Abstract
Renin-Angiotensin System (RAS) is a vital system regulating blood pressure and maintaining sodium homeostasis in the human body. It consists of Angiotensin I (Ang I), Angiotensin II (Ang II), Angiotensin-converting enzyme (ACE), Angiotensin II type 1 receptor (AT1R), and angiotensin II type 2 receptor (AT2R), which functions in both normal and pathological conditions including cancer. Besides, the effectors of RAS are also included, such as Angiotensin-(1-7). This review focuses on the pre-clinical studies and clinical trials assessing the roles of RAS in regulating tumor progression as well as the underlying mechanisms.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Renin-Angiotensin System (RAS) is a complex systemic hormonal cascade of interacting peptides and enzymes, which plays a vital role in regulating blood pressure and maintaining normal sodium homeostasis of the human body [1]. It contains Angiotensin I (Ang I), Ang II, Ang-(1-7), MAS Receptor (MASR), Angiotensin-converting enzyme (ACE), ACE-2, Angiotensin II type 1 receptor (AT1R), and angiotensin II type 2 receptor (AT2R) [2]. Angiotensinogen (AGT), a precursor peptide related to the family of serine protease inhibitors (serpins), is the unique substrate for the protease renin (EC 3.4.23.15). The hydrolysis of AGT by renin is rate-limiting for the whole system and results in the production of des(Ang I)-AGT and of the vasoinactive peptide Ang I, which is converted to the vasoactive peptides Ang II and Ang III by ACE (EC 3.4.15.1) and aminopeptidase A (EC 3.4.11.7), respectively [3]. Angiotensin-(1-7), converted from Ang II by ACE and ACE2, has been reported to counteract the function of Ang II in many aspects, serving as a biologically active intermediate of the vasodilatory arm of the renin-angiotensin system [4]. RAS plays a pivotal role in the maintenance of normal physiological state in the human body, whose dysregulation has been reported to lead to the onset of various diseases, such as hypertension [5], diabetes [5], stroke [6], chronic obstructive pulmonary disease (COPD) [7], cancer [8], etc.
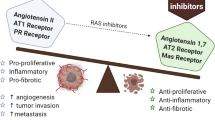
Cancer is one of the leading causes of death in the world and acquires the malignancy depending on several abilities: constant proliferation signals, sustained angiogenesis, tissue invasion and metastases, and evasion of apoptosis [9]. It has been reported that upregulation of the components of RAS is in close relationship with some types of cancers, such as colorectal cancer [10], prostate cancer [11], renal clear cell carcinoma [12], and gliomas [13], etc. The importance of RAS and its components in cancer development merits attentions to cancer researchers. Here, this article reviews the role of RAS in tumor progression including cell proliferation, angiogenesis, metastasis, and the potential strategies for cancer treatment through modulation of RAS functions (Fig. 16.1).
The role of RAS in tumor progression. Main members of RAS include Ang I, Ang II, Ang-(1-7), MASR, ACE, ACE-2, AT1R, and AT2R. Binding of Ang II to AT1R increases angiogenesis, tumor proliferation, migration, invasion, and metastasis. On the contrary, binding of Ang II to AT2R inhibits angiogenesis, cell proliferation, migration, invasion, and metastasis. Binding of Ang-(1-7) with MASR exerts similar anti-tumor effects
RAS and Tumor Progression
RAS and Cell Proliferation
RAS has been first revealed as the regulator in proliferation of tumor cells. Among the members of RAS, Ang II and ACE have a close association with tumor cell proliferation, which are involved in multiple signaling pathways including AT1R/PI3K/Akt/mTOR, AT1R/Raf/ERK1/2, and MAPK signaling pathways (Fig. 16.2). Insulin resistance induces the secretion of insulin-like growth factor (IGF)-1 in the liver, which promotes cell proliferation and inhibits cell apoptosis through Akt signaling pathway [14]. Besides, IGF-1 also triggers MAPK signaling and promotes mitosis in a variety of cancer cells [15, 16]. Ang II can activate the AT1R-mediated PI3K/Akt/mTOR pathway, promoting the occurrence, survival, and growth of cancer cells [17, 18], which can be blocked by antagonizing AT1R [19]. Ang II is able to upregulate CyclinD1, GSK3β, and downregulate p27 in hepatocellular carcinoma via AT1R-induced activation of PKC and MAPK signaling pathways [20].
The ACE inhibitor elicits outside-in signaling in endothelial cells, enhancing the activity of ACE-associated kinase CK2 and increasing the phosphorylation of the intracellular tail of ACE [21]. Researchers have found ACE activation might control the expression of diverse proteins besides ACE itself. Upon binding to ACE, Ang II internalizes with a faster onset compared to the binding of Ang II to its classical AT1 receptor [22]. Ang II and ACE can form a complex, which translocates to the nucleus through a clathrin-mediated process, and interacts with β3 isoform of PLC, triggering a nuclear Ca2+ signal resulting in induced cell proliferation [23, 24]. In melanoma TM-5 cells, Ang II induced cell proliferation through ACE activation, which was confirmed by ACE inhibitor (Lisinopril) or by the silencing of ACE [22].
Different RAS receptors either activate or inactivate various signaling pathways related to cancer development. AT1R activation through binding of Ang II leads to activation of PI3K/AKT/mTOR, NF-κB, MAPK, JNK, Ras/ERK pathways. Transcription of growth factors and formation of matrix proteins are then triggered and lead to increased cell proliferation. Ang-(1-7) bind to MASR and is mediated through TGF-β, PAK1/NF-κB/Snail pathways.
RAS and Tumor Angiogenesis
Tumor angiogenesis produced by RAS is mainly induced by vascular endothelial growth factor (VEGF) and its related pathways. As opposed to the systemic RAS, recently, the concept of a localized RAS has been reported, such as in the central nervous system (CNS) [13] and female reproductive organs [25] (Fig. 16.3).
