Abstract
The global soil stabilisation market is forecast to grow to $35 billion by 2027 driven primarily by infrastructure and construction activities and exacerbated by the increasingly urgent need to adapt to climate change, flood risk and sea-level rise. Additionally there is anticipated to be increasing demand for stabilised-earth based building products due to their low carbon impact. Cement and lime are widely used to stabilise soil but suffer from significant carbon and energy costs. Naturally sourced biopolymers are a promising low carbon ‘green’ substitute, achieving higher strength in stabilised soils than cement and at similar cost. However, widespread uptake of biopolymers is impeded by the fact that they typically suffer from (a) poor water resistance and (b) poor resistance to biodegradation over time.
This paper presents an overview of design concepts and pilot results from a research study into novel biopolymer treatment processes which are applied at the soil/biopolymer mixing stage with the aim of enhancing resistance to water and biodegradation. The approaches include investigation of (i) acetylation, whereby vulnerable hydrophilic functional groups in the biopolymers are replaced by natural hydrophobic acetyl groups, (ii) binding vulnerable hydrophilic groups to specific inorganic minerals, thereby blocking interactions with water. The study design includes a comprehensive series of laboratory tests guided by detailed characterisation of the underlying chemical binding processes with the aim of involving the addition of only small volumes of natural materials as part of the treatment process.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The global soil stabilisation market is forecast to grow from $25 billion in 2019 to $35 billion by 2027 (Emergen Research, 2020) driven primarily by infrastructure and construction activities and exacerbated by the increasingly urgent need to adapt to climate change, flood risk and sea-level rise. Soil chemical stabilisation (where soil is mixed with an additive to enhance its strength) is forecast to be the fastest growing sector of the market, taking just under half the share. In parallel there is anticipated to be a significantly increased demand for stabilised-earth based building products due to their low carbon impact. However, the most widely used additives, cement and lime, can suffer from significant carbon and energy costs and substantial environmental impacts e.g. highly alkaline conditions, toxic cement dust, polluting run-off. Geopolymers offer reduced environmental impacts but still require significant processing of raw materials and the use of highly concentrated alkali activator solutions.
Such concerns have stimulated interest in biologically derived alternatives as a low-carbon sustainable solution. There is existing evidence that naturally sourced biopolymers have significant potential, achieving higher strength in stabilised soils with lower additive mass than cement and at similar cost. However, widespread uptake of biopolymers is impeded by the fact that they often suffer from (a) poor water resistance and (b) poor resistance to biodegradation over time. If these challenges can be addressed, biopolymers could achieve a transformational impact on soil stabilisation. This paper presents design concepts and pilot results from a research study into novel biopolymer treatment processes which are applied at the soil/biopolymer mixing stage.
2 Biopolymer Binding, Water Resistance and Biodegradation
A significant body of literature exists investigating soil stabilisation using biopolymers. Production of soil/biopolymer composites normally involves dissolution of the biopolymer in water to allow thorough mixing with soil and to expose functional groups for binding to soil. When dry (or cured), the biopolymer functional groups within a stabilised soil will bind strongly to other biopolymers or to soil particles and it is typically found that on drying, treated soils display significant gains in strength and stiffness. Chang et al. (2015) used 1% xanthan gum resulting in significant strength gains for soils ranging from sands to clays including an 86% increase in Unconfirmed Compressive Strength (UCS) compared to 10% cement addition for a residual soil. Muguda et al. (2017) identified a 30% higher UCS using 3% guar gum compared to 8% cement addition for a gravel/sand/clay soil. This level of strength gain is attributed to the biopolymer binding to soil particles and the high tensile strength and stiffness of dried biopolymer. There is thus a significant potential for using such materials in environments where design detailing can maintain low humidity and exposure to water.
However, for applications where exposure to high humidity or liquid water is anticipated there are potential challenges since many of the beneficial binding/strengthening mechanisms can be easily reversed on exposure to water. Biopolymer tensile strength and stiffness can diminish significantly with increased water content (Yakimets et al. 2007).
Soil/biopolymer binding mechanisms depend significantly on the soil mineralogy and biopolymer chemistry. Mechanisms proposed in the literature include for example: hydrogen and ionic binding to electrically charged clay particles (Chang 2016); electrostatic adsorption of biopolymer additives to negatively charged SiO2 surfaces facilitated by aqueous molecules; covalent interactions through acid/base reactions, forming C–O–Fe bonds with Fe2O3 particles (Armistead et al. 2020). Some of these binding mechanisms can be weakened or outcompeted by water molecules. Experimental measurements on xanthan gum stabilised soils have previously demonstrated a loss of compressive strength upon exposure to water (Chang et al. 2015; Chang et al. 2020). Elsewhere the addition of chitin - with subsequent reacetylation - has been shown to improve the mechanical properties of either clay-based soils, however this effect is significantly reduced in the presence of water due hydrodegradation (Hataf et al. 2018).
