Abstract
Melanin is a group of ubiquitous biological pigments. It has excellent photonic properties such as broadband light absorption and high refractive index, combined with other physicochemical functions like free radical quenching and metal chelating capabilities. In this chapter, we focus on the optical functions of melanin. We will first discuss chemical and physical structures in five different types of melanin. Next, we divide melanin’s photonic properties into two parts. One is about broadband absorption, where we explore the mechanism behind it and how absorption leads to various applications such as UV protection, solar desalination, and photothermal therapy. The other is related to scattering, which includes the scattering from single particle and aggregates of particles. In both scenarios, structural colors can be produced. We believe this chapter will provide a clear understanding of melanin’s optical properties and insights into the rational design of melanin-based optically active materials.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Melanin is a family of dark polymeric pigments widely found in nature, ranging from animals, plants, and fungi to prehistoric organisms. It possesses superior multifunctional properties despite the fact that its precise hierarchical chemical structure remains unclear. These include a high refractive index, broadband absorption across UV to near IR region, radical quenching ability, strong metal chelating ability, and protection against high-energy radiation. In this chapter, we focus on the optical properties of melanin-based materials and, broadly speaking, how melanin interacts with electromagnetic waves. First, we introduce the chemical structures of melanin and make classifications based on the chemical structure of monomer precursors. In the next two sections, we discuss absorption- and scattering-related properties. In the absorption section, we summarize the fundamentals of melanin’s broadband absorption and how melanin dissipates absorbed energy, which is related to different applications, like UV protection, photothermal therapy, and fluorescent imaging. The scattering section includes the scattering from single particles and assemblies of particles, with detailed summary on summarize how melanin particles assemble into structurally colored materials. In the end, we present our views on the challenges and opportunities in this field.
2 Chemical Structure
In contrast to proteins or nucleic acids, melanin biosynthesis is not a genetic or sequence-controlled process. It starts with various monomeric precursors and ends with heterogeneous, hierarchical polymeric structures (Cao et al. 2021). The unique optical properties of melanin rely on the complexity of its molecular units, chemical structures, and physical packings. In this section, we will discuss five types of melanin and their complex hierarchical structures.
2.1 Classifications of Melanin
There are five categories of melanin based on molecular units in their macromolecular structures: eumelanin, pheomelanin, neuromelanin, pyomelanin, and allomelanin (Fig. 1). They share similarities such as displaying dark colors (brown to black), containing conjugated aromatic structures, and being insoluble in most solvents. However, their chemical structures are quite different from each other, which will be discussed later.
Eumelanin, pheomelanin, and neuromelanin are mostly found in animals. They are produced initially from tyrosine by a series of enzymatic reactions in cells (commonly melanocytes). In their biosynthetic paths, tyrosinase oxidizes tyrosine to dopaquinone (DQ) and DQ polymerizes to eumelanin with two main building blocks 5,6-dihydroxyindole (DHI) or 5,6-dihydroxyindole-2-carboxylic acid (DHICA). The cysteine in the cell promotes the production of pheomelanin by forming cysteinyldopa and then benzothiazine intermediates, which prohibits the formation of eumelanin (Wakamatsu et al. 2021). Neuromelanin also originates from tyrosine, which is produced in the substantia nigra and locus coeruleus of brains. Neuromelanin is often mixed with other living tissues and it contains both indole and benzothiazine as molecular units. Pyomelanin and allomelanin are nitrogen-free melanins, which are produced mostly in fungi, bacteria, or plants. Pyromelanin biosynthetic process involves catabolism of tyrosine to homogentisic acid, while allomelanin is produced directly from nitrogen-free precursors such as 1,8-dihydroxynaphthalene (DHN).
In addition to biosynthesis in living tissues, melanin can be made in the laboratory through chemical oxidation or enzymatic oxidation using corresponding monomeric precursors. Synthetic melanin has dark coloration similar to natural melanin. Eumelanin, pheomelanin, and neuromelanin can be synthesized starting from various precursors such as tyrosine, dopamine, L-3,4-dihydroxyphenylalanine (L-DOPA), DHI, and DHICA via enzymatic reaction or oxidations (d’Ischia et al. 2013; Li et al. 2019; Napolitano et al. 2013; Wakamatsu et al. 2003). Both allomelanin and pyomelanin, can be synthesized in vitro from DHN and homogentisic acid, respectively (Zhou et al. 2019; Schmaler-Ripcke et al. 2009). More details on the synthesis of different types of synthetic melanin can be referred to in a recent review from Gianneschi group (Cao et al. 2021).
Although the chemical structure of main molecular units in melanin has been intensively studied, melanin’s polymeric structure has not been fully revealed. Melanin’s monomeric precursors have more than two reactive sites, making its polymerization complicated. In the synthesis of eumelanin, the rearrangement of dopachrome to form DHI or DHICA not only diversifies the monomeric structures but also provides various arrangements of units in the polymeric chains (Swift 2009). In the pheomelanin, the cysteine not only attaches to a different site on DOPA, but also splits a fraction of the monomers to form benzothiazines, rather than indole-like eumelanin, which adds another level of complexity. Neuromelanin is produced from two monomeric precursors and has a more chemically disordered structure. Pyomelanin can be made from the polymerization of homogentisic acid, which is a degradation product from tyrosine (Schmaler-Ripcke et al. 2009; Frases et al. 2007). However, the chemical structure of pyomelanin is less studied compared to other types of melanin. Both natural and synthetic allomelanin can be made from a universal monomeric precursor, DHN. Unlike dopachrome, DHN has fewer chances to rearrange, but allomelanin structure is also disordered due to various linkages in DHN dimers and mutations of DHN by reduction, oxidation, and dehydration (Zhou et al. 2019; Jackson et al. 2009).
2.2 Complex Hierarchical Structure of Melanin
Melanin often comes in the form of particles ranging from tens of nanometers to a few microns. Although its insolubility in most solvents makes it a challenge to quantify its chemical structure, it is clear that melanin has a complex hierarchical structure ranging from molecular to micrometer scales. The structure of eumelanin is the most widely studied. As the monomer precursor, the tyrosine converts to molecular units, DHI and DHICA. Several molecular units form planar oligomers that are called protomolecules and some models suggest that protomolecules are porphyrin-like tetramer structures (Chen et al. 2013; Kaxiras et al. 2006). These planar protomolecules stack with a spacing of 3–4 Å and stacked protomolecules pack with ordered or disordered structures to form primary nanoparticles 10–60 nm. Higher geometric packing order of these protomolecules will lead to broadening and red-shifts of the absorption band (Ju et al. 2018). The primary particles assemble into submicron-sized particles, ranging from solid spheres, solid rods, or hollow rods in natural melanin (Fig. 2) (Xiao et al. 2018).