In CNS, the predominant cells expressing AGT are astrocytes [26], whereas renin, another key member of RAS system, is expressed in both astrocytes and neuron cells [27]. In glioblastoma, ACE activity cannot be detected in glioblastoma cells; however, ACE is highly expressed in the aberrant vasculature of human glioblastoma [28]. It was postulated that a complete angiotensin system existed in the brain, independent from the circulatory system and that its role in the regulation of vascular functions was crucial in tumors. In the vasculature, AGT, the unique and specific substrate of renin, is demonstrated to be antiangiogenic [29], while angiotensin peptides, in particular Ang II, have been shown to be proangiogenic and to be involved in vascular growth [3].
The mammalian VEGF family is comprised of five members: VEGFA, VEGFB, VEGFC, VEGFD, and placenta growth factor (PGF). VEGFA is the most functional factor, which exerts angiogenic effects by activating VEGFR2 expressed in endothelial cells [30]. It has been revealed that ACE2 inhibits angiogenesis via suppressing the VEGFA/VEGFR2/ERK pathway in breast cancer [31]. Hypoxia leads to hypoxia-inducible factor 1α (HIF-1α) stabilization, increasing VEGF production in tumor cells. Besides, activation of the PI3K/AKT pathway also increases VEGF secretion in both HIF-1 dependent [32] and independent [33] manners. Moreover, the PI3K/AKT pathway also modulates the expression of other angiogenic factors such as nitric oxide [34] and angiopoietins [35, 36].
In the female reproductive system, activated RAS exists in the uterine endometrium, ovary, and placenta under both physiologic and pathological conditions [25, 37,38,39]. In ovarian carcinoma, VEGF is induced by Angiotensin II via AT1R, which is significantly correlated with poor prognosis [40]. AT1R upregulation in different cancers performs the function of promoting angiogenesis, contributing to cancer progression [25]. Angiotensin II also stimulated cell proliferation, invasion, and VEGF secretion via AT1R in cervical cancer [41, 42], endometrial cancer [43], choriocarcinoma [20], and ovarian cancer [44]. Besides, the host AT1R pathway supports tumor-associated macrophage infiltration, which results in enhanced VEGF levels [45]. Under neoplastic conditions, ACE inhibitors could inhibit the function of Ang II in angiogenesis. Serving as the most important cytokine affecting angiogenesis, VEGF is in a positive relationship with ERO1α, which promotes angiogenesis through the S1PR1/STAT3/VEGF pathway [46, 47]. Candesartan, an AT1R antagonist, has been reported to inhibit angiogenesis by downregulating AT1R/VEGF pathway [17, 48].
Angiogenesis generated by RAS system is mainly due to the binding of Ang II and AT1R. Activation of the PI3K/AKT pathway increases VEGF secretion in both HIF-1 dependent and independent manners. VEGFA exerts angiogenic effects by activating VEGFR2 expressed in endothelial cells through SIPR1/STAT3 and Raf/ERK pathways.
RAS and Tumor Metastasis
Metastasis is a multi-step process that accelerates tumor spread in the whole body, which starts with proliferation and acquires the escape capability from the primary tumor, then enters into the body circulation system, seeds into adjacent tissue cavities [49]. Growing evidence indicates that components of RAS are involved in tumor metastasis, especially Ang II [49]. Clinical research has also found that the use of renin-angiotensin system inhibitors (RASIs) can reduce cancer metastasis in several different cancer types, including hepatocellular carcinoma (HCC) [50] and bladder cancer [51].
Preliminary research in one murine renal cancer model has found that the blockade of Ang II with ACEI or ARB alone or in combination reduced tumor growth and the number of lung metastases [52]. NF-κB, a multi-regulatory transcription factor, can upregulate the expression of VEGF and matrix metalloproteinase-9 (MMP-9) genes, and accelerate the occurrence, invasion, and metastasis of liver tumors [53]. RAS inhibitors, such as perindopril, fosinopril, and losartan, could inhibit the activation of NF-κB and further downregulate the levels of VEGF and MMP, thereby inhibiting metastasis [17, 54]. ACE2/Ang-(1-7)/MAS axis is silenced in human breast cancer; its downregulated expression critically promotes breast cancer metastasis to the lungs through activating PAK1/NF-κB/Snail signaling pathway by increasing SOCE-mediated Ca2+ influx, leading to decreased E-cadherin expression [55]. MAS receptor antagonist A-779 blocks the ACE2/Ang-(1-7)/Mas axis, leading to anti-metastatic effects [55].
Epithelial-mesenchymal transition (EMT) is one major process in metastasis, which can be indicated by downregulation of E-cadherin and upregulation of Vimentin. In A549 lung cancer cells, overexpression of ACE suppressed metastasis in vivo with increased level of E-cadherin and decreased level of Vimentin both in vitro and in vivo [56]. Dysregulation of TGF-β may contribute to the metastasis and invasiveness of cancerous pancreatic cells in advanced cancers [57]. ACE2 attenuated TGF-β1-mediated EMT in A549 cells. DX600, an inhibitor of ACE2, could reverse the sensitivity to TGF-β1 [56]. Ang II-treatment exacerbated hematogenous cancer metastasis by promoting E-selectin-mediated adhesion of cancer cells to vascular endothelial cells [58]. 20(S)-protopanaxadiol (PPD), the final metabolite of protopanaxadiol-type ginsenosides, effectively prevented Ang II-induced EMT via upregulation of the class III deacetylase sirtuin 1 (SIRT1). Downregulation of SIRT1 was involved in the suppression of Ang II-induced EMT by PPD [59].
Inhibition of the AT1R via angiotensin-converting enzyme inhibitors (ACE-Is) has demonstrated a decrease in solid tumor development and metastasis. In colorectal cancer (CRC) liver metastases, decreased Ang II and increased Ang-(1-7) were detected [60]. Captopril not only inhibits ACE, but also reduces the level of AGT in the host liver, leading to a distinct anti-metastatic effect [60, 61]. A study demonstrated an inverse relationship between the history of hypertension with the prescription of ACE-Is and the risk of distant metastasis in stage 2 CRC patients, implicating ACE-Is as a potential chemo-preventative option [61] (Fig. 16.4).
