Abstract
Elevated lipoprotein(a) [Lp(a)] is a valuable risk factor for development of atherosclerotic cardiovascular diseases (ASCVD). Within this chapter, we discuss the molecular and cellular pathways related to the pro-inflammatory and pro-atherogenic activity of Lp(a). Lp(a) is considered as a potential auto-antigen and DAMP-containing particle. Attention is paid to its capacity to bind to and transport several mediators, involved in the initiation and progression of arterial wall inflammation. We put in this chapter currently available information about Lp(a) and autoantibodies with different structure against Lp(a); innate immunity cells; pro-inflammatory status in ASCVD pathogenesis and Lp(a) as a carrier of inflammatory and repair mediators.
The effect of Lp(a) on the maturation, phenotype, and effector functions of immune cells participating in atherogenesis, particularly monocytes and macrophages, is also discussed. Age-associated chronic pro-inflammatory status, or inflammaging, accompanied by immune perturbations may contribute to the realization of pathophysiological effects of Lp(a).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Lipoprotein(a) [Lp(a)] is an atherothrombogenic lipoprotein particle that differs in its composition and physicochemical and biological properties from other lipoproteins and contains a unique apolipoprotein(a) molecule [apo(a)]. The relationship between the immune system and lipid metabolism has been evaluated for many decades. An increased blood Lp(a) concentration is a proven risk factor for atherosclerotic cardiovascular disease (ASCVD). Lawn’s hypothesis about Lp(a) as a repair factor remains relevant until today (Lawn et al. 1992). Recent studies suggest participation of humoral and cell immunity in wound healing and regeneration and in inflammatory diseases (Masoomikarimi and Salehi 2022; Eming et al. 2017). An elevated Lp(a) level in long-living persons suggests possible participation of immunological factors in both the physiological and pathophysiological Lp(a) pathways (Panza et al. 2007). It is assumed that with increased life expectancy and in the presence of “inflammaging,” [Inflammaging is the long-term result of the chronic physiological stimulation of the innate immune system, which can become damaging during ageing—a period of life largely unpredicted by evolution (Franceschi et al. 2018)] Lp(a) may become a factor contributing to atherosclerosis and other inflammatory diseases (Franceschi et al. 2018).
Lipoprotein(a) and IgG and IgM Autoantibodies (AAbs)
The production of immunoglobulins (Ig) by B cells is necessary for the recognition, neutralization, and removal of exogenous and endogenous pathogens and for maintaining homeostasis. The concept of “natural” antibodies synthesized by B1 cells with specificity to alien and native proteins was first proposed in 1908 by Ehrlich (Piro et al. 2008). Natural IgM antibodies are encoded by the germline cells, and they are present in the umbilical cord blood of newborns. It is assumed that the level of natural IgM antibodies is maintained constant throughout life (Holodick et al. 2017). The main biological functions of natural IgM are removal of apoptotic cells, protection from infection, and maintenance of tissue homeostasis (Reyneveld et al. 2020; Wang et al. 2016). The protective effect of IgM AAbs to oxidized LDL (low-density lipoprotein) (oxLDL) produced by B1 cells has been described in several studies and literature reviews (Tsimikas et al. 2012; van den Berg et al. 2018; Pattarabanjird et al. 2021). The presence of circulating Lp(a)-containing immune complexes in the plasma of patients with coronary heart disease (CHD), healthy donors, and patients with autoimmune diseases has been reported in several studies. Most of the immune complexes found in the plasma of healthy donors contained IgM AAbs against Lp(a), unlike patients with CHD (Wang et al. 2003; Sabarinath and Appukuttan 2015; Klesareva et al. 2016).
Recently, we have shown that the levels of IgM AAbs against Lp(a) were higher in patients without atherosclerosis or non-stenosing lesions of the coronary arteries (Afanasievа et al. 2016b). Such a protective function of these IgM AAbs was also present in patients with severe hypercholesterolemia (Klesareva et al. 2018). In a retrospective study of 1228 patients, the lower the IgM level of Lp(a) AAbs and the higher the concentration of Lp(a), the more vascular beds there were with stenosing atherosclerotic lesions (Tmoyan et al. 2021).
The autoimmune theory of atherosclerosis was formulated by Klimov more than 40 years ago. He showed that modified lipoproteins acquire autoantigenic properties and trigger an immune response to the “altered self” (Klimov 1990). The role of autoantigens is played by modified LDL, as well as lipoproteins containing oxidized phospholipids (Virella and Lopes-Virella 2008). Elevated plasma levels of IgG AAbs to oxLDL are associated with angiographically verified coronary atherosclerosis and progression of carotid lesions (Salonen et al. 1992). Previously, a direct relationship between the level of IgG AAbs against Lp(a) and the number of affected coronary arteries was demonstrated (Afanas’eva et al. 2014). The content of IgG AAbs against MDA (malondialdehyde)-LDL in the upper quartile was associated with the risk of cardiovascular events at a 10-year follow-up (Prasad et al. 2017). However, the role of Lp(a), as well as oxLDL, as possible specific autoantigen for B2 cells remains controversial (Ravandi et al. 2011). Nevertheless, studies aimed at using immunoglobulins specific to oxidized epitopes present on lipoproteins’ and apoptotic cells’ surfaces for the treatment of ASCVD are in progress (de Vries et al. 2021; Pluijmert et al. 2021; Ståhle et al. 2020).
Lp(a), such as LDL-like particles, also could be affected by modification of their protein and/or lipid compounds; such modifications activate humoral immune responses and create AAbs formation. Lp(a) AAbs immune complexes removed by macrophages can be transferred to foam cells.