A hierarchical structure model for eumelanin: (a) melanin molecular units, (b) stacked protomolecules, (c) primary particles, and (d) melanin particles with different morphologies. Adapted from Xiao et al. (2018)
As a model synthetic eumelanin, polydopamine has been utilized to aid in revealing the mysterious structure of natural eumelanin. Polydopamine, made via oxidation polymerization of dopamine, contains various molecular units, including uncyclized dopamine and DHI, which provide various chromophores to absorb light (Della Vecchia et al. 2013; Bisaglia et al. 2007). Although there is still some debate, it is mostly agreed that polydopamine particles are composed of linear polymer chains that are bound together via physical interactions such as hydrogen bonding, cation-π interaction, and π-π stacking (Büngeler et al. 2017; Li et al. 2015; Hong et al. 2018). The DHI units propagate through the six-membered ring to increase the molecular sizes in polydopamine (Li et al. 2019). Using matrix-assisted laser desorption/ionization mass spectroscopy, Reale et al. found that oligomers made from DHI can reach up to 30 units (Reale et al. 2012).
3 Absorption-Related Properties and Applications
Melanin can absorb light broadly from UV up to near-infrared (200–1700 nm) with decayed absorptivity at longer wavelengths (Li et al. 2020). As organic polymers, their broadband absorption is unique, making them ideal for black pigments and UV-protecting agents. Most of the light absorbed by melanin is dissipated as heat, and this photothermal effect can be used for saltwater desalination or photothermal therapy. In addition, melanin can be modified to fluoresce, expanding its applications. In this section, we will discuss the broadband absorption of melanin, the underlying mechanism of absorption, and related applications.
3.1 Optical Absorption
Melanin has a broadband absorption, unlike other pigments that often have significant absorption peaks at certain wavelengths. Its absorption spectrum varies with its chemical structure. Quantifying melanin absorption is challenging for several reasons. First, melanin is insoluble in most solvents without chemical degradation, so it is difficult to conduct conventional absorption measurements on solvent-casting melanin films. Second, melanin from biological systems is often mixed with other cellular components, making the purification process tremendously difficult. Third, melanin, often in the form of micron-scale or even larger particles, scatters light, which overestimates light absorption. Therefore, there is no perfect way to accurately measure the absorption of natural melanin. Stavenga et al. measured the refractive index of intact barbule cells that contained multilayered natural melanin particles and used an optical model to obtain a best-fitting value (Stavenga et al. 2015). Based on this method, they reported that the imaginary index varies from 0.127 to 0.029 at wavelengths of 400–800 nm (Fig. 3a).
Refractive indices of various types of melanin. (a) The imaginary part of refractive indices of melanin in bird feathers (Stavenga et al. 2015), PDOPA melanin films (Bothma et al. 2008), PDA and PDD melanin films (Li et al. 2020), and PDA melanin nanoparticles (Xiao et al. 2015). (b) Real part of refractive indices of melanin in beetle scales (Yoshioka and Kinoshita 2011), bird feathers (Stavenga et al. 2015), PDA and PDD films (Li et al. 2020), and PDA melanin nanoparticles (Xiao et al. 2015)
Synthetic melanin can be chemically prepared in a more controlled manner, removing some barriers to absorption measurements. Xiao et al. measured melanin nanoparticles made from dopamine and the imaginary index decreased from 0.33 to 0.09 at 400–800 nm (Fig. 3a). To greatly reduce the scattering effect of the particles, they used dilute solutions (10−4–10−3% v/v) and small particle sizes (120 nm) (Xiao et al. 2015). In addition to melanin nanoparticles, there are a few reports on quantifying the absorption of synthetic melanin films. Bothma et al. fabricated device-quality melanin films with DOPA and used an integrating sphere to measure absorption. We can calculate the imaginary indices of the PDOPA melanin film as 0.286–0.083 based on the absorption coefficient (Fig. 3a) (Bothma et al. 2008; Xiao et al. 2020). Li et al. made two types of smooth melanin films through oxidation polymerization at the water-air interface. One is polydopamine (PDA) and the other is poly(dopamine-L-DOPA) (PDD). They used ellipsometry to measure imaginary indices of PDA and PDD melanin films to be 0.185–0.0096 and 0.222–0.0094 (Fig. 3a) (Li et al. 2020). The reported synthetic melanin shows higher absorption than natural melanin, probably because natural melanin is mixed with non-absorbing components like proteins, lipids, or polysaccharides.
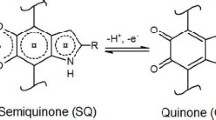
Understanding the mechanism behind melanin’s broadband absorption will guide the rational design of novel black pigments. Different levels of heterogeneities contribute to melanin’s broadband absorption. The first level is the chemical structure variation as mentioned in Sect. 2. Take eumelanin, for example, it is made of mixtures of DHI and DHICA units. These molecular units make protomolecules with different degrees of polymerization. DHI and DHICA have different connecting sites, leading to different chemical structures in protomolecules. Thus, the eumelanin contains different protomolecules that have absorption peaks at different wavelengths and the supposition of these peaks leads to broadband absorption (Tran et al. 2006). The second level of heterogeneity is the variation in redox states. The indole groups in melanin have a fully reduced state (hydro-quinone), half-reduced state (semi-quinone), and oxidized state (quinone). Density functional theory calculations show that the absorption peak of the reduced protomolecules is red-shifted as compared to the oxidized form and the half-reduced form exhibits intermediate behaviors with a broader absorption (Stark et al. 2005). On the third level, the stacking of planar melanin protomolecules plays a significant role in its absorption behavior. Eumelanin protomolecules pack with a spacing of 3–4 Å. Chen et al. combined experiments and simulations to demonstrate excitonic coupling from geometric packing is a key reason for broadband optical absorption (Chen et al. 2014). Later, Ju et al. demonstrated that higher geometric packing ordering efficiently broadens and red-shifts the absorption band (Ju et al. 2018). Taken together, the unique blackness of melanin is a synergistic effect of heterogeneity in chemical structure, redox states, and geometric packing.