Angiotensin-(1-7), converted from Ang II by ACE2, binds to MasR and activates TGF-β and PAK1/NF-κB/Snail pathways. Dysregulation of these signalling pathways contributes to the transcription of targeted genes, including E-cadherin, Vimentin, and SIRT1, etc., which regulates tumor metastasis. RAS inhibitors, such as perindopril, fosinopril, and losartan, inhibit the activation of NF-κB. A-779 blocks the ACE2/Ang-(1-7)/Mas axis.
RAS and Cell Apoptosis
Research investigating the relationship between RAS and apoptosis has been focused on the AT2R which plays an opposite role of AT1R, protecting the normal function of RAS. It is reported that AT2R could stimulate apoptosis in various cancer cell lines, including vascular smooth muscle cells, such as cardiomyocytes, endothelial cells, prostate cancer cells, and lung cancer cells [62,63,64,65,66,67]. Depending on the cell type, AT2R-mediated apoptosis involves distinct biological processes. In INS-1O rat insulinoma cells, overexpression of AT2R induced cleavage of caspase-8, caspase-9, and caspase-3, and decreased Bcl-2, p-AKT, and p-ERK levels [68]. In intestinal epithelial cells, Ang II signals upregulate GATA-6 expression through AT2R, which in turn upregulates the expression of Bax and eventually leads to apoptosis in these cells [63]. Increased apoptosis appears to be caused by INOs upregulation following enhanced AT2R expression in HL-1 cardiomyocytes [64]. Moreover, AT2R signaling stimulates the MAPK tyrosine phosphatase, which inhibits MAPK activation and consequently inactivates Bcl-2 and induces apoptosis in proximal tubular cells [69]. AT2R-mediated apoptosis was mediated by p38 MAPK and downregulation of Gadd45a, TRAIL-R2, and harakiri Bcl-2-interacting protein (HRK) in prostate cancer cells [65, 70]. In HCC cells, researchers found that apoptosis caused by AT2R overexpression is due to the activation of p38 MAPK, phosphorylated c-Jun N-terminal kinase (p-JNK), caspase-8, and caspase-3, and inactivation of pp42/44 MAPK (ERK1/2) [66]. Similarly, AT2R overexpression-induced apoptosis in BCA cells is mediated via an extrinsic cell death signaling pathway that is dependent on activation of p38 MAPK, caspase-8, and caspase-3 and downregulation of ERK/MAPK [71]. AT2R overexpression also leads to upregulation of 2 apoptosis-related genes (BCL2A1, TNFSF25) and downregulation of 8 apoptosis-related genes (CASP 6, CASP 9, DFFA, IGF1R, PYCARD, TNF, TNFRSF21, TNFSF10, NAIP) in transduced EJ cells [71] (Fig. 16.5).
Ang II binds to the AT2R, which activates P38 MAPK, GATA-6, INOS, PI3K/AKT/mTOR, and Ras/ERK pathways. Signals in the cytoplasm are then transported into the nucleus to upregulate anti-apoptotic genes and downregulate pro-apoptotic genes, leading to apoptosis.
Angiotensin-(1-7) and Tumor Progression
In addition to RAS system itself, the main effector peptide of RAS, Ang-(1-7), has been identified as a biologically active mediator of the RAS [72]. Ang-(1-7), converted from Ang II by ACE and ACE2, has been reported to counteract the function of Ang II in many aspects, which has attracted a lot of attention in recent years. Ang-(1-7), acting mainly through the MAS receptor, abolishes Ang II-induced migration, invasion, VEGF expression, and MMP-9 activity in breast cancer cells [72]. Moreover, Ang-(1-7) has been reported to completely block Ang II-induced EMT in breast cancer [72].
The angiotensin II receptor family members include AGTR1 and AGTR2, which belong to the G-protein-coupled receptor superfamily. AGTR1, overexpressed in many primary and metastatic tumors [73, 74], enhances tumor growth and angiogenesis in breast cancer cells, as evidenced by upregulation of nuclear accumulation of phospho-Smad3, Snail, increased Smad4 and N-cadherin levels [75]. By enhancing adhesion of epithelial to the extracellular matrix, Ang-(1-7) can partially prevent the EMT process [72]. Besides, Ang II promotes survival and proliferation in breast cancer cells through activating PI3K/Akt pathway [72]. Ang II induces AKT phosphorylation in mammary epithelial cells at a very early time point (1 min). In contrast, Ang-(1-7) can blunt the AKT phosphorylation by the Ang II at a later time point (15 min) [72]. Recent studies have reported that AT1 stimulation by Ang II induces EMT via the Smad signaling pathway in renal epithelial cells and vascular smooth muscle cells in vitro [76, 77]. Ang-(1-7) reduced the cell migratory and invasive abilities by reducing the expression and activity of MMP-2 and MMP-9 mediated through inactivation of the PI3K/Akt, P38, and JNK signal pathways [78, 79].
Ang-(1-7) hampers the angiogenesis caused by the activation of Ang II [80]. In MDA-MB-231 cells, knockdown of MMP-2 and MMP-9 by siRNA significantly suppressed Ang II-induced cell migration [80, 81]. Enhanced expression and enzymatic activity of MMP-9 by Ang II are significantly abolished by Ang-(1-7). Besides, Ang-(1-7) suppresses Ang II-induced VEGF expression in breast cancer cells, which is consistent with prior research showing considerable VEGF reduction in nasopharyngeal carcinoma cells overexpressing Ang-(1-7) [82]. It has been discovered that Ang-(1-7) inhibits cancer cell invasion. Induced activation of ACE2/Ang-(1-7)/Mas axis attenuates breast cancer cell invasion by upregulating E-cadherin expression through downregulating SOCE-induced NF-κB and PAK signal pathways [55].
