Abstract
Bile acids have several effects on fat and glucose metabolism in many tissues, including the liver, skeletal muscle, and white and brown adipose tissue. Several studies have shown that bile acid levels and composition are markedly altered after bariatric surgery. The hormonal roles of bile acids in metabolic regulation make them prime candidates as mediators of the beneficial effects of bariatric surgery. Fasting and postprandial total bile acid concentrations have been reported increased in patients after DS (duodenal switch). The release of bile in a long biliopancreatic that is not exposed to other nutrients and delivery of the bile to the distal intestine in duodenal switch and in other biliopancreatic derivations can be associated with increased weight loss, increased serum bile acids, improved glucose tolerance, and increased postprandial secretion of glucagon-like peptide-1 (GLP1).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Bile Acids
Bile acids (BAs) are synthesized from cholesterol in the liver and are components of bile. Traditionally, BAs have the function of emulsifying lipids and assisting in the absorption and digestion of dietary fats. They play a key role in the absorption of fat-soluble vitamins such as vitamins A, D, E, and K. After taking part in small intestine digestion processes, bile acids are almost completely (95%) resorbed in the distal ileum and then are taken up from portal blood through the liver (enterohepatic circulation). Excess cholesterol in the body is also converted into bile acids and eliminated by bile, thus maintaining cholesterol hemostasis [1]. Bile acids not only play a role in the absorption of lipids in the intestine but also seem to be part of a larger physiological system in response to ingested nutrients which involves glucose metabolism [1, 2]. Bile salts induce hepatic glycogen synthesis, inhibit gluconeogenesis, improve insulin sensitivity, and control glucose metabolism [2].
It has been suggested that BAs are important mediators of weight loss and metabolic changes after bariatric surgery and different BA fractions have been associated with different characteristics of glucose metabolism [3,4,5]. They perform as signaling hormones, activating nuclear and membrane-coupled receptors in the intestine, liver, muscle, and adipose tissue. Furthermore, they regulate the balance of bacterial flora, while the latter reciprocally regulate the metabolism and composition of Bas [4].
Thus, understanding the physiology of BA regulation after bariatric surgery is an important therapeutic target in treating severe obesity and its metabolic sequelae.
2 Bile Salt Physiology
Bile is predominantly composed of water and several dissolved substances, including cholesterol, amino acids, enzymes, vitamins, heavy metals, bile salts, bilirubin, phospholipids, and other constituents such as drugs and toxins. The cells that line the intrahepatic and extrahepatic bile ducts have the function of altering and refining the content of hepatically synthesized bile through a complex mechanism, controlled by a multitude of molecules, hormones, and neurotransmitters [1, 2]. Bile acids undergo chemical modification through conjugation in the liver and dehydroxylation by intestinal bacteria [6]. It is important to stress the fact that bariatric surgery also results in significant changes in intestinal microbiome and the dynamic interactions between bile acids and intestinal microbiota after bariatric surgery may contribute to metabolic improvements, although the detailed mechanisms leading to these effects require further investigation [6].
Bile salts participate in enterohepatic circulation in two types of chemical structure—primary, cholic acid and chenodeoxycholic acid, and secondary, deoxycholic acid and lithocholic acid—which are formed in the terminal ileum from the primary bile salts as a result of a structural change called 7-alpha dihydroxylation, by the action of microflora bacteria, especially the anaerobic ones. In humans, the reuptake of conjugated Bas is performed by transport protein Na+ taurocholate cotransporting polypeptide (NTCP), while the non-conjugated bile acids are absorbed by organic anion transporters which also absorb bilirubin and other anions. The total pool of bile acids in humans is tightly controlled by coordinated regulation of the expression of genes involved in the synthesis, secretion, reabsorption, and reuptake of bile acids by the liver [7, 8].
3 Increased Bile Acid Levels and the Improvement of Metabolic Syndrome After Bariatric Surgery
For over four decades, it has been known that deficiencies in insulin signaling and glycemic control can precipitate multiple disruptions in BA physiology. Several studies have shown that bile acid levels and composition are altered after bariatric surgery [2, 5, 9].
Creating a long bilioprancreatic limb that is then not exposed to other nutrients other than lipids from bile and also allowing bile to reach the distal without mixing with other nutrients, which takes place, for example, in DS and RYGB surgeries, is associated with weight loss, increased serum bile acid levels, improved glucose tolerance, and increased postprandial GLP-1 secretion. The altered anatomy leading to changes in bile acids contact and interaction with the small intestines and other nutrients have been implicated as one of the main mediators for the effect on GLP-1 levels after surgery. GLP-1 is a incretin hormone secreted by the L cells of the distal intestine usually in response to intestinal nutrients and stimulates insulin secretion [10].
4 FXR and TGR5 Bile Acid Nuclear Receptors as Molecular Targets of Surgery Bariatric
In the twenty-first century, there was the identification of BA receptors: the farnesoid receiver X receptor (FXR) and the G protein-coupled bile acid receptor 5 (TGR5). They perform as signaling molecules to regulate the lipid and glucose homeostasis [2, 9].
Animal model studies have shown that the effects of bile acids on glucose metabolism can be mediated by the activation of L cells via the TGR5 receptor and the FXR signaling [5, 9, 11]. FXR and TGR5, along with BAs, participate in the insulin release signaling mechanism. It is important to consider that BAs and their targets are present and involved in cellular bioenergetic control within the same organs and tissues impaired by insulin resistance in severe obesity. Strong evidence links markedly anomalous BA metabolism to the pathophysiology of severe obesity, insulin resistance, NAFLD (nonalcoholic fatty liver disease), and T2D (type 2 diabetes) [9].
FXR is expressed at high levels in the liver and intestine. Similar to other nuclear receptors, once activated, FXR is translocated to the cell nucleus, where it forms a dimer (in this case a heterodimer, with) and binds to hormone response elements on the DNA, which regulates the expression of certain. BAs function as endogenous ligands for FXR, so that enteric and systemic release of BAs induces FXR-driven changes in gene expression networks. The complex role of FXR in metabolic homeostasis is evident in studies in mice [11, 12]. In the liver, FXR activation suppresses hepatic BA synthesis, changes BA composition, and contributes to liver regeneration, as well as to glucose, lipid, and cholesterol homeostasis.
In addition to expression in the liver, FXR is also expressed in the intestine, where it regulates the production of the endocrine hormone FGF19 which, along with the hepatic FXR, seems to be involved in the control of the synthesis, transport, and metabolism of BA [11,12,13]. FXR plays a central role in mediating the negative feedback regulation of BA synthesis. Bile acid biosynthesis is tightly controlled by intrahepatic negative feedback signaling elicited by bile acid binding to FXR, as well as by enterohepatic communication involving ileal bile acid reabsorption [3, 8]. In severely obese non-diabetic adults, the body mass index can be positively correlated with FXR mRNA expression in the liver and ileum, and inversely related to hepatic NTCP expression [9, 14]. That reduces fasting or postprandial BA reuptake in the liver [14]. In fact, the expected inhibitory effects of FXR positively regulate BA synthesis [9, 14, 15].
BAs in the small and large intestine partly regulate the intestinal microbiota, incretin secretion, and FGF15/19 production, which then assists modulating whole-body lipid, glucose, and energy homeostasis. As a primary bile acid receptor, FXR has been investigated for its role in bariatric surgery [11]. Absence of FXR in animals results in those being unable to maintain lower body weights after vertical gastroplasty; rather, they increase energy intake to compensate for early postsurgical weight loss [2, 16].
Conversely, TGR5 functions as a cell surface receptor for bile acids. The receptor is implicated in the suppression of macrophage function and in the regulation of energy homeostasis by bile acids [5]. Bile acids, through the activation of TGR5 in muscle and brown adipose tissue, are capable of increasing energy expenditure and preventing—or even reversing—induced obesity in mice [5, 9, 17]. These changes resulted in increased TGR5 signaling in the ileum and brown adipose tissues, concomitant with improved glucose control and increased energy expenditure. It is accepted that bariatric surgery achieves its postoperative therapeutic effects through improving TGR5 signaling.
5 Bile Acid and Duodenal Switch and Its Derivatives
T2D resolution occurs most commonly and rapidly after Roux-en-Y gastric bypass (RYGB) and biliopancreatic bypass (BPD), which share the common feature of a proximal small intestine bypass. Surgically induced decrease in caloric intake, weight and fat mass loss, changes in carbohydrate, fat and protein absorption, or changes in intestinal hormone release all combined promote the dramatic effect of bariatric surgery on T2D [9, 10, 18].
Research in humans indicate that gene expression of the farnesoid X receptor (FXR), which is a target of the BAs, is increased in the liver but decreased in the small intestine after RYGB. In contrast, intestinal expression of the transmembrane G protein-coupled BA receptor (TGR5) is upregulated after surgery. These changes were followed by NAFLD and/or T2D regression after 1 year [9]. Animal models suggest that the control of T2D after gastrointestinal bypass surgery may result directly from the redirection of nutrients in the intestine [4, 15].
Systemic BA concentrations can begin to increase as early as 1 week after RYGB and VSG (vertical gastrectomy), which contrasts directly with the reduced BAs after non-surgical diet [2, 6, 15]. Interestingly, higher systemic concentrations may help explain why intestinal FXR and TGR5 are differentially impacted by RYGB. Whereas FXR is a nuclear receptor and requires intracellular BA transport for ligand-dependent activation, TGR5 is a basolateral receptor that, unlike FXR, shows an increased signaling potential in response to increased systemic BAs. Growing evidence indicate that altered BA physiology and signaling through FXR and TGR5 may support or enhance metabolic improvements related to weight loss [15].
Since FXR inhibits glycolysis, downregulation of FXR after DS and other derivations may support the improvement in glucose uptake and use in the small intestine, observed after bariatric surgery [11, 14, 15]. In addition to the direct effects of FXR signaling in hepatic BA, glucose, and lipid metabolism, the inhibition of intestinal FXR after RYGB is also associated with upregulation of TGR5 [5, 9], which probably contributes to increased postprandial levels of hormones such as GLP1 and YY peptide (PYY) by means of enteroendocrine L cells after bariatric surgery [9, 15].
BAs stimulate the secretion of gastrointestinal hormones (such as GLP-1, PYY, and GIP) through the activation of TGR5 receptors located on the basolateral membrane of enterocytes. Therefore, the higher GLP-1 postprandial levels observed in individuals who underwent BPD/DS (biliopancreatic diversion with duodenal switch) can be attributed to bile acid interactions across a longer intestinal length, leading to greater stimulation of GLP-1-producing cells [19].
Importantly, DS, VSG, and RYGB result in greater weight loss and changes in BA concentrations, as well as greater changes in FXR and TGR5 signaling and higher cardiometabolic improvements as compared to the restrictive-only adjustable gastric band procedure [2, 4, 9].
Total bile acid concentrations increased substantially at 5 years after RYGB and DS with greater increases in total and primary bile acids after DS; however this effect was greater in patients with prior cholecystectomy. Higher levels of total bile acid in 5 years have been associated with decreased body mass index (BMI), greater weight loss, and lower total serum cholesterol [4]. A long biliopancreatic loop may be important for metabolic improvement after bariatric surgery and suggests that BAs are involved in this process [4, 12].
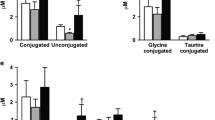
The mechanisms explaining the higher BA concentrations after BPD/DS (biliopancreatic diversion with duodenal switch), when compared to RYGB, are unknown and may have several different origins. Differences in intestinal absorption may contribute to it. The longer biliopancreatic loop in BPD/DS leads to the transport of high concentrations of primary BAs through a longer segment of small intestine that sees no other form of luminal nutrients than lipids of bile and only mixing with food and promoting digestion and absorption of fat in the common loop. The higher primary BA concentrations after BPD/DS were predominantly due to an increase in unconjugated and glycine-conjugated primary Bas, but the site of greater absorption (BP or common channel) and whether differences in liver BA reuptake from portal circulation occurs are still under investigation [4, 9, 15].
In normal anatomy, BAs in the proximal intestine are mostly conjugated primary BAs. A possible explanation for the increase in unconjugated BAs could be the microbial contamination in the small intestine due to changes in intestinal anatomy, resulting in higher unconjugated BA production by the intestinal microflora bacteria and the subsequent absorption at the biliopancreatic loop [4, 14, 15]. This may mean that the higher BA levels we observed after BPD/DS (and also RYGB) does not necessarily reflect an actual increase in the size of the bile salt reservoir, but may be a result of shorter enterohepatic cycles [4, 15, 20]. Altered microbial metabolism may be involved, since it may modulate the BA pool. Changes in the BA synthesis or excretion may also be involved [14, 15].
6 Conclusions
Several studies have shown that fasting and postprandial bile acid levels and composition are altered after bariatric surgery. The hormonal roles of bile acids in metabolic regulation make them prime candidates for mediators of the beneficial effects of bariatric surgery. Growing evidence indicates that altered BA physiology and signaling through FXR and TGR5 may support or enhance metabolic improvements related to weight loss. BAs stimulate the secretion of gastrointestinal hormones (such as GLP-1, PYY, and GIP) through activation of TGR5 receptors located in the basolateral membranes of enterocytes.
