Abstract
Recently, nano-engineered two-dimensional (2D) materials have gained immense interest in various applications, including CO2 capture. The precise atomic structure of 2D nanomaterials introduced various significant characteristics required for specific applications. Increasing levels of CO2 in the environment is a concerning topic for surviving a sustainable life on Earth. Therefore, CO2 capture and conversion into useful products have been recognized as the best approach to reduce the CO2 level in the atmosphere. To capture CO2, several materials have been studied and emphasised about their advantages and disadvantages. The recent progress in 2D materials, especially graphene-based materials, has shown their potential in CO2 capture. Graphene-based materials, transition metal dichalcogenides (TMDCs), 2D transition metal oxides (TMOs), MXenes, boron nitrides, carbon nitrides, 2D metal–organic frameworks (MOFs) etc., are the various examples of 2D materials, which have been investigated for CO2 capture. This chapter aims to provide a brief overview of the recent advantages in the nano-engineering of the various 2D materials for CO2 capture. In particular, the recent development of emerging strategies such as doping, defects engineering, hetero-structural designing, and architectural functionalization of 2D nanomaterials for enhanced CO2 capture are discussed thoroughly. The challenges and future outcomes have also been highlighted, which will open the directions for future research.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
14.1 Introduction
Industrial modernisation and increased living standards have deteriorated environmental health. The ever-increasing demand and consumption of energy leading to the sharp rise of CO2 concentration in the environment globally, which is directly or indirectly responsible for the deteriorating climate situation and global warming. CO2 is one of the prime greenhouse gases (GHGs), and its high levels are not beneficial for the health of planet Earth. Several agencies noted that the CO2 level in the atmosphere has surpassed the 415 ppm, which is the maximum record yet and alarming the current situation [1]. IPCC (International Panel on Climate Change) has also predicted that by 2100, the level of CO2 will reach 590 ppm, which will raise the global temperature by 1.90 °C [2]. This exponential rise of CO2 in the atmosphere can cause severe adverse effects on the planet, such as glaciers melting, and ice melting on Earth’s poles, which further raise the sea level [3]. The constant rise in global temperature can significantly affect the living survival on Earth. Therefore, there is a need to control the emission of CO2 in the atmosphere [4] and to improve the research and development techniques for CO2 capture and storage (CCS) [5] and convert the captured CO2 into value-added products via different conversion routes [6].
Industrial CO2 emission is the major source of CO2 in the environment via pre-combustion or post-combustion treatments. To control the environmental CO2 concentration, there is a foremost need to develop technologies to capture it from the atmosphere. Adsorption, absorption and membrane separation are the most applicable methods for CO2 capture. However, except for adsorption, all other methods feature drawbacks such as corrosion, long processing period, high cost, and the generation of other harmful side products [7, 8]. Therefore, CO2 capture via adsorption has attracted the attention of researchers globally. Several materials such as ionic liquids [9], polymers [10], carbon-based materials [11], zeolites [12], metal oxides [13], MXenes [14] and MOFs [15] have been proposed as favourable sorbent materials to capture the CO2 from the atmosphere. Among all the class of 2D nanomaterials, for example, graphene oxide (GO), metal dichalcogenides, carbon nitrides, metal–organic frameworks, boron nitrides, MXenes etc., has gained immense interest in various applications, including CO2 capture due to its outstanding characteristics such as high active surface area, high aspect ratio, excellent optical and mechanical properties and ease of functionalization [16]. Figure 14.1 represents the various examples of 2D nano-engineered materials used in CO2 adsorption with their unique characteristics. In the last few decades, there is an exponential growth in the advancement of the nano-engineering of 2D materials using new strategies. 2D materials were fabricated with tunable characteristics, controlled orientations, and doped or combined with several other nanomaterials to synthesize 2D based nanocomposite materials with improved CO2 capturing capacity.
Several review articles have been published on CO2 capture and conversion with a prime focus on the various technologies or using specific materials [5, 17,18,19,20]. For instance, the progress in using various 2D nano-engineered materials for CO2 capture has been addressed. Recently, the significance of 2D nanomaterials such as graphene, boron nitrides, carbon nitrides, MXenes and metal chalcogenides have been proven to be successful candidates for the CO2 capture. Further, in several reports, the analysis of the engineering aspects of 2D materials for improved CO2 capture has also been reported [16]. Therefore, there is a need to put the application of various 2D nano-engineered materials in one frame for better understanding for the researchers. This chapter aims to methodically review the critical developments in applying different nano-engineered 2D materials in CO2 capture. The functionalization of 2D nanomaterials or preparation of 2D nanomaterial-based nanocomposites can potentially improve the CO2 capture aptitude of parent 2D nanomaterials. Additionally, the current scenario, opportunities, and challenges of applying nano-engineered 2D materials have also been discussed.
14.2 CO2: Sources and Hazardous Effect
CO2 gas is one of the major gases of GHGs emissions and causes global warming and climate change. CO2 is one of the significant environmental emissions that can be through natural sources or anthropogenic activities. Figure 14.2 shows the possible sources of CO2 emission in the environment [3]. Major natural sources of CO2 emission include the CO2 release from decomposition of biomass and vegetation, respiration, venting volcanos, ocean release and natural wildfires. Other than this, most of the CO2 is released into the environment through human activities such as burning fossil fuels, deforestation, burning solid waste and biological matters, transportation, power generation, agricultural practices and industrial release. In one survey (2014), total global CO2 emission was noticed to be 32.4 Gt CO2, out of which the energy sector contributed majorly (82%), and the burning of fossil fuels was the prime cause. Amongst fossil fuels, oil consumption was the leading cause of CO2 emission (34%), followed by coal (46%), gas fuels (1%), and the remaining was from the geothermal, nuclear, solar, hydro, biofuels, wind and waste [21]. Therefore, the ever-increasing demand for energy with the increase in population is one of the major causes of CO2 release. Increasing CO2 levels can be life-threatening to the planet, and there is a need to curb the CO2 emissions or GHGs emissions to save life on Earth.

Reproduced with permission from ref. [3]. Copyright 2022 CRC PRESS, Taylor, and Francis
Examples of several sources of CO2 emission in the environment.
The regular CO2 emission via natural and human activities is causing global warming and increasing the global temperature. IIPCC has stated that to avoid any devastating cost of CO2 emission, the increase in global temperature should be restricted to 2 °C and it is most likely achieved 1.5 °C already [22]. Global warming and drastic climate changes can negatively impact the terrestrial and ocean ecosystem, water supply, food chain, health, weather condition and economic growth (Fig. 14.3). Acid rain is one of the disastrous consequences of increased CO2 levels and can damage trees and the environment [23,24,25]. Global warming aids in melting the glaciers, particularly in Greenland and Antarctica, which adds water to oceans and seas. This exercise increases the sea level, which can cause floods, agricultural soil contamination with salt, destructive erosion and destruct habitat for living creatures such as fish, plants and birds. It is also apparent that the gaseous exchange between air and oceans will increase the ocean CO2 concentration with the increase in atmospheric CO2. This will increase the dissolved inorganic carbon and consequently decrease the ocean surface pH, resulting in ocean acidification [26]. Ocean acidification is threatening the habitat of marine creatures and will make a negative impact on the food chain. In one study, it has been noticed that the CO2 concentration has risen from 378 to 410 ppm in the span of 14 years (i.e. 2005–2020), which is a clear indication that more drastic weather conditions are about to come [27, 28].
Therefore, there is an urgent need to curb CO2 or GHGs emissions into the environment. Nowadays, several strategies are adopted to reduce atmospheric CO2 levels, such as (a) absorption/adsorption of atmospheric CO2, (b) progressive adaptation of renewable energy sources, (c) CCS and (c) CO2 capture and utilization (CCU). Various porous materials, such as zeolites, can be considered as possible adsorbents for CO2 capture, but the poor selectivity limits their industrial applications. In this regard, 2D nanomaterials have been fabricated to improve CO2 adsorption/absorption and selectivity. The following sections will describe the fundamentals and mechanism of CO2 capture using various 2D nanomaterials.
14.3 Mechanism of Carbon Dioxide Capture
Carbon dioxide capture is an important process that has been developed to reduce the amount of carbon dioxide emissions released into the atmosphere. Carbon dioxide capture involves trapping and storing carbon in various ways, including capturing it from industrial sources before it is emitted into the atmosphere or by directly removing it from ambient air. This technology can help mitigate climate change and reduce our dependence on fossil fuels for energy production. The most widely used form of carbon dioxide capture is direct air capture (DAC). DAC systems use filters to trap CO2 molecules as they pass through them, which are then stored in tanks or other containers until they can be processed further for reuse or disposal. Other methods include pre-combustion techniques and oxyfuel combustion, which uses chemical absorption processes like amine scrubbing. These technologies have proven effective at reducing CO2 emissions but require significant investment in infrastructure costs due to their complexity and costliness relative to other emission reduction strategies like renewable energy sources or efficiency improvements within existing facilities.
Overall, while there are many different mechanisms available for capturing carbon dioxide emissions before they enter Earth’s atmosphere, each one requires careful consideration when evaluating its potential effectiveness against environmental goals while also taking into account any associated economic implications related with implementation costs & benefits over time. Currently, adsorption is the most viable technology for CO2 capture due to its straightforward operation and low cost. Adsorption occurs in two ways- strong chemical interactions (chemisorption) and weak physical interactions (physisorption) [29, 30]. Solid adsorbents benefit from the lack of substantial amounts of water/solvent compared to aqueous absorbents (use amine-based solvents), which results in less energy being used during CO2 desorption. In addition, solid adsorbents have lower heat capacities than aqueous ones, reducing the energy needed to heat the adsorbent to desorb the CO2. The solid adsorbents also offer fast kinetics of CO2 adsorption/desorption and effortless operation [29]. Because of these advantages, development of novel adsorbents with high efficiency are utmost importance for remediation of CO2 from the atmosphere. Figure 14.4 illustrates the various kinds of interactions taking place during CO2 adsorption.