However, in contrast to the anti-cancer properties, there are also reports showing Ang-(1-7) exerted a growth-stimulatory effect on glioma cells [83]. Ang-(1-7)/Mas axis has also been shown to mediate Ang II-stimulated epithelial-to-mesenchymal transformation (EMT) in tubule cells [84]. Ang-(1-7) is also reported to promote the migration and invasion of human renal cell carcinoma cells via MAS-mediated AKT signaling pathway [85]. The reasons for the different functions of Ang-(1-7) in different cancer types are unknown, which are possibly due to experimental conditions or cell-specific signaling.
Regulation of RAS in Clinical Utilization
Currently, two types of regulators of RAS are being studied for clinical utilization. One is ACEIs that suppress signaling of Ang II receptors by reducing Ang II synthesis; the other is ARB, blockers of AT1R signaling, which have been tested in clinical studies (Table 16.1).
Angiotensin-Converting Enzyme Inhibitors (ACEIs)
ACEIs have been shown to block solid tumor development and metastasis. Captopril, a classic ACEI, not only inhibits ACE, but also lowers ACE levels and angiotensinogen expression in the host liver, leading to inhibition of metastasis and further inhibitory mechanisms [60]. In lung cancer, Captopril also exerted anti-metastatic and growth inhibitive effects [86]. However, in renal carcinoma, the effects of Captopril are controversial. One group reported that Captopril inhibited tumor growth [87], while another group showed the induction of tumor by Captopril [88]. Independently of VEGF expression, ACE inhibition promoted neovascularization through activation of bradykinin B2 receptor signaling, whereas it reduced blood vessel growth through inhibition of the Ang II pathway [89, 90]. Besides, Perindopril was tested targeting breast cancer and found that Perindopril reduced tumor volume and downregulated the level of VEGF [91], leading to blockage of angiogenesis and metastasis in hepatocellular carcinoma [92].
AT1R Blockers (ARB)
ARBs are drugs that target the AT1R receptor. The effects of ARBs on cancer are not consistent currently. ARBs may suppress the promotional effect on proliferation by antagonizing AT1R caused by Ang II [19]. The blockade of Ang II with ACEI and ARB alone or in combination reduced tumor proliferation and metastatic capacity of RCC [52]. Losartan, the most commonly used ARB, was found to inhibit tumor growth and promote apoptosis in glioma [68]. However, Losartan was revealed to induce cancer progression and angiogenesis in lung cancer [93, 94]. Besides, it was also found to promote cell proliferation in human melanoma [95]. One research found that Losartan slowed pancreatic tumor progression by abrogating aberrant TGF-β activation [96].
Clinical Study of ACEI/ARB
In clinical studies, the efficacy of ACEI/ARB in the treatment of cancer patients is also controversial currently. It has been revealed that in 13 projects with breast cancer, only two studies showed beneficial effects of ACEI/ARB, whereas three studies reported poor outcomes [57]. Evaluating the tumor subtype information may be helpful to understand the different responsiveness of patients to the ACEI/ARB treatment [74]. Similarly, population-based studies failed to show any association or risk reduction, in patients receiving ACEI/ARB treatment [97,98,99]. However, a retrospective study in stage I-II colorectal cancer (CRC) patients showed that ACEI/ARB treatment reduced tumor recurrence in left-sided CRC and early-stage CRC [99]. Improved survival was also observed in non-metastatic pancreatic ductal adenocarcinoma (PDAC) in a large-scale study [100]. Hence, multiple strategies and strict criteria should be applied to identify and include the studies to reveal the factors that may influence the association between RAS inhibitors and cancer progression [101]. Response to ACEI/ARB treatment may not only vary with tumor types but also depend on certain tumor characteristics, cancer treatment, and RASI type and dosing [50].
Conclusion
RAS participates in tumor progression in many cancer types and its dysregulation leads to increased malignancy of cancer. Although the role of RAS and the underlying mechanisms have been well studied, there are also controversies in its functions in cancer due to different environments and tissue conditions. ACE inhibitors and ARB have been investigated in preclinical research, and their roles in restricting the development of cancer is promising. However, limitations of the in vivo experiments with animal models indicate that there is still a possibility that the mechanism will be different in the human body. How to accelerate the transformation from experimental animals to clinics still needs further exploration.
