Abstract
Transfusion related acute lung injury (TRALI) is a clinical syndrome with non-cardiogenic pulmonary edema and hypoxemia. In the final criteria (2019), TRALI was divided into type 1 (without ARDS risk factor) and type 2 (with ARDS risk factor or diagnosis). TRALI is mainly caused by the accumulation of neutrophils in the pulmonary vascular bed and their migration to the interstitium. Inflammatory processes that may exist in the patient due to various reasons (such as surgery, infection) significantly increase the likelihood of TRALI development. TRALI treatment mainly consists of supportive treatments. As TRALI causes acute hypoxemic respiratory failure, respiratory support can be provided with noninvasive mechanical ventilation as in other causes of respiratory failure.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
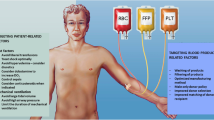
Transfusion related acute lung injury (TRALI), one of the most common complications of blood and blood product transfusions, is a clinical syndrome with non-cardiogenic pulmonary edema and hypoxemia occurring during or after transfusion. TRALI was first described by Bernard in 1951 [1]. A standardized definition was made at the Canadian Consensus Conference (CCC) in 2004, and standardization was achieved in the diagnosis of the disease and in research with the determined diagnostic criteria. [2]. In the conference, it was divided into TRALI and possible TRALI (pTRALI) according to whether there is a risk factor for ARDS. The CCC criteria were reviewed in 2019 by an international expert panel and new diagnostic criteria were published [3]. In the final criteria, the term “possible TRALI” was removed and instead divided into TRALI type 1 (without ARDS risk factor) and TRALI type 2 (with ARDS risk factor or diagnosis).
2 Pathogenesis of TRALI
TRALI is mainly caused by the accumulation of neutrophils in the pulmonary vascular bed and their migration to the interstitium. Normally, the passage of neutrophils through the lung capillary network takes place within a certain period of time, as the cells change shape and compress themselves, making their size suitable for passage. Neutrophils must be activated to slow their passage through the lung capillaries and to be sequestered. Activated neutrophils lose their ability to change their shape for passage and become bound to capillary endothelium. However, they can also be activated by stimulated endothelium and subsequently attached to the endothelium. As a result, the attachment of neutrophils to the lung capillary endothelium initiates the development of TRALI. Triggers that activate neutrophils and endothelial cells are human leucocyte antigen (HLA) antibodies, human neutrophil antigen (HNA) antibodies, bioactive lipids, chemokines/cytokines, and neutrophil binding antibodies in the donor blood. Their presence and amount are directly proportional to the probability of TRALI development. However, inflammatory processes that may exist in the patient due to various reasons (such as surgery, infection) significantly increase the likelihood of TRALI development [4, 5].
3 Risk Factors
3.1 Conditions Associated with TRALI [3]
Sepsis, noncardiogenic shock, massive transfusion, cardiac surgery, increased pretransfusion plasma IL-8, mechanical ventilation with peak airway pressure >30 cmH2O, chronic alcohol abuse, current smoker, positive fluid balance, higher APACHE II score, increased age, end-stage liver disease, postpartum hemorrhage, liver transplantation surgery, thrombotic microangiopathy, surgery requiring multiple transfusions, and hematologic malignancy.
3.2 Transfusion Risk Factors for TRALI [3]
Cognate HLA Class II antibody, cognate HNA antibody, granulocyte antibody positive by granulocyte immunofluorescence test (GIFT), cognate anti-HLA Class I that activates cells as shown (for example, by granulocyte aggregation in vitro or at least by a positive by GIFT result), higher volume of female plasma.
4 Diagnosis and Clinical Manifestations
TRALI remained a clinical diagnosis in the last definition [3]. The 2019 diagnostic criteria, for which the term pTRALI has been abandoned and replaced by the terms type 1 and type 2 TRALI, are given in Table 48.1. Accordingly, type 1 TRALI defines patients without ARDS risk factors, while type 2 TRALI defines TRALI cases in patients with ARDS risk factors or a diagnosis of mild ARDS. Patients with acute onset of hypoxemia (P/F ≤ 300 or SpO2 < 90% on room air) and bilateral pulmonary infiltrates during or within 6 h of transfusion, no signs of left atrial hypertension (even if present, not the main factor) and no temporal relationship with other ARDS risk factors are diagnosed with TRALI type 1. In the presence of respiratory distress and bilateral lung infiltrates (not of cardiac origin) that are stable for 12 h before transfusion and worsen in the next 6 h, TRALI type 2 is diagnosed in cases with ARDS risk factors but without ARDS or in cases with mild ARDS.
It is important to make the differential diagnosis of respiratory distress developing after transfusion. Before TRALI is diagnosed, other causes of post-transfusion respiratory distress (transfusion-associated circulatory overload (TACO), transfusion-associated dyspnea (TAD), transfusion-related bacterial sepsis, and severe allergic reactions) should be excluded.
5 Management
TRALI treatment mainly consists of supportive treatments. Hypoxemia, which is the cause of respiratory distress, should be eliminated with oxygen support, nasal high-flow oxygen therapy, noninvasive ventilation and invasive mechanical ventilation when necessary. However, to prevent secondary damage due to mechanical ventilation, treatment should be arranged in accordance with lung protective ventilation rules. Ventilation should be managed so that the plateau pressure does not exceed 30 cmH2O.
6 Physiological Basis for Noninvasive Ventilation in TRALI
As TRALI causes acute hypoxemic respiratory failure, respiratory support can be provided with noninvasive mechanical ventilation as in other causes of respiratory failure. With the application of noninvasive mechanical ventilation, respiratory effort and work of breathing decrease, lung compliance and tidal volume increase, and alveolocapillary gas exchange and arterial blood oxygen content can improve.