3 Design Concepts
3.1 Background
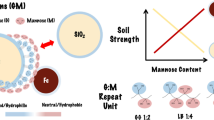
It is hypothesised that the biopolymer functional groups within a stabilised soil which bind strongly to other biopolymers or to soil particles can be treated post mixing to negate their vulnerability to water and biodegradation without affecting the soil-biopolymer binding. Two different approaches as illustrated in Fig. 1 are being investigated:
-
conversion of ‘vulnerable’ functional groups utilising acetylation,
-
binding ‘vulnerable’ functional groups to inorganic minerals.
3.2 Acetylation
Acetylation is a chemical process that has been employed as a non-toxic ‘green’ timber treatment technology, enabling replacement of vulnerable hydrophilic functional groups in the wood biopolymer with natural hydrophobic acetyl groups (Mantanis 2017). Elimination of the hydrophilic groups in this way inhibits the enzymatic digestion of wood and has been demonstrated to achieve exceptional timber lifetimes of >50 years in exposed outdoor conditions. The potential for such a treatment process to be applied to biopolymers employed in soil-strengthening will be investigated.
A strong candidate biopolymer for initial investigation for the potential of this process is chitosan, which has shown significant strengthening potential in soil stabilisation (Hataf et al. 2018; Shariatmadari et al. 2020; Adamczuk and Jozefaciuk 2022). Chitosan is already the product of the deacetylation of chitin, a natural material commonly used by insects and crustaceans for exoskeletons and which has inherent water resistant properties. It is hypothesised that re-acetylation of chitosan, post soil treatment, will revert chemical bonds unused for soil binding to the chitin form and benefit from its associated properties such as high stability.
Re-acetylation can be carried out through the addition of acetic anhydride, acetic acid and mixtures of the two. Acetylation of wood typically avoids use of catalysts, but operates at relatively high temperatures (100 to 120 ℃). However previous studies have shown effective (partial) acetylation of chitosan can occur at ambient conditions e.g. Lavertu et al. (2011), Qun et al. (2007). The quantity of acetic acid/acetic anhydride employed will be minimised in order to limit any residue. The degree of deacetylation and subsequent re-acetylation will be a key metric by which the success of the chemical process is evaluated. This will be quantified through Fourier transform infrared (FTIR) spectroscopy; a well-established technique for this purpose. X-ray diffraction (XRD) will be employed to identify changes to the crystal structure of chitin/chitosan effected by the de/re-acetylation procedures.
3.3 Inorganic Binding
The alternative, ‘binding’ approach will evaluate the potential to bulk bind the vulnerable hydrophilic groups to specific inorganic minerals, thereby blocking interactions with water. This would provide a potentially transformative approach to design bespoke biopolymer mixes for specific soil mineralogies, enhanced where required by addition of specific minerals. The challenge and associated risk is to identify whether the most advantageous binding is achievable in a multi mineral/biopolymer functional group system. Methods developed in previous work (e.g. Armistead et al. 2020) will allow the systematic exploration of the nature of the binding. Exploration of the interactions between naturally occurring silica, alumna and iron compounds and biopolymers containing 'hard' cationic/anionic and 'soft' neutral ligand functional groups will establish the understanding required for this approach.
For example, a recent study by Lalonde et al. (2012) found that 21% ± 8.6% of organic carbon within sediments are associated with reactive iron phases. Further investigation by Barber et al. (2017) found that this phenomena was driven by the formation of irreversible covalent C–O–Fe complexes, which reduce both the biopolymer enzymatic hydrolysis and biodegradation. The implications of this phenomena are promising areas for further study.
4 Study Design
The core aim of the current research is to demonstrate that the hypothesized approaches work under controlled laboratory conditions. Once achieved, the aim is then to refine the process to minimise process complexity, volume of additional additives and residual chemicals, and to investigate the viability for field application. A brief overview of the planned testing programme is given below.
4.1 Materials
Five standardised soil types will be investigated covering a range of mineralogies and specific surface areas: silica sand, calcareous sand, silt (silica based), kaolinite and montmorillonite.
