Abstract
Cesarean scar pregnancy (CSP) is a serious, iatrogenic, and late consequence of a previous cesarean delivery (CD), which may result in clinical complications throughout all three trimesters of the pregnancy when unrecognized or untreated. Its rate of occurrence parallels the number of CDs. If untreated, it may progress and result in a live offspring; however, it is the main cause of morbidly adherent placentae in the late second and in the third trimesters of the pregnancy.
The last 40 years witnessed a steady increase in cesarean deliveries (CD). Very few paid attention that hand-in-hand with the already high but somewhat stabilized rate of CDs, cases of placenta accreta/increta/percreta lately mentioned under a general term placenta accreta spectrum (PAS) as well as cases of cesarean scar pregnancy (CSP) earned increasing attention. Even fewer realized the connection between CSP and PAS. Slowly, during the last 20 years, but definitely in the last 10 years, the connection between CSP and degrees of PAS was recognized and became the subject of increased scrutiny and research. Understanding CSP as the proven precursor of PAS can lead to its planned management, reducing their numbers and maybe even preventing its occurrence. In the last several years, the subject of CSP and PAS constituted the fastest increase in the number of publications in the obstetrical and gynecologic literature. This chapter addresses the diagnostic, differential diagnostic algorithms and sonographic definitions of CSP. It also provides a detailed overview of the treatment protocols found in the literature emphasizing their success as well as their failure rates, which are reflected by their possible but avoidable complications. The text also includes the suggested counseling approach to patients with early diagnosis of CSP that may be useful in patient management.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction/Terminology
Before discussing epidemiology, it is important to touch upon the diagnostic issues and management of cesarean scar pregnancy (CSP). The terms and names used to define this entity and special form of early pregnancy are often referred to as “cesarean ectopic pregnancy,” “cesarean scar ectopic,” or “cesarean delivery scar pregnancy.” Other terms may also include the word “ectopic.” In fact, there is a heated discussion among societies and interested professional groups about the subject of whether CSP is a form of intrauterine pregnancy or is it, by definition, an ectopic gestation. Recently in an opinion article, convincing arguments were voiced to discourage all clinicians to use the term “cesarean ectopic pregnancy” and employ what we consider the anatomically as well as clinically correct term: CSP [1].
In fact, there are three main reasons to avoid using the term “ectopic.” First, CSP is well within the uterine cavity. The placenta at times (but not always) is squeezed into the niche or dehiscence created by the cesarean delivery in the lower segment of the uterus or at the level of the internal os. If untreated, the gestational sac and the embryo/fetus will develop within the uterine cavity, which is within the well-defined and widely accepted anatomic boundaries of the uterus. Second, a CSP can lead to a live offspring as opposed to any kind of true ectopic pregnancy, which rarely, if ever, results in a viable neonate. Last, treatments devised for true ectopic pregnancies and applied for a CSP may not work or may even cause complications.
Our analysis of 751 cases of CSP reviewed until 2012 found that almost a third (30%) were misdiagnosed or diagnosed at a late gestational age, significantly contributing to a large number of treatment complications that could have been avoided by an early and correct diagnosis. Although an exact number cannot be quoted, it seems that, due to a higher awareness of the disease, among 1223 cases found in the literature published between 2012 and 2014, the number of misdiagnoses appeared to have dropped significantly.
Background
Due to the close and causal relationship between a previous CD and CSP, we have to discuss the gradual but steady increasing rate of CD in the USA and the rest of the world. In the USA, the rate of CD slowly increased from 5% in 1970 to 32.9% in 2009 [2]. Recent national statistics by the Centers for Disease Control and Prevention report a leveling off of CD rate, which in 2012 reached 32.8% [3]. To our knowledge, no updated CDC communications to update the previous ones were published. Rates ranging from 35% to 80% were reported in other parts of the world [4], leading us to believe that the incidence of CSP is higher in those countries than in the USA. Expect an increase in incidence nationally and internationally mostly due to increased awareness and more accurate diagnosis.
Keeping in mind the causative connection between CD and its recognized consequences, such as the placenta previa and placenta accrete spectrum (PAS) in the last decade, many Ob/Gyn practitioners became increasingly exposed to the clinical picture of PAS. Most have rarely, if ever, faced a patient with a first- or early second-trimester CSP. The learning process was traumatic resulting in misdiagnosed patients with CSP as “aborting gestations,” “ectopic pregnancies,” and “cervical pregnancies.” Also, obstetricians were confronted with diagnostic and management dilemmas. When “traditional” treatments, such as D&C and systemic methotrexate (MTX), were employed, practitioners experienced severe and almost unmanageable vaginal bleeding that, at times, led to hysterectomy. If “low-lying” pregnancies were left to continue, many resulted in second-trimester uterine ruptures and profuse internal or vaginal bleeding, causing loss of the pregnancy and requiring hysterectomy. Even in reviewing the literature, one could usually find reports of single or sporadic cases or a series of one to two dozen cases that would fit the clinical picture. It is clear that it was impossible to learn from the numerous, previously used treatments, “tested” on few patients (sometimes only one). The published review compiling 751 patients diagnosed with CSP [5] may have helped to shed light on the various treatments and their complications; however, to date, there is no universally recognized treatment protocol adopted by professional societies. Our chapter will discuss the pathogenesis, diagnosis, counseling, and management options to treat CSP based upon the evidence in the literature as well as our own clinical experience.
What Is a Cesarean Scar Pregnancy?
Cesarean scar pregnancy develops if a blastocyst implants on the uterine scar or in the dehiscence (otherwise known as a “niche”) resulting from repair of the uterine incision at the previous CD. Implantation of the fertilized oocyte in the faulty anterior uterine wall will give rise to the CSP.
While the definition and diagnostic issues of CSP will be expanded on later, it may be useful to define the main features of it here:
-
Cesarean scar pregnancy (CSP) is a potentially dangerous, man-made consequence of a previous cesarean delivery (CD).
-
It is detected after a previous cesarean delivery.
-
It features an empty uterine cavity and closed, empty endocervical canal.
-
It is detected as a low/anterior gestational sac and/or placenta in close proximity (or previa) of the hysterotomy scar/niche with fetal or embryonic pole and/or yolk sac with/without heartbeats.
-
It usually but not always demonstrates abundant blood flow around the gestational sac determined by color or power Doppler interrogation.
-
An anteflexed/retroverted uterus strengthens the diagnosis.
Before engaging in the diagnosis of CSP, we also have to devote an additional paragraph to discuss the two ways an incision made at the time of the CD heals and appears after it was repaired. Normally, we expect that healing tissues generate a thick scar without leaving behind a defect. At times, a dehiscence or as it is usually referred a niche, with a certain depth and width, marks the area of the previous CD and can be seen with or without a saline infusion sonohysterography [6]. The niche can be triangular or rectangular and can be filled with fluid (Fig. 18.1a). The size of the niche on a sagittal section of the uterus may be misleading; therefore, the area should always be looked at in the transverse plane on which the real size of the dehiscence can be appreciated (Fig. 18.1b). This is logical, since most primary cesarean incisions are performed from side to side, e.g., in the transverse plane. Bij deVaate et al. [7] published an extensive review analyzing 21 articles dealing with the prevalence, potential risk factors for development, and symptoms related to the presence of uterine niches following CD. The prevalence of a niche after a CD was found to vary between 56% and 84%. Several risk factors for the development of niches were found: the technique of repair, location of the incision, wound healing, and probably number of layers included in the closure as well as multiple CDs and uterine retroflexion. The dehiscence left behind by the previous CD may be extensive and reaches the anterior uterine wall or the area below the bladder in the shape of a fistulous connection between the uterine cavity and the abovementioned areas (Fig. 18.1c, d).
Niche/defect left behind by the previous CD. (a) Sagittal image of the niche marked by an arrow (Cx cervix). (b) Three-dimensional orthogonal images of the uterus showing the niche (arrows). The width of the dehiscence should always be looked at on a transverse or coronal view since that is the real size of it. Unenhanced images. (c, d) At times, the niche/dehiscence extends all the way from the uterine cavity to the anterior surface of the uterus. Saline infusion sonographic images
At times, the niche is deep and wide (Fig. 18.2a), explaining the deep insertion of the tiny placenta with its rich blood supply (Fig. 18.2b, c). Since the prevalence of niches is relatively high, it can be expected that the possibility of such deep implantation is realistic; therefore, a careful scrutiny of the small placenta and its vessels should be performed in all first-trimester diagnoses of CSP.
Placental implantation into the niche of a previous CD. Sagittal images (Cx cervix). (a) Saline infusion sonohysterography of a uterus with a large niche. (b) Grayscale sagittal image of a CSP. Note the implantation of the placenta IN the niche outlined by small arrows. (c) Color Doppler image of the same CSP demonstrating the invasion of the placenta (outlined by small arrows) with its blood vessels into the myometrium
Incidence/Risk Factor
Estimated incidence rates of CSP appear to be stable and range between 1/1800 and 1/2500 of all CDs performed [8,9,10,11]. Seow et al. [12] state that CSP was seen in 0.15% of all pregnancies with a history of a previous CD. The above numbers appear unrealistic; however, their true incidence is unknown due to the lack of population-based statistics (registries). As pointed out before, it seems that the actual rate did not increase in the last year since CD rates plateaued, and the increase in publications increased due to the actual awareness and its more accurate diagnosis.
The only risk factor for CSP is one or more [13] previous CDs.
It must be emphasized that “scar pregnancy” was described as associated with or caused by previous myomectomy [14], and also after in vitro fertilization [15]. In these two instances, the pathophysiology seems to be identical.
Pathogenesis of CSP
Later in this chapter, we will provide evidence that the histology of the tiny placental insertion or myometrial invasion of a CSP in the first trimester of the gestation is identical with the histologic findings of a PAS in the second and third trimesters of pregnancy. The previously and widely accepted explanation for the pathophysiology of PAS was that, in both diseases (CSP and PAS), intervening fibrinoid layer between the myometrium and the cytotrophoblastic shell in the placenta is naturally present between the endometrium in normally attached placentae when thinned or missing. This fibrin layer (fibrinoid material) is known by the name of Nitabuch layer. Previous uterine surgery or uterine interventions lead to thin or absent decidua basalis in scarred areas, as well as the abovementioned protective layer of the lower uterine segment. In CSP and in MAP, this membrane is missing and the placental villi attach itself and penetrate between the myometrial fibers into the depth of the uterine wall. These descriptions have prevailed for over 50 years and form the basis for diagnosis and grading of accreta placentation.
