Abstract
Lymnaeid snails are simultaneous hermaphrodites that have a worldwide distribution, inhabiting freshwater areas from almost all continents ranging from tropical to arctic regions and from sea level to very high altitudes. In this chapter, we review the reproductive anatomy, behavioral and physiological traits, and mating strategies associated with increased survival and invasiveness of lymnaeids across different ecosystems around the globe. We also discuss the biotic and abiotic factors that can affect mating systems in this family, and how they have expanded their geographical range by natural, as well as human-mediated ways, likely promoting the spread of infectious diseases. Finally, we discuss why we believe that lymnaeids are suitable model organisms for studying mechanisms and processes involved in the ecology and evolution of mating systems and biological invasions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
The family Lymnaeidae is a worldwide-distributed group that originated and diversified 200 Myr ago, which now inhabits freshwater areas from all continents (except for Antarctica) ranging from tropical to arctic regions and from sea level to very high altitudes. Researchers have studied genetic diversity, life-history traits, and reproductive strategies to better understand their ecological and evolutionary history, and how they are related to the current distribution of the family. Yet, compared to other zoological groups (e.g., insects, vertebrates), relatively few studies have been conducted on this group, and they have focused mainly on a handful of species.
In this chapter, we review the life-history traits associated with lymnaeid survival and invasion by focusing on evolutionary and ecological studies performed in this family. We also call upon studies performed in other animal groups, and sometimes in plants, in an attempt to understand lymnaeid ecological and evolutionary success. We review the morphological, behavioral, and physiological traits involved in lymnaeid reproduction. We also discuss the factors that can affect lymnaeid mating systems, and how lymnaeids have expanded their geographical range by natural as well as human-mediated ways. Finally, we explain why we believe that lymnaeids are suitable model organisms for studying mechanisms and processes involved in the ecology and evolution of mating systems and biological invasions. In the whole chapter, we draw heavily on results obtained in the most studied species—that is, the great pond snail Lymnaea stagnalis and the Galba genus, putting emphasis on the mating system (selfing vs. outcrossing).
10.2 Reproductive Anatomy and Traits Potentially Enhancing Invasion in Lymnaeids
In this section, we first provide an overview of the reproductive anatomy of lymnaeids. Next, we move on to describing the morphological, behavioral, and physiological features that could enhance their invasiveness. Most of the following insights are from studies of Lymnaea stagnalis, as this species is extensively studied in multiple research fields (e.g., neurophysiology, ecotoxicology). Previous reviews in the Hygrophila, i.e. the superorder including the Lymnaeidae family, have developed this matter in greater detail (Geraerts and Joosse 1984; Jarne et al. 2010, 1993).
10.2.1 Reproductive Anatomy of Lymnaeids
As in other Hygrophila species, the reproductive morphology of Lymnaeids is rather complex with enough variation among species to serve as a rather accurate tool in systematics (Geraerts and Joosse 1984; Jarne et al. 2010; Fig. 10.1). Lymnaeids, as all Hygrophila and a significant fraction of gastropods, are simultaneous hermaphroditic organisms, having male and female functions within a single body at the same time (Jarne and Auld 2006). Thus, they have two choices of sperm for fertilization: autosperm (own sperm) for self-fertilization (or selfing), and allosperm (partners’ sperm) for cross-fertilization (or outcrossing). Sperm and eggs are produced in a single hermaphroditic organ, called the ovotestis. After spermatogenesis, mature spermatozoa are stored in the seminal vesicles after descending through the hermaphroditic duct. The latter separates into female and male tracts at the level of the carrefour where the fertilization pouch is positioned. Mature oocytes also exit the ovotestis and go down the hermaphroditic duct. The exact sites for both cross- and self-fertilization remain elusive, but should occur upstream of the carrefour. Then, fertilized eggs move down through several glands (albumen gland, pars contorta [oviduct], muciparous gland [oviduct pouch], oothecal gland) packaging eggs into egg capsules and an egg mass, which can contain from a few to a few hundred eggs. The egg mass is then laid through the female gonopore and deposited onto some surface in the environment to which they adhere.
(a) Schematic representation of the reproductive apparatus of Lymnaeidae (modified from Jarne et al. 2010). Reproductive apparatus drew using a camera lucida attachment. (b) from the outcrossing species Ampullaceana balthica and (c) from the selfing species Pseudosuccinea columella. Hermaphrodite, female and male organs are colored in green, yellow and blue, respectively. The route of oocytes, fertilized eggs, allo- and autosperm is also indicated. Abbreviations: ad, allosperm duct; ag, albumen gland; bc, bursa copulatrix; bt, bursa copulatrix tract; c, carrefour (= fertilization pouch); hd, hermaphroditic duct; m, muciparous gland; o, oothecal or nidamental gland; ot, ovotestis; otd, ovotestis duct; pco, pars contorta (= oviduct); pe, penis; pg, prostate gland; pp preputium; ps, penis sheath; sd, sperm duct; sv, seminal vesicles; u, uterus; v, vagina; vd, vas deferens
When an individual plays the male role, it everts the preputium. The penis is usually invaginated within the preputium. During copulation, the male-acting snail sticks the preputium into the partner’s female gonopore, then its penis devaginates from within the preputium to inside the partner’s vagina. Mature spermatozoa stored in the seminal vesicles are transferred, furnished with seminal fluids in the prostate gland, into the vagina of the female-acting partner. The ejaculate is transferred to the partner via the vas deferens and the preputium (that stays outside) and the penis (inside the partner) toward the female part of the partner’s apparatus. There, most of the ejaculate is directed toward the bursa copulatrix where it supposedly gets digested (data are from a species from another gastropod family, the garden snail Cornu aspersum (Rogers and Chase 2001)), and a small fraction of spermatozoa travels up the female tracts toward the carrefour to get stored and used for later fertilization (Koene et al. 2009). In contrast, the trajectory of the autosperm used for self-fertilization is different. It is highly unlikely that the autosperm travel through the male ducts of the donor and then female ducts of the recipient up to the carrefour for fertilization, meaning that autosperm does not come with seminal fluid. Thus, autosperm and allosperm may be in different states, allowing for differential success in fertilization, and leaving room for a potential control over selfing rate by the mother.