Solid adsorbents are further classified into three ways: low-temperature adsorbents (less than 200 °C), mid-temperature adsorbents (200–400 °C), and high-temperature adsorbents (greater than 400 °C) [31]. The low temperature adsorbents follow physisorption mechanism through electrostatic or van der Waals forces. Because of low interaction energy, desorption and active site regeneration can be done at low temperature conditions, resulting in lower energy and cost requirements. However, the major drawback of low temperature adsorbent is poor selectivity towards CO2 [30]. Moreover, flue gas stream contaminants (such as NOX, SOX, particulate matter and water vapour) can deactivate the sorption site for CO2 or out-compete CO2. Furthermore, water vapours can selectively adsorb over CO2 on low-temperature adsorbents, resulting in poor adsorbent stability due to deactivation [30]. Contrarily, high-temperature adsorbents offer better CO2 selectivity and thus, they have been used in DAC conditions [32]. However, to replenish these adsorbents, a cyclic carbonation/decarbonation process is needed at high temperatures. In contrast, mid-temperature adsorbents provide reasonable selectivity as well as high performance for CO2 capture. Additionally, these adsorbents are suitable for effective CO2 capture and sequential catalytic conversion as they operate in the same temperature window [33].
14.4 Capture of CO2 Using Nano-engineered 2D Materials
Regular emission of toxic and greenhouse gases into the environment has become a threat to the healthy planet. CO2 is one of the major greenhouse gases released into the atmosphere via several natural and anthropogenic sources. The healthy management of these gases in the atmosphere is one of the essential requirements to balance survival. Several routes have been adopted to lower the CO2 level, such as (a) control of the CO2 emission, (b) CCS and (c) CCU conversion and utilization. Various 2D nano-engineered materials have been proven as suitable candidates for CO2 capture and conversion. Lately, 2D materials such as graphene and graphene-based nanomaterials, 2D TMDCs, h-BN, 2D TMOs, MXenes, 2D MOFs, 2D covalent organic frameworks (COFs), g-C3N4, borophene, phosphorenes, nanoclays, etc. have been employed for CO2 capture, (Fig. 14.1) [34]. The subsequent sections will discuss role of these 2D materials for CO2 capture in details.
14.4.1 Graphene and Graphene-Based Nanomaterials
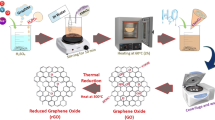
Since the discovery of graphene, it has been considered as an active and exciting material in wide range of applications such as lubrication, catalysis, supercapacitors, tissue engineering, 3D printing, sensors, desalination, water and air purification, drug delivery, solar cells and molecular imaging [35,36,37,38]. Graphene based materials such as graphene oxide (GO) and reduced graphene oxide (rGO) have also been widely used in the CO2 capture as adsorbents and absorbents due to its favourable properties [39]. Graphene exhibit a 2D hexagonal morphology, which resembles more like a honeycomb network and provides extraordinary physicochemical, mechanical, thermal and electrical properties for its high efficiency in a wide range of applications [40, 41]. Theoretical graphene nanosheets demonstrate a very high active surface area (~2630 m2.g−1), which is one of the key characteristics for adsorption/absorption applications. Additionally, the high porosity of graphene-based materials, high thermal and chemical stability, and ease of functionalization of graphene nanosheets open the infinite possibilities to construct an adsorbent/absorbent with high CO2 capture efficiency and selectivity [42]. The oxygen functionalities on the GO make its surface basic in nature, which attracts the CO2, which is acidic in nature and helps in CO2 adsorption. Moreover, the oxygen functionalities on GO surface open the prominent platform for easy modification of the adsorbent surface to enhance CO2 adsorption.
Graphite and GO have been modified via several routes with different functional groups to enhance their capability for gas storage. Exfoliated rGO is one of the popular graphene derivatives in CO2 capture and exists in the stacked few layers of defected graphene sheets with fewer oxygen functionalities. The route of GO reduction to prepare rGO also significantly affects the CO2 adsorption capacity. As chemical/thermal reduction reduces the CO2 adsorption capacity of rGO by 1.5 mmol.g−1 than GO at ambient conditions [43], whereas on reducing GO hydrothermally, the CO2 adsorption capacity enhances by 2.4 mmol.g−1 at ambient pressure and 0 °C [44]. Additionally, on exfoliating the graphene or graphene derivatives sheets, the CO2 adsorption efficiency increases due to exposure to a more active surface. Molecular dynamics simulation studies have also proven that under the assistance of H2O molecules and the presence of active functional groups on the GO, surface enhances the CO2 adsorption from the environment [45]. Further, the adsorption of CO2 can be promoted by the functionalization and doping of the graphene sheets. CO2 adsorption efficiency of rGO was compared with boron-doped rGO (B/rGO) under identical conditions. Doped boron significantly improves the adsorption characteristics of rGO from 1.3 mmol.g−1 to 1.8 mmol.g−1 [43]. Theoretical simulation studies reveal that the CO2 molecule adsorbed on the graphene sheets with a parallel orientation and the distance between the graphene sheets and CO2 was found to be 0.345–0.36 nm, which is attributed to the van der Waals interactions.
Doping or functionalizing graphene and its derivatives with nitrogen or nitrogen-based functional groups is another way to improve CO2 adsorption efficiency. Polyaniline/hydrogen exfoliated graphene (PANI/HEG) based nanocomposite materials have excellent applications as CO2 adsorbents [46]. PANI/HEG could adsorb 75 mmol.g−1 CO2 at room temperate and 11 bar pressure, which was almost 3.5 times higher than the CO2 adsorbed by HEG (21.6 mmol.g−1) only [47]. FTIR studies of CO2-adsorbed PANI/HEG revealed that the interactions between the CO2 and nitrogen atom present on adsorbent are majorly chemical interactions. Additionally, PANI/HEG also showed excellent recyclability, with 2–3% less adsorption efficiency. Amine functionalized graphene is one of the most popular graphene based adsorbent material, which has been investigated to capture the CO2. Nucleophilic nature of amines strongly interacts with the electrophilic CO2 and improve the CO2 adsorption efficiency of GO. Shin et al. used polyethyleneimine (PEI) as an amine source to functionalize the GO and used it as an adsorbent material for CO2 capture [48]. The amine-functionalized GO has shown an excellent adsorption capacity (84 mg.g−1) for CO2 adsorption, which was attributed to the appropriate PEI concentration and improved the electron donor and acceptor interaction between the adsorbate and adsorbent. Additionally, the loading of PEI on GO surface also enhances the selectivity of CO2 adsorption over N2 (Fig. 14.5). However, the excess loading of PEI further reduces the CO2 capacity, which might be due to the unavailability of the active sites of the GO surface.

Reproduced with permission from ref. [48]. Copyright 2016, Elsevier Publications
Adsorption curves of a GO and b PEI-GO for CO2 and N2 gases and c amount of CO2 and N2 gases adsorbed on GO and various PEI functionalized GO.
In another report, TEPA (Tetraethylenepentamine) was employed as an amine precursor to functionalize the GO to improve the CO2 uptake [49]. Ultrasonic waves were used to activate the TEPA functionalized GO for CO2 adsorption studies. Ultrasonic-activated TEPA-GO has shown higher CO2 adsorption capacity (1.2 mmol.g−1) than bare GO (0.3 mmol.g−1) under identical conditions. This might be due to ultrasonic waves assisted in the exfoliation of graphene layers in the adsorbent material and provided a large number of active sites with functional groups for CO2 adsorption. Consequently, the nucleophilic amine in the adsorbent material (TEPA-GO) interacts with electrophilic CO2 efficiently and performs excellently. TEPA-GO also display excellent recycling stability, which is suitable for practical applications.
Various graphene-based nanocomposite materials have also been investigated for CO2 capture. In one study, Pokhrel et al. designed novel nanocomposite compromises of GO, Zeolite imidazolate framework (ZIF-8), and amine functional groups, which was present due to functionalization with 3-aminopropyl triethylsilane (APTES), PEI and ethylene diamine (ED) [50]. The amine groups inserted in the GO layers increased the interlayer spacing, which helped in improving CO2 adsorption. Among all amine, functional groups precursors, APTES-modified GO-based nanocomposite showed the highest adsorption efficiency with a 36% increase in CO2 adsorption capacity compared to unmodified GO. Additionally, the adsorption efficiency of APTES-modified GO nanocomposite increased in pre-adsorbed water conditions (10% RH), and successfully adsorbed 33% extra CO2 than in dry adsorption conditions. This might be due to the generation of bicarbonates in the presence of moisture which provides more active sites of CO2 adsorption. On comparing the ZIF-8/GO amine functionalized adsorbent with amine-modified ZIF-8,
Other than functionalized and doped graphene, 3D graphene, such as graphene-based hydrogels and aerogels, has also been studied for CO2 adsorption. The 3D structure of the graphene-based adsorbent provides a more porous architecture for the adsorption studies. Hsan et al. prepared the chitosan (CS) embedded GO aerogels (CSGO) for the adsorption of CO2 [51]. To construct crosslinking between the CS and GO, (1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride and N-hydroxy succinimide crosslinkers were used and followed by the freeze-drying process to obtain aerogel structure. CSGO was found to be excellent adsorbent material with 0.25 mmol.g−1 adsorption capacity for CO2 at 298 K and 1 bar pressure in comparison to raw CS (0.05 mmol.g−1). The presence of nitrogen contents on the aerogel skeleton improves the selective CO2 adsorption capacity. Therefore, introducing the nitrogen functionalities on the exfoliated graphene and its derivatives improved the CO2 capture efficiency.
14.4.2 2D Transition Metal Oxide-Based Nanomaterials
2D transition metal oxide (TMOs)-based nanomaterials have been explored for various applications including electrochemistry, photochemistry, energy storage, tribology, catalysis, and environmental remediation [52,53,54,55,56]. Due to their remarkable properties such as high surface area, a large number of active sites, cost-effectiveness, ease of modification, abundance availability and microstructural features, metal oxide-based nanomaterials have been considered potent competitors in adsorption applications [53]. Additionally, oxide-based adsorbent material have been found highly selective towards CO2 adsorption at high temperature conditions [57]. In dry atmospheric conditions, TMOs surface is usually terminated with O2− anions, which are typically bigger than the cationic M+. This mismatching of size creates a lower coordination of cations on the surface and results in plenty of adsorption sites [58]. Therefore, several metal oxide-based nanomaterials are used in gas adsorption. TMOs can adsorb the CO2 via physisorption or chemisorption or both (Fig. 14.6) [33]. Metal oxides usually exhibit a stronger affinity towards CO2 and form a strong chemical interaction with CO2 in the presence of other co-existing gases, which are adsorbed by weak physio-sorption only. This improves the selective adsorption application, which is indispensable for practical approaches.