Other theories, such as the role of a low oxygen tension at the area of the scar providing a stimulus to help the invading cytotrophoblast [13, 16], as well as the in vitro studies of Kliman et al. [17] with trophoblast and EM explants, showing a strong propensity for attaching to exposed extracellular matrix and then to endometrial epithelial cells, are the most frequently quoted. Both theories support the observation that the more CDs a patient has, the higher the risk of placenta previa and placenta accrete.
While we duly mentioned the above explanation for the generation of CSP, we are also cognizant of the fact that there are several theories of the pathophysiological implantation process for the faulty placenta invasion. At this time, the relevant current hypothesis is the one by Eric Jauniaux, which theorizes that large cesarean scar defects in the lower uterine segment are associated with failure of normal decidualization and loss of the sub-decidual myometrium, and this secondary defect of the endometrium-myometrium interface leads to abnormally deep placental anchoring villi and trophoblast infiltration into the myometrium [18].
Diagnosis of Cesarean Scar Pregnancy
The two diagnostic modalities used are ultrasound and MRI; however, ultrasound is the best modality. Transvaginal sonography (TVS) presents an advantage over transabdominal ultrasound (TAS), since it has a higher resolution and can be placed in close proximity to the low, anterior gestational sac. MRI has been used for imaging and is expensive. In addition, it requires moving the patient to a radiology site. Also, MRI lacks the color Doppler flow that provides a high-resolution image, which is important in establishing a correct diagnosis. The authors of this chapter do not use or encourage the use of MRI in the diagnosis of cases suspected of CSP. The Society for Maternal Fetal Medicine also supports the use of transvaginal and/or transabdominal ultrasound to diagnose CSP [19, 20].
The diagnosis of CSP requires a high clinical index of suspicion. We reiterate that every woman with a history of a previous CD and a positive pregnancy test, presenting in the first trimester of the pregnancy, should be considered a “rule out CSP” until proven otherwise. Stirnemann et al. [21, 22] published studies to lay the basics for such screening if proven significant. Until that time, this should be strongly considered, since there is no downside to that first early scan. Godin et al. [23], Vial et al. [24], and Seow et al. [25] published similar sonographic criteria they used to define a CSP; however, other authors used additional characteristics, relying mostly on single cases.
Our diagnostic criteria of CSP [5, 26] took into consideration a history of previous CD, a positive pregnancy test, and the following sonographic criteria (Fig. 18.3):
-
Endometrial and endocervical canal devoid of a gestational sac
-
Placenta and/or a gestational sac embedded on or in the hysterotomy scar/niche
-
In early gestations, a triangular gestational sac that fills a niche of the scar (Fig. 18.4)
-
Thin or absent myometrial layer between the gestational sac and the bladder
-
The presence of a chorionic sac, with or without embryonic/fetal pole and/or yolk sac and with or without heart activity
-
The presence of a prominent and at times rich vascular pattern at or in the area of a CD scar. As a rule, detection of peri-trophoblastic blood flow, detected by the most sensitive Doppler settings around a low, anteriorly situated chorionic sac, in a patient with a previous CD, is a reliable sign of CSP.
-
It is remarkable that, at very early stages of the pregnancy (4–5 weeks), the blood vessels tend to concentrate on the anterior side of the chorionic sac (Fig. 18.5) “marking” the site of the placental implantation.
-
The usefulness of 3D ultrasound in the diagnosis is debated. However, it furnishes information regarding the exact location of the sac, its vascularity, and volume, the latter two in a quantitative fashion (Fig. 18.6). We use the above measurements to follow the healing process of the treated cases or for the early warning signs of an impending enhanced myometrial vascularity (EMV) or also previously known as arteriovenous malformation (AVM) developing at the site of a conservatively treated or even an untreated, but spontaneously failing CSP [27, 28].
Sonographic markers of CSP (Cx cervix, Bl bladder, UC uterine cavity). (a) Empty uterine cavity and cervical canal. Low anterior triangular gestational sac with yolk sac in close proximity to the bladder (long arrow). (b) Triangular gestational sac with close proximity to the bladder. (c) The developing vascularity between the sac and the bladder. (d) Arteriovenous malformation in a CSP that required UAE
The developing vascular grid of the early CSP. (a, b) 2D color Doppler of the vessels surrounding the chorionic sac. (c) Three-dimensional, orthogonal planes and 3D rendering (lower right picture) of the vascularity that start to concentrate on the anterior side of the sac, the future site of the placenta. We suspect that the future placenta will invade the myometrium in the anterior direction. (d) Thick-slice 3D rendering of the sac with its vessels clearly more prominent anteriorly
The use of 3D ultrasound in the diagnosis and follow-up of treatment of CSP. (a) 3D orthogonal planes with power Doppler used in segmentation (marking the perimeter of the sac) to obtain the volume of it. (b) After the volume of the sac is obtained, a special algorithm is applied to compute and display the quantitative vessel content of the above volume. (c) Visual display of the three-dimensional vascular angiogram that can be used qualitatively for follow-up purposes after local injection of UAE treatments
If an EMV (a.k.a. AVM) was suspected (at times, this may be the presenting sonographic picture), Doppler measurements of the blood velocity were measured and expressed by the peak systolic velocity (PSV) in cm/s. Velocities above 39 cm/s were considered for uterine artery embolization (UAE) by the interventional radiologist. This evaluation is best done when the region of interest of the Doppler interrogation is constricted to the questionable area, using the appropriate pulse repetition frequency and filter settings.
These are pathological, high-velocity, low-resistance “short circuits” of the bloodstream between an organ’s arterial and venous supply. Ultrasound presents a valuable tool for the diagnosis of AVM and guideline for their treatment [29]. Although uncommon, they may cause dangerous hemorrhages due to disrupted blood vessels, after miscarriage or uterine instrumentation [30]. The acquired form, seen in CSP, is usually traumatic, resulting from prior dilation and curettage (D&C), therapeutic abortion, uterine surgery, or direct uterine trauma. Their incidence is about 1% of CSPs. In our series of 60 CSPs, 5 patients had EMV [31].
Differential Diagnosis of CSP
There are two main differential diagnostic entities to consider: first, a cervical pregnancy, which is rare and has no history of a prior CDs, and second, a miscarriage in progress, which can be seen in the cervical canal or close to the internal os and “on its way out” having no heart activity. Also, under pressure on the cervix with the vaginal probe, the sac will slide back-and-forth, while a true CSP will stay fixed. It should be noted that misdiagnosis has, at times, severe consequences. The proof is in the literature: 107 of the 751 cases of CSP reviewed (13.6%) were missed or misdiagnosed leading to complications (e.g., hysterectomy and loss of fertility) [5]. Figure 18.7 demonstrates a simple method to distinguish between the two, abovementioned, differential diagnostic entities and a true CSP.
However, it is extremely important to realize that this simplified diagnostic aid is valid and reliable only while the gestational sac is small (e.g., 5–6 mm in diameter or 5–6 postmenstrual weeks) and remains “local,” close to the niche or above the scar. In other words, the sac did not start to elongate and move/expand cranially to fill the uterine cavity. In this case, the sac will be found increasingly in the uterine cavity misleading the uninitiated observer to think that it is an intrauterine sac. In such cases, one should shift the attention from the sac and concentrate upon the blood vessels of the tiny placenta, which stay in their original site of implantation, thereby holding the most important diagnostic feature of CSP: the true site of placental implantation. Figures 18.2, 18.3, 18.4, and 18.5 clearly demonstrate the abovementioned diagnostic principle.
Lately, clinicians and clinical researchers have started to pay attention to the exact location of placental implantation in the area of the scar/niche left behind by the previous CD. Vial et al. [24] suggested that there are two kinds of CSPs, based on the depth of implantation. The question is whether a deeply implanted chorionic sac in a niche or dehiscence, close to the bladder with very thin or no visible myometrium (Fig. 18.8a, b) recently termed type 2 CSP, will result in a worse outcome than if inserted on top of a scar, also called type 1 CSP, that has some thickness (Fig. 18.8c, d). Comstock et al. [32] and personal communication with Cali G. refer to “on-the-scar implantations” as “low-lying sacs” and assume that these are the CSPs that may proceed to third trimester giving rise to PAS. Deeply implanted in the niche, surrounded by myometrium, and seldom reach term is a “true” or type 2 scar pregnancy. We slightly differ about the latter form of CSP since we have witnessed the reaching delivery of a live offspring.
The issue of distance between the anterior uterine surface and the gestational sac: “in the niche/scar” or “on the scar” (Bl bladder). (a, b) These two are examples of a close proximity of the sac to the bladder (2.1 mm and 3.2 mm, respectively). (c, d) Depicts two CSPs in which the sac is 6 and 7 mm remote from the bladder
Rac et al. [33] studied 39 patients, of which 14 had histologically confirmed PAS. The smallest myometrial thickness measurement was one of the variables associated with invasion. More research is needed before the gestational-sac-to-bladder distance (Fig. 18.8) can become useful in counseling patients with CSP in the first trimester of pregnancy.
The Connection Between CSP and PAS
The connection or continuity between CSP and CSP has gradually become evident through clinical observation [34, 35]. We studied placental implantation in the early (second trimester) placenta accreta and in CSP, to find out if they represent different stages in the disease continuum leading to morbidly adherent placenta in the third trimester [36]. Two pathologists, blinded to the diagnosis, evaluated their histologic slides on the basis of these microscopic slides. They could not tell the difference between the two clinical entities and found that both had one thing in common: neither had intervening deciduae between the villi and the myometrium, consistent with the classic definition of morbidly adherent placenta. Therefore, our conclusion is that CSP and an early second-trimester placenta accreta are histopathologically identical and represent different stages in the disease continuum leading to PAS in the third trimester.
