, it is tempting to assume that most, if not all, neurons adjacent to a particular cardiac effector tissue, for instance, the SAN, would be involved in the control of that tissue (e.g., pacemaker discharge rate [63]). However, this assumption has proven to be an oversimplification [64]. Certainly, neurons exist in right atrial ganglionated plexi near the SAN that do in fact affect heart rate, but subpopulations of neurons with their somata in most other ganglionated plexi in the heart can also modify heart rate via axons coursing in the plexus nerves to the SAN region. More generally, it has been firmly established that the ICNS constitutes a functionally as well as anatomically distributed system, with neurons in all ganglionated plexi projecting to effector tissues in the atria and the ventricles [62, 65,66,67,68].
3.1.1 Properties of Intracardiac Neurons
3.1.1.1 Electrophysiological Properties
The first recordings of transmembrane potential in intracardiac neurons were made from cells in the cardiac ganglion in the interatrial septum of amphibian hearts in vitro [69, 70]. Those studies investigated the synaptophysiology of inputs to the neurons from vagal preganglionic axons. Two categories of neurons were described: (1) large principal cells projecting axons to the myocardium and to other nearby principal cells, releasing acetylcholine from their terminals; and (2) a population of catecholamine-containing intercalated cells with somata smaller than those of principal cells and with processes that terminate exclusively on nearby principal cells [71, 72]. Principal cells from the cardiac ganglion of mudpuppy, studied in an isolated tissue preparation, exhibited repetitive action potential (AP) firing behavior when depolarized by long-duration intracellular current injection [73, 74]. A major advantage of such reduced preparations of the amphibian heart is that cardiac ganglia are readily visualized in the thin wall of the interatrial septum and can be easily accessed for electrical recording and stimulation. In contrast, in the mammalian ICNS, it has proven difficult to obtain intracellular recordings from ICN somata in situ since these are embedded in subepicardial tissue throughout both atria and near the atrioventricular border. Two general approaches have been taken to solve this problem. In the first, tissue containing atrial ganglionated plexi are removed from the heart and the neurons are enzymatically dissociated and cultured in preparations in which sharp intracellular microelectrodes or patch pipettes are used to record membrane potentials and ion channel currents. In the second approach, atrial tissues containing ganglionated plexi are isolated and dissected to expose individual ganglia for the impalement of neurons with sharp electrodes. Both of these approaches have yielded insights into the properties of individual ICN, but the scope of information obtainable from each type of preparation is limited.
Dissociated and cultured ICN are free of associated connective tissue and are not subject to movement artifacts resulting from the contraction of substrate cardiomyocytes; however, these cells no longer have synaptic connections. This type of preparation has been used extensively to analyze passive and active membrane voltage properties as well as ion channel currents, AP firing behavior and membrane receptor-mediated responses to neurotransmitters, agonists and antagonists (see [75] for review). A key electrophysiological property in understanding neuronal function is AP firing behavior during long-duration current pulses (usually 0.2–1 s), delivered through the recording electrode to depolarize the membrane above the threshold for AP initiation. This type of stimulation at least partially mimics the effect of strong excitatory synaptic drive on neurons in situ and has been used to classify cells into functional categories. Based on this test, cultured neurons from a variety of mammalian models display three main classes of AP discharge in response to prolonged depolarization, illustrated in the example from guinea pig intracardiac neurons shown in Fig. 8.4 [76]. The most common neuronal type (65–75% of neurons sampled) typically discharged one AP at the start of prolonged (>1 s duration) depolarization (Fig. 8.4a, right panel). Furthermore, this type of neuron, when depolarized with a short (5 ms duration) intrasomal stimulus pulse, displayed a high-amplitude, prolonged afterhyperpolarization (AHP) following the AP (Fig. 8.4a, left panel), so was designated “AHs” after these characteristics. A second neuron type also displayed a prolonged AHP following single APs (Fig. 8.4b, left panel), but when challenged with long-duration depolarizing current injection, these cells produced multiple APs, initially at a high frequency but accommodating to a lower discharge rate as the stimulus continued (Fig. 8.4b, right panel). This type, making up 10–15% of the neurons, was designated “AHm” to reflect a prolonged AHP along with multiple AP discharges. The third type of neuron (10–15%) displayed continuous, high-frequency AP discharge during sustained depolarization (Fig. 8.4c, right panel) but had a short-duration AHP following a single AP (Fig. 8.4c, left panel). This type was classed as “M,” reflecting its multiple and rapidly repeating AP discharge. Similar results were obtained by Xu and Adams [77] in neurons dissociated and cultured from the neonatal rat heart; those authors described neurons with two patterns of AHP time course and AP firing behavior that resembled those of the guinea pig AHs and M type neurons shown in Fig. 8.4. In cultured guinea pig and neonatal rat intracardiac neurons, the distinct AHP time courses and firing behaviors of the different neuron types were shown to result from differential membrane ion channel complements, thus establishing the ionic basis for the functional differences among these types [75].
Intracellular recordings of action potential firing properties of identified intracardiac neurons dissociated from guinea pig heart and cultured. In a, b and c, left panels show responses to single, short intrasomally injected depolarizing current pulse; right panels show responses to 1 s duration current pulses. (a) Responses of AHs neuron subclass. (b) Responses of AHm subclass. (c) Responses of M subclass (see text for explanation of subclasses) ([75] used by permission)
Cultured neurons lack the interconnections present in intact ganglia, so studies such as those cited above cannot address questions concerning the organization and properties of synaptic connections between cells. These questions and others have been addressed using isolated, perfused preparations of atrial ganglionated plexi in vitro, taken from the hearts of a variety of mammalian species. Electrophysiological properties of ICN in these studies show trends generally similar to those in cultured ICN. Passive membrane properties (transmembrane potential, whole-cell input resistance, membrane capacitance and characteristics of evoked APs) of ICN in vitro (summarized in [78]) appear to be within the range of those of autonomic neurons in other peripheral ganglia (summarized in [79]), so these characteristics may not be particularly useful in distinguishing between functional neuron types (or their roles) in the heart. However, by extension, from studies in cultured ICN, properties such as the time course and amplitude of AHP and AP firing behavior appear to provide distinctions among neuronal types in the heart that may be correlated with their functions. When analysis of these electrophysiological properties is combined with investigation of other neuronal features, such as sources of synaptic input or neurochemical phenotype of axon terminals and somata, a clearer picture of the role of specific classes of neurons in intracardiac function-specific pathways may result. To address progress on this issue, I focus on several schemes for classifying ICN based on data from cells in ganglia with intact synaptic connections and what these schemes may contribute to a unified perspective relating the properties of neurons to their network functions.
Figure 8.5 shows examples typical of the classification schemes that have been proposed based on active membrane properties of ICN from in vitro studies of intact ganglion preparations in four mammalian species. In the adult rat heart, Selyanko [80] proposed two general neuronal types: Type I and Type II (Fig. 8.5a). Type I neurons had relatively short AHP duration following a single brief stimulus that evoked an AP (Fig. 8.5a, trace designated “a”). These neurons were further categorized into two subtypes, Ib and Im, based on responses to long-duration depolarizing intracellular current injection (Fig. 8.5a, traces designated “b” and “c”). Type Ib neurons generated a short burst of APs only at the start of this depolarization, while Im displayed multiple but frequency-decrementing APs for the duration of depolarization. Type II neurons had a relatively long-duration AHP following a single AP and produced one or a few low-frequency APs during long-duration depolarization (Fig. 8.5a, traces designated “a” and “b” respectively, bottom panels). No information, however, was provided on synaptic inputs to any of these neuron types.
Comparison of classification systems for intracardiac neurons based on intracellular recordings of membrane potentials from in vitro preparations of mammalian hearts (see text for details). Action potentials were evoked by intracellular stimulus protocols similar to those used in Fig. 8.4. (a) Rat (after [80]). (b) Canine [82]. (c) Guinea pig [84]. (d) Pig [85] (all images used by permission)
Xi et al. [81, 82] identified R, S and N neurons in the canine right atrial ganglionated plexus based on AP firing behavior during long-duration depolarization (Fig. 8.5b; note, no data on AHP were given). Type R (“repetitive”) neurons fired APs for the duration of depolarization (Fig. 8.5b, upper left), usually with an accommodating firing pattern (decrementing in frequency), but a few cells maintained a high firing frequency throughout. The authors indicated that nearly two-thirds of R neurons also generated spontaneous APs (that is, APs appearing in the absence of external stimulation). Type S (“single AP”) neurons generated one AP at the start of depolarization (Fig. 8.5b, center traces, top); half of these neurons showed spontaneous APs. Type N (“nonresponding”) neurons (Fig. 8.5b, traces at top right) did not respond to hyperpolarizing or depolarizing currents injected intracellularly at any intensity. These authors noted that, overall, AP discharge patterns of these classes of neurons paralleled those reported in other peripheral autonomic ganglia [83], such that type R neuronal discharge resembled that of tonic neurons, type S resembled that of phasic neurons, and the lack of responses of type N neurons was also found in other ganglia. Xi et al. [82] also injected a neuromarker into some of the cells they sampled to determine if there was any correlation between electrophysiological class and morphology of ICN. Examples of the typical somatic morphology and patterns of processes of each cell type are shown in Fig. 8.5b under their respective membrane response traces. Some differences in somatic morphology were reported among these types, but the processes of some samples of each cell type were observed either to terminate entirely within the ganglion under study, or to leave the ganglion.
In the guinea pig, Edwards et al. [84] also reported three classes of neurons, S, SAH, and P cells (Fig. 8.5c). S cells (Fig. 8.5c, upper trace) had a relatively short AHP and displayed frequent spontaneously arising postsynaptic depolarizations (hence the designation). These cells were reported to respond with only a single AP at the start of long-duration intracellular depolarization (responses not illustrated in their article). They described this cell type as discharging phasically. S cells displayed ongoing, spontaneous postsynaptic depolarizations, some of which exceeded the threshold for AP generation. All of these cells responded synaptically to electrical stimulation of local interganglionic nerves attached to the sampled ganglion, but very few of these cells were even weakly responsive to stimulation of the vagosympathetic trunk. SAH cells had long AHPs (Fig. 8.5c) and maintained AP discharge throughout the duration of long intracellular depolarizing pulses. These cells showed strong postsynaptic depolarizations and AP discharge from vagosympathetic trunk stimulation. P cells had long AHPs (Fig. 8.5c) and displayed repetitive AP discharges under long-duration depolarization, as well as showing spontaneous and rhythmic AP discharge without apparent postsynaptic depolarizations. No postsynaptic responses could be elicited by stimulating the vagosympathetic trunk or local nerves in this cell type.
In pigs, ICN have been classified into three categories: phasic, accommodating, and tonic (Fig. 8.5d [85]). Phasic neurons, constituting 40% of the neurons sampled, showed only one AP at the start of long-duration depolarization and had a relatively short-duration AHP after the AP (Fig. 8.5d, top panel, main trace and insert). These neurons all received cholinergic synaptic inputs from vagal preganglionic axons; some also received adrenergic inputs from postganglionic axons in cardiopulmonary nerves. Accommodating neurons (33%) displayed multiple AP firing that decremented during long-duration depolarization and had short AHPs following the AP (Fig. 8.5c, middle panel, main trace and insert). Few of these neurons received inputs from either the vagosympathetic trunk or the cardiopulmonary nerves. Tonically discharging neurons (27%) showed long AHP duration and fired APs continuously at a high frequency throughout the duration of long depolarizing pulses (Fig. 8.5d, bottom main trace and insert). These cells had neither vagosympathetic nor cardiopulmonary inputs.