A long biliopancreatic loop seems to be related to an increase in unconjugated BA through a higher concentration of primary BAs, and this mechanism may be one of the driving forces of metabolic improvement after bariatric surgery.
References
Dave HD, Al Obaidi NM. Physiology, biliary. Treasure Island (FL): StatPearls; 2021.
Nguyen N, Brethauer SA, Morton JM, Ponce J, Rosenthal RJ. The ASMBS textbook of bariatric surgery. Cham: Springer; 2020.
Staels B, Fonseca VA. Bile acids and metabolic regulation: mechanisms and clinical responses to bile acid sequestration. Diabetes Care. 2009;32(Suppl 2):S237.
Risstad H, Kristinsson JA, Fagerland MW, le Roux CW, Birkeland KI, Gulseth HL, et al. Bile acid profiles over 5 years after gastric bypass and duodenal switch: results from a randomized clinical trial. Surg Obes Relat Dis. 2017;13(9):1544–53. https://doi.org/10.1016/j.soard.2017.05.024.
Duboc H, Tolstanova G, Yuan PQ, Wu VS, Kaji I, Biraud M, et al. Reduction of epithelial secretion in male rat distal colonic mucosa by bile acid receptor TGR5 agonist, INT-777: role of submucosal neurons. Neurogastroenterol Motil. 2016;28(11):1663–76.
Browning MG, Campos GM. Bile acid physiology as the potential driver for the sustained metabolic improvements with bariatric surgery. Surg Obes Relat Dis. 2017;13(9):1553–4. https://doi.org/10.1016/j.soard.2017.06.005.
National Institute of Diabetes and Digestive and Kidney Diseases. Bile acids. In: LiverTox: clinical and research information on drug-induced liver injury. Bethesda: National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
Hundt M, Basit H, John S. Physiology, bile secretion. Treasure Island (FL): StatPearls; 2020.
Browning MG, Pessoa BM, Khoraki J, Campos GM. Changes in bile acid metabolism, transport, and signaling as central drivers for metabolic improvements after bariatric surgery. Curr Obes Rep. 2019;8(2):175–84.
Rubino F, Forgione A, Cummings DE, Vix M, Gnuli D, Mingrone G, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244(5):741–9.
Cully M. Obesity and diabetes: FXR and JAK step up to BAT. Nat Rev Drug Discov. 2015;14(2):91. https://doi.org/10.1038/nrd4543.
Fang S, Suh JM, Reilly SM, Yu E, Osborn O, Lackey D, et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015;21(2):159–65. https://doi.org/10.1038/nm.3760.
Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, et al. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81(5):687–93.
Haeusler RA, Camastra S, Nannipieri M, Astiarraga B, Castro-Perez J, Xie D, et al. Increased bile acid synthesis and impaired bile acid transport in human obesity. J Clin Endocrinol Metab. 2016;101(5):1935–44.
Steven MS, Fink MY. Bile acid signaling and bariatric surgery. Physiol Behav. 2019;176(3):139–48.
Mazzini GS, Khoraki J, Dozmorov M, Browning MG, Wijesinghe D, Wolfe L, et al. Concomitant PPARα and FXR activation as a putative mechanism of NASH improvement after gastric bypass surgery: a GEO datasets analysis. J Gastrointest Surg. 2019;23(1):51–7.
Buchwald H. The evolution of metabolic/bariatric surgery. Obes Surg. 2014;24(8):1126–35.
Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Nanni G, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386(9997):964–73. https://doi.org/10.1016/S0140-6736(15)00075-6.
Pereira SS, Guimarães M, Almeida R, Pereira AM, Lobato CB, Hartmann B, et al. Biliopancreatic diversion with duodenal switch (BPD-DS) and single-anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S) result in distinct post-prandial hormone profiles. Int J Obes. 2019;43(12):2518–27.
Sousa CS, Bispo DM, Cunha ALSM. Capacitação em cirurgia robótica no programa de residência em enfermagem perioperatória. Rev SOBECC. 2016;21(4):198.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kreimer, F., Chaves, F.K.P., Campos, G.M. (2023). Metabolic Syndrome and the Influence of Bile Acids. In: Teixeira, A., et al. Duodenal Switch and Its Derivatives in Bariatric and Metabolic Surgery. Springer, Cham. https://doi.org/10.1007/978-3-031-25828-2_67
Download citation
DOI: https://doi.org/10.1007/978-3-031-25828-2_67
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-25827-5
Online ISBN: 978-3-031-25828-2
eBook Packages: MedicineMedicine (R0)