10.2.2 Mating Strategies and Behavioral and Physiological Traits
10.2.2.1 Selfing
In common with all Hygrophila, the species of the Lymnaeid family can self-fertilize, meaning that a single adult snail can initiate a population without any mates (“reproductive assurance”; Baker’s law: Baker 1955; Cheptou 2012). In freshwater snails, selfing is associated with the modification of traits associated with reproduction, including morphological ones, which has been referred to as the selfing syndrome (Doums et al. 1996). This syndrome encompasses the wide range of traits associated with preferential selfing, such as decreased inbreeding depression, limited male copulatory behavior, and reduced heterozygosity. Further, the selfing syndrome is supported by the predictions from the sex allocation theory: selection would drive the adaptive optima toward allocating fewer resources to the male function because of reduced sexual selection in selfing species (Charnov 1982; Schärer 2009). In species prone to selfing, it is therefore expected a lower investment into the prostate gland and prostatic fluids relative to outcrossing ones. Such variation in sex allocation is exemplified in Fig. 10.1, which compares the reproductive morphology of Pseudosuccinea columella (a selfer) and Ampullaceana (Radix) balthica (an outcrosser) where the prostate gland exhibits a simpler internal structure in selfing relative to an outcrossing species (Jarne et al. 2010; Swart et al. 2020). However, the analysis of the relationship between selfing rates and reproductive morphology is limited to few species in lymnaeids (Escobar et al. 2011). Thus, a comparative study including a sufficient number of species, associated with phylogenetic analysis to account for phylogenetic correlations, would be needed to confirm if selfing syndrome is indeed affecting the evolution of reproductive morphology.
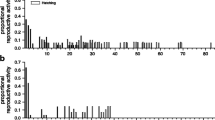
As Hygrophila in general, the Lymnaeid family displays a diversity of reproductive strategies with respect to selfing rate, which generally falls into two well-separated groups: preferential outcrossers and preferential selfers (strongly U-shaped distribution, based on molecular estimates; Escobar et al. 2011). On the one hand, preferential outcrossers (including, as far as we know, Lymnaea stagnalis and the Radix clade) show selfing rates below 20% in natural populations, and self-fertilization is generally associated with inbreeding depression (Fig. 10.2). Hence, these species outcross their eggs whenever possible, and they self-fertilize only when they are unable to find a mate, after waiting some time for potential mates. The latter strategy is likely because it is better paying the price of depressed fitness with selfing than not reproducing at all (see review in Escobar et al. 2011). Note that the pond snail L. stagnalis is an exception (and a puzzle): this species is an outcrosser, they do wait for a potential mate, exhibiting little inbreeding depression, though initiating selfing at an older age than outcrossing (Coutellec and Caquet 2011; Koene et al. 2008; Puurtinen et al. 2007). On the other hand, preferential selfers, including Pseudosuccinea columella (Lounnas et al. 2017a; Nicot et al. 2008) and the Galba spp. clade (Chapuis et al. 2007; Hurtrez-Boussès et al. 2010; Meunier et al. 2004; Trouvé et al. 2003; Lounnas et al. 2018, 2017b), show natural selfing rates in excess of 80% and there is no evidence for the existence of any significant delay in egg production upon the lack of a partner, or of inbreeding depression (see Fig. 10.2). In both preferential outcrossers and preferential selfers, selfed progeny is readily obtained and raised in laboratory conditions (Alba et al. 2019; Nakadera et al. 2019). Accordingly, selfing could serve, for instance, as a (temporary or permanent) strategy to successfully invade new areas and overcome difficulties associated with low densities and low population increase. Regular selfers, or species like L. stagnalis, that lack inbreeding depression and/or reproductive delays associated with self-fertilization, may pay even lower costs during founding events, which occur repeatedly when an invasive species spreads through fragmented freshwater habitats.
Phylogeny of the Lymnaeidae indicating the preferentially outcrossing (pink) and preferentially selfing species (green). The tree was obtained by concatenating the 16S, ITS1, and ITS2 sequences and includes 50 species and Physa acuta as an outgroup (modified from Correa et al. 2010). The embedded table shows the mean and the standard deviation of the selfing rate, the number of studied populations (N), and the references that have been used to estimate the selfing rate for each lymnaeid species
10.2.2.2 Multiple Mating
Among outcrossing lymnaeids, multiple matings with different male and female partners are frequent. This behavior has largely been documented in L. stagnalis under laboratory conditions with up to two inseminations per day in the male role for some individuals (Koene and Ter Maat 2007). The male mating drive is determined by mating history, and virgin individuals or individuals that have been isolated for several days are eager to copulate as male (Van Duivenboden and Ter Maat 1985). This motivation is associated with the fullness of the prostate gland (De Boer et al. 1997). Field investigations using genetic markers support that multiple mating is indeed prevalent in natural populations of outcrossing lymnaieds, although this approach would underestimate the degree of multiple mating due to the potential matings did not yield any offspring (L. stagnalis, Nakadera et al. 2017; A. balthica, Coutellec-Vreto et al. 1997; Bürkli and Jokela 2017). One of the consequences of promiscuity and multiple paternity is increased genetic variance among offspring, which could be beneficial to cope with environmental challenges. Also, promiscuity is likely associated with strong male–male competition, both precopulatory (access to mating partners) and postcopulatory (sperm competition), and, consequently, sexual selection on the male function (e.g., Hoffer et al. 2017 for study of sexual selection in L. stagnalis). Such selective pressures on the male function in outcrossing lymnaeids would promote purging deleterious mutations and limiting genetic load in populations, as shown in other freshwater snail species (Bonel et al. 2018; Noël et al. 2019).
10.2.2.3 Mate Preference and Mating Behavior
Contrasting with separate-sex species, in which individuals need the opposite sex to reproduce, simultaneous hermaphrodites can reproduce with any conspecific, thus increasing the potential to initiate a population and overcoming the Allee effect in low-density populations (see above for the further advantage of selfing). Lymnaea stagnalis prefers to mate with a new partner than a previously mated one, which is called the Coolidge effect (Koene and Ter Maat 2007), which would increase the genetic diversity of offspring by getting access to new partners. Moreover, other types of mate choice, depending on the relatedness or infectious status, would influence their capability of invading a new area, but it remains to be evaluated in lymnaeids.
Sex role preference is associated with unilateral mating, meaning that in a given mating event, one snail plays the male role while the other plays the female role (the most common mating pattern in Hygrophila; Jarne et al. 2010). We already mentioned that individuals without access to partners for several days are very eager to mate in outcrossing species (Coolidge effect), as shown experimentally in L. stagnalis (van Duivenboden 1983). Such a role preference and mating eagerness may generate a conflict in mating encounters because smaller individuals are more likely to play the male role but bigger ones are also motivated to copulate as males (Nakadera et al. 2014). After the first copulation, individuals may exchange roles where the female-acting snail often extends its body to grasp the male-acting partner to exchange role, leading to bilateral sperm exchange in a unilaterally-copulating species (Koene and Ter Maat 2005). The male mating behavior has been extensively examined in L. stagnalis, but recent studies on the female behavior of this species elucidated the unexplored dimension of mating interaction to examine the sex role preferences (Daupagne and Koene 2020; Moussaoui et al. 2018).
10.2.2.4 Sperm Storage
Gastropods have developed efficient sperm storage capacity (Backeljau et al. 2007), and lymnaeids are no exception, even if this capacity has been studied only in L. stagnalis. From a single mating event, individuals can store allosperm up to 2.5 months, a rather long period given their reproduction is initiated at 3 months after hatching in this species (Nakadera et al. 2014). This prolonged sperm storage ability would be beneficial to start a new population. We still ignore, however, where and how freshwater snails store allosperm, how sperm choice occurs, and how allosperm and autosperm are interacting (Koene et al. 2009). Sperm choice might indeed be critical in the context of range shift, provided that the partner’s quality can be evaluated based on sperm characteristics (e.g., motility).