The above data begs the question: are there common features among these schemes that may be used to determine what the roles of the identified neurons might be in controlling the heart? First, it appears that different AP firing characteristics during prolonged depolarization are determined by specific membrane ion channel compositions that vary among the neuron types (see [75] for discussion), so a categorization founded on AP discharge patterns likely has a physiological basis. Second, neurons that could be identified with “phasic,” “accommodating” (equivalent to “adapting”) or “tonic” firing behaviors occur in all of the classification schemes discussed here. Third, there are large variations among these schemes in terms of correlations between neuronal firing behavior and accompanying characteristics such as patterns of synaptic input or AHP duration, within each neuronal class. Regarding synaptic connectivity, all neuron types in the classification system of Xi et al. [82] and Smith [85] appeared to have synaptic inputs. However, while SAH and P neurons in the scheme of Edwards et al. [84] had mixed accommodating and tonic firing patterns, only SAH neurons received synaptic inputs. There was thus no clear correlation between the source of synaptic drive and AP discharge behavior in that study. In the scheme of Smith [85], phasic neurons predominantly received inputs from extracardiac nerves while accommodating and tonic neurons were innervated primarily by axons of intracardiac origin. Conversely, in the scheme of Edwards et al. [84], S (phasic) neurons received primarily intracardiac inputs, while SAH (accommodating or tonic) neurons received inputs from the vagosympathetic trunk. It is likely that at least some of the differences in connectivity of ICN among these schemes resulted from interspecies variations in the organization of the ICNS, given that data were derived from four different species. Additionally, many details of the features of ICN and their integration into the ICNS are still unexplored within each of these experimental models.
In the ongoing quest for a consistent neuronal classification scheme, more recent studies of the properties of ICN at the cellular level in both cultured cells and intact ganglia preparations have used AP firing behavior as a primary characteristic in categorizing cells. In cultured rat ICN, Xu and Adams [86] found that the majority of neurons sampled were phasic (85%), and the rest were tonic. Cuevas et al. [87], also working with cultured rat ICN, reported that while more than 90% of neurons were accommodating (termed “multiple adaptive firing”) at room temperature, when the temperature was increased to 37 °C more than a third of the accommodating neurons were converted to phasic discharge. The temperature of the preparation is thus a factor in the firing behavior of ICN, so should be considered in experimental designs. In in vitro studies of ICN in rat atria by Rimmer and Harper [88] and Dyavanapalli et al. [89] phasic, accommodating and tonic neurons were identified, all with synaptic inputs from both extracardiac and intracardiac sources. In a refinement of the technique of recording from ICN in situ, McAllen et al. [90] used a working heart-brainstem preparation in the rat to record intracellularly from ICN in the innervated heart. They identified neurons responding to activation of centrally-mediated cardiorespiratory reflexes that displayed either tonic or phasic discharge characteristics. Tonic neurons, which they termed “principal cells,” responded predominantly to reflex-driven vagal inputs; these neurons were proposed to be the major intracardiac relays from preganglionic neurons in the medulla to cardiac effectors. Phasic neurons had synaptic inputs from intracardiac sources but not from the vagus; these ICN were proposed to represent “interneurons” that fulfill the role of LCN [8]. One potential limitation of this study was that intracellular recordings were made only from a limited subset of cardiac ganglia located near the sinoatrial node, so generalizing these results to the whole ICNS may be problematic. In a recent study by Ashton et al. [91] in atrial tissue isolated from rat, ICN with intact synaptic connections were accessed through whole-cell patch electrodes to record synaptic currents. No phasic cells were found; all voltage-clamp records were made from tonic neurons that received synaptic inputs. The authors reported that there was considerable ongoing, spontaneous synaptically driven activity in these ICN but were unable to identify the source of the activity.
It is thus clear from the studies cited above that knowledge of the basic AP discharge properties of ICN is an important component of the overall characterization of the roles of these neurons in the intracardiac network. Inherent AP firing patterns of specific neuron types will control the frequency-dependent properties of information throughput by the network. However, while this component is a necessary factor, it is not sufficient to evaluate how ICN work in function-specific pathways in the heart. In addition to the inherent membrane properties of ICN, their synaptic connections, axonal projections, neurotransmitter phenotype and receptor complement remain to be established to complete the profile of these neuron types.
3.1.1.2 Neurochemical Complexity
In the peripheral autonomic nervous system, parasympathetic postganglionic neurons generally employ acetylcholine as their main excitatory neurotransmitter at neuroeffector terminals while sympathetic postganglionic neurons release norepinephrine from their terminals (some also release epinephrine). Activation of subtype-specific receptors for these neurotransmitters on effector cells in the visceral organs then determines the nature of the influence of the limbs of the autonomic nervous system on these organs. However, as studies of the neurochemical constitutions of various populations of peripheral autonomic neurons have progressed, it has become clear that there is a wide variety of additional neurotransmitters and neuromodulators that may be expressed by a given population of neurons. In fact, in some systems, these so-called “nonadrenergic, noncholinergic” (NANC) neurochemicals may have a greater influence on visceral effectors than the “main” neurotransmitter [92]. The patterns of co-localization of neurotransmitters or neuromodulators in autonomic neurons (termed “chemical coding” [37, 39, 40, 93]) are thus part of the overall profile identifying discrete populations of these cells with their roles in function-specific pathways.
In the ICNS, primary parasympathetic efferent postganglionic neurons have been identified as cholinergic by the presence of choline acetyltransferase (ChAT), an enzyme in the synthesis pathway for this neurotransmitter, or by the occurrence of vesicular acetylcholine transporter (VAChT), in their somata and processes. Similarly, sympathetic postganglionic efferent neurons are usually identified as adrenergic by their expression of one or more enzymes involved in the synthesis of NE (most commonly tyrosine hydroxylase, TH). Numerous studies over the last three decades have used immunohistochemical detection of these markers to explore the distribution of parasympathetic and sympathetic ICN in a wide variety of mammalian hearts. Immunohistochemical techniques have also been used to detect the coexpression of NANC neurotransmitters and neuromodulators, such as peptides, nitric oxide (NO) and glutamate in these neurons. Much of this work has recently been summarized by Wake and Brack [13].
In all species examined to date, the majority of ICN appears to be cholinergic, and many receive synaptic inputs from cholinergic axon terminals (see for example guinea pig intracardiac ganglia in Fig. 8.6: map, Fig. 8.6a; ChAT immunohistochemistry, Fig. 8.6b [54]). At least a portion of this population appears to represent principal parasympathetic postganglionic neurons in the ICNS. However, many subpopulations of cholinergic ICN display differential coexpression of NANC neurochemicals. For most of these subpopulations, data on their synaptic connectivity, target projections within the heart and the physiological effects of neurochemicals released by these cells are still lacking. The specific details of neurochemicals co-localized in cholinergic neurons also vary greatly depending on the species examined [13]. The most common peptides co-localized in neurons expressing ChAT or VAChT in the ICNS across species are vasoactive intestinal polypeptide (VIP) and neuropeptide Y (NPY); in addition, ICN in many species commonly express neuronal nitric oxide synthetase (nNOS), an enzyme in the synthetic pathway for generating NO, a gaseous transmitter with widespread effects on many visceral organs. In the ICNS of some mammals, cholinergic neurons have been shown to express multiple neuropeptides, thus defining a series of subpopulations with distinct neurochemical profiles. For instance, in the guinea pig ICNS Steele and coworkers reported that neurons expressing combinations of the peptides somatostatin, dynorphin, substance P, NPY and VIP constituted at least seven neuronal subclasses; this picture is further complicated by the fact that many but not all of these cells also expressed nNOS [52, 94]. Furthermore, in an extensive immunohistochemical study, Steele et al. [95] established the axonal termination patterns within the heart of multiple peptide-expressing subpopulations of ICN, showing that the sinoatrial node, the atrioventricular node and the cardiac valves were each innervated by several of these subpopulations. In human ICN, the most common somatic phenotype was cholinergic, with the majority of cells expressing nNOS. However, none of the somata in humans showed labeling for VIP, SP or calcitonin gene-related peptide (CGRP, commonly associated with the somata of sensory neurons), but these peptides were abundant in axons and terminals throughout the ganglia [96]. The latter finding suggests that these peptide-containing elements were derived from neurons with their somata extrinsic to the heart.
Neuroanatomy of intracardiac nervous system in guinea pig heart. (a) Flattened wholemount preparation of both atria; neuronal network identified by antibodies directed against the pan-neuronal marker protein gene product 9.5 (white lines). Edges of tissue mark atrioventricular border (AV), interrupted by areas labeled PV (pulmonary veins), IVC (inferior vena cava) and SVC (superior vena cava). LAA and RAA: left and right atrial appendages; LVN, RVN: left and right vagosympathetic nerve trunks (arrowheads); LCPN, RCPN: left and right cardiopulmonary nerves (arrowheads). Small arrows indicate ganglia containing intracardiac neuronal cell bodies; dotted oval shows the location of sinoatrial node pacemaker. A line taken between the large arrows at top and bottom of the tissue marks the position of the junction of the external atrial wall with the interatrial septum (not shown). (b) Ganglion with labeled neuronal somata (oval-shaped cells; protein gene product 9.5) adjacent to two interganglionic nerves. Smaller, lighter-staining cells and processes expressing tyrosine hydroxylase (arrows and arrowheads) are also shown; these do not overlap with the neuronal label. (c) Catecholamine-containing processes with varicosities (arrowheads) associated with ganglion neurons (diffuse background staining). Scale bars represent 2.5 mm in a, 50 μm in b, 25 μm in c ([54] with permission)
The number of neurons (<10–15% of the total number) in the ICNS that express TH is relatively small. Members of that population should have the capability for synthesizing and releasing NE (and possibly epinephrine) [53, 97,98,99,100,101] so they may represent sympathetic postganglionic neurons with their somata located in the ICNS. Certainly, when intracardiac ganglia in most parts of the mammalian heart are stimulated chemically to activate neuronal somata (but not axons that may be of extracardiac origin), cardio-augmentation occurs similar to the effects of activating sympathetic postganglionic neurons in the intrathoracic ganglia via axons projecting to the heart [32]. On the basis of these findings, sympathetic efferent neurons have been represented at the intracardiac level of the schematic diagrams of neuronal hierarchy for cardiac control (Figs. 8.1 and 8.2). Putative sympathetic postganglionic efferent neurons in the ICNS have also been shown to co-express peptides such as NPY [53].
Many reports have also identified TH-positive cells in the ganglia of the ICNS that do not express markers typical of neurons; such cells have a smaller average soma size than ICN and may release catecholaminergic neurotransmitters [54]. The function of these cells in the heart is not known, but it is possible that they may represent a form of “interneuron” as proposed by Parsons et al. [102] in the cardiac ganglion of the amphibian. In some studies of the mammalian heart, these cells are reported to have pericellular baskets of cholinergic axon terminals. They also give rise to short projections that appear to contact nearby cholinergic ganglion neurons (Fig. 8.6), reinforcing the idea that these cells could release catecholamines under the influence of cholinergic synaptic input. There is also the possibility that these TH-positive cells may be a type of “small, intensely fluorescent cell” (SIF cell), common in other peripheral autonomic ganglia (for instance, pelvic ganglia [103]).
There is a small subpopulation of ICN in which individual somata may contain both cholinergic and adrenergic neuromarkers, reported in several species (guinea pig [53]; mouse [47, 99]; human [98]; rats, mice and human [104]). As yet, it is unclear what the function of such neurons might be, since ACh and NE have different influences (i.e., inhibitory versus excitatory) on those effectors expressing receptors for both neurotransmitters. It has been proposed that the function of such neurons may be switched, perhaps by fluctuating local concentrations of neurotrophic factors that control neurotransmitter gene expression, to work in either sympathetic or parasympathetic pathways at different times and under different physiological conditions [98, 104]. However, the connectivity and physiology of these neurons have not been established and their roles in the control of the heart are still unknown, leaving an intriguing gap in our knowledge of this system.
Nerve fibers containing neuropeptides (SP, CGRP) that are expressed by visceral afferent neuronal populations with their somata in the nodose and petrosal ganglia and the dorsal root ganglia (DRG) have also been reported within the ICNS [13, 34, 47, 99, 105, 106]. In addition, DRG neurons express markers for glutamate-handling biochemistry and there are numerous intracardiac plexus components containing these markers [107]. Furthermore, mechanical and chemical manipulation of most parts of the myocardium evoke rapid changes in ICNS activity that correlate with changes in the behavior of cardiac effectors. It has been proposed that collaterals of afferent axons within the heart terminate on some ICN, constituting local sensory feedback to intracardiac circuitry about the dynamic state of the heart [20, 32, 33]. There is also evidence that the somata of some ICN contain sensory neuronal markers (CGRP, SP [53]; glutamate [107]). These neurons may thus serve as intracardiac afferent neurons with their processes entirely within the ICNS [32] (see Sect. 8.3.1.2.2 for further discussion). Such afferent neurons are therefore represented within the ICNS in the hierarchy for cardiac control (Figs. 8.1 and 8.2).