Reproduced with permission from ref [33]. Copyright 2022, Elsevier Publications
CO2 adsorption on metal oxides via a chelation, b chemisorption, and c physisorption.
Magnesium oxide (MgO) and Calcium oxide (CaO) are the two most successful metal oxides for CO2 capture. Other metal oxides such as CuO, MnO2, and CeO2 were also used in CO2 capture but were comparatively less explored. For the adsorption of CO2, MgO exhibits appropriate surface morphology with oxygen, which improves the adsorption capacity and lowers the regeneration energy consumption [59, 60]. Recently Hu et al. published a detailed review focused on the strategy development of MgO-based adsorbents for CO2 capture [61]. CO2 adsorption on MgO is majorly an acid–base type reaction followed by physical interactions. The acidic CO2 reacts with the basic sites of MgO to form a complex (MgO–O-C = O) to get chemically adsorbed. Othman et al. have incorporated several metal oxide nanoparticles (MgO, MnO2, ZnO, and CaO) on activated carbon nanofibers (ACNFs) via electrospinning and pyrolysis process and examined for the CO2 capture [62]. MgO incorporated ACNFs were found to be with highest surface area (413 m2.g−1) and micropore volume (0.1777 cm3.g−1) than other metal oxide ACNFs and pristine ACNFs. CO2 adsorption capacity of MgO-ACNFs was also the highest (60 cm3.g−1) at 298 K. Also, the incorporation of MgO nanoparticles on the activated carbon-based bamboo (BAC) enhanced the BAC CO2 adsorption capacity by 112% [63]. The major driven force for the adsorption of CO2 on MgO-BAC was physical adsorption forces than chemical interactions because the chemical interactions between MgO and CO2 to form MgO–O-C = O (MgCO3) was slow than physical attractions.
Recently, alkali metal nitrates have been mixed with MgO-based nanoadsorbents to improve their efficiency in CO2 capture. Alkali metals in MgO melt during the CO2 capture process and avoid the MgCO3 formation of MgO surface [64]. Figure 14.7 demonstrates the application of molten alkali nitrates in MgO to enhance CO2 capture via carbonation [64]. NaNO3 and KNO3 are the popular nitrate promoters used in MgO as they do not react with CO2 directly [65]. However, alkali metal carbonates can also be used in place of alkali metal nitrates to improve the CO2 capture efficiency of MgO-based adsorbents. Carbonate promotors in MgO enable easier melting during the CO2 capture process, allowing the faster transport of CO2 through the carbonate layer. Kwak et al. mixed the triple eutectic alkali carbonate (TEC) (Li2CO3, Na2CO3 and K2CO3) with MgO for enhanced CO2 capture [66]. The TEC amount in MgO significantly affects the kinetics of CO2 capture. The CO2 capture process was classified into two key steps: (a) fast and large and (b) slow and small. Higher the amount of TEC promotes the step ‘a’ but does not affect step ‘b’.

Reproduced with permission from ref. [64]. Copyright 2022, Royal Society of Chemistry
Diagrammatic illustration of MgO sorbent role with nitrate salts in enhancing the CO2 capture capacity.
Other than MgO, CaO is also extensively examined metal oxide material for CO2 capture due to its fast carbonation and easy regeneration. CaO is generally used for CO2 storage and reacts with CO2 reversibly, as shown in Eq. 14.1
The adsorption of CO2 takes place at 500 °C, and the exothermic reaction knows as carbonation. However the desorption of CO2 takes place at higher temperature (800–950 °C) and the endothermic reaction is known as calcination or decarbonation [67]. The reversible adsorption and desorption of CO2 using CaO are known as chemical looping. CaO-based adsorbents are commercially used to capture the CO2 from cement plants. However, mechanical failure and excessive sintering are the significant drawbacks of CaO in CO2 capture. Once CO2 uses the initial layer of CaO to form the CaCO3, the adsorption process slows down. Several works have been proposed to improve the adsorption efficiency of CaO. On using nanosized CaO, the number of active surface sites is increased for the adsorption studies. Florin et al. derived the CaO from nanosized CaCO3 and used it for CO2 capture [68]. The prepared CaO was exposed for 5 carbonation cycles (each cycle was run for 24 h) with no morphological impediment. Also, the material was used for 100 CO2 carbonation and decarbonisation cycles (20 min for each cycle), representing the potential of nano CaO for CO2 capture [68]. In summary, MgO and CaO are the most explored transition metal oxide-based nanomaterials for CO2 capture, whereas there is a need to examine other metal oxides also.
14.4.3 MXenes
Beyond graphene, transition metal carbon/nitride (MXene) is another class of 2D material that has drawn a lot of interest from research communities. By carefully etching the A layer from MAX phases, where M is a transition metal, A is a IIIA or IVA element, and X is C or N, MXenes have been synthesized [69]. Due to the high corrosivity of hydrofluoric acid, it has been employed to etch the covalently bonded IIIA or IVA element [70]. To avoid the toxicity of hydrofluoric acid, alternative environmentally friendly processes have also been implemented, including alkali treatment, Lewis acid etching, electrochemical etching and so on [71]. MXenes have received enormous attention for their functionality and characteristics in electrochemical charge storage, environmental remediation, electromagnetic interference shielding, catalysts and other applications [72]. Owing to high chemical and mechanical stability with outstanding thermal and electrical conductivities, it offers requisite features for CO2 activation/conversion, capture and storage. Various termination functionalities (−O, OH, halogens, chalcogens or their mixtures) of MXenes make them attractive materials for adsorption processes. Additionally, these materials can be produced with high surface areas in range of 250–1000 m2/g that provide high reactive sites and porous functionalities for CO2 capture and conversion [70]. Importantly, it was noticed that MXenes have higher adsorption energies compare to other 2D materials [73].
CO2 adsorption on MXenes depends on many factors such as the thickness of sheets, defects, specific surface area, lone pair electrons and presence of CO2-philic groups on the surface [74]. For example, Viñes et al. used 2D MXene, which has the general formula Mn+1XnTx (M = Ti, Zr, Hf, V, Nb, Ta, Cr, Mo, W, n = 1, 2, 3, 4), for effective CO2 removal [75]. The maximum CO2 abatement rate could be 8.25 mol CO2 per kg MXene. Studied MXenes followed the given order for CO2 capture: Ti2CTx > V2CTx > Zr2CTx > Nb2CTx > Mo2CTx > Hr2CTx > Ta2CTx > W2CTx. They exhibited high adsorption capacity despite of low CO2 partial pressure and high temperature. The higher adsorption energy, large surface area, MXene/CO2 charge transfer, and abundant adsorption sites could be the causes of the greater ability of MXenes to capture CO2. The study summarized that the adsorption strength and conformation had the greatest influence on CO2 desorption. In another study, dimethylsulfoxide intercalated Ti3C2Tx was prepared via wet chemistry using sodium fluoride and hydrochloric acid [76]. It demonstrated a high surface area of 66 m2.g−1 with a high volume capacity of 502 V.v−1 and exhibited a high CO2 adsorption uptake of 5.79 mmol.g−1. However, this adsorption capacity was lower than the theoretical capacity (44.2 mmol.g−1) of Ti3C2Tx with a specific surface area of 496 m2.g−1 [76]. Pristine Ti3C2Tx showed a lower surface area (21 m2.g−1) and lower CO2 adsorption capacity. It is noteworthy that tailoring the surface area of MXenes is a potential approach for enhancing the CO2 uptake. In another study, individual sheets of Ti3C2Tx carbides were employed to achieve efficient CO2 adsorption (12 mol/kg) [77].
Numerous theoretical studies have been conducted to understand the insights of the CO2 adsorption process on MXenes. Using density functional theory, Morales-Garcia et al. proposed that there was insignificant effect of a number of MXene sheets on CO2 adsorption [78]. Using first-principles calculations, Sun et al. proposed the surface lone pair of MXenes account for high CO2 absorption [79]. They selected nine MXene for the study such as Ti2CTx, Zr2CTx, Hf2CTx, V2CTx, Nb2CTx, Ta2CTx, Cr2CTx, Mo2CTx, and W2CTx. The CO2 adsorption by MXenes followed the IVB > VB > VIB trend, which was linked to the lone pair electrons of MXene that shift to CO2 during the absorption process. Thus, the occurrence of surface lone pairs on metals of MXenes could play a significant role in CO2 adsorption. Another study used DFT calculations to examine the CO2 adsorption and desorption on 2D M2N materials (M = Ti, Zr, Hf, V, Nb, Ta, Cr, Mo, W) [80]. M2N is better than Mo2C for effective CO2 uptake due to the presence of high electronegativity N atom layers and substantial adsorption energy (−3.13 eV).
Furthermore, MXene-based composites have been prepared to enhance the adsorption capacity for capturing CO2 gas at the different sources. For example, MXene-based solid nanocomposite (Ti3C2/PEI/BO) adsorbent was fabricated using Ti3C2(OH)x, polyethyleneimine and 1,2-butylene Oxide [81]. Ti3C2/PEI/BO adsorbent demonstrated high CO2 adsorption capacity (2.60 mmol/g) during CO2 (100%) as a purge gas at a high temperature (120 ℃). Furthermore, adsorbent also showed long-term cyclic stability and superior attrition resistance. In another study, thin-film matrix membranes were prepared by incorporating Ti3C2Tx nanosheets in Pebax-1657 (Pebax is the trade name of polyether block amide) for effective mixed gases (CO2/N2 and CO2/H2) separation [82]. The separation performance of the composite membranes exceeds Robeson’s upper bounds, while working within the target range for economical CO2 remediation. The uniform galleries of MXene produced via H-bonding interaction between MXene and rubbery Pebax might be responsible for the high CO2 capacity. Fast and specialized CO2 transport was made possible by interfacial interactions and selective Ti3C2Tx composite nanochannels. The performance mechanism of MXene/Pebax membrane for selective capturing of CO2 and N2 can be depicted in Fig. 14.8. Because CO2 has a higher quadrupole moment than N2, it more easily affiliates with the MXene surface. The surface hydroxyl groups of MXene improved CO2 capture efficiency and also resulted in highly selective adsorption of CO2. MXene galleries were ~0.35 nm in diameter, bigger than CO2 but smaller than N2 [82]. It demonstrated that N2 was constrained while CO2 was qualified to infiltrate and diffuse across the membrane. Additionally, the adsorption capacity of various 2D materials-based adsorbents at different conditions is discussed in Table 14.1.