10.2.2.5 Egg Production
It is crucial to decide when to produce eggs and egg capsules, and how much to invest in them. Mature individuals in lymnaeid species continuously produce eggs, packaged in egg mass, and deposited them on various types of surfaces in the environment (e.g., branches or stones in the wild, box wall in the laboratory). The number of eggs and egg masses depend on the species. For instance, adult L. stagnalis lay, on average, 1–3 egg masses per week, and each egg mass can contain from 50 to 150 eggs, whereas this figure has been reported to be much lower in Galba species (2 eggs per egg capsule; e.g., Chapuis et al. 2007). Lymnaeid species show a stereotyped egg-laying behavior, including extensive cleaning on the surface to lay egg mass and inspection after laying (Jarne et al. 2010). Moreover, it has been reported in the same species that the exposure to clean water and surface immediately triggers egg-laying within 2 h (clean water stimulus; Ter Maat et al. 1983), indicating that environmental conditions play a role in egg-laying strategy. That is, lymnaeid species do not provide any parental care after depositing egg masses. Hence, it is a critical choice where and when eggs masses should be laid for successful juvenile development before and after hatching. Note that high fecundity also allows “not to put all eggs in the same basket,” which can be interpreted as a some sort of reproductive assurance.
Mating does not result only in the transfer of spermatozoa, but also of seminal fluids, including seminal fluid protein (SFP). Such proteins have been shown to strongly affect the behavior and fecundity of receiving individuals in a wide range of animal species (e.g., Avila et al. 2011; Herndon and Wolfner 1995; Patlar et al. 2020). Their effects on egg production have been primarily studied in L. stagnalis. For instance, receiving Ovipostatin (an SFP also called LyAcp10) during copulation triggers a significant delay of egg laying (Koene et al. 2010) and a reduction in the number of eggs laid associated with a higher investment per egg (Swart et al. 2020). These results need to be set in a broader fitness analysis to assess the extent to which transferring ovipostatin is adaptive and how receivers have developed a counter-adaptation. Whether the production of SFP is reduced in selfing species, as expected from the theory of sex allocation, remains unknown.
In addition, the role of the gelatinous substance in egg mass has been well speculated but has yet not been systematically evaluated. Eggs indeed do not hatch synchronously with a hatching range of 34 days for an incubation time of ca. 1 week as in Lymnaea peregra, for instance (Jarne and Delay 1990). However, Marois and Croll (1991) reported for L. stagnalis that removing the gelatinous substance largely increases the synchronicity of hatching timing. It is therefore likely that the access to oxygen and other essential substances from the outer environment that are required for egg development is heterogeneous within a capsule. This gelatinous substance might also protect against bacterial or fungal infection, and slow down desiccation, a useful characteristic both in case of drought and of transportation by birds or other larger animals; this certainly opens the possibility of long-distance migration to reach new habitats (Van Leeuwen et al. 2013).
10.2.2.6 Sperm Digestion
Gastropod reproductive apparatus includes an organ called bursa copulatrix (Fig. 10.1), and it has been shown in the land snail Cornu aspersum that the major part of the received ejaculate ends up in this organ where it is digested (Rogers and Chase 2001). This suggests that the ejaculate could be interpreted as a “nuptial gift,” since mating partners can extract energy from received ejaculates. This seems rather unlikely, at least in L. stagnalis, since the energy brought in by ejaculates is very small compared to the energy required for egg production (Lodi et al. 2017). However, it might play a role in limiting the effect of SFPs received during copulation, or even of sexually-transmitted diseases. These ideas are purely speculative and need to be tested based on experiments in the great pond snail.
10.3 Is Selfing an Evolutionary Dead End?
Biologists have studied transitions between predominantly outcrossing and predominantly selfing systems for a long time in plants, a literature dominated by the concept of predominant selfing as an evolutionary dead end (Burgarella and Glémin 2017; Igic and Busch 2013). As illustrated by the case of Arabidopsis thaliana (Durvasula et al. 2017; Tang et al. 2007), predominantly selfing species sometimes rapidly evolve from outcrossing ancestors. Indeed, as mentioned above, keeping ovules for self-fertilization has some short-term advantages, including higher fidelity of gene transmission and reproductive assurance, which may be especially relevant during the establishment of new populations or demographic bottlenecks. If not opposed by high inbreeding depression, selfing variants may therefore rapidly spread through populations (Burgarella and Glémin 2017; Charlesworth and Willis 2009). Increased selfing may also promote purging of some deleterious mutations (e.g., recessive semi-lethals), reducing inbreeding depression (Noël et al. 2019). This, in turn, would increase the advantage of selfing in a self-sustained loop, leading to very high selfing rates (Burgarella and Glémin 2017; Charlesworth and Willis 2009). This evolutionary loop between mutation load and selfing rate may reinforce the stability of extreme states—predominant outcrossers with high inbreeding depression, and predominant selfers with low inbreeding depression—and explain the rarity of transitions.
The asymmetry in transitions (more transitions from outcrosser to selfer than the reverse) may be explained by both short-term and long-term processes. On the short term, reproductive assurance may favor selfing in outcrossing populations despite high inbreeding depression under particular conditions such as lack of pollinators. By contrast, there are no obvious conditions that would give an advantage to an outcrossing mutant in a selfing population with low inbreeding depression. On the long term, highly selfing taxa get extinct with higher probability than outcrossing ones, because high selfing rates reduce effective recombination, decreasing evolutionary potential and facilitating the fixation of deleterious mutations in genomes over time scales in the order of 1–2 Myr (Goldberg et al. 2010; Slotte et al. 2013).
Lymnaeids have been much less studied than plants, and we know relatively little about the frequency of transitions from predominant outcrosser to predominant selfer in their evolutionary history. They offer though an interesting situation that is not found in plants: the Galba genus, which age is estimated at 20 Mya (Burgarella et al. 2015), is essentially made of highly selfing species (see Fig. 10.2), suggesting that selfing has been the main mating system for a long time (Alda et al. 2021). Although the genomes of selfing Galba tend to accumulate relatively high numbers of mutations, as predicted from the long-term effects of selfing (Burgarella et al. 2015), the long-term persistence of these species contradicts the “dead-end claim” for selfing, but the circumstances under which this is possible remain unclear. Burgarella et al. (2015) suggested that this might be due to the fact that Galba species have colonized a new adaptive zone. They are indeed more amphibious than aquatic, being able to survive on mud banks or swamped meadows, when competition with other Hygrophila is limited. This idea, however, remains to be evaluated adequately.
