Abstract
By testing some of the most known ecogeographic rules, such as Rapoport’ rule, and Bergmann’s rule, we expand our knowledge on the spatial patterns governing the distribution of biodiversity across scales. This knowledge may then be translated into effective measures aimed to preserve endangered species and environments. In this chapter, we investigate how suitable are the Bergmann’s rule, and the Rapoport’s effect in explaining latitudinal trends in scorpion diversity in the Brazilian Atlantic Forest – one of the global biodiversity hotspots. To this end, we compiled occurrence data, and body size information of Atlantic Forest scorpion from public repositories, and scientific literature. A total of 2,429 occurrences for 28 species grouped in two families (Bothriuridae, and Buthidae) were obtained. We observed a positive and significant correlation between latitude and both species’ distribution ranges, and focal species richness. On the other hand, we did not find any apparent latitudinal pattern in the geographical distribution of body size in these animals. In summary, this study proposes that 1) scorpion richness in the Atlantic Forest does not adhere to the Rapoport’s effect, being more similar to the assumptions of the Inverse Rapoport’ rule, and latitudinal diversity gradient hypothesis; and 2) a comprehensive pattern in the spatial distribution of scorpion body sizes in the Atlantic Forest seems to be absent, or at least, its complexity cannot be explained by well-known ecogeographical rules such as the Bergmann’s rule. The potential associations between such biodiversity patterns and the biogeographical backgrounds of the Atlantic Forest are discussed. We strongly believe that what was highlighted here will improve our overall understanding concerning the spatial drivers of species richness in the Atlantic Forest, which may help future studies and conservation planning.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
7.1 Effects of Latitudinal Gradients in Biodiversity
Considering that species are not evenly distributed across space (Stevens et al. 2013), researchers have attempted to describe and understand broad scale patterns of biodiversity that characterize the world’s biota (Darwin 1859; Wallace 1878; LaManna et al. 2017). Few species possess a widespread distribution across habitats, while most species are adapted to a limited range of environmental conditions, referred to as the ecological niche (Peterson 2011). Although niche breadths are generally thought to vary positively with latitude (e.g., Sunday et al. 2011; Papacostas and Freestone 2016; Sexton et al. 2017), a better comprehension of such geographic patterns in species diversity is mandatory for ecological studies with conservation backgrounds (Gaston 2000; Whittaker et al. 2005). In such a context, one of the best-known patterns of species distribution is the latitudinal gradient expressed by an overall increase in species diversity toward the equator (e.g., Brown 2014; Fine 2015; Roll et al. 2017). This geographical trend has been reported for several taxa, including birds, mammals, reptiles, arthropods, and mollusks (Willig et al. 2003; Hillebrand 2004; Kinlock et al. 2018). However, others such as parasitic species and taxa with narrow latitudinal ranges may show a positive or non-significant relationship with latitude (Visser et al. 2014; Timms et al. 2016; Brocklehurst et al. 2017).
Different hypotheses have been formulated to explain the effect of latitudinal gradient in biodiversity (reviewed in Pontarp et al. 2019), these hypotheses can be divided into three main categories: (i) ecological limits, (ii) diversification rates, and (iii) time for species accumulation (Mittelbach et al. 2007). In the first, competition for limited resources is a key factor that limits the number of species co-occurring in the same locality (e.g., Mittelbach et al. 2007; Rabosky 2009), and the niche conservatism is often evoked to explain the differential dispersal rates across latitudes (Wiens et al. 2010). In the diversification rate hypothesis, variation in speciation or extinction rates, which may occur faster in the tropics than temperate regions, exert an important role (Allen and Gillooly 2006; Weir and Schluter 2007). Finally, in the third category, historical colonization rates assume an unbalanced diversity dynamic, implying that regions that were colonized first are expected to harbor long periods of diversification, resulting in high levels of species richness (Stephens and Wiens 2003).
Interestingly, the processes behind latitudinal diversity gradients are still under debate (e.g., Fine 2015; Lomolino et al. 2016; Willig and Presley 2018). Several factors may explain the lack of consensus on the relative importance of the processes that generate and maintain such gradients in biodiversity (e.g., Latham and Ricklefs 1993; Willig et al. 2003; Weiser et al. 2017). It is well known, for example, that biodiversity gradients are expected to be driven by spatial ranges in species distribution (e.g., Cardillo et al. 2005; Mittelbach et al. 2007) and the taxonomic resolution (Weiser et al. 2007; Kreft and Jetz 2007). In some cases, latitudinal patterns in species richness are not generally attributed to latitude per se, but to the variation in bioclimatic conditions across latitudinal ranges. In this way, the macroecological approach evokes a set of biotic and abiotic covariables to explain the described patterns of biodiversity (e.g., Currie et al. 2004; Kaspari et al. 2004). For this, explanations for the latitudinal gradient of diversity make implicit assumption that these covariables of latitude are not taxon scale-dependent. For example, the area of a continent or the temperature of a habitat is independent of the taxonomic rank of the focal taxon (Weiser et al. 2018). Therefore, variation in species richness along geographical gradients may help us to understand the evolutionary history of organisms through the interplay between ecological adaptability and dispersal capabilities (Albuquerque et al. 2011; Silveira et al. 2019).
7.2 Rapoport and Bergmann Rules
Species' ecological traits and their tolerance to environmental factors act as a complex interplay constraining their dispersion and thus, determining their geographical ranges (Gaston 2003). In their monograph study, Rapoport (1975) found that latitude correlates with species distribution ranges. This author found that the range size of species was positively correlated with latitude, with smaller ranges for species occurring closer to the equator. Since the description of Rapoport’s rule, many studies have tested Rapoport effects in different taxa (e.g., Liu et al. 2020; Murphy et al. 2020; Pie et al. 2021). Ruggiero and Werenkraut (2007) demonstrated that Rapoport’s rule was applied particularly for the large, continental landmasses of the Northern Hemisphere and less so in other geographic regions. Applications of Rapoport rule in tropical regions and the Southern Hemisphere appear to be less well defined (Rohde 1996). Many studies in these regions support the existence of complex regional patterns, providing only partial support for Rapoport’s rule (e.g., Fortes and Absalão 2004; Pintor et al. 2015; Núñez-Flores et al. 2019).
In addition, species range size is fundamental for understanding of many life sciences disciplines, such as ecology, biogeography, and conservation, and has received significant research attention throughout the decades (e.g., Jablonski and Roy 2003; Böhm et al. 2017). For example, through the determination of drivers of range size we can measure the level of extinction risk of species. Large-ranged species generally possess a large environmental tolerance (Jablonski and Roy 2003) and lower extinction risk than small-ranged species that exhibits a higher environmental specificity, which may result in elevated extinction risks (Purvis et al. 2000; Lee and Jetz 2010; Böhm et al. 2017). Thus, understanding the drivers of range size in species can provide valuable information about appropriate conservation actions for range-restricted species. Therefore, studies at macro-scale are particularly important for testing the universality of latitudinal Rapoport’s rule, principally in the Southern Hemisphere.
Furthermore, to richness and range size, latitudinal gradient may affect the ecological traits of species with many of these traits being influenced by body size (Peters and Wassenberg 1983; Smith and Lyons 2013). Body size mediates several aspects of an animal’s life history, such as life span, clutch size and growth rate, and its variability across spatial gradients is a prominent topic in biogeography and ecology (Brown et al. 2004; Peters and Wassenberg 1983; Smith and Lyons 2013). The most known ecogeographical rule is the Bergmann’s rule (BR), which predicts that closely related endothermic vertebrates increase in body size toward cold environments (Bergmann 1847; Blackburn et al. 1999). The mechanism proposed to explain this pattern toward colder sites is that heat generation capacity increases with body volume, whereas heat loss increases with surface area in endotherms (Blackburn et al. 1999). However, there is increasing evidence pointing that this mechanism is not universal (Blackburn et al. 1999; Meiri 2011). For ectotherm animals, on the other hand, there are comparatively few studies addressing whether variation in body size can be properly represented by latitudinal trends (Blanckenhorn and Demont 2004), with more evidences suggesting mixed indirect effects of latitude on body size variation in arthropods, as an example (Chown and Gaston 2010; Entling et al. 2010; Tseng et al. 2018). For these animals, previous studies have demonstrated that either an increase, or decrease in body size may associate to colder climates, as expected by Bergmann’s rule and the so-called converse BR, respectively (Shelomi 2012; ; Scriven et al. 2016). The application of BR to ectotherms is justified by the dependence of environmental temperatures required for these animals to thermoregulatory activities. This implies that thermoregulation in larger ectotherms is expected to be less efficient in cooler climates because they absorb heat more slowly than the smaller ones (Aragon and Fitze 2014). In contrast, the converse BR posits that colder sites limit the time available for foraging and growth, resulting in smaller organisms (Mousseau 1997; Blanckenhorn and Demont 2004). Therefore, these mixed results in the application of BR to ectotherms has been hotly debated, because both geographical patterns have been found (Chown and Gaston 2010).
7.3 Scorpions as Organism Model in Ecological Studies
Scorpions constitute one of the oldest groups of terrestrial arthropods, with their first records dated to the Silurian (Kjelleswig-Waering 1986; Jeram 1998; Dunlop 2010). Due to the long evolutionary period and low modification in the basic bauplan, these animals are considered living fossils (Polis 1990). Corresponding to the fifth highest order of Arachnida in terms of species richness, scorpions are currently represented by about 2600 species (Brazil and Porto 2011; Rein 2021), with a total estimated diversity of around 7000 species (Coddington and Colwell 2001). In general, these arachnids present a wide spectrum of ecological requirements, being distributed in a plethora of habitats (e.g., tropical forests, dry forests, mountain tops, deserts, caves, and urban centers) across almost all landmasses (Polis 1990; Lourenço 2015). Scorpion richness seems to be maximized in arid and semiarid environments, with local assemblages being usually represented by an average of 5–6 species (Polis 1990, 1993; Porto et al. 2014), although neotropical forests can eventually harbor a comparable number of species at local scales (Lourenço 1994; Brito and Borges 2015). Despite their wide distribution, scorpions cannot be considered as ecologically generalist, as the vast majority of species have specific requirements regarding habitat and microhabitat (Polis 1990).
Several evidences suggest that scorpion assemblages are, in part, driven by environmental temperature, precipitation, type of substrate (e.g., soil hardness, litter quantity), vegetation structure, as well as by the complex interaction among these factors (Warburg and Ben-Horin 1981; Prendini 2005; Foord et al. 2015; Lira et al. 2019a, 2021a). In some cases, the responses of scorpion assemblages to the environmental variation may be detected even at very small spatial scales (Raz et al. 2009; Foerster et al. 2020). For example, a strong correlation of species composition with climate variation was described by Foord et al. (2015) when investigating the effect of the altitudinal gradient on a mountain system in South Africa. Species of the genus Hottentotta Birulla, 1908 and Parabuthus Pocock, 1890 were absent in areas where rainfall was above 600 mm. Similar results were described by Prendini (2005) when investigating the scorpion assemblage along a longitudinal gradient in the southern region of Africa, finding that species of Afroisometrus Kovarik, 1997, Lychas C. L. Koch, 1845, Cheloctonus Pocock, 1892, Pseudolychas Kraepelin, 1911 and Opistacanthus Peters, 1861 were restricted to areas with high rainfall such as montane forests. In a recent study, Lira et al. (2019a) investigated the effects of bioclimatic variables across a longitudinal gradient on Brazilian scorpions. These authors found that variables associated with precipitation and temperature had a strong effect on scorpion distribution.
On a local scale, even within the habitat, scorpions are not randomly distributed within the environment. Different substrates exert different selective pressures on scorpions, resulting in microhabitat-specialist species (Polis 1990; Prendini 2001). In a study carried out in a humid forest enclave in northeastern Brazil, no niche overlap was detected between the three scorpion species (Tityus brazilae Lourenço and Eickstedt 1984; T. neglectus Mello-Leitão 1932 and T. pusillus Pocock 1893) that co-occur in the area with each one collected exclusively in specific microhabitats (Lira and DeSouza 2014). Similarly, when analyzing the use of microhabitat in Colombian species from a dry forest fragment, Álvarez et al. (2013) described that Opisthacanthus elatus (Gervais 1844) and T. tayrona Lourenço 1991 preferentially use fallen objects (trunks and stones) while T. ashtenes Pocock 1893 and Ananteris columbiana Lourenço 1991 were frequently found in vegetation and litter, respectively. Such specificity in microhabitat selectivity can be observed even in species that coexist on the same substrate. Lira et al. (2013, 2018) investigating the use of microhabitat in Atlantic Forest scorpions, found a vertical stratification in two litter species, with T. pusillus colonizing the upper layers, while Ananteris mauryi Lourenço 1982 uses the lower layers of the litter. Due to environmental requirements, whether regional (climate variation) or local (microhabitat specificity), scorpions are responsive to habitat modification. Smith (1995) studying the effect of habitat fragmentation in Australia, reported that larger fragments support higher abundance and richness of scorpions than smaller fragments. Similar results with higher scorpion richness observed in larger fragments have been described for a hyper-fragmented Brazilian Atlantic Forest landscape (Lira et al. 2016). These authors suggested that larger fragments have a greater number of microhabitats available in relation to smaller ones, thus supporting a greater diversity of these arachnids. In a study conducted on locations with different usage histories in the Caatinga, Foerster et al. (2020) corroborates the idea that environmental complexity is crucial for the maintenance of local species pool. In this work, the authors found more species in areas that presented greater environmental complexity than in monotonous landscapes. Therefore, the sensitivity of scorpion species to environmental variations at different scales (regional or local) make these animals good candidates for environmental bioindicators. Polis (1990) argues that scorpions meet a series of criteria that frame them as bioindicators, such as being easily sampled, classified and stored. In addition, Gerlach et al. (2013) point out that these arachnids have a high potential as bioindicators, and may even be used as flagship species, due to their charisma. However, the authors emphasize that the low diversity and abundance in many areas can act as a limiting factor for their potential use as bioindicators. Within this context, scorpions have been used as models for ecological studies that address environmental changes, especially in Neotropics (e.g., Nime et al. 2014; Lira et al. 2020, 2021a).
7.4 The Brazilian Atlantic Forest
As the second largest rainforest biome on the American continent, the Brazilian Atlantic Forest is one of the 36 global hotspots in biodiversity conservation (Conservation International 2009). The exuberant biodiversity contained in Brazilian Atlantic Forest has been continuously threatened by habitat loss and fragmentation due to anthropogenic activities (Laurance 2009; Haddad et al. 2015). Such human activities were (and still are) one of the major drivers affecting the current spatial configuration of the Atlantic Forest, represented today by small islands of vegetation inserted in a matrix of areas converted to the human settlements (Ribeiro et al. 2009; Joly et al. 2014; Haddad et al. 2015; Rezende et al. 2018). These factors, together with other threats to the biome (e.g., hunting and illegal logging), have led to the extinction of numerous species (Marini and Garcia 2005), compromising regions where important centers of endemism are located. Nowadays, the estimated fraction of original vegetation cover in the Atlantic Forest is estimated in only 28%, which includes native forest and non-forest formations (Rezende et al. 2018). Those original formations are extremely important to assure ecological corridors aimed to improve the connectivity among Atlantic Forest patches (i.e., fragments) within the landscape, preventing populations from deleterious effects of genetic isolation and improving their capacity to remain viable over time (Rezende et al. 2018).
High levels of endemism in the Brazilian Atlantic Forest were detected for several taxa, such as bees (Garraffoni et al. 2017), harvestmen (DaSilva et al. 2015), birds (Marini and Garcia 2005; Carvalho et al. 2021), and anuran (Vasconcelos et al. 2014). Different hypotheses have been formulated to explain the causes of the high diversity and endemism in the Atlantic Forest. For example, tectonic activities, marine introgressions, Pleistocene refuges (Martins 2011), and environmental gradients shaped the distribution of lineages in this biome. In the latter case, environmental gradients along the same biome would favor speciation (Silva and Casteleti 2003) and would limit the distribution of species in time and space.
The Atlantic Forest has a wide geographic distribution extending along the entire Atlantic coast of Brazil (Galindo-Leal and Câmara 2003) originally ranged between latitudes 6° S and 30° S and covered approximately 1.1 million km2. It is characterized by strong environmental gradients, seasonality (Ribeiro et al. 2009), and complex topography. Their latitudinal gradient reveals a biome with a vegetational complex, described below, and climate heterogeneity (Fiaschi and Pirani 2009; Oliveira-Filho et al. 2015). Some researchers have demonstrated the role of climate on floristic differentiation, along altitudinal gradients and between geographic regions in the biome (Oliveira-Filho and Fontes 2000; Joly et al. 2012; Eisenlohr et al. 2013). Temperature and precipitation, for example, varies in a north–south gradient (Oliveira-Filho and Fontes 2000), where the decreasing temperatures toward higher latitudes and altitudes along the coast (Oliveira-Filho et al. 2015) which influence several species. In addition to historical factors, these climatic variations contribute to the high turnover of species in the biome (Condit et al. 2002; Silva et al. 2014; Nogueira et al. 2019).
This complex biome is composed of three forest types influenced by rainfall regime: the Dense Rainforest, the Mixed Rainforest, and the Seasonal Forest (Oliveira-Filho and Fontes 2000). The Dense Rain forests (or ombrophilous forest) are located on the Atlantic coast and are distributed in lowlands (generally the climate is hot and wet) and slopes (climate cold and wetter) from northeastern to southern Brazil (Oliveira-Filho and Fontes 2000), where rainfall is increased by oceanic winds and mountain ranges, especially in the south (Cancello et al. 2014). Associated with the vegetation in lowlands it presents the “restinga,” characterized by drier climate and sand soil (Marques et al. 2011). Mixed Rain forests, also known as Araucaria forests because of the presence of the conifer Araucaria angustifolia (Bertol.) Kuntze, constitute the main forest type on the highland plateau in southern Brazil. The mixed rain forest is characterized by tropical and sub-tropical humid climates without pronounced dry periods. Seasonal forests are characterized by two distinct seasons with marked seasonality (summer with intense rainfalls and winter with low temperatures and scarce precipitation) and extends across the plateau (usually 600 m elevation) in the center and southeastern interior of Brazil (Oliveira-Filho and Fontes 2000) and are influenced by Cerrado biome.
Several mountain ranges and cliffs compose the Atlantic Forest landscape along the latitudinal gradient. Originally, more than 80% of their area occurred at elevations from 200 to 1200 m (Tabarelli et al. 2010). In some high altitudes can be observed an increase in precipitation and humidity (Camara 2003). For example, in southeastern Brazil, the remnants located in “Serra do Mar” and “Serra da Mantiqueira” stand out where the occurrence of orographic rains provides greater humidity in these forests (Por 1992). Due to variations in altitude, we found in Atlantic Forest differences in the phytophysiognomy along its extension that, in addition to lowland forests, montane, and submontane forests. Differences in species composition in relation to the latitudinal gradient in the Atlantic Forest, which are influenced by climate and historical factors, have been the subject of several studies (e.g., Almeida-Neto et al. 2006; Martins et al. 2015; Nogueira et al. 2019; Shimabukuro and Trivinho-Strixino 2021). In this context, this chapter is intended to provide insights into the scorpion assemblages in a latitudinal gradient at Brazilian Atlantic Forest through application of Rapoport effect and Bergmann rule.
7.5 Case Study: Scorpion Assemblage Along Latitudinal Gradient in Brazilian Atlantic Forest
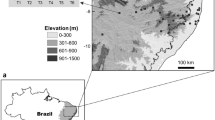
In order to investigate the applicability of Rapoport and Bergman’s rules on scorpion assemblages along the latitudinal gradient in the Brazilian Atlantic Forest, we first georeferenced scorpion occurrence data were primarily extracted from public repositories, notably the Global Biodiversity Information Facility (GBIF 2021) and the SpeciesLink platform (CRIA 2021). All georeferenced data available for the order Scorpiones until 18 September 2021 were downloaded from both repositories. Additional information on scorpion occurrence in Atlantic Forest was also added from scientific literature (Galiano and Maury 1979; Lourenço 2002, 2005; Yamaguti and Pinto-da-Rocha 2003; Lourenço and Giupponi 2004; Dias et al. 2006; Santos et al. 2006; Giupponi et al. 2009; Quintela et al. 2014; Lira et al. 2021a). Combined datasets resulted in a raw amount of 66,976 occurrences, of which, 2429 occurrences were used in downstream analyses as a result of the data cleaning process. This consisted in the exclusion of taxa that were not identified to Linnean species names, as well as those occurrences that fell outside the boundaries of a spatial grid of 0.5° × 0.5° pixel resolution, intersecting the spatial extent of the Brazilian Atlantic forest (Fig. 7.1). The spatial grid was drawn on QGis 3.18 (QGIS Development Team 2021) using a shapefile of the Brazilian Atlantic Forest provided by the MapBiomas project (MaPBiomas Project 2021) as a reference for the delineation of its spatial extent. Spatial operations (i.e., overlapping the spatial grid over the Atlantic Forest boundaries and scorpion occurrences) were performed in the R environment (R Core Team 2021) using the ‘sf’ (Pebesma 2018) and ‘dplyr’ (Wickham et al. 2021) packages and assuming the SIRGAS 2000 (EPSG: 4674) map projection. Species with dubious distributions within the Atlantic Forest were also excluded from the dataset based on our expertise about their ecological requirements and scientific information from the literature when available. Based on such criteria, the following species were removed from the dataset: Bothriurus rochai Mello-Leitão 1932, Jaguajir rochae (Borelli 1910), Physoctonus debilis (C. L. Koch 1840) – both typical species of Caatinga forests (Foerster et al. 2019; Lira et al. 2020); Tityus obscurus (Gervais 1843), T. metuendus Pocock 1897, T. raquelae Lourenço 1988 – Amazonian species (Martins et al. 2021); Ananteris franckei Lourenço 1982 and B. araguayae Vellard 1934 commonly found in montane forests of northeastern Brazil and Cerrado formations, respectively (Lourenço 2002; Foerster et al. 2019). The list of scorpion species of the Brazilian Atlantic Forest recovered in this study is presented in Appendix 7.1.
After this, the geographic range of each species was initially calculated as the latitudinal distance (in kilometers) between the northernmost and the southernmost location that each species was found (Lear et al. 2017). To this end, species with a single occurrence (singletons) were excluded from the dataset (Appendix 7.1). As described in the previous section, an average geographic species range per latitudinal band was obtained from the computation of a mean value of the geographic ranges of the species present in each latitudinal band. The average geographic species range per latitudinal band was then adjusted by the number of valid grid cells in each band (thereafter referred to as “latitudinal species range”). Species richness per latitudinal band was obtained by summing the number of species present in each latitudinal band (i.e., latitudinal species richness). The latitudinal species range, latitudinal species richness, and the absolute latitude of each latitudinal band were correlated in a pairwise scheme through the Spearman’s rank correlation coefficient with Holm’s adjustment of p-values. Statistical analyses (i.e., mean arithmetic adjustments and Spearman’s rank correlations) were performed in R using the ‘correlation’, dplyr, and ‘base’ (R Core Team 2021) packages.
A second dataset containing the carapace length of scorpion species listed in Appendix 7.1 was assembled based on direct specimen measurements and additional data from the literature. Previous studies have demonstrated that carapace length is a reliable proxy of body size in scorpions (e.g., Outeda-Jorge et al. 2009; Lira et al. 2021b). Moreover, the use of a single corporal structure (carapace) to represent body size in these animals is expected to minimize the error inherent to the measurement process, especially if compared to a more complex metric of body size, such as the total length. This is because total length can be obtained by both measuring the specimen at once – usually a straight line ranging from the anterior tip of the carapace to the distal tip of the telson – or by summing the length of its body parts separately. For the information extracted from the literature, the most recent publications were prioritized (Appendix 7.1) and an average carapace length was calculated when this metric was available for more than one specimen and sex, allowing the incorporation of a potential sexual dimorphism in this character.
To test the presence of latitudinal trends in body size of scorpion species distributed along the Atlantic Forest, the grid cells containing scorpion occurrences were initially identified. After that, those grid cells were consistently grouped according to the latitudinal position of their centroids, resulting in latitudinal bands across the Atlantic Forest (Fig. 7.1). All the species found in each of the latitudinal bands were translated to its measurements of body size, allowing the calculation of an average body size per latitudinal band (c.f. de Menezes et al. 2018). To account for the inevitable difference in the number of grid cells per latitudinal band (Fig. 7.1), the average body size calculated for each latitudinal band was adjusted by the number of valid grid cells (i.e., cells with scorpion occurrence) through the mean arithmetic approach:
where, wBS corresponds to the mean body size per latitudinal band weighted by the number of valid grid cells in that band (thereafter referred as to “latitudinal body size”); VCi is the number of valid grid cells in the latitudinal band i; and BSi is the mean body size calculated for the latitudinal band i. The latitudinal body size was then correlated to the absolute latitude of each latitudinal band through the Spearman’s rank correlation coefficient (ρ), thus, avoiding any assumptions about the shape of the relationship between these two variables or the potential noise due to the presence of skewed data (McDonald 2014; de Menezes et al. 2018). P-values for Spearman’s correlation were adjusted using Holm’s correction as implemented in the ‘correlation’ R package (Makowski et al. 2020).
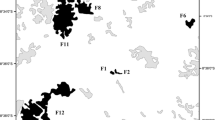
We found a total of 28 species belonging to seven genera and two families (Bothriuridae and Buthidae) for the Atlantic Forest (Appendix 7.1, Fig. 7.2). Buthid scorpions are represented by four genera and 21 species, while bothriurids were represented by eight species grouped into three genera. Buthidae species show a widespread distribution in the Brazilian Atlantic Forest, species from Tityus C. L. Koch 1836 genera were found in practically all latitudinal gradient (Fig. 7.3). In contrast, other buthid genera such as Zabius Thorell 1893 showed a more limited distribution being found on the southern side of the latitudinal gradient, respectively (Fig. 7.3). Bothriurids scorpions were represented by genera Bothriurus Peters 1861, Thestylus Simon 1880, and Urophonius Pocock 1893, with Urophonius represented by one species and Bothriurus being more specious with four species. Scorpions from this family are also found throughout the latitudinal gradient (Fig. 7.3).
Representatives of scorpion species found in Brazilian Atlantic Forest. (a) Bothriurus asper Pocock 1893, (b) Thestylus aurantiurus Yamaguti and Pinto-da-Rocha 2003, (c) Ananteris mauryi Lourenço 1982, (d) Tityus adrianoi Lourenço 2003, (e) Tityus bahiensis (Perty 1833), (f) Tityus brazilae Lourenço and Eickstedt 1984, (g) Tityus neglectus Mello-Leitão 1932 and (h) Tityus pusillus Pocock 1893. (Photos A, B and G by Dr. Leonardo Carvalho, C, F and H by Dr. Adriano DeSouza and D and E by Dr. Pedro Martins)
Species with the highest number of occurrences were also the widest distributed ones in terms of number of occupied grid cells (Fig. 7.4). In terms of genera, Tityus, Bothriurus, and Ananteris Thorell 1891 were the most abundant in number of occurrences, and together with genus Isometrus Ehrenberg 1828 presented the wider latitudinal ranges within the Atlantic Forest (Fig. 7.3). The latitudinal range of the scorpion species used to test the Rapoport’s effect in Atlantic Forest ranged from 85.25 to 2625.46 km (Fig. 7.5). Although the largest latitudinal ranges were reported to the genera Isometrus, Tityus, Bothriurus, and Ananteris (Fig. 7.3), there was a considerable variation in the latitudinal range sizes among species within these genera (Fig. 7.5), except for Isometrus, represented here by a single species I. maculatus (DeGeer 1778). Latitudinal species range increased toward the equator, as evidenced by the positive correlation (ρ = 0.390, p = 0.016) found between absolute latitude and latitudinal species range. Yet, there was also a strong positive correlation between the latitudinal species range and the latitudinal species richness (ρ = 0.787, p < 0.001). To a lesser extent, the latitudinal species richness was positively correlated to the absolute latitude (ρ = 0.339, p = 0.023), increasing toward the equator. At family level, buthid scorpions from the Atlantic Forest were the most variable in terms of body size (carapace length) than bothriurid species. Most of 50% of Atlantic Forest scorpion assemblage body size was composed by large-mid species (Fig. 7.6). Tityus species being overall larger than the species from the other genera in this environment (Fig. 7.6). Nevertheless, body size in Atlantic Forest scorpions was notably conserved among congeneric species (Fig. 7.6) and no latitudinal trends in this trait could be detected, as indicated by the Spearman rank correlation coefficient (ρ = 0.227, p = 0.130).
Our study was a first attempt to help in understanding the patterns behind scorpion diversity in an Atlantic Forest. The scorpions recorded from this biome belong to either Buthidae (70%) or Bothriuridae (30%), the two most diverse scorpion families in the Brazilian fauna (Lourenço 2002). Considering the higher degradation level of this biome (e.g., Ribeiro et al. 2009; Haddad et al. 2015), the Brazilian Atlantic Forest exhibited a greater number of species, corresponding to 20% of the Brazilian scorpion fauna. Most of this fauna is composed of forest-specialist scorpions, such as T. brazilae, T. pusillus, and A. mauryi (Porto et al. 2018; Lira et al. 2020). However, species considered typical of other biomes can occur in ecotonal regions with characteristics of the Atlantic Forest, such as the species T. fasciolatus, considered typical of the Cerrado (Savana-like vegetation: Lourenço 2002). We also found records of I. maculatus, an Indo-Malayan species, possibly introduced in several coastal regions of the world through ships (Kovařík 2003). In addition, Atlantic Forest scorpion fauna is composed also by species adapted to disturbed environments, such T. serrulatus and T. stigmurus, considered as synanthropic because of their intimate relationship with urban areas (Amado et al. 2021).
We found that scorpion diversity can be explained by the latitudinal gradient along the Brazilian Atlantic Forest with species richness increasing toward the equator. Although there have been no previous studies on the influence of latitudinal gradient on Atlantic Forest scorpion diversity, studies with other organisms, such as termites (Cancello et al. 2014) and epiphytic lichens (Menezes et al. 2018), indicate effects of latitudinal gradient on species richness similar to those found in this study. In contrast, studies with other animals such as harvestmen (Nogueira et al. 2019), bats (Stevens 2013), and leaf litter ants (Silva and Brandão 2014) described an opposite pattern with species richness increasing toward the southern portion of Atlantic Forest. In latitudinal gradients we found a clinal variation in climatic factors, mainly temperature and precipitation, that are the major predictors of floristic structure (Prata et al. 2018). Habitat complexity generated by vegetation structure in a determined area, influencing the habitat structure and providing keystone structure (Tews et al. 2004). Therefore, habitats with greater microhabitat availability are associated with a high species richness once scorpion assemblages are composed of niche specialists, due to aggressive behavior between scorpion heterospecifics (Polis 1990; Lira et al. 2013, 2018). Alternatively, another possible and non-exclusive explanation for the increase in the scorpion species richness toward the equator is related to the species range size. In contrast to the proposed by Rapoport rule, our results pointed up an increase in range size toward the equator. Therefore, the higher species richness found on the north portion of Atlantic Forest may be an artificial artifact resulting from the overlapping distribution of species with a large range size.
Scorpion species range in Atlantic Forest may be related to two main factors: (1) biological traits of these arachnids and (2) historical biome formation. In the first, scorpions are considered invertebrates with poor dispersal capacity (Polis et al. 1985) and dependent on forested habitats (Dionisio et al. 2018; Lira et al. 2019b). For example, Lira et al. (2019b) investigated the scorpion fauna in Atlantic Forest remnants and in their neighboring cropland. These authors found that scorpion species are unable to colonize the croplands being restricted to forested remnants. Although some scorpions may be found in disturbed environments such as urban areas (Brazil et al. 2009; Bertani et al. 2018), these species are exceptions and the majority of Atlantic Forest scorpions are forest-dwelling species (Lira et al. 2021a). In the second main factor, the historical process of Atlantic Forest formation was marked by successive forest reduction during climatic fluctuations and global cooling since the Pliocene or late Miocene (Holbourn et al. 2014; Ravelo et al. 2004). According to Carnaval et al. (2014), the northern portion of Atlantic Forest exhibited a more stable climate. In this way, larger mesic refugia were formed when compared to southern and southeastern portions. This complex process led to the proposal to delimit 12 areas of endemism for the biome, corresponding to two areas in the northern region and ten in the southern region (DaSilva et al. 2015). Therefore, these two main factors may have acted synergistically for the range size pattern found for Atlantic Forest scorpions. Contrary to our expectations, we found any effect of latitude on scorpion body size. In addition, the body size of these arachnids seems to be conserved within genus and also within the subgenera (Tityus and Archeotityus) that subdivide the genus Tityus (Lourenço 2006) found in Atlantic Forest. Therefore, in Atlantic Forest, scorpions may be a result of a synergistic interaction encompassing both ancestry and environmental constraints, which, in many cases, may lead to the emergence of ecomorphotypes (Polis 1990; Prendini 2001; Koch et al. 2017).
In summary, this chapter provides a first insight of diversity patterns from Atlantic Forest scorpions. Overall, we found the three main results: (1) the species richness of these arachnids increases toward the equator as postulated in latitudinal diversity gradient hypothesis; (2) species range size follows an inverse that was proposed in the Rapoport rule with range size increasing toward equator; and (3) no latitudinal effects was detected on scorpion body size. These findings may reflect that scorpion natural history traits and historical processes of Atlantic Forest exerts a key role on diversity patterns of these arachnids along a latitudinal gradient in Brazilian Atlantic Forest. However, these findings may be interpreted with some caution as new collection expeditions and taxonomic revisions in progress may alter knowledge about the diversity of Atlantic Forest scorpions.
References
Acosta LE, Candido DM, Buckup EH, Brescovit AD (2008) Description of Zabius gaucho (Scorpiones, Buthidae), a new species from southern Brazil, with an update about the generic diagnosis. J Arachnol 36:491–501
Albuquerque FS, Castro-Díez P, Rueda M, Hawkins BA, Rodríguez MÁ (2011) Relationship of climate, residence time and biogeographical origin with the range sizes and species richness patterns of exotic plants in Great Britain. Plant Ecol 212:1901
Allen AP, Gillooly JF (2006) Assessing latitudinal gradients in speciation rates and biodiversity at the global scale. Ecol Lett 9:947–954
Almeida-Neto M, Machado G, Pinto-da-Rocha R, Giaretta AA (2006) Harvestman (Arachnida: Opiliones) species distribution along three Neotropical elevational gradients: an alternative rescue effect to explain Rapoport’s rule? J Biogeogr 33:361–375
Álvarez D, Gómez D, Martínez J, Mendoza H, Ruiz S (2013) Microhabitat use by scorpions in a tropical dry forest relict of the Colombian Caribbean. Revista Colombiana de Entomologia 39:301–304
Amado TF, Moura TA, Riul P, Lira AFA, Badillo-Montaño R, Martinez PA (2021) Vulnerable áreas to acidentes with scorpions in Brazil. Tropical Med Int Health 26:591–601
Aragon P, Fitze PS (2014) Geographical and temporal body size variation in a reptile: roles of sex, ecology, phylogeny and ecology structured in phylogeny. PLoS One 9:e104026
Bergmann C (1847) Ueber die Verhaltnisse der Warmeokonomie der Thiere zu ihrer Grosse. Gottinger Studien 1:595–708
Bertani R, Bonini RK, Toda MM, Isa LS, Figueiredo JVA, Santos MR, Ferraz SC (2018) Alien scorpions in the municipality of São Paulo, Brazil: evidence of successful establishment of Tityus stigmurus (Thorell, 1876) and first records of Broteochactas parvulus Pocock, 1897, and Jaguajir rochae (Borelli, 1910). Bioinvasion Rec 7:89–94
Blackburn TM, Gaston KJ, Loder N (1999) Geographic gradients in body size: a clarification of Bergmann’s rule. Divers Distrib 5:165–174
Blanckenhorn WU, Demont M (2004) Bergmann and converse Bergmann latitudinal clines in arthropods: two ends of a continuum. Integr Comp Biol 44:413–424
Böhm M, Kemp R, Williams R, Davidson AD, Garcia A, McMillan KM, Bramhall HR, Collen B (2017) Rapoport’s rule and determinants of species range in snakes. Divers Distrib 23:1472–1481
Brazil TK, Porto TJ (2011) Os escorpiões. Edufba, Salvador
Brazil TK, Lira-da-Silva RM, Porto TJ, Amorim AMD, Silva TFD (2009) Escorpiões de importância médica do estado da Bahia, Brasil. Gazeta Médica da Bahia 79:38–72
Brito G, Borges A (2015) A checklist of the scorpions of Ecuador (Arachnida: Scorpiones), with notes on the distribution and medical significance of some species. J Venom Anim Toxins Incl Trop Dis 21:23–40
Brocklehurst N, Day MO, Rubidge BS, Fröbisch J (2017) Olson’s extinction and the latitudinal biodiversity gradient of tetrapods in the Permian. Proc R Soc B Biol Sci 284:20170231
Brown JH, Gillooly JF, Allen AP, Savage VM, West GB (2004) Toward a metabolic theory of ecology. Ecology 85:1771–1789
Brown JH (2014) Why are there so many species in the tropics? J Biogeogr 41:8–22
Câmara IG (2003) Brief history of conservation in the Atlantic Forest. In: Galindo–Leal C, Câmara IG (eds) The Atlantic forest of South America: biodiversity status, threats, and outlook. CABS and Island Press, Washington, pp 31–42
Cancello EM, Silva RR, Vasconcellos A, Reis YT, Oliveira LM (2014) Latitudinal variation in termite species richness and abundance along the Brazilian Atlantic Forest hotspot. Biotropica 46:441–450
Candido DM, Lucas S, Souza CAR, Diaz D, Lira-da-Silva RM (2005) Uma nova espécie de Tityus C. L. Koch, 1836 (Scorpiones, Buthidae) do estado da Bahia. Brasil Biota Neotropica 5:193–200
Cardillo M, Orme CDL, Owens IPF (2005) Testing for latitudinal bias in diversification rates: an example using new world birds. Ecology 86:2278–2287
Carnaval AC, Waltari E, Rodrigues MT, Rosauer D, VanDerWal J, Damasceno R, Prates I, Strangas M, Spanos Z, Rivera D, Pie MR, Firkwski CR, Bornschein MR, Ribeiro LF, Moritz C (2014) Prediction of phylogeographic endesmism in na environmentally complex biome. Proc R Soc B Biol Sci 281:20141461
Carvalho CS, Martello F, Galetti M, Pinto F, Francisco MR, Silveira LF, Galetti PM Jr (2021) Environmental heterogeneity and sampling relevance areas in an Atlantic Forest endemism region. Perspect Ecol Conserv 19:311–318
Chown SL, Gaston KJ (2010) Body size variation in insects: a macroecological perspective. Biol Rev 85:139–169
Coddington JA, Colwell RK (2001) Arachnida. In: Levin SC (ed) Encyclopedia of biodiversity. Academic Press, New York, pp 199–218
Condit R, Pitman N, Leigh EG Jr (2002) Beta-diversity in tropical forest trees. Science 295:666–669
Conservation International (2009) Biodiversity hotspots-resources-maps and GIS data. https://www.conservation.org/priorities/biodiversity-hotspots. Accessed 27 Sept 2021.
CRIA (2021) Species link (http://splink.cria.org.br/project?criaLANG=pt). Accessed on 18 Sept 2021
Currie DJ, Mittelbach GG, Cornell HV, Field R, Guégan J-F, Hawkins BA, Kaufman DM, Kerr JT, Oberdorff T, O’Brien E, Turner JRG (2004) Predictions and tests of climate-based hypothesis of broad-scale variation in taxonomic richness. Ecol Lett 7:1121–1134
Darwin CR (1859) On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. Murray, London
DaSilva MB, Pinto-Da-Rocha R, DeSouza AM (2015) A protocol for the delimitation of areas of endemism and the historical regionalization of the Brazilian Atlantic rain forest using harvestmen distribution data. Cladistics 31:692–705
Dias SC, Candido DM, Brescovit AD (2006) Scorpions from Mata do Buraquinho, João Pessoa, Paraíba, Brazil, with ecological notes on a population of Ananteris mauryi Lourenço (Scorpiones, Buthidae). Revista Brasileira de Zoologia 23:707–710
Dionisio-da-Silva W, Lira AFA, Albuquerque CMR (2018) Distinct edge effects and reproductive periods of sympatric litter-dwelling scorpions (Arachnida: Scorpiones) in a Brazilian Atlantic Forest. Zoology 129:17–24
Dunlop JA (2010) Geological history and phylogeny of Chelicerata. Arthropod Struct Dev 39:124–142
Eisenlohr PV, Alves LF, Bernacci LC, Padgurschi MCG, Torres RB, Prata EMB, Santos FAM, Assis MA, Ramos E, Rochelle ALC, Martins FR, Campos MCR, Pedroni F, Sanchez M, Pereira LS, Vieira SA, Gomes JAMA, Tamashiro JY, Scaranello MAS, Caron CJ, Joly CA (2013) Disturbances, elevation, topography and spatial proximity drive vegetation patterns along an altitudinal gradient of a top biodiversity hotspot. Biodiver Conserv 22:2767–2783
Entling W, Schmidt-Entling MH, Bacher S, Brandl R, Nentwig W (2010) Body size-climate relationships of European spiders. J Biogeogr 37:477–485
Fiaschi P, Pirani JR (2009) Review of plant biogeographic studies in Brazil. J Syst Evol 47:477–496
Fine PVA (2015) Ecological and evolutionary drivers of geographic variation in species diversity. Annu Rev Ecol Evol Syst 46:369–392
Foerster SÍA, DeSouza AM, Lira AFA (2019) Macroecological approach for scorpions (Arachnida, Scorpiones): β-diversity in Brazilian montane forests. Can J Zool 97:914–921
Foerster SÍA, Lira AFA, Almeida CG (2020) Vegetation structure as the main source of variability in scorpion assemblages at small spatial scales and further considerations for the conservation of Caatinga landscapes. Neotrop Biol Conserv 15:533–550
Foord SH, Gelebe V, Prendini L (2015) Effects of aspect and altitude on scorpion diversity along an environmental gradient in the Soutpansberg, South Africa. J Arid Environ 113:114–120
Fortes RR, Absalão RS (2004) The applicability of Rapoport’s rule to the marine molluscs of the Americas. J Biogeogr 31:1909–1916
Galiano ME, Maury EA (1979) Lista de los ejemplares tipicos de Arachnida (Araneae, Opiliones, Scorpiones y Solifugae) depositados en el Museo Argentino de Ciencias Naturales Bernadino Rivadavia. Revista Del Museo Argentino de Ciencias Naturales Bernadino Rivadavia 5:301–334
Galindo-Leal C, Câmara IG (2003) The Atlantic forest of South America: biodiversity status, threats, and outlook. CABS and Island Press, Washington
Garraffoni AR, Moura FR, Lourenço AP (2017) Areas of endemism in the Atlantic Forest: quantitative biogeography insights from orchid bees (Apidae: Euglossini). Apidologie 48:513–522
Gaston KJ (2000) Global patterns in biodiversity. Nature 405:220–227
Gaston KJ (2003) The how and why of biodiversity. Nature 421:900–901
GBIF.org (2021) GBIF home page. Disponible at: https://www.gbif.org. 18 Sept 2021
Gerlach J, Samways M, Pryke J (2013) Terrestrial invertebrates as bioindicators: an overview of available taxonomic groups. J Insect Conserv 17:831–850
Giupponi APL, Vasconcelos EG, Lourenço WR (2009) The genus Ananteris Thorell, 1891 (Scorpiones, Buthidae) in southeast Brazil, with the description of three new species. ZooKeys 13:29–41
Haddad NM, Brudvig LA, Clobert J, Davies KF, Gonzalez A, Holt RD, Cook WM (2015) Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci Adv 1(2):e1500052
Hillebrand H (2004) On the generality of the latitudinal diversity gradient. Am Nat 163:192–211
Holbourn A, Kuhnt W, Lyle M, Schneider L, Romero O, Andersen N (2014) Middle Miocene climate cooling linked to intensification of eastern equatorial Pacific upwelling. Geology 42:19–22
Jablonski D, Roy K (2003) Geographical range and speciation in fossil and living molluscs. Proc R Soc B Biol Sci 270:401–406
Jeram AJ (1998) Phylogeny, classification and evolution of Silurian and Devonian scorpions. In: Brownell P, Polis GA (eds) Scorpion biology and research. Oxford University Press, Oxford
Joly CA, Assis MA, Bernacci LC, Tamashiro JY, Campos MCR, Gomes JAMA, Lacerda MS, Santos FAM, Pedroni F, Pereira LS, Padgurschi MCG, Prata EMB, Ramos E, Torres RB, Rochelle A, Martins FR, Alves LF, Vieira SA, Martinelli LA, Camargo PB, Aidar MPM, Eisenlohr PV, Simões E, Villani JP, Belinello R (2012) Florística e fitossociologia em parcelas permanentes da Mata Atlântica do sudeste do Brasil ao longo de um gradiente altitudinal. Biota Neotrop 12:123–145
Joly CA, Metzger JP, Tabarelli M (2014) Experiences from the Brazilian Atlantic Forest: ecological findings and conservation initiatives. New Phytol 204:459–473
Kaspari M, Ward PS, Yuan M (2004) Energy gradients and the geographic distribution of local ant diversity. Oecologia 140:407–413
Kinlock NL, Prowant L, Herstoff EM, Foley CM, Akin-Fajiye M, Bender N, Umarani M, Ryu HY, Sen B, Gurevitch J (2018) Explaining global variation in the latitudinal diversity gradient: meta-analysis confirms known patterns and uncovers new ones. Glob Ecol Biogeogr 27:125–141
Kjelleswig-Waering EN (1986) A restudy of the fossil Scorpionida of the world. Paleontol Res 55:1–287
Koch NM, Ceccarelli FS, Ojanguren-Affilastro AA, Ramirez MJ (2017) Discrete and morphometric traits reveal contrasting patterns and processes in the macroevolutionary history of a clade of scorpions. J Evol Biol 30:814–825
Kovarik F (2003) A review of the genus Isometrus Ehrenberg, 1828 (Scorpiones: Buthidae) with description of four new species from Asia and Australia. Euscorpius 10:1–19
Kreft H, Jetz W (2007) Global patterns and determinants of vascular plant diversity. Proc Natl Acad Sci U S A 104:5925–5930
LaManna JA, Belote RT, Burkle LA, Catano CP, Myers JA (2017) Negative density dependence mediates biodiversity productivity relationship across scales. Nat Ecol Evol 1:1107–1115
Latham RE, Ricklefs RE (1993) Global patterns of tree species richness in moist forests: energy-diversity theory does not account for variation in species richness. Oikos 67:325–333
Laurance WF (2009) Conserving the hottest of the hotspots. Biol Conserv 142:1137
Lear G, Lau K, Perchec A-M, Buckley HL, Case BS, Neale M, Fierer N, Leff JW, Handley KM, Lewis G (2017) Following Rapoport’s rule: the geographic range and genome size of bacterial taxa decline at warmer latitudes. Environ Microbiol 19:3152–3162
Lee TM, Jetz W (2010) Unravelling the structure of species extinction risk for predictive conservation science. Proc R Soc B: Biol Sci 278:1329–1338
Lira AFA, DeSouza AM (2014) Microhabitat use by scorpion species (Arachnida: Scorpiones) in the montane Atlantic rainforest, Brazil. Revista Ibérica de Aracnologia 24:107–108
Lira AFA, DeSouza AM, Silva Filho AAC, Albuquerque CMR (2013) Spatio-temporal micro-habitat use by two co-occurring Species of scorpions in Atlantic rainforest in Brazil. Zoology 116:182–185
Lira AFA, Araújo VLN, DeSouza AM, Rego FNAA, Albuquerque CMR (2016) The effect of habitat fragmentation on the scorpion assemblage of a Brazilian Atlantic Forest. J Insect Conserv 20:457–466
Lira AFA, DeSouza AM, Albuquerque CMR (2018) Environmental variation and seasonal changes as determinants of the spatial distribution of scorpions (Arachnida: Scorpiones) in Neotropical forests. Can J Zool 96:963–972
Lira AFA, Salomão RP, Albuquerque CMR (2019a) Pattern of scorpion diversity across a bioclimatic dry-wet gradient in Neotropical forests. Acta Oecol 96:10–17
Lira AFA, Pordeus LM, Salomão RP, Badillo-Montaño R, Albuquerque CMR (2019b) Effects of anthropogenic land-use on scorpions (Arachnida: Scorpiones) in Neotropical forests. Int J Trop Insect Sci 39:211–218
Lira AFA, Badillo-Montaño R, Lira-Noriega A, Albuquerque CMR (2020) Potential distribution patterns of scorpion in north-eastern Brazil under scenarios of future climate change. Austral Ecol 45:215–228
Lira AFA, Foerster SÍA, Salomão RP, Porto TJ, Albuquerque CMR, Moura GJB (2021a) Understanding the effects of human disturbance on scorpion diversity in Brazilian tropical forests. J Insect Conserv 25:147–158
Lira AFA, Foerster SÍA, Albuquerque CMR, Moura GJB (2021b) Contrasting patterns at interspecific and intraspecific levels in scorpion body size across a climatic gradient from rainforest to dryland vegetation. Zoology 146:125908
Liu H, Yu R, Huang J, Liu Y, Zang R, Guo Z, Ding Y, Lu X, Li Q, Chen HYH (2020) Latitudinal diversity gradients and Rapoport effects in Chinese endemic woody seed plants. Forests 11:1–9
Lomolino MV, Riddle BR, Whittaker RJ (2016) Biogeography: biological diversity across space and time. Oxford University Press, Oxford
Lourenço WR (1980) Contribution à la connaissance systématique des Scorpions appartenant au “complexe” Tityus trivittatus Kraepelin, 1898 (Buthidae). Bulletin Du Muséum National d’Histoire Naturelle Paris 4:793–843
Lourenço WR (1994) Diversity and endemism in tropical versus temperate scorpion communities. Biogeographica 70:155–160
Lourenço WR (2002) Scorpion of Brazil. Les Éditions de I’If, Paris
Lourenço WR (2003) Description of a new species of Tityus (Scorpiones, Buthidae) from Serra do Cipo in the State of Minas Gerais, Brazil. Rev Suisse Zool 110:427–435
Lourenço WR (2005) Description of a new species of Tityus (Scorpiones, Buthidae) from the Parque Estadual de Vila Velha in the state of Paraná (Brazil). Acta Biológica Paranaense 34:15–26
Lourenço WR (2006) Nouvelle proposition de découpage sous-générique du genre Tityus C.L. Koch, 1836 (Scorpiones, Buthidae). Boletín SEA 39:55–67
Lourenço WR (2015) Comments on the Ananterinae Pocock, 1900 (Scorpiones: Buthidae) and description of a new remarkable species of Ananteris from Peru. C R Biol 338:134–139
Lourenço WR, Giupponi APL (2004) Description of a new species of “Tityus” Koch, 1836 (Scorpiones, Buthidae) from the States of Espirito Santo and Rio de Janeiro in Brazil. Revista Ibérica de Aracnología 10:237–243
Makowski D, Ben-Shachar MSC, Patil I, Ludecke D (2020) Methods and algorithms for correlation analysis in R. J Open Source Softw 5:1–4
MapBiomas Project (2021) Collection of Brazilian land cover & use map series (http://mapbiomas.org/). Accessed on 18 Sept 2021
Marini MA, Garcia FI (2005) Conservação de aves no Brasil. Megadiversidade 1:95–102
Martins FM (2011) Historical biogeography of the Brazilian Atlantic Forest and the Carnaval–Moritz model of Pleistocene refugia: what do phylogeographical studies tell us? Biol J Linn Soc 104:499–509
Martins MA, De Carvalho WD, Dias D, França DDS, De Oliveira MB, Peracchi AL (2015) Bat species richness (Mammalia, Chiroptera) along an elevational gradient in the Atlantic forest of Southeastern Brazil. Acta Chiropterol 17:401–409
Martins JG, Santos GC, Procópio REL, Arantes EC, Bordon KC (2021) Scorpion species of medical importance in the Brazilian Amazon: a review to identify knowledge gaps. J Venom Anim Toxins Incl Trop Dis 27:1–32
Marques MCM, Swaine MD, Liebsch D (2011) Diversity distribution and floristic differentiation of the coastal lowland vegetation: implications for the conservation of the Brazilian Atlantic Forest Biodivers Conserv 20:153–168
McDonald JH (2014) Handbook of biological statistics, 3rd edn. Sparky House Publishing, Baltimore
Meiri S (2011) Bergmann’s rule – What’s in a name? Glob Ecol Biogeogr 20:203–207
Menezes AA, Cáceres MES, Bastos CJP, Lucking R (2018) The latitudinal diversity gradient of epiphytic lichens in the Brazilian Atlantic Forest: does Rapoport’s rule apply? Bryologist 121:480–497
Mittelbach GG, Schemske DW, Cornell HV, Allen AP, Brown JM, Bush MB, Harrison SP, Hurlbert AH, Knowlton N, Lessios HA, McCain CM, McCune AR, McDade LA, McPeek MA, Near TJ, Price TD, Ricklefs RE, Roy K, Sax DF, Schluter D, Sobel JM, Turelli M (2007) Evolution and the latitudinal diversity gradient: speciation, extinction and biogeography. Ecol Lett 10:315–331
Mousseau TA (1997) Ectotherms follow the converse to Bergmann’s rule. Evolution 51:630–632
Murphy K, Carvalho P, Efremov A, Grimaldo JT, Molina-Navarro E, Davidson TA, Thomaz SM (2020) Latitudinal variation in global range-size of aquatic macrophyte species shows evidence ford a Rapoport effect. Freshw Biol 65:1622–1640
Nime MF, Casanoves F, Mattoni CI (2014) Scorpion diversity in two different habitats in the Arid Chaco, Argentina. J Insect Conserv 18:373–384
Nogueira AA, Bragagnolo C, DaSilva MB, Martins TK, Lorenzo EP, Perbiche-Neves G, Pinto-da-Rocha R (2019) Historical signatures in the alpha and beta diversity patterns of Atlantic Forest harvestman communities (Arachnida: Opiliones). Can J Zool 97:631–643
Núñez-Flores M, Solórzano A, Hernández CE, López-González PJ (2019) A latitudinal diversity gradiente of shallow-water gorgonians (Cnidaria: Octocorallia: Alcyonacea) along the Tropical Eastern Pacific Ocean: testing for underlying mechanisms. Mar Biodivers 49:2787–2800
Oliveira-Filho A, Fontes MAL (2000) Patterns of floristic differentiation among Atlantic forests in southeastern Brazil, and the influence of climate. Biotropica 32:793–810
Oliveira-Filho AT, Budke JC, Jarenkow JA, Eisenlohr PV, Neves DRM (2015) Delving into the variations in tree species composition and richness across South American subtropical Atlantic and Pampean forests. J Plant Ecol 8:242–260
Olivero PA, Mattoni CI, Peretti AV (2012) Morphometry and geographical variation of Bothriurus bonariensis (Scorpiones: Bothriuridae). J Arachnol 40:113–122
Outeda-Jorge S, Mello T, Pinto-da-Rocha R (2009) Litter size, effects of maternal body size, and date of birth in South American scorpions (Arachnida: Scorpiones). Zoologia 26:43–53
Papacostas KJ, Freestone AL (2016) Latitudinal gradient in niche breadth of brachyuran crabs. Glob Ecol Biogeogr 25:207–217
Pebesma E (2018) Simple features for R: standardized support for spatial vector data. R J 10:439–446
Peters RH, Wassenberg K (1983) The effect of body size on animal abundance. Oecologia 60:89–96
Peterson AT (2011) Ecological niche conservatism: a time-structured review of evidence. J Biogeogr 38:817–827
Pie MR, Divieso R, Caron FS, Siqueira AC, Barneche DR, Luiz OJ (2021) The evolution of latitudinal ranges in reef-associated fishes: heritability limits and inverse Rapoport’s rule. J Biogeogr 48:2121–2132
Pintor AFV, Schwarzkpof L, Krockenberger AK (2015) Rapoport’s rule: do climatic variability gradients shape range extent? Ecol Monogr 85:643–659
Pocock RI (1893) Notes on the classification of Scorpions, followed by some observations upon synonymy, with descriptions of new genera and species. Ann Mag Nat Hist 12:303–330
Polis GA (1990) The biology of scorpions. Stanford University Press, Stanford
Polis GA (1993) Scorpions as model vehicles to advance theories of population and community ecology: the role of scorpions in desert communities. Mem Queensl Mus 33:401–410
Polis GA, McReynolds CN, Ford RG (1985) Home range geometry of the desert scorpion Paruroctonus mesaensis. Oecologia 67:273–277
Pontarp M, Bunnefeld L, Cabral JS, Etienne RS, Fritz SA, Gillespie R, Graham CH, Hagen O, Hartig F, Huang S, Jansson R, Maliet O, Munkemuller T, Pellissier L, Rangel TF, Storch D, Wiegand T, Hurlbert A (2019) The latitudinal diversity gradient: novel understanding through mechanistic eco-evolutionary models. Trends Ecol Evol 34:211–223
Por FD (1992) Sooretama - the Atlantic rain forest of Brazil. SPB Academic Publishing, The Hague, p 130
Porto TJ, Carvalho LS, De Souza CAR, Oliveira U, Brescovit AD (2014) Escorpiões da Caatinga: conhecimento atual e desafios. In: Bravo F, Calor AR (eds) Artrópodes do semiárido: biodiversidade e conservação. Printmídia, Feira de Santana
Porto TJ, Pinto-da-Rocha R, Rocha PLB (2018) Regional distribution patterns can predict the local habitat specialization of arachnids in heterogeneous landscapes of the Atlantic Forest. Divers Distrib 24:375–386
Prata BEM, Teixeira AP, Joly CA, Assis MA (2018) The role of climate on floristic composition in a latitudinal gradient in the Brazilian Atlantic Forest. Plant Ecol Evol 151:303–313
Prendini L (2001) Substratum specialization and speciation in southern African scorpion: the effect hypothesis revisited. In: Fet V, Selden PA (eds) Memoriam Gary A. Polis. British Arachnological Society, Burnham Beeches
Prendini L (2005) Scorpion diversity and distribution in southern Africa: pattern and process. In: Hubner BA, Sinclair B, Lampe KH (eds) African biodiversity: molecules, organisms, ecosystems. Springer, New York
Purvis A, Gittleman JL, Cowlishaw G, Mace GM (2000) Predicting extinction risk in declining species. Proc R Soc B Biol Sci 267:1947–1952
QGIS Development Team (2021) QGIS geographic information system. Open source geospatial foundation project. http://qgis.osgeo.org
Quintela FM, Iob G, Artioli LGS (2014) Diet of Procyon cancrivorus (Carnivora, Procyonidae) in restinga and estuarine environments of southern Brazil. Iheringia 104:143–149
R Core Team (2021) R: a language and environment for statistical computing. Foundation for Statistical Commuting, Vienna, Austria
Rabosky DL (2009) Ecological limits and diversification rate: alternative paradigms to explain the variation in species richness among clades and regions. Ecol Lett 12:735–743
Rapoport A (1975) Toward a redefinition of density. Environ Behav 7:133–158
Ravelo AC, Andreasen DH, Lyle M, Lyle AO, Wara MW (2004) Regional climate shifts caused by gradual global cooling in the Pliocene epoch. Nature 429:263–267
Raz S, Retzkin S, Pavlicek T, Hoffman A, Kimchi H, Zehavi D, Beiles A, Nevo E (2009) Scorpion biodiversity and interslope divergeat evolution canyon, lower Nahal Oren microsite, Mt Carmel, Israel. PLoS One 4:e5214
Rein JO (2021) The scorpion files. Accessed at: https://www.ntnu.no/ub/scorpion-files/
Rezende CL, Scarano FR, Assad ED, Joly CA, Metzger JP, Strassburg BBN, Mittermeier RA (2018) From hotspot to hotspot: an opportunity for the Brazilian Atlantic Forest. Perspect Ecol Conserv 16:208–214
Ribeiro MC, Metzger JP, Martensen AC, Ponzoni FJ, Hirota MM (2009) The Brazilian Atlantic Forest: how much is left, and how is the remaining forest distributed? Implications for conservation. Biol Conserv 142:1141–1153
Rohde K (1996) Rapoport’s rule is a local phenomenon and cannot explain latitudinal gradients in species diversity. Biodivers Lett 3:10–13
Roll U, Feldman A, Novosolov M, Allison A, Bauer AM, Bernard R, Bohm M, Castro-Herrera F, Chirio L, Collen B, Colli GR, Dabool L, Das I, Doan TM, Grismer LL, Hoogmoed M, Itescu Y, Kraus F, LeBreton M, Lewin A, Martins M, Maza E, Meirte D, Nagy ZT, Nogueira CC, Pauwels OSG, Pincheira-Donoso D, Powney GD, Sindaco R, Tallowin OJS, Torres-Carvajal O, Trape J-F, Vidan E, Uetz P, Wagner P, Wang Y, Orme CDL, Grenyer R, Meiri S (2017) The global distribution of tetrapods reveals a need for targeted reptile conservation. Nat Ecol Evol 1:1677–1682
Ruggiero A, Werenkraut V (2007) One-dimensional analyses of Rapoport’s rule reviewed through meta-analysis. Glob Ecol Biogeogr 16:401–414
San Martín PR (1966) Escorpiofauna Brasileña III (Bothriuridae). Una nueva forma de “Bothriurus” del Brasil. Rev Bras Biol 26:181–190
San Martín PR (1967) Escorpiofauna Brasileña. II. Bothriuridae Redescripcin de Bothriurus moojeni Mello-Leitão, 1945. Physis 74:135–141
San Martín PR (1968) Bothriurus vachoni, n. sp. del Brasil (Scorpionida, Bothriuridae). Acta Biologica Venezuelica 6:38–51
Santos RL, Almeida EA, Almeida MG, Coelho MS (2006) Biogeography of the Bromeliad-dwelling scorpion Tityus neglectus Mello-Leitão (Buthidae) in Rio Grande do Norte, Brazil. J Bromel Soc 56:201–207
Scriven JJ, Whitehorn PR, Goulson D, Tinsley MC (2016) Bergmann’s body size rule operates in facultatively endothermic insects: evidence from a complex of cryptic bumblebee species. PLoS One 11:e0163307
Sexton JP, Montiel J, Shay JE, Stephens MR, Slatyer RA (2017) Evolution of ecological niche breadth. Annu Rev Ecol Evol Syst 48:183–206
Shelomi M (2012) Where are we now? Bergmann’s rule sensu lato in insects. Am Nat 180:511–519
Shimabukuro EM, Trivinho-Strixino S (2021) Elevational boundaries influence richness patterns at large spatial scales evinced by madicolous insects of the Brazilian Atlantic Forest. Ecol Entomol 46:1036–1046
Silva RR, Brandão CRF (2014) Ecosystem-wide morphological structure of leaf-litter ant communities along a tropical latitudinal gradient. PLoS One 9:e93049
Silva JMC, Casteleti CHM (2003) Status of the biodiversity of the Atlantic forest of Brazil. In: Galindo-Leal C, Câmara IG (eds) The Atlantic forest of South America: biodiversity status, threats, and outlook. CABS and Island Press, Washington, pp 43–59
Silva FR, Almeida-Neto M, Arena MVN (2014) Amphibian beta diversity in the Brazilian Atlantic forest: contrasting the roles of historical events and contemporary conditions at different spatial scales. PLos One 9:e109642
Silveira FAO, Barbosa M, Beiroz W, Callisto M, Macedo DR, Morellato LPC, Neves FS, Nunes YRF, Solar RR, Fernandes GW (2019) Tropical mountains as natural laboratories to study global changes: a long-term ecological research project in a megadiverse biodiversity hotspot. Perspect Plant Ecol Evol Syst 38:64–73
Smith GT (1995) Species richness, habitat and conservation of scorpions in the Western Australian wheatbelt. Rec West Aust Mus 52:55–66
Smith FA, Lyons SK (2013) Introduction. on being the right size: the importance of size in life history, ecology, and evolution. In: Smith FA, Lyons SK (eds) Animal body size. University of Chicago Press, Chicago
Stephens PR, Wiens JJ (2003) Explaining species richness from continents to communities: the time-for-speciation effect in emydid turtles. Am Nat 161:112–128
Stevens RD (2013) Gradients of bat diversity in Atlantic forest of South America: environmental seasonality, sampling effort and spatial autocorrelation. Biotropica 45:764–770
Stevens RD, Tello JS, Gavilanez SS (2013) Stronger tests of mechanisms underlying geographic gradients of biodiversity: insights from the dimensionality of biodiversity. PLoS One 8:e56853
Sulakhe S, Dandekar N, Padhye A, Bastawade D (2020) Two new cryptic species of Isometrus (Scorpiones: Buthidae) from the northern Western Ghats, India. Euscorpius 305:1–24
Sunday JM, Bates AE, Dulvy NK (2011) Global analysis of thermal tolerance and latitude in ectotherms. Proc Royal Soc B 278(1713): 1823–1830. https://doi.org/10.1098/rspb.2010.1295
Tabarelli M, Aguiar AV, Ribeiro MC, Metzger JP, Peres CA (2010) Prospects for biodiversity conservation in the Atlantic forest: lessons from aging human-modified landscapes. Biol Conserv 143:2328–2340
Tews J, Brose U, Grimm V, Tielbörger K, Wichmann MC, Schwager M, Jeltsch F (2004) Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. J Biogeogr 31:79–92
Timms LL, Schwarzfeld M, Sääksjärvi IE (2016) Extending understanding of latitudinal patterns in parasitoid wasp diversity. Insect Conserv Divers 9:74–86
Tseng M, Kaur KM, Pari SS, Sarai K, Chan D, Yao CH, Porto P, Toor A, Toor HJ, Fograscher K (2018) Decreases in beetle body size linked to climate change and warming temperatures. J Anim Ecol 87:647–659
Vasconcelos TS, Prado VH, da Silva FR, Haddad CF (2014) Biogeographic distribution patterns and their correlates in the diverse frog fauna of the Atlantic forest hotspot. PLoS One 9:e104130
Visser V, Clayton WD, Simpson DA, Freckleton RP, Osborne CP (2014) Mechanisms driving an unusual latitudinal diversity gradient for grasses. Glob Ecol Biogeogr 23:61–75
Wallace AR (1878) Tropical nature, and other essays. Macmillan and Company, London
Warburg MR, Ben-Horin A (1981) The response to temperature gradients of scorpions from mesic and xeric habitats. Comp Biochem Physiol 68:277–279
Weir JT, Schluter D (2007) The latitudinal gradient in recent speciation and extinction rates of birds and mammals. Science 315:1574–1576
Weiser MD, Enquist B, Boyle B, Killenn T, Jøgensen P, Fonseca G, Jennings MD, Kerkhoff AJ, Lacher TE Jr, Monteagudo A, Vargas MPN, Phillips OL, Swenson NG, Vasquez Martınez R (2007) Latitudinal patterns of range size and species richness of New World woody plants. Glob Ecol Biogeogr 16:679–688
Weiser MD, Buzzard V, Deng Y, He Z, Michaletz S, Shen L, Enquist BJ, Waide RB, Zhou J, Kaspari M (2017) Toward a theory for diversity gradients: the abundance-adaptation hypothesis. Ecography 41:255–264
Weiser WD, Swenson NG, Enquist BJ, Michaletz ST, Waide RB, Zhou J, Kaspari M (2018) Taxonomic decomposition of the latitudinal gradient in species diversity of North American floras. J Biogeogr 45:418–428
Whittaker RJ, Araújo MB, Jepson P, Ladle RJ, Watson JE, Willis KJ (2005) Conservation biogeography: assessment and prospect. Divers Distrib 11:3–23
Wickham H, François R, Henry L, Müller K (2021) Dplyr: a grammar of data manipulation. R package version 1.0.7. (1.0.7) [Computer software]. https://CRAN.R-project.org/package=dplyr
Wiens JJ, Ackerly DD, Allen AP, Anacker BL, Buckley LB, Cornell HV, Damschen EI, Davies TJ, Grytnes J-A, Harrison SP, Hawkins BA, Holt RD, McCain CM, Stephens PR (2010) Niche conservatism as an emerging principle in ecology and conservation biology. Ecol Lett 13:1310–1324
Willig MR, Presley SJ (2018) Latitudinal gradients of biodiversity: theory and empirical patterns. Encycl Anthr 3:13–19
Willig MR, Kaufman DM, Stevens RD (2003) Latitudinal gradients of biodiversity: pattern, process, scale and synthesis. Annu Rev Ecol Evol Syst 34:273–309
Yamaguti HY, Pinto-da-Rocha R (2003) Taxonomic review of the genus Thestylus Simon, 1880 (Scorpiones: Bothriuridae). Revista Ibérica de Aracnología 7:157–171
Acknowledgments
We thank Dr. Randall W. Myster for inviting us to participate in the book through this chapter. We also thank Andria de Paula, Adriano DeSouza, Adriano Kury, Carlos Toscano-Gadea and Leonardo Carvalho for providing scientific literature. We would also like to thank the Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE) for the postdoctoral scholarship (BFP -0121-2.05/20) to André F.A. Lira. Finally, we thanks the Estonian Research Council for providing financial support (PRG741) for Stênio Í.A. Foerster.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Appendix 7.1
Appendix 7.1
Scorpion species listed to the Brazilian Atlantic Forest. The occupancy refers to the number of 0.5° × 0.5° grid cells in which the species were present. “Rapoport” column states if the species were used to test the Rapoport’s Effect in the Brazilian Atlantic Forest; all species listed below were used to test the adequacy of Bergmann’s rule to the scorpion assemblages in the Brazilian Atlantic Forest. Bergmann’s reference states the source of the carapace length was obtained.
Species | Records | Occupancy | Rapoport | Bergmann’s reference |
|---|---|---|---|---|
Bothriuridae | ||||
Bothriurus asper Pocock, 1893 | 147 | 18 | yes | |
Bothriurus bonariensis (C.L. Koch 1842) | 246 | 10 | yes | Olivero et al. (2012) |
Bothriurus moojeni Mello-Leitao, 1945 | 1 | 1 | No | San Martin (1967) |
Bothriurus sooretamensis San Martín, 1966 | 1 | 1 | No | San Martin (1966) |
Bothriurus vachoni San Martín, 1968 | 1 | 1 | No | San Martin (1968) |
Thestylus aurantiurus Yamaguti & Pinto-da-Rocha, 2003 | 8 | 7 | yes | Yamaguti and Pinto-da-Rocha (2003) |
Thestylus glasioui Bertkau, 1880 | 16 | 11 | yes | Yamaguti and Pinto-da-Rocha (2003) |
Urophonius iheringi Pocock, 1893 | 1 | 1 | No | Pocock (1893) |
Buthidae | ||||
Ananteris balzanii Thorell, 1891 | 16 | 5 | yes | Outeda-Jorge et al. (2009) |
Ananteris bernabei Giupponi, Vasconcelos & Lourenço, 2009 | 1 | 1 | No | Giupponi et al. (2009) |
Ananteris kuryi Giupponi, Vasconcelos & Lourenço, 2009 | 1 | 1 | No | Giupponi et al. (2009) |
Ananteris mauryi Lourenço, 1982 | 39 | 5 | Yes | |
Isometrus maculatus (DeGeer, 1778) | 26 | 7 | Yes | Sulakhe et al. (2020) |
Tityus aba Candido, Lucas, de Souza, Diaz & Lira-da-Silva, 2005 | 8 | 3 | Yes | Candido et al. (2005) |
Tityus adrianoi Lourenço, 2003 | 6 | 2 | yes | Lourenço (2003) |
Tityus bahiensis (Perty, 1833) | 46 | 16 | yes | Outeda-Jorge et al. (2009) |
Tityus brazilae Lourenço & Eickstedt, 1984 | 222 | 19 | yes | |
Tityus costatus (Karsch, 1879) | 25 | 12 | yes | Outeda-Jorge et al. (2009) |
Tityus fasciolatus Pessôa, 1935 | 2 | 1 | no | Lourenço (1980) |
Tityus kuryi Lourenço, 1997 | 1 | 1 | no | Outeda-Jorge et al. (2009) |
Tityus mattogrossensis Borelli, 1901 | 47 | 8 | yes | Outeda-Jorge et al. (2009) |
Tityus neglectus Mello-Leitao, 1932 | 24 | 10 | yes | |
Tityus pintodarochai Lourenço, 2005 | 1 | 1 | no | Lourenço (2005) |
Tityus potameis Lourenço & Leao Giupponi, 2004 | 7 | 7 | yes | Lourenço and Giupponi (2004) |
Tityus pusillus Pocock, 1893 | 12 | 6 | yes | |
Tityus serrulatus Lutz & Mello, 1922 | 1063 | 58 | yes | Outeda-Jorge et al. (2009) |
Tityus stigmurus (Thorell, 1876) | 460 | 24 | yes | |
Zabius gaucho Acosta, Candido, Buckup & Brescovit, 2008 | 1 | 1 | no | Acosta et al. (2008) |
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lira, A.F.A., Andrade, A.R.S., Foerster, S.I.A. (2023). Latitudinal Trends in Scorpion Assemblages of Brazilian Atlantic Forest: Do the Rapoport’s and Bergmann’s Rules Apply?. In: Myster, R.W. (eds) Neotropical Gradients and Their Analysis. Springer, Cham. https://doi.org/10.1007/978-3-031-22848-3_7
Download citation
DOI: https://doi.org/10.1007/978-3-031-22848-3_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-22847-6
Online ISBN: 978-3-031-22848-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)