The next logical question is whether, left untreated, a CSP would result in a live-born offspring. We followed ten patients diagnosed with CSPs who opted to continue the pregnancy declining early termination [37]. The diagnosis of CSP was made before 10 weeks. All ten had sonographic signs of PAS by the second trimester. Nine of the ten patients delivered live-born neonates, between 32 and 37 weeks. One patient had progressive intractable vaginal bleeding, leading to hysterectomy, at 20 weeks. The other nine patients underwent hysterectomy at the CD. Blood loss ranged from 300 to 6000 mL. Histopathological diagnosis of all placentae was placenta percreta.
Above, we provided reliable data regarding two clinical issues: (a) CSP is a precursor of PAS, both sharing the same histopathology, and (b) pregnancies diagnosed as CSP in the first trimester may proceed to deliver live offspring, risking premature delivery and loss of uterus and fertility. This data can be used to counsel patients with CSP, to make an evidence-based and informed choice between first-trimester termination of an early pregnancy or continuation, risking premature delivery, and loss of uterus and fertility.
The societal recognition of the connection between CSP and PAS, in the USA, was the SMFM Consensus Statement published recently [20]. In this document, several PAS ultrasound markers have been described in the first trimester. Their prevalence and type of markers of PAS in the first trimester were shown to vary between early first trimester (6–9 weeks) and later first trimester (11–14 weeks). It also reinforces that implantation of a gestational sac in the lower uterine segment is one of the most common US markers for PAS in the first trimester. Finally, it draws attention to the fact that in high-risk women, a gestational sac implanted in close proximity to a uterine scar was identified in 82.4% of women (95% CI, 85.8–95.7) with confirmed PAS.
PAS in the First Trimester?
PAS can exist in the first trimester of pregnancy. For beginners, Comstock et al. [32] described seven patients after sonographic examination at 10 weeks or earlier with placenta accreta, increta, and percreta, not only by their clinical course but, more importantly, by pathologic examination of the uterus. In six, at the time of the early ultrasound, the chorionic sac was located in the lower uterine segment, in the scar area of the previous CD. Two patients underwent D&C, at which time severe bleeding led to hysterectomy. The remaining four had sonographic findings typical of placenta accreta during subsequent scans but delivered at term. The author’s conclusion suggested that, in a patient with a previous CD, a chorionic sac detected by a 10 week or less ultrasound, located in the lower uterine segment, suggests the possibility of placenta accreta. A similar article was published by Ballas et al. [34].
Using our material, Fig. 18.9 depicts the early sonographic markers of a MAP: placenta previa, focal loss of the clear space, and focally increased vascularity. The patient in this example delivered at 34 weeks and had placenta accreta. In ten patients, we reported [37] that the early sonographic markers of MAP could be detected at the end of the first and beginning of the second trimesters.
CSP is a precursor of MAP. This is a 9 weeks and 5 days gestation (Cx cervix, Pl placenta). (a) Sagittal, grayscale image of a CSP with an anterior placenta previa. (b) Power Doppler reveals two areas of vessel proximity to the bladder with loss of the myometrium (arrows). (c) Another plane showing the same findings as in b. (d) A more lateral section concentrates on an area with clear vessel invasion of the myometrium (arrow)
Counseling Patients with a First-Trimester CSP
Prior to treatment and after the reliable diagnosis of CSP, one has to determine if fetal heartbeats are seen. If no yolk sac and/or no embryo and/or no heartbeats are seen, re-scan every 2–3 days. If, after a week, no heart activity, no yolk sac, and/or no embryo are detected, a sonography and biochemistry-based follow-up should be planned. Only after this time should the gestation be considered live or a pregnancy failure and the serum hCG should be followed until nonpregnant levels are reached. Some management protocols call for systemic administration of methotrexate (MTX), even with the absence of heartbeats for early drug effect. While such an approach is not contraindicated, the patient and the provider must be sure that under no circumstances is this a wanted pregnancy.
In the case of positive heart activity, counseling should enumerate the two main clinical management options to reach a decision as early as possible. The two options before further growth of the gestation are (a) termination or (b) continuation of the pregnancy. Our counseling of patients with a CSP diagnosed in the first trimester of pregnancy underwent a fundamental change. Several years ago, we would counsel toward termination of the pregnancy without delay. Recent studies on the natural history of the CSP, with the possibility of reaching term or near-term delivery of a live offspring, have changed our counseling [38, 39]. We provide the patient with evidence that this is possible and that the patient should understand that second- and third-trimester PAS may be complicated by severe hemorrhage and also necessitate hysterectomy. Management in the above case should be based on the patient’s age, number of previous CDs, desired number of children, and expertise of the clinicians giving the care. If the patient decides to continue the pregnancy, bleeding precautions should be given. The management should be based upon serial ultrasounds, until a safe gestational age is reached. The SMFM guidelines detail the approach to CSP and discourage continuing the pregnancy unless proper, evidence-based consultation is well understood by the patient, a multidisciplinary team can be involved in the pregnancy management and the delivery, and blood products are available, since ultrasound cannot predict the blood loss at surgery [40].
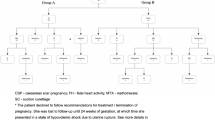
Our general guidelines in counseling and managing the patient with a CSP are shown in Fig. 18.10.
Management of Cesarean Scar Pregnancy
Treatment regimens and their combinations can be classified as one of the following:
-
1.
Major surgery (these require general anesthesia):
-
(a)
Laparotomy (hysterectomy or local excision)
-
(b)
Excision by laparoscopy, hysteroscopy, or transvaginal surgery
-
(c)
Dilatation of the cervix and sharp or blunt curetting
-
(d)
Suction aspiration without dilatation of the cervix
-
(e)
Excision performed by the vaginal route
-
(a)
The last two can be guided by continuous, real-time ultrasound.
-
2.
Minimally invasive surgery (does not involve general anesthesia):
-
(a)
Local injection of MTX or KCl
-
(b)
Vasopressin locally was also used
-
(a)
-
3.
Systemic medication
-
(a)
Single or repeated doses of methotrexate (MTX) and etoposide (some articles originating from China advocate intravenous use of MTX claiming reasonable success)
-
(b)
Uterine artery embolization (UAE)
-
(a)
-
4.
Combination of the above treatments: A large number of articles report on combining treatments in a planned, simultaneous, or sequential fashion. Treatments are also changed, mostly after the first-line therapy failed. As a matter of fact, it is rare to find a recently (2012–2014) published case or case series in which the patients were managed only by one single treatment agent or protocol.
-
5.
Adjuvant measures: Most recently, single Foley balloon and Cook cervical ripening double-balloon® placement and inflation to prevent and/or control bleeding, following or replacing local treatments such as aspiration, curettage, and local injection, have been used.
-
6.
It is beneficial for the patient with CSP to be referred to a facility that provides evidence-based care as well as experienced in managing cases, in response to developing emergency situations [41]. Such centers should be able to provide operating rooms and interventional radiology procedures and have blood transfusion/blood products immediately available. The latter is important since bleeding complications are typical of this dangerous clinical entity.
Treatment Options Available for CSP
Based upon the in-depth and available literature, analyzing the different aspects of CSP, in 2012, there were about 33 published treatment modalities with their results and complications [5]. No preferred treatment became apparent; however, of the 751 patients, D&C (305), surgical excision (laparoscopic, hysteroscopic, and transvaginal) (261), UAE (142), MTX (92), and local, intragestational sac injection (86) were the most used.
Between 2012 and 2022, no less than 70 peer-reviewed articles on CSP were published. Not surprising is the fact that Chinese authors contributed to the overwhelming number of cases, describing their various and different treatment modalities and their combinations. This is due to their large population and over 40% CD rate. At least 36 primary or combination treatments were found; however, the number is not substantially different from the list of treatment approaches described in our review of 751 cases. No wonder one cannot draw a clear conclusion as to which treatment was the most effective, resulting in the least or no complications. This large number underlines the fact that, in 2015, there is no nationally/internationally agreed-upon or suggested management protocol published with a set of guidelines to manage CSP or early first-trimester placenta accreta. While the distribution of the various treatments and their rates of use are found in the tables of our previous review [5], the somewhat different distribution of treatment choices is detailed in Table 18.1.
Some of the general guidelines at counseling a patient diagnosed with CSP are the following:
We start with an evidence-based counseling and consider the best management methods by gestational age (most published management-related articles do not take into consideration the gestational age at which the termination is suggested). We do take into consideration the CSP type and the practitioner’s own experience with the treatment. We emphasize that CSP is a rare and dangerous clinical entity regardless of the management, and if TOP is desired, the patient should sign a detailed informed consent and her understanding of this. We emphasize that the decision of termination is time sensitive since the gestation grows every day along with its blood supply, which exposes the patient to a higher rate of possible complications. We stress and explain the importance of “early decision” to the patient without putting excess pressure on the patient, but we expect a decision one day after the diagnosis. We also emphasize that even the best treatment may endanger life. During the counseling, we also touch upon the usually pertinent question of patients: if expectant measurement is indeed an option to retain the pregnancy. We stress that while this is a possibility depending on the nature and the type of the CSP, however continuing the gestation may expose the patient to sever hemorrhage, uterine rupture, severe consequences of PAS, and even maternal mortality [38]. Despite the above, some CSPs may progress to/close to term; therefore, TOP should NOT be the only option offered. If continuation of CSP is entertained, we describe possible complications specific to PAS in each trimester. To remind the reader: the SMFM guidelines do not recommend expectant management.
Evaluating the global experience regarding the different treatment modalities of CSP, in addition to the one mentioned before [40], we include here the two detailed reviews on management, which also include their success and complication rates [42, 43].
Despite several treatments for CSP, our detailed discussion will be limited to the most used. A much more detailed analysis is found in our in-depth review [5], complete with their efficacy and complication rates. We now add the pertinent data resulting from the review of the 1223 cases published after 2012.
-
1.
Suction aspiration or D&C, alone or in combination
Based on our first review of treating 305 cases with D&C only or in combination with other means as a “first line” or a backup, therapy had a mean complication rate of about 62% (range, 29–86%) [5]. The main complication was unanticipated bleeding, forcing an emergency second- or third-line treatment that, almost always, was surgical. At times, hysterectomy became necessary. This option requires general anesthesia.
There were some changes between the results of the two reviews. If D&C was used as a sole treatment, in 69 cases, 24 (34.7%) resulted in complication as opposed to first-line or secondary treatment combined with other treatments. Only 52 of 413 (12.2%) had complications. If UAE was combined with systemic MTX, it caused 35% complications, while combined with other means (e.g., suction evacuation or hysteroscopic excision among others), the rate was only 11.3%.