Reproduced with permission from ref. [82]. Copyright 2020, American Chemical Society
Proposed mechanism for transporting and separating CO2 and N2 by Ti3C2Tx/Pebax membranes.
14.4.4 Hexagonal Boron Nitride (h-BN)
Hexagonal boron nitride (h-BN) nanosheet is made of sp2-conjugated boron and nitrogen atoms that create a 2D structure. It has high thermal stability (up to 800 °C), excellent thermal conductivity (300–2000 Wm.K−1), high Young’s modulus (0.8 TPa) and superior fracture strength (165 GPa) [99]. h-BN nanosheet has been prepared using both top-down and bottom-up approaches. Tunning and functionalization of pores and structures of BN via selecting appropriate precursors during synthesis is a crucial strategy to achieve excellent CO2 adsorbent. For instance, a few layered h-BN (1–4 layers) with a high surface area (927 m2.g−1) were produced chemically using boric acid and urea and found to be advantageous for CO2 adsorption [100]. Yang et al. synthesized three-dimensional (3D) flower-like BN nanosheets (FBNNSs) by template-free cylinder compressing route using boron oxide and guanidine hydrochloride as starting precursors [101]. FBNNSs demonstrated efficient CO2 adsorption up to 74.4 mg.g−1 at 1 bar, which was ascribed to the large surface area (1114 m2.g−1), pore volume (0.7 cm3.g−1), hierarchical pore availability, and plentiful edge functionalities (−OH/−NH2). The CO2 adsorption mechanism on nanosheets was governed by hydrogen bonding, and weak van der Waals interaction. FBNNSs showed high stability and performance even after 10 cycles of recyclability. Additionally, in another study, a new type of micropore-rich BN fibres was produced by introducing a hexamethylenetetramine surfactant in the conventional porous BN precursor (melamine-diborate) [93]. Because of excessive micropores, micropore-rich BN fibres showed better CO2 capture capacity (2.85 mmol.g−1) than original porous BN fibres (3.19). These thermally stable BN fibres can be reused in several cycles without loss their adsorption property. Moreover, large surface area (1900 m2.g−1) BN can be produced using multiple N precursors (mixing biuret or melamine with urea), exhibiting high adsorption capacity of 1.6 mmol.g−1 at 1 bar, 25 °C and 8.3 mmol.g−1 at 20 bar, 25 °C. More notably, CO2 adsorption capacity decreased to 1.1 mmol.g−1 (1 bar and 25 °C) after palletization [90]. C-doped BN fibre was also prepared using melamine and boric acid with final treatment at 1100 °C [102]. Excellent sorption property (3.71 mmol.g−1 at 273 K) of doped fibre was attributed to micropores (formed by doping of C) and more negative charges on BN (induced by structural defects), resulting in high CO2 affinity via chemisorption interaction. Thus, it is noteworthy that the adsorption performance of BN sheets/fibres can be improved by slight modification in their structure and composition.
To improve CO2 capture performance, BN can be engineered with metallic nanoparticles. For instance, in-situ Cu-incorporated BN nanofibers (BNNF) were prepared using BN precursors (melamine and boric acid) and Cu source (Copper dichloride dihydrate) for efficient uptake of CO2 (Fig. 14.9a) [94]. It was observed that Cu2+ ions speed up the nucleation process during the reaction between melamine and boric acid, which is discovered to be a crucial component for producing Cu@BNNF with uniform and tiny diameters (Fig. 14.9b–d). Interestingly, the presence of Cu on BNNF improves the CO2 affinity, the adsorption capacity of the BN nanofibers enhanced from 1.34 to 2.77 mmol.g−1 (1 bar and 273 K) (Fig. 14.9e). The Cu@BNNF also exhibited high recycling stability, unlike several other BN-based absorbents (Fig. 14.9f). Moreover, CO2 adsorption mechanism on Cu-loaded BNNS was investigated with density-functional theory calculations. It was discovered that adding Cu on BNNF can lead to enhanced electron interaction between BNNF and the CO2 molecule, resulting in better CO2 affinity and adsorption capacity. In another study, Yang et al. synthesized 3D hierarchically cubic/spherical morphologies of functionalized BN nanosheets (FBNNSs)/ZnO superstructures using the evaporation-mediated solvothermal approach [95]. These materials offer several benefits, including scalability, inexpensive technology and high throughput. As-produced FBNNSs/ZnO superstructures showed a high CO2 adsorption performance of 124.5 mg.g−1 from 0 to 1 bar at 273 K and presented reasonable reusability of 10 cycles with an average adsorption capacity up to 115.7 mg.g−1. The superior adsorption performance of superstructure for CO2 removal was described by van der Waals interaction, chemisorption and hydrogen bonds from the ZnO and amino groups of FBNNs.

Reproduced with permission from ref. [94]. Copyright 2020, American Chemical Society
a Synthesis of Cu@BNNF; SEM b and TEM images c–d exhibit uniform and smaller diameter of Cu@BNNF; e adsorption quantity of Cu@BNNF and BNNF; f CO2 adsorption and desorption cycling for the Cu@BNNF.
14.4.5 Transition Metal Dichalcogenides
Transition metal dichalcogenides (TMDCs) have recently gained attention for their potential application in carbon dioxide and other gases capture [103]. TMDCs possess unique physical and chemical properties, such as high surface area and strong adsorption abilities, which make them ideal candidates for capturing CO2 from air or exhaust streams. In addition to their excellent adsorptive properties, they also exhibit a low cost of production compared to other materials used in carbon capture technologies [104, 105].
The most common type of TMDC used for CO2 capture is molybdenum disulfide (MoS2). This material has been found to be highly effective at trapping CO2 molecules due to its large surface area and strong affinity towards the gas molecule [106]. MoS2 can be produced synthetically via chemical vapour deposition methods and direct chemical synthesis using S and Mo precursors or extracted from natural sources like shale deposits [107,108,109,110]. The synthesis approaches are relatively inexpensive, reducing the cost of CO2 adsorption compared with traditional methods used in CO2 removal systems such as cryogenic distillation or amine scrubbing processes [111]. Additionally, this material does not require any post-treatment steps after use, making it an attractive option for industrial applications where cost savings is essential. Recently, various studies have been reported for CO2 capture using pristine or modified MoS2. According to Sun et al., the strength of the applied electric field affects how strongly CO2 adsorb on the MoS2 [112]. When an electric field is applied, CO2 is adsorbed on the material and desorbed when the electric force is relaxed. It becomes a possible carbon capture material because of this distinctive quality. The Cu-modified MoS2 nanosheets were prepared for the CO2 reduction process by Shi et al. [113]. The Cu/MoS2 hybrid (0.44 cm3/g) showed better adsorption capacity compared to pristine MoS2 (0.22 cm3.g−1). Thus, Cu nanoparticles have improved the adsorption capacity since CO2 can now be adsorbed on both Cu and host MoS2 nanosheets.
In addition, the surface of MoS2 can be tuned to obtain high reactive edge sites via doping or creating defects for the adsorption of a specific gas. Wu et al. studied the role of nitrogen doping on MoS2 (defect-rich and defect-free structure) reactivity towards CO2 adsorption [114]. N-doped MoS2 with Mo defects demonstrated the highest CO2 adsorption capacity because of more electrostatic and covalent interactions with the CO2. On the contrary, the defect-free MoS2 followed weak van der Waals interactions, which resulted in weak CO2 adsorption. Also, the defective and N-doped MoS2 showed high selectivity of CO2 over N2 [114]. In another study, MoS2 nanocomposites were prepared using rGO and polypyrrole (PPy) to enhance the reactive sites for CO2 adsorption [96]. Nanocomposite with the highest PPy concentration exhibited the highest CO2 adsorption capacity (13 cm3.g−1) compared to MoS2 (6 cm3.g−1) due to the presence of excessive nitrogen binding sites (Fig. 14.10). Therefore, the adsorption capability of MoS2 nanosheets can be improved by structural modifications using doping/defect or making composite with other active species.

Reproduced with permission from ref. [96]. Copyright 2015, American Chemical Society
CO2 adsorption of the MoS2 nanosheets, rGO–MoS2, rGO–MoS2/PPy-600, rGO–MoS2/PPy-300, and rGO–MoS2/PPy-150 nanocomposites.