Abbreviations
- ACE:
-
Angiotensin-converting enzyme
- Ang II:
-
Angiotensin II
- AT1R:
-
Angiotensin II type 1 receptor
- AT2R:
-
Angiotensin II type 2 receptor
- Ang (1-7):
-
Angiotensin 1-7
- MASR:
-
MAS Receptor
- ACE-2:
-
Angiotensin-converting enzyme 2
- VEGF:
-
Vascular endothelial growth factor
- EMT:
-
Epithelial mesenchymal transition
- MMPs:
-
Matrix metalloproteinases
- ECM:
-
Extracellular matrix
- ACE-Is:
-
ACE inhibitors
- ARBs:
-
AT1R blockers
- EC:
-
Endometrial cancer
- PC:
-
Prostate cancer
- PTK:
-
Protein tyrosine kinase
- ROS:
-
Reactive oxygen species
- AMPK:
-
AMP-activated protein kinase
- mTOR:
-
Mammalian target of rapamycin
- EGFR:
-
Epidermal growth factor receptor
- MAPK/STAT:
-
Mitogen-activated protein kinase/signal transducer and activator of transcription
- PI3K/AKT:
-
Phosphoinositide 3-kinase/Akt
- RCC:
-
Renal cell carcinoma
- HCC:
-
Hepatocellular carcinoma
- CRC:
-
Colorectal cancer
- NSCLC:
-
Non-small cell lung cancer
References
Patel S, Rauf A, Khan H, Abu-Izneid T (2017) Renin-angiotensin-aldosterone (RAAS): the ubiquitous system for homeostasis and pathologies. Biomed Pharmacother 94:317–325
Takimoto-Ohnishi E, Murakami K (2019) Renin-angiotensin system research: from molecules to the whole body. J Physiol Sci 69:581–587
Juillerat-Jeanneret L, Celerier J, Chapuis Bernasconi C et al (2004) Renin and angiotensinogen expression and functions in growth and apoptosis of human glioblastoma. Br J Cancer 90:1059–1068
Medina D, Arnold AC (2019) Angiotensin-(1-7): translational avenues in cardiovascular control. Am J Hypertens 32:1133–1142
Hsueh WA, Wyne K (2011) Renin-angiotensin-aldosterone system in diabetes and hypertension. J Clin Hypertens (Greenwich) 13:224–237
Marcheselli S, Micieli G (2008) Renin-angiotensin system and stroke. Neurol Sci 29(Suppl 2):S277–S278
Shrikrishna D, Astin R, Kemp PR, Hopkinson NS (2012) Renin-angiotensin system blockade: a novel therapeutic approach in chronic obstructive pulmonary disease. Clin Sci (Lond) 123:487–498
Afsar B, Afsar RE, Ertuglu LA et al (2021) Renin-angiotensin system and cancer: epidemiology, cell signaling, genetics and epigenetics. Clin Transl Oncol 23:682–696
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70
Beitia M, Solano-Iturri JD, Errarte P et al (2019) Altered expression of renin-angiotensin system receptors throughout colorectal adenoma-adenocarcinoma sequence. Int J Med Sci 16:813–821
Uemura H, Hoshino K, Kubota Y (2011) Engagement of renin-angiotensin system in prostate cancer. Curr Cancer Drug Targets 11:442–450
Siljee S, Milne B, Brasch HD et al (2021) Expression of components of the renin-angiotensin system by cancer stem cells in renal clear cell carcinoma. Biomolecules 11
Perdomo-Pantoja A, Mejía-Pérez SI, Gómez-Flores-Ramos L et al (2018) Renin angiotensin system and its role in biomarkers and treatment in gliomas. J Neurooncol 138:1–15
Siddique A, Kowdley KV (2011) Insulin resistance and other metabolic risk factors in the pathogenesis of hepatocellular carcinoma. Clin Liver Dis 15:281–296, vii–x
Price JA, Kovach SJ, Johnson T et al (2002) Insulin-like growth factor I is a comitogen for hepatocyte growth factor in a rat model of hepatocellular carcinoma. Hepatology 36:1089–1097
Boissan M, Beurel E, Wendum D et al (2005) Overexpression of insulin receptor substrate-2 in human and murine hepatocellular carcinoma. Am J Pathol 167:869–877
Zhang HF, Gao X, Wang X et al (2021) The mechanisms of renin-angiotensin system in hepatocellular carcinoma: from the perspective of liver fibrosis, HCC cell proliferation, metastasis and angiogenesis, and corresponding protection measures. Biomed Pharmacother 141:111868
Zhao Y, Chen X, Cai L et al (2010) Angiotensin II/angiotensin II type I receptor (AT1R) signaling promotes MCF-7 breast cancer cells survival via PI3-kinase/Akt pathway. J Cell Physiol 225:168–173
Du N, Feng J, Hu LJ et al (2012) Angiotensin II receptor type 1 blockers suppress the cell proliferation effects of angiotensin II in breast cancer cells by inhibiting AT1R signaling. Oncol Rep 27:1893–1903
Ino K, Uehara C, Kikkawa F et al (2003) Enhancement of aminopeptidase A expression during angiotensin II-induced choriocarcinoma cell proliferation through AT1 receptor involving protein kinase C- and mitogen-activated protein kinase-dependent signaling pathway. J Clin Endocrinol Metab 88:3973–3982
Kohlstedt K, Brandes RP, Müller-Esterl W et al (2004) Angiotensin-converting enzyme is involved in outside-in signaling in endothelial cells. Circ Res 94:60–67
Alvarenga EC, Fonseca MC, Carvalho CC et al (2016) Angiotensin converting enzyme regulates cell proliferation and migration. PLoS One 11:e0165371
Guimarães PB, Alvarenga ÉC, Siqueira PD et al (2011) Angiotensin II binding to angiotensin I-converting enzyme triggers calcium signaling. Hypertension 57:965–972
Rodrigues MA, Gomes DA, Nathanson MH, Leite MF (2009) Nuclear calcium signaling: a cell within a cell. Braz J Med Biol Res 42:17–20
Suganuma T, Ino K, Shibata K et al (2005) Functional expression of the angiotensin II type 1 receptor in human ovarian carcinoma cells and its blockade therapy resulting in suppression of tumor invasion, angiogenesis, and peritoneal dissemination. Clin Cancer Res 11:2686–2694
Humpel C, Lippoldt A, Strömberg I et al (1994) Human angiotensinogen is highly expressed in astrocytes in human cortical grafts. Glia 10:186–192
Ariza A, Fernandez LA, Inagami T et al (1988) Renin in glioblastoma multiforme and its role in neovascularization. Am J Clin Pathol 90:437–441