3.2 Black Additives
The basic function of melanin is to provide dark colorations in animals and fungi and the colors include black, brown, or grey colors (Evayanti and Artaria 2019; Itou et al. 2019; Surmacki et al. 2021; Mattoon et al. 2021). Dark pigmentations induced with melanin have significant biological functions such as warning color, camouflage, and courtship. Some animals like insects generate black patterns with melanin for aposematic signals, (Liu et al. 2014) while some like cephalopods generate dynamic patterns for camouflage by controlling the melanin density and distribution rapidly (Mäthger et al. 2009). Melanin patterns are also critical in sexual selection for animals like butterflies (Wiernasz 1995). In addition to exterior dark patterns, cuttlefish ejects inks for anti-predator defense (Derby 2014). The inks are a primary source of natural eumelanin used in research.
Melanin can be used as biocompatible hair dyes due to its excellent binding ability. Through the deposition of polydopamine or co-deposition of polydopamine and cysteine, one can obtain melanin-dyed dark hair with different hues (Battistella et al. 2020; Dong et al. 2019). The colors of the dyes can be tuned by doping different types of metals or melanin precursors (Fig. 4a). The melanin hair dye can be even made permanent using a pre-grafting method (Zheng et al. 2022).
Absorption-related applications of melanin in various fields. (a) Hairs are dyed with polydopamine melanin catalyzed by different metal ions. Reproduced with permission (Im et al. 2017). Copyright 2017, the American Chemical Society. (b) The absorption curves of rhodamine solutions change with the time of UV irradiation. The solutions are covered by PVA film that contains different concentrations of sepia melanin. Reproduced with permission (Wang et al. 2016). Copyright 2016, the American Chemical Society. (c) 4 T1 tumor growth curves of the mice after different treatments show the efficacy of photothermal therapy. Reproduced with permission (Liu et al. 2013). Copyright 2013, WILEY-VCH. (d) Confocal microscope images of A549 Cells stained with in situ synthesized PDA nanoparticles. Reproduced with permission (Ding et al. 2017). Copyright 2017, the American Chemical Society. Scale bars, 10 μm
Melanin can enhance color saturation by absorbing incoherent backscattering light. Advantages of melanin over other conventional broadband absorbers include biocompatibility, defined and controllable particle sizes, and tunable absorption. Non-iridescent structural colors in bird feathers are caused by coherent light scattering in the spongy layer, and the underlying amorphous melanin layer absorbs incoherent light scattering and enhances color saturation (Saranathan et al. 2012; Shawkey and Hill 2006). Synthetic melanin particles have been extensively doped into photonic colloidal packings to enhance color saturation, and the required doping ratio is as low as 0.5% w/w (Cai et al. 2014; Lee et al. 2019).
3.3 Protection from Electromagnetic Radiation
Melanin has a gradually descending absorption band from UV (200 nm) (Wang et al. 2017) to near-infrared (NIR, 900 nm) (Li et al. 2020) with two broad absorption humps in the mid-infrared range (MIR, 2.5–25 μm). Although some melanin can provide protection from high energy radiation like X-ray and γ-ray (Cao et al. 2020; Rageh et al. 2015), there is still a lack of more reliable quantitative evidence of absorption in such a high energy range.
UV light that reaches the earth’s surface is mostly UVA and UVB (290–400 nm), which can cause DNA damage and even skin cancer in some extreme cases. Humans with darker skin pigmentation have less oxidative stress and a lower chance of sun-induced skin cancers (Shih et al. 2020; Brenner and Hearing 2008). Birds with melanin-enriched skins tend to be more common in regions with abundant sunshine, suggesting that UV protection is related to dark bird skins (Nicolaï et al. 2020). Melanin in skin, hair, and eye can absorb UV light and quench free radicals generated by UV radiation (Shih et al. 2020; Herrling et al. 2008; Hu et al. 2008). Melanin can release its absorbed energy from UV radiation to heat at a time scale of 160 fs, reducing the chance of excited state splitting to free radicals that will cause damage to the living tissues (Corani et al. 2014; Qu et al. 2000). Corani et al. and Panzella et al. reported that DHICA melanin dissipated UV and scavenged free radicals faster than DHI melanin, suggesting the DHICA component played a major role in eumelanin’s UV protection (Corani et al. 2014; Panzella et al. 2013). Melanin is doped into polymer films to enhance the resistance to UV exposure. A polyvinyl alcohol film doped with sepia melanin can efficiently protect rhodamine dyes from photodegradation (Fig. 4b) (Wang et al. 2016). In addition, doping sepia melanin largely decreases the UV damage to polymer mechanical properties.
Along with the photoprotection of surrounding tissues, the melanin structure may also undergo photodegradation when exposed to visible and UV light. Though melanin releases hydroxyl radicals to quench other free radicals upon visible and UV irradiation (Qu et al. 2000), a study showed that visible light can disrupt the structure of melanin and reduce the antioxidative capacity (Zareba et al. 2006). Li et al. studied the UV degradation behaviors of dopamine melanin, L-DOPA melanin, and sepia melanin. They demonstrated that strong UVA irradiation (2.43 ± 0.02 W/cm2) degraded a fraction of the six-membered benzyl ring to furo[3,4-b]pyrrole and CO2 while the pyrrole ring of the indole unit was still intact for all three types of melanin (Li et al. 2019).
3.4 Photothermal Effect
The absorption of light by melanin mainly converts into heat, and this photothermal effect leads to many applications. Liu et al. made a photothermal therapeutic (PPT) agent based on melanin nanoparticles for in vivo cancer therapy. With a photothermal conversion efficiency of 40%, the PPT agent can damage tumor cells under 808 nm lasering without affecting healthy tissues (Fig. 4c) (Liu et al. 2013). Later, Yang et al. developed a new type of synthetic melanin by copolymerizing arginine and dopamine, which significantly increased the photothermal conversion efficiency at 808 nm light (Yang et al. 2019).
In addition, the photothermal effect of melanin can be used for solar desalination. The solar energy absorbed by melanin can be harvested to evaporate brine to produce pure water. Zou et al. constructed donor-accepter pairs in synthetic melanin molecules to boost the absorption and used optimized melanin nanoparticles for solar desalination (Zou et al. 2020). When applied to thermoelectric devices, melanin can convert solar energy into heat, generating voltages up to 229 mV through the thermoelectric effect (Bai et al. 2022).
3.5 Fluorescence
Melanin is usually not fluorescent because it can absorb self-emitted light. Synthetic melanin made from dopamine can efficiently quench various fluorescent dyes that are attached to the surface of melanin nanoparticles (Qiang et al. 2014; Ma et al. 2016). The fluorescence quenching mechanism is assumed to involve Forster resonance energy transfer and/or photoinduced electron transfer. To make melanin fluorescent, one needs to eliminate the aggregation-induced quenching effect by disrupting the stacking of planar melanin protomolecules. Researchers have used chemical oxidation (H2O2, NaIO4, KMnO4) to disrupt the stacking or degrade melanin particles in alkaline solutions to decrease particle sizes (Lin et al. 2015; Yin et al. 2018; Zhang et al. 2012; Xue et al. 2018).