3.1.2 Functional Roles
At the level of individual neurons within the ICNS, what practical criteria might be used during experimental studies to identify the cell types labeled “efferent,” “local circuit” and “afferent” in Figs. 8.1 and 8.2? This question is considered in the discussion of function-specific neuronal types below.
3.1.2.1 Efferent Neurons
(i) Parasympathetic Postganglionic Efferent Neurons
There is no doubt that cholinergic ICN are the primary parasympathetic postganglionic neurons in the two-neuron pathway for signaling from medullary preganglionic neurons to the cardiac effectors. However, as a number of studies have shown over the last four decades, not all, and perhaps not even the majority of ICN (depending at least partly on species) subserve this function. Even determining how many neurons are in this category in any species is still problematic. What would constitute a reasonable set of necessary and sufficient characteristics to define parasympathetic efferent ICN, and what is the evidence for neurons that would fit this set?
-
1.
Axonal projection pattern. Each neuron in this category should project an axon directly to at least one class of cardiac effector cell (pacemaker, conducting system, myocytes, smooth muscle of vasculature) in a region of the heart (or multiple regions if the axon branches) that may lie adjacent to, or more distant from, the location of the ganglion in which the soma is located. There is anatomical and physiological evidence in every species examined so far indicating that all regions of the heart receive parasympathetic postganglionic innervation [13, 34, 95, 108] and that stimulation of ICN in ganglia located in all of the major intracardiac plexi can affect cardiac function via activation of effector cells both adjacent to and remote from the stimulated ganglia [62, 65,66,67,68]. To date, there have been few attempts to analyze the intracardiac axonal projection patterns of ICN with the use of neurotracers. In an intracellular study of the morphology-function relationships of physiologically identified ICN [82], a neurotracer was injected through the recording microelectrode into the somata of sampled neurons. The processes of some of these neurons exited the ganglion under study via interganglionic nerves, but the distal targets of these projections could not be determined. In the reverse experiment, in which the neurotracer cholera toxin subunit B (CTB) was applied to the right or left atrial wall of the canine heart, the tracer was transported from axonal terminals to neuronal somata in both local and remotely located ganglia. In some cases, individual somata were labeled by CTB transport from axonal branches innervating multiple, well-separated injection sites [109]. However, somata in this study were not characterized on the basis of their physiological properties. While these findings reinforce the general idea of the distributed nature of ICN projections to local and distant cardiac effectors, it is not clear what the specific pattern of “fanout” of axonal projections of specific efferent parasympathetic neurons may be within the heart.
-
2.
Transmitter release at neuroeffector junctions. Parasympathetic efferent neurons release ACh from axon terminals in the vicinity of cardiac effectors, representing the fulcrum for parasympathetic control of cardiac functions. Endogenously released ACh acts postjunctionally at primarily M2-type muscarinic receptors, with consequent negative chronotropic, inotropic and dromotropic effects. In addition to cholinergic neuroeffector transmission, these neurons may also release one or more of a constellation of neurotransmitters and neuromodulators (e.g., NO, VIP and NPY) from their axonal terminals (Sect. 8.3.1.1.2), which can modify the postjunctional effects of muscarinic receptor activation. However, there have as yet been no definitive studies in functioning cardiac preparations on the nature of neuroeffector transmission from cholinergic efferent neurons identified either by their neurochemical profiles or by their specific axonal projection patterns to particular cardioeffectors.
-
3.
Convergence of synaptic inputs. Some efferent postganglionic parasympathetic neurons receive direct synaptic input from one (or more) vagal preganglionic axons coursing into the heart from extrinsic nerves, although the proportion of neurons receiving such inputs is known to be only a small fraction of the total number of ICN [26, 110]. Vagal preganglionic axon terminals release ACh, causing excitatory postsynaptic potentials (EPSPs) in the postsynaptic membrane via activation of nicotinic receptors. The simplest arrangement for information transfer in this system, assuming that preganglionic input is represented by one axon terminating on a single postganglionic cell, would therefore consist of strong synaptic drive (one AP arriving at the preganglionic terminal evoking a high-amplitude EPSP) that is suprathreshold for AP generation in the postganglionic neuron. This would fit the “classical” concept of the two-neuron peripheral autonomic pathway from central neurons to the viscera. However, data from studies recording extracellular AP discharge within the ICNS in the in situ heart show that this condition is rare: trains of high-frequency, high-intensity stimuli delivered to vagal preganglionic nerves are normally required to evoke even minimal AP discharge in ICN in efferent pathways [26, 110]. The major implication of this is that the majority of postganglionic parasympathetic efferent ICN do not receive direct synaptic inputs from extracardiac preganglionic axons. Other investigations using intracellular recordings from ICN in isolated atrial preparations in vitro have identified a population of neurons with preganglionic vagal inputs [85, 111]. Some of these neurons display high-strength, unitary synaptic excitation, but most show evidence of polysynaptic innervation, requiring summation of inputs from multiple presynaptic terminals to reach the threshold for AP generation. In contrast, McAllen et al. [90] have reported that vagal preganglionic inputs to individual ICN, driven both by cardiorespiratory reflexes and vagosympathetic trunk stimulation, usually evoke strong, unitary postsynaptic responses and APs in these neurons. However, it was not possible in any of these studies to identify where in the intracardiac control pathways the sampled neurons resided so their identity as potential parasympathetic postganglionic neurons could not be confirmed.
While details of the number, source and neurochemical phenotypes of synaptic terminals on efferent ICN remain unknown, it is likely that most, if not all, of these cells, will receive inputs from several different populations of ICN involved in local processing and possibly from cardiac afferent axon collaterals or axons from afferent ICN. These inputs may originate from cells within the ganglion in which the efferent neuron resides or from neurons in other intracardiac ganglia. In any case, given the potential complexity of convergence of inputs onto efferent ICN, if there are specific subsets of these inputs forming differentiable patterns, these have not yet been established (see further discussion in Sect. 8.3.1.2.3 below).
-
4.
AP discharge properties. The separation of ICN into discrete populations based on their AP firing behaviors (Sect. 8.3.1.1.1) was originally done in the hope that these behaviors would correlate with other characteristics such as input pattern or output projection to enable the identification of neurons in each category with a particular functional pathway. So far, however, this has not proven to be the case. While there appears to be general consensus that neurons with phasic and multiple-firing (accommodating or tonic) behaviors actually represent physiologically distinct subtypes (based on discrete sets of membrane ion channel complements), there are as yet no straightforward correlations between the firing behavior of a neuron and its position within a specific pathway or its functional role in cardiac control. This may partly be due to interspecies differences and partly to the fact that the anatomical identification of efferent neurons is still lacking in any species (see above). Therefore studies in which the somata of neurons are retrogradely labeled with neurotracers applied to various cardioeffectors, combined with intracellular recordings to determine the firing properties of such neurons, would represent the most direct way to address the question of physiological to pathway-position correlation. Such studies have been performed in investigations of the enteric nervous system to help establish the functional characteristics of myenteric and submucosal neuronal populations (see [112]), but these techniques have not yet been applied to the heart.
-
5.
Integrative properties. The capability of a single efferent parasympathetic postganglionic ICN to generate APs that modulate the activity of a cardiac effector will be determined by integration of the combination of factors discussed above pertaining to that particular neuron: the origins of synaptic input and postsynaptic receptor types present, synaptic strength of different inputs and the firing behavior of the cell. Given that some of these characteristics can also be modified by ongoing changes in the cardiac milieu (such as metabolite levels, extracellular ion concentrations, availability of nutritive substrates, local ischemia and pH [33]), the role of any efferent neuron will likely also adapt at least in the degree of its effectiveness if not in principle, during such variations.
(ii) Sympathetic Postganglionic Efferent Neurons
While the somata of the vast majority of sympathetic neurons in the peripheral autonomic innervation targeting the heart are located in the extracardiac intrathoracic ganglia [101], there is evidence that some somata lie within the ICNS [32, 101]. These neurons may have been displaced from the intrathoracic ganglia into the heart during development, so they could have the same operating characteristics as those in the thoracic ganglia, or they may represent a different subpopulation of sympathetic postganglionic neurons with their own set of characteristics. This has not been established. There is in fact very little known about these neurons at the cellular level. Their neuroeffector terminals contain catecholamines (chiefly NE), so these cells should be distinguishable as a distinct subpopulation from the other neuron types in the ICNS by their expression of amine-synthesizing enzymes, capability for storing and releasing adrenergic neurotransmitters and affinity for pan-neuronal markers.
-
1.
Axonal projection pattern. By analogy with parasympathetic efferent neurons in the heart (Sect. 8.3.1.2.1, i(1)), sympathetic efferent ICN should project axons to neuroeffectors in one or more regions of the heart (diverging through axonal branching) and their axon terminals will release NE at neuroeffector junctions to augment effector function. Physiological experiments support this supposition. Activation of these ICN with focal application of small volumes of excitatory neurochemicals to individual ganglia located in all parts of the heart evokes neurally mediated increases in cardiac indices [67, 113]. Those results demonstrate that sympathetic postganglionic ICN are present and project axons to a range of neuroeffectors throughout the heart. It thus appears that, while sympathetic postganglionic neurons within the ICNS appear to be much more rare than parasympathetic neurons, adrenergic ICN also constitute a distributed control system capable of driving local and remote cardio-augmentatory responses. However, despite several studies that have identified ICN somata with adrenergic characteristics, thus making them likely candidates for sympathetic postganglionic ICN [53, 98, 100], there have been no studies of the neuroanatomy of the axonal projections of these neurons.
-
2.
Transmitter release at neuroeffector junctions. Sympathetic postganglionic neurons with their somata located in the ICNS will likely have axon terminal-cardiac effector relationships similar to those of postganglionic neurons with their somata in the intrathoracic ganglia. Release of NE from the terminals of sympathetic ICN would thus be expected to augment chronotropic, dromotropic and inotropic cardiac functions. The neuropeptide NPY is co-localized with the adrenergic marker TH in the somata and terminals of some sympathetic ICN [53] and release of this peptide can modulate the effects of NE on cardiac myocytes [114] as well as reduce the negative chronotropic effect of parasympathetic cholinergic inputs to the pacemaker [115].
-
3.
Convergence of synaptic inputs. The major synaptic input on sympathetic postganglionic ICN, as for postganglionic neurons in the intrathoracic ganglia, is likely from preganglionic neurons with their cell bodies in the intermediolateral column of the spinal cord. Preganglionic terminals release ACh at the synaptic junction, acting at nicotinic cholinergic receptors on the postsynaptic membrane (Sect. 8.3.1.2.1, i(1)). The cardiac augmentation that results from chemical activation of these neurons was eliminated by propranolol, a β-adrenergic antagonist that blocks postjunctional effects of NE, as well as by the application of hexamethonium, a nicotinic cholinergic channel blocker that interrupts cholinergic neurotransmission to these cells [67].
There is as yet no information about noncholinergic synapses on sympathetic postganglionic neurons in the ICNS. Studies identifying putative sympathetic ICN by their primary expression of TH [53, 99] have not provided details of the neurotransmitter phenotypes of synaptic terminals on these cells.
-
4.
AP discharge properties. No intracellular records have yet been made from cells identified as intracardiac sympathetic postganglionic neurons, so the firing behavior of these neurons is not established.
-
5.
Integrative properties. Given that sympathetic postganglionic ICN will have synaptic inputs from one or more sympathetic preganglionic axons and that the neurochemical profiles of any additional synaptic contacts on these cells remain unclear, it is tempting to speculate that their capability for integration at the level of the soma may be limited. In the simplest case, these cells may receive only one strong synaptic preganglionic input, thus acting as simple relays from the autonomic motor neurons in the spinal cord to the cardiac effectors. However, intracellular studies of sympathetic postganglionic neurons in the intrathoracic ganglia have shown that neurons here typically integrate multiple preganglionic inputs and may receive additional inputs from afferent axons and possibly from intraganglionic processing neurons [8, 29, 116]. The capability for processing information through the network of intracardiac sympathetic postganglionic neurons is, therefore, likely to be no less complex than that for their counterparts in the intrathoracic ganglia.