Initial work will target polysaccharide biopolymers, due to their high-volume commercial availability (e.g. a co-product of shellfish processing) and ease of extraction/co-production, focusing on chitosan and galactomannans in the first instance.
4.2 Sample Preparation
Biopolymer solutions will be mixed mechanically with the relevant soil and the soil compacted using a cylindrical drop hammer within a 202 mm × 42 mm hollow cylindrical sample mould. These will then be extruded and allowed to cure under controlled temperature and humidity and at a range of time durations. The treatment agents may be combined into the original mix prior to soil mixing, after soil mixing over a range of time intervals or (if liquid) allowed to permeate a sample once cured in order to assess the feasibility of implementing this process in the field.
4.3 Characterisation Tests
Following curing, samples will undergo a range of characterisation tests, as follows:
-
Water resistance. Samples will undergo either immersion tests followed by weight and volume change measurement or erodibility tests using the jet index method (ASTM 2017).
-
Biodegradation. Biodegradation tests will be carried using an approach based on ISO (2019), originally developed for determining the aerobic biodegradability of plastics in soil. Specifically, mass spectrometry will be used to qualitatively monitor the evolution of CO2 from soil/biopolymer samples over time.
-
Compression testing. Full stress strain/response (pre- and post-water immersion and biodegradation) will be carried out on unconfined specimens. Selected samples will undergo confined triaxial testing.
-
Chemical/physical characterisation Both initial reagents/soils and final samples will undergo selected chemical/physical characterisation as appropriate using particle sizing, Atterberg limit determination, optimum moisture content for geotechnical interpretation and XRD, GPC, zeta potential, FTIR spectroscopy, TGA, SEM to identify key chemical binding characteristics.
In addition to the final performance of the treated soils, additional process related issues to be investigated include:
-
Required and residual volumes of treatment agents:
-
Effect of treatment reagents on curing rates.
4.4 Initial Results
An initial pilot study (Siripanich 2023) investigated the strength of re-acetylated chitosan in the presence of coarse grained SiO2 sand particles using two different approaches:
-
1.
Re-acetylation with sand present in the solution
-
2.
Re-acetylation before mixing with sands, i.e. conducting re-acetylation process first to obtain a chitin gel, followed by mixing of the gel with sands
Figure 2 shows the uniaxial stress-strain response of the two samples. It can be seen that Approach 1 where the re-acetylation agents were mixed with the chitosan and sand gave improved strength. However further testing is required to confirm the observed trends. Figure 3 presents a more direct comparison of strength (UCS) and stiffness (E50). The incorporation of the biopolymer into the sand was confirmed by FTIR spectroscopy, Fig. 4, where characteristic peaks for chitin (e.g. C-H stretching peaks, 2900–3000 cm-1) are observed. Figure 5 shows an example of one of the generated samples.
For these tests, the re-acetylation was conducted using different proportions of methanol-water mixture and molar ratios of acetic anhydride to (chitosan) amine. While the amine group reacts with acetic anhydride, this reaction is not 100% selective as the hydroxyl group may also undergo conversion. The addition of methanol however improves yields of the reacetylation process through modulating the pKa of acetic acid and reducing the degree of ionisation of the biopolymer. Therefore, methanol was used as a co-solvent in this process.
Preliminary observations indicate that the chitin-sand samples are resistant to immersion in water however this requires confirmation by a more comprehensive testing programme.
5 Discussion
The proposed investigation is high risk. While the concept is attractive, the proposed solutions may simply not function in the soil chemical environment, may inhibit the desired soil-biopolymer binding, or leave significant residues. One or more of the techniques investigated may work, but either only partially or only in carefully controlled laboratory conditions that are not scalable to the field. This may limit application to factory produced bricks and panels or to specific applications under specific environmental conditions. However it is anticipated that any demonstration of the viability of the core concepts would catalyse a new field for further research and innovation.
6 Conclusions
-
1.
Biopolymer treated soils typically display high gains in strength but conversely low resistance to biodegradation and water.
-
2.
Conversion of ‘vulnerable’ functional groups utilising acetylation, and binding ‘vulnerable’ functional groups to inorganic minerals, have significant potential to enhance biopolymer resistance to biodegradation and water. Selected potential mechanisms and candidate materials have been discussed.
-
3.
An outline test methodology for the investigation of techniques for the enhancement of water and biodegradation resistance of biopolymer-soil mixes is described.
-
4.
Pilot tests involving the re-acetylation of chitosan have shown promising results. However further studies are required.