Although we have currently no example of recent or ongoing transitions between mating systems in Lymnaeids (and Hygrophila in genera), the forces involved in such transitions could be experimentally approached, as has been done in Physa acuta (belonging to the Hygrophila) with continuous or recurrent selfing (Noël et al. 2019, 2017). This study has confirmed a loss of evolutionary potential after increasing selfing rates (Noël et al. 2017), with potential consequences on the capacity to adapt to changing environmental conditions.
Lymnaea stagnalis is a good candidate for similar investigations because this outcrossing species has a low inbreeding depression (a characteristic that may favor selfing variants) and can be inbred for tens of generations without lethality (Colton and Pennypacker 1934; J. Koene, unpublished data). In New Zealand, invasive populations of L. stagnalis have practically no polymorphism (Kopp et al. 2012), as would be the case after a few recurrent generations of selfing. The lack of variation precludes the estimation of spontaneous selfing rates in extant New Zealand populations. In this case, however, self-fertilization (compared to outcrossing) is expected to have no particular effect on heterozygosity and fitness. This creates a favorable context to invasion by predominantly selfing strategies and should be studied in more detail.
10.4 Mating Systems in a Changing World
Human-induced environmental changes are altering ecosystems by triggering a wide array of irreversible consequences. These environmental changes are predicted to affect ecological processes across several levels of organization, likely over the coming decades, by altering the performance of individual organisms, the dynamics of populations, and the distribution of species, ultimately modifying ecosystem properties and functioning (Lonhart et al. 2019). If organisms have difficulties in adapting and fail to track projected environmental changes, populations become vulnerable to decline and extinction (Hill et al. 2011). Research has documented several mechanisms allowing rapid adaptation, which is crucial for the long-term persistence of populations facing new and stressful environment conditions brought about by global change and range shifting (Hill et al. 2011).
As mentioned above, mating systems have a deep impact on individual fitness and may therefore contribute to understanding how species respond to stressful environmental conditions (Aanen et al. 2016; Bijlsma and Loeschcke 2005). Some lymnaeid species, for instance, can survive drought conditions for several weeks and even months by adhering their aperture to the soil surface or burying themselves into deeper ground where humidity is conserved (Chapuis et al. 2007). When moist conditions return, one or a few individuals can self-fertilize and recolonize the site. Individuals can also colonize a new habitat if they have been transported by flooding, birds, mammals, or humans (Kappes and Haase 2012; Van Leeuwen et al. 2013, 2012). In this sense, self-fertilization facilitates and ensures colonization success, which may explain how two lymnaeid selfing species such as P. columella and G. schirazensis have successfully established across different ecosystems worldwide (Lounnas et al. 2017a, 2018). Indeed, human activities drastically accelerated lymnaeid invasion worldwide. The aquarium trade has a long history of transporting and introducing snails, among plants and fish, into regions where they are not native (Duggan 2010). This is particularly interesting in long-range invasions because it may intensify selection for selfing species. In other words, aquarium populations are typically recurrently bottlenecked and re-seeded with a few eggs or individuals on a plant. Such populations may selectively propagate selfing species such as P. columella or may also force preferential outcrossers through several cycles of selfing, which would be, for instance, the case of L. stagnalis introduced in New Zealand (Kopp et al. 2012).
A drastic loss of genetic diversity was observed in the invaded regions in the selfing species P. columella (Lounnas et al. 2017a), G. schirazensis (Lounnas et al. 2018), and G. truncatula (Meunier et al. 2001). Populations in invaded areas analyzed in these studies are characterized by an extremely low genetic diversity, with sometimes a single (or a few) genotype. Selfing explains much of this depletion because species are indeed made of little-recombining genotypes and have lower variation. This could result from bottlenecks (associated with a low propagule pressure), which efficiently sieve out a few genotypes and then proliferate through selfing. All of these studies suggest that multiple ecological and evolutionary factors shape the distribution of genetic variation throughout species ranges. Yet, the only aspect that has been studied is neutral genetic variation, and we lack data on many aspects related to the invasion. The question that inevitably arises is how lymnaeids succeed in their invasion process with genetically depleted populations that originated by selfing. This dilemma is known as the genetic paradox of invasion (Allendorf and Lundquist 2003).
A growing number of studies propose different mechanisms that allow invasive populations to increase population fitness and compensate for the loss in genetic diversity during introduction events and at the invasion front (see Estoup et al. 2016 for further details). Purging of the genetic load is one possibility (Crnokrak and Barrett 2002). Recurrent introductions of different genotypes can also increase the genetic variance and adaptability (Facon et al. 2008). However, the same single genotype can repeatedly invade new regions, resulting in low genetic variability in those regions. This would be the case of P. columella because an extensive sampling coupled with population genetic analysis showed that the depletion of genetic variation is extremely strong and that this species has a unique “worldwide invader” genotype (Lounnas et al. 2017a). Adaptive phenotypic plasticity is another possible mechanism that could compensate for genetic diversity loss and explain the high invasiveness of populations (Estoup et al. 2016; Ghalambor et al. 2007). Future work should focus on investigating which possible mechanisms, processes, or strategies that facilitate genetically depleted populations of lymnaeids to rapidly respond to novel stressful environmental conditions when shifting their distribution and colonizing new areas.
10.5 Can Parasites Shift Host Mating Systems?
The Red Queen Hypothesis (RQH) suggests that hosts continuously evolve to evade strategies developed by their parasites and, in turn, parasites must counter-adapt to host changes. Parasites therefore have a profound impact on their hosts as they can impose selection against common host genotypes, resulting in an advantage to producing outcrossed, genetically variable progeny (Hamilton 1980). The original version of the RQH was developed for the evolution of sex in plants and animals (Lively 1987; Verhoeven and Biere 2013) but has been extended for the evolution of selfing. Coevolution, in this case, leads to selection for outcrossing of both parasites and hosts (Agrawal and Lively 2001; Hurtrez-Boussès et al. 2001). Recent laboratory experiments have shown that coevolving parasites can favor increased rates of outcrossing in mixed-mating (outcrossing and selfing) host populations of the nematode Caenorhabditis elegans (Slowinski et al. 2016). Computer simulations have also predicted that if selective pressure imposed by parasites is high enough to favor outcrossing in hosts, it could promote the occurrence of resistant mutants among the host population (e.g., Agrawal and Lively 2001).
Lymnaeids and the parasites that infect them, such as trematodes like Fasciola hepatica, are excellent models to study the evolution of mating systems because both are usually simultaneous hermaphrodites that reproduce by selfing or outcrossing (Chapuis et al. 2007; Hurtrez-Boussès et al. 2004; Trouvé et al. 1996). Yet, there is limited or no evidence that the mating system evolved in hosts and parasites as a response to their interaction. Further research in simultaneously hermaphroditic animals is necessary to evaluate which and how different stressors affect selfing and outcrossing rates, considering the underlying genetic and phenotypic basis of both mating systems and the basis for infection and virulence in parasites.