3.1.2.2 Afferent Neurons
Primary afferent neurons associated with the heart may be defined as those that respond to one or more sensory modalities (mechanical stretch or change in the local chemical environment) with a receptive field limited to a restricted cardiac region [117]. Some of these afferent neurons can transduce both mechanical and chemical stimuli [32]. The somata of such neurons may be located outside the heart in the DRG, vagal sensory ganglia, intrathoracic ganglia or within the ICNS [8, 33]. Thus, within the heart, there are two apparent sources of sensory signaling: (1) afferent neurons with their somata in extracardiac ganglia with axons that give rise to intracardiac collaterals terminating on neurons within the ICNS; and (2) afferent neurons with their somata resident within the ICNS and with axons that project intracardially. Activation of receptor endings of either of these neuron types by an adequate stimulus of the correct modality results in the generation of trains of APs. These in turn activate other ICN involved in a variety of integrative processes that eventually contribute to cardiac command signals by which the heart responds to the original stimulus [20]. Such afferent input into LCN and efferent neurons within the ICNS would thus provide the necessary drive for short-latency intracardiac reflexes [33].
The observation of ongoing, spontaneous intracardiac neuronal activity, recorded in the absence of inputs from the central nervous system or intrathoracic ganglia (heart decentralized), was the first convincing physiological evidence that afferent neurons could be driving activity within the ICNS [26, 110, 118]. The fact that spontaneous activity could be recorded in the hearts of dogs up to a year after cardiac transplant (in those animals that did not show signs of regrowth of extracardiac nerves into the heart) further indicated that afferent neurons resident in the ICNS were driving this activity [119]. In these, and in virtually all other studies of ICN in the beating heart, neuronal activity was recorded extracellularly. It has thus not been possible to determine whether the source of afferent discharge was the primary afferent neurons themselves or activity that was induced in secondary or higher-order neurons entrained by such primary afferent inputs [110]. The characteristics of primary afferent ICN at the level of individual cells have not been established.
Primary cardiac afferent neurons with their somata in the DRG that project to the dorsal horn of the spinal cord express CGRP and SP along with markers for the synthesis of glutamate [107, 120] and have a stereotypical bipolar morphology [55, 105]. Cardiac afferent information is also conveyed to the nucleus solitarius in the medulla via primary afferent neurons with their somata in the nodose and petrosal ganglia of the vagus. The occurrence and distribution of the axons of these afferent neurons within the heart are identifiable by the patterns of expression of their sensory neuromarkers. It is reasonable to suppose that resident cardiac afferent ICN would have similar profiles of neuromarkers; in fact, cell bodies expressing neuromarkers typical of afferent neurons have been identified within the ICNS [53], although these are relatively rare. Some afferent terminals and axons in the heart also express the transient receptor potential vanilloid 1 (TRPV1) ion channel [121,122,123,124]; these terminals are activated by a variety of chemical stimuli including capsaicin and bradykinin [121, 122, 125]. Recently the TRPV1 agonist resiniferatoxin has been used to overstimulate and thus partially deplete these channels, providing a method to selectively impair some intracardiac afferent terminals [121, 123]. This has allowed the contribution of these receptors to cardio-cardiac reflexes to be evaluated [123, 124]. It is, therefore, possible that the characteristics typical of vagal and DRG afferent neurons may also apply to resident afferent ICN.
There is physiological evidence for the presence of intracardiac afferent neurons in multiple mammalian species. Spontaneous, ongoing activity from ICN in isolated atrial tissues in vitro has been reported during intracellular recording by several authors [84, 126, 127]. This activity generally consists of EPSPs occurring either irregularly or in rhythmic trains; some of these depolarizations exceeded the threshold for AP discharge. In this situation, it is not clear whether these neurons are themselves primary sensory cells or are responding to activity in a primary neuron with a synapse on the sampled neuron. In some cells, APs rose directly from the baseline membrane potential without a preceding EPSP. Such activity may represent ongoing discharge of primary afferent neurons, perhaps responding to small contractions of the underlying myocardial substrate.
An attempt to correlate the firing pattern of ICN with other indicators of a potential afferent function was made by Edwards et al. [84], who recorded intracellularly from several classes of neurons in the guinea pig heart (see Sect. 8.3.1.1.1 for details). They described one class, P-type neurons, that had no synaptic inputs, showed bipolar or pseudounipolar morphology after labeling with neurotracer injected via the recording electrode and displayed multiple AP discharges in response to long intracellular depolarizing current injection. P-type neurons also had consistent patterns of spontaneous, frequent short depolarizations that were subthreshold for AP generation and occasionally discharged APs that rose rapidly from the baseline membrane potential without a preceding EPSP. Selyanko [80] also reported that some ICN with the firing properties of type Im (Fig. 8.5) showed regular spontaneous AP discharges that rose directly from the baseline membrane potential without evidence of preceding EPSPs. This author discounted the possibility that injury potentials resulting from poor cell impalement were responsible, suggesting that this activity was “intrinsic in origin”. Such activity may well have arisen from the transduction of mechanical stimuli from elements of the myocardium underlying the ganglia containing the sampled neurons.
Given the attributes of extracardiac afferent neurons innervating the atria and ventricles, it would be appropriate to speculate that afferent neurons with their somata within the ICNS had similar attributes, as a starting point for identifying these cells. The most useful criteria for this may be: (1) somata and processes that exhibit immunohistochemistry for one or more of the neuropeptides associated with extracardiac afferent cells (CGRP and SP) with markers of glutamate expression also a possibility; (2) expression of TRPV family channels or stretch-activated channels in the cell membrane; (3) lack of synaptic inputs; (4) pseudounipolar or possibly bipolar cell morphology; (5) receptor terminals associated with the myocardium or cardiac connective tissue; (6) membrane potential depolarization in response to activation of chemosensory or mechanosensory transduction mechanisms (or both), with such depolarizations reaching the threshold for AP discharge upon delivery of an adequate stimulus to the membrane; (7) capability for firing APs repetitively under strong sensory stimulation (some of these neurons may show adaptation of AP firing rate); (8) synaptic transmission, with a high safety factor, to one or more secondary neurons. It is possible, in regard to item 8, that those afferent neurons make connections with nearby secondary neurons or with those in more distant ganglia; ultimately, such afferents may even project axons centripetally via extracardiac nerves.
3.1.2.3 Local-Circuit Neurons
Evidence from numerous studies employing extracellular recordings of ICN activity in in situ hearts indicates that the majority of activity originates from neither principal postganglionic efferent neurons nor primary afferent neurons [32]. Instead, such activity is proposed to result from the potentially very large number of interactions between neurons of as yet indeterminate function within the intracardiac ganglia; these neurons are represented in the hierarchical schematics of the ICNS as LCN (Figs. 8.1 and 8.2).
Beaumont et al. [110] showed that, in spontaneously beating hearts in the open-chest, anesthetized canine, many ICN discharged APs spontaneously at varying rates. Their activity could be modulated by manipulations including mechanical stimulation of the myocardium, short periods of left ventricular ischemia, great vessel occlusion, induction of atrial arrhythmia or extracardiac nerve stimulation. However, it is salutary that none of the neurons sampled in that study responded directly to extracardiac inputs. Instead, approximately half of the neurons responded indirectly to electrical stimulation of either vagal or sympathetic extracardiac nerves. A proportion of these ICN showed responses to stimulation of both extrinsic input pathways. The authors interpreted these results as showing that most ICN with responses to extrinsic inputs was located in intracardiac processing pathways that were involved in integrating information from central neurons but were not directly driven by those inputs. Many of the sampled ICN, whether or not they received extrinsic inputs, showed spontaneous activity that was locked to specific phases of the cardiac cycle, implying that this aspect of their activity originated from intracardiac afferent neurons or axon collaterals innervating the myocardium. In keeping with this, about one-quarter of neurons sampled in this study responded to mechanical manipulation of the myocardium or to multiple cardiac stressors.
Given the apparent complexity of neuronal pathways within the ICNS and the finding that the majority of ICN within this system appears to consist of subpopulations of LCN that presumably perform a variety of functions within these pathways, it has been proposed that LCN be subdivided into three classes on the basis of experimental evidence [110, 128].
-
(i)
Secondary afferent local-circuit neurons. These neurons are defined as receiving inputs from one or more primary afferents mediating mechanosensory or chemosensory modalities (or both). The designation “secondary” refers to ICN contacted either monosynaptically or through a multisynaptic pathway by primary afferents. In the study of Beaumont et al. [110], the majority of neurons that preferentially received afferent inputs certainly belonged to this class, as they commonly responded to multimodal sensory signals and had broad receptive fields.
-
(ii)
Secondary efferent local-circuit neurons. Neurons of this type would be entrained in the cardiomotor pathways between extrinsic vagal or sympathetic preganglionic inputs and cardiac effectors. They would process inputs derived from extracardiac sources, and their activity would presumably be subject to modification by intracardiac integrative processes controlling the routing of cardiomotor information through these pathways.
-
(iii)
Convergent local-circuit neurons. These neurons are perhaps the least well-defined of the three LCN categories but may be the largest population in the ICNS. A substantial portion of ICN responding indirectly to extrinsic nerve stimulation was activated by more than one input source. These ICN could respond to inputs from the right or left vagus, the right or left sympathetic nerves, or from both sets of nerves [110, 128]. These findings are corroborated by intracellular microelectrode studies in isolated atrial tissue in vitro, in which membrane potential responses of single ICN were recorded during electrical stimulation of the proximal ends of extracardiac nerves [85, 111].
In the rat heart stimulation of the stump of the vagosympathetic trunk attached to the preparation evoked EPSPs and APs in ICN (Fig. 8.7a, top panel [111]); bath application of mecamylamine, a nicotinic cholinergic antagonist, eliminated the nicotinic component of the EPSP, revealing a small noncholinergic postsynaptic depolarization (Fig. 8.7a, bottom panel). In this context, it is important to note that the stimulated nerve branch contains both vagal preganglionic (cholinergic) and sympathetic postganglionic (adrenergic) axons. In the pig heart, more than one-third of the neurons sampled responded with EPSPs to vagosympathetic trunk stimulation; depolarizations usually appeared at latencies of 20–40 ms or longer from the stimulation pulse ([85]; Fig. 8.7b, top trace). Some of those inputs exceeded the threshold for AP discharge (not shown). Roughly half of the ICN responding to vagosympathetic trunk input also responded to cardiopulmonary nerve stimulation with EPSPs (Fig. 8.7b, second trace from the top; none of these reached the threshold for AP discharge). In the example shown here, when both nerves were stimulated together, the combined EPSP exceeded the threshold for the AP (Fig. 8.7b, third trace from top). Bath application of hexamethonium, a nicotinic cholinergic antagonist, eliminated the effect of vagosympathetic trunk stimulation (Fig. 8.7b, second trace from bottom) and application of timolol, a β-adrenergic antagonist, blocked depolarizations from cardiopulmonary nerve stimulation (Fig. 8.7b, bottom trace). In both studies, ICN were identified that responded to extrinsic nerve stimulation over a range of latencies, and in many of these cells, increasing the stimulus amplitude recruited multiple temporally- and spatially-summated EPSP components. It is, therefore, arguable that the ICN recorded in these intracellular studies could be examples of convergent LCN. Unfortunately, in neither study was a response to local, interganglionic nerve stimulation tested, so whether these neurons received synaptic inputs from intracardiac sources is not known.