7 Evidence for NIV Use in TRALI
Specific clinical studies investigating the effect of NIV use in TRALI are lacking.
Patel et al. [6] investigated the effect of NIV applications (face mask vs. helmet) on intubation rates in ARDS patients. The randomized controlled trial, which was planned to include 206 patients, was terminated early when 83 patients were reached, as it was found that patients who received NIV with a helmet significantly reduced intubation rates compared to patients who used a face mask. Patients with helmets had better results in terms of number of ventilator free days, length of stay in intensive care unit, hospital and 90-day mortality. The 1-year results of these patients were published in a separate article by the same group [7]. In the face mask group, higher rates of ICU acquired weakness and lower rates of functional independence were found at discharge from the hospital. One-year mortality was found to be higher in the face mask group. In the first year, it was determined that the patients in the helmet group had better functional independence and the number of days away from the hospital.
In a prospective, randomized, controlled, multicenter study conducted by He et al. [8], NIV administration in patients with early mild ARDS was compared with conventional oxygen therapy and the need for invasive ventilation was investigated. In the study in which 21 centers participated and 200 patients were included, no significant difference was found between the groups in terms of invasive ventilation requirement. There was no difference between the groups in terms of intensive care mortality.
Xu et al. [9] reviewed studies comparing noninvasive ventilation and standard oxygen therapy in patients diagnosed with acute lung injury. A total of 1886 patients were evaluated in the meta-analysis, which included 17 studies published between 2000 and 2015. In the studies, noninvasive ventilation modes were applied as CPAP or BiPAP. After the analysis, it was concluded that NIV reduces intubation rates, shortens the length of intensive care stay, and decreases intensive care and hospital mortality.
Coudroy et al. [10] investigated the effect of the difference between NIV administration protocols on the outcome in patients with respiratory failure. Seven hundred fifty patients from 14 studies were analyzed, and they concluded that longer NIV duration did not make a difference in intubation rates. It was concluded that the ventilator type did not improve intubation rates; however, lower intubation rates were obtained in cases with high PEEP compared to low PEEP.
In the multicenter randomized controlled study conducted by Zhan et al. [11], a total of 40 patients from 10 centers were randomized. The effect of NIV and conventional oxygen therapy on intubation rates in early stage ARDS patients was investigated. Intubation rates and organ failure were found to be lower in the NIV group.
8 Conclusions
The ICU management of a critically ill patient with TRALI should include a lung protective strategy. Noninvasive ventilation is safe for selected patients with acute lung injury and might have potential beneficial effects by reducing the incidence of organ failure. Early identification, timely NIV approach, and careful hemodynamic monitoring should be warranted in complicated cases.
References
Barnard RD. Indiscriminate transfusion: a critique of case reports illustrating hypersensitivity reactions. N Y State J Med. 1951;51(20):2399–402.
Kleinman S, Caulfield T, Chan P, Davenport R, McFarland J, McPhedran S, et al. Toward an understanding of transfusion-related acute lung injury: statement of a consensus panel. Transfusion. 2004;44(12):1774–89.
Vlaar APJ, Toy P, Fung M, Looney MR, Juffermans NP, Bux J, et al. A consensus redefinition of transfusion-related acute lung injury. Transfusion. 2019;59(7):2465–76.
Bux J, Sachs UJ. The pathogenesis of transfusion-related acute lung injury (TRALI). Br J Haematol. 2007;136(6):788–99.
Roubinian NH, Triulzi DJ. Transfusion-associated circulatory overload and transfusion-related acute lung injury: etiology and prevention. Hematol Oncol Clin North Am. 2019;33(5):767–79.
Patel BK, Wolfe KS, Pohlman AS, Hall JB, Kress JP. Effect of noninvasive ventilation delivered by helmet vs. face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2016;315(22):2435–41.
Patel BK, Wolfe KS, MacKenzie EL, Salem D, Esbrook CL, Pawlik AJ, et al. One-year outcomes in patients with acute respiratory distress syndrome enrolled in a randomized clinical trial of helmet versus facemask noninvasive ventilation. Crit Care Med. 2018;46(7):1078–84.
He H, Sun B, Liang L, Li Y, Wang H, Wei L, et al. A multicenter RCT of noninvasive ventilation in pneumonia-induced early mild acute respiratory distress syndrome. Crit Care. 2019;23(1):300.
Xu X, Yuan B, Liang Q, Hu J, Shi Z, Huang H, et al. Noninvasive ventilation for acute lung injury a meta-analysis of randomized controlled trials. Heart Lung. 2016;45(3):249–57.
Coudroy R, Hoppe MA, Robert R, Frat JP, Thille AW. Influence of noninvasive ventilation protocol on intubation rates in subjects with De novo respiratory failure. Respir Care. 2020;65(4):525–34.
Zhan Q, Sun B, Liang L, Yan X, Zhang L, Yang J, et al. Early use of noninvasive positive pressure ventilation for acute lung injury: a multicenter randomized controlled trial. Crit Care Med. 2012;40(2):455–60.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kayhan, O., Demirkiran, O. (2023). Noninvasive Ventilation in Transfusion Related Acute Lung Injury. In: Esquinas, A.M. (eds) Noninvasive Mechanical Ventilation. Springer, Cham. https://doi.org/10.1007/978-3-031-28963-7_48
Download citation
DOI: https://doi.org/10.1007/978-3-031-28963-7_48
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-28962-0
Online ISBN: 978-3-031-28963-7
eBook Packages: MedicineMedicine (R0)