Fluorescent melanin can be used as a novel biocompatible and surface-active dye for sensors and biomedicine. Melanin has strong chelating capabilities with metal ions, and its fluorescence disappears after binding with metal ions. Taking advantage of this pheonomenon, researchers have designed fluorescent probes to detect Fe3+ or Pb2+ (Yin et al. 2018; Zhang et al. 2020). Another important application is to use melanin nanoparticles for cell imaging (Yang et al. 2016; Ding et al. 2017; Zhang et al. 2019). Ding et al. demonstrated that polydopamine nanoparticle (5–10 nm) was synthesized in situ within the nucleus of living cells, and this biomimetic nuclear dye showed high quantum yield (~35.8%), high photostability, low cytotoxicity, and long-term fluorescence tracking property (Fig. 4d) (Ding et al. 2017).
4 Scattering-Related Properties and Applications
Natural and synthetic melanins are often particulate shapes. When the size of melanin particles is comparable to the wavelength of light, they can scatter light and produce various optical effects. By controlling the size and packing of melanin particles, one can manipulate their scattering for different applications, for example, producing structural colors, monitoring the state of melanin-containing cells, or making a nanoprobe for Raman imaging.
The scattering property strongly depends on a material’s refractive index. The refractive index of melanin typically reported to be as high as 2.0, is much higher than most common polymers (1.4–1.6). Recent efforts have been made to measure the refractive index of melanin, however, the reported index varies depending on the morphology and chemistry of melanin and measurement methods (Fig. 3b). Yoshinoka and Kinoshita determined the real refractive index of melanin in the Jewel beetle’s elytra to be 1.65–1.78 from a typical multilayered model using experimental data (Yoshioka and Kinoshita 2011). Later, Stavenga et al. reported 1.7–1.8 for melanin in damselfly wings and bird feathers by fitting several parameters to match theoretical calculation with experimental data from interference microscopy (Stavenga et al. 2012, 2015). Xiao et al. measured the refractive index of synthetic melanin (PDA) nanoparticles to be 1.74 at 589 nm (Xiao et al. 2015). Li et al. used ellipsometry to measure indices of films for two different synthetic melanin: PDA melanin from only dopamine and PDD melanin from a mixture of dopamine and L-DOPA. PDA film has a maximum index of 1.81 at 485 nm and PDD film has a maximum index of 1.86 at 550 nm (Li et al. 2020). Despite some discrepancies among different measurements (Fig. 3b), the real part of the refractive index ranges from 1.65 to1.85 across the visible wavelengths, which is higher than common polymers.
4.1 Single Particle Scattering
Melanin particles cannot dissolve in solvents completely without a degradation reaction catalyzed by a strong base like NaOH. When measuring the absorption of melanin, the scattering of melanin particles cannot be completely avoided. Riesz et al. quantified that the contribution of scattering in dilute eumelanin solutions (0.0025% w/w) is less than 6% to the total optical attenuation in the UV regions (210–325 nm), and contribution at wavelengths of 325–800 nm is negligible (Riesz et al. 2006). They obtained a perfect match between calculations and experiments when fitting the scattering of eumelanin to Raileigh scattering with assuming particle sizes to be 38 nm. This suggested that small particle size and a very dilute solution is the key to minimizing the scattering effect when measuring the real absorption. When particles become larger or concentrations are not dilute enough, the scattering contributes more to the total extinction.
One can exploit the scattering of individual melanin particles for new applications. For example, Song et al. simulated wavelength-dependent scattering of melanosomes in retinal pigmented epithelial cells and found that optical properties of melanin particles changed when they were bleached. This method can be used to detect the changes in retinal pigmented epithelial cells (Song et al. 2017). When melanin particles are monodisperse and fall in sizes comparable to wavlengths of visible light, they can generate structural colors by Mie scattering from individual particles (Cho et al. 2017). The high absorption of melanin attenuates multiple scattering so that resonate Mie scattering of monodisperse melanin particles dominates. The Mie scattering makes structural colors and different colors are obtained depending on the size of melanin particles (Fig. 5a).
Structural colors due to scattering from individual and assembled melanin particles. (a) Dilute melanin nanoparticle solutions (0.2% w/w) show different colors depending on the particle sizes, which is due to Mie scattering. Reproduced with permission (Cho et al. 2017). Copyright 2017, WILEY-VCH. (b) Photos and transmission electron microscopy images of structurally colored bird feathers that contain multilayered melanin particles. From left to right, common bronzewing (Reproduced with permission (Xiao et al. 2014). Copyright 2014, the Optical Society) and bird of paradise (Reproduced with permission (Stavenga et al. 2011). Copyright 2011, the Royal Society). (c) Photos and transmission electron microscopy images of structurally colored bird feathers where melanin particles form 2D ordered structures. From left to right, 2D hexagonal packing in mallard feathers (Reproduced with permission (Eliason and Shawkey 2012). Copyright 2012, the Royal Society) and 2D square packing in peacock feathers (Reproduced with permission (Zi et al. 2003). Copyright 2003, the National Academy of Sciences, USA). (d) Structural colors from films made of synthetic melanin particles. Reproduced with permission (Xiao et al. 2015). Copyright 2015, the American Chemical Society. (e) Core-shell melanin particles (melanin as cores) assemble into 3D photonic balls to produce bright and noniridescent structural colors (Xiao et al. 2017)
4.2 Scattering from Assemblies of Particles
The assemblies of melanin particles can generate more interesting optical phenomena. In bird feathers, natural melanin particles organize into various ordered packings, like multilayered structures, square packing, or hexagonal packing. These ordered structures made from melanin particles can selectively reflect light at certain wavelengths, producing structural colors. These types of colors depend only on the nanostructures, but not the chemical structure, in contrast to colors of pigments or dyes. We will summarize different types of assembled photonic structures that are made of melanin particles.
-
1.
Layered Structures
Melanin particles assemble into a single layer or multiple layers in some bird feathers. When the layer thickness and spacing are hundreds of nanometers, they can reflect light with interference, producing structural colors. The simplest case is the iridescent colors in feathers of blue-black grassquits (Volatinia jacarina), where a 127-nm-thick keratin layer and a 422-nm-thick-melanosome layer form a two-layered structure (Maia et al. 2009). Another example is the tree swallow mantle feathers containing a keratin layer (148 nm) and a melanosome layer (173 nm). The keratin layer can absorb water and its thickness increases under high humid conditions, leading to color changes in these feathers (Eliason and Shawkey 2010).
In some bird feathers, there are multilayers of melanin particles dispersed in the keratin matrix with ~100 nm spacing. For example, common bronzewing (Phaps chalcoptera) feathers contain 6–7 layers of melanin particles with equal spacings in the keratin matrix (Fig. 5b) (Xiao et al. 2014). The slight variations in both thickness and spacing of the melanin layer lead to a full-color spectrum from blue to red (462–647 nm) across a single feather. The bird of paradise (Parotia lawesii) has uniquely boomerang-shaped barbules in which reflectors are composed of multilayer melanin particles crossing at a 30 ° angle (Fig. 5b) (Stavenga et al. 2011). This boomerang-shaped geometry increases the angle dependence of the colors, which is biologically beneficial to mating.
Bird feathers have inspired researchers to assemble synthetic melanin particles into structurally colored films. Xiao et al. synthesized monodisperse melanin nanoparticles from dopamine and assembled them into hundreds of nanometers of films to produce structural colors (Xiao et al. 2015). Their colors vary with the film thickness (Fig. 5d). The high color saturation is due to the broadband absorption of melanin. In addition, they found the melanin nanoparticle films could reversibly absorb and desorb water, and melanin films change colors dramatically upon humidity variations (Xiao et al. 2016).
-
2.
2D Packing of Particles
Natural melanin particles in bird feathers are often rod-like shaped with hundreds of nanometers in diameter and several microns in length. They can be monodisperse with controlled aspect ratios (Xiao et al. 2018), however, it remains challenging to synthesize such rod-like melanin particles with controlled size and aspect ratios in the laboratory. In bird feathers, the rod-like melanin particles prefer to align parallel to the inner surface of barbules due to depletion attraction (Maia et al. 2011). Being monodisperse and rod-like shaped, natural melanin particles readily self-organize into 2D crystalline structures, square or hexagonal lattices, producing bright and iridescent structural colors (Fig. 5c). For example, iridescent peacock barbules contain 2D square packed melanin rod-like particles and different colors of these feathers originate from the variation in lattice spacing (Zi et al. 2003). In mallard feathers, melanin particles form a non-close packed 2D hexagonal lattice (Fig. 5c), resulting in a broader and larger reflectance peak, and thus brighter colors (Eliason and Shawkey 2012).
-
3.
3D Packing of Particles
Spherical particles can pack in crystalline or short-range correlated structures that can produce structural colors (Takeoka 2012). Natural melanin particles are spherical, rod-like, or irregular shapes, however, monodisperse spherical melanin particles are rarely found in nature. Nowadays, there are quite a few approaches to synthesizing monodisperse melanin particles from different monomers (Xiao et al. 2015; Cho and Kim 2015). This fundamentally supports recent research where synthetic melanin particles are assembled in various ways to produce structural colors.
Melanin has high broadband absorption with a reported absorption coefficient of about 1/μm (Bothma et al. 2008). Based on the beer-lambert’s law, a melanin film of 5 μm absorbs more than 99% of light. This suggests that if one prepares 3D packing of pure melanin particles, black colors will be obtained due to too much absorption. This issue has been resolved by using core-shell melanin particles, co-assembling melanin and other non-absorbing particles, or copolymerization melanin with other polymers.
There are two types of core-shell melanin particles. The first type is with melanin shells. Kishikawa and co-authors coated synthetic melanin (polydopamine) onto polystyrene nanoparticles with melanin shell thickness from 2.5 to 22 nm. They drop cast solutions of core-shell particles to 3D ordered assemblies with structural colors (Kawamura et al. 2016). When the melanin shell thickness increased, the surface of core-shell particles became rougher, prohibiting the formation of crystalline structures. Later, they assembled those core-shell particles to different morphologies, micron-sized photonic balls via membrane emulsification method and photonic fibers via a microfluidic approach (Kohri et al. 2018). Kohri et al. made ellipsoidal melanin particles by stretching uncross-linked polystyrene core and melanin shell particles. When depositing structurally colored films using these particles, they found higher aspect ratios caused a blue shift of reflected spectra (Kohri et al. 2019).
The second type is with melanin cores. Xiao et al. used optical simulations to demonstrate that higher reflectance and brighter colors could be achieved from 3D photonic assemblies made of core-shell nanoparticles with high refractive index core and low index shell. Based on this theory, they used a sol-gel method to coat a silica layer (index = 1.45) onto the surface of synthetic melanin nanoparticles (index = 1.74) to make core-shell nanoparticles with high index core and low index shell. They used a one-pot emulsion method to assemble these core-shell particles into photonic balls that showed bright colors (Fig. 5e). The colors were tuned either by varying core size and shell thickness or by mixing two sizes of core-shell nanoparticles.
Co-assembling melanin with other non-absorbing particles can avoid too much absorption. Random mixing is not always obtained when assembling two types of particles. Xiao et al. demonstrated that melanin particles completely segregate at the outmost layer of photonic balls during co-assembly of melanin and silica particles in an emulsion approach (Xiao et al. 2019). With interfacial tension measurements and molecular dynamic simulations, they revealed that the surface enrichment was caused by the larger interfacial contact angle of melanin than that of silica particles. Inspired by this, Patil et al. used molecular dynamic simulations and numerical optical calculations to systematically explore how the degree of surface enrichment influences the colors of photonic balls (Patil et al. 2021). By decreasing the surface enrichment of melanin particles, the reflectance peak not only broadens but also shifts to shorter wavelengths.
Liu et al. synthesized melanin-doped polymeric nanoparticles by adding dopamine chloride to mixtures of monomers (methyl methacrylate, divinylbenzene, and 2-hydroxyehtyl methacrylate) during emulsion polymerization. To create a non close packing, they dispersed these melanin-doped nanoparticles in 2-hydroxyethyl methacrylate (HEMA) solvent and crosslinked HEMA to produce a photonic hydrogel film. By controlling the melanin concentration in these nanoparticles, they obtained sufficient absorption to produce high color saturation without disrupting the packing order (Liu et al. 2020).
4.3 Inelastic Scattering
When the light interacts with melanin, most light is scattered without changing frequency. If the frequency of the scattered light is different from that of the incident light, inelastic scattering (also called Raman scattering) happens. The frequency of Raman scattered light is often smaller than the incident light. The energy difference between incident light and scattered light is determined by the molecular vibration energy, which represents the characteristic information of molecular structures. Raman spectroscopy can detect functional groups, just like IR spectroscopy. Melanin is Raman active with two Raman peaks at around 1380 cm−1 and 1580 cm−1, similar to G and D bands in graphene and graphite (Huang et al. 2004; Capozzi et al. 2005). The first peak originates from the linear stretching of C-C bonds within the aromatic rings, and the second peak originates from the in-plane stretching of the rings. These peaks are distinguishable from other biological materials, like proteins, polysaccharides, and carotenoids, which provides an invasive method to identify the existence of eumelanin in feathers, hairs or even fossils (Galván et al. 2013; Li et al. 2018; Peteya et al. 2017). Noble metal substrates (like gold, and silver) can largely enhance Raman signal intensity of melanin due to plasmonic resonances. Ju et al. recently coated melanin onto gold nanoparticles and used the core-shell particles as nanoprobes for Raman imaging (Ju et al. 2015).
5 Outlook
Melanin is a group of dark pigments and it interacts with electromagnetic waves in a unique way. Its special optical properties include broadband absorption across UV to near IR, the capability to rapidly dissipate most absorbed energy to heat, and a high refractive index. These properties make melanin widely used in fields like biocompatible hair dyes, UV protection, photothermal therapy, and structural coloration. Here we conclude by highlighting the challenges and opportunities in the study of melanin’s photonic properties and optic-related applications. First, there is still a lack of understanding in the quantitative relations between melanin’s hierarchical chemical structure and its broad absorption spectra. This includes the challenges of precisely quantifying melanin’s chemical structure and revealing the complex supramolecular interactions in melanin. This quantitative relation will provide a roadmap to developing novel melanin-like materials with tuned absorption. Second, it is still unclear how melanin particles organize into ordered or disordered photonic structures in bird feathers and other living creatures. Addressing this challenge may change the current approaches to fabricating photonic materials. Third, we need to integrate melanin’s excellent photonic properties with its biocompatibility, radical quenching, and metal binding capabilities so that we can create multifunctional materials for broader applications. To this end, we believe further development of this field requires joint efforts from biologists, chemists, physicists, and materials scientists.
References
Bai W et al (2022) Boosting the optical absorption of melanin-like polymers. Macromolecules 55:3493–3501
Battistella C et al (2020) Mimicking natural human hair pigmentation with synthetic melanin. ACS Cent Sci 6:1179–1188
Bisaglia M, Mammi S, Bubacco L (2007) Kinetic and structural analysis of the early oxidation products of dopamine. J Biol Chem 282:15597–15605
Bothma JP, De Boor J, Divakar U, Schwenn PE, Meredith P (2008) Device-quality electrically conducting melanin thin films. Adv Mater 20:3539–3542
Brenner M, Hearing VJ (2008) The protective role of melanin against UV damage in human skin†. Photochem Photobiol 84:539–549
Büngeler A, Hämisch B, Strube O (2017) The supramolecular buildup of eumelanin: structures, mechanisms, controllability. Int J Mol Sci 18:1901
Cai Z et al (2014) 2D photonic crystal protein hydrogel coulometer for sensing serum albumin ligand binding. Anal Chem 86:4840–4847
Cao W et al (2020) Selenomelanin: an abiotic selenium analogue of pheomelanin. J Am Chem Soc 142:12802–12810
Cao W et al (2021) Unraveling the structure and function of melanin through synthesis. J Am Chem Soc 143:2622–2637
Capozzi V et al (2005) Raman and optical spectroscopy of eumelanin films. J Mol Struct 744:717–721
Chen C-T et al (2013) Self-assembly of tetramers of 5,6-dihydroxyindole explains the primary physical properties of eumelanin: experiment, simulation, and design. ACS Nano 7:1524–1532
Chen C-T et al (2014) Excitonic effects from geometric order and disorder explain broadband optical absorption in eumelanin. Nat Commun 5:3859
Cho S, Kim S-H (2015) Hydroxide ion-mediated synthesis of monodisperse dopamine–melanin nanospheres. J Colloid Interface Sci 458:87–93
Cho S, Shim TS, Kim JH, Kim D-H, Kim S-H (2017) Selective coloration of melanin nanospheres through resonant Mie scattering. Adv Mater 29:1700256
Corani A et al (2014) Superior photoprotective motifs and mechanisms in eumelanins uncovered. J Am Chem Soc 136:11626–11635
d’Ischia M et al (2013) Melanins and melanogenesis: methods, standards, protocols. Pigment Cell Melanoma Res 26:616–633
Della Vecchia NF et al (2013) Building-block diversity in polydopamine underpins a multifunctional eumelanin-type platform Tunable through a quinone control point. Adv Funct Mater 23:1331–1340
Derby C (2014) Cephalopod ink: production, chemistry, functions and applications. Mar Drugs 12:2700–2730
Ding P et al (2017) In situ live-cell nucleus fluorescence labeling with bioinspired fluorescent probes. Anal Chem 89:7861–7868
Dong Y et al (2019) Melanin-mimetic multicolor and low-toxicity hair dye. RSC Adv 9:33617–33624
Eliason CM, Shawkey MD (2010) Rapid, reversible response of iridescent feather color to ambient humidity. Opt Express 18:21284–21292
Eliason CM, Shawkey MD (2012) A photonic heterostructure produces diverse iridescent colours in duck wing patches. J R Soc Interface 9:2279–2289
Evayanti LG, Artaria MD (2019) Understanding the characteristics of physical color in human–an article review. In: Proceedings of the international conference of social science. https://doi.org/10.4108/eai.21-9-2018.2281158
Frases S, Salazar A, Dadachova E, Casadevall A (2007) Cryptococcus neoformans can utilize the bacterial melanin precursor Homogentisic acid for fungal melanogenesis. Appl Environ Microbiol 73:615–621