Juillerat-Jeanneret L, Lohm S, Hamou MF, Pinet F (2000) Regulation of aminopeptidase A in human brain tumor vasculature: evidence for a role of transforming growth factor-beta. Lab Invest 80:973–980
Célérier J, Cruz A, Lamandé N et al (2002) Angiotensinogen and its cleaved derivatives inhibit angiogenesis. Hypertension 39:224–228
Ferrara N (1999) Vascular endothelial growth factor: molecular and biological aspects. Curr Top Microbiol Immunol 237:1–30
Zhang Q, Lu S, Li T et al (2019) ACE2 inhibits breast cancer angiogenesis via suppressing the VEGFa/VEGFR2/ERK pathway. J Exp Clin Cancer Res 38:173
Zhong H, Chiles K, Feldser D et al (2000) Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res 60:1541–1545
Maity A, Pore N, Lee J et al (2000) Epidermal growth factor receptor transcriptionally up-regulates vascular endothelial growth factor expression in human glioblastoma cells via a pathway involving phosphatidylinositol 3′-kinase and distinct from that induced by hypoxia. Cancer Res 60:5879–5886
Fukumura D, Gohongi T, Kadambi A et al (2001) Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci USA 98:2604-2609
Kim I, Kim HG, So JN et al (2000) Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3′-Kinase/Akt signal transduction pathway. Circ Res 86:24–29
Kim I, Kim JH, Moon SO et al (2000) Angiopoietin-2 at high concentration can enhance endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Oncogene 19:4549–4552
Ando H, Nagasaka T, Nomura M et al (2002) Premenstrual disappearance of aminopeptidase A in endometrial stromal cells around endometrial spiral arteries/arterioles during the decidual change. J Clin Endocrinol Metab 87:2303–2309
Ito M, Itakura A, Ohno Y et al (2002) Possible activation of the renin-angiotensin system in the feto-placental unit in preeclampsia. J Clin Endocrinol Metab 87:1871–1878
Ando H, Furugori K, Shibata D et al (2003) Dual renin-angiotensin blockade therapy in patients at high risk of early ovarian hyperstimulation syndrome receiving IVF and elective embryo cryopreservation: a case series. Hum Reprod 18:1219–1222
Yamamoto S, Konishi I, Mandai M et al (1997) Expression of vascular endothelial growth factor (VEGF) in epithelial ovarian neoplasms: correlation with clinicopathology and patient survival, and analysis of serum VEGF levels. Br J Cancer 76:1221–1227
Kikkawa F, Mizuno M, Shibata K et al (2004) Activation of invasiveness of cervical carcinoma cells by angiotensin II. Am J Obstet Gynecol 190:1258–1263
Suganuma T, Ino K, Shibata K et al (2004) Regulation of aminopeptidase A expression in cervical carcinoma: role of tumor-stromal interaction and vascular endothelial growth factor. Lab Invest 84:639–648
Watanabe Y, Shibata K, Kikkawa F et al (2003) Adipocyte-derived leucine aminopeptidase suppresses angiogenesis in human endometrial carcinoma via renin-angiotensin system. Clin Cancer Res 9:6497–6503
Ino K, Shibata K, Kajiyama H et al (2006) Angiotensin II type 1 receptor expression in ovarian cancer and its correlation with tumour angiogenesis and patient survival. Br J Cancer 94:552–560
Egami K, Murohara T, Shimada T et al (2003) Role of host angiotensin II type 1 receptor in tumor angiogenesis and growth. J Clin Invest 112:67–75
Yang S, Yang C, Yu F et al (2018) Endoplasmic reticulum resident oxidase ERO1-Lalpha promotes hepatocellular carcinoma metastasis and angiogenesis through the S1PR1/STAT3/VEGF-A pathway. Cell Death Dis 9:1105
Wang J, Liu Q, Xiao H et al (2019) Suppressive effects of Momordin Ic on HepG2 cell migration and invasion by regulating MMP-9 and adhesion molecules: involvement of p38 and JNK pathways. Toxicol In Vitro 56:75–83
Fan F, Tian C, Tao L et al (2016) Candesartan attenuates angiogenesis in hepatocellular carcinoma via downregulating AT1R/VEGF pathway. Biomed Pharmacother 83:704–711
Cao H, Xu E, Liu H et al (2015) Epithelial-mesenchymal transition in colorectal cancer metastasis: a system review. Pathol Res Pract 211:557–569
Pinter M, Jain RK (2017) Targeting the renin-angiotensin system to improve cancer treatment: implications for immunotherapy. Sci Transl Med 9
Yoshida T, Kinoshita H, Fukui K et al (2017) Prognostic impact of renin-angiotensin inhibitors in patients with bladder cancer undergoing radical cystectomy. Ann Surg Oncol 24:823–831
Araújo WF, Naves MA, Ravanini JN et al (2015) Renin-angiotensin system (RAS) blockade attenuates growth and metastatic potential of renal cell carcinoma in mice. Urol Oncol 33:389.e1–389.e7
Feng Y, Zu LL, Zhang L (2018) MicroRNA-26b inhibits the tumor growth of human liver cancer through the PI3K/Akt and NF-κB/MMP-9/VEGF pathways. Oncol Rep 39:2288–2296
Saber S, Mahmoud AAA, Goda R et al (2018) Perindopril, fosinopril and losartan inhibited the progression of diethylnitrosamine-induced hepatocellular carcinoma in mice via the inactivation of nuclear transcription factor kappa-B. Toxicol Lett 295:32–40
Yu C, Tang W, Wang Y et al (2016) Downregulation of ACE2/Ang-(1-7)/Mas axis promotes breast cancer metastasis by enhancing store-operated calcium entry. Cancer Lett 376:268–277
Qian YR, Guo Y, Wan HY et al (2013) Angiotensin-converting enzyme 2 attenuates the metastasis of non-small cell lung cancer through inhibition of epithelial-mesenchymal transition. Oncol Rep 29:2408–2414
Sankpal UT, Maliakal P, Bose D et al (2012) Expression of specificity protein transcription factors in pancreatic cancer and their association in prognosis and therapy. Curr Med Chem 19:3779–3786