References
Adamczuk, A., Jozefaciuk, G.: Impact of Chitosan on the mechanical stability of soils. Molecules 27, 2273 (2022). https://doi.org/10.3390/molecules27072273
Armistead, S.J., Rawlings, A.E., Smith, C.C., Staniland, S.S.: Biopolymer stabilization/solidification of soils: a rapid, micro-macro, cross-disciplinary approach. Environ. Sci. Technol. 54(21), 13963–13972 (2020)
ASTM: Standard Test Method for Erodibility Determination of Soil in the Field or in the Laboratory by the Jet Index Method 2017. D5852-95, https://www.astm.org/d5852-95.html
Barber, A., et al.: Preservation of organic matter in marine sediments by inner-sphere interactions with reactive iron. Sci. Rep. 7(1) (2017). https://doi.org/10.1038/s41598-017-00494-0
Chang, I., Im, J., Prasidhi, A.K., Cho, G.-C.: Effects of Xanthan gum biopolymer on soil strengthening. Constr. Build. Mater. 74, 65–72 (2015). https://doi.org/10.1016/j.conbuildmat.2014.10.026
Chang, I., Im, J., Cho, G.C.: Introduction of microbial biopolymers in soil treatment for future environmentally-friendly and sustainable geotechnical engineering. Sustainability 8(3), 251 (2016). https://doi.org/10.3390/su8030251
Chang I., et al.: Review on biopolymer-based soil treatment (BPST) technology in geotechnical engineering practices, Transportation Geotechnics 24, 100385 (2020)
Emergen Research, Soil Stabilization Market By Application (Agriculture, Industrial, Non-Agriculture), By Method (Chemical, Mechanical), By Additive (Mineral & Stabilizing agents, Polymers), Forecasts to 2027 (2023). https://www.emergenresearch.com/industry-report/soil-stabilization-market
Hataf, N., Ghadir, P., Ranjbar, N.: Investigation of soil stabilization using chitosan biopolymer. J. Clean. Prod. 170, 1493 (2018). https://doi.org/10.1016/j.jclepro.2017.09.256
ISO 17556, Plastics — Determination of the ultimate aerobic biodegradability of plastic materials in soil by measuring the oxygen demand in a respirometer or the amount of carbon dioxide evolved (2019). https://www.iso.org/standard/74993.html
Lalonde, K., Mucci, A., Ouellet, A., Gelinas, Y.: Preservation of organic matter in sediments promoted by iron. Nature 483(7388), 198–200 (2012). https://doi.org/10.1038/nature10855
Lavertu, M., Darras, V., Buschmann, M.D.: Kinetics and efficiency of chitosan reacetylation. Carbohydrate Polymers 87(2), 1192–1198 (2011)
Mantanis, G.I.: Chemical modification of wood by acetylation or furfurylation: a review of the present scaled-up technologies. BioRes 12(2), 4478–4489 (2017)
Muguda, S., et al.: Mechanical properties of biopolymer-stabilised soil-based construction materials. Géotech. Lett. 7(4), 309–314 (2017). https://doi.org/10.1680/jgele.17.00081
Qun, G., Ajun, W., Yong, Z.: Effect of reacetylation and degradation on the chemical and crystal structures of chitosan 2007. J. Appl. Polym. Sci. 104, 2720–2728 (2007)
Shariatmadari, N., Reza, M., Tasuji, A., Ghadir, P., Javadi, A.: Experimental study on the effect of chitosan biopolymer on sandy soil stabilization. In: E3S Web of Conferences, vol. 195, pp. 06007 (2020)
Siripanich, N.: Application of chitin as a sustainable material for soil stabilization process. M.Sc thesis. King Mongkut’s University of Technology Thonburi, Thailand (2023)
Yakimets, I., Paes, S.S., Wellner, N., Smith, A.C., Wilson, R.H., Mitchell, J.R.: Effect of water content on the structural reorganization and elastic properties of biopolymer films: a comparative study. Biomacromol 8, 1710–1722 (2007)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Smith, C., McGregor, J., Martsinovich, N., Armistead, S., Yu, X., Siripanich, N. (2023). Enhancing the Water and Biodegradation Resistance of Biopolymer Stabilised Soils – Design Concepts. In: Amziane, S., Merta, I., Page, J. (eds) Bio-Based Building Materials. ICBBM 2023. RILEM Bookseries, vol 45. Springer, Cham. https://doi.org/10.1007/978-3-031-33465-8_23
Download citation
DOI: https://doi.org/10.1007/978-3-031-33465-8_23
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-33464-1
Online ISBN: 978-3-031-33465-8
eBook Packages: EngineeringEngineering (R0)