Intracellular postsynaptic responses of intracardiac neurons to electrical stimulation of extrinsic cardiac nerves. (a) Isolated heart of neonatal rat. Upper panel: neuron responds with action potential to single presynaptic stimulus. Lower panel: upper trace (“control”) shows excitatory postsynaptic response in another neuron to single presynaptic stimulus; response did not reach threshold for action potential discharge. Lower trace (“mecamylamine”) shows that depolarizing response to a repeated presynaptic stimulus was eliminated by exposure to this nicotinic cholinergic antagonist. (b) Isolated heart of pig. From top of panel downward: Postsynaptic depolarizing responses of the same neuron to single-pulse stimuli delivered to attached stumps of extrinsic cardiac nerves (“VAGUS,” upper trace) and cardiopulmonary nerve (“CPN,” second trace). Stimulation of either nerve alone evoked excitatory postsynaptic potentials (EPSPs) that did not reach the threshold for action potential discharge. Middle trace, simultaneous stimulation of both nerves (“VAGUS+CPN”) evoked multiple EPSPs that summated to exceed threshold for action potential. Second trace from bottom: hexamethonium, a nicotinic antagonist, blocked postsynaptic responses from vagosympathetic trunk stimulation (“VAGUS”). Bottom trace: timolol, a B-adrenergic antagonist, blocked responses from stimulating the cardiopulmonary nerve (“CPN”). In a and b, the stimulus artifact is represented by small pulse on baseline trace preceding responses (a: [111]; b: [85] with permission)
The concept of classification of LCN by a combination of (1) their responses to events transduced by primary afferent inputs; (2) their connectivity (or lack of it) with extrinsic autonomic inputs; and (3) their spontaneous discharge profiles entrained by the process of integrative network signal processing as proposed by Beaumont et al. [110] makes a compelling framework to begin to understand how information may be processed in the ICNS. Furthermore, it appears that there is some evidence of support for this system at the level of single ICN, at least for the category of convergent LCN, as in the examples above. However, the characteristics of potential LCN of all types must be further investigated at the level of the single cell.
4 Future Directions in Analysis of Intracardiac Neuronal Circuitry
A critical factor in the anatomical and functional analysis of circuitry within the ICNS is access to the system for visualization and experimental manipulation in preparations in which in vivo levels of integration are maintained. In most mammalian models studied to date, the ICNS is embedded in relatively thick cardiac walls, so not accessible in its entirety. Functional studies on the physiology and synaptic interconnections of ICN have thus largely been done in explanted cardiac segments studied in vitro. This has limited the scope for gleaning information about the circuitry in which individual ICN are involved. Similarly, anatomical studies of the ICNS have been hindered by the lack of capability for visualizing labeled neuronal elements in relatively thick, nontransparent cardiac tissue. The development of new tools for tissue clearance to facilitate visualization of the three-dimensional organization of cardiac innervation in whole mammalian hearts with relatively low volumes, such as the mouse heart, has begun to solve the problem of lack of tissue transparency at depth. There are new techniques becoming available for identifying neurotransmitter-specific populations of ICN in whole, spontaneously beating hearts in situ and in vitro. Furthermore, enabling the membrane expression of light-activated channels in identified neuronal subpopulations of the ICNS will allow these populations to be selectively stimulated and their effects on cardioeffectors assessed with high precision. In addition, exploration of the ICNS is being extended to encompass alternative, nonmammalian experimental models, such as zebrafish, that provide advantages for analyzing the fundamental principles of neural control in hearts that are dually innervated by sympathetic and parasympathetic nerves, representing archetypes for control of the mammalian heart. Such “simple” hearts face the same basic challenges of controlling cardiac output as mammalian hearts.
4.1 “Low-Volume” Mammalian Hearts
The development of genetically engineered animal models in which defined lines of neurons are identified by their expression of specific neurotransmitters has begun to clarify our understanding of function-specific pathways within the ICNS. This approach has been facilitated by the advent of the parallel development of advanced tissue-clearing protocols so that fluorescent markers genetically incorporated into neuronal somata and processes can be visualized at the single-cell level in whole organs [129,130,131,132]. Recent advances in microscopy techniques, such as light-sheet microscopy [133] and improvements in laser confocal microscopy, are also contributing to the process of visualizing details of the ICNS in three dimensions in the whole heart [34]. A prime example of the recent use of these techniques is the publication of an atlas of the ICNS in the whole rat heart: this study has, for the first time, provided a detailed three-dimensional map of the intracardiac neuroanatomy in the mammalian heart as well as elaborating the organization and distribution of neurotransmitter-specific neuronal populations at the single-cell level [51]. The rich detail provided by such advances is now facilitating the analysis of the functional roles of specific populations of ICN in cardiac control.
A very powerful tool for identifying and genetically manipulating specific neuronal populations is the use of recombinant adenoassociated viruses (AAV) as vectors for delivering genetic material into these populations [134, 135]. Such techniques have been used for inducing neurons to express fluorescent marker proteins, optogenetic actuators such as channelrhodopsins (ChR, see below) and a variety of cytosolic calcium indicators and membrane voltage-sensitive agents. These viruses can be used to deliver genetic material into adult organs and tissues including the heart, and once delivered, the resulting expression of the cellular products is long-lasting. This technique raises the possibility of identifying and manipulating whole populations of neurotransmitter-identified ICN to help define the circuitry in which these neurons are embedded.
A recent example of the use of a combination of these new techniques to explore the wiring of the ICNS is a study by Rajendran et al. [136] in the mouse heart. Here these authors have mapped the broad outlines of sympathetic and parasympathetic intracardiac and intrathoracic circuitry controlling pacemaker and other cardioeffector functions using neuroanatomical and optogenetic techniques. In the ICNS, they first defined the detailed three-dimensional distribution of ganglia and nerves using a pan-neuronal, fluorescently-tagged marker, whole-organ tissue-clearing procedure [130, 132] and visualization with confocal and light-sheet microscopy. They then used AAV-based labeling of cholinergic ICN to identify parasympathetic efferent neurons projecting to the SAN, the AVN and the conducting system, thus anatomically defining a portion of the function-specific neuronal pathway controlling heart rate and conduction (Fig. 8.8). To evaluate the function of this neuronal circuitry the authors developed a transgenic mouse line expressing ChR in the cell membrane of cholinergic ICN. Neurons expressing ChR, when exposed to pulses of light of the correct wavelength to open these channels, become depolarized and generate APs. Light-associated opening of ChR channels thus emulates excitation of these neurons by suprathreshold synaptic input. In an isolated preparation of the spontaneously beating heart, light application to the inferior pulmonary ganglionated plexus, adjacent to the SAN, evoked varying degrees of bradycardia that depended on the light intensity (Fig. 8.9). Activation of these ganglionic neurons also altered the electrocardiogram waveform (prolonging the P-wave) and caused ectopic atrial beats. These results illustrate the potential for the use of genetic engineering and optogenetic techniques to dissect the circuitry of the ICNS to unravel the roles of defined subpopulations of neurons in cardiac control. The authors were thus able to explore the cardiac effects of activating a population of cells that could represent primary efferent parasympathetic neurons, thus defining the output segment of one of the function-specific reflex pathways within the heart that controls rate. It is anticipated that the combination of such new circuitry-defining techniques with established intracellular and patch-clamp recording methodology will allow the next step to be taken in understanding the organization and physiology of intracardiac reflexes.
Intracardiac nervous system in transgenic mouse heart. Left panel: schematic of dorsal aspect of heart showing the location of major cardiac ganglionated plexi. AV node, atrioventricular node; IVC, inferior vena cava; IPV-GP, inferior pulmonary vein ganglionated plexus; LA, left atrium; LV, left ventricle; PVs, pulmonary veins; RA, right atrium; RV, right ventricle; SA node, sinoatrial node; SVC, superior vena cava. Right panel shows enlarged image of region in dotted box in left panel. Cholinergic neurons in the IVP-GP and their processes were labeled using an adenovirus-associated vector-based system (see text for details) showing cholinergic axons innervating the SA and AV nodes (dotted circles, nodal tissue identified with antibodies against hyperpolarization-activated, cyclic nucleotide-gated ion channel 4, HCN4). Scale bar in the photomicrograph represents 200 μm ([136] with permission)
Chronotropic effects of light activation of channelrhodopsin-expressing intracardiac neurons. Left panel: photograph of Langendorff preparation of mouse heart: light pulses from optical fiber were directed toward inferior pulmonary vein ganglionated plexus (IVP-GP, dotted oval). ECG, bath electrodes placed for surface electrocardiogram recording; EP (electrophysiology) catheter site for recording intracardiac ECG. Right panel: bradycardia resulting from the application of light pulses to IVP-GP neurons. Upper trace: light pulses delivered at 10 Hz; lower trace, 20 Hz. Scale bar in the left panel represents 1 mm ([136], with permission)
4.2 Ancestral Vertebrate Hearts: Alternative Models for Neurocardiology
The ICNS in several amphibian and teleost fish species has been studied using a variety of in vitro experimental approaches. The earliest studies of vertebrate ICN were done in amphibians to determine the nature of synaptic inputs from vagal preganglionic neurons (see discussion in Sect. 8.3.1.1.1). At approximately the same time, Saito and Tenma [137] evaluated the vagal control of pacemaker discharge in the heart of the carp, a cyprinid teleost fish, demonstrating that the principles of neural control of pacemaker discharge rate were broadly similar to those in the mammalian heart. The fish heart has one atrium and one ventricle, while the amphibian heart has a divided atrium supplying blood to a single ventricle; these organisms thus represent different evolutionary stages in progress toward the four-chambered hearts of mammals. Yet even the most ancestral version of the vertebrate heart, that of teleosts, is governed by dual sympathetic and parasympathetic autonomic inputs and has broadly distributed postganglionic innervation of the myocardium [42,43,44]. The single atrium and ventricle, operating in series in these hearts, face the same issues in controlling cardiac output to perfuse the vascular beds in the body as do mammalian hearts. The fish heart also has several advantages for neuroanatomical and structure-function studies of the heart, including the hardiness of explanted, perfused hearts and ready access to the entire ICNS in spontaneously beating in vitro preparations.
The most powerful nonmammalian model emerging for investigating many fundamental research questions is the zebrafish, a cyprinid teleost. Studies into a broad range of questions have burgeoned over the last three decades, and a number of reviews have covered the use of zebrafish in these investigations (i.e., [138] and works cited therein). This model was originally employed for studies of the genetics and molecular biology of embryonic development and organogenesis [139, 140] and the effects of mutagenesis (see the special issue of the journal Development 123 [1996]) as well as organ function in adults [141]. The zebrafish genome has been sequenced and is highly conserved to that of humans; there may be one or more orthologues in zebrafish for about 70% of human genes [142]. There is now extensive literature on studies taking advantage of zebrafish to investigate many aspects of basic cardiac function (e.g., [138, 143, 144]). This model has many applications in studies of organ function: zebrafish are readily available and have modest breeding and upkeep requirements; fertilization is external and animals in early developmental stages are transparent; they reproduce quickly and plentifully and form an ideal platform for mutagenesis studies [145]. Moreover, the physiological range of normal heart rate, operation of the pacemaker, cardiac electrophysiology, myocyte AP morphology, contractile protein function and some aspects of calcium handling strongly resemble those of human hearts [146,147,148,149,150]. Transgenic lines of zebrafish have been created with modified orthologues of human genes that are responsible for many heart defects ranging from channelopathies to cardiac conduction. These transgenics have provided clear and profound insights into the physiological mechanisms underlying such defects [144, 151,152,153,154,155] .
Given these factors, our research group has investigated the possibility that the zebrafish ICNS could illuminate fundamental questions of organization and operation of this system. We have shown that all parts of the zebrafish heart are innervated, neurons in the ICNS are mainly localized at the venous pole within a plexus surrounding the sinoatrial valves and the vast majority of neuronal somata are cholinergic with some adrenergic and nitrergic somata also present (Fig. 8.10 [156]). There are approximately 200 ICN somata within the zebrafish heart. Application of a neuronal tracer to the ends of the left and right vagosympathetic trunks attached to the heart showed that axons in these nerves merge with the sinoatrial plexus. Extrinsic adrenergic axons terminate either on ICN somata within the plexus or continue through the plexus into the heart, diverging to innervate targets at the junction of the sinoatrial valve leaflets with the atrium and throughout the atrial and ventricular walls. Extrinsic cholinergic axons terminate extensively on the somata of ICN throughout the plexus. An intriguing finding from the application of neurotracer to extrinsic nerves was that a few neuronal somata were labeled within the sinoatrial plexus. These somata potentially represent afferent neurons with their axons projecting cardiofugally. Axons arising from some plexus ICN expressing the cholinergic phenotype terminated proximal to cells that were immunoreactive for antibodies against hyperpolarization-activated, cyclic nucleotide-gated ion channel 4 (HCN4, a marker for pacemaker cells in mammalian hearts [157]). These cells were located at the junctions of the sinoatrial valve leaflets with the atrium, forming a ring around the valve ostium, and were adjacent to plexus ICN (Fig. 8.10). This study shows that the ICNS can be visualized in the whole heart, that this system is neurochemically complex, that separate populations of ICN express markers indicating cholinergic, adrenergic or NANC phenotypes, that there may be afferent ICN present and that postganglionic axons from both parasympathetic and sympathetic limbs of the autonomic nervous system innervate all cardiac regions. Our results thus provide details of the neuroanatomical substrate involved in control of cardiac function in the zebrafish.
Image (panel a) and schematic (panel b) of wholemount preparation of zebrafish heart; intracardiac nervous system labeled with pan-neuronal markers acetylated tubulin (Act, axons) and human neuronal protein C/D (Hu, somata). A, atrium; AVP, atrioventricular plexus; BA, bulbus arteriosus; SAP, sinoatrial plexus; V, ventricle. (c) Enlarged view of SAP region surrounding sinoatrial valve leaflets. Arrows indicate the locations of some of the ganglia containing intracardiac neurons. dSAP, vSAP: dorsal and ventral components of the plexus; LX, RX: entry into the plexus of left and right vagosympathetic trunks; O: ostium of sinoatrial valve between its leaflets. Scale bar in a represents 1 mm; in c, 100 μm ([156] with permission)
We developed an isolated preparation of the spontaneously beating, perfused zebrafish heart to study the relationship between the ICNS and pacemaker function (Fig. 8.11 [158]). This preparation provides access to both extrinsic and intrinsic neural elements to dissect their roles in the control of heart rate. Electrical stimulation of attached vagosympathetic trunks activated parasympathetic preganglionic axons evoking bradycardia during the duration of stimulation (Fig. 8.11b). Blockade of cholinergic drive to the pacemaker with atropine eliminated the bradycardia. A delayed, adrenergically mediated tachycardia occurred following the cessation of nerve stimulation (Fig. 8.11b), produced by activation of sympathetic postganglionic axons also present in the vagosympathetic trunks. Blockade of β-adrenergic neurotransmission eliminated this response. Optical imaging of the spread of electrical activity in the myocardium demonstrated that excitation originated in the region of the sinoatrial valve, confirming anatomical results showing that the HCN4-positive cells clustered in this area likely constitute the primary pacemaker. These cells express both β2-adrenergic and muscarinic cholinergic receptors on their membranes, consistent with the influence of sympathetic and parasympathetic activation on pacemaker discharge rate.
Effects of vagosympathetic trunk electrical stimulation on cardiac chronotropy in isolated zebrafish heart. (a) Photograph of preparation showing placement of electrocardiogram electrodes on atrial (aE) and ventricular (vE) walls. Nerve stimulation electrode is not shown. (b) Simultaneous electrical stimulation of both attached vagosympathetic trunks (0.5 ms, 300 μA pulses, 15 Hz pulse rate in 10 s train) evoked bradycardia during stimulation, followed by poststimulus tachycardia. Scale bar in a represents 1 mm ([158] with permission)
Our results from studies of the neuroanatomy and activation of the ICNS in the isolated zebrafish heart indicate that this preparation has the capability to provide additional insight into the principles governing neural control of the heart. The easy accessibility of the ICNS in this preparation for physiological experiments, as well as the tractability of the zebrafish for genetic manipulation, provides an opportunity to use the same advanced techniques for analyzing ICNS properties already in use for studies in mammalian hearts. Studies designed to identify neurotransmitter-specific neuron populations in whole, living zebrafish hearts and to insert genes coding for light-activated ion channels into the membranes of these neurons to demonstrate the effects of optically activating ICN would begin to clarify the circuitry of intracardiac reflexes, leading to an improved understanding of the operating principles of the ICNS.
5 Conclusions
The neural control of the heart has been proposed to depend upon a set of feedback loops involving populations of neurons in the central and peripheral compartments of the autonomic nervous system, illustrated in Figs. 8.1 and 8.2. At the level of the heart, the ICNS is the final common pathway for cardiac control. Intracardiac neurons integrate inputs from higher levels in the hierarchy along with a range of afferent signals from within the heart to generate motor outputs to the effector cells that determine cardiac output on a beat-by-beat basis. The ICNS appears to be composed of collections of locally driven reflex circuits whose activity is constantly and rapidly being modified by changes sensed by local afferents to modulate motor outputs. These reflex circuits are subject to modulation over longer time scales by higher-order reflexes relayed through intrathoracic and central loops. It is thus not surprising that the majority of the ICNS appears to consist of populations of LCN involved in the integration of information that ultimately sets the discharge rates of efferent neurons controlling the cardiac effectors. This, coupled with the distributed nature of intracardiac ganglia throughout the heart and the capability for ICN to influence both local and remote cardiac regions, provides this system with a highly flexible control capability. However, at the level of individual ICN, there remain large gaps in our knowledge related to their physiological types and membrane properties, connectivity, neurotransmitter phenotypes and organization into local reflexes. New technologies for analyzing the neuroanatomical organization of this system combined with novel and established methodologies for studying neuronal circuitry within the heart will ultimately yield a better understanding of the integrated function of the ICNS in controlling cardiac output in the face of varying demands for whole-body perfusion. This understanding is essential to guide the development of effective, neurally based therapies for managing cardiovascular diseases.
References
Gaskell W. The involuntary nervous system. London, UK: Longmans; 1916.
Langley G. The autonomic nervous system. Cambridge: Cambridge University Press; 1921.
Cannon W, Rosenblueth A. Autonomic effector systems. New York: The MacMillan Company; 1937.
Sheehan D. Discovery of the autonomic nervous system. Arch Neurol Psychiatry. 1936;35:1081–115.
Randall WC. Changing perspectives concerning neural control of the heart. In: Armour JA, Ardell JL, editors. Neurocardiology. New York: Oxford University Press; 1994. p. 3–17.
Armour JA, Ardell JL, editors. Neurocardiology. New York: Oxford University Press; 1994.
Armour JA, Ardell JL, editors. Basic and clinical neurocardiology. New York: Oxford University Press; 2004.
Armour JA. Potential clinical relevance of the “little brain” the mammalian heart. Exp Physiol. 2008;93(2):165–76.
Shivkumar K, Ardell JL. Cardiac autonomic control in health and disease. J Physiol. 2016;594(14):3851–2.
Coote JH, Gilbey MP. Special issue, ‘Central and peripheral nerve influence on cardiac function in health and disease’. Auton Neurosci Basic Clin. 2016;199:1–2.
Osteraas ND, Lee VH. Neurocardiology. In: Wijdicks EFM, Kramer AH, editors. Handbook of Clinical Neurology vol 140 (3rd series): Critical care neurology, Part 1. Edinburgh: Elsevier; 2017. p. 49–65.
Durães Campos I, Pinto V, Sousa N, Pereira VH. A brain within the heart: A review on the intracardiac nervous system. J Mol Cell Cardiol. 2018;119:1–9.
Wake E, Brack K. Characterization of the intrinsic cardiac nervous system. Auton Neurosci. 2016;199:3–16.
Loewy A, Spyer K, editors. Central regulation of autonomic functions. New York: Oxford University Press; 1990.
Taggart P, Critchley H, van Duijvendoden S, Lambiase PD. Significance of neuro-cardiac control mechanisms governed by higher regions of the brain. Auton Neurosci. 2016;199:54–65.
Hopkins DA, Armour JA. Ganglionic distribution of afferent neurons innervating the canine heart and cardiopulmonary nerves. J Auton Nerv Syst. 1989;26(3):213–22.
Armour JA, Huang MH, Pelleg A, Sylvén C. Responsiveness of in situ canine nodose ganglion afferent neurones to epicardial mechanical or chemical stimuli. Cardiovasc Res. 1994;28(8):1218–25.
Vance WH, Bowker RC. Spinal origins of cardiac afferents from the region of the left anterior descending artery. Brain Res. 1983;258(1):96–100.
Armour JA. Anatomy and function of the intrathoracic neurons regulating the mammalian heart. In: Zucker I, Gilmore J, editors. Reflex control of the circulation. Boca Raton, FL: CRC Press; 1991. p. 1–37.
Kember G, Ardell JL, Shivkumar K, Armour JA. Recurrent myocardial infarction: mechanisms of free-floating adaptation and autonomic derangement in networked cardiac neural control. PLoS One. 2017;12(7):e0180194.
Norris JE, Foreman RD, Wurster RK. Responses of the canine heart to stimulation of the first five ventral thoracic roots. Am J Physiol. 1974;227(1):9–12.
Mizeres NJ. The anatomy of the autonomic nervous system in the dog. Am J Anat. 1955;96(2):285–318.
Hopkins DA, Armour JA. Localization of sympathetic postganglionic and parasympathetic preganglionic neurons which innervate different regions of the dog heart. J Comp Neurol. 1984;229(2):186–98.
Levy MN. Sympathetic-parasympathetic interactions in the heart. Circ Res. 1971;29(5):437–45.
Armour JA. Anatomy and function of thoracic cardiac neurons. In: Ciriello J, Calaresu F, Renaud L, Polosa C, editors. Organization of the autonomic nervous system: central and peripheral mechanisms. New York: Alan R Liss; 1987. p. 67–77.
Gagliardi M, Randall WC, Bieger D, Wurster RD, Hopkins DA, Armour JA. Activity of in vivo canine cardiac plexus neurons. Am J Physiol. 1988;255(4):H789–800.
Armour JA. Activity of in situ stellate ganglion neurons of dogs recorded extracellularly. Can J Physiol Pharmacol. 1986;64(2):101–11.
Armour JA. Neuronal activity recorded extracellularly in chronically decentralized in situ canine middle cervical ganglia. Can J Physiol Pharmacol. 1986;64(7):1038–46.
Bosnjak ZJ, Kampine JP. Intracellular recordings from the stellate ganglion of the cat. J Physiol. 1982;324:273–83.
Kalia M, Mesulam MM. Brain stem projections of sensory and motor components of the vagus complex in the cat: I. The cervical vagus and nodose ganglion. J Comp Neurol. 1980;193(2):435–65.
Kalia M, Mesulam MM. Brain stem projections of sensory and motor components of the vagus complex in the cat: II. Laryngeal, tracheobronchial, pulmonary, cardiac, and gastrointestinal branches. J Comp Neurol. 1980;193(2):467–508.
Ardell JL, Armour JA. Neurocardiology: structure-based function. Compr Physiol. 2016;6(4):1635–53.
Shivkumar K, Ajijola OA, Anand I, Armour JA, Chen P-S, Esler M, et al. Clinical neurocardiology defining the value of neuroscience-based cardiovascular therapeutics. J Physiol. 2016;594(14):3911–54.
Hanna P, Rajendran PS, Ajijola OA, Vaseghi M, Andrew Armour J, Ardell JL, et al. Cardiac neuroanatomy—imaging nerves to define functional control. Auton Neurosci. 2017;207:48–58.
Rajendran PS, Nakamura K, Ajijola OA, Vaseghi M, Armour JA, Ardell JL, et al. Myocardial infarction induces structural and functional remodelling of the intrinsic cardiac nervous system. J Physiol. 2016;594(2):321–41.
Jänig W. The integrative action of the autonomic nervous system: neurobiology of homeostasis. Cambridge: Cambridge University Press; 2006.
Jänig W, McLachlan EM. Specialized functional pathways are the building blocks of the autonomic nervous system. J Auton Nerv Syst. 1992;41(1–2):3–13.
Jänig W, McLachlan EM. Neurobiology of the autonomic nervous system. In: Mathias C, Bannister R, editors. Autonomic failure: a textbook of clinical disorders of the autonomic nervous system. 5th ed. Oxford: Oxford University Press; 2013. p. 21–34.
Jänig W, McLachlan EM. Characteristics of function-specific pathways in the sympathetic nervous system. Trends Neurosci. 1992;15(12):475–81.
Jänig W. Neurocardiology: a neurobiologist’s perspective. J Physiol. 2016;594:3955–62.
Farrell A, Smith F. Cardiac form, function and physiology. In: Gamperl A, Gillis T, Farrell A, Brauner C, editors. The cardiovascular system: morphology, control and function. Cambridge, MA: Academic; 2017. p. 155–264.
Nilsson S. Autonomic nerve function in the vertebrates. Berlin: Springer; 1983.
Nilsson S. Comparative anatomy of the autonomic nervous system. Auton Neurosci. 2011;165:3–9.
Nilsson S, Holmgren S, editors. Comparative physiology and evolution of the autonomic nervous system. Chur, Switzerland: Harwood Academic Publishers; 1994.
Nonidez J. Studies on the innervation of the heart: 1. Distribution of the cardiac nerves, with special reference to the identification of the sympathetic and parasympathetic postganglionics. Am J Anat. 1939;65(3):361–413.
King TS, Coakley JB. The intrinsic nerve cells of the cardiac atria of mammals and man. J Anat. 1958;92(3):353–76.
Rysevaite K, Saburkina I, Pauziene N, Noujaim SF, Jalife J, Pauza DH. Morphologic pattern of the intrinsic ganglionated nerve plexus in mouse heart. Heart Rhythm. 2011;8(3):448–54.
Pauza DH, Saburkina I, Rysevaite K, Inokaitis H, Jokubauskas M, Jalife J, et al. Neuroanatomy of the murine cardiac conduction system: a combined stereomicroscopic and fluorescence immunohistochemical study. Auton Neurosci. 2013;176(1–2):32–47.
Pardini BJ, Patel KP, Schmid PG, Lund DD. Location, distribution and projections of intracardiac ganglion cells in the rat. J Auton Nerv Syst. 1987;20(2):91–101.
Richardson RJ, Grkovic I, Anderson CR. Immunohistochemical analysis of intracardiac ganglia of the rat heart. Cell Tissue Res. 2003;314(3):337–50.
Achanta S, Gorky J, Leung C, Moss A, Robbins S, Eisenman L, et al. A comprehensive integrated anatomical and molecular atlas of rat intrinsic cardiac nervous system. iScience. 2020;23(6):101140.
Steele PA, Gibbins IL, Morris JL, Mayer B. Multiple populations of neuropeptide-containing intrinsic neurons in the guinea-pig heart. Neurosci. 1994;62(1):241–50.
Horackova M, Armour JA, Byczko Z. Distribution of intrinsic cardiac neurons in whole-mount guinea pig atria identified by multiple neurochemical coding. A confocal microscope study. Cell Tissue Res. 1999;297(3):409–21.
Leger J, Croll RP, Smith FM. Regional distribution and extrinsic innervation of intrinsic cardiac neurons in the guinea pig. J Comp Neurol. 1999;407(3):303–17.
Pauza DH, Skripkiene G, Skripka V, Pauziene N, Stropus R. Morphological study of neurons in the nerve plexus on heart base of rats and guinea pigs. J Auton Nerv Syst. 1997;62(1–2):1–12.
Pauza DH, Pauziene N, Pakeltyte G, Stropus R. Comparative quantitative study of the intrinsic cardiac ganglia and neurons in the rat, guinea pig, dog and human as revealed by histochemical staining for acetylcholinesterase. Ann Anat. 2002;184(2):125–36.
Arora RC, Waldmann M, Hopkins DA, Armour JA. Porcine intrinsic cardiac ganglia. Anat Rec Part A Discov Mol Cell Evol Biol. 2003;271(1):249–58.
Saburkina I, Rysevaite K, Pauziene N, Mischke K, Schauerte P, Jalife J, et al. Epicardial neural ganglionated plexus of ovine heart: anatomic basis for experimental cardiac electrophysiology and nerve protective cardiac surgery. Heart Rhythm. 2010;7(7):942–50.
Yuan BX, Ardell JL, Hopkins DA, Losier AM, Armour JA. Gross and microscopic anatomy of the canine intrinsic cardiac nervous system. Anat Rec. 1994;239(1):75–87.
Armour JA, Murphy DA, Yuan BX, Macdonald S, Hopkins DA. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec. 1997;247(2):289–98.
Pauza DH, Skripka V, Pauziene N, Stropus R. Morphology, distribution, and variability of the epicardiac neural ganglionated subplexuses in the human heart. Anat Rec. 2000;259(4):353–82.
Randall DC, Brown DR, McGuirt AS, Thompson GW, Armour JA, Ardell JL. Interactions within the intrinsic cardiac nervous system contribute to chronotropic regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):R1066–75.
Gatti PJ, Johnson TA, Phan P, Jordan IK 3rd, Coleman W, Massari VJ. The physiological and anatomical demonstration of functionally selective parasympathetic ganglia located in discrete fat pads on the feline myocardium. J Auton Nerv Syst. 1995;51(3):255–9.
Armour JA. Cardiac neuronal hierarchy in health and disease. Am J Physiol Regul Integr Comp Physiol. 2004;287(2):R262–71.
Butler CK, Smith FM, Cardinal R, Murphy DA, Hopkins DA, Armour JA. Cardiac responses to electrical stimulation of discrete loci in canine atrial and ventricular ganglionated plexi. Am J Physiol. 1990;259(5 Pt 2):H1365–73.
Butler CK, Smith FM, Nicholson J, Armour JA. Cardiac effects induced by chemically activated neurons in canine intrathoracic ganglia. Am J Physiol. 1990;259(4 Pt 2):H1108–17.
Yuan BX, Ardell JL, Hopkins DA, Armour JA. Differential cardiac responses induced by nicotine sensitive canine atrial and ventricular neurones. Cardiovasc Res. 1993;27(5):760–9.
Cardinal R, Pagé P, Vermeulen M, Ardell JL, Armour JA. Spatially divergent cardiac responses to nicotinic stimulation of ganglionated plexus neurons in the canine heart. Auton Neurosci. 2009;145(1–2):55–62.
Dennis MJ, Harris AJ, Kuffler SW. Synaptic transmission and its duplication by focally applied acetylcholine in parasympathetic neurons in the heart of the frog. Proc Roy Soc London Ser B Biol Sci. 1971;177(1049):509–39.
Harris AJ, Kuffler SW, Dennis MJ. Differential chemosensitivity of synaptic and extrasynaptic areas on the neuronal surface membrane in parasympathetic neurons of the frog, tested by microapplication of acetylcholine. Proc Roy Soc London Ser B Biol Sci. 1971;177(1049):541–53.
McMahan UJ, Purves D. Visual identification of two kinds of nerve cells and their synaptic contacts in a living autonomic ganglion of the mudpuppy (Necturus maculosus). J Physiol. 1976;254(2):405–25.
Hartzell HC, Kuffler SW, Stickgold R, Yoshikami D. Synaptic excitation and inhibition resulting from direct action of acetylcholine on two types of chemoreceptors on individual amphibian parasympathetic neurones. J Physiol. 1977;271(3):817–46.
Konopka LM, McKeon TW, Parsons RL. Galanin-induced hyperpolarization and decreased membrane excitability of neurones in mudpuppy cardiac ganglia. J Physiol. 1989;410:107–22.
Parsons RL, Barstow KL, Scornik FS. Spontaneous miniature hyperpolarizations affect threshold for action potential generation in mudpuppy cardiac neurons. J Neurophysiol. 2002;88(3):1119–27.
Allen T, Hassall C, Burnstock G. Mammalian intrinsic cardiac neurons in cell culture. In: Armour JA, Ardell JL, editors. Neurocardiology. New York: Oxford University Press; 1994. p. 115–38.
Allen TG, Burnstock G. Intracellular studies of the electrophysiological properties of cultured intracardiac neurones of the guinea-pig. J Physiol. 1987;388:349–66.
Xu ZJ, Adams DJ. Voltage-dependent sodium and calcium currents in cultured parasympathetic neurones from rat intracardiac ganglia. J Physiol. 1992;456:425–41.
Adams DJ, Cuevas J. Electrophysiological properties of intrinsic cardiac neurons. In: Armour JA, Ardell JL, editors. Basic and clinical neurocardiology. New York: Oxford University Press; 2004. p. 1–60.
Adams DJ, Harper AA. Electrophysiological properties of autonomic ganglion neurons. In: McLachlan EM, editor. Autonomic ganglia. London: Harwood Academic Publishers; 1995. p. 153–212.
Selyanko AA. Membrane properties and firing characteristics of rat cardiac neurones in vitro. J Auton Nerv Syst. 1992;39(3):181–9.
Xi XH, Thomas JXJ, Randall WC, Wurster RD. Intracellular recordings from canine intracardiac ganglion cells. J Auton Nerv Syst. 1991;32(2):177–82.
Xi X, Randall WC, Wurster RD. Morphology of intracellularly labeled canine intracardiac ganglion cells. J Comp Neurol. 1991;314(2):396–402.
Griffith WH 3rd, Gallagher JP, Shinnick-Gallagher P. An intracellular investigation of cat vesical pelvic ganglia. J Neurophysiol. 1980;43(2):343–54.
Edwards FR, Hirst GD, Klemm MF, Steele PA. Different types of ganglion cell in the cardiac plexus of guinea-pigs. J Physiol. 1995;486(2):453–71.
Smith FM. Extrinsic inputs to intrinsic neurons in the porcine heart in vitro. Am J Physiol. 1999;276(2):R455–67.
Xu ZJ, Adams DJ. Resting membrane potential and potassium currents in cultured parasympathetic neurones from rat intracardiac ganglia. J Physiol. 1992;456:405–24.
Cuevas J, Harper AA, Trequattrini C, Adams DJ. Passive and active membrane properties of isolated rat intracardiac neurons: regulation by H- and M-currents. J Neurophysiol. 1997;78(4):1890–902.
Rimmer K, Harper AA. Developmental changes in electrophysiological properties and synaptic transmission in rat intracardiac ganglion neurons. J Neurophysiol. 2006;95(6):3543–52.
Dyavanapalli J, Rimmer K, Harper AA. The action of high K+ and aglycaemia on the electrical properties and synaptic transmission in rat intracardiac ganglion neurones in vitro. Exp Physiol. 2009;94(2):201–12.
McAllen RM, Salo LM, Paton JFR, Pickering AE. Processing of central and reflex vagal drives by rat cardiac ganglion neurones: an intracellular analysis. J Physiol. 2011;589(Pt 23):5801–18.
Ashton JL, Argent L, Smith JEG, Jin S, Sands GB, Smaill BH, et al. Evidence of structural and functional plasticity occurring within the intracardiac nervous system of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2020;318(6):H1387–400.
Gibbins IL. Chemical neuroanatomy of sympathetic ganglia. In: McLachlan EM, editor. Autonomic Ganglia. Luxembourg: Harwood Academic Publishers; 1995. p. 73–122.
Morris JL, Gibbins IL, Holmgren S. Galanin is more common than NPY in vascular sympathetic neurons of the brush-tailed possum. Regul Pept. 1992;37(2):101–9.
Steele PA, Choate JK. Innervation of the pacemaker in guinea-pig sinoatrial node. J Auton Nerv Syst. 1994;47(3):177–87.
Steele PA, Gibbins IL, Morris JL. Projections of intrinsic cardiac neurons to different targets in the guinea-pig heart. J Auton Nerv Syst. 1996;56(3):191–200.
Hoover DB, Tompkins JD, Parsons RL. Differential activation of guinea pig intrinsic cardiac neurons by the PAC1 agonists maxadilan and pituitary adenylate cyclase-activating polypeptide 27 (PACAP27). J Pharmacol Exp Ther. 2009;331(1):197–203.
Hoard JL, Hoover DB, Mabe AM, Blakely RD, Feng N, Paolocci N. Cholinergic neurons of mouse intrinsic cardiac ganglia contain noradrenergic enzymes, norepinephrine transporters, and the neurotrophin receptors tropomyosin-related kinase A and p75. Neurosci. 2008;156(1):129–42.
Hoover DB, Isaacs ER, Jacques F, Hoard JL, Pagé P, Armour JA. Localization of multiple neurotransmitters in surgically derived specimens of human atrial ganglia. Neurosci. 2009;164(3):1170–9.
Rysevaite K, Saburkina I, Pauziene N, Vaitkevicius R, Noujaim SF, Jalife J, et al. Immunohistochemical characterization of the intrinsic cardiac neural plexus in whole-mount mouse heart preparations. Heart Rhythm. 2011;8(5):731–8.