14.4.6 Carbon Nitride
Like graphene, graphitic carbon nitride (g-C3N4) is made of hexagonally ordered heptazine (tri-s-triazine) units bonded across tertiary amines. The carbon nitride term is basically used for CN material with elemental compositions C/N (x) of less than 1.0, in which the main constituting elements are carbon and nitrogen atoms are either substituted for carbon atoms or bonded as nitrogen functionalities. In the literature, the materials with CNx with x > 1.0 are frequently referred to as N-rich carbon nitrides [115]. Because of its excellent physicochemical characteristics, g-C3N4 has gained significant attention for energy and environmental applications [116, 117]. Unlike other 2D materials, g-C3N4 can be commonly synthesized by direct thermal decomposition of N-rich precursors such as urea or melamine. Conventional top-down exfoliation of g-C3N4 is not popular because of densely packed heptazine units. The affinity of pristine g-C3N4 and CO2 is low due to the poor alkalinity of N species in g-C3N4, which result in limited utilization in CO2 removal. Nevertheless, amine functionalization of carbon nitride is the way to enhance CO2 uptake through improving adsorbent-adsorbate interactions. For instance, polyethylenimine (PEI)-functionalized g-C3N4 was synthesized using the physical impregnation method to enhance the adsorption capacity and selectivity of g-C3N4 towards CO2 [118]. Due to the presence of excessive amine groups and stacked pores, the highest CO2 adsorption capacity of PEI-g-C3N4 was found to be 3.77 mmol.g−1 at 100 °C and ambient pressure, which was better than pristine g-C3N4. In another study, N-rich ionic liquid functionalization of g-C3N4 was performed with ionic liquid 1-Butyl-3-Methylimidazolium bis(trifluoromethyl sulfonyl)imide ([BMIM][TFSI]) for enhanced uptake of CO2 [83]. The NS-g-C3N4/[BMIM][TFSI] demonstrated excellent adoption capacity to 42.93 mmol.g−1 under nearly ideal conditions, better than bulk g-C3N4 (8.54 mmol.g−1). The high sorption uptake was ascribed to high N content (56.6 atomic%) that acts as reactive sites for CO2 interactions. At the same time, ionic liquid molecules on the g-C3N4 surface offer extra physicochemical adsorption mechanism thus increasing the CO2 uptake.
Additionally, more active sites on g-C3N4 can be generated by making a composite of it with other nanomaterials. Carbon nitride-ZnO hybrid adsorbent was prepared for CO2 adsorption. The hybrid material showed a remarkable adsorption capacity of 6.11 mmol.g−1 (1 atm and 25 °C), which was three times better than g-C3N4 [85]. It was summarized that the high CO2 affinity of hybrid material is governed by basicity, amount of amine precursors, framework stability, surface area and surface porosity. However, Kim et al. used RGO aerogel as a template for the evolution of carbon nitride with a large surface area for CO2 uptake [84]. The schematic of the preparation of carbon nitride aerogels for CO2 capture is described in Fig. 14.11. The as-prepared structure exhibited high adsorption properties (0.43 mmol.g−1) at ambient conditions with high selectivity against the N2 and excellent regeneration capacity. The increased CO2 capture capacity of carbon nitride aerogels was attributed to the microporous edges of carbon nitride that provide the optimal CO2 affinity via dipole-induced dipole interactions.

Reproduced with permission from ref. [84]. Copyright 2015, American Chemical Society
Synthesis of carbon nitride aerogels for the selective adsorption of CO2.
14.4.7 2D Metal–Organic Frameworks
Metal–organic frameworks (MOFs) are periodic network-structured porous coordination polymers, created by carefully coordinating the congregation of metal ions or clusters with organic ligands. Due to the diversities of metal ions/clusters and organic ligands, MOFs have shown a large surface area, high porosity, unique framework structure and tailorable pore, and open metal sites, which make them suitable for married applications, including CO2 capture [119]. When the MOFs dimension is reduced to two-dimension, ultrathin 2D MOFs layers have more outward remarkable characteristics, offering various scientific applications [120]. Like graphene, 2D MOFs can be prepared using either top-down or bottom-up methods [41, 120]. For example, Zhang et al. applied a bottom-up room temperature approach for preparing 2D MOFs using Cu(II) propeller and different ligands in a dimethylformamide/water solvent system [97]. The size and surface functionalization can be adjusted by changing the solvent ratio and ligands, respectively (Fig. 14.12). The findings of the gas sorption experiments indicated that nitro and amine-functionalized 2D MOFs exhibited better CO2 sorption selectivity over CH4 and N2, indicating that these materials may also be applied for carbon capture from flue gas (CO2/N2). The highest CO2 adsorption capacity was found to be 1.78 mmol.g−1 (1 atm, 273 K) for [Cu2(nbdc)2(dabco)]n. Sun et al. synthesized Co based 2D Co-MOF-UPC-32 with persistent porosity for high CO2 and H2 adsorption [121].

Reproduced with permission from ref. [97]. Copyright 2018, American Chemical Society
a Schematic shows the structure of 2D MOFs; b TEM images (with electron diffraction patterns in insets) and AFM topographic images of synthesized 2D MOFs; c CO2 sorption capacity for CO2, N2 and CH4.
Particularly in hot and humid environments, structural instability of MOFs is a major issue because of brittle coordination bonds. This restricts their capability to capture CO2, especially in pre- or post-combustion processes. On the other hand, covalent organic frameworks (COFs) are joined by covalent bonds and are structurally stable to be used in harsh CO2 capture settings. In fabricating membranes for the capture/separation of CO2 gas, 2D COFs are primarily used as active constituents [122,123,124,125]. Lately, 3D MOFs and 2D COFs have been applied to fabricate dual-layered membranes for CO2/H2 separation [122]. 2D COFs were accommodated on vertical binding sites of MOFs, producing composite membranes with exceptional H2/CO2 selectivity (32.9) and superior permeability.
14.4.8 Other 2D Materials
Nanoclays consist of layered mineral silicate with excellent mechanical properties. Due to its high abundance and low cost, it has been used as a solid adsorbent for environmental remediation. Clay-based materials have also been studied for their potential to adsorb CO2, but they have a major drawback [98, 126, 127]. The efficiency of CO2 uptake under wet conditions is low due to the presence of diffused H2O, which avert the trap of gas molecules. In dry conditions, however, high uptake can be expected because of nano-channels present in clay that allow easy intercalation of CO2. The moisture issue has led researchers to investigate ways in which this could be overcome, such as by modification or impregnation with other compounds like polymers and surfactants or by making composites with other nanomaterials such as carbon nanotubes and graphene oxide sheets. For example, montmorillonite (MMT) clay was first modified with polyphosphoric acid and used to make a composite with rGO for CO2 capture [98]. The MMT/rGO hybrid exhibited a reasonable CO2 adsorption capacity of 0.5 mmol.g−1 at 1 bar and 25 °C. In another method, porous MMT was modified with octadecylamine to adsorb CO2, which showed excellent adsorption of CO2 (7.16 mmol.g−1) compared to pristine MMT (only 3.47 mmol.g−1) at room temperature and 50 bar [127].
Other 2D materials, such as borophene [128, 129], phosphorene [130] and graphyne (comprised of both sp and sp2 carbons) [131], have mainly been studied theoretically for CO2 capture and separation. The adsorption of gas molecules (CO, CO2, NH3, NOx, and CH4) on these 2D materials was investigated by DFT and grand canonical Monte Carlo (GCMC) calculations. The findings suggest they are potential candidates for excellent adsorption of CO2 at ambient conditions.
14.5 Conclusion and Future Directions
The distinct and extraordinary properties of 2D materials (e.g., high surface area and porosity, tailorable structure, surface functionalities, chemical and thermal stability) have been fundamental driving forces for researchers to explore their potential in CO2 capture and utilisation. Lately, 2D materials such as graphene and graphene-based nanomaterials, TMDCs (e.g., MoS2), h-BN, 2D TMOs, MXenes, 2D MOFs, 2D COFs, g-C3N4, borophene, phosphorene, and nanoclays have been employed for CO2 capture. This chapter summarised an overview of various sources of CO2 and commonly employed mechanisms of CO2 removal technologies, as well as recent developments related to the use of 2D materials for capturing CO2. Usually, the CO2 adsorption efficiency is controlled by external factors, including pressure, temperature, and contaminations. Various strategies, such as doping, defect engineering, and the formation of composites and hybrids, have been employed to improve the CO2 adsorption capability of 2D materials. The importance of controlling and functionalising pores of adsorbent for CO2 uptake is discussed thoroughly. Adsorption performance could also be improved by functionalizing 2D materials and their composites with CO2-philic components. Moreover, new 2D materials such as phosphenes, borophene, 2D MOFs/COFs, and graphyne are mostly theoretically studied for CO2 capture. Therefore, more research is required to establish their CO2 capture and conversion potential.
There are still many limitations of commercializing 2D materials-based adsorbents technology for CO2 capture. For CO2-containing mixed gases, more focus should be placed on the high selectivity and high-performance CO2 adsorption at moderate temperatures and low CO2 concentration. The design and development of multi-functional 2D materials are critical to future technologies, where materials can concurrently capture CO2 from gas streams and convert it into value-added chemicals using in-situ heterogeneous catalysis. The selectivity over other gases and low performance are the major troublesome for industrial adoption of 2D materials for CO2 capture and conversion. Much research efforts are needed to match the industrial scale efficiencies. Still, there is a high preparation cost and non-uniform quality of the materials at large-scale production. Therefore, dedicated research is needed for the sustainable, affordable synthesis and processing of 2D materials-based technologies. Additionally, machine learning tools can be employed to fast-track the discovery of advanced 2D materials, experimental designs and to achieve the best possible performance.
References
T. Zhang, W. Zhang, R. Yang, Y. Liu, M. Jafari, CO2 capture and storage monitoring based on remote sensing techniques: a review. J. Clean. Prod. 281, 124409 (2021)
B. Netz, O.R. Davidson, P.R. Bosch, R. Dave, L.A. Meyer, Climate change 2007: Mitigation. Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Summary for Policymakers. Climate change 2007: Mitigation Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change Summary for Policymakers (2007)
R. Gusain, N. Kumar, S.S. Ray, Metal oxide-based nanocomposites for photocatalytic reduction of CO2 Adv. Mater. Sustain. Environ., pp. 293–315 (2022). CRC Press
S. Dey, G.C. Dhal, Materials progress in the control of CO and CO2 emission at ambient conditions: an overview. Mater. Sci. Energy Technol. 2(3), 607–623 (2019)
N.S. Sifat, Y. Haseli, A critical review of CO2 capture technologies and prospects for clean power generation. Energies 12(21), 4143 (2019)
A. Saravanan, D.-V.N. Vo, S. Jeevanantham, V. Bhuvaneswari, V.A. Narayanan, P. Yaashikaa, et al. A comprehensive review on different approaches for CO2 utilization and conversion pathways. Chem. Eng. Sci., 236, 116515 (2021).
A.A. Olajire, CO2 capture and separation technologies for end-of-pipe applications–a review. Energy 35(6), 2610–2628 (2010)
J. Pires, F. Martins, M. Alvim-Ferraz, M. Simões, Recent developments on carbon capture and storage: an overview. Chem. Eng. Res. Des. 89(9), 1446–1460 (2011)
S. Lian, C. Song, Q. Liu, E. Duan, H. Ren, Y. Kitamura, Recent advances in ionic liquids-based hybrid processes for CO2 capture and utilization. J. Environ. Sci. 99, 281–295 (2021)
A. Sattari, A. Ramazani, H. Aghahosseini, M.K. Aroua, The application of polymer containing materials in CO2 capturing via absorption and adsorption methods. J. CO2 Utilization; 48, 101526 (2021)
U. Kamran, S.-J. Park, Chemically modified carbonaceous adsorbents for enhanced CO2 capture: a review. J. Clean. Prod. 290, 125776 (2021)
D. Bonenfant, M. Kharoune, P. Niquette, M. Mimeault, R. Hausler, Advances in principal factors influencing carbon dioxide adsorption on zeolites. Sci. Technol. Adv. Mater. 9(1), 013007 (2008)
N. Mat, S.N. Timmiati, L.P. The, Recent development in metal oxide-based core–shell material for CO2 capture and utilisation. Appl. Nanosci., 1–21 (2022)
Y. Chen, C. Liu, S. Guo, T. Mu, L. Wei, Y. Lu, CO2 capture and conversion to value-added products promoted by MXene-based materials. Green Energy Environ.
C.A. Trickett, A. Helal, B.A. Al-Maythalony, Z.H. Yamani, K.E. Cordova, O.M. Yaghi, The chemistry of metal–organic frameworks for CO2 capture, regeneration and conversion. Nat. Rev. Mater. 2(8), 1–16 (2017)
E. Jelmy, N. Thomas, D.T. Mathew, J. Louis, N.T. Padmanabhan, V. Kumaravel et al., Impact of structure, doping and defect-engineering in 2D materials on CO2 capture and conversion. Reaction Chem. Eng.g. 6(10), 1701–1738 (2021)
E.I. Koytsoumpa, C. Bergins, E. Kakaras, The CO2 economy: review of CO2 capture and reuse technologies. J. Supercritical Fluids. 132, 3–16 (2018)
T.N. Borhani, M. Wang, Role of solvents in CO2 capture processes:tThe review of selection and design methods. Renew. Sustain. Energy Rev. 114, 109299 (2019)
B. Shao, Y. Zhang, Z. Sun, J. Li, Z. Gao, Z. Xie et al., CO2 capture and in-situ conversion: recent progresses and perspectives. Green Chem. Eng. 3(3), 189–198 (2022)
M. Usman, N. Iqbal, T. Noor, N. Zaman, A. Asghar, M.M. Abdelnaby et al., Advanced strategies in metal-organic frameworks for CO2 capture and separation. Chem. Rec. 22(7), e202100230 (2022)
M. Aghaie, N. Rezaei, S. Zendehboudi, A systematic review on CO2 capture with ionic liquids: Current status and future prospects. Renew. Sustain. Energy Rev. 96, 502–525 (2018)
M. Haaf, R. Anantharaman, S. Roussanaly, J. Ströhle, B. Epple, CO2 capture from waste-to-energy plants: Techno-economic assessment of novel integration concepts of calcium looping technology. Resour. Conserv. Recycl. 162, 104973 (2020)
E. Paoletti, F. Manes, Effects of elevated carbon dioxide and acidic rain on the growth of holm oak. Developments in Environmental Science. 3: Elsevier; p. 375–89 (2003)
M. Cellura, F. Guarino, S. Longo, G. Tumminia, Climate change and the building sector: modelling and energy implications to an office building in southern Europe. Energy Sustain. Dev. 45, 46–65 (2018)
J. Kahl, Effect of acid rain on building material of the El Tajín archaeological zone in Veracruz. Mexico. Environ. Pollut. 144(2), 655–660 (2006)
H. Kurihara, Y. Shirayama, Effects of increased atmospheric CO2 on sea urchin early development. Mar. Ecol. Prog. Ser. 274, 161–169 (2004)
J.M. Kolle, M. Fayaz, A. Sayari, Understanding the effect of water on CO2 adsorption. Chem. Rev. 121(13), 7280–7345 (2021)
W. Gao, S. Liang, R. Wang, Q. Jiang, Y. Zhang, Q. Zheng et al., Industrial carbon dioxide capture and utilization: state of the art and future challenges. Chem. Soc. Rev. 49(23), 8584–8686 (2020)
B.P. Spigarelli, S.K. Kawatra, Opportunities and challenges in carbon dioxide capture. J. CO2 Utilization 1, 69–87 (2013)
M. Younas, M. Sohail, L.K. Leong, M.J. Bashir, S. Sumathi, Feasibility of CO2 adsorption by solid adsorbents: a review on low-temperature systems. Int. J. Environ. Sci. Technol. 13(7), 1839–1860 (2016)
L.-P. Merkouri, T.R. Reina, M.S. Duyar, Closing the carbon cycle with dual function materials. Energy Fuels 35(24), 19859–19880 (2021)
H. Sun, C. Wu, B. Shen, X. Zhang, Y. Zhang, J. Huang, Progress in the development and application of CaO-based adsorbents for CO2 capture—a review. Mater. Today Sustain. 1–2, 1–27 (2018)
L. Yang, J. Heinlein, C. Hua, R. Gao, S. Hu, L. Pfefferle et al., Emerging dual-functional 2D transition metal oxides for carbon capture and utilization: a review. Fuel 324, 124706 (2022)
E.J. Jelmy, N. Thomas, D.T. Mathew, J. Louis, N.T. Padmanabhan, V. Kumaravel et al., Impact of structure, doping and defect-engineering in 2D materials on CO2 capture and conversion. Reaction Chem. Eng. 6(10), 1701–1738 (2021)
A. Razaq, F. Bibi, X. Zheng, R. Papadakis, S.H.M. Jafri, H. Li, Review on graphene-, graphene oxide-, reduced graphene oxide-based flexible composites: from fabrication to applications. Materials 15(3), 1012 (2022)
R. Gusain, P. Kumar, O.P. Sharma, S.L. Jain, O.P. Khatri, Reduced graphene oxide–CuO nanocomposites for photocatalytic conversion of CO2 into methanol under visible light irradiation. Appl. Catal. B 181, 352–362 (2016)
R. Gusain, H.P. Mungse, N. Kumar, T.R. Ravindran, R. Pandian, H. Sugimura et al., Covalently attached graphene–ionic liquid hybrid nanomaterials: synthesis, characterization and tribological application. J. Mater. Chem. A 4(3), 926–937 (2016)
N. Mukwevho, R. Gusain, E. Fosso-Kankeu, N. Kumar, F. Waanders, S.S. Ray, Removal of naphthalene from simulated wastewater through adsorption-photodegradation by ZnO/Ag/GO nanocomposite. J. Ind. Eng. Chem. 81, 393–404 (2020)
A. Ali, R. Pothu, S.H. Siyal, S. Phulpoto, M. Sajjad, K.H. Thebo, Graphene-based membranes for CO2 separation. Mater. Sci. Energy Technol. 2(1), 83–88 (2019)
P. Li, H.C. Zeng, Hierarchical nanocomposite by the integration of reduced graphene oxide and amorphous carbon with ultrafine MgO nanocrystallites for enhanced CO2 capture. Environ. Sci. Technol. 51(21), 12998–13007 (2017)
N. Kumar, R. Salehiyan, V. Chauke, O. Joseph Botlhoko, K. Setshedi, M. Scriba et al., Top-down synthesis of graphene: a comprehensive review. FlatChem. 27, 100224 (2021)
L. Jiang, Z. Fan, Design of advanced porous graphene materials: from graphene nanomesh to 3D architectures. Nanoscale 6(4), 1922–1945 (2014)
J. Oh, Y.-H. Mo, V.-D. Le, S. Lee, J. Han, G. Park et al., Borane-modified graphene-based materials as CO2 adsorbents. Carbon 79, 450–456 (2014)
Z.-Y. Sui, B.-H. Han, Effect of surface chemistry and textural properties on carbon dioxide uptake in hydrothermally reduced graphene oxide. Carbon 82, 590–598 (2015)
D. Kim, D.W. Kim, H.-K. Lim, J. Jeon, H. Kim, H.-T. Jung et al., Intercalation of gas molecules in graphene oxide interlayer: The role of water. Jo. Phys. Chem. C. 118(20), 11142–11148 (2014)
A.K. Mishra, S. Ramaprabhu, Nanostructured polyaniline decorated graphene sheets for reversible CO2 capture. J. Mater. Chem. 22(9), 3708–3712 (2012)
A.K. Mishra, S. Ramaprabhu, Carbon dioxide adsorption in graphene sheets. AIP Adv. 1(3), 032152 (2011)
G.-J. Shin, K. Rhee, S.-J. Park, Improvement of CO2 capture by graphite oxide in presence of polyethylenimine. Int. J. Hydrogen Energy 41(32), 14351–14359 (2016)
Y. Liu, B. Sajjadi, W.-Y. Chen, R. Chatterjee, Ultrasound-assisted amine functionalized graphene oxide for enhanced CO2 adsorption. Fuel 247, 10–18 (2019)
J. Pokhrel, N. Bhoria, S. Anastasiou, T. Tsoufis, D. Gournis, G. Romanos et al., CO2 adsorption behavior of amine-functionalized ZIF-8, graphene oxide, and ZIF-8/graphene oxide composites under dry and wet conditions. Microporous Mesoporous Mater. 267, 53–67 (2018)
N. Hsan, P. Dutta, S. Kumar, R. Bera, N. Das, Chitosan grafted graphene oxide aerogel: Synthesis, characterization and carbon dioxide capture study. Int. J. Biol. Macromol. 125, 300–306 (2019)
