Abstract
The effect of chemisorption of molybdenum atom (Mo) on single walled (8, 0) zigzag carbon nanotube (CNT) is studied with band structure, density of state, charge transfer, charge isosurface and HOMO–LUMO molecular orbitals. The binding energy of chemisorption of molybdenum atom on carbon nanotube is found using band structure and density of state. The molybdenum is strongly chemisorbed on carbon nanotube with binding energy range from 0.196 to 0.906 eV. The band structure and density of states clearly illustrates the creation of extra states and reduction in the band gap. The amount of charge transfer is found using mulliken population analysis and it is in the range from 0.508 to 0.603 electron depending upon site of chemisorption. The nature of bonding between molybdenum atom and carbon atom of carbon nanotube is explained with molecular orbitals and the charge density analysis. It shows the formation of sigma bond between Mo and carbon atoms.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The ongoing research to miniaturize electronic devices is an effort to make our life simpler which has posed the material science researchers with newer challenges every day. To solve these problems of integration of nanoscale components on the IC, researchers have resorted to carbon nanotubes (CNTs) [1, 2]. As these carbon nanotubes have been one of the major materials under consideration in the field of nano-electronics, which has attracted lot of attention due to their excellent and unique electrical [3], thermal, physical and mechanical properties. According to their chirality and size of diameter, there exist a vast number of different carbon nanotubes with different electronic properties [4].
Carbon nanotubes studies do not end with the pristine models; much research work carried out which shows that carbon nanotubes exhibit much better properties when interacted with metal atoms [6, 7]. Semiconducting carbon nanotubes with small band gaps can be applied in CNT-based transistors, while metallic semiconductors are useful in interconnect systems [5]. A number of potential applications include catalytic sensors, fabrication of nanostructures, nano-electronics [8], nano-electro mechanical systems (NEMS) and spintronics [6] have been proposed. CNT–metal interactions are essential in the formation of nanowires and by continuously coating the sidewalls of these carbon nanotubes with metals atoms, metallic or superconducting nanowires could be obtained [9, 10].
The depth knowledge and understanding of how CNT–metal interactions alter the electronic properties of pristine carbon nanotubes can prove beneficial to the scientific community and the electronics industry [11, 12]. For example, interaction of carbon nanotubes with transition metals possessing different electronic properties could be engineered to have similar electronic properties [5] and such knowledge would be useful in the fabrication of various devices. Therefore, the aim of this study is to investigate the geometrical, electronic and adsorption properties of molybdenum atom when interacted with single walled carbon nanotube zigzag (8, 0) using density functional theory (DFT).
2 Computational Details
All calculations were performed based pseudopotential density functional theory. The generalized gradient approximation (GGA) [13,14,15] with Perdew–Burke and Ernzerhof (PBE) function [16] is adopted as the exchange–correlation interaction. GGA function trends to improve total energies, atomization energies and energy barriers. In our calculations, the ultrasoft pseudopotential by a DNP basis set with 3.5 Å and Brillouin zone 1 × 1 × 1 k points were used. All the calculations were performed using boundary conditions with 64 atoms within the supercell. The tetragonal unit cell of 20 × 20 × 8.4 Å dimensions and kept sufficient separation between tubes is used to avoid interaction between the atoms.
The internal co-ordinate for all the atoms in unit cell is fully relaxed without any constraint; the spin unrestricted calculations were performed. Atomic positions are determined with the energy convergence 2.72 × 10−6 eV/atom and maximum force and displacement to 0.0272 eV/Å and 0.005 Å, respectively.
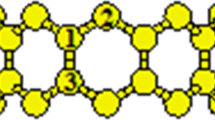
In this study, we have selected (8, 0) zigzag CNT of diameter 6.26 Å and the length of tube is 8.52 Å as a model to study the adsorption of molybdenum atom. We have examined different four sites for adsorption of transition metal (Mo) such as (1) carbon Atom (Site A), (2) carbon–carbon chiral bond (site B), (3) carbon–carbon axial bond (site C) and (4) hexagon (site D) as shown in Fig. 1. In all calculations, the carbon nanotubes along with molybdenum atom were first optimized to occupy their minimize energy state. For each site we kept at a finite distance of 3.0 Å to optimize the system to get stable structure. The binding energy (Eb) of adsorption of transition metals on nanotube for all ground state structures were calculated by
where ET(adsorbent+adsorbate) is the total energy of atom and CNT system, ET(adsorbent) is the total energy of CNT and ET(adsorbate) is the total energy of atom.
To verify the computational accuracy of the structure, we have calculated the binding energy of CNTs, density of state, band gap charge density, HOMO–LUMO energy, HOMO–LUMO gap, Fukui function, Mulliken charge and spin.
3 Results and Discussions
The (8, 0) zigzag SWCNTs presented in Fig. 2 has a carbon atom arrangement with an average C–C bond length of 1.432 Å at chiral bond length and 1.398 Å for axial bond length. One carbon atom were chosen for exo-interaction of atom as shown in Fig. 2.
Molybdenum is a chemical element with symbol Mo and atomic number 42. The electronic configuration of molybdenum is 1s22s22p63s23p63d104s24p64d55s1. Molybdenum does not occur naturally as a free metal on Earth, it is found only in various oxidation states in minerals. The free element, a silvery metal with a gray cast, has the sixth-highest melting point of any element. It readily forms hard, stable carbides in alloys, and for this reason most of world production of the element (about 80%) is used in steel alloys, including high-strength alloys and super alloys.
The metal has found recent application as electrodes for electrically heated glass furnaces and foreheaths. The metal is also used in nuclear energy applications and for missile and aircraft parts. Molybdenum is valuable as a catalyst in the refining of petroleum. It has found applications as a filament material in electronic and electrical applications. The radius of molybdenum atom is 139 pm (0.139 nm) which is greater than atom of a carbon 70 pm. Thus carbon atom is highlighted on the CNT for reference, where we will interact transition metal Mo.
3.1 Geometrical Properties
We have first performed geometry optimization for Mo adatom—SWCNT system and then found most stable configuration to get geometrical properties of the system. We performed first principal calculated for total energy to investigate electronic property and to observe the nature of adsorption of Mo atom on nanotube. The four different interactive sites were tested as mentioned above for the absorption of adatom. For all sites it is observed that Mo adatom get chemisorbed with slight change bond length as in Fig. 3(a–d).
After performing geometry optimization, Mo adatom get attached to carbon atom of nanotube by covalent bond of bond length 2.180 Å at atomic, axial; 2.181–2.202 Å at chiral; and 2.616–2.613 Å at hexagon. From bond length, we have observe that at all four position Mo atom is chemisorbed as shown in Fig. 3 and after calculating bond it making bond at axial position and chiral position but the most suitable site for interaction is axial position.
Due to adsorption of Mo atom the bond lengths between C–C atom of both chiral bond at absorption, B-site is changed from 1.432 to 1.47 Å at atomic and axial position; from 1.458 to 1.519 Å for chiral position; and from 1.473 to 1.429 Å for hexagon position. For axial site the bond length of C–C atom is changed from 1.398 to 1.453 Å for atomic and axial positions; to 1.423 Å for chiral position; and to 1.391 Å for hexagon position. So slight variation in bond lengths at absorption sites were observed for chiral and axial bonds as shown in Fig. 4(a–d) and optimized bond length of CNT is present in Table 1.
The bond angles between C–C–C bonds were ranging from 112.435 to 119.79°, which is less than 120°, indicates trigonal structure of bonding and prefers SP2 hybridization as shown in Table 2.
3.2 Electronic Properties
To investigate the effects of interaction on the electