Abstract
The relationship between cloud forest regeneration processes and fauna recovery is poorly understood. We assessed the species diversity of amphibians, dung beetles, and ants, considering habitat specialization type, along a forest successional gradient in Mexico. We found that forest specialist amphibians and ants increased in diversity as the forest ages, but dung beetle diversity is already high from the open areas or early stages (13 years) of this gradient. Species compositions of the cattle pasture were mostly dominated by generalist species, while forest specialist species were abundant in the forests. Faunal response to succession was taxon-specific, but generally defined by the replacement of generalist by specialist species. Following 23 years of succession, a composition similar to that of the reference forest had not yet been re-established, demonstrating the great importance of protecting mature and secondary forests.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
8.1 Introduction
Forests cover 31% of the global land surface, but only about one-third of this is primary forest because we have lost more than 80 million ha of primary forests since 1990 (FAO and PNUMA 2020). Habitat loss is the main threat to biodiversity; it is estimated that insect populations have decreased by 9% globally every decade since 1980 (van Klink et al. 2020). For vertebrate groups such as mammals, birds, reptiles, amphibians, and freshwater fishes, a population decrease of 68% has been estimated worldwide from 1970 up to the present. This loss is relatively higher in the Neotropical region, where populations of the groups mentioned have been reduced in greater proportion than in other regions of the planet (WWF 2020).
Forest restoration practices have increased worldwide in response to concerns about deforestation and its socio-ecological consequences (Bullock et al. 2011; Gatica-Saavedra et al. 2017). One of the strategies most widely implemented to recover forests is natural regeneration (or passive restoration), which occurs when stressors are eliminated or controlled, and a natural process of secondary succession can begin (Rey-Benayas et al. 2008). It is estimated that 36.2 million ha of secondary forest regrew between 2000 and 2010 in the Neotropics (Aide et al. 2013) as a result of land abandonment, exclusion and/or the implementation of restoration practices (Davies et al. 2020).
Species must be able to colonize the successional forests from the regional pool. In this context, Suganuma and Durigan (2021) categorized each plant species according to dispersal syndrome, seed traits, growth rate, shade tolerance, and rarity in the communities. They found that successful colonizers were mostly zoochorous, dispersed by birds or bats, shade tolerant, and of moderate or fast growth. The lack of slow-growing species dispersed by large mammals indicates the absence of certain faunal groups in the secondary forests.
Despite the increase in area of secondary forests, their value in terms of maintaining or recovering animal populations is poorly understood (Thompson and Donnelly 2018; Davies et al. 2020). It has long been assumed that fauna will recolonize naturally in secondary forests once the suitable vegetation and environmental characteristics, such as microclimate or food availability, have re-established (Catterall 2018). However, studying the responses of different groups of fauna to secondary succession is important since animals can significantly modulate the ecological trajectory and the rate of regeneration of forests through their participation in ecological processes such as pollination, seed dispersal, herbivory, and energy flow through trophic webs (Ortega-Álvarez et al. 2013; Fraser et al. 2015; Díaz-García et al. 2017).
To study how the species diversity of faunal communities varies, chronosequences of forest successional processes can be used since they comprise different vegetation conditions that represent a recovery gradient. These recovery gradients should be formed by the following vegetation conditions: a degraded ecosystem, which represents the conditions prior to initiation of natural succession, areas in natural succession of different ages, and an ecological reference ecosystem (Wortley et al. 2013; Marchand et al. 2021). Different ecological and functional attributes can be compared among the vegetation conditions in order to assess the response of faunal communities along the successional and environmental gradients. The components of species diversity, such as species richness, abundance, and composition, are the attributes most commonly used to measure the progress of faunal communities in forest regeneration processes (Gatica-Saavedra et al. 2017).
In studies that have evaluated the response of fauna to restoration strategies, it has been reported that species richness is the first attribute to recover, while this process takes more time for species composition and abundance (Catterall 2018). For example, Hernández-Ordóñez et al. (2015) found that ~25 years of natural succession are sufficient to recover the species richness of amphibians and reptiles, but not the species composition or abundance of these animals. In general, the recovery of species composition and abundance of faunal communities seems to be slower than the recovery of species richness, since they depend on re-establishment of the carrying capacity of secondary forests necessary in order to support stable populations (Chazdon 2008; Catterall 2018).
It has recently been proposed that evaluation of the richness and abundance of faunal communities, considering the habitat specialization type of the species (e.g. forest specialists vs. generalists), can be an effective indicator with which to evaluate changes in forest restoration processes (Díaz-García et al. 2020b). Depending on the ecological traits intrinsic to each habitat specialization type, species can respond differentially to environmental filters generated by the re-establishment of habitat characteristics, including microclimate, refuge and resource availability, and biotic interactions (Catterall 2018; Díaz-García et al. 2020b). To assess the recovery of forest specialist species in particular, it is important to determine the value of secondary forests for the conservation and recovery of vulnerable groups, since these forest specialist species are usually in danger of extinction (Hanski 2011; Pyron 2018; Luther et al. 2020).
The responses of faunal communities to forest regeneration also depend on extrinsic factors of the species, such as land use history, age, and proximity to mature forest (Lamb and Gilmour 2003; Smith et al. 2015). In addition, it has been found that the response may vary within and among taxonomic groups, for which reason the use of multi-taxonomic studies has been recommended (Gatica-Saavedra et al. 2017; Díaz-García et al. 2020b). Amphibians, ants, and dung beetles have been proposed as focal taxa for the evaluation of changes in Neotropical forest regeneration processes (Aguilar-Garavito and Ramírez 2015). Communities of these three faunal groups are highly sensitive to environmental changes and their responses can be detected at the local scale. Furthermore, subsets of species within each group present specific requirements that are positively associated with the heterogeneity and structural complexity of the habitat (Atauri and de Lucio 2001; Brodman et al. 2006; Nichols et al. 2008). Likewise, these taxa perform important functions in the ecosystems. For example, ants favour the flow of nutrients in the soil, promote microbial activity, and efficiently disperse seeds (Del Toro et al. 2012), while dung beetles are involved in nutrient recycling, secondary seed dispersal, and soil aeration (Nichols et al. 2008), and amphibians participate in the control of aquatic algae and herbivorous insects, as well as in energetic exchange between aquatic and terrestrial environments (Cortéz-Gómez et al. 2015). In this chapter, we assessed the species diversity of amphibians, dung beetles, and ants, considering their habitat specialization type, along a successional gradient formed by a cattle pasture, a 13-year-old regenerating secondary forest, a 23-year-old regenerating secondary forest, and a cloud forest remnant located in a mountainous landscape of central Mexico.
8.2 Materials and Methods
8.2.1 Study Area
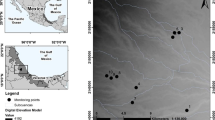
Fieldwork was carried out in the municipality of Huatusco (19° 11′ 23″ N, 96° 59′ 11″ W, 1300 m a.s.l.) in the mountainous region of central Veracruz, Mexico (Fig. 8.1). The climate of the study site is subhumid with a mean annual temperature of 17.1 °C and mean annual precipitation of 1850 mm (CONAGUA 2016). There are three distinct seasons in the area: cold-dry (November–March), hot-dry (April–May) and hot-humid (June–October). This region currently presents ~10,000 ha of tropical montane cloud forest but, of these, only 30% are mature patches immersed in a matrix of agricultural crops, agroforestry systems, cattle pastures, and secondary forests (CONABIO 2010).
8.2.2 Forest Successional Gradient
To make valid and efficient comparisons of the ecological results generated by forest recovery processes, it is necessary to control certain factors such as the land use history of the intervened areas and their proximity to the reference ecosystem (Reid et al. 2018). In 2018, we determined a secondary successional gradient in a landscape of Huatusco, Mexico, formed by four vegetation conditions: a cattle pasture, a 13-year-old regenerating secondary forest, a 23-year-old regenerating secondary forest, and a mature cloud forest remnant. Both of the secondary forests were adjacent, and presented the same land use history and similar mean distance (~1 km) from the cloud forest remnant (Fig. 8.1, Díaz-García et al. 2020a).
The degraded ecosystem represented by cattle pasture was an open area dominated by exotic grasses, with some isolated trees of native species. The 13-year-old regenerating secondary forest had recovered 47% of the tree density and 87% of the canopy cover values presented in the cloud forest, and had also reduced the value of the exotic grass cover recorded in the cattle pasture by 76%. The 23-year-old regenerating secondary forest had recovered 57% of the tree density and 93% of the canopy cover recorded in the cloud forest, and had reduced the value of the exotic grass cover recorded in the cattle pasture by 82%. The two secondary forest conditions shared similar values of some variables of vegetation structure, such as tree height and basal area. The reference ecosystem, represented by the cloud forest remnant, had the highest values of tree density, basal area, tree height, canopy cover, and leaf litter cover. Finally, the species composition of mature cloud forests is dominated by forest specialist tree species. In contrast, the cattle pasture and both of the regenerating forest sites under natural succession presented a combination of trees from primary forests and secondary succession stages (Table 8.1).
8.2.3 Faunal Sampling
We established nine plots (10 × 50 m) with a north-south orientation in each vegetation condition of the natural succession gradient. All plots were located between 1330 and 1450 m a.s.l. in order to avoid an effect of elevation on the species diversity of the faunal communities. All plots were separated from each other and from other vegetation types by a minimum distance of 100 m (Fig. 8.1).
For the amphibians, we conducted three samplings during June, August, and October (hot-humid season) of 2018. In each sampling, two people searched for amphibians in each plot using a visual encounter survey with manual capture (Crump and Scott 1994) between the hours of 20:00 and 01:00. We identified captured individuals to species level and released them after 24 h at the same site where they had been captured. The sampled area in each vegetation type was 4500 m2 (500 m2 × 9 plots) and the total sampling area was 18,000 m2 (4500 m2 × 4 vegetation conditions).
We sampled ants and dung beetles once during the hot-dry season (May 2018) and again in the hot-humid season (September 2018). For the ants, we established four sampling stations along the central line of each plot at 0, 17, 34, and 50 m. Each sampling station comprised one pitfall trap (500 ml) buried to ground level, and one trap (200 ml) placed on trees at a height of 2 m from the ground. The two trap types were located at a distance of 10 m apart (the plot width). The resulting eight traps per plot were filled to one quarter of their capacity with soapy water and protected from the rain by plastic plates (García-Martínez et al. 2015). We used different bait types; pitfall traps were either unbaited or baited with ~20 g of tuna. The tree traps were baited with either ~20 g of tuna or honey. Trap and bait types were alternated from right to left along each plot to avoid traps with the same type of bait being too close to each other or all on the same side of the plot. We checked the traps after 72 h and collected all captured ants. In addition, in the centre of each plot, we collected all leaf litter in 1 m2 and processed it with Winkler sacks in order to extract the ants contained within (Bestelmeyer et al. 2000). Ant specimens were identified to species level in the laboratory.
For the dung beetles, we established three sampling stations along the central line of each plot at 0.25 and 50 m. Each sampling station comprised two pitfall traps (500 ml) buried to ground level and located at a distance of 10 m apart (the width of the plot). The traps were filled to one quarter of their capacity with soapy water and protected from the rain by plastic plates. We used three different bait types: human faeces, carrion (tilapia fish that had been decomposing for two days), and a mixture of guava and banana (1,1). We used ~20 g of bait placed in a small plastic cup suspended inside the pitfall traps. As with the ant traps, bait type was alternated between pitfall traps from right to left along each plot. We checked the traps after 72 h and collected all captured dung beetles. Specimens were identified to species level in the laboratory.
We grouped amphibian, ant, and dung beetle species into two habitat specialization categories: (1) forest-specialist species; those with populations that thrive better in mature forests and present limited tolerance to environmental changes, and (2) generalist species; those with a broader environmental tolerance, and with thriving populations to be found in a variety of natural and modified habitats (MacArthur and Levins 1964; Devictor et al. 2010). To assign a species to one or the other category, we consulted the specialized literature for each group (see Díaz-García et al. 2020a). Ants not identified to species level were not categorized and not used for analysis.
8.2.4 Data Analysis
To evaluate the efficiency of the inventory and make valid comparisons of the species diversity among the vegetation conditions, we calculated the sample coverage (\( {\hat{\textrm{C}}}_n \)) for each vegetation condition, using the formula:
where f1 is the number of singletons, f2 is the number of doubletons, and n is the number of individuals of the vegetation condition (Chao and Jost 2012). We calculated species richness per habitat specialization category for each faunal group and compared these among vegetation conditions using generalized linear models (GLM) with a Poisson distribution and log link function, and post hoc tests of contrasts. These analyses were conducted with the package ‘iNEXT’ (Hsieh et al. 2016), ‘gmodels’ (Warnes et al. 2018), and ‘fifer’ (Fife 2014) in R version 1.1.383 (R Core Team 2017).
We calculated abundance per habitat specialization category by pooling the number of individuals collected in all samplings for amphibians and dung beetles. To compare abundances between vegetation conditions, we used generalized linear models (GLM) with a Poisson distribution and log link function, and post hoc tests of contrasts. Since ants exhibit social behaviour, we calculated occurrence-frequency as the number of plots in which the species was present and summed the occurrences recorded in each of the two samplings (i.e. maximum occurrence frequency for a species that was present in all plots in every sampling would be 9 × 2 = 18). We used a Chi-square test to evaluate goodness-of-fit and a post hoc test to compare the occurrence-frequency of ants among vegetation conditions. These analyses were conducted with the packages ‘gmodels’ (Warnes et al. 2018) and ‘fifer’ (Fife 2014) in R version 1.1.383 (R Core Team 2017).
We constructed a dendrogram using the Bray-Curtis similarity index for each faunal community in order to compare species composition among vegetation conditions. To reduce the influence of the most abundant species, we used the chord transformation on the abundance matrix of amphibians and dung beetles (Legendre and Legendre 2012). We then ran a permutational multivariate analysis of variance (Permanova: 999 permutations) of Bray-Curtis indices. These analyses were conducted with the packages ‘vegan’ (Oksanen et al. 2016) and ‘ggplot2’ (Wickham 2016) in R version 1.1.383 (R Core Team 2017). Finally, we compared the structure of each faunal community among vegetation conditions using rank abundance curves (Feinsinger 2001).
8.3 Results
We found a total of 13 amphibian species (7 forest specialists and 6 generalists), 41 ant species (21 forest specialists, 17 generalists, and 3 undefined), and 15 dung beetle species (5 forest specialists and 10 generalists). Since the sample coverage in each vegetation type was >90% for all faunal communities, we were able to compare our variables with the observed values.
8.3.1 Species Richness
For amphibians, the richness of forest specialist species varied among vegetation conditions (Residual Deviance = 30.5, df = 32, p < 0.001). The richness of forest specialist amphibian species was similar between the two regenerating secondary forests (13- and 23-year-old), but was lower than in the mature cloud forest. The richness of generalist amphibian species was similar among vegetation conditions (Residual Deviance = 36.5, df = 32, p = 0.9; Fig. 8.2a).
Species richness and abundance or occurrence frequency of amphibians, ants, and dung beetles recorded along a natural succession gradient: P = cattle pasture, 13 years SF = 13-year-old regenerating secondary forest, 23 years SF = 23-year-old regenerating secondary forest, and CF = cloud forest. Mean values and standard error are shown in the graphs
For ants, the richness of the forest specialist (Residual Deviance = 23.2, df = 32, p < 0.01) and generalist (Residual Deviance = 25.6, df = 32, p = 0.01) species varied among vegetation conditions. The richness of forest specialist ants was greater in the cloud forest and 23-year-old regenerating secondary forest than in the other vegetation conditions. The richness of generalist ants was greater in cattle pastures and 13-year-old regenerating secondary forest than in the other vegetation conditions (Fig. 8.2b).
For dung beetles, the richness of forest specialist (Residual Deviance = 16.6, df = 32, p < 0.001) and generalist (Residual Deviance = 11.4, df = 32, p < 0.001) species varied among vegetation conditions. The richness of forest specialist dung beetles was lower in cattle pasture than in the other vegetation conditions, among which no significant differences were found. The richness of generalist dung beetles was greater in cattle pasture than in the other vegetation conditions, among which no significant differences were found (Fig. 8.2c).
8.3.2 Abundance or Occurrence Frequency
For amphibians, the abundance of forest specialist (Residual Deviance = 120.7, df = 32, p < 0.001) and generalist (Residual Deviance = 224.9, df = 32, p < 0.001) species varied among vegetation conditions. The abundance of forest specialist amphibians was similar between both secondary forest conditions, but was lower than in the mature cloud forest. The abundance of generalist amphibians was lower in 13-year-old regenerating secondary forest than in the other vegetation conditions, among which no significant differences were found (Fig. 8.2d).
For ants, the occurrence frequency of forest specialist (X2 = 25.8, df = 7, p < 0.001) and generalist (X2 = 26.1, df = 7, p < 0.001) species varied among vegetation conditions. The occurrence frequency of forest specialist ants was greater in cloud forest and 23-year-old regenerating secondary forest than in the other vegetation conditions. The occurrence frequency of generalist ants was greater in cattle pastures than in the other vegetation conditions, among which no significant differences were found (Fig. 8.2e).
For dung beetles, the abundance of forest specialist (Residual Deviance = 211.5, df = 32, p < 0.001) and generalist (Residual Deviance = 273.6, df = 32, p < 0.001) species varied among vegetation conditions. The abundance of forest specialist dung beetles was similar between the two secondary forests, but was higher in the mature cloud forest. The abundance of generalist dung beetles was greater in cattle pasture than in the other vegetation conditions, among which no significant differences were found (Fig. 8.2f).
8.3.3 Species Composition
The species composition of amphibians (F = 12.7, p = 0.001), ants (F = 10.8, p = 0.01), and dung beetles (F = 15.3, p = 0.001) varied among vegetation conditions. For all taxa, the similarity in species composition of the two secondary forests was closer to that recorded in the cloud forest (47–62%), than to that of the cattle pasture (24–38%). For the amphibian and ant communities, the highest similarity values were observed between the two secondary forests (Fig. 8.3a, b). For dung beetles, the highest similarity values were observed between the cloud forest and 13-year-old regenerating secondary forest (Fig. 8.3c).
Dendrogram of similarity (Bray-Curtis index) based on amphibian abundance (a), ant occurrence frequency (b), and dung beetle abundance (c) recorded along a natural succession gradient: P = cattle pasture, 13 years SF = 13-year-old regenerating secondary forest, 23 years SF = 23-year-old regenerating secondary forest, and CF = cloud forest. In all dendrograms: 0 = completely different and 1 = completely identical
8.3.4 Community Structure
For amphibians, two forest specialist species were dominant in the cloud forest (Craugastor rhodopis and Parvimolge townsendi). The dominant species in both successional forests was the forest specialist C. rhodopis, while the dominant species in the cattle pasture was the generalist species Rheohyla miotympanum. Other forest specialist species, such as Charadrahyla taeniopus, Aquiloeurycea cafetalera, and Thorius pennatulus were recorded in both successional forests, but with abundance values lower than those of the cloud forest (Fig. 8.4a).
Rank abundance curves of the amphibian (a), ant (b), and dung beetle (c) communities recorded along a successional gradient: P = cattle pasture, 13 years SF = 13-year-old regenerating secondary forest, 23 years SF = 23-year-old regenerating secondary forest, and CF = cloud forest. For amphibians: Ac Aquiloeurycea cafetalera, Bp Bolitoglossa platydactyla, Cm Craugastor mexicanus, Cp Craugastor pygmaeus, Cr Craugastor rhodopis, Ct Charadrahyla taeniopus, Ec Eleutherodactylus cystignathoides, Hv Hyalinobatrachium viridissimum, Iv Incilius valliceps, Lb Lithobates berlandieri, Pt Parvimolge townsendi, Rm Rheohyla miotympanum, and Tp Thorius pennatulus. For ants: At Adelomyrmex tristani, Cat Camponotus atriceps, Cr Cyphomyrmex rimosus, Gs Gnamptogenys strigata, Na Nylanderia austroccidua, Nb Nylanderia bourbonica, Pi Pheidole insipida, Px Pheidole xyston, Sb Strumigenys brevicornis, and Sg Solenopsis geminata. For dung beetles: Ch Canthidium hespenheidei, Cl Canthon leechi, Ci Copris incertus, Cc Coprophanaeus corythus, Cg Coprophanaeus gilli, Dm Deltochilum mexicanum, Dc Dichotomius colonicus, Ds Dichotomius satanas, Ob Onthophagus belorhinus, Oc Onthophagus corrosus, Ocy Onthophagus cyanellus, Oi Onthophagus incensus, Or Onthophagus rhinolophus, Pe Phanaeus endymion, and So Scatimus ovatus
For ants, the habitat-generalists Solenopsis geminata and Camponotus atriceps were the dominant species in the cattle pasture, but their abundance decreased in the other vegetation conditions along the successional gradient. In the 13-year-old secondary forest, the dominant species was the habitat-generalist species Gnamptogenys strigata. In the 23-year-old secondary forest, the dominant species were the forest specialists Adelomyrmex tristani and Pheidole xyston. In the cloud forest, the dominant species was the forest specialist A. tristani. The abundance of some forest specialist species, such as A. tristani, increased from the 13-year-old forest to the cloud forest (Fig. 8.4b).
For dung beetles, the habitat-generalist species Onthophagus corrosus and Phanaeus endymion were the species with the highest number of individuals in the cattle pasture. However, these species were absent or presented low abundance in the forested sites. In both successional forests, the forest specialist species Deltochilum mexicanum was the dominant species. In the cloud forest, the forest specialist species D. mexicanum and Onthophagus cyanellus were the most abundant. In the vegetation conditions along the successional gradient, the abundance of habitat-generalist species such as Coprophanaeus corythus and Onthophagus incensus decreased from the cattle pasture to the cloud forest. Some forest specialist species, such as Canthidium hespenheidei, Coprophanaeus gilli, and Onthophagus rhinolophus, were recorded in both regenerating forest sites, but did not reach the values recorded in the mature forest (Fig. 8.4c).
8.4 Discussion
Successional forest gradients could theoretically also impose environmental gradients (i.e. canopy or litter cover, etc.) that gradually change as forest ages. Along our successional gradient, the environmental variation was represented by increased tree density, tree height, canopy cover, and leaf litter cover and decreased grass cover, as the forest ages. There was also a decrease in the variability of these conditions as the forest recovered. It is considered that a large proportion of species may coexist under intermediate conditions along an environmental gradient (Flynn et al. 2009; Bitencourt et al. 2019). In our study, to evaluate species turnover along the natural regeneration gradient, we analysed the response of generalist and forest specialist species separately. In the case of the amphibians and ants, a general trend was found; forest specialist species increased in richness and abundance as the forest ages. However, recovery of the species richness of forest specialist dung beetles occurred in the early successional stages (13 years). Our results show that biodiversity recovery during natural forest regrowth is taxon-specific and depends on the habitat specialization type of the species.
8.4.1 Species Richness and Abundance in Forest Specialists Across Forest Chronosequences
8.4.1.1 Amphibians
Studies that have assessed changes in amphibian communities across forest succession chronosequences in Neotropical landscapes have shown that species richness can be recovered in a relatively short period following agricultural abandonment (5–25 years; Herrera-Montes and Brokaw 2010; Basham et al. 2016). Acevedo-Charry and Aide (2019) conducted a meta-analysis of amphibian recovery along tropical forest succession gradients and found that secondary forests reached the levels of species richness found in the reference forests after 5–30 years of succession. It has been reported that secondary forests can maintain the same abundance of amphibians as mature forests after 10–30 years of natural succession (Hilje and Aide 2012; Hernández-Ordóñez et al. 2015).
Acevedo-Charry and Aide (2019) reported that forest specialist amphibians colonize secondary forests gradually, and only secondary forest of 15–30 years of age reached values similar to those of the mature forests used as a reference. In this study, the species richness and abundance of forest specialist species were still not reached even after 23 years of forest regeneration, probably because the strict habitat requirements of these species had not completely recovered in the secondary forests (Thompson and Donnelly 2018). Studies that have related environmental factors to the recovery of forest specialist amphibians suggest that forest cover and connections between forests appear to help species to colonize sites, regardless of age. Moreover, forests with closed canopy, high humidity, deep leaf litter, and abundant arthropods provide favourable microhabitat conditions and resources for forest specialist amphibians (Hilje and Aide 2012; Smith et al. 2015; Díaz-García et al. 2017). In the study area, proximity to water bodies, as well as high canopy and leaf litter cover, had a strong positive influence on the recovery of forest specialist amphibians [Díaz-García et al. 2020a].
Along the successional gradient, the richness and abundance of amphibian generalist species remained intact, with only a small decrease in their abundance found in the youngest forest. However, it is important to highlight the significant increase in the species richness and abundance of forest specialist amphibians, although, for these species, a period of 23 years is still insufficient to recover the conditions of the mature forest. Given the current state of danger of extinction of the forest specialist amphibians, this result may point to the need to intervene in forests undergoing natural succession with complementary actions based on the particular requirements of the species (i.e. artificial ponds, introduction of tree trunks or translocation of epiphytic plants [Fernandez-Barrancos et al. 2017]).
8.4.1.2 Ants
Studies that have evaluated the response of ant communities to natural regeneration in Neotropical landscapes have obtained contrasting results. It has been found that secondary forests can entirely recover the species richness recorded in mature forests after 30–50 years of natural succession (Dunn 2004; Bihn et al. 2008). Higher species richness has also been found in mid-secondary forest under natural regeneration (25–35 years) compared to that of mature forests (Osorio-Pérez et al. 2007), as well as a decline in species richness with increasing forest age (5–30 years; Hethcoat et al. 2019). Tiede et al. (2017) found similar species richness and occurrence among primary and secondary forests of different ages. However, the increase in forest specialist species with advancing forest succession can be considered a general pattern (Dunn 2004; Hethcoat et al. 2019).
In this study, the species richness and occurrence of forest specialist ants reached values similar to those recorded in the mature forest fragment after 23 years of forest regeneration. It is interesting to note that the 13-year-old regenerating forest and cattle pasture sites had similar values of forest specialist ant species, indicating either that their recovery is faster than that of the forest specialist amphibians and dung beetles, or that there are processes of persistence despite the environmental conditions of the cattle pasture. The persistence or recovery of forest specialist species may be due to the proximity of secondary forests and cattle pastures to the mature forest remnant (Domínguez-Haydar and Armbrecht 2011; Gilroy and Edwards 2017), and also to the re-establishment of microhabitat conditions such as higher canopy cover and fallen trunk density in secondary forests, or the presence of isolated trees and shrubs in cattle pastures (Díaz-García et al. 2020b).
Ant generalist species showed a sharp decrease in the 23-year-old regenerating forest stand. At the beginning of the successional gradient (pasture and early successional forest), it is expected to find generalist and forest specialist species that were able to resist forest transformation due to the existence of forest remnants, availability of nesting resources and favourable soil conditions (Rocha-Ortega and García-Martínez 2018). This latter subset of species is expected to increase in secondary forests in the middle portion of the gradient. This pattern of high species substitution contributes to the β-diversity for different organisms (Hernández‐Ordóñez et al. 2019; Bitencourt et al. 2019), particularly in the heterogeneous region of Central Veracruz, where montane cloud forest landscapes present a high β-diversity of woody plants, ants, amphibians, and dung beetles (Arellano and Halffter 2003; Williams-Linera et al. 2013; Meza-Parral and Pineda 2015; García-Martínez et al. 2016, 2017).
8.4.1.3 Dung Beetles
In the Neotropics, it has been reported that the species richness and abundance of dung beetles can be lower in early and mid-secondary forests (0–18 years) than in old-secondary (40 years) and mature forests (Audino et al. 2014). In contrast, Bitencourt et al. (2019) reported a similar species richness of dung beetles in young successional (15–16 years), mid-successional (25–26 years), and mature forests. In addition, Davies et al. (2020) reported similar species richness in secondary (35 years) and primary forest, which was higher than that of cattle pastures. In general, dung beetle abundance has been reported to be lower in secondary forests than in mature forests, even after 18, 26, or 35 years of forest regeneration (Audino et al. 2014; Bitencourt et al. 2019; Davies et al. 2020).
For forest specialist dung beetles in particular, an increase in species richness and abundance has been found after ~10 years of natural succession (Audino et al. 2014). Similarly, the forest specialist dung beetle species richness and abundance in our study reached the mature forest values from 13 years of forest regeneration onwards, demonstrating the resilience of this group and the importance of even young forests stands for this group (Davies et al. 2020; Whitworth et al. 2021). They also found that tree density was the main factor determining the recovery of specialist dung beetles in their study sites. Other important factors that maximize the recovery of forest specialist dung beetles during secondary succession include a closed canopy, and a high cover of leaf litter, fallen trunks, and non-grass herbaceous plants (Díaz-García et al. 2020b).
The values of abundance and richness of generalist dung beetle species were almost two times higher in cattle pasture than in the forested stands, but were similar across the secondary forests. Generalist dung beetle communities can persist in cattle pastures with isolated trees because the livestock present provides sufficient resources for feeding and nesting (Huerta et al. 2018). The different responses between generalist and specialist forest dung beetles in the studied successional gradient may also be due to the fact that both species types have different biogeographic origins (Nearctic and Neotropical), which acts to determine the level of tolerance to habitat modifications (Gómez-Cifuentes et al. 2018).
8.4.2 Composition Similarity and Community Structure Along the Successional Forest Gradient
In several natural regeneration processes in the Neotropical region, species composition is an attribute that presents slower recovery compared to species richness and abundance (Catterall 2018). For amphibians, ants, and dung beetles, most studies have found a transformation from open environment generalist species to forest specialist species along different secondary successional gradients (Dent and Wright 2009; Audino et al. 2014; Thompson and Donnelly 2018; Acevedo-Charry and Aide 2019; Bitencourt et al. 2019; Hethcoat et al. 2019; Díaz-García et al. 2020b).
In the same study area, Trujillo-Miranda et al. (2018) reported that woody plant species composition differed significantly from the adjacent old-growth forests even after 23 years of natural regeneration. Seedling density of late-successional, barochorous-synzoochorous species was much higher in mature forests than in the secondary forests, highlighting the lack of efficient seed dispersal (few seed sources and/or vertebrate seed dispersers) of these species in successional forests (Toledo-Aceves et al. 2021). Biological traits have also been found that can determine the response of faunal groups to habitat modifications. For example, hot climate specialist ants are dominant in open areas and scarce in secondary and primary forests (Gómez and Abril 2011). Forest specialist salamanders from our study (belonging to the Plethodontidae Family) are skin-breathers and depend on the restoration of high levels of atmospheric humidity and a constant temperature (Díaz-García et al. 2020b). Large paracoprid dung beetles decreased with increasing temperature (Gómez-Cifuentes et al. 2017).
Along our studied gradient, a greater similarity of amphibian, ant, and dung beetle composition (66–74%) was recorded between the successional forests (13 and 23 years-old) than when compared to the mature forest (54–68%). The successional forest stands presented similarity in some average values related to vegetation structural variables such as tree density, tree height, basal area, and canopy cover. However, the early secondary forest (13 years) presented higher variation in these attributes than the older (23 years) and mature forests, since forest regeneration is not spatially homogeneous. The highly variable environmental conditions during the early successional stages could act to promote the abundance of generalist species, as occurred for the ant species.
As expected, the active cattle pasture presented a different composition, mostly dominated by generalist species. However, a subset of species was similar to those that occurred in forested stands; similarity varied from 24% in the case of dung beetles to 38% for ants. The studied cattle pasture had an average tree density of 25 trees per ha and presented highly variable canopy cover. This tree density is low compared to forested stands, and the average basal area is about 33% of that recorded in natural regenerated forests. Isolated large and mature trees remain in non-intensive cattle pastures, providing a refuge to several other species such as the taxa studied. When these sites are abandoned, these isolated trees may act as regeneration nuclei, thus assisting forest recovery (Sandor and Chazdon 2014).
The changes from generalist to forest specialist species can also be noted in the place that the species occupied in the community structure of each vegetation condition along the successional gradient studied. Generalist ants, dung beetles, and amphibians were dominant in the cattle pastures, while forest specialist species were abundant in the secondary and reference forests. Moreover, some generalist species were unique to cattle pastures and/or early successional forest, while some forest specialist species appeared with natural regeneration and increased in abundance with forest age. For example, the same forest specialist frog (Craugastor rhodopis) was dominant in the forested sites but scarce in the cattle pasture, probably due to that fact that this species requires a high cover of leaf litter in which to lay its eggs and hunt its arthropod prey (Luría-Manzano et al. 2019).
For ants, the habitat-generalist species Solenopsis geminata was dominant in the cattle pasture, but its abundance decreased in the other vegetation conditions along the successional gradient. Species of the Solenopsis genera are common ants that persist at higher abundances in cattle pastures, and are considered generalist foragers (Hethcoat et al. 2019). This is in contrast to Adelomyrmex tristani, the dominant species in the 23-year-old and mature forests, a ground-dwelling specialist predator (Groc et al. 2014). For dung beetles, Onthophagus corrosus was the species with the highest number of individuals in the cattle pasture. The diet of O. corrosus is based on dung, a resource provided by livestock. In the forested sites, the forest specialist species Deltochilum mexicanum was dominant. This is a copro-necrophagous species that can exploit the high availability of faeces and dead animals in the forest (Huerta et al. 2016).
8.5 Perspectives
Forest successional gradients are a useful framework with which to understand how plant and animal communities could recover as forest ages, and to determine which local and landscape factors modulate the recolonization or persistence processes. Exploring the recovery patterns of a range of taxa during secondary forest succession is critical for conservation and restoration strategies.
Few studies include a degraded system as part of their forest successional gradients, to act as a reference system with environmental and land management characteristics similar to those of the historic initial point of the secondary succession process (Reid et al. 2018). Our study demonstrates that, besides the abundance of generalist species in the cattle pasture, this land use harboured a subset of low abundance species that were present in forested ecosystems. Remnant large trees (such as Quercus insignis trees) in these modified habitats must be conserved since they could play an important role in facilitating forest recovery once these sites are passively or actively restored. Tree species from the Quercus genus provide acorns that are consumed by many insects, birds, and mammals, and their dense canopy creates a favourable microhabitat for many other species (Nsibi et al. 2006; Magee 2019). Oak trunks provide shelter for birds, bats, and a diversity of epiphytic plants (i.e. orchids, bromeliads, ferns, mosses, and lichens; Alzate-Q et al. 2019).
Along forest successional gradients, the gradual gradient of vegetation structure recovery is expected to dictate the fauna recovery. However, this study shows that biodiversity recovery during natural forest regrowth is taxon-specific and depends on the particular habitat specialization of the species. The expected gradual change in environmental gradient across the studied chronosequences does not always take place, since forest regeneration is spatially heterogeneous, especially in the earlier stages. Each animal group therefore responds to the creation of permanent and ephemeral microhabitats according to its own habitat requirements. The three studied fauna groups play crucial roles in ecosystem functioning and the evaluation of their responses in a single study therefore provides us with valuable information regarding the regeneration success of the ecosystem.
This study also highlights the need to complement traditional metrics such as species richness with others such as the richness of forest specialist or functional groups, which can help us to understand the species turnover along these forest successional gradients. The slow recovery of species compositional similarity in several taxa confirms the need to conserve mature forests as a habitat for forest specialists and a potential source for passive forest restoration.
Understanding the factors that modulate fauna recovery is important to the establishment of restoration actions to accelerate the recovery of compositional similarity. In the studied region, the intervention of early successional forest stands by the establishment of artificial ponds and the introduction of broad-leaved tree species in canopy gaps could facilitate fauna colonization and biodiversity recovery. It has been also documented that, at the landscape level, biodiversity recovery through natural forest regrowth varies according to ecological, cultural, and socioeconomic factors (Chazdon et al. 2020; Crouzeilles et al. 2017). In several regions, secondary forests are under tremendous pressure from selective logging, edge effects, grazing, invasive species, etc., which could prevent the recovery of biodiversity (Guariguata and Ostertag 2001). Nevertheless, secondary forests clearly provide important environmental services and habitat for many species, some of which are currently endangered (Chazdon et al. 2020). In this study, both of the secondary forests evaluated are under the protection of the landowners and are located relatively close to the mature forest fragment, and the forest specialist recovery rate could therefore have been higher compared to that of other less well-conserved landscapes.
References
Acevedo-Charry O, Aide M (2019) Recovery of amphibian, reptile, bird and mammal diversity during secondary forest succession in the tropics. Oikos 128(8):1065–1078. https://doi.org/10.1111/oik.06252
Aguilar-Garavito M, Ramírez W (2015) Monitoreo a procesos de restauración ecológica, aplicado a ecosistemas terrestres. Instituto de Recursos Biológicos Alexander von Humboldt (IAvH), Bogotá
Aide TM, Clark ML, Grau HR, López-Carr D, Levy MA, Redo D, Bonilla-Moheno M, Riner G, Andrade-Núñez MJ, Muñiz M (2013) Deforestation and reforestation of Latin America and the Caribbean (2001–2010). Biotropica 45:262–271. https://doi.org/10.1111/j.1744-7429.2012.00908.x
Alzate-Q NF, García-Franco JG, Flores-Palacios A, Krömer T, Laborde J (2019) Influence of land use types on the composition and diversity of orchids and their phorophytes in cloud forest fragments. Flora 260:151463. https://doi.org/10.1016/j.flora.2019.151463
Arellano L, Halffter G (2003) Gamma diversity: derived from and a determinant of alpha diversity and beta diversity. An analysis of three tropical landscapes. Acta Zool Mex 90:27–76
Atauri JA, de Lucio JV (2001) The role of landscape structure in species richness distribution of birds, amphibians, reptiles and lepidopterans in Mediterranean landscapes. Landsc Ecol 16:147–159. https://doi.org/10.1023/A:1011115921050
Audino LD, Louzada J, Comita L (2014) Dung beetles as indicators of tropical forest restoration success: is it possible to recover species and functional diversity? Biol Conserv 169:248–257. https://doi.org/10.1016/j.biocon.2013.11.023
Basham EW, González del Pliego P, Acosta-Galvis AR, Woodcock P, Medina-Uribe CA, Haugaasen T, Gilroy JJ, Edwards DP (2016) Quantifying carbon and amphibian co-benefits from secondary forest regeneration in the Tropical Andes. Anim Conserv 19(6):548–560. https://doi.org/10.1111/acv.12276
Bestelmeyer BT, Agosti D, Alonso LE, Brandão CRF, Brown WR, Delabie JHC, Silvestre R (2000) Field techniques for the study of ground-dwelling ants, an overview, description, and evaluation. In: Agosti D, Majer JD, Alonso LE, Schultz TR (eds) Ants: standard methods for measuring and monitoring biodiversity. Smithsonian Institution Press, Washington/London, pp 122–144
Bitencourt BS, da Silva PG, Morato EF, de Lima YG (2019). Dung beetle responses to successional stages in the Amazon rainforest. Biodivers Conserv 28(10):2745–2761. https://doi.org/10.1007/s10531-019-01791-y
Bihn JH, Verhaagh M, Brändle M, Brandl R (2008) Do secondary forests act as refuges for old growth forest animals? Recovery of ant diversity in the Atlantic forest of Brazil. Biol Conserv 141(3):733–743. https://doi.org/10.1016/j.biocon.2007.12.028
Bitencourt BS, da Silva PG, Morato EF, de Lima YG (2019) Dung beetle responses to successional stages in Amazon rainforest. Biodivers Conserv 28:2745–2761. https://doi.org/10.1007/s10531-019-01791-y
Brodman R, Parrish M, Kraus H, Cortwright S (2006) Amphibian biodiversity recovery in a large-scale ecosystem restoration. Herpetol Conserv Biol 2:101–108
Bullock JM, Aronson J, Newton AC, Pywell RF, Rey-Benayas JM (2011) Restoration of ecosystem services and biodiversity: conflicts and opportunities. Trends Ecol Evol 26:541–549. https://doi.org/10.1016/j.tree.2011.06.011
Catterall CP (2018) Fauna as passengers and drivers in vegetation restoration: a synthesis of processes and evidence. Ecol Manage Restor 19:54–62. https://doi.org/10.1111/emr.12306
Chao A, Jost L (2012) Coverage-based rarefaction and extrapolation: standardizing samples by completeness rather than by size. Ecology 93(12):2533–2547. https://doi.org/10.1890/11-1952.1
Chazdon RL (2008) Beyond deforestation: restoring forest and ecosystem services on degraded lands. Science 320:1458–1460. https://doi.org/10.1126/science.1155365
Chazdon RL, Lindenmayer D, Guariguata MR, Crouzeilles R, Rey-Benayas JR, Lazos-Chavero E (2020) Fostering natural forest regeneration on former agricultural land through economic and policy interventions. Environ Res Lett 15(4):043002. https://doi.org/10.1088/1748-9326/ab97cc
Comisión Nacional del Agua (CONAGUA) (2016). Available from: https://smn.conagua.gob.mx/
Comisión Nacional para el conocimiento y uso de la Biodiversidad (CONABIO) (2010) El bosque mesófilo de montaña en México: amenazas y oportunidades para su conservación y manejo sostenible. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, Distrito Federal, México
Cortéz-Gómez AM, Ruiz-Agudelo CA, Valencia-Aguilar A, Ladle RJ (2015) Ecological functions of Neotropical amphibians and reptiles: a review. Univ Sci 20(2):229–245. https://doi.org/10.11144/Javeriana.SC20-2.efna
Crouzeilles R, Ferreira MS, Chazdon RL, Lindenmayer DB, Sansevero JBB, Monteiro L, Iribarrem A, Latawiec A, Strassburg BBN (2017) Ecological restoration success is higher for natural regeneration than for active restoration in tropical forests. Sci Adv 3(11):e1701345. https://doi.org/10.1126/sciadv.1701345
Crump ML, Scott NJ (1994) Visual encounter surveys. In: Heyer WR, Donnelly MA, McDiarmid RW, Hayek LAC, Foster MS (eds) Measuring and monitoring biological diversity. Standard methods for amphibians. Smithsonian Institution Press, Washington, DC, pp 84–92
Davies RW, Edwards DP, Edwards FA (2020) Secondary tropical forests recover dung beetle functional diversity and trait composition. Anim Conserv 23(5):617–627. https://doi.org/10.1111/acv.12584
Del Toro I, Ribbons RR, Pelini SL (2012) The little things that run the world revisited: a review of ant-mediated ecosystem services and disservices (Hymenoptera: Formicidae). Myrmecol News 17:133–146
Dent DH, Wright SJ (2009) The future of tropical species in secondary forests: a quantitative review. Biol Conserv 142(12):2833–2843. https://doi.org/10.1016/j.biocon.2009.05.035
Devictor V, Clavel J, Julliard R, Lavergne S, Mouillot D, Thuiller W, Venail P, Villéger S, Mouquet N (2010) Defining and measuring ecological specialization. J Appl Ecol 47:15–25. https://doi.org/10.1111/j.1365-2664.2009.01744.x
Díaz-García JM, Pineda E, López-Barrera F, Moreno CE (2017) Amphibian species and functional diversity as indicators of restoration success in tropical montane forest. Biodivers Conserv 26:2569–2589. https://doi.org/10.1007/s10531-017-1372-2
Díaz-García JM, López-Barrera F, Pineda E, Toledo-Aceves T, Andresen E (2020a) Comparing the success of active and passive restoration in a tropical cloud forest landscape: a multi-taxa fauna approach. PLoS One 15(11):e0242020. https://doi.org/10.1371/journal.pone.0242020
Díaz-García JM, López-Barrera F, Toledo-Aceves T, Andresen E, Pineda E (2020b) Does forest restoration assist the recovery of threatened species? A study of cloud forest amphibian communities. Biol Conserv 242:108400. https://doi.org/10.1016/j.biocon.2019.108400
Domínguez-Haydar Y, Armbrecht I (2011) Response of ants and their seed removal in rehabilitation areas and forests at El Cerrejon coal mine in Colombia. Restor Ecol 19(201):178–184. https://doi.org/10.1111/j.1526-100X.2010.00735.x
Dunn RR (2004) Recovery of faunal communities during tropical forest regeneration. Conserv Biol 18(2):302–309. https://doi.org/10.1111/j.1523-1739.2004.00151.x
FAO and PNUMA (2020) El estado de los bosques del mundo 2020. Los bosques, la biodiversidad y las personas. Roma, Italy. https://doi.org/10.4060/ca8642es
Feinsinger P (2001) Designing field studies for biodiversity conservation. Island Press, Washington, DC
Fernandez-Barrancos EP, Reid JL, Aronson J (2017) Tank bromeliad transplants as an enrichment strategy in southern Costa Rica. Restor Ecol 25(4):569–576. https://doi.org/10.1111/rec.12463
Fife DA (2014) An introduction to the “fifer” package in R. R package version 1.0. Available at: https://CRAN.R-project.org/package=fifer
Flynn DF, Gogol-Prokurat M, Nogeire T, Molinari N, Richers BT, Lin BB, Simpson N, Mayfield MM, DeClerck F (2009) Loss of functional diversity under land use intensification across multiple taxa. Ecol Lett 12:22–33. https://doi.org/10.1111/j.1461-0248.2008.01255.x
Fraser LH, Harrower WL, Garris HW, Davidson S, Hebert PDN, Howie R, Moody A, Polster D, Schmitz OJ, Sinclair ARE, Starzomski BM, Sullivan TP, Turkington R, Wilson D (2015) A call for applying trophic structure in ecological restoration. Restor Ecol 23:503–507. https://doi.org/10.1111/rec.12225
García-Martínez MA, Martínez-Tlapa DL, Pérez-Toledo GR, Quiroz-Robledo LN, Castaño-Meneses G, Laborde J, Valenzuela-González JE (2015) Taxonomic, species and functional group diversity of ants in a tropical anthropogenic landscape. Trop Conserv Sci 8(4):1017–1032. https://doi.org/10.1177/194008291500800412
García-Martínez MA, Martínez-Tlapa DL, Pérez-Toledo GR, Quiroz-Robledo LN, Valenzuela-González JE (2016) Myrmecofauna (Hymenoptera: Formicidae) response to habitat characteristics of tropical montane cloud forest in Central Veracruz. Mexico Fla Entomol 99(2):248–256
García-Martínez MA, Valenzuela-González JE, Escobar-Sarria F, López-Barrera F, Castaño-Meneses G (2017) The surrounding landscape influences the diversity of leaf-litter ants in riparian cloud forest remnants. PLoS One 12(2):e0172464. https://doi.org/10.1371/journal.pone.0172464
Gómez C, Abril S (2011). Selective logging in public pine forests of the central Iberian Peninsula: Effects of the recovery process on ant assemblages. For Ecol Manag 262(6):1061–1066. https://doi.org/10.1016/j.foreco.2011.05.043
Gatica-Saavedra P, Echeverría C, Nelson CR (2017) Ecological indicators for assessing ecological success of forest restoration: a world review. Restor Ecol 25(6):850–857. https://doi.org/10.1111/rec.12586
Gilroy JJ, Edwards DP (2017) Source-sink dynamics: a neglected problem for landscape-scale biodiversity conservation in the tropics. Curr Landsc Ecol Rep 2(1):51–60. https://doi.org/10.1007/s40823-017-0023-3
Gómez-Cifuentes A, Munevar A, Gimenez VC, Gatti MG, Zurita GA (2017) Influence of land use on the taxonomic and functional diversity of dung beetles (Coleoptera: Scarabaeinae) in the southern Atlantic forest of Argentina. J Insect Conserv 21(1):147–156. https://doi.org/10.1007/s10841-017-9964-4
Gómez-Cifuentes A, Giménez-Gómez VC, Moreno CE, Zurita GA (2018) Tree retention in cattle ranching systems partially preserves dung beetle diversity and functional groups in the semideciduous Atlantic forest: the role of microclimate and soil conditions. Basic Appl Ecol 34:64–74. https://doi.org/10.1016/j.baae.2018.10.002
Groc S, Delabie JHC, Fernández F, Leponce M, Orivel J, Silvestre R, Vasconcelos HL, Dejean A (2014) Leaf–litter ant communities (Hymenoptera: Formicidae) in a pristine Guianese rainforest: stable functional structure versus high species turnover. Myrmecol News 19:43–51
Guariguata MR, Ostertag R (2001) Neotropical secondary forest succession: changes in structural and functional characteristics. For Ecol Manag 148(1–3):185–206. https://doi.org/10.1016/S0378-1127(00)00535-1
Hanski I (2011) Habitat loss, the dynamics of biodiversity, and a perspective on conservation. Ambio 40:248–255. https://doi.org/10.1007/s13280-011-0147-3
Hernández-Ordóñez O, Urbina-Cardona N, Martinez-Ramos M (2015) Recovery of amphibian and reptile assemblages during old-field succession of tropical rain forests. Biotropica 47:377–388. https://doi.org/10.1111/btp.12207
Hernández‐Ordóñez O, Santos BA, Pyron RA, Arroyo‐Rodríguez V, Urbina‐Cardona JN, Martínez‐Ramos M, Parra-Olea G, Reynoso VH (2019) Species sorting and mass effect along forest succession: Evidence from taxonomic, functional, and phylogenetic diversity of amphibian communities. Ecol Evol 9(9):5206–5218. https://doi.org/10.1002/ece3.5110
Herrera-Montes A, Brokaw N (2010) Conservation value of tropical secondary forest: a herpetofaunal perspective. Biol Conserv 143(6):1414–1422. https://doi.org/10.1016/j.biocon.2010.03.016
Hethcoat MG, King BJ, Castiblanco FF, Ortiz-Sepúlveda CM, Prada-Achiardi FC, Edwards FA, Medina C, Gilroy JJ, Haugaasen T, Edwards DP (2019) The impact of secondary forest regeneration on ground-dwelling ant communities in the Tropical Andes. Oecologia 191:475–482. https://doi.org/10.1007/s00442-019-04497-8
Hilje B, Aide TM (2012) Recovery of amphibian species richness and composition in a chronosequence of secondary forests, northeastern Costa Rica. Biol Conserv 146(1):170–176. https://doi.org/10.1016/j.biocon.2011.12.007
Hsieh TC, Ma KH, Chao A (2016) iNEXT: an R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol Evol 7(12):1451–1456. https://doi.org/10.1111/2041-210X.12613
Huerta C, Arellano-Gámez L, Cruz-Rosales M, Escobar-Sarria F, Martínez-Morales I (2016) Los escarabajos del estiércol en los potreros ganaderos de Xico. Instituto de Ecología, A. C, Xalapa/Veracruz, p 20
Huerta C, Arellano-Gámez L, Cruz-Rosales M (2018) Dung beetles (Coleoptera: Scarabaeidae, Scarabaeinae) and dung removal in Mexican livestock pastures. Rev Mex Biodivers 89:1280–1292. https://doi.org/10.22201/ib.20078706e.2018.4.2495
Lamb D, Gilmour D (2003) Rehabilitation and restoration of degraded forests. IUCN, Gland, Switzerland and Cambridge, UK and WWF, Switzerland
Legendre P, Legendre L (2012) Numerical ecology. Elsevier Press, Amsterdam
Luría-Manzano R, Oropeza-Sánchez MT, Aguilar-López JL, Díaz-García JM, Pineda E (2019) Dieta de la rana de hojarasca Craugastor rhodopis (Anura: Craugastoridae): una especie abundante en la región montañosa del este de México. Rev Biol Trop 67(1):196–205. https://doi.org/10.15517/rbt.v67i1.33135
Luther D, Beatty CR, Cooper J, Cox N, Farinelli S, Foster M, Lamoreux J, Stephenson PJ, Brooks TM (2020) Global assessment of critical forest and landscape restoration needs for threatened terrestrial vertebrate species. Glob Ecol Conserv 24:e01359. https://doi.org/10.1016/j.gecco.2020.e01359
MacArthur R, Levins R (1964) Competition, habitat selection, and character displacement in a patchy environment. Proc Natl Acad Sci U S A 51(6):1207–1210. https://doi.org/10.1073/pnas.51.6.1207
Magee R (2019) Effects of leaf litter on amphibian site selection. College of Applied Natural Sciences. Louisiana Tech University. Master’s Theses
Marchand L, Castagneyrol B, Jiménez JJ, Rey-Benayas JM, Benot ML, Martínez-Ruiz C, Alday JC, Jaunatre R, Dutoit T, Buisson E, Mench M, Alard D, Corcket E, Comin F (2021) Conceptual and methodological issues in estimating the success of ecological restoration. Ecol Indic 123:107362. https://doi.org/10.1016/j.ecolind.2021.107362
Meza-Parral Y, Pineda E (2015) Amphibian diversity and threatened species in a severely transformed neotropical region in Mexico. PLoS One 10(3):e0121652. https://doi.org/10.1371/journal.pone.0121652
Nichols E, Spector S, Louzada J, Larsen T, Amezquita S, Favila ME, The Scarabaeinae Research Network (2008) Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biol Conserv 141(6):1461–1474. https://doi.org/10.1016/j.biocon.2008.04.011
Nsibi R, Souayah N, Khouja LM, Bouzid S (2006) La régénération naturelle par semis de la suberaie de Tabarka - Aïn Draham face aux facteurs écologiques et anthropiques. Geo-Eco-Trop 30(1):35–48
Oksanen J, Guillaume-Blanchet F, Friendly M, Kindt R, Legendre P, McGlinn, D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2016) Community ecology package “Vegan” for R. https://cran.r-project.org/web/packages/vegan/index.html
Ortega-Álvarez R, Lindig-Cisneros R, MacGregor-Fors I, Renton K, Schondube JE (2013) Avian community responses to restoration efforts in a complex volcanic landscape. Ecol Eng 53:275–283. https://doi.org/10.1016/j.ecoleng.2012.12.058
Osorio-Pérez K, Barberena-Arias MF, Aide TM (2007) Changes in ant species richness and composition during plant secondary succession in Puerto Rico. Caribb J Sci 43(2):244–253. https://doi.org/10.18475/cjos.v43i2.a9
Pyron RA (2018) Global amphibian declines have winners and losers. Proc Natl Acad Sci 115:3739–3741. https://doi.org/10.1073/pnas.1803477115
R Development Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reid LJ, Fagan ME, Zahawi RA (2018) Positive site selection bias in meta-analyses comparing natural regeneration to active forest restoration. Sci Adv 4:eaas9143. https://doi.org/10.1126/sciadv.aas9143
Rey-Benayas JM, Bullock JM, Newton AC (2008) Creating woodland islets to reconcile ecological restoration, conservation and agricultural land use. Front Ecol Environ 6:329–336. https://doi.org/10.1890/070057
Rocha-Ortega M, García-Martínez MA (2018) Importance of nesting resources and soil conditions for the recovery of ant diversity during secondary succession in a tropical rainforest. Trop Conserv Sci 11:1–14. https://doi.org/10.1177/1940082918787063
Sandor ME, Chazdon RL (2014) Remnant trees affect species composition but not structure of tropical second-growth forest. PLoS One 9(1):e83284. https://doi.org/10.1371/journal.pone.0083284
Smith GC, Lewis T, Hogan LD (2015) Fauna community trends during early restoration of alluvial open forest/woodland ecosystems on former agricultural land. Restor Ecol 23:787–799. https://doi.org/10.1111/rec.12269
Suganuma MS, Durigan G (2021) Build it and they will come, but not all of them in fragmented Atlantic Forest landscapes. Restor Ecol:e13537. https://doi.org/10.1111/rec.13537
Thompson ME, Donnelly MA (2018) Effects of secondary forest succession on amphibians and reptiles: a review and meta-analysis. Copeia 106(1):10–19. https://doi.org/10.1643/CH-17-654
Tiede Y, Schlautmann J, Donoso DA, Wallis CIB, Bendix J, Brandl R, Farwig N (2017) Ants as indicators of environmental change and ecosystem processes. Ecol Indic 83:527–537. https://doi.org/10.1016/j.ecolind.2017.01.029
Toledo-Aceves T, Trujillo-Miranda A, López-Barrera F (2021) Tree regeneration in active and passive cloud forest restoration: functional groups and timber species. For Ecol Manag 489(1):119050. https://doi.org/10.1016/j.foreco.2021.119050
Trujillo-Miranda AL, Toledo-Aceves T, López-Barrera F, Gerez-Fernandez P (2018) Active versus passive restoration: recovery of cloud forest structure, diversity and soil condition in abandoned pastures. Ecol Eng 117:50–61. https://doi.org/10.1016/j.ecoleng.2018.03.011
van Klink R, Bowler DE, Gongalsky KB, Swengel AB, Gentile A, Chase JM (2020) Meta-analysis reveals declines in terrestrial but increases in freshwater insect abundances. Science 368:417–420. https://doi.org/10.1126/science.aax9931
Warnes GR, Bolker B, Lumley T, Johnson RC (2018) Package ‘gmodels’: various R programming tools for model fitting for R. Available at: https://CRAN.R-project.org/package=gmodels
Whitworth A, Beirne C, Flatt E, Froese G, Nuñez C, Forsyth A (2021) Recovery of dung beetle biodiversity and traits in a regenerating rainforest: a case study from Costa Rica’s Osa Peninsula. Insect Conserv Divers 14:439–454. https://doi.org/10.1111/icad.12470
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer, New York, p 260
Williams-Linera G, Toledo-Garibaldi M, Gallardo-Hernández C (2013) How heterogeneous are the cloud forest communities in the mountains of central Veracruz, Mexico? Plant Ecol 214(5):685–701. https://doi.org/10.1007/s11258-013-0199-5
Wortley L, Hero JM, Howes M (2013) Evaluating ecological restoration success: a review of the literature. Restor Ecol 21:537–543. https://doi.org/10.1111/rec.12028
WWF (2020) In: Almond REA, Grooten M, Petersen T (eds) Living planet report 2020 - bending the curve of biodiversity loss. WWF, Gland
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Díaz-García, J.M., López-Barrera, F., Pineda, E., Valenzuela-González, J.E., Sandoval-Comte, A. (2023). Species Diversity of Three Faunal Communities Along a Successional Cloud Forest Gradient. In: Myster, R.W. (eds) Neotropical Gradients and Their Analysis. Springer, Cham. https://doi.org/10.1007/978-3-031-22848-3_8
Download citation
DOI: https://doi.org/10.1007/978-3-031-22848-3_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-22847-6
Online ISBN: 978-3-031-22848-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)