R. Gusain, K. Gupta, P. Joshi, O.P. Khatri, Adsorptive removal and photocatalytic degradation of organic pollutants using metal oxides and their composites: a comprehensive review. Adv. Coll. Interface. Sci. 272, 102009 (2019)
K. Gupta, P. Joshi, R. Gusain, O.P. Khatri, Recent advances in adsorptive removal of heavy metal and metalloid ions by metal oxide-based nanomaterials. Coord. Chem. Rev. 445, 214100 (2021)
R. Gusain, O.P. Khatri, Ultrasound assisted shape regulation of CuO nanorods in ionic liquids and their use as energy efficient lubricant additives. J. Mater. Chem. A 1(18), 5612–5619 (2013)
Y. Ren, Z. Ma, P.G. Bruce, Ordered mesoporous metal oxides: synthesis and applications. Chem. Soc. Rev. 41(14), 4909–4927 (2012)
M.S.S. Danish, A. Bhattacharya, D. Stepanova, A. Mikhaylov, M.L. Grilli, M. Khosravy et al., A systematic review of metal oxide applications for energy and environmental sustainability. Metals 10(12), 1604 (2020)
A. Azmi, A. Ruhaimi, M. Aziz, Efficient 3-aminopropyltrimethoxysilane functionalised mesoporous ceria nanoparticles for CO2 capture. Mater. Today Chem. 16, 100273 (2020)
J.C. Védrine, Metal oxides in heterogeneous oxidation catalysis: State of the art and challenges for a more sustainable world. Chemsuschem 12(3), 577–588 (2019)
Y. Guo, C. Tan, P. Wang, J. Sun, W. Li, C. Zhao et al., Magnesium-based basic mixtures derived from earth-abundant natural minerals for CO2 capture in simulated flue gas. Fuel 243, 298–305 (2019)
P. Li, R. Chen, Y. Lin, W. Li, General approach to facile synthesis of MgO-based porous ultrathin nanosheets enabling high-efficiency CO2 capture. Chem. Eng. J. 404, 126459 (2021)
Y. Hu, Y. Guo, J. Sun, H. Li, W. Liu, Progress in MgO sorbents for cyclic CO2 capture: a comprehensive review. J. Mater. Chem. A 7(35), 20103–20120 (2019)
F.E.C. Othman, N. Yusof, S. Samitsu, N. Abdullah, M.F. Hamid, K. Nagai, et al. Activated carbon nanofibers incorporated metal oxides for CO2 adsorption: Effects of different type of metal oxides. J. CO2 Utilization 45, 101434 (2021)
W.N.R. Wan Isahak, Z.A.C. Ramli, M.W. Mohamed Hisham, M.A. Yarmo (eds.), Magnesium oxide nanoparticles on green activated carbon as efficient CO2 adsorbent. AIP Conference Proceedings; 2013: American Institute of Physics
R. Chang, X. Wu, O. Cheung, W. Liu, Synthetic solid oxide sorbents for CO2 capture: state-of-the art and future perspectives. J. Mater. Chem. A (2022)
B.W. Hwang, J.H. Lim, H.J. Chae, H.-J. Ryu, D. Lee, J.B. Lee et al., CO2 capture and regeneration properties of MgO-based sorbents promoted with alkali metal nitrates at high pressure for the sorption enhanced water gas shift process. Process Saf. Environ. Prot. 116, 219–227 (2018)
J.-S. Kwak, K.-R. Oh, K.-Y. Kim, J.-M. Lee, Y.-U. Kwon, CO 2 absorption and desorption characteristics of MgO-based absorbent promoted by triple eutectic alkali carbonate. Phys. Chem. Chem. Phys. 21(37), 20805–20813 (2019)
L.K.G. Bhatta, U.M. Bhatta, K. Venkatesh, Metal oxides for carbon dioxide capture, in Inamuddin, Asiri A.M., Lichtfouse, E. (eds.), Sustainable Agriculture Reviews 38: Carbon Sequestration Vol 2 Materials and Chemical Methods. Cham: Springer International Publishing, p. 63–83 (2019)
N.H. Florin, A.T. Harris, Reactivity of CaO derived from nano-sized CaCO3 particles through multiple CO2 capture-and-release cycles. Chem. Eng. Sci. 64(2), 187–191 (2009)
O. Folorunso, N. Kumar, Y. Hamam, R. Sadiku, S.S. Ray, Recent progress on 2D metal carbide/nitride (MXene) nanocomposites for lithium-based batteries. FlatChem. 29, 100281 (2021)
M. Naguib, O. Mashtalir, J. Carle, V. Presser, J. Lu, L. Hultman et al., Two-dimensional transition metal carbides. ACS Nano 6(2), 1322–1331 (2012)
M.W. Barsoum, Y. Gogotsi, Removing roadblocks and opening new opportunities for MXenes. Ceramics Int. (2022)
L. Wang, M. Han, C.E. Shuck, X. Wang, Y. Gogotsi, Adjustable electrochemical properties of solid-solution MXenes. Nano Energy 88, 106308 (2021)
V. Parey, B.M. Abraham, S.H. Mir, J.K. Singh, High-throughput screening of atomic defects in Mxenes for CO2 capture, activation, and dissociation. ACS Appl. Mater. Interfaces. 13(30), 35585–35594 (2021)
Y. Chen, C. Liu, S. Guo, T. Mu, L. Wei, Y. Lu, CO2 capture and conversion to value-added products promoted by MXene-based materials. Green Energy Environ 7(3), 394–410 (2022)
Á. Morales-García, A. Fernández-Fernández, F. Viñes, F. Illas, CO2 abatement using two-dimensional MXene carbides. J. Mater. Chem. A 6(8), 3381–3385 (2018)
B. Wang, A. Zhou, F. Liu, J. Cao, L. Wang, Q. Hu, Carbon dioxide adsorption of two-dimensional carbide MXenes. J. Adv. Ceramics 7(3), 237–245 (2018)
I. Persson, J. Halim, H. Lind, T.W. Hansen, J.B. Wagner, L.-Å. Näslund et al., 2D transition metal carbides (MXenes) for carbon capture. Adv. Mater. 31(2), 1805472 (2019)
Á. Morales-García, M. Mayans-Llorach, F. Viñes, F. Illas, Thickness biased capture of CO2 on carbide MXenes. Phys. Chem. Chem. Phys. 21(41), 23136–23142 (2019)
Z. Guo, Y. Li, B. Sa, Y. Fang, J. Lin, Y. Huang et al., M2C-type MXenes: Promising catalysts for CO2 capture and reduction. Appl. Surf. Sci. 521, 146436 (2020)
R. Morales-Salvador, Á. Morales-García, F. Viñes, F. Illas, Two-dimensional nitrides as highly efficient potential candidates for CO2 capture and activation. Phys. Chem. Chem. Phys. 20(25), 17117–17124 (2018)
F.-Q. Liu, X. Liu, L. Sun, R. Li, C.-X. Yin, B. Wu, MXene-supported stable adsorbents for superior CO2 capture. J. Materials Chem. A 9(21), 12763–12771 (2021)
A.A. Shamsabadi, A.P. Isfahani, S.K. Salestan, A. Rahimpour, B. Ghalei, E. Sivaniah et al., Pushing rubbery polymer membranes to be economic for CO2 separation: embedment with Ti3C2Tx MXene nanosheets. ACS Appl. Mater. Interfaces 12(3), 3984–3992 (2020)
S. Ghosh, S. Ramaprabhu, High-pressure investigation of ionic functionalized graphitic carbon nitride nanostructures for CO2 capture. J. CO2 Utilization 21, 89–99 (2017)
Y. Oh, V.-D. Le, U.N. Maiti, J.O. Hwang, W.J. Park, J. Lim et al., Selective and regenerative carbon dioxide capture by highly polarizing porous carbon nitride. ACS Nano 9(9), 9148–9157 (2015)
S.A. Anuar, K.N. Ahmad, A. Al-Amiery, M.S. Masdar, W.N.R. Wan Isahak, Facile preparation of carbon nitride-ZnO hybrid adsorbent for CO2 capture: the significant role of amine source to metal oxide ratio. Catalysts 11(10), 1253 (2021)
B. Szczęśniak, S. Borysiuk, J. Choma, M. Jaroniec, Mechanochemical synthesis of highly porous materials. Mater. Horiz. 7(6), 1457–1473 (2020)
A.K. Mishra, S. Ramaprabhu, Enhanced CO2 capture in Fe3O4-graphene nanocomposite by physicochemical adsorption. J. Appl. Phys. 116(6), 064306 (2014)
J. Xiao, Y. Wang, T.C. Zhang, S. Yuan, rGO/N-porous carbon composites for enhanced CO2 capture and energy storage performances. J. Alloy. Compd. 857, 157534 (2021)
S. Jin, Y. Guo, J. Wang, L. Wang, Q. Hu, A. Zhou, Carbon dioxide adsorption of two-dimensional Mo2C MXene. Diam. Relat. Mater. 128, 109277 (2022)
S. Marchesini, C.M. McGilvery, J. Bailey, C. Petit, Template-free synthesis of highly porous boron nitride: insights into pore network design and impact on gas sorption. ACS Nano 11(10), 10003–10011 (2017)
S. Chen, P. Li, S. Xu, X. Pan, Q. Fu, X. Bao, Carbon doping of hexagonal boron nitride porous materials toward CO2 capture. J. Mater. Chem. A 6(4), 1832–1839 (2018)
R. Shankar, M. Sachs, L. Francàs, D. Lubert-Perquel, G. Kerherve, A. Regoutz et al., Porous boron nitride for combined CO2 capture and photoreduction. J. Mater. Chem. A 7(41), 23931–23940 (2019)
D. Wang, Y. Xue, C. Wang, J. Ji, Z. Zhou, C. Tang, Improved capture of carbon dioxide and methane via adding micropores within porous boron nitride fibers. J. Mater. Sci. 54(14), 10168–10178 (2019)
J. Liang, Q. Song, J. Lin, G. Li, Y. Fang, Z. Guo et al., In Situ Cu-loaded porous boron nitride nanofiber as an efficient adsorbent for CO2 capture. ACS Sustain. Chem. Eng. 8(19), 7454–7462 (2020)
C. Yang, D. Liu, Y. Chen, C. Chen, J. Wang, Y. Fan et al., Three-dimensional functionalized boron nitride nanosheets/ZnO superstructures for CO2 capture. ACS Appl. Mater. Interfaces. 11(10), 10276–10282 (2019)