The mating strategy resulting from environment or parasite pressure is likely to have an impact on the transmission of infectious diseases. Theoretical models indeed show that low host genetic diversity—as a result of the type of mating system and of the rate of migration—can promote disease spread (e.g., Lively 2010). It is widely accepted that genetically homogenous host populations, resulting from selfing or low propagule pressure, are more vulnerable to infection than genetically diverse ones (King and Lively 2012). This has been observed in plants (Mundt, 2002; Zhu et al. 2000) and in animals (Altermatt and Ebert 2008; Ellison et al. 2011). For instance, the rapid expansion of the selfing freshwater snail Biomphalaria pfeifferi, an intermediate host for human schistosomiasis, might result in the establishment of genetically homogenous populations in pristine water bodies in Zimbabwe that were susceptible to the parasite thus promoting the resurgence of the disease in this country (Campbell et al. 2010). As most invasive host populations have no genetic variability to evolve resistance mechanisms, the parasite could therefore freely expand without opposition, representing a health threat (Lounnas et al. 2017a).
10.6 What’s Next?
Lymnaeids are suitable model organisms for tackling issues that are of general interest in ecology and evolution of mating systems and invasion. Up to now, only three lymnaeid species groups have monopolized studies regarding these issues: L. stagnalis, Galba species and the sister species P. columella, and to a lesser extent some Radix species. There are tens of other lymnaeid species that have never or very little been studied that could bring light in our current understanding about the ecology and evolution of mating systems and invasion in lymnaeids.
Experimental evolution approaches and genomic and phylogenetic analyses that explore the genetic architecture of lymnaeid reproductive traits should be performed to better understand the evolution of mating systems. A good starting point for digging into lymnaeid mating systems would be a thorough phylogenetic comparison of selfing rates in lymnaeid species. Our current knowledge about the evolution of mating systems in lymnaeids (see Fig. 10.2) is far from being sufficient because, in some cases, genetic evidence for self-fertilization in natural population is hard to be obtained. This is because it is difficult to estimate selfing rates in highly selfing populations showing very low variation (Lounnas et al. 2018). In these cases, genetic approaches such as RAD seq that use hundreds of genetic markers must be applied (Miller et al. 2007). This phylogenetic approach could also include a comparison of morphological reproductive traits at a family level. The hypothesis that outcrossing species show a complex reproductive anatomy and that selfing species a simplified one needs to be tested in lymnaeids. In addition, it would be interesting to investigate whether there are genetic differences in SFPs between selfing and outcrossing species.
Lymnaea stagnalis and A. balthica are good lymnaeid species for conducting experimental approaches since they are easy to breed under experimental conditions, unlike other lymnaeid species. Yet, the distribution of selfing rates among populations of both species is still unclear, and it should be studied at the species scale. Galba species are difficult to breed under experimental conditions, but they are a very promising species group for studying the evolution of selfing. Recently, Alda et al. (2021) suggested that a group of Galba snails would have reverted its mating system from selfing to outcrossing—something that would contradict Stebbins’ law that claims: “the transition from outcrossing to selfing is unidirectional.” This seemingly unexpected reversion in Galba cousini, a species that inhabits high altitudes in the north-western Andes, needs to be properly tested. If true, it would be the first evidence in the history of the study of mating systems of hermaphrodites that would have evolved from selfing to outcrossing. Further studies should explore which are the genetic architecture and morphological traits that accompanied this reversion, and which are the ecological factors that could prompt it.
Lymnaeid selfing species could be used as model organisms in systematics to test species concepts in selfing species. Most species-delimitation models currently used in systematics are based on Multispecies Coalescence that assumes that gene flow occurs within species though not between species (Sukumaran and Knowles 2017). Thus, the use of these models would be inappropriate in a fully selfing species groups. The selfing rate in lymnaeid species and populations is high (often ~0.9), but outcrossing does occur (Chapuis et al. 2007; Lounnas et al. 2017a, 2017b). Further research could explore how selfing may affect speciation (Cutter 2019). Lymnaeid species would be excellent models to investigate such questions, since selfing rates vary among species and populations.
Studies of population genetics, demography, and phylogeography of both source and invading populations will be useful for understanding the underlying mechanisms driving the success of lymnaeid invasive species, their geographic patterns of invasion and range expansion and potential for evolutionary responses to new environments and their parasites and other ecological challenges. Compiling the existing data about the effect of pesticides, herbicides, fertilizers, pharmaceuticals, and other pollutants in many lymnaeids species could help us to predict which species could potentially become urban invaders.
Finally, it is imperative to conduct studies investigating the consequences of lymnaeid invasions in the spread of infectious diseases. For that purpose, an investigation of the interaction between invader genotypes and local populations of parasites is needed. Such population genetic studies in snail hosts and parasites would bridge the gap between biological invasions and epidemiology; research areas that are interconnected but rarely studied together. The snail-parasite system constituted by P. columella, the species from the genus Galba, and the trematode F. hepatica would be an appropriate model to investigate what are the mechanisms and processes behind the interactions between hosts, parasites, and environment that enhance the spread of infection diseases in wildlife, domestic animals, and humans. This goal would completely match the scope of the One Health Initiative—an approach that seeks to understand infectious diseases to then apply programs, policies, and legislation in which multiple sectors work together to achieve better public health outcomes (e.g., Destoumieux-Garzón et al. 2018).