Galván I et al (2013) Raman spectroscopy as a non-invasive technique for the quantification of melanins in feathers and hairs. Pigment Cell Melanoma Res 26:917–923
Herrling T, Jung K, Fuchs J (2008) The role of melanin as protector against free radicals in skin and its role as free radical indicator in hair. Spectrochim Acta A Mol Biomol Spectrosc 69:1429–1435
Hong S, Wang Y, Park SY, Lee H (2018) Progressive fuzzy cation-π assembly of biological catecholamines. Sci Adv 4(9):eaat7457
Hu D-N, Simon JD, Sarna T (2008) Role of ocular melanin in ophthalmic physiology and pathology. Photochem Photobiol 84:639–644
Huang Z et al (2004) Raman spectroscopy of in vivo cutaneous melanin. J Biomed Opt 9:1198–1205
Im KM, Kim T-W, Jeon J-R (2017) Metal-chelation-assisted deposition of polydopamine on human hair: a ready-to-use eumelanin-based hair dyeing methodology. ACS Biomater Sci Eng 3:628–636
Itou T, Ito S, Wakamatsu K (2019) Effects of aging on hair color, melanosome morphology, and melanin composition in Japanese females. Int J Mol Sci 20:3739
Jackson JC, Higgins LA, Lin X (2009) Conidiation color mutants of aspergillus fumigatus are highly pathogenic to the heterologous insect host Galleria mellonella. PLoS One 4:e4224
Ju K-Y, Fischer MC, Warren WS (2018) Understanding the role of aggregation in the broad absorption bands of eumelanin. ACS Nano 12:12050–12061
Ju K-Y, Lee S, Pyo J, Choo J, Lee J-K (2015) Bio-inspired development of a dual-mode nanoprobe for MRI and Raman imaging. Small 11:84–89
Kawamura A et al (2016) Full-color biomimetic photonic materials with iridescent and non-iridescent structural colors. Sci Rep 6:33984
Kaxiras E, Tsolakidis A, Zonios G, Meng S (2006) Structural model of eumelanin. Phys Rev Lett 97:218102
Kohri M et al (2018) Polydopamine-based 3D colloidal photonic materials: structural color balls and fibers from melanin-like particles with polydopamine shell layers. ACS Appl Mater Interfaces 10:7640–7648
Kohri M et al (2019) Ellipsoidal artificial melanin particles as building blocks for biomimetic structural coloration. Langmuir 35:5574–5580
Lee GH et al (2019) Colloidal photonic inks for mechanochromic films and patterns with structural colors of high saturation. Chem Mater 31:8154–8162
Li Q, Clarke JA, Gao K-Q, Peteya JA, Shawkey MD (2018) Elaborate plumage patterning in a cretaceous bird. PeerJ 6:e5831
Li Y et al (2015) Mass spectrometric and spectrophotometric analyses reveal an alternative structure and a new formation mechanism for melanin. Anal Chem 87:7958–7963
Li W et al (2019) Mechanism of UVA degradation of synthetic eumelanin. Biomacromolecules 20:4593–4601
Li W et al (2020) Characterization of broadband complex refractive index of synthetic melanin coatings and their changes after ultraviolet irradiation. Appl Phys Lett 117:203701
Lin J-H, Yu C-J, Yang Y-C, Tseng W-L (2015) Formation of fluorescent polydopamine dots from hydroxyl radical-induced degradation of polydopamine nanoparticles. Phys Chem Chem Phys 17:15124–15130
Liu J, Lemonds TR, Popadić A (2014) The genetic control of aposematic black pigmentation in hemimetabolous insects: insights from Oncopeltus fasciatus: the genetic control of black pigmentation in Oncopeltus. Evol Dev 16:270–277
Liu Y et al (2013) Dopamine-melanin colloidal nanospheres: an efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv Mater 25:1353–1359
Liu P et al (2020) A highly colorimetric photonic film composed of non-close-packed melanin-like colloidal arrays. J Colloid Interface Sci 580:573–582
Ma S et al (2016) Selective and sensitive monitoring of cerebral antioxidants based on the dye-Labeled DNA/polydopamine conjugates. Anal Chem 88:11647–11653
Maia R, Caetano JVO, Báo SN, Macedo RH (2009) Iridescent structural colour production in male blue-black grassquit feather barbules: the role of keratin and melanin. J R Soc Interface 6:S203–S211
Maia R, D’Alba L, Shawkey MD (2011) What makes a feather shine? A nanostructural basis for glossy black colours in feathers. Proc R Soc B Biol Sci 278:1973–1980
Mäthger LM, Denton EJ, Marshall NJ, Hanlon RT (2009) Mechanisms and behavioural functions of structural coloration in cephalopods. J R Soc Interface 6(Suppl 2):S149–S163
Mattoon ER, Cordero RJB, Casadevall A (2021) Fungal melanins and applications in healthcare bioremediation and industry. J Fungi 7:488
Napolitano A, Panzella L, Leone L, d’Ischia M (2013) Red hair benzothiazines and benzothiazoles: mutation-inspired chemistry in the quest for functionality. Acc Chem Res 46:519–528
Nicolaï MPJ, Shawkey MD, Porchetta S, Claus R, D’Alba L (2020) Exposure to UV radiance predicts repeated evolution of concealed black skin in birds. Nat Commun 11:2414
Panzella L et al (2013) Atypical structural and π-electron features of a melanin polymer that lead to superior free-radical-scavenging properties. Angew Chem Int Ed 52:12684–12687
Patil A et al (2021) Structural color production in melanin-based disordered colloidal nanoparticle assemblies in spherical confinement. Adv Opt Mater 10:2102162. https://doi.org/10.1002/adom.202102162
Peteya JA, Clarke JA, Li Q, Gao K-Q, Shawkey MD (2017) The plumage and colouration of an enantiornithine bird from the early cretaceous of China. Palaeontology 60:55–71
Qiang W, Li W, Li X, Chen X, Xu D (2014) Bioinspired polydopamine nanospheres: a superquencher for fluorescence sensing of biomolecules. Chem Sci 5:3018–3024
Qu X, Kirschenbaum LJ, Borish ET (2000) Hydroxyterephthalate as a fluorescent probe for hydroxyl radicals: application to hair melanin. Photochem Photobiol 71:307–313
Rageh MM, EL-Gebaly RH, Abou-Shady H, Amin DG (2015) Melanin nanoparticles (MNPs) provide protection against whole-body ɣ-irradiation in mice via restoration of hematopoietic tissues. Mol Cell Biochem 399:59–69
Reale S, Crucianelli M, Pezzella A, d’Ischia M, De Angelis F (2012) Exploring the frontiers of synthetic eumelanin polymers by high-resolution matrix-assisted laser/desorption ionization mass spectrometry: Eumelanins at their mass limits studied by MALDI mass spectrometry. J Mass Spectrom 47:49–53
Riesz J, Gilmore J, Meredith P (2006) Quantitative scattering of melanin solutions. Biophys J 90:4137–4144
Saranathan V et al (2012) Structure and optical function of amorphous photonic nanostructures from avian feather barbs: a comparative small angle X-ray scattering (SAXS) analysis of 230 bird species. J R Soc Interface 9:2563–2580
Schmaler-Ripcke J et al (2009) Production of pyomelanin, a second type of melanin, via the tyrosine degradation pathway in Aspergillus fumigatus. Appl Environ Microbiol 75:493–503
Shawkey MD, Hill GE (2006) Significance of a basal melanin layer to production of non-iridescent structural plumage color: evidence from an amelanotic Steller’s jay(Cyanocitta stelleri). J Exp Biol 209:1245–1250
Shih BB et al (2020) Influence of skin melanisation and ultraviolet radiation on biomarkers of systemic oxidative stress. Free Radic Biol Med 160:40–46
Song W, Zhang L, Ness S, Yi J (2017) Wavelength-dependent optical properties of melanosomes in retinal pigmented epithelium and their changes with melanin bleaching: a numerical study. Biomed Opt Express 8:3966
Stark KB et al (2005) Effect of stacking and redox state on optical absorption spectra of melanins−comparison of theoretical and experimental results. J Phys Chem B 109:1970–1977
Stavenga DG, Leertouwer HL, Hariyama T, De Raedt HA, Wilts BD (2012) Sexual dichromatism of the damselfly Calopteryx japonica caused by a melanin-chitin multilayer in the male wing veins. PLoS One 7:e49743
Stavenga DG, Leertouwer HL, Marshall NJ, Osorio D (2011) Dramatic colour changes in a bird of paradise caused by uniquely structured breast feather barbules. Proc R Soc B Biol Sci 278:2098–2104
Stavenga DG, Leertouwer HL, Osorio DC, Wilts BD (2015) High refractive index of melanin in shiny occipital feathers of a bird of paradise. Light Sci Appl 4:e243–e243
Surmacki A, Minias P, Kudelska K (2021) Occurrence and function of melanin-based grey coloration in Western Palaearctic songbirds (Aves: Passeriformes). Ibis 163:390–406
Swift JA (2009) Speculations on the molecular structure of eumelanin. Int J Cosmet Sci 31:143–150
Takeoka Y (2012) Angle-independent structural coloured amorphous arrays. J Mater Chem 22:23299–23309
Tran ML, Powell BJ, Meredith P (2006) Chemical and structural disorder in eumelanins: a possible explanation for broadband absorbance. Biophys J 90:743–752
Wakamatsu K, Fujikawa K, Zucca FA, Zecca L, Ito S (2003) The structure of neuromelanin as studied by chemical degradative methods: chemical structure of neuromelanin. J Neurochem 86:1015–1023
Wakamatsu K, Zippin JH, Ito S (2021) Chemical and biochemical control of skin pigmentation with special emphasis on mixed melanogenesis. Pigment Cell Melanoma Res 34:730–747
Wang Y et al (2016) Simultaneous enhancements of UV-shielding properties and photostability of poly(vinyl alcohol) via incorporation of sepia eumelanin. ACS Sustain Chem Eng 4:2252–2258
Wang Y et al (2017) A Novel UV-Shielding and transparent polymer film: when bioinspired dopamine–melanin hollow nanoparticles join polymers. ACS Appl Mater Interfaces 9:36281–36289
Wiernasz DC (1995) Male choice on the basis of female melanin pattern in Pieris butterflies. Anim Behav 49:45–51
Xiao M, Dhinojwala A, Shawkey M (2014) Nanostructural basis of rainbow-like iridescence in common bronzewing Phaps chalcoptera feathers. Opt Express 22:14625
Xiao M, Shawkey MD, Dhinojwala A (2020) Bioinspired melanin-based optically active materials. Adv Opt Mater 8:2000932
Xiao M et al (2015) Bio-inspired structural colors produced via self-assembly of synthetic melanin nanoparticles. ACS Nano 9:5454–5460
Xiao M et al (2016) Stimuli-responsive structurally colored films from bioinspired synthetic melanin nanoparticles. Chem Mater 28:5516–5521
Xiao M et al (2017) Bioinspired bright noniridescent photonic melanin supraballs. Sci Adv 3:e1701151
Xiao M et al (2018) Elucidation of the hierarchical structure of natural eumelanins. J R Soc Interface 15:20180045
Xiao M et al (2019) Experimental and theoretical evidence for molecular forces driving surface segregation in photonic colloidal assemblies. Sci Adv 5:eaax1254
Xue Q, Cao X, Zhang C, Xian Y (2018) Polydopamine nanodots are viable probes for fluorometric determination of the activity of alkaline phosphatase via the in situ regulation of a redox reaction triggered by the enzyme. Microchim Acta 185:1–9
Yang L et al (2016) Fluorescent nanocomposite for visualizing cross-talk between microRNA-21 and hydrogen peroxide in ischemia-reperfusion injury in live cells and in vivo. Anal Chem 88:11886–11891
Yang P et al (2019) Tailoring synthetic melanin nanoparticles for enhanced photothermal therapy. ACS Appl Mater Interfaces 11:42671–42679
Yin H et al (2018) Redox modulation of polydopamine surface chemistry: a facile strategy to enhance the intrinsic fluorescence of polydopamine nanoparticles for sensitive and selective detection of Fe3+. Nanoscale 10:18064–18073
Yoshioka S, Kinoshita S (2011) Direct determination of the refractive index of natural multilayer systems. Phys Rev E 83:051917
Zareba M et al (2006) Effects of photodegradation on the physical and antioxidant properties of melanosomes isolated from retinal pigment epithelium. Photochem Photobiol 82:1024
Zhang X et al (2012) Biocompatible polydopamine fluorescent organic nanoparticles: facile preparation and cell imaging. Nanoscale 4:5581
Zhang M et al (2019) Polydopamine-based tumor-targeted multifunctional reagents for computer tomography/fluorescence dual-mode bioimaging-guided photothermal therapy. ACS Appl Bio Mater 2:630–637
Zhang S et al (2020) Mimicking neuromelanin nanoparticles as a selective Pb2+ probe. Anal Chim Acta 1105:208–213
Zheng C et al (2022) Permanent low-toxicity hair dye based on Pregrafting melanin with cystine. ACS Biomater Sci Eng 8:2858–2863
Zhou X et al (2019) Artificial allomelanin nanoparticles. ACS Nano 13:10980–10990
Zi J et al (2003) Coloration strategies in peacock feathers. Proc Natl Acad Sci 100:12576–12578
Zou Y et al (2020) Regulating the absorption spectrum of polydopamine. Sci Adv 6:eabb4696
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Xiao, M., Li, W. (2023). Fundamentals and Applications of Optically Active Melanin-Based Materials. In: Gosset, G. (eds) Melanins: Functions, Biotechnological Production, and Applications. Springer, Cham. https://doi.org/10.1007/978-3-031-27799-3_7
Download citation
DOI: https://doi.org/10.1007/978-3-031-27799-3_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-27798-6
Online ISBN: 978-3-031-27799-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)