Ishikane S, Hosoda H, Nojiri T et al (2018) Angiotensin II promotes pulmonary metastasis of melanoma through the activation of adhesion molecules in vascular endothelial cells. Biochem Pharmacol 154:136–147
Wang Y, Xu H, Fu W et al (2019) 20(S)-Protopanaxadiol inhibits angiotensin II-induced epithelial-mesenchymal transition by downregulating SIRT1. Front Pharmacol 10:475
Neo JH, Ager EI, Angus PW et al (2010) Changes in the renin angiotensin system during the development of colorectal cancer liver metastases. BMC Cancer 10:134
Childers WK (2015) Interactions of the renin-angiotensin system in colorectal cancer and metastasis. Int J Colorectal Dis 30:749–752
Qi Y, Li H, Shenoy V et al (2012) Moderate cardiac-selective overexpression of angiotensin II type 2 receptor protects cardiac functions from ischaemic injury. Exp Physiol 97:89–101
Sun L, Wang W, Xiao W et al (2012) Angiotensin II induces apoptosis in intestinal epithelial cells through the AT2 receptor, GATA-6 and the Bax pathway. Biochem Biophys Res Commun 424:663–668
Wang X, Lu J, Khaidakov M et al (2012) Delineation of the effects of angiotensin type 1 and 2 receptors on HL-1 cardiomyocyte apoptosis. Apoptosis 17:908–915
Li H, Qi Y, Li C et al (2009) Angiotensin type 2 receptor-mediated apoptosis of human prostate cancer cells. Mol Cancer Ther 8:3255–3265
Du H, Liang Z, Zhang Y et al (2013) Effects of angiotensin II type 2 receptor overexpression on the growth of hepatocellular carcinoma cells in vitro and in vivo. PLoS One 8:e83754
Kawabata A, Baoum A, Ohta N et al (2012) Intratracheal administration of a nanoparticle-based therapy with the angiotensin II type 2 receptor gene attenuates lung cancer growth. Cancer Res 72:2057–2067
Arrieta O, Guevara P, Escobar E et al (2005) Blockage of angiotensin II type I receptor decreases the synthesis of growth factors and induces apoptosis in C6 cultured cells and C6 rat glioma. Br J Cancer 92:1247–1252
Tejera N, Gómez-Garre D, Lázaro A et al (2004) Persistent proteinuria up-regulates angiotensin II type 2 receptor and induces apoptosis in proximal tubular cells. Am J Pathol 164:1817–1826
Pei N, Jie F, Luo J et al (2014) Gene expression profiling associated with angiotensin II type 2 receptor-induced apoptosis in human prostate cancer cells. PLoS One 9:e92253
Pei N, Mao Y, Wan P et al (2017) Angiotensin II type 2 receptor promotes apoptosis and inhibits angiogenesis in bladder cancer. J Exp Clin Cancer Res 36:77
Cambados N, Walther T, Nahmod K et al (2017) Angiotensin-(1-7) counteracts the transforming effects triggered by angiotensin II in breast cancer cells. Oncotarget 8:88475–88487
Ateeq B, Tomlins SA, Chinnaiyan AM (2009) AGTR1 as a therapeutic target in ER-positive and ERBB2-negative breast cancer cases. Cell Cycle 8:3794–3795
Rhodes DR, Ateeq B, Cao Q et al (2009) AGTR1 overexpression defines a subset of breast cancer and confers sensitivity to losartan, an AGTR1 antagonist. Proc Natl Acad Sci U S A 106:10284–10289
Oh E, Kim JY, Cho Y et al (2016) Overexpression of angiotensin II type 1 receptor in breast cancer cells induces epithelial-mesenchymal transition and promotes tumor growth and angiogenesis. Biochim Biophys Acta 1863:1071–1081
Yang F, Huang XR, Chung AC et al (2010) Essential role for Smad3 in angiotensin II-induced tubular epithelial-mesenchymal transition. J Pathol 221:390–401
Carvajal G, Rodríguez-Vita J, Rodrigues-Díez R et al (2008) Angiotensin II activates the Smad pathway during epithelial mesenchymal transdifferentiation. Kidney Int 74:585–595
Miyajima A, Kosaka T, Asano T et al (2002) Angiotensin II type I antagonist prevents pulmonary metastasis of murine renal cancer by inhibiting tumor angiogenesis. Cancer Res 62:4176–4179
Ni L, Feng Y, Wan H et al (2012) Angiotensin-(1-7) inhibits the migration and invasion of A549 human lung adenocarcinoma cells through inactivation of the PI3K/Akt and MAPK signaling pathways. Oncol Rep 27:783–790
Zhao Y, Wang H, Li X et al (2014) Ang II-AT1R increases cell migration through PI3K/AKT and NF-κB pathways in breast cancer. J Cell Physiol 229:1855–1862
Rodrigues-Ferreira S, Abdelkarim M, Dillenburg-Pilla P et al (2012) Angiotensin II facilitates breast cancer cell migration and metastasis. PLoS One 7:e35667
Pei N, Wan R, Chen X et al (2016) Angiotensin-(1-7) decreases cell growth and angiogenesis of human nasopharyngeal carcinoma xenografts. Mol Cancer Ther 15:37–47
Fogarty DJ, Sánchez-Gómez MV, Matute C (2002) Multiple angiotensin receptor subtypes in normal and tumor astrocytes in vitro. Glia 39:304–313
Burns WC, Velkoska E, Dean R et al (2010) Angiotensin II mediates epithelial-to-mesenchymal transformation in tubular cells by ANG 1-7/MAS-1-dependent pathways. Am J Physiol Renal Physiol 299:F585–F593
Khanna P, Soh HJ, Chen CH et al (2021) ACE2 abrogates tumor resistance to VEGFR inhibitors suggesting angiotensin-(1-7) as a therapy for clear cell renal cell carcinoma. Sci Transl Med 13
Attoub S, Gaben AM, Al-Salam S et al (2008) Captopril as a potential inhibitor of lung tumor growth and metastasis. Ann N Y Acad Sci 1138:65–72
Hii SI, Nicol DL, Gotley DC et al (1998) Captopril inhibits tumour growth in a xenograft model of human renal cell carcinoma. Br J Cancer 77:880–883
Wysocki PJ, Kwiatkowska EP, Kazimierczak U et al (2006) Captopril, an angiotensin-converting enzyme inhibitor, promotes growth of immunogenic tumors in mice. Clin Cancer Res 12:4095–4102
Silvestre JS, Bergaya S, Tamarat R et al (2001) Proangiogenic effect of angiotensin-converting enzyme inhibition is mediated by the bradykinin B(2) receptor pathway. Circ Res 89:678–683