References
Aanen D, Beekman M, Kokko H (2016) Weird sex: the underappreciated diversity of sexual reproduction. Phil Trans R Soc B 371:20160262. https://doi.org/10.1098/rstb.2016.0262
Agrawal AF, Lively CM (2001) Parasites and the evolution of self-fertilization. Evolution 55:869–879. https://doi.org/10.1554/0014-3820(2001)055[0869:PATEOS]2.0.CO;2
Alba A, Vázquez AA, Sánchez J et al (2019) Patterns of distribution, population genetics and ecological requirements of field-occurring resistant and susceptible Pseudosuccinea columella snails to Fasciola hepatica in Cuba. Sci Rep-UK 9:14359. https://doi.org/10.1038/s41598-019-50894-7
Alda P, Lounnas M, Vázquez AA et al (2021) Systematics and geographical distribution of Galba species, a group of cryptic and worldwide freshwater snails. Molec Phyl Evol 157:107035. https://doi.org/10.1016/j.ympev.2020.107035
Allendorf FW, Lundquist LL (2003) Introduction: population biology, evolution, and control of invasive species. Conserv Biol 17:24–30. https://doi.org/10.1046/j.1523-1739.2003.02365.x
Altermatt F, Ebert D (2008) Genetic diversity of Daphnia magna populations enhances resistance to parasites. Ecol Lett 11:918–928. https://doi.org/10.1111/j.1461-0248.2008.01203.x
Avila FW, Sirot LK, LaFlamme BA et al (2011) Insect seminal fluid proteins: identification and function. Annu Rev Entomol 56:21–40. https://doi.org/10.1146/annurev-ento-120709-144823
Backeljau T, Jordaens K, Dillen L (2007) Effects of mating, breeding system and parasites on reproduction in hermaphrodites: pulmonate gastropods (Mollusca). Anim Biol 57:137–195. https://doi.org/10.1163/157075607780377965
Baker HG (1955) Self-compatibility and establishment after “long-distance” dispersal. Evolution 9:347–349. https://doi.org/10.2307/2405656
Bijlsma R, Loeschcke V (2005) Environmental stress, adaptation and evolution: an overview. J Evol Biol 18:744–749. https://doi.org/10.1111/j.1420-9101.2005.00962.x
Boer P, Jansen R, Koene J, Maat M (1997) Nervous control of male sexual drive in the hermaphroditic snail. J Exp Biol 200:941–951
Bonel N, Noël E, Janicke T et al (2018) Asymmetric evolutionary responses to sex-specific selection in a hermaphrodite. Evolution 72:2181–2201. https://doi.org/10.1111/evo.13565
Burgarella C, Glémin S (2017) Population genetics and genome evolution of selfing species. In: John Wiley & Sons Ltd (ed) eLS. John Wiley & Sons Ltd, Chichester, pp 1–8. https://doi.org/10.1002/9780470015902.a0026804
Burgarella C, Gayral P, Ballenghien M et al (2015) Molecular evolution of freshwater snails with contrasting mating systems. Mol Biol Evol 32:2403–2416. https://doi.org/10.1093/molbev/msv121
Bürkli A, Jokela J (2017) Increase in multiple paternity across the reproductive lifespan in a sperm-storing, hermaphroditic freshwater snail. Mol Ecol 26:5264–5278. https://doi.org/10.1111/mec.14200
Campbell G, Noble LR, Rollinson D et al (2010) Low genetic diversity in a snail intermediate host (Biomphalaria pfeifferi Krauss, 1848) and schistosomiasis transmission in the Senegal River basin. Mol Ecol 19:241–256. https://doi.org/10.1111/j.1365-294X.2009.04463.x
Chapuis E, Trouve S, Facon B et al (2007) High quantitative and no molecular differentiation of a freshwater snail (Galba truncatula) between temporary and permanent water habitats. Mol Ecol 16:3484–3496. https://doi.org/10.1111/j.1365-294X.2007.03386.x
Charlesworth D, Willis JH (2009) The genetics of inbreeding depression. Nat Rev Genet 10:783–796. https://doi.org/10.1038/nrg2664
Charnov EL (1982) The theory of sex allocation. Monogr Popul Biol 18:1–355
Cheptou PO (2012) Clarifying Baker’s Law. Ann Bot-London 109:633–641. https://doi.org/10.1093/aob/mcr127
Colton HS, Pennypacker M (1934) The results of twenty years of self-fertilization in the pond snail Lymnaea columella say. Am Nat 68:129–136. https://doi.org/10.1086/280532
Correa AC, Escobar JC, Durand P et al (2010) Bridging gaps in the molecular phylogeny of the Lymnaeidae (Gastropoda: Pulmonata), vectors of fascioliasis. BMC Evol Biol 10:381. https://doi.org/10.1186/1471-2148-10-381
Coutellec MA, Caquet T (2011) Heterosis and inbreeding depression in bottlenecked populations: a test in the hermaphroditic freshwater snail Lymnaea stagnalis: genetic load in a freshwater snail. J Evol Biol 24:2248–2257. https://doi.org/10.1111/j.1420-9101.2011.02355.x
Coutellec-Vreto MA, Madec L, Guiller A (1997) Selfing and biparental inbreeding: a mating system analysis in Lymnaea peregra (Gastropoda: Lymnaeidae). Heredity 79:277–285. https://doi.org/10.1038/hdy.1997.155
Crnokrak P, Barrett SCH (2002) Perspective: purging the genetic load: a review of the experimental evidence. Evolution 56:2347–2358. https://doi.org/10.1111/j.0014-3820.2002.tb00160.x
Cutter AD (2019) Reproductive transitions in plants and animals: selfing syndrome, sexual selection and speciation. New Phytol 224(3):1080–1094
Daupagne L, Koene JM (2020) Disentangling female postmating responses induced by semen transfer components in a simultaneous hermaphrodite. Anim Behav 166:147–152. https://doi.org/10.1016/j.anbehav.2020.06.009
Destoumieux-Garzón D, Mavingui P, Boetsch G et al (2018) The one health concept: 10 years old and a long road ahead. Front Vet Sci 14
Doums C, Viard F, Pernot AF et al (1996) Inbreeding depression, neutral polymorphism, and copulatory behavior in freshwater snails: a self-fertilization syndrome. Evolution 50:1908–1918. https://doi.org/10.1111/j.1558-5646.1996.tb03578.x
Duggan IC (2010) The freshwater aquarium trade as a vector for incidental invertebrate fauna. Biol Invasions 12:3757–3770. https://doi.org/10.1007/s10530-010-9768-x
Durvasula A, Fulgione A, Gutaker RM et al (2017) African genomes illuminate the early history and transition to selfing in Arabidopsis thaliana. Proc Natl Acad Sci U S A 114:5213–5218. https://doi.org/10.1073/pnas.1616736114
Ellison A, Cable J, Consuegra S (2011) Best of both worlds? Association between outcrossing and parasite loads in a selfing fish. Evolution 65:3021–3026. https://doi.org/10.1111/j.1558-5646.2011.01354.x
Escobar JS, Auld JR, Correa AC et al (2011) Patterns of mating-system evolution in hermaphroditic animals: correlations among selfing rate, inbreeding depression, and the timing of reproduction: mating-system evolution in hermaphroditic animals. Evolution 65:1233–1253. https://doi.org/10.1111/j.1558-5646.2011.01218.x