The IgM and IgG antibody classes against Lp(a) detected in human serum appear to have not only different origins but also different functions. Natural IgM implies an evolutionary advantage to neutralize Lp(a) and to eliminate it. The appearance of autoantibodies of different IgG subclasses indicates the activation of adaptive immunity, which perceives Lp(a) as the antigen, and causes subsequent development of inflammatory reactions.
Lipoprotein(a) and Innate Immunity Cells
Monocytes and macrophages play a critical role in innate immunity (Libby et al. 2013) and have been the subject of numerous studies in connection with Lp(a). Lp(a) was detected in macrophage cell-rich areas of atherosclerotic plaques in humans according to morphology and immunohistochemistry studies (Sotiriou et al. 2006). On the other hand, individuals with elevated Lp(a) level exhibit enhanced accumulation of peripheral blood mononuclear cells in the arterial wall compared to individuals with normal levels of Lp(a) (van der Valk et al. 2016). Apo(a) stimulates the production of reactive oxygen species and matrix metalloproteinase-9 by collagen-adherent monocytes, and this effect was inversely associated with the molecular weight of apo(a) (Sabbah et al. 2019). Apo(a) also caused increased secretion of IL-8 by macrophages of the THP-1 and U-937 cell lines (Scipione et al. 2015). Monocytes isolated from subjects with elevated Lp(a) demonstrated an enhanced cell surface expression of chemokine receptors, adhesion molecules, and scavenger receptors (CCR7, CD62L, CD11b, CD11c, CD29, CD36, SR-A). Apo(a) upregulates the expression of the β2-integrin Mac-1 (CD11b/CD18), thereby facilitating cell adhesion and migration capacity. Several signaling cascades leading to altered gene expression profiles were found to contribute to Lp(a)-induced monocyte chemotactic activity (Scipione et al. 2015; Dzobo et al. 2022).
Besides displaying an activated and proinflammatory phenotype, monocytes isolated from individuals with elevated Lp(a) exhibited an increased secretion of proinflammatory cytokines (IL-1β, IL-6, TNFα) and a decrease in the anti-inflammatory cytokine IL-10 after stimulation via toll-like receptors. OxPLs associated with apo(a) as potent danger-associated molecular patterns (DAMPs) could be responsible for these effects (Koschinsky and Boffa 2022).
Apo(a) antisense treatment resulted in downregulation of proinflammatory gene expression in monocytes, including interferon (IFN)α, IFNγ, and toll-like receptor (TLR) pathways, and subsequent changes in monocyte phenotype and function, that is, a reduction in chemokine receptors CCR2 and CX3CR1 and transendothelial migratory capacity (Stiekema et al. 2020).
The number of circulating monocytes in apo(a) transgenic mice was four times higher than in wild-type mice and remained elevated for 3 weeks after Ca2+-induced vascular damage (Huang et al. 2014). Also, Lp(a) affects the maturation of monocytes in humans (Schnitzler et al. 2020).
Monocytes are divided into three subpopulations, depending on the content of CD14 and CD16 surface markers, classical CD14++CD16−, intermediate CD14++CD16+, and nonclassical CD14+CD16++, while the latter two populations have the most pronounced proinflammatory and profibrotic potential. The participation of circulating monocytes in atherogenesis has been proven (Vergallo and Crea 2020), but the contribution of various subpopulations of monocytes to chronic inflammatory states is currently under discussion (Yang et al. 2014; Ożańska et al. 2020).
The high content of CD16+ monocytes is associated with unstable atherosclerotic plaques in the coronary arteries (Kashiwagi et al. 2010) and predicts the risk of cardiovascular events (Rogacev et al. 2012). In CHD patients, an increased content of intermediate monocytes CD14++CD16+ occurs with hyperlipoproteinemia(a) (Krychtiuk et al. 2015a), atherogenic dyslipidemia (Krychtiuk et al. 2015b), and dysfunctional high-density lipoproteins (Krychtiuk et al. 2014). The association of elevated Lp(a) concentration with absolute and relative content of CD14+CD16++ was shown in a retrospective study (Afanasieva et al. 2021). Since the function of this subpopulation is to remove “cellular debris,” it is assumed that it contributes to elimination of excess Lp(a).
Neutrophil granulocytes are the largest population of circulating phagocytizing leukocytes capable of synthesizing a wide range of substances. Neutrophils and “neutrophil extracellular traps” (NETs) formed by them were found in atherosclerotic plaques of laboratory animals and humans (Afanasieva et al. 2021). NETs stimulate the production of IL-1 by macrophages and activate IL-17-producing T-helpers (Th17) in apoE-deficient mice, contributing to inflammation in the vessel wall (Döring et al. 2017). There are no data on the effect of Lp(a) or apo(a) on the formation of neutrophil traps. The absolute number of neutrophils and the neutrophil-lymphocyte index, as well as the concentration of Lp(a), was significantly higher in patients with stenosing atherosclerosis of various vascular beds (Tmoyan et al. 2021). The evaluation of the effect of Lp(a) on neutrophil activation is a promising avenue of further research.
The Role of Lipoprotein(a) and Proinflammatory Status in ASCVD Pathogenesis
Data on the association of increased Lp(a) concentration with systemic inflammation and its markers are ambiguous (Pirro et al. 2017). The risk of cardiovascular events associated with Lp(a) was significantly higher in the presence of “proinflammatory” genotype IL-1 (Naka et al. 2018) or elevated C-reactive protein level (Puri et al. 2020).
A higher lymphocyte count is associated with a higher apoB level; Lp(a) was inversely associated with basophil count in men but not in women according to a population study with 417,132 participants (Tucker et al. 2021). Low molecular weight apo(a) phenotype, reduced lymphocyte count, and increases in neutrophil granulocytes potentiated the risk of CHD in patients with type 2 diabetes (Suzuki et al. 2013).
The combination of a higher absolute monocyte count (>0.54 × 109 cells/mL) with elevated Lp(a) (≥30 mg/dL) is associated with higher risk of major adverse cardiovascular events (MACE) in patients with premature CHD manifestation (Afanasieva et al. 2022) (Fig. 16.1). An increase of Lp(a) concentration and the percentage of CD14++CD16+ monocytes potentiated risk of multivessel coronary disease (Afanasieva et al. 2021; Filatova et al. 2022) (Fig. 16.2).
The proportion of major adverse cardiovascular events (MACE) in patients with premature coronary heart disease depending on blood monocyte count and lipoprotein(a) concentration. Two-hundred adult patients with early coronary heart disease manifestation (before 55 years in men and 60 years in women) were enrolled, median follow-up 12 years. MACE, nonfatal myocardial infarction, ischemic stroke, coronary artery bypass grafting, and hospitalization for unstable angina (Afanasieva et al. 2022)
Association of lipoprotein(a), CD14++CD16+ intermediate monocyte subpopulation, and their association with coronary atherosclerosis severity (n = 150). Odds ratio (OR) of triple vessel disease vs no significant, and 1–2-vessel disease was calculated according to logistic regression analysis adjusted for age, sex, type 2 diabetes, and hypertension (Afanasieva et al. 2021)
A lower level of IgM AAbs against Lp(a) is negatively correlated with the concentration of sCD25 [the soluble form of the IL-2 receptor and a surrogate marker of T-cell activation (Brusko et al. 2009)] and associated with stenosing coronary atherosclerosis (Afanasievа et al. 2016b). This fact may serve as a confirmation of participation of both Lp(a) and T-cells in atherogenesis and also the immunomodulatory ability of IgM AAbs against Lp(a) (Wang et al. 2016).
Systemic inflammation accompanies age-related changes in lymphocyte subpopulations (Thomas et al. 2020). In patients with ASCVD, the number of naïve lymphocytes, including regulatory cells, decreases with age, while the level of effector populations, that is, Th1 and Th17, remains constant (Filatova et al. 2021). T-Lymphocytes with predominating Th1 are detected in atherosclerotic plaques (Saigusa et al. 2020). Th17, a subpopulation of CD4+ lymphocytes producing IL-17, also has a proatherogenic effect. Th17 cells participate in the immune response against their own and alien antigens by attracting myeloid cells to a place of inflammation, activating lymphocytes and secreting proinflammatory cytokines (Gao et al. 2010; Park et al. 2005). On the contrary, regulatory T-cells have anti-inflammatory activity and inhibit atherogenesis (Albany et al. 2019). Thus, age-related deficiency of regulatory cells and a shift of the immune balance toward effector populations may contribute to atherosclerosis progression.
Activation and increased amounts of Th17 are related to the progression of atherosclerosis and risk of coronary events (Liuzzo et al. 2013). The ratio of circulating Treg/Th17 is reduced in patients with severe coronary atherosclerosis (Potekhina et al. 2015). The concentration of Lp(a) is not associated with the content of various T-cell subpopulations (Afanasieva et al. 2016a, b). However, an increased content of circulating Th17 (% of CD4+ lymphocytes), as well as a reduced content of Treg or IL-10 CD4+-producing cells along with Lp(a) concentrations above 12 mg/dL, is associated with severe coronary atherosclerosis (Afanasievа et al. 2016b) and carotid atherosclerosis progression (Afanasieva et al. 2016a). Thus, the increased concentration of Lp(a) and proinflammatory status with some shifts in immunity could potentiate atherosclerosis progression.
Lipoprotein(a) as a Carrier of Inflammatory Mediators
Differences in the physicochemical and immunochemical properties of LDL and Lp(a) have been noted for a long time (Zawadzki et al. 1988). The apo(a) moiety has a binding site for oxidized phospholipid (oxPL) that determines its proinflammatory effects on immune cells (Koschinsky and Boffa 2022).
Proteomic analysis shows that Lp(a) may serve as a carrier of many protein molecules, and their spectrum differs in Lp(a) and LDL (Bourgeois et al. 2020a; von Zychlinski et al. 2011, 2014). These proteins can participate in the processes of oxidation, cell proliferation and intercellular interactions, immunomodulation and activation of the complement system, and blood clotting (Bourgeois et al. 2021).
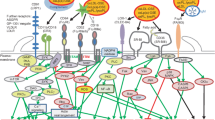
Such a variety of proteins can provide Lp(a) particles with the ability to participate in the response to injury or damage. Possible ways that Lp(a) participates in activation of the immune system via its plasma components are shown in Fig. 16.3.
Possible mechanisms of lipoprotein(a) contribution to immune cell activation. lysoPC lysophosphatidylcholines, LPARs LPA receptors or G-protein-coupled receptors, IL-1β interleukin 1β, IL-6 interleukin 6, TNF tumor necrosis factor, CXCL2 chemokine (C–X–C motif) ligand 2, MCP-1 monocyte chemoattractant protein 1, mRNA messenger RNA, LRP-1 low-density lipoprotein receptor-related protein 1, TGFβ transforming growth factor beta, IFNγ interferon γ, PRRs pattern recognition receptors, oxPL oxidized phospholipids, PLA2 phospholipase A2, α2M alpha-2-macroglobulin, PCSK9 proprotein convertase subtilisin/kexin type 9, C3 and C4 complement components 3 and 4, and apo(a) apolipoprotein(a)
The complement components C3 and C4 associated with Lp(a) could determine the interaction of Lp(a) with innate and acquired immunity. The complex of Lp(a) with α2 macroglobulin can interact with low-density lipoprotein receptor-related protein 1 (LRP-1) and can not only contribute to the internalization of Lp(a) with high molecular weight isoforms of apo(a) (März et al. 1993) but also induce the migration of myeloid cells, such as monocytes and neutrophils.
Lp(a) constitutes the main pool of lipoprotein-associated proprotein convertase subtilisin/kexin type 9 PCSK9 (Tavori et al. 2016). There is evidence of the modulating effect of PCSK9 on cell immunity (Liu and Frostegard 2018; Kim et al. 2019). Also, PCSK9 can regulate the number of CD36 and LRP-1 receptors (Shapiro et al. 2018), which are expressed by hematopoietic cells, participating in the processes of hemostasis, inflammation, and tissue regeneration. The binding of PCSK9 to CD36 (Qi et al. 2021) can be recognized as a “danger signal” of innate immunity (Silverstein 2021).
Lp(a) as a possible carrier of autotaxin and a source of lysophosphatidic acid is associated with calcification and aortic valve stenosis (Bouchareb et al. 2015; Bourgeois et al. 2020b). The lysophosphatidic acid participates in the differentiation and homing of T-lymphocytes (Zhang et al. 2012; Knowlden and Georas 2014). Both facts suggest another possible mechanism of Lp(a) action on the immune system.
An association of MCP-1 with Lp(a) via oxidized phospholipids of Lp(a) has been described (Wiesner et al. 2013). The attachment of Lp(a) containing MCP-1 at the site of injury can lead to increased recruitment of monocytes. Thus, proteins associated with the Lp(a) particle as well as oxPL may explain its proinflammatory properties.
Many properties of Lp(a), as well as its biological roles, remain a mystery despite more than 50 years of research. Lp(a) is able to carry affected areas not only the cholesterol necessary for the synthesis of new cells but also biologically active components that attract phagocytes of the innate immune system. It can be assumed that the original role of Lp(a) as a factor in damage repair and transport systems has largely been lost at the present time. An increased concentration of Lp(a) set against the background of genetic, epigenetic, and environmental variables has become a powerful risk factor for atherosclerotic cardiovascular diseases. We designed an immunosorbent for specific Lp(a) apheresis and proved that specific Lp(a), but not LDL, removal by extracorporeal treatment can lead to stabilization and even regression of atherosclerotic lesions in coronary and carotid arteries (Pokrovsky et al. 2016, 2020). This study was the first direct clinical observation and confirmation of Lp(a) atherogenicity in humans (Pokrovsky et al. 2017). The elucidation of molecular and cellular mechanisms of Lp(a) involvement in inflammatory remodeling of the arterial wall engaging the Lp(a) immunity axis is a promising direction for the development of new therapeutic approaches.
Lp(a) is an extremely interesting polymolecular complex, and as we learn more about it, it is clear the less we understand about its enormous functional range and its capacity to interact with and influence important pathways, such as immunity, inflammation, thrombosis, and oxidation.
References
Afanas’eva OI, Klesareva EA, Levashov PA, Berestetskaya YV, Ezhov MV, Artem’eva NV, Pokrovskii SN. Autoantibodies against lipoprotein(a) in patients with coronary heart disease. Kardiologiya. 2014;54(6):4–8.
Afanasieva OI, Pylaeva EA, Arefieva TI, Klesareva EA, Afanasieva MI, Potekhina AV, Shchinova AM, Balakhonova TV, Pogorelova OA, Tripoten MI, Pokrovsky SN. Lipoprotein(a) and T-helper cells as independent predictors of rapid progression of carotid atherosclerosis. Atherosclerosis. 2016a;252:e126–7.
Afanasieva OI, Pylaeva EA, Klesareva EA, Potekhina AV, Provatorov SI, Afanasieva MI, Krasnikova TL, Masenko VP, Arefieva TI, Pokrovsky SN. Lipoprotein(a), its autoantibodies, and circulating T lymphocyte subpopulations as independent risk factors for coronary artery atherosclerosis. Ter Arkh. 2016b;88(9):31–8.
Afanasieva OI, Filatova AY, Arefieva TI, Klesareva EA, Tyurina AV, Radyukhina NV, Ezhov MV, Pokrovsky SN. The association of lipoprotein(a) and circulating monocyte subsets with severe coronary atherosclerosis. J Cardiovasc Dev Dis. 2021;8(6):63.
Afanasieva OI, Tyurina AV, Klesareva EA, Arefieva TI, Ezhov MV, Pokrovsky SN. Lipoprotein(a), immune cells and cardiovascular outcomes in patients with premature coronary heart disease. J Pers Med. 2022;12(2):269.
Albany CJ, Trevelin SC, Giganti G, Lombardi G, Scottà C. Getting to the heart of the matter: the role of regulatory T-cells (Tregs) in cardiovascular disease (CVD) and atherosclerosis. Front Immunol. 2019;10:2795.
Bouchareb R, Mahmut A, Nsaibia MJ, Boulanger MC, Dahou A, Lépine JL, Laflamme MH, Hadji F, Couture C, Trahan S, Pagé S, Bossé Y, Pibarot P, Scipione CA, Romagnuolo R, Koschinsky ML, Arsenault BJ, Marette A, Mathieu P. Autotaxin derived from lipoprotein(a) and valve interstitial cells promotes inflammation and mineralization of the aortic valve. Circulation. 2015;132(8):677–90.
Bourgeois R, Girard A, Perrot N, Guertin J, Mitchell PL, Couture C, Gotti C, Bourassa S, Poggio P, Mass E, Capoulade R, Scipione CA, Després AA, Couture P, Droit A, Pibarot P, Boffa MB, Thériault S, Koschinsky ML, Mathieu P, Arsenault BJ. A comparative analysis of the lipoprotein(a) and low-density lipoprotein proteomic profiles combining mass spectrometry and Mendelian randomization. CJC Open. 2020a;3(4):450–9.
Bourgeois R, Devillers R, Perrot N, Després AA, Boulanger MC, Mitchell PL, Guertin J, Couture P, Boffa MB, Scipione CA, Pibarot P, Koschinsky ML, Mathieu P, Arsenault BJ. Interaction of autotaxin with lipoprotein(a) in patients with calcific aortic valve stenosis. JACC Basic Transl Sci. 2020b;5(9):888–97.
Bourgeois R, Bourgault J, Despres AA, Perrot N, Guertin J, Girard A, Mitchell PL, Gotti C, Bourassa S, Scipione CA, Gaudreault N, Boffa MB, Koschinsky ML, Pibarot P, Droit A, Thériault S, Mathieu P, Bossé Y, Arsenault BJ. Lipoprotein proteomics and aortic valve transcriptomics identify biological pathways linking lipoprotein(a) levels to aortic stenosis. Metabolites. 2021;11(7):459.
Brusko TM, Wasserfall CH, Hulme MA, Cabrera R, Schatz D, Todd Atkinson MA. Influence of membrane CD25 stability on T lymphocyte activity: implications for immunoregulation. PLoS One. 2009;4(11):e7980.
de Vries MR, Ewing MM, de Jong RCM, MacArthur MR, Karper JC, Peters EAB, Nordzell M, Karabina SAP, Sexton D, Dahlbom I, Bergman A, Mitchell JR, Frostegård J, Kuiper J, Ninio E, Jukema JW, Pettersson K, Quax PHA. Identification of IgG1 isotype phosphorylcholine antibodies for the treatment of inflammatory cardiovascular diseases. J Intern Med. 2021;290(1):141–56.
Döring Y, Soehnlein O, Weber C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ Res. 2017;120(4):736–43.
Dzobo KE, Kraaijenhof JM, Stroes E, Nurmohamed NS, Kroon J. Lipoprotein(a): an underestimated inflammatory mastermind. Atherosclerosis. 2022;349:101–9.
Eming SA, Wynn TA, Martin P. Inflammation and metabolism in tissue repair and regeneration. Science. 2017;356(6342):1026–30.
Filatova AY, Potekhina AV, Aref'eva TI. Age-associated characteristics of CD4+ T-cell composition in patients with atherosclerosis. Immuno. 2021;1(3):277–84.
Filatova AY, Potekhina AV, Radyukhina NV, Ruleva NY, Provatorov SI, Aref'eva TI. Circulating monocyte populations in patients with coronary atherosclerosis. Future Cardiol. 2022;18(6):455–60.
Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14(10):576–90.
Gao Q, Jiang Y, Ma T, Zhu F, Gao F, Zhang P, Guo C, Wang Q, Wang X, Ma C, Zhang Y, Chen W, Zhang L. A critical function of Th17 proinflammatory cells in the development of atherosclerotic plaque in mice. J Immunol. 2010;185(10):5820–7.
Holodick NE, Rodríguez-Zhurbenko N, Hernández AM. Defining natural antibodies. Front Immunol. 2017;8:872.
Huang M, Gong Y, Grondolsky J, Hoover-Plow J. Lp(a)/apo(a) modulate MMP-9 activation and neutrophil cytokines in vivo in inflammation to regulate leukocyte recruitment. Am J Pathol. 2014;184(5):1503–17.
Kashiwagi M, Imanishi T, Tsujioka H, Ikejima H, Kuroi A, Ozaki Y, Ishibashi K, Komukai K, Tanimoto T, Ino Y, Kitabata H, Hirata K, Akasaka T. Association of monocyte subsets with vulnerability characteristics of coronary plaques as assessed by 64-slice multidetector computed tomography in patients with stable angina pectoris. Atherosclerosis. 2010;212(1):171–6.
Kim YU, Kee P, Danila D, Teng B-B. A critical role of PCSK9 in mediating IL-17-producing T cell responses in hyperlipidemia. Immune Netw. 2019;19(6):e41.
Klesareva EA, Afanas’eva OI, Donskikh VV, Adamova IY, Pokrovskii SN. Characteristics of lipoprotein(a)-containing circulating immune complexes as markers of coronary heart disease. Bull Exp Biol Med. 2016;162(2):231–6.
Klesareva EA, Afanasieva OI, Kononova EV, Utkina EA, Ezhov MV, Balakhonova TV, Afanasieva MI, Pokrovsky SN. Raised IgM autoantibody titer to lipoprotein(a) as antiatherogenic factor in severe hypercholesterolemia patients. Russ J Cardiol. 2018;8:13–20.
Klimov AN. Autoimmune theory of atherogenesis and the concept of modified lipoproteins. Vestn Akad Med Nauk SSSR. 1990;11:30–6.
Knowlden S, Georas SN. The autotaxin-LPA axis emerges as a novel regulator of lymphocyte homing and inflammation. J Immunol. 2014;192(3):851–7.
Koschinsky ML, Boffa MB. Oxidized phospholipid modification of lipoprotein(a): epidemiology, biochemistry and pathophysiology. Atherosclerosis. 2022;349:92–100.
Krychtiuk KA, Kastl SP, Pfaffenberger S, Pongratz T, Hofbauer SL, Wonnerth A, Katsaros KM, Goliasch G, Gaspar L, Huber K, Maurer G, Dostal E, Oravec S, Wojta J, Speidl WS. Small high-density lipoprotein is associated with monocyte subsets in stable coronary artery disease. Atherosclerosis. 2014;237(2):589–96.
Krychtiuk KA, Kastl SP, Hofbauer SL, Wonnerth A, Goliasch G, Ozsvar-Kozma M, Katsaros KM, Maurer G, Huber K, Dostal E, Binder CJ, Pfaffenberger S, Oravec S, Wojta J, Speidl WS. Monocyte subset distribution in patients with stable atherosclerosis and elevated levels of lipoprotein(a). J Clin Lipidol. 2015a;9(4):533–41.
Krychtiuk KA, Kastl SP, Pfaffenberger S, Lenz M, Hofbauer SL, Wonnerth A, Koller L, Katsaros KM, Pongratz T, Goliasch G, Niessner A, Gaspar L, Huber K, Maurer G, Dostal E, Wojta J, Oravec S, Speidl WS. Association of small dense LDL serum levels and circulating monocyte subsets in stable coronary artery disease. PLoS One. 2015b;10(4):e0123367.
Lawn RM. Lipoprotein(a) in heart disease. Sci Am. 1992;266(6):54–60.
Libby P, Nahrendorf M, Swirski FK. Monocyte heterogeneity in cardiovascular disease. Semin Immunopathol. 2013;35(5):553–62.
Liu A, Frostegard J. PCSK9 plays a novel immunological role in oxidized LDL-induced dendritic cell maturation and activation of T cells from human blood and atherosclerotic plaque. J Intern Med. 2018;284:193–210.
Liuzzo G, Trotta F, Pedicino D. Interleukin-17 in atherosclerosis and cardiovascular disease: the good, the bad, and the unknown. Eur Heart J. 2013;34(8):556–9.
März W, Beckmann A, Scharnagl H, Siekmeier R, Mondorf U, Held I, Schneider W, Preissner KT, Curtiss LK, Gross W, Hüttinger M. Heterogeneous lipoprotein(a) size isoforms differ by their interaction with the low-density lipoprotein receptor and the low-density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. FEBS Lett. 1993;325(3):271–5.
Masoomikarimi M, Salehi M. Modulation of the immune system promotes tissue regeneration. Mol Biotechnol. 2022;64(6):599–610.
Naka KK, Bechlioullis A, Marini A, Sionis D, Vakalis K, Triantis G, Wilkins L, Rogus J, Kornman KS, Witztum JL, Doucette-Stamm L, Michalis LK, Tsimikas S. Interleukin-1 genotypes modulate the long-term effect of lipoprotein(a) on cardiovascular events: the Ioannina study. J Clin Lipidol. 2018;12(2):338–47.
Ożańska A, Szymczak D, Rybka J. Pattern of human monocyte subpopulations in health and disease. Scand J Immunol. 2020;92(1):e12883.
Panza F, D’introno A, Capurso C, Colacicco AM, Seripa D, Pilotto A, Santamato A, Capurso A, Solfrizzi V. Lipoproteins, vascular-related genetic factors, and human longevity. Rejuvenation Res. 2007;10(4):441–58.
Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–41.
Pattarabanjird T, Li C, McNamara C. B cells in atherosclerosis: mechanisms and potential clinical applications. JACC Basic Transl Sci. 2021;6(6):546–63.
Piro A, Tagarelli A, Tagarelli G, Lagonia P, Quattrone A. Paul Ehrlich: the Nobel Prize in physiology or medicine 1908. Int Rev Immunol. 2008;27(1–2):1–17.
Pirro M, Bianconi V, Paciullo F, Mannarino MR, Bagaglia F, Sahebkar A. Lipoprotein(a) and inflammation: a dangerous duet leading to endothelial loss of integrity. Pharmacol Res. 2017;119:178–87.
Pluijmert NJ, de Jong RCM, de Vries MR, Pettersson K, Atsma DE, Jukema JW, Quax PHA. Phosphorylcholine antibodies restrict infarct size and left ventricular remodelling by attenuating the unreperfused post-ischaemic inflammatory response. J Cell Mol Med. 2021;25(16):7772–82.
Pokrovsky SN, Afanasieva OI, Ezhov MV. Lipoprotein(a) apheresis. Curr Opin Lipidol. 2016;27(4):351–8.
Pokrovsky SN, Afanasieva OI, Safarova MS, Balakhonova TV, Matchin YG, Adamova IY, Konovalov GA, Ezhov MV. Specific Lp(a) apheresis: a tool to prove lipoprotein(a) atherogenicity. Atheroscler Suppl. 2017;30:166–73.
Pokrovsky SN, Afanasieva OI, Ezhov MV. Therapeutic apheresis for management of Lp(a) hyperlipoproteinemia. Curr Atheroscler Rep. 2020;22(11):68.
Potekhina AV, Pylaeva EA, Provatorov SI, Ruleva N, Masenko V, Noeva E, Krasnikova T, Arefieva T. Treg/Th17 balance in stable CAD patients with different stages of coronary atherosclerosis. Atherosclerosis. 2015;238(1):17–21.
Prasad A, Clopton P, Ayers C, Khera A, de Lemos JA, Witztum JL, Tsimikas S. Relationship of autoantibodies to MDA-LDL and ApoB-immune complexes to sex, ethnicity, subclinical atherosclerosis, and cardiovascular events. Arterioscler Thromb Vasc Biol. 2017;37(6):1213–21.
Puri R, Nissen SE, Arsenault BJ, St John J, Riesmeyer JS, Ruotolo G, McErlean E, Menon V, Cho L, Wolski K, Lincoff AM, Nicholls SJ. Effect of C-reactive protein on lipoprotein(a)-associated cardiovascular risk in optimally treated patients with high-risk vascular disease: a prespecified secondary analysis of the ACCELERATE trial. JAMA Cardiol. 2020;5(10):1136–43.
Qi Z, Hu L, Zhang J, Yang W, Liu X, Jia D, Yao Z, Chang L, Pan G, Zhong H, Luo X, Yao K, Sun A, Qian J, Ding Z, Ge J. PCSK9 (Proprotein Convertase Subtilisin/Kexin 9) enhances platelet activation, thrombosis, and myocardial infarct expansion by binding to platelet CD36. Circulation. 2021;143(1):45–61.
Ravandi A, Boekholdt SM, Mallat Z, Talmud PJ, Kastelein JJ, Wareham NJ, Miller ER, Benessiano J, Tedgui A, Witztum JL, Khaw KT, Tsimikas S. Relationship of IgG and IgM autoantibodies and immune complexes to oxidized LDL with markers of oxidation and inflammation and cardiovascular events: results from the EPIC-Norfolk Study. J Lipid Res. 2011;52(10):1829–36.
Reyneveld GI, Savelkoul H, Parmentier HK. Current understanding of natural antibodies and exploring the possibilities of modulation using veterinary models. A review. Front Immunol. 2020;11:2139.
Rogacev KS, Cremers B, Zawada AM, Seiler S, Binder N, Ege P, Große-Dunker G, Heisel I, Hornof F, Jeken J, Rebling NM, Ulrich C, Scheller B, Böhm M, Fliser D, Heine GH. CD14++CD16+ monocytes independently predict cardiovascular events: a cohort study of 951 patients referred for elective coronary angiography. J Am Coll Cardiol. 2012;60(16):1512–20.
Sabarinath PS, Appukuttan PS. Immunopathology of desialylation: human plasma lipoprotein(a) and circulating anti-carbohydrate antibodies form immune complexes that recognize host cells. Mol Cell Biochem. 2015;403(1–2):13–23.
Sabbah N, Jaisson S, Garnotel R, Anglés-Cano E, Gillery P. Small size apolipoprotein(a) isoforms enhance inflammatory and proteolytic potential of collagen-primed monocytes. Lipids Health Dis. 2019;18(1):166.
Saigusa R, Winkels H, Ley K. T cell subsets and functions in atherosclerosis. Nat Rev Cardiol. 2020;17(7):387–401.
Salonen JT, Ylä-Herttuala S, Yamamoto R, Butler S, Korpela H, Salonen R, Nyyssönen K, Palinski W, Witztum JL. Autoantibody against oxidized LDL and progression of carotid atherosclerosis. Lancet. 1992;339(8798):883–7.
Schnitzler JG, Poels K, Stiekema LCA, Yeang C, Tsimikas S, Kroon J, Stroes ESG, Lutgens E, Seijkens TTP. Short-term regulation of hematopoiesis by lipoprotein(a) results in the production of pro-inflammatory monocytes. Int J Cardiol. 2020;315:81–5.
Scipione CA, Sayegh SE, Romagnuolo R, Tsimikas S, Marcovina SM, Boffa MB, Koschinsky ML. Mechanistic insights into Lp(a)-induced IL-8 expression: a role for oxidized phospholipid modification of apo(a). J Lipid Res. 2015;56(12):2273–85.
Shapiro MD, Tavori H, Fazio S. PCSK9: from basic science discoveries to clinical trials. Circ Res. 2018;122(10):1420–38.
Silverstein RL. PCSK9 (Proprotein Convertase Subtilisin/Kexin 9) goes “DAMP”. Circulation. 2021;143(1):62–4.
Sotiriou SN, Orlova VV, Al-Fakhri N, Ihanus E, Economopoulou M, Isermann B, Bdeir K, Nawroth PP, Preissner KT, Gahmberg CG, Koschinsky ML, Chavakis T. Lipoprotein(a) in atherosclerotic plaques recruits inflammatory cells through interaction with Mac-1 integrin. FASEB J. 2006;20(3):559–61.
Ståhle M, Silvola JMU, Hellberg S, de Vries M, Quax PHA, Kroon J, Rinne P, de Jong A, Liljenbäck H, Savisto N, Wickman A, Stroes ESG, Ylä-Herttuala S, Saukko P, Abrahamsson T, Pettersson K, Knuuti J, Roivainen A, Saraste A. Therapeutic antibody against phosphorylcholine preserves coronary function and attenuates vascular 18F-FDG uptake in atherosclerotic mice. JACC Basic Transl Sci. 2020;5(4):360–73.
Stiekema LCA, Prange KHM, Hoogeveen RM, Verweij SL, Kroon J, Schnitzler JG, Dzobo KE, Cupido AJ, Tsimikas S, Stroes ESG, de Winther MPJ, Bahjat M. Potent lipoprotein(a) lowering following apolipoprotein(a) antisense treatment reduces the pro-inflammatory activation of circulating monocytes in patients with elevated lipoprotein(a). Eur Heart J. 2020;41(24):2262–71.
Suzuki T, Futami-Suda S, Igari Y, Watanabe K, Ouchi M, Suzuki K, Sekimizu K, Kigawa Y, Nakano H, Oba K. Low-molecular-weight lipoprotein(a) and low relative lymphocyte concentration are significant and independent risk factors for coronary heart disease in patients with type 2 diabetes mellitus: Lp(a) phenotype, lymphocyte, and coronary heart disease. Lipids Health Dis. 2013;12:31.
Tavori H, Christian D, Minnier J, Plubell D, Shapiro MD, Yeang C, Giunzioni I, Croyal M, Duell PB, Lambert G, Tsimikas S, Fazio S. PCSK9 association with lipoprotein(a). Circ Res. 2016;119(1):29–35.
Thomas R, Wang W, Su DM. Contributions of age-related thymic involution to immunosenescence and inflammaging. Immun Ageing. 2020;17:2.
Tmoyan NA, Afanasieva OI, Ezhov MV, Klesareva EA, Balakhonova TV, Pokrovsky SN. Lipoprotein(a), immunity, and inflammation in polyvascular atherosclerotic disease. J Cardiovasc Dev Dis. 2021;8(2):11.
Tsimikas S, Willeit P, Willeit J, Santer P, Mayr M, Xu Q, Mayr A, Witztum JL, Kiechl S. Oxidation-specific biomarkers, prospective 15-year cardiovascular and stroke outcomes, and net reclassification of cardiovascular events. J Am Coll Cardiol. 2012;60:2218–29.
Tucker B, Sawant S, McDonald H, Rye KA, Patel S, Ong KL, Cochran BJ. The association of serum lipid and lipoprotein levels with total and differential leukocyte counts: results of a cross-sectional and longitudinal analysis of the UK Biobank. Atherosclerosis. 2021;319:1–9.
van den Berg VJ, Haskard DO, Fedorowski A, Hartley A, Kardys I, Caga-Anan M, Akkerhuis KM, Oemrawsingh RM, van Geuns RJ, de Jaegere P, van Mieghem N, Regar E, Ligthart JMR, Umans VAWM, Serruys PW, Melander O, Boersma E, Khamis RY. IgM anti-malondialdehyde low density lipoprotein antibody levels indicate coronary heart disease and necrotic core characteristics in the Nordic Diltiazem (NORDIL) study and the Integrated Imaging and Biomarker Study 3 (IBIS-3). EBioMedicine. 2018;36:63–72.
van der Valk FM, Bekkering S, Kroon J, Yeang C, Van den Bossche J, van Buul JD, Ravandi A, Nederveen AJ, Verberne HJ, Scipione C, Nieuwdorp M, Joosten LA, Netea MG, Koschinsky ML, Witztum JL, Tsimikas S, Riksen NP, Stroes ES. Oxidized phospholipids on lipoprotein(a) elicit arterial wall inflammation and an inflammatory monocyte response in humans. Circulation. 2016;134(8):611–24.
Vergallo R, Crea F. Atherosclerotic plaque healing. N Engl J Med. 2020;383(9):846–57.
Virella G, Lopes-Virella MF. Atherogenesis and the humoral immune response to modified lipoproteins. Atherosclerosis. 2008;200(2):239–46.
von Zychlinski A, Kleffmann T, Williams MJ, McCormick SP. Proteomics of lipoprotein(a) identifies a protein complement associated with response to wounding. J Proteome. 2011;74(12):2881–91.
von Zychlinski A, Williams M, McCormick S, Kleffmann T. Absolute quantification of apolipoproteins and associated proteins on human plasma lipoproteins. J Proteome. 2014;106:181–90.
Wang J, Qiang H, Zhang C, Liu X, Chen D, Wang S. Detection of IgG-bound lipoprotein(a) immune complexes in patients with coronary heart disease. Clin Chim Acta. 2003;327(1–2):115–22.
Wang H, Coligan JE, Morse HC 3rd. Emerging functions of natural IgM and its Fc receptor FCMR in immune homeostasis. Front Immunol. 2016;7:99.
Wiesner P, Tafelmeier M, Chittka D, Choi SH, Zhang L, Byun YS, Almazan F, Yang X, Iqbal N, Chowdhury P, Maisel A, Witztum JL, Handel TM, Tsimikas S, Miller YI. MCP-1 binds to oxidized LDL and is carried by lipoprotein(a) in human plasma. J Lipid Res. 2013;54(7):1877–83.
Yang J, Zhang L, Yu C, Yang XF, Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res. 2014;2(1):1.
Zawadzki Z, Tercé F, Seman LJ, Theolis RT, Breckenridge WC, Milne RW, Marcel YL. The linkage with apolipoprotein(a) in lipoprotein(a) modifies the immunochemical and functional properties of apolipoprotein B. Biochemistry. 1988;27(22):8474–81.
Zhang Y, Chen YC, Krummel MF, Rosen SD. Autotaxin through lysophosphatidic acid stimulates polarization, motility, and transendothelial migration of naive T cells. J Immunol. 2012;189(8):3914–24.
Acknowledgements
The authors are grateful to Professor Gilbert Thompson for his help in proofreading text of the manuscript.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Afanasieva, O.I., Arefieva, T.I., Ezhov, M.V., Pokrovsky, S.N. (2023). Lipoprotein(a) and Immunity. In: Kostner, K., Kostner, G.M., Toth, P.P. (eds) Lipoprotein(a). Contemporary Cardiology. Humana, Cham. https://doi.org/10.1007/978-3-031-24575-6_16
Download citation
DOI: https://doi.org/10.1007/978-3-031-24575-6_16
Published:
Publisher Name: Humana, Cham
Print ISBN: 978-3-031-24574-9
Online ISBN: 978-3-031-24575-6
eBook Packages: MedicineMedicine (R0)