Ebrahimian TG, Tamarat R, Clergue M et al (2005) Dual effect of angiotensin-converting enzyme inhibition on angiogenesis in type 1 diabetic mice. Arterioscler Thromb Vasc Biol 25:65–70
Rasha F, Ramalingam L, Gollahon L et al (2019) Mechanisms linking the renin-angiotensin system, obesity, and breast cancer. Endocr Relat Cancer 26:R653–R672
Yoshiji H, Kuriyama S, Kawata M et al (2001) The angiotensin-I-converting enzyme inhibitor perindopril suppresses tumor growth and angiogenesis: possible role of the vascular endothelial growth factor. Clin Cancer Res 7:1073–1078
Sipahi I, Debanne SM, Rowland DY et al (2010) Angiotensin-receptor blockade and risk of cancer: meta-analysis of randomised controlled trials. Lancet Oncol 11:627–636
Xie Y, Xu P, Wang M et al (2020) Antihypertensive medications are associated with the risk of kidney and bladder cancer: a systematic review and meta-analysis. Aging (Albany NY) 12:1545–1562
Renziehausen A, Wang H, Rao B et al (2019) The renin angiotensin system (RAS) mediates bifunctional growth regulation in melanoma and is a novel target for therapeutic intervention. Oncogene 38:2320–2336
Arnold SA, Rivera LB, Carbon JG et al (2012) Losartan slows pancreatic tumor progression and extends survival of SPARC-null mice by abrogating aberrant TGFβ activation. PLoS One 7:e31384
Friis S, Sørensen HT, Mellemkjaer L et al (2001) Angiotensin-converting enzyme inhibitors and the risk of cancer: a population-based cohort study in Denmark. Cancer 92:2462–2470
Htoo PT, Stürmer T, Jonsson-Funk M et al (2019) Renin-angiotensin-aldosterone system-based antihypertensive agents and the risk of colorectal cancer among Medicare beneficiaries. Epidemiology 30:867–875
Mandilaras V, Bouganim N, Yin H et al (2017) The use of drugs acting on the renin-angiotensin system and the incidence of pancreatic cancer. Br J Cancer 116:103–108
Liu H, Naxerova K, Pinter M et al (2017) Use of Angiotensin system inhibitors is associated with immune activation and longer survival in nonmetastatic pancreatic ductal adenocarcinoma. Clin Cancer Res 23:5959–5969
Sun H, Li T, Zhuang R et al (2017) Do renin-angiotensin system inhibitors influence the recurrence, metastasis, and survival in cancer patients? Evidence from a meta-analysis including 55 studies. Medicine (Baltimore) 96:e6394
Nakai Y, Isayama H, Ijichi H et al (2010) Inhibition of renin-angiotensin system affects prognosis of advanced pancreatic cancer receiving gemcitabine. Br J Cancer 103:1644–1648
Aydiner A, Ciftci R, Sen F (2015) Renin-Angiotensin system blockers may prolong survival of metastatic non-small cell lung cancer patients receiving erlotinib. Medicine (Baltimore) 94:e887
Röcken C, Röhl FW, Diebler E et al (2007) The angiotensin II/angiotensin II receptor system correlates with nodal spread in intestinal type gastric cancer. Cancer Epidemiol Biomarkers Prev 16:1206–1212
Keizman D, Huang P, Eisenberger MA et al (2011) Angiotensin system inhibitors and outcome of sunitinib treatment in patients with metastatic renal cell carcinoma: a retrospective examination. Eur J Cancer 47:1955–1961
McKay RR, Rodriguez GE, Lin X et al (2015) Angiotensin system inhibitors and survival outcomes in patients with metastatic renal cell carcinoma. Clin Cancer Res 21:2471–2479
Izzedine H, Derosa L, Le Teuff G et al (2015) Hypertension and angiotensin system inhibitors: impact on outcome in sunitinib-treated patients for metastatic renal cell carcinoma. Ann Oncol 26:1128–1133
Wilop S, von Hobe S, Crysandt M et al (2009) Impact of angiotensin I converting enzyme inhibitors and angiotensin II type 1 receptor blockers on survival in patients with advanced non-small-cell lung cancer undergoing first-line platinum-based chemotherapy. J Cancer Res Clin Oncol 135:1429–1435
Miao L, Chen W, Zhou L et al (2016) Impact of angiotensin I-converting enzyme inhibitors and angiotensin II Type-1 receptor blockers on survival of patients with NSCLC. Sci Rep 6:21359
Menter AR, Carroll NM, Sakoda LC et al (2017) Effect of angiotensin system inhibitors on survival in patients receiving chemotherapy for advanced non-small-cell lung cancer. Clin Lung Cancer 18:189–197.e3
Wei J, Zhou Z, Xu Z et al (2019) Retrospective clinical study of renin-angiotensin system blockers in lung cancer patients with hypertension. PeerJ 7:e8188
O'Rawe M, Wickremesekera AC, Pandey R et al (2022) Treatment of glioblastoma with re-purposed renin-angiotensin system modulators: results of a phase I clinical trial. J Clin Neurosci 95:48–54
Zaher H, Rasheed H, El-Komy MM et al (2016) Propranolol versus captopril in the treatment of infantile hemangioma (IH): a randomized controlled trial. J Am Acad Dermatol 74:499–505
Makar GA, Holmes JH, Yang YX (2014) Angiotensin-converting enzyme inhibitor therapy and colorectal cancer risk. J Natl Cancer Inst 106:djt374
Acknowledgement
This work was financially supported by the National Natural Science Foundation of China (81973341 to Q.Q.).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Su, J., Zou, Q., Li, S., Qi, Q. (2023). Renin-Angiotensin System and Cancer: From Laboratory to Clinics. In: Bhullar, S.K., Tappia, P.S., Dhalla, N.S. (eds) The Renin Angiotensin System in Cancer, Lung, Liver and Infectious Diseases. Advances in Biochemistry in Health and Disease, vol 25. Springer, Cham. https://doi.org/10.1007/978-3-031-23621-1_16
Download citation
DOI: https://doi.org/10.1007/978-3-031-23621-1_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-23620-4
Online ISBN: 978-3-031-23621-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)