Estoup A, Ravigné V, Hufbauer R et al (2016) Is there a genetic paradox of biological invasion? Annu Rev Ecol Evol Syst 47:51–72. https://doi.org/10.1146/annurev-ecolsys-121415-032116
Facon B, Pointier JP, Jarne P (2008) High genetic variance in life-history strategies within invasive populations by way of multiple introductions. Curr Biol 18:363–367. https://doi.org/10.1016/j.cub.2008.01.063
Geraerts WPM, Joosse J (1984) Freshwater snails (Basommatophora). In: Wilbur KM (ed) The Mollusca, Reproduction, vol Vol. 7. Academic Press, New York, pp 141–207
Ghalambor CK, McKay JK, Carroll SP et al (2007) Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct Ecol 21:394–407. https://doi.org/10.1111/j.1365-2435.2007.01283.x
Goldberg EE, Kohn JR, Lande R et al (2010) Species selection maintains self-incompatibility. Science 330:493–495. https://doi.org/10.1126/science.1194513
Hamilton WD (1980) Sex versus non-sex versus parasite. Oikos 35:282–290. https://doi.org/10.2307/3544435
Herndon LA, Wolfner MF (1995) A drosophila seminal fluid protein, Acp26Aa, stimulates egg laying in females for 1 day after mating. Proc Natl Acad Sci U S A 92:10114–10118. https://doi.org/10.1073/pnas.92.22.10114
Hill JK, Griffiths HM, Thomas CD (2011) Climate change and evolutionary adaptations at species’ range margins. Annu Rev Entomol 56:143–159. https://doi.org/10.1146/annurev-ento-120709-144746
Hoffer JN, Mariën J, Ellers J et al (2017) Sexual selection gradients change over time in a simultaneous hermaphrodite. eLife 6:e25139. https://doi.org/10.7554/eLife.25139
Hurtrez-Boussès S, Meunier C, Durand P et al (2001) Dynamics of host–parasite interactions: the example of population biology of the liver fluke (Fasciola hepatica). Microbes Infect 3:841–849. https://doi.org/10.1016/S1286-4579(01)01442-3
Hurtrez-Bousses S, Durand P, Jabbour-Zahab R (2004) Isolation and characterization of microsatellite markers in the liver fluke (Fasciola hepatica). Molec Ecol Notes 4:689–690. https://doi.org/10.1111/j.1471-8286.2004.00786.x
Hurtrez-Boussès S, Hurtrez JE, Turpin H et al (2010) Hydrographic network structure and population genetic differentiation in a vector of fasciolosis, Galba truncatula. Infect Genet Evol 10:178–183. https://doi.org/10.1016/j.meegid.2010.01.005
Igic B, Busch JW (2013) Is self-fertilization an evolutionary dead end? New Phytol 198:386–397. https://doi.org/10.1111/nph.12182
Jarne P, Auld JR (2006) Animals mix it up too: the distribution of self-fertilization among hermaphroditic animals. Evolution 60:1816–1824. https://doi.org/10.1111/j.0014-3820.2006.tb00525.x
Jarne P, Delay B (1990) Inbreeding depression and self-fertilization in Lymnaea peregra (Gastropoda: Pulmonata). Heredity 64:169–175. https://doi.org/10.1038/hdy.1990.21
Jarne P, Vianey-Liaud M, Delay B (1993) Selfing and outcrossing in hermaphrodite freshwater gastropods (Basommatophora): where, when and why. Biol J Linn Soc 49:99–125. https://doi.org/10.1111/j.1095-8312.1993.tb00893.x
Jarne P, Pointier JP, David P et al (2010) Basommatophoran gastropods. In: Leonard J, Cordoba-Aguilar A (eds) The evolution of primary sexual characters in animals. Oxford University Press, Oxford and New York, pp 173–196
Kappes H, Haase P (2012) Slow, but steady: dispersal of freshwater molluscs. Aquat Sci 74:1–14. https://doi.org/10.1007/s00027-011-0187-6
King KC, Lively CM (2012) Does genetic diversity limit disease spread in natural host populations? Heredity 109:199–203. https://doi.org/10.1038/hdy.2012.33
Koene JM, Ter Maat A (2005) Sex role alternation in the simultaneously hermaphroditic pond snail Lymnaea stagnalis is determined by the availability of seminal fluid. Anim Behav 69:845–850. https://doi.org/10.1016/j.anbehav.2004.07.012
Koene JM, Ter Maat A (2007) Coolidge effect in pond snails: male motivation in a simultaneous hermaphrodite. BMC Evol Biol 7:212. https://doi.org/10.1186/1471-2148-7-212
Koene JM, Loose MJ, Wolters L (2008) Mate choice is not affected by mating history in the simultaneously hermaphroditic snail Lymnaea stagnalis. J Mollus Stud 74:331–335. https://doi.org/10.1093/mollus/eyn020
Koene JM, Montagne-Wajer K, Roelofs D et al (2009) The fate of received sperm in the reproductive tract of a hermaphroditic snail and its implications for fertilisation. Evol Ecol 23:533–543. https://doi.org/10.1007/s10682-008-9253-5
Koene JM, Sloot W, Montagne-Wajer K et al (2010) Male accessory gland protein reduces egg laying in a simultaneous hermaphrodite. PLoS One 5:e10117. https://doi.org/10.1371/journal.pone.0010117
Kopp KC, Wolff K, Jokela J (2012) Natural range expansion and human-assisted introduction leave different genetic signatures in a hermaphroditic freshwater snail. Evol Ecol 26:483–498. https://doi.org/10.1007/s10682-011-9504-8
Lively CM (1987) Evidence from a New Zealand snail for the maintenance of sex by parasitism. Nature 328:519–521. https://doi.org/10.1038/328519a0
Lively CM (2010) The effect of host genetic diversity on disease spread. Am Nat 175:E149–E152. https://doi.org/10.1086/652430
Lodi M, Meijer FW, Koene JM (2017) Ejaculates are not used as nuptial gifts in simultaneously hermaphroditic snails. Zoology 123:30–36. https://doi.org/10.1016/j.zool.2017.05.007
Lonhart SI, Jeppesen R, Beas-Luna R (2019) Shifts in the distribution and abundance of coastal marine species along the eastern Pacific Ocean during marine heatwaves from 2013 to 2018. Mar Biodivers Rec 12:13. https://doi.org/10.1186/s41200-019-0171-8
Lounnas M, Correa AC, Vázquez AA et al (2017a) Self-fertilization, long-distance flash invasion and biogeography shape the population structure of Pseudosuccinea columella at the worldwide scale. Mol Ecol 26:887–903. https://doi.org/10.1111/mec.13984
Lounnas M, Vázquez AA, Alda P (2017b) Isolation, characterization and population-genetic analysis of microsatellite loci in the freshwater snail Galba cubensis (Lymnaeidae). J Mollus Stud 83:63–68. https://doi.org/10.1093/mollus/eyw041
Lounnas M, Correa AC, Alda P et al (2018) Population structure and genetic diversity in the invasive freshwater snail Galba schirazensis. Can J Zool 96(5):425–435. https://doi.org/10.1139/cjz-2016-0319
Marois R, Croll RP (1991) Hatching asynchrony within the egg mass of the pond snail, Lymnaea stagnalis. Invertebr Reprod Dev 19:139–146. https://doi.org/10.1080/07924259.1991.9672167