Singh S, Johnson PI, Javed A, Gray TS, Lonchyna VA, Wurster RD. Monoamine- and histamine-synthesizing enzymes and neurotransmitters within neurons of adult human cardiac ganglia. Circulation. 1999;99(3):411–9.
Coote JH, Chauhan RA. The sympathetic innervation of the heart: important new insights. Auton Neurosci. 2016;199:17–23.
Parsons RL, Neel DS, McKeon TW, Carraway RE. Organization of a vertebrate cardiac ganglion: a correlated biochemical and histochemical study. J Neurosci. 1987;7(3):837–46.
de Groat WC, Booth AM. Inhibition and facilitation in parasympathetic ganglia of the urinary bladder. Fed Proc. 1980;39(12):2990–6.
Weihe E, Schütz B, Hartschuh W, Anlauf M, Schäfer MK, Eiden LE. Coexpression of cholinergic and noradrenergic phenotypes in human and nonhuman autonomic nervous system. J Comp Neurol. 2005;492(3):370–9.
Hoover DB, Shepherd AV, Southerland EM, Armour JA, Ardell JL. Neurochemical diversity of afferent neurons that transduce sensory signals from dog ventricular myocardium. Auton Neurosci. 2008;141(1–2):38–45.
Hardwick JC, Mawe GM, Parsons RL. Evidence for afferent fiber innervation of parasympathetic neurons of the guinea-pig cardiac ganglion. J Auton Nerv Syst. 1995;53(2–3):166–74.
Wang T, Miller KE. Characterization of glutamatergic neurons in the rat atrial intrinsic cardiac ganglia that project to the cardiac ventricular wall. Neurosci. 2016;329:134–50.
Pauza DH, Rysevaite K, Inokaitis H, Jokubauskas M, Pauza AG, Brack KE, et al. Innervation of sinoatrial nodal cardiomyocytes in mouse. A combined approach using immunofluorescent and electron microscopy. J Mol Cell Cardiol. 2014;75:188–97.
Lee S-R, Cho Y, Cha M-J, Choi E-K, Seo J-W, Oh S. Atrial innervation patterns of intrinsic cardiac autonomic nerves. J Korean Med Sci. 2018;33(39):e253.
Beaumont E, Salavatian S, Southerland EM, Vinet A, Jacquemet V, Armour JA, et al. Network interactions within the canine intrinsic cardiac nervous system: implications for reflex control of regional cardiac function. J Physiol. 2013;591(18):4515–33.
Seabrook GR, Fieber LA, Adams DJ. Neurotransmission in neonatal rat cardiac ganglion in situ. Am J Physiol. 1990;259(4 Pt 2):H997–1005.
Furness JB, Callaghan BP, Rivera LR, Cho H-J. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol. 2014;817:39–71.
Huang MH, Smith FM, Armour JA. Modulation of in situ canine intrinsic cardiac neuronal activity by nicotinic, muscarinic, and beta-adrenergic agonists. Am J Physiol. 1993;265(3):R659–69.
Masliukov PM, Moiseev K, Emanuilov AI, Anikina TA, Zverev AA, Nozdrachev AD. Development of neuropeptide Y-mediated heart innervation in rats. Neuropeptides. 2016;55:47–54.
Herring N, Paterson DJ. Neuromodulators of peripheral cardiac sympatho-vagal balance. Exp Physiol. 2009;94(1):46–53.
Bosnjak ZJ, Seagard JL, Kampine JP. Peripheral neural input to neurons of the stellate ganglion in dog. Am J Physiol. 1982;242(3):R237–43.
Brown AM. Cardiac reflexes. In: Berne RM, Sperelakis N, Geiger SR, editors. Handbook of physiology, section 2: The cardiovascular system Vol 1, The heart. Washington, DC: American Physiological Society; 1979. p. 677–89.
Ardell JL, Butler CK, Smith FM, Hopkins DA, Armour JA. Activity of in vivo atrial and ventricular neurons in chronically decentralized canine hearts. Am J Physiol. 1991;260(3):H713–21.
Murphy DA, Thompson GW, Ardell JL, McCraty R, Stevenson RS, Sangalang VE, et al. The heart reinnervates after transplantation. Ann Thorac Surg. 2000;69(6):1769–81.
Spencer NJ, Kyloh M, Beckett EA, Brookes S, Hibberd T. Different types of spinal afferent nerve endings in stomach and esophagus identified by anterograde tracing from dorsal root ganglia. J Comp Neurol. 2016;524(15):3064–83.
Wang H-J, Rozanski GJ, Zucker IH. Cardiac sympathetic afferent reflex control of cardiac function in normal and chronic heart failure states. J Physiol. 2017;595(8):2519–34.
Lázár BA, Jancsó G, Oszlács O, Nagy I, Sántha P. The insulin receptor is colocalized with the TRPV1 nociceptive ion channel and neuropeptides in pancreatic spinal and vagal primary sensory neurons. Pancreas. 2018;47(1):110–5.
Yoshie K, Rajendran PS, Massoud L, Kwon O, Tadimeti V, Salavatian S, et al. Cardiac vanilloid receptor-1 afferent depletion enhances stellate ganglion neuronal activity and efferent sympathetic response to cardiac stress. Am J Physiol. 2018;314(5):H954–66.
Shanks J, de Morais SDB, Gao L, Zucker IH, Wang H-J. TRPV1 (transient receptor potential vanilloid 1) cardiac spinal afferents contribute to hypertension in spontaneous hypertensive rat. Hypertension. 2019;74(4):910–20.
Liu X, Zhang Q, Han M, Du J. Intrapericardial capsaicin and bradykinin induce different cardiac-somatic and cardiovascular reflexes in rats. Auton Neurosci. 2016;198:28–32.
Xi X, Randall WC, Wurster RD. Intracellular recording of spontaneous activity of canine intracardiac ganglion cells. Neurosci Lett. 1991;128(1):129–32.
Smith FM, Vermeulen M, Cardinal R. Long-term spinal cord stimulation modifies canine intrinsic cardiac neuronal properties and ganglionic transmission during high-frequency repetitive activation. Physiol Rep. 2016;4(13):e12855.
Salavatian S, Beaumont E, Longpré J-P, Armour JA, Vinet A, Jacquemet V, et al. Vagal stimulation targets select populations of intrinsic cardiac neurons to control neurally induced atrial fibrillation. Am J Physiol. 2016;311(5):H1311–20.
Treweek JB, Gradinaru V. Extracting structural and functional features of widely distributed biological circuits with single cell resolution via tissue clearing and delivery vectors. Curr Opin Biotechnol. 2016;40:193–207.
Renier N, Wu Z, Simon DJ, Yang J, Ariel P, Tessier-Lavigne M. iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell. 2014;159(4):896–910.
Richardson DS, Lichtman JW. Clarifying tissue clearing. Cell. 2015;162(2):246–57.
Treweek JB, Chan KY, Flytzanis NC, Yang B, Deverman BE, Greenbaum A, et al. Whole-body tissue stabilization and selective extractions via tissue-hydrogel hybrids for high-resolution intact circuit mapping and phenotyping. Nat Protoc. 2015;10(11):1860–96.
Mano T, Albanese A, Dodt H-U, Erturk A, Gradinaru V, Treweek JB, et al. Whole-brain analysis of cells and circuits by tissue clearing and light-sheet microscopy. J Neurosci. 2018;38(44):9330–7.
Bedbrook CN, Deverman BE, Gradinaru V. Viral strategies for targeting the central and peripheral nervous systems. Annu Rev Neurosci. 2018;41:323–48.
Chan KY, Jang MJ, Yoo BB, Greenbaum A, Ravi N, Wu W-L, et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat Neurosci. 2017;20(8):1172–9.
Rajendran PS, Challis RC, Fowlkes CC, Hanna P, Tompkins JD, Jordan MC, et al. Identification of peripheral neural circuits that regulate heart rate using optogenetic and viral vector strategies. Nat Commun. 2019;10(1):1944.
Saito T, Tenma K. Effects of left and right vagal stimulation on excitation and conduction of the carp heart (Cyprinus carpio). J Comp Physiol. 1976;111:39–53.
Gut P, Reischauer S, Stainier DYR, Arnaout R. Little fish, big data: zebrafish as a model for cardiovascular and metabolic disease. Physiol Rev. 2017;97(3):889–938.
Woo K, Shih J, Fraser SE. Fate maps of the zebrafish embryo. Curr Opin Genet Dev. 1995;5(4):439–43.
Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203(3):253–310.
Briggs JP. The zebrafish: a new model organism for integrative physiology. Am J Physiol. 2002;282(1):R3–9.
Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496(7446):498–503.
Genge CE, Lin E, Lee L, Sheng X, Rayani K, Gunawan M, et al. The zebrafish heart as a model of mammalian cardiac function. Rev Physiol Biochem Pharmacol. 2016;171:99–136.
Shrestha R, Lieberth J, Tillman S, Natalizio J, Bloomekatz J. Using zebrafish to analyze the genetic and environmental etiologies of congenital heart defects. Adv Exp Med Biol. 2020;1236:189–223.
Rafferty SA, Quinn TA. A beginner’s guide to understanding and implementing the genetic modification of zebrafish. Prog Biophys Mol Biol. 2018;138:3–19.
Vornanen M, Hassinen M. Zebrafish heart as a model for human cardiac electrophysiology. Channels. 2016;10(2):101–10.
Nemtsas P, Wettwer E, Christ T, Weidinger G, Ravens U. Adult zebrafish heart as a model for human heart? An electrophysiological study. J Mol Cell Cardiol. 2010;48(1):161–71.
Brette F, Luxan G, Cros C, Dixey H, Wilson C, Shiels HA. Characterization of isolated ventricular myocytes from adult zebrafish (Danio rerio). Biochem Biophys Res Commun. 2008;374(1):143–6.
Ravens U. Ionic basis of cardiac electrophysiology in zebrafish compared to human hearts. Prog Biophys Mol Biol. 2018;38:38–44.
Rayani K, Lin E, Craig C, Lamothe M, Shafaattalab S, Gunawan M, et al. Zebrafish as a model of mammalian cardiac function: optically mapping the interplay of temperature and rate on voltage and calcium dynamics. Prog Biophys Mol Biol. 2018;138:69–90.
Dvornikov AV, de Tombe PP, Xu X. Phenotyping cardiomyopathy in adult zebrafish. Prog Biophys Mol Biol. 2018;138:116–25.
Haverinen J, Hassinen M, Korajoki H, Vornanen M. Cardiac voltage-gated sodium channel expression and electrophysiological characterization of the sodium current in the zebrafish (Danio rerio) ventricle. Prog Biophys Mol Biol. 2018;138:59–68.
Hull CM, Genge CE, Hobbs Y, Rayani K, Lin E, Gunawan M, et al. Investigating the utility of adult zebrafish ex vivo whole hearts to pharmacologically screen hERG channel activator compounds. Am J Physiol. 2019;317(6):R921–31.
Verkerk AO, Remme CA. Zebrafish: a novel research tool for cardiac (patho)electrophysiology and ion channel disorders. Front Physiol. 2012;3:255.
Lin E, Shafaattalab S, Gill J, Al-Zeer B, Craig C, Lamothe M, et al. Physiological phenotyping of the adult zebrafish heart. Mar Genomics. 2020;49:100701.
Stoyek MR, Croll RP, Smith FM. Intrinsic and extrinsic innervation of the heart in zebrafish (Danio rerio). J Comp Neurol. 2015;523(11):1683–700.
Christoffels VM, Smits GJ, Kispert A, Moorman AFM. Development of the pacemaker tissues of the heart. Circ Res. 2010;106(2):240–54.
Stoyek MR, Quinn TA, Croll RP, Smith FM. Zebrafish heart as a model to study the integrative autonomic control of pacemaker function. Am J Physiol. 2016;311(3):H676–88.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Smith, F.M. (2023). Fundamental Neurocardiology: The Intracardiac Nervous System. In: Tripathi, O.N., Quinn, T.A., Ravens, U. (eds) Heart Rate and Rhythm. Springer, Cham. https://doi.org/10.1007/978-3-031-33588-4_8
Download citation
DOI: https://doi.org/10.1007/978-3-031-33588-4_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-33587-7
Online ISBN: 978-3-031-33588-4
eBook Packages: MedicineMedicine (R0)