As opposed to a spontaneous delivery or spontaneous abortion, where the uterine myometrial grid constricts the bleeding after placental separation, in CSP, the sharp curettage exposes vessels of the gestational sac leading to severe and sometimes unstoppable bleeding since there is less or no adequate muscle grid to contain the bleeding. A sharp curettage might injure the thin myometrium leading to bleeding or even perforation.
If D&C or suction aspiration is still the preferred treatment, blood and blood products as well as a Foley balloon catheter should be readily available [44]. Foley balloon catheters or a cervical ripening Cook cervical ripening double-balloon catheter® [41] was successfully used to stop and tamponade possible bleeding [45, 46]. Cali et al. [47] successfully used the following sequential treatment approach in eight of their patients. At admission to the hospital, the patient undergoes UAE and, after 5 days, a gentle suction aspiration under continuous, real-time ultrasound is performed by immediate insertion and inflation of a Foley balloon catheter for bleeding prevention and control [44].
A number of recent articles advocate the safe and uncomplicated use of blunt sac aspiration; however, all were followed or preceded by other treatment methods [48]. Interestingly, no complications were seen in 81 suction aspirations in our review of the cases between 2012 and 2014. This probably is attributed to its blunt, as opposed to a sharp curetting at the time of D&C, and therefore, it is less prone to disrupt blood vessels.
-
2.
Uterine artery embolization, alone or in combination
This treatment requires general anesthesia. If used as a primary and only treatment, the complication rate among the 64 cases described in the review of 751 cases of CSP was 47%. It is difficult to evaluate the real complication rates, due to partial or incomplete data in the published articles. In another 78 cases, UAE was used in combination with other treatments. It seems that UAE is not the best first-line treatment, if administered alone as a single-agent therapy, since it allows the pregnancy, with its vascularity, to grow and increase. For this reason, Cali et al. [47] delayed suction aspiration in their patients with CSP for 5 days after UAE. Uterine artery embolization works better combined with other noninvasive and invasive (suction aspiration) treatments [49,50,51]. In our 60 cases of CSP, UAE was used as a secondary treatment in 4 patients with persistent vaginal bleeding or developing enhanced myometrial vascularity (EMV), also known as arteriovenous malformation (AVM). Embolization failed to stop the bleeding in one of the patients with EMV/AVM; therefore, hysterectomy was performed [31].
If UAE fails, which may be the case, the clinician must contend with a larger gestation applying a secondary treatment. However, it is hard to evaluate its actual complication rates, since some articles have insufficient data to rely on. As stated previously, in our 60 cases of CSP, one of the patients required (and finally agreed to) AVM embolization to stop her continuing vaginal bleeding (as well as her high PSV on Doppler), 122 days after her initial local MTX injection (Fig. 18.11).
In a recent article, we reported on a more serious kind/variant of EMV/AVM in terms of its difficult management (TIMOR-Tritsch IE insert enhanced) since all of the 13 patients in this series required one or more UAEs.
Updating this treatment approach with the review of 1223 patients published after 2012, UAE was used alone or in combination in 309 cases with a mean complication rate of 28%, with its highest rate if combined with intra-arterial injection of MTX, at the time of the catheterization: 18 of 52 (34.6%).
In a recent review of 142 patients treated with UAE, the authors concluded that the treatment of CSP patients with UAE can reduce the amount of intraoperative bleeding and the duration of vaginal bleeding, promote the improvement of patients’ clinical symptoms, have less impact on the disruption of patients’ sex hormone balance, reduce patients’ surgical risks to a greater extent, preserve patients’ normal fertility, and have better application [52].
-
3.
Excision by hysteroscopy and/or laparoscopy
Hysteroscopic and laparoscopic surgery requires general anesthesia. The overall complication rate for 108 cases managed by hysteroscopy was 13.8% [5]. However, no complications were noted if hysteroscopy was combined with transabdominal ultrasound guidance (9 cases were published). The rate of complications increased to 17% if hysteroscopy was combined with mifepristone. In the hands of an experienced clinician, guided by transabdominal ultrasound, hysteroscopy may be a reasonable way of treatment for CSP [48, 53,54,55,56,57,58,59]. The use of an inflatable balloon catheter, after treatment with hysteroscopic excision, may prevent (or treat) possible bleeding from the operative site.
Laparoscopic surgery, alone or in combination, was used to excise the site of the scar pregnancy and repair the anterior uterine wall. Fifty-four such cases were published up to 2012 in the reviewed literature, with complication rates between 20% and 30% [5]. Since 2012, there have been several other laparoscopically treated case reports [51, 60,61,62,63,64].
Robotic assisted laparoscopic removal of CSP was also published [65]. We speculate that the complicated, time-consuming, and probably costly robotic surgery involving dedicated staff and its availability only in selected medical centers make the use of this operative approach to CSP questionable, since it can be replaced with several office-based, simple, and less involved treatments.
One of the latest publications favors laparoscopic excision of the CSP combined with the site repair claiming that primary laparoscopic management is not only the most effective method with the lowest complication rates but also an approach that allows for simultaneous repair and revision of the cesarean scar defect. The authors demonstrate easily adaptable techniques for maintaining hemostasis, minimizing injury to normal myometrium, and creating multilayer closures that lead to successful revisions with minimal impact to subsequent fertility [66].
-
4.
Methotrexate
One of the most frequently used therapies to treat CSP is undoubtedly methotrexate (MTX). It is administered in single or multiple, successive doses, intramuscularly, injected locally into the gestational sac, as intravenous slow drip, and finally injected into the umbilical artery at the time of a UAE. It was reported to be administered as a first-line or a secondary or backup medication, as a single agent and/or combined with any other conceivable treatment as an adjunct.
Systemic, “first-line,” single-dose MTX is administered as an intramuscular, single injection. The usual protocol was 1 mg/kg of body weight or 50 mg/m2 of body surface area. Its complication rate is 62.1% due to a required second-line treatment, when the fetal heartbeat fails to cease after several days [5]. Bodour et al. [67] challenged this result, which prompted a reevaluation of the reviewed material; however, after the more rigorous recounting of the cases, an even higher (66.1%) complication rate was found [68].
The reason for this, we suspect, may be caused by its slow action and the fact that the results may take days to be seen. We also suspect that it may not be able to stop cardiac activity and placental invasion. During these several days (or entire week), the gestational sac, the embryo or fetus, and its vascularity continue to grow, forcing a secondary treatment that must be able to handle a larger gestation with more abundant vascularization. The slow action of systemic MTX treatment is echoed, among others, in the series of Yin et al. [69]. It is true that there are also proponents of the use of systemic MTX as a single agent; however, it is impossible to attribute the cessation of the heart activity to the effect of MTX, since at least 10% of first-trimester intrauterine pregnancies undergo a spontaneous demise.
Based upon our recent review of 1223 cases of CSP, there were 236 cases in which MTX was administered as a single agent or in a combined fashion with other treatments, with a mean of 21.4% complications. Methotrexate used alone (as single or multidose) leads to 38% of the cases needing a secondary treatment [48, 70]. Combined with D&C (26 cases), another therapy with high complication rate, all needed a secondary treatment.
The guidelines of the Society of Maternal Fetal Medicine clearly discourage treating CSP using systemic MTX alone and encourage combining MTX with other treatment modalities [19].
Systemic, sequential, multidose use of MTX. The injected amounts of MTX are similar to the dose for the single-dose regimen. However, 2–3 intramuscular injections (1 mg/kg of body weight or 50 mg/m2 of surface area) are given at an interval of 2 or 3 days over the course of a week. In this case, one should be aware of the cumulative, adverse effects of this drug on the liver and bone marrow, since the total amount is higher than that in the single-dose regimen. In fact, even multidose treatments have failed [71]. Some combine it with different doses of leucovorin, which protects against unwanted and adverse systemic effects (termed “rescue” regimen). Several articles expressed their authors’ confidence in support of systemic multidose MTX treatment [72].
It is difficult to assess the complication rate associated with the above approach because it was often used in conjunction with or after “first-line” or even after “secondary” treatments [69]. It is clear that MTX can successfully be applied as an adjunct and combined with other mostly nonsurgical treatments. The drawback of both treatments is the long waiting time to observe their effect. If they fail to stop the heart and quickly lower the levels of hCG, a secondary treatment has to deal with a larger gestation and vascular supply.
While multidose MTX was reported to be useful in tearing CSP, it still had a failure rate of 13.4% in using it in 29 patients needing a secondary intervention by local, intragestational sac injection [73].
Intra-arterial or intravenous MTX treatment. Adopted and used in China—a total of 193 patients were treated using intravenous or intra-arterial administration of MTX solution. The intra-arterial route is used at the time of UAE. Most intravascular treatments were combined with other methods such as suction aspiration laparoscopy, hysteroscopy, and D&C. Li et al. [74] treated 33 patients with CSP out of 13 patients treated with intravenous MTX. Three of the 13 required hysterectomy for profuse bleeding. Zhang et al. [75] have a series of 96 patients of which 33 had intravenous MTX treatment. Since most patients, however, were treated in combination with other methods, their outcome is unclear from the English abstract. Another method is to infuse MTX solution into the uterine artery at the time of UAE. An et al. [76] treated 22 patients with UAE and intra-arterial MTX infusion: 6 patients had severe hemorrhage, 12 had abdominal pain, and 4 hysterectomies were necessary. As opposed to this, Lan et al. [77] successfully used 50 mg MTX infused into the uterine artery at the time of UAE in 79 patients.
-
5.
Excision by hysteroscopic guidance alone or in combination
In our first review [5], hysteroscopic excision was used alone or with other treatments in 113 cases, with a mean complication rate of 18.4%, which is reasonably low in comparison to other treatment methods. General anesthesia is required for the procedure.
In the literature published after 2012, we found 63 cases managed by this method alone or combined, usually, with laparoscopy [60, 75, 78,79,80,81].
-
6.
Excision by laparoscopic guidance
It is mostly used as the sole, standalone treatment, since it provides a final solution removing the gestational sac and the tiny placenta. General anesthesia is required. Fifteen of the 49 cases (30.6%) described in the literature published before 2013 involved complications, as opposed to the 94 cases published in or after 2012 [48, 51, 60, 61, 63, 64, 80, 81], which experienced only 7.7% in complications when hysteroscopy and laparoscopy were combined. The small numbers may not allow meaningful evaluation of the latter two approaches.
-
7.
Excision by laparotomy
Only a handful of articles were published. Fifteen patients undergoing excision of the gestational sac using this, relatively involved, surgery procedure, which is usually performed under general anesthesia [60, 81,82,83]. At times, elective laparotomy was the treatment of choice to perform hysterectomy, or it was used as a solution to treat bleeding complications [76, 84,85,86,87]. Figure 18.12a depicts the closed suture line after the excision of a CSP, while Fig. 18.12b shows the local results after 1 year.
-
8.
Transvaginal surgical excision
Scarce and mostly single case reports are in the literature. This procedure requires a skilled surgeon and is used electively in 119 patients with a relatively low (mean 9.7%) complication rate [88,89,90,91]. Li et al. [48] described this surgical approach, which elevates the bladder, excising the gestational sac after curetting and, finally, suturing the area. They managed 49 cases, reporting that, despite 18% minor complications, the procedure is easy and safe. Three patients had intrauterine pregnancies at 6 and 12 months postoperatively. One patient had a recurrent CSP and repeat transvaginal surgical excision. Another patient had an intrauterine pregnancy 5 months postoperatively; however, D&C was performed to prevent uterine rupture.
-
9.
Intragestational sac injection of methotrexate or potassium chloride, with continuous, real-time ultrasound guidance
No anesthesia is required. This approach (Fig. 18.13) had the fewest and least involved complications. In certain cases, we completed the local injection by an immediate placement of a Foley balloon catheter that, after inflation with several milliliters of saline solution, can be kept in place for several days to prevent vaginal bleeding (Fig. 18.14a–f). Of the 83 cases, only 9 (10.8%) involved complications. Cases performed with transabdominal sonography guidance had a slightly higher complication rate (15%) than those using TVS guidance. Since 2012, several authors used this simple treatment in 53 patients.
Since the publication of our review, a handful of articles reported on the successful use of the local, intragestational sac injection of ethanol [80], MTX [71, 92,93,94,95], and KCl [70] in a total of 53 patients with a complication rate of 5.8%. Yin et al. [69] treated 20 of 34 patients with CSP by local, transvaginal ultrasound-guided intragestational sac injection of MTX, without complications. Yamaguchi et al. [95] treated 8 CSP cases, using intragestational injection of MTX, guided by TVS. Two of the patients needed additional local or systemic MTX injection. The time to hCG normalization was a mean of 78.5 days (range, 42–166 days). Four of the five patients went on and had pregnancies after the treatment and had uneventful parturition; however, another CSP was diagnosed in one patient. Pang et al. [93] successfully treated three patients with local, intragestational MTX injection. Some providers prefer the use of KCl for all their local injections in all types of ectopic pregnancies including CSP [96]. KCl is exclusively used to inject heterotopic pregnancies to enable the normal development of the intrauterine gestation.
Local, intragestational sac injections render a desired solution by stopping the heart activity, and it appears to be an effective and simple intervention for first-trimester CSP between 6 and 8–9 weeks and can be performed by TAS or TVS guidance. A single-balloon Foley or a double-balloon Cook catheter should be handy if bleeding is encountered. These treatments may be even more relevant for patients desiring future fertility.
In a recent publication, this treatment was used in 14 cases and the authors report that direct MTX injection into the gestational sac for NTEP treatment is safe and effective. The failure rate of 7% is considerably lower than what was previously reported for a failure of systemic MTX in similar cases (25%). Resolution of serum hCG after treatment can be quite prolonged even in uncomplicated cases [97]. No other larger series were found to add more information on this formally relatively widespread treatment.
-
10.
Shirodkar suture in the treatment of CSP
This was used by Jurkovic et al. [98], during the evacuation of a cesarean scar pregnancy, which is an effective method for securing hemostasis. In their view, it minimized the need for blood transfusion and ensured preservation of fertility.
-
11.
Foley single-balloon and Cook double-balloon catheters as an adjuvant to other treatments to prevent/control bleeding
A creative and relatively new approach to the treatment is inserting a Foley balloon catheter that is inflated at the site of the CSP, like the Bakri balloon in cases of obstetrical hemorrhage [45, 99,100,101]. We used this approach as an adjuvant to treatments of CSP [41, 44]. Even so, these approaches are almost always used in a planned fashion, in conjunction with another treatment or as backup, if bleeding occurs (Fig. 18.15a–h). Catheters may be kept in place for as long as 3–4 days, according to the individual case, provided that antibiotic coverage is prescribed. As stated above, this approach is almost always used in a pre-planned case of a patient who restarted bleeding 23 days after local injection of MTX, with a relatively large gestation of 9 weeks 3 days. Inflating the balloon to 20 mL controlled bleeding (Fig. 18.16).
-
12.
Recurrent CSP
Patients treated in the first trimester for CSP should be informed that such a gestation may not happen again in a future pregnancy since the risk is about 1% for reoccurrence. In the literature reviewed through 2012, seven recurrent cases of CSP were described [5]. Gupta et al. [102] provided an additional case, with a patient who had four consecutive CSPs within 2 years. Please note that this patient became pregnant with the fifth CSP, decided to continue the pregnancy, and at the time of this writing is 16 weeks pregnant.
Two series are worth mentioning including relatively larger series with recurrence rates between 12% and 34% [103]. Forty-four studies (3598 women with CSP) were included. CSP recurred in 17.6% of women. Miscarriage, preterm birth, and placenta accreta spectrum disorders complicated 19.1% (65/341), 10.3% (25/243), and 4.0% of pregnancies, and 67.0% were uncomplicated. When stratifying the analysis according to the type of management, CSP recurred in 21% of women undergoing surgical and in 15.2% of those undergoing nonsurgical management. PAS disorders complicated 4.0% and 12.0% of cases, respectively.
-
13.
Multifetal CSP
Rare but possible, two gestational sacs with two embryos can be present as a twin CSP (Fig. 18.17). There was also a triplet CSP published. Their treatment, so far, was to terminate the pregnancies.
-
14.
Heterotopic CSP
Several heterotopic pregnancies were reported. In these cases, the intrauterine pregnancy can result in live offspring (Fig. 18.18). Several articles reported heterotopic IUP and CSP. The best review, however, containing detailed information is by Ugurlucan et al. [104]. Heterotopic CSP after CS may occur especially when a pregnancy follows assisted reproductive technology. These pregnancies are usually managed by selective injection of the scar pregnancy by local intragestational injection of KCl and laparoscopic excision [105, 106]. Fortunately, most intrauterine pregnancies can be preserved after treatment. A triplet heterotopic pregnancy was also reported by Hsieh et al. [107]. They reported a case of IVF-induced triplet heterotopic pregnancy of early gestational age that was diagnosed as early as 6 weeks’ gestation. Treatment with embryo aspiration under vaginal ultrasonography for selective embryo reduction was given, and the concurrent intrauterine twin pregnancy was preserved successfully.
Late development of an AVM after local intragestational injection of MTX with sonographic follow-up of the vascularization on days 7, 14, 67, 97, and 122 following the treatment (a–f). The patient refused an UAE after 4 weeks; however, the continuous vaginal spotting and slight bleeding finally led to the acceptance of the bilateral embolization of the uterine arteries, which was successful (g, h)
Sequential images of treating a 5–6-week live CSP using local injection followed by insertion of a Foley balloon. (a) Sagittal image showing the gestational sac in an anteverted/anteflexed uterus. (b) The vascularization is evident. (c) The needle was inserted under transvaginal ultrasound guidance, and MTX was injected. (d) The inflated balloon in situ creating pressure on the surrounding tissues. (e) Transverse image of the inflated balloon with barely detectable blood vessels. (f) The area 3 days later after removal of the balloon. Minimal vascularity was seen, and the minimal vaginal bleeding stopped after 1 week
Sequential, pictorial demonstration of the treatment of a 4-week 5-day CSP and use of a Foley balloon catheter. (a) The sagittal power Doppler image at 4 weeks 5 days. The patient selected to wait if systemic MTX would suffice as treatment. (b) At 5 weeks 4 days, embryonic heartbeats were seen. (c) A transverse section demonstrates the anterior placenta with its vessels between the sac and the bladder. (d) 3D Doppler angiography clearly shows the rich vascular web below the bladder. (e, f) After local, intragestational injection of MTX, a Foley balloon was inserted. The compressed sac is seen. (g, h) Two hours after balloon insertion, diminished blood flow was observed around the sac by Doppler interrogation
The use of Foley balloon catheter in a patient with a relatively advanced CSP of 9+ weeks with a gestational sac of 4.4 × 4.3 cm treated by local intragestational injection of MTX and who started to bleed late, 25 days after treatment. (a–e) Sequential power Doppler ultrasound images from diagnosis and immediately after the local injection of MTX stopping the heartbeats and throughout days 1, 16, and 21 after treatment. No vaginal bleeding was reported; however, no real decrease of the sac size occurred and the small embryo was still visible in the sac. (f) On day 25, after the initial treatment, vaginal bleeding occurred, which was successfully treated by insertion of a Foley balloon catheter and inflated to about 4 cm diameter by about 20
Heterotopic CSP and IUP at 7 weeks and 4 days. (a) Panoramic sagittal view of the two sacs. Both embryos were alive. The intrauterine sac (b) is filling the available space in the uterine cavity (Cx cervix). (b) Image of the embryo (a) in the lower anterior sac. (c) Image of the intrauterine embryo (b) in the upper sac. (d) Proof of the heartbeats of the intrauterine embryo moments after the injection of scar pregnancy. The patient delivered at term a healthy neonate
Summary and Conclusion
Cesarean scar pregnancy (CSP) is not an ectopic pregnancy by definition. Contrary to real ectopic pregnancies, the CSP is in the uterine cavity and if not terminated (based upon the recently available literature) can result in a live offspring [108]. CSP is a relatively rare but dangerous and complication-ridden clinical entity, closely related to a consequence of cesarean deliveries (CD).
The best diagnostic tool for its detection, and at times for treatment, is transvaginal sonography. In addition, transabdominal and color Doppler and lately also microvascular color Doppler ultrasound provide satisfactory diagnostic information. The main differential diagnostic entities of a CSP are cervical pregnancy and a miscarriage in progress. Patients with CSP should be counseled based upon new, peer-reviewed evidence published in the latest literature. In addition, patients must be informed of the possible second- and third-trimester complications.
There is mounting evidence that every patient with previous CD should be screened for CSP, as soon as possible [39, 108]. Also, there has been evidence that first-trimester CSP and PAS share the same histologic picture, as CSP is a precursor of PAS. Most patients with a CSP diagnosed in the first trimester will by the third trimester have PAS. And a large number of repeat CD will have a very high risk of hysterectomy.
There is no single best treatment approach to terminate CSP with positive heart activity. Therefore, the procedure with the least complications should be considered and performed without delay. Single-dose systemic MTX injection is a lengthy and usually ineffective first-line therapy, delaying the final treatment. MTX, however, as an adjuvant to other treatments has a proven efficacy. Ultrasound-guided local, intragestational sac injection of MTX/KCl is simple and has low complication rates. Sharp curetting of the CSP site can cause severe bleeding. Uterine artery embolization (UAE) alone is less effective as a single, first-line treatment but has proven useful as an adjunct to other therapies and in cases of emergency due to sustained vaginal bleeding. Insertion and inflation of a Foley balloon or a Cook cervical ripening double-balloon catheter are effective to terminate a CSP or to prevent bleeding from the site of a CSP, or following local injection or endoscopic treatment of CSP. Attention should be given to the possibility of recurrent multifetal and heterotopic CSP.
To evaluate the present practices pertinent to diagnosing, counseling, and treating CSP, an international registry was created (www.CSP-registry.com). The aim of the registry was to investigate safety and efficacy of the different treatment options for termination of CSP from an international large registry and compare these findings with physician’s views gained from the results of an international survey [109].
Teaching Points
-
Diagnose a cesarean scar pregnancy by the diagnostic criteria and differentiate it from cervical pregnancy and/or a spontaneous abortion.
-
Realize that there is a common histologic basis of cesarean scar pregnancy and morbidly adherent placenta (accreta, increta, and percreta).
-
Construct a counseling and a management plan for the CSP taking into consideration patients’ obstetrical goals and evidence-based management.
References
Timor-Tritsch IE. A cesarean scar pregnancy is not an ectopic pregnancy. Ultrasound Obstet Gynecol. 2022;59(4):424–7. https://doi.org/10.1002/uog.24877. Epub 2022 Mar 10. PMID: 35266211
Spong CY, Berghella V, Wenstrom KD, Mercer BM, Saade GR. Preventing the first cesarean delivery: summary of a joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, and American College of Obstetricians and Gynecologists Workshop. Obstet Gynecol. 2012;120(5):1181–93.
Hamilton BE, Hoyert DL, Martin JA, Strobino DM, Guyer B. Annual summary of vital statistics: 2010–2011. Pediatrics. 2013;131(3):548–58.
Arnold J. World cesarean rates: OECD countries 2012. http://www.cesareanrates.com/blog/2012/12/8/world-cesarean-rates-oecd-countries.html.
Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. Am J Obstet Gynecol. 2012;207(1):14–29.
Monteagudo A, Carreno C, Timor-Tritsch IE. Saline infusion sonohysterography in nonpregnant women with previous cesarean delivery: the “niche” in the scar. J Ultrasound Med. 2001;20(10):1105–15.
Bij de Vaate AJ, van der Voet LF, Naji O, Witmer M, Veersema S, Brolmann HA, et al. Prevalence, potential risk factors for development and symptoms related to the presence of uterine niches following Cesarean section: systematic review. Ultrasound Obstet Gynecol. 2014;43(4):372–82.
Jauniaux E, Jurkovic D. Placenta accreta: pathogenesis of a 20th century iatrogenic uterine disease. Placenta. 2012;33(4):244–51.
Jurkovic D, Hillaby K, Woelfer B, Lawrence A, Salim R, Elson CJ. Cesarean scar pregnancy. Ultrasound Obstet Gynecol. 2003;21(3):310.
Jurkovic D, Hillaby K, Woelfer B, Lawrence A, Salim R, Elson CJ. First-trimester diagnosis and management of pregnancies implanted into the lower uterine segment cesarean section scar. Ultrasound Obstet Gynecol. 2003;21(3):220–7.
Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol. 2006;107(6):1373–81.
Seow KM, Hwang JL, Tsai YL, Huang LW, Lin YH, Hsieh BC. Subsequent pregnancy outcome after conservative treatment of a previous cesarean scar pregnancy. Acta Obstet Gynecol Scand. 2004;83(12):1167–72.
Norwitz ER. Defective implantation and placentation: laying the blueprint for pregnancy complications. Reprod Biomed Online. 2006;13(4):591–9.
Toro-Bejarano M, Mora R, Timor-Tritsch IE, Vernon J, Monteagudo A, D’Antonio F, Duncan K. Myomectomy scar pregnancy – a serious, but scarcely reported entity: literature review and an instructive case. Case Rep Perinat Med. 2021;10(1):20210071. https://doi.org/10.1515/crpm-2021-0071.
Matsuzaki S, Nagase Y, Takiuchi T, Kakigano A, Mimura K, Lee M, Matsuzaki S, Ueda Y, Tomimatsu T, Endo M, Kimura T. Antenatal diagnosis of placenta accreta spectrum after in vitro fertilization-embryo transfer: a systematic review and meta-analysis. Sci Rep. 2021;11(1):9205. https://doi.org/10.1038/s41598-021-88551-7. PMID: 33911134; PMCID: PMC8080594
Rosen T. Placenta accreta and cesarean scar pregnancy: overlooked costs of the rising cesarean section rate. Clin Perinatol. 2008;35(3):519–29, x.
Kliman HJ, Feinberg RF, Haimowitz JE. Human trophoblast-endometrial interactions in an in vitro suspension culture system. Placenta. 1990;11(4):349–67.
Jauniaux E, Jurkovic D, Hussein AM, Burton GJ. New insights into the etiopathology of placenta accreta spectrum. Am J Obstet Gynecol. 2022; https://doi.org/10.1016/j.ajog.2022.02.038. Epub ahead of print. PMID: 35248577
Society for Maternal-Fetal Medicine (SMFM), Miller R, Timor-Tritsch IE, Gyamfi-Bannerman C. Society for Maternal-Fetal Medicine (SMFM) Consult Series #49: Cesarean scar pregnancy. Am J Obstet Gynecol. 2020;222(5):B2–B14. https://doi.org/10.1016/j.ajog.2020.01.030. Epub 2020 Jan 21. Erratum in: Am J Obstet Gynecol. 2020 Oct 6; PMID: 31972162
Shainker SA, Coleman B, Timor-Tritsch IE, Bhide A, Bromley B, Cahill AG, Gandhi M, Hecht JL, Johnson KM, Levine D, Mastrobattista J, Philips J, Platt LD, Shamshirsaz AA, Shipp TD, Silver RM, Simpson LL, Copel JA, Abuhamad A, Society for Maternal-Fetal Medicine. Special report of the Society for Maternal-Fetal Medicine Placenta Accreta Spectrum Ultrasound Marker Task Force: consensus on definition of markers and approach to the ultrasound examination in pregnancies at risk for placenta accreta spectrum. Am J Obstet Gynecol. 2021;224(1):B2–B14. https://doi.org/10.1016/j.ajog.2020.09.001. Erratum in: Am J Obstet Gynecol. 2021 Jul;225(1):91. PMID: 33386103
Stirnemann JJ, Chalouhi GE, Forner S, Saidji Y, Salomon LJ, Bernard JP, et al. First-trimester uterine scar assessment by transvaginal ultrasound. Am J Obstet Gynecol. 2011;205(6):551.e1–6.
Stirnemann JJ, Mousty E, Chalouhi G, Salomon LJ, Bernard JP, Ville Y. Screening for placenta accreta at 11–14 weeks of gestation. Am J Obstet Gynecol. 2011;205(6):547.e1–6.
Godin PA, Bassil S, Donnez J. An ectopic pregnancy developing in a previous caesarian section scar. Fertil Steril. 1997;67(2):398–400.
Vial Y, Petignat P, Hohlfeld P. Pregnancy in a cesarean scar. Ultrasound Obstet Gynecol. 2000;16(6):592–3.
Seow KM, Hwang JL, Tsai YL. Ultrasound diagnosis of a pregnancy in a cesarean section scar. Ultrasound Obstet Gynecol. 2001;18(5):547–9.
Timor-Tritsch IE, Monteagudo A, Santos R, Tsymbal T, Pineda G, Arslan AA. The diagnosis, treatment, and follow-up of cesarean scar pregnancy. Am J Obstet Gynecol. 2012;207(1):44.e1–e13.
Timor-Tritsch IE, Haynes MC, Monteagudo A, Khatib N, Kovács S. Ultrasound diagnosis and management of acquired uterine enhanced myometrial vascularity/arteriovenous malformations. Am J Obstet Gynecol. 2016;214(6):731.e1–731.e10. https://doi.org/10.1016/j.ajog.2015.12.024. Epub 2016 Feb 9. PMID: 26873276
Timor-Tritsch IE, McDermott WM, Monteagudo A, Calί G, Kreines F, Hernandez S, Stephenson C, Bryk H, D’Antonio F. Extreme enhanced myometrial vascularity following cesarean scar pregnancy: a new diagnostic entity. J Matern Fetal Neonatal Med. 2021;17:1–12. https://doi.org/10.1080/14767058.2021.1897564. Epub ahead of print. PMID: 33730990
Timmerman D, Wauters J, Van Calenbergh S, Van Schoubroeck D, Maleux G, Van Den Bosch T, et al. Color Doppler imaging is a valuable tool for the diagnosis and management of uterine vascular malformations. Ultrasound Obstet Gynecol. 2003;21(6):570–7.
Polat P, Suma S, Kantarcy M, Alper F, Levent A. Color Doppler US in the evaluation of uterine vascular abnormalities. Radiographics. 2002;22(1):47–53.
Timor-Tritsch IE, Khatib N, Monteagudo A, Ramos J, Berg R, Kovacs S. Cesarean scar pregnancy (CSP): experience of sixty cases. J Ultrasound Med. 2015;34(4):601–10.
Comstock CH, Lee W, Vettraino IM, Bronsteen RA. The early sonographic appearance of placenta accreta. J Ultrasound Med. 2003;22(1):19–23; quiz 4–6.
Rac M, Moschos E, Wells E, McIntire DD, Dashe JS, Twickler DM. Ultrasound (US) findings of placenta accreta in the first trimester. Ultrasound Obstet Gynecol. 2014;44(Suppl. 1):62–180.
Ballas J, Pretorius D, Hull AD, Resnik R, Ramos GA. Identifying sonographic markers for placenta accreta in the first trimester. J Ultrasound Med. 2012;31(11):1835–41.
Sinha P, Mishra M. Caesarean scar pregnancy: a precursor of placenta percreta/accreta. J Obstet Gynaecol. 2012;32(7):621–3.
Timor-Tritsch IE, Monteagudo A, Cali G, Palacios-Jaraquemada JM, Maymon R, Arslan AA, et al. Cesarean scar pregnancy and early placenta accreta share common histology. Ultrasound Obstet Gynecol. 2014;43(4):383–95.
Timor-Tritsch IE, Monteagudo A, Cali G, Vintzileos A, Viscarello R, Al-Khan A, et al. Cesarean scar pregnancy is a precursor of morbidly adherent placenta. Ultrasound Obstet Gynecol. 2014;44(3):346–53.
Timor-Tritsch IE, Monteagudo A, Calì G, D’Antonio F, Agten AK. Cesarean scar pregnancy: patient counseling and management. Obstet Gynecol Clin North Am. 2019;46(4):813–28. https://doi.org/10.1016/j.ogc.2019.07.010. PMID: 31677756
Timor-Tritsch I, Buca D, Di Mascio D, Cali G, D’Amico A, Monteagudo A, Tinari S, Morlando M, Nappi L, Greco P, Rizzo G, Liberati M, Jose-Palacios-Jaraquemada, D’Antonio F. Outcome of cesarean scar pregnancy according to gestational age at diagnosis: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;258:53–9. https://doi.org/10.1016/j.ejogrb.2020.11.036. Epub 2020 Nov 12. PMID: 33421811. PMID: 31677756
Miller R, Timor-Tritsch IE, Gyamfi-Bannerman C. Society for Maternal-Fetal Medicine (SMFM) Consult Series #49: Cesarean scar pregnancy. Am J Obstet Gynecol. 2020;222(5):B2–B14. https://doi.org/10.1016/j.ajog.2020.01.030. Epub 2020 Jan 21. Erratum in: Am J Obstet Gynecol. 2020 Oct 6: PMID: 31972162
Timor-Tritsch IE, Monteagudo A, Bennett TA, Foley C, Ramos J, Kaelin AA. A new minimally invasive treatment for cesarean scar pregnancy and cervical pregnancy. Am J Obstet Gynecol. 2016;215(3):351.e1–8. https://doi.org/10.1016/j.ajog.2016.03.010. Epub 2016 Mar 12. Erratum in: Am J Obstet Gynecol. 2020 Jun;222(6):618. PMID: 26979630
Maheux-Lacroix S, Li F, Bujold E, Nesbitt-Hawes E, Deans R, Abbott J. Cesarean scar pregnancies: a systematic review of treatment options. J Minim Invasive Gynecol. 2017;24(6):915–25. https://doi.org/10.1016/j.jmig.2017.05.019. Epub 2017 Jul 18. PMID: 28599886
Birch Petersen K, Hoffmann E, Rifbjerg Larsen C, Svarre NH. Cesarean scar pregnancy: a systematic review of treatment studies. Fertil Steril. 2016;105(4):958–67. https://doi.org/10.1016/j.fertnstert.2015.12.130. Epub 2016 Jan 18. PMID: 26794422
Timor-Tritsch IE, Cali G, Monteagudo A, Khatib N, Berg R, Forlani F, et al. The use of a Foley balloon catheter as an adjuvant therapy in preventing or managing bleeding during treatment for cesarean scar and cervical pregnancies. Ultrasound Obstet Gynecol. 2014;46:118–23.
Jiang T, Liu G, Huang L, Ma H, Zhang S. Methotrexate therapy followed by suction curettage followed by Foley tamponade for caesarean scar pregnancy. Eur J Obstet Gynecol Reprod Biol. 2011;156(2):209–11.
Yu XL, Zhang N, Zuo WL. Cesarean scar pregnancy: an analysis of 100 cases. Zhonghua Yi Xue Za Zhi. 2011;91(45):3186–9.
Cali G, Giambanco L, Puccio G, Forlani F. Morbidly adherent placenta: evaluation of ultrasound diagnostic criteria and differentiation of placenta accreta from percreta. Ultrasound Obstet Gynecol. 2013;41(4):406–12.
Li JB, Kong LZ, Fan L, Fu J, Chen SQ, Yao SZ. Transvaginal surgical management of cesarean scar pregnancy: analysis of 49 cases from one tertiary care center. Eur J Obstet Gynecol Reprod Biol. 2014;182C:102–6.
Cao S, Zhu L, Jin L, Gao J, Chen C. Uterine artery embolization in cesarean scar pregnancy: safe and effective intervention. Chin Med J. 2014;127(12):2322–6.
Gao L, Huang Z, Gao J, Mai H, Zhang Y, Wang X. Uterine artery embolization followed by dilation and curettage within 24 hours compared with systemic methotrexate for cesarean scar pregnancy. Int J Gynaecol Obstet. 2014;127(2):147–51.
Wu X, Xue X, Wu X, Lin R, Yuan Y, Wang Q, et al. Combined laparoscopy and hysteroscopy vs. uterine curettage in the uterine artery embolization-based management of cesarean scar pregnancy: a cohort study. Int J Clin Exp Med. 2014;7(9):2793–803.
Zhu W, Zhang X, Liu C, Liu Y, Xu W. Uterine artery embolization on serum β-HCG levels, fertility function and clinical efficacy in patients with cesarean uterine scar pregnancy. Front Surg. 2022;9:838879. https://doi.org/10.3389/fsurg.2022.838879. PMID: 35187063; PMCID: PMC8847222
Chang Y, Kay N, Chen YH, Chen HS, Tsai EM. Resectoscopic treatment of ectopic pregnancy in previous cesarean delivery scar defect with vasopressin injection. Fertil Steril. 2011;96(2):e80–2.
Chao A, Wang TH, Wang CJ, Lee CL, Chao AS. Hysteroscopic management of cesarean scar pregnancy after unsuccessful methotrexate treatment. J Minim Invasive Gynecol. 2005;12(4):374–6.
Chen ZY, Zhang XM, Xu H, Zhang J, Huang XF. Management of cesarean scar pregnancy by hysteroscopy combined with uterine artery embolism. Zhonghua Fu Chan Ke Za Zhi. 2011;46(8):591–4.
Deans R, Abbott J. Hysteroscopic management of cesarean scar ectopic pregnancy. Fertil Steril. 2010;93(6):1735–40.
Gubbini G, Centini G, Nascetti D, Marra E, Moncini I, Bruni L, et al. Surgical hysteroscopic treatment of cesarean-induced isthmocele in restoring fertility: prospective study. J Minim Invasive Gynecol. 2011;18(2):234–7.
Ozkan S, Caliskan E, Ozeren S, Corakci A, Cakiroglu Y, Coskun E. Three-dimensional ultrasonographic diagnosis and hysteroscopic management of a viable cesarean scar ectopic pregnancy. J Obstet Gynaecol Res. 2007;33(6):873–7.
Robinson JK, Dayal MB, Gindoff P, Frankfurter D. A novel surgical treatment for cesarean scar pregnancy: laparoscopically assisted operative hysteroscopy. Fertil Steril. 2009;92(4):1497.e13–6.
Wang G, Liu X, Bi F, Yin L, Sa R, Wang D, et al. Evaluation of the efficacy of laparoscopic resection for the management of exogenous cesarean scar pregnancy. Fertil Steril. 2014;101(5):1501–7.
He Y, Wu X, Zhu Q, Wu X, Feng L, Wu X, et al. Combined laparoscopy and hysteroscopy vs. uterine curettage in the uterine artery embolization-based management of cesarean scar pregnancy: a retrospective cohort study. BMC Womens Health. 2014;14:116.
Hudecek R, Ivanova Z, Smerdova M, Pankova S, Krajcovicova R. Effect of GnRH analogues pre-treatment on myomectomy outcomes in reproductive age women. Ceska Gynekol. 2012;77(2):109–17.
Jiang S, Zhao S. Laparoscopic surgery for ectopic pregnancy within a cesarean scar. Clin Exp Obstet Gynecol. 2013;40(3):440–4.
Wang YL, Weng SS, Huang WC, Su TH. Laparoscopic management of ectopic pregnancies in unusual locations. Taiwan J Obstet Gynecol. 2014;53(4):466–70.
Siedhoff MT, Schiff LD, Moulder JK, Toubia T, Ivester T. Robotic-assisted laparoscopic removal of cesarean scar ectopic and hysterotomy revision. Am J Obstet Gynecol. 2015;212(5):681.e1–4.
Yoon R, Sasaki K, Miller CE. Laparoscopic excision of cesarean scar pregnancy with scar revision. J Minim Invasive Gynecol. 2021;28(4):746–7. https://doi.org/10.1016/j.jmig.2020.06.017. Epub 2020 Jun 27. PMID: 32603870
Bodur S, Gun I, Guido R. What is the role of primary methotrexate treatment in scar ectopic pregnancy? Am J Obstet Gynecol. 2014;210(4):379–80.
Timor-Tritsch I, Monteagudo A. Correction: Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. Am J Obstet Gynecol. 2014;210(4):371–4.
Yin XH, Yang SZ, Wang ZQ, Jia HY, Shi M. Injection of MTX for the treatment of cesarean scar pregnancy: comparison between different methods. Int J Clin Exp Med. 2014;7(7):1867–72.
Uysal F, Uysal A. Spontaneous heterotopic cesarean scar pregnancy: conservative management by transvaginal sonographic guidance and successful pregnancy outcome. J Ultrasound Med. 2013;32(3):547–8.
Berhie SH, Molina RL, Davis MR, Anchan RM, Wang KC. Beware the scar: laparoscopic hysterectomy for 7-week cesarean delivery scar implantation pregnancy. Am J Obstet Gynecol. 2015;212(2):247.e1–2.
Kutuk MS, Uysal G, Dolanbay M, Ozgun MT. Successful medical treatment of cesarean scar ectopic pregnancies with systemic multidose methotrexate: single-center experience. J Obstet Gynaecol Res. 2014;40(6):1700–6.
Levin G, Zigron R, Dior UP, Gilad R, Shushan A, Benshushan A, Rottenstreich A. Conservative management of Caesarean scar pregnancies with systemic multidose methotrexate: predictors of treatment failure and reproductive outcomes. Reprod Biomed Online. 2019;39(5):827–34. https://doi.org/10.1016/j.rbmo.2019.05.015. Epub 2019 May 24. PMID: 31530445
Li C, Feng D, Jia C, Liu B, Zhan X. Transcatheter arterial chemoembolization versus systemic methotrexate for the management of cesarean scar pregnancy. Int J Gynaecol Obstet. 2011;113(3):178–82.
Zhang Y, Chen YS, Wang JJ, Lu ZY, Hua KQ. Analysis of 96 cases with cesarean scar pregnancy. Zhonghua Fu Chan Ke Za Zhi. 2010;45(9):664–8.
An X, Ming X, Li K, Wang J. The analysis of efficacy and failure factors of uterine artery methotrexate infusion and embolization in treatment of cesarean scar pregnancy. TheScientificWorldJOURNAL. 2013;2013:213603.
Lan W, Hu D, Li Z, Wang L, Yang W, Hu S. Bilateral uterine artery chemoembolization combined with dilation and curettage for treatment of cesarean scar pregnancy: a method for preserving the uterus. J Obstet Gynaecol Res. 2013;39(6):1153–8.
Fylstra DL. Cervical pregnancy: 13 cases treated with suction curettage and balloon tamponade. Am J Obstet Gynecol. 2014;210(6):581.e1–5.
Mollo A, Alviggi C, Conforti A, Insabato L, De Placido G. Intact removal of spontaneous twin ectopic caesarean scar pregnancy by office hysteroscopy: case report and literature review. Reprod Biomed Online. 2014;29(5):530–3.
Shao MJ, Hu MX, Xu XJ, Zhang L, Hu M. Management of caesarean scar pregnancies using an intrauterine or abdominal approach based on the myometrial thickness between the gestational mass and the bladder wall. Gynecol Obstet Investig. 2013;76(3):151–7.
Li Y, Xiang Y, Wan X, Feng F, Ren T. Clinical study on 39 cases with caesarean scar pregnancy with sonographic mass. Zhonghua Fu Chan Ke Za Zhi. 2014;49(1):10–3.
Abdelkader MA, Fouad R, Gebril AH, El Far MA, Elyassergi DF. Caesarean scar pregnancy: hysterotomy is rapid and safe management option. Arch Gynecol Obstet. 2014;290(2):381–3.
Kai K, Shimamoto K, Matsumoto H, Narahara H. Conservative surgical treatment for caesarean scar pregnancy. J Obstet Gynaecol. 2014;34(1):91–2.
Nankali A, Ataee M, Shahlazadeh H, Daeichin S. Surgical management of the cesarean scar ectopic pregnancy: a case report. Case Rep Obstet Gynecol. 2013;2013:525187.
Sorbi F, Sisti G, Pieralli A, Di Tommaso M, Livi L, Buccoliero AM, et al. Cervicoisthmic choriocarcinoma mimicking cesarean section scar ectopic pregnancy. J Res Med Sci. 2013;18(10):914–7.
Wozniak S, Pyra K, Kludka-Sternik M, Czuczwar P, Szkodziak P, Paszkowski T, et al. Uterine artery embolization using gelatin sponge particles performed due to massive vaginal bleeding caused by ectopic pregnancy within a cesarean scar: a case study. Ginekol Pol. 2013;84(11):966–9.
Zhang B, Jiang ZB, Huang MS, Guan SH, Zhu KS, Qian JS, et al. Uterine artery embolization combined with methotrexate in the treatment of cesarean scar pregnancy: results of a case series and review of the literature. J Vasc Interv Radiol. 2012;23(12):1582–8.
Shi J, Qin J, Wang W, Zhang H. Clinical study on 57 cases with caesarean scar pregnancy. Zhonghua Fu Chan Ke Za Zhi. 2014;49(1):18–21.
Wang DB, Chen YH, Zhang ZF, Chen P, Liu KR, Li Y, et al. Evaluation of the transvaginal resection of low-segment cesarean scar ectopic pregnancies. Fertil Steril. 2014;101(2):602–6.
Wang Z, Shan L, Xiong H. Transvaginal removal of ectopic pregnancy tissue and repair of uterine defect for cesarean scar pregnancy. Clin Exp Obstet Gynecol. 2013;40(4):546–7.
Le A, Shan L, Xiao T, Zhuo R, Xiong H, Wang Z. Transvaginal surgical treatment of cesarean scar ectopic pregnancy. Arch Gynecol Obstet. 2013;287(4):791–6.
Nguyen-Xuan HT, Lousquy R, Barranger E. Diagnosis, treatment, and follow-up of cesarean scar pregnancy. Gynecol Obstet Fertil. 2014;42(7–8):483–9.
Pang YP, Tan WC, Yong TT, Koh PK, Tan HK, Ho TH. Caesarean section scar pregnancy: a case series at a single tertiary centre. Singap Med J. 2012;53(10):638–42.
Seow KM, Wang PH, Huang LW, Hwang JL. Transvaginal sono-guided aspiration of gestational sac concurrent with a local methotrexate injection for the treatment of unruptured cesarean scar pregnancy. Arch Gynecol Obstet. 2013;288(2):361–6.
Yamaguchi M, Honda R, Uchino K, Tashiro H, Ohba T, Katabuchi H. Transvaginal methotrexate injection for the treatment of cesarean scar pregnancy: efficacy and subsequent fecundity. J Minim Invasive Gynecol. 2014;21(5):877–83.
Doubilet PM, Benson CB, Frates MC, Ginsburg E. Sonographically guided minimally invasive treatment of unusual ectopic pregnancies. J Ultrasound Med. 2004;23(3):359–70.
Gilbert SB, Alvero RJ, Roth L, Polotsky AJ. Direct methotrexate injection into the gestational sac for nontubal ectopic pregnancy: a review of efficacy and outcomes from a single institution. J Minim Invasive Gynecol. 2020;27(1):166–72. https://doi.org/10.1016/j.jmig.2019.03.016. Epub 2019 Mar 28. PMID: 30930212
Jurkovic D, Ben-Nagi J, Ofilli-Yebovi D, Sawyer E, Helmy S, Yazbek J. Efficacy of Shirodkar cervical suture in securing hemostasis following surgical evacuation of cesarean scar ectopic pregnancy. Ultrasound Obstet Gynecol. 2007;30(1):95–100.
Atilgan R, Celik A, Boztosun A, Ilter E, Yalta T, Ozercan R. Evaluation of cervical cytological abnormalities in Turkish population. Indian J Pathol Microbiol. 2012;55(1):52–5.
Tsui KH, Lin LT, Yu KJ, Chen SF, Chang WH, Yu S, et al. Double-balloon cervical ripening catheter works well as an intrauterine balloon tamponade in post-abortion massive hemorrhage. Taiwan J Obstet Gynecol. 2012;51(3):426–9.
Shao HJ, Ma JT, Yang XE, Xu LP, Yang CL. Diagnosis and treatment of cesarean scar pregnancy. Zhonghua Yi Xue Za Zhi. 2010;90(37):2616–9.
Gupta S, Pineda G, Rubin S, Timor-Tritsch IE. Four consecutive recurrent cesarean scar pregnancies in a single patient. J Ultrasound Med. 2013;32(10):1878–80.
Timor-Tritsch IE, Horwitz G, D’Antonio F, Monteagudo A, Bornstein E, Chervenak J, Messina L, Morlando M, Cali G. Recurrent cesarean scar pregnancy: case series and literature review. Ultrasound Obstet Gynecol. 2021;58(1):121–6. https://doi.org/10.1002/uog.23577. PMID: 33411387
Ugurlucan FG, Bastu E, Dogan M, Kalelioglu I, Alanya S, Has R. Management of cesarean heterotopic pregnancy with transvaginal ultrasound-guided potassium chloride injection and gestational sac aspiration, and review of the literature. J Minim Invasive Gynecol. 2012;19(5):671–3.
Demirel LC, Bodur H, Selam B, Lembet A, Ergin T. Laparoscopic management of heterotopic cesarean scar pregnancy with preservation of intrauterine gestation and delivery at term: case report. Fertil Steril. 2009;91(4):1293.e5–7.
Wang CJ, Tsai F, Chen C, Chao A. Hysteroscopic management of heterotopic cesarean scar pregnancy. Fertil Steril. 2010;94(4):1529.e15–8.
Hsieh BC, Hwang JL, Pan HS, Huang SC, Chen CY, Chen PH. Heterotopic caesarean scar pregnancy combined with intrauterine pregnancy successfully treated with embryo aspiration for selective embryo reduction: case report. Hum Reprod. 2004;19(2):285–7. https://doi.org/10.1093/humrep/deh080. PMID: 14747168
Timor-Tritsch IE. Cesarean scar pregnancy is not an ectopic pregnancy. Ultrasound Obstet Gynecol. 2022; https://doi.org/10.1002/uog.24877.
Agten AK, Monteagudo A, Timor-Tritsch IE, Thilaganathan B. Cesarean Scar Pregnancy Registry: an international research platform. Ultrasound Obstet Gynecol. 2020;55(4):438–40. https://doi.org/10.1002/uog.21952. PMID: 31840910
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Timor-Tritsch, I.E., Monteagudo, A., Bennett, TA. (2023). Cesarean Scar Pregnancy: A Baby Placenta Accreta. In: Abramowicz, J.S., Longman, R.E. (eds) First-Trimester Ultrasound. Springer, Cham. https://doi.org/10.1007/978-3-031-24133-8_18
Download citation
DOI: https://doi.org/10.1007/978-3-031-24133-8_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-24132-1
Online ISBN: 978-3-031-24133-8
eBook Packages: MedicineMedicine (R0)