N. Kumar, S. Kumar, R. Gusain, N. Manyala, S. Eslava, S.S. Ray, Polypyrrole-Promoted rGO–MoS2 nanocomposites for enhanced photocatalytic conversion of CO2 and H2O to CO, CH4, and H2 products. ACS Appl. Energy Mater. 3(10), 9897–9909 (2020)
J. Zha, X. Zhang, Room-Temperature synthesis of two-dimensional metal-organic frameworks with controllable size and functionality for enhanced CO2 sorption. Cryst. Growth Des. 18(5), 3209–3214 (2018)
S. Stanly, E.J. Jelmy, C.P.R. Nair, H. John, Carbon dioxide adsorption studies on modified montmorillonite clay/reduced graphene oxide hybrids at low pressure. J. Environ. Chem. Eng. 7(5), 103344 (2019)
M.G. Rasul, A. Kiziltas, B. Arfaei, R. Shahbazian-Yassar, 2D boron nitride nanosheets for polymer composite materials. npj 2D Mater. Appl. 5(1), 56 (2021)
A. Nag, K. Raidongia, K.P.S.S. Hembram, R. Datta, U.V. Waghmare, C.N.R. Rao, Graphene analogues of BN: novel synthesis and properties. ACS Nano 4(3), 1539–1544 (2010)
C. Yang, J. Wang, Y. Chen, D. Liu, S. Huang, W. Lei, One-step template-free synthesis of 3D functionalized flower-like boron nitride nanosheets for NH3 and CO2 adsorption. Nanoscale 10(23), 10979–10985 (2018)
Y. Li, L. Liu, H. Yu, Y. Zhao, J. Dai, Y. Zhong et al., Synergy of developed micropores and electronic structure defects in carbon-doped boron nitride for CO2 capture. Sci. Total Environ. 811, 151384 (2022)
X. Zhang, S.Y. Teng, A.C.M. Loy, B.S. How, W.D. Leong, X. Tao, Transition metal dichalcogenides for the application of pollution reduction: a review. Nanomaterials 10(6), 1012 (2020)
N. Kumar, E. Fosso-Kankeu, S.S. Ray, Achieving Controllable MoS2 nanostructures with increased interlayer spacing for efficient removal of Pb(II) from aquatic systems. ACS Appl. Mater. Interfaces. 11(21), 19141–19155 (2019)
S. Pandey, E. Fosso-Kankeu, M.J. Spiro, F. Waanders, N. Kumar, S.S. Ray et al., Equilibrium, kinetic, and thermodynamic studies of lead ion adsorption from mine wastewater onto MoS2-clinoptilolite composite. Mater. Today Chem. 18, 100376 (2020)
N. Aguilar, S. Aparicio, Theoretical insights into CO2 adsorption by MoS2 nanomaterials. J. Phys. Chem. C 123(43), 26338–26350 (2019)
N. Kumar, B.P.A. George, H. Abrahamse, V. Parashar, J.C. Ngila, Sustainable one-step synthesis of hierarchical microspheres of PEGylated MoS2 nanosheets and MoO3 nanorods: their cytotoxicity towards lung and breast cancer cells. Appl. Surf. Sci. 396, 8–18 (2017)
L. Seravalli, M. Bosi, A review on chemical vapour deposition of two-dimensional MoS2 flakes. Materials 14(24), 7590 (2021)
R. Gusain, N. Kumar, F. Opoku, P.P. Govender, S.S. Ray, MoS2 Nanosheet/ZnS composites for the visible-light-assisted photocatalytic degradation of oxytetracycline. ACS Appl. Nano Mater. 4(5), 4721–4734 (2021)
A. Parviainen, K. Loukola-Ruskeeniemi, Environmental impact of mineralised black shales. Earth Sci. Rev. 192, 65–90 (2019)
T.E. Rufford, S. Smart, G.C.Y. Watson, B.F. Graham, J. Boxall, J.C. Diniz da Costa et al., The removal of CO2 and N2 from natural gas: A review of conventional and emerging process technologies. J. Petroleum Sci. Eng., 94–95,123–54 (2012)
Q. Sun, G. Qin, Y. Ma, W. Wang, P. Li, A. Du et al., Electric field controlled CO2 capture and CO2/N2 separation on MoS2 monolayers. Nanoscale 9(1), 19–24 (2017)
G. Shi, L. Yu, X. Ba, X. Zhang, J. Zhou, Y. Yu, Copper nanoparticle interspersed MoS2 nanoflowers with enhanced efficiency for CO2 electrochemical reduction to fuel. Dalton Trans. 46(32), 10569–10577 (2017)
F.M. Enujekwu, Y. Zhang, C.I. Ezeh, H. Zhao, M. Xu, E. Besley et al., N-doping enabled defect-engineering of MoS2 for enhanced and selective adsorption of CO2: A DFT approach. Appl. Surf. Sci. 542, 148556 (2021)
M. Inagaki, M. Toyoda, Y. Soneda, T. Morishita, Nitrogen-doped carbon materials. Carbon 132, 104–140 (2018)
S.S. Ray, R. Gusain, N. Kumar, Chapter ten—Two-dimensional carbon nanomaterials-based adsorbents, in Ray S.S., Gusain, R., Kumar, N. (eds.) Carbon Nanomaterial-Based Adsorbents for Water Purification: Elsevier; pp. 225–73 (2020)
N. Mukwevho, N. Kumar, E. Fosso-Kankeu, F. Waanders, J.R. Bunt, R.S. Sinha, Visible light-excitable ZnO/2D graphitic-C3N4 heterostructure for the photodegradation of naphthalene. Desalin. Water Treat. 163, 286–296 (2019)
H.-L. Peng, F.-Y. Zhong, J.-B. Zhang, J.-Y. Zhang, P.-K. Wu, K. Huang et al., Graphitic carbon nitride functionalized with polyethylenimine for highly effective capture of carbon dioxide. Ind. Eng. Chem. Res. 57(32), 11031–11038 (2018)
Z. Li, P. Liu, C. Ou, X. Dong, Porous metal-organic frameworks for carbon dioxide adsorption and separation at low pressure. ACS Sustain. Chem. Eng. 8(41), 15378–15404 (2020)
W. Wang, Y. Yu, Y. Jin, X. Liu, M. Shang, X. Zheng et al., Two-dimensional metal-organic frameworks: from synthesis to bioapplications. J. Nanobiotechnol. 20(1), 207 (2022)
W. Fan, Y. Wang, Z. Xiao, Z. Huang, F. Dai, R. Wang et al., Two-dimensional cobalt metal-organic frameworks for efficient C3H6/CH4 and C3H8/CH4 hydrocarbon separation. Chin. Chem. Lett. 29(6), 865–868 (2018)
S. Das, T. Ben, S. Qiu, V. Valtchev, Two-Dimensional COF–three-dimensional MOF dual-layer membranes with unprecedentedly high H2/CO2 selectivity and ultrahigh gas permeabilities. ACS Appl. Mater. Interfaces. 12(47), 52899–52907 (2020)
Z. Kang, Y. Peng, Y. Qian, D. Yuan, M.A. Addicoat, T. Heine et al., Mixed matrix membranes (MMMs) comprising exfoliated 2D covalent organic frameworks (COFs) for efficient CO2 separation. Chem. Mater. 28(5), 1277–1285 (2016)
Y.B. Apriliyanto, N. Darmawan, N. Faginas-Lago, A. Lombardi, Two-dimensional diamine-linked covalent organic frameworks for CO2/N2 capture and separation: theoretical modeling and simulations. Phys. Chem. Chem. Phys. 22(44), 25918–25929 (2020)
Y. Cheng, X. Wang, C. Jia, Y. Wang, L. Zhai, Q. Wang et al., Ultrathin mixed matrix membranes containing two-dimensional metal-organic framework nanosheets for efficient CO2/CH4 separation. J. Membr. Sci. 539, 213–223 (2017)
N. Chouikhi, J.A. Cecilia, E. Vilarrasa-García, S. Besghaier, M. Chlendi, F.I. Franco Duro et al., CO2 adsorption of materials synthesized from clay minerals: a review. Minerals [Internet] 9(9) (2019)
M. Atilhan, S. Atilhan, R. Ullah, B. Anaya, T. Cagin, C.T. Yavuz et al., High-pressure methane, carbon dioxide, and nitrogen adsorption on amine-impregnated porous montmorillonite nanoclays. J. Chem. Eng. Data 61(8), 2749–2760 (2016)
X. Tan, H.A. Tahini, S.C. Smith, Borophene as a promising material for charge-modulated switchable CO2 capture. ACS Appl. Mater. Interfaces. 9(23), 19825–19830 (2017)
T. Liu, Y. Chen, M. Zhang, L. Yuan, C. Zhang, J. Wang et al., A first-principles study of gas molecule adsorption on borophene. AIP Adv. 7(12), 125007 (2017)
T. Kaewmaraya, L. Ngamwongwan, P. Moontragoon, W. Jarernboon, D. Singh, R. Ahuja et al., Novel green phosphorene as a superior chemical gas sensing material. J. Hazard. Mater. 401, 123340 (2021)
S. Zhou, M. Wang, S. Wei, S. Cao, Z. Wang, S. Liu et al., First-row transition-metal-doped graphyne for ultrahigh-performance CO2 capture and separation over N2/CH4/H2. Materials Today Physics. 16, 100301 (2021)
Acknowledgements
The authors are thankful to the Council for Scientific and Industrial Research (C6ACH20), and the University of Johannesburg (086310) for their financial support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kumar, N., Gusain, R., Ray, S.S. (2023). Nano-engineered 2D Materials for CO2 Capture. In: Kumar, N., Gusain, R., Sinha Ray, S. (eds) Two-Dimensional Materials for Environmental Applications. Springer Series in Materials Science, vol 332. Springer, Cham. https://doi.org/10.1007/978-3-031-28756-5_14
Download citation
DOI: https://doi.org/10.1007/978-3-031-28756-5_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-28755-8
Online ISBN: 978-3-031-28756-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)