Meunier C, Tirard C, Hurtrez-Boussès S et al (2001) Lack of molluscan host diversity and the transmission of an emerging parasitic disease in Bolivia. Mol Ecol 10:1333–1340. https://doi.org/10.1046/j.1365-294x.2001.01284.x
Meunier C, Hurtrez-Boussès S, Durand P et al (2004) Small effective population sizes in a widespread selfing species, Lymnaea truncatula (Gastropoda: Pulmonata). Mol Ecol 13:2535–2543. https://doi.org/10.1111/j.1365-294X.2004.02242.x
Miller MR, Dunham JP, Amores A, Cresko WA, Johnson EA (2007) Rapid and cost-effective polymorphism identification and genotyping using restriction site associated DNA (RAD) markers. Genome Res 17(2):240–248. https://doi.org/10.1101/gr.5681207. Epub 2006 Dec 22. PMID: 17189378; PMCID: PMC1781356
Moussaoui R, Verdel K, Benbellil-Tafoughalt S et al (2018) Female behaviour prior to additional sperm receipt in the hermaphroditic pond snail Lymnaea stagnalis. Invertebr Reprod Dev 62:82–91. https://doi.org/10.1080/07924259.2017.1415988
Mundt CC (2002) Use of multiline cultivars and cultivar mixtures for disease management. Annu Rev Phytopathol 40:381–410. https://doi.org/10.1146/annurev.phyto.40.011402.113723
Nakadera Y, Blom C, Koene JM (2014) Duration of sperm storage in the simultaneous hermaphrodite Lymnaea stagnalis. J Mollus Stud 80:1–7. https://doi.org/10.1093/mollus/eyt049
Nakadera Y, Mariën J, Van Straalen NM et al (2017) Multiple mating in natural populations of a simultaneous hermaphrodite, Lymnaea stagnalis. J Mollus Stud 83:56–62. https://doi.org/10.1093/mollus/eyw043
Nakadera Y, Giannakara A, Ramm SA (2019) Plastic expression of seminal fluid protein genes in a simultaneously hermaphroditic snail. Behav Ecol 30(4):904–913. https://doi.org/10.1093/beheco/arz027
Nicot A, Dubois MP, Debain C (2008) Characterization of 15 microsatellite loci in the pulmonate snail Pseudosuccinea columella (Mollusca, Gastropoda). Mol Ecol Resour 8:1281–1284. https://doi.org/10.1111/j.1755-0998.2007.02065.x
Noël E, Jarne P, Glémin S et al (2017) Experimental evidence for the negative effects of self-fertilization on the adaptive potential of populations. Curr Biol 27:237–242. https://doi.org/10.1016/j.cub.2016.11.015
Noël E, Fruitet E, Lelaurin D et al (2019) Sexual selection and inbreeding: two efficient ways to limit the accumulation of deleterious mutations. Evolution Letters 3:80–92. https://doi.org/10.1002/evl3.93
Patlar B, Weber M, Temizyürek T et al (2020) Seminal fluid-mediated manipulation of post-mating behavior in a simultaneous hermaphrodite. Curr Biol 30:143–149.e4. https://doi.org/10.1016/j.cub.2019.11.018
Puurtinen M, Emily Knott K, Suonpää S et al (2007) Predominance of outcrossing in Lymnaea stagnalis despite low apparent fitness costs of self-fertilization. J Evol Biol 20:901–912. https://doi.org/10.1111/j.1420-9101.2007.01312.x
Rogers D, Chase R (2001) Dart receipt promotes sperm storage in the garden snail Helix aspersa. Behav Ecol Sociobiol 50:122–127. https://doi.org/10.1007/s002650100345
Schärer L (2009) Tests of sex allocation theory in simultaneously hermaphroditic animals. Evolution 63:1377–1405. https://doi.org/10.1111/j.1558-5646.2009.00669.x
Slotte T, Hazzouri KM, Ågren JA et al (2013) The Capsella rubella genome and the genomic consequences of rapid mating system evolution. Nat Genet 45:831–835. https://doi.org/10.1038/ng.2669
Slowinski SP, Morran LT, Parrish RC (2016) Coevolutionary interactions with parasites constrain the spread of self-fertilization into outcrossing host populations. Evolution 70:2632–2639. https://doi.org/10.1111/evo.13048
Sukumaran J, Knowles LL (2017) Multispecies coalescent delimits structure, not species. Proc Natl Acad Sci U S A 114:1607–1612. https://doi.org/10.1073/pnas.1607921114
Swart EM, Starkloff NC, Ypenburg S et al (2020) The effect of mating on female reproduction across hermaphroditic freshwater snails. Invertebr Biol 139(1):e12275. https://doi.org/10.1111/ivb.12275
Tang C, Toomajian C, Sherman-Broyles et al (2007) The evolution of Selfing in Arabidopsis thaliana. Science 317:1070–1072. https://doi.org/10.1126/science.1143153
Ter Maat A, Lodder JC, Wilbrink M (1983) Induction of egg-laying in the pond snail Lymnaea stagnalis by environmental stimulation of the release of ovulation hormone from the caudo-dorsal cells. Int J Invert Repr 6:239–247. https://doi.org/10.1080/01651269.1983.10510048
Trouvé S, Renaud F, Durand P (1996) Selfing and outcrossing in a parasitic hermaphrodite helminth (Trematoda, Echinostomatidae). Heredity 77:1–8. https://doi.org/10.1038/hdy.1996.101
Trouvé S, Degen L, Renaud F et al (2003) Evolutionary implications of a high selfing rate in the freshwater snail Lymnaea truncatula. Evolution 57:2303–2314. https://doi.org/10.1554/02-452
van Duivenboden YA (1983) Transfer of semen accelerates the onset of egg-laying in female copulants of the hermaphrodite freshwater snail, Lymnaea stagnalis. Int J Invert Repr 6:249–257. https://doi.org/10.1080/01651269.1983.10510049
Van Duivenboden YA, Ter Maat A (1985) Masculinity and receptivity in the hermaphrodite pond snail, Lymnaea stagnalis. Anim Behav 33:885–891. https://doi.org/10.1016/S0003-3472(85)80022-1
Van Leeuwen CHA, Van der Velde G, Van Groenendael JM et al (2012) Gut travellers: internal dispersal of aquatic organisms by waterfowl. J Biogeogr 39:2031–2040. https://doi.org/10.1111/jbi.12004
Van Leeuwen CHA, Huig N, Van Der Velde G et al (2013) How did this snail get here? Several dispersal vectors inferred for an aquatic invasive species. Freshw Biol 58:88–99. https://doi.org/10.1111/fwb.12041
Verhoeven KJ, Biere A (2013) Geographic parthenogenesis and plant-enemy interactions in the common dandelion. BMC Evol Biol 13:23. https://doi.org/10.1186/1471-2148-13-23
Zhu Y, Chen H, Fan J (2000) Genetic diversity and disease control in rice. Nature 406:718–722. https://doi.org/10.1038/35021046
Acknowledgments
We thank Jean-Pierre Pointier for providing drawings, references, and insights to this chapter.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bonel, N. et al. (2023). Reproductive Strategies, Genetic Diversity, and Invasive Ability in Lymnaeidae. In: Vinarski, M.V., Vázquez, A.A. (eds) The Lymnaeidae. Zoological Monographs, vol 7. Springer, Cham. https://doi.org/10.1007/978-3-031-30292-3_10
Download citation
DOI: https://doi.org/10.1007/978-3-031-30292-3_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-30291-6
Online ISBN: 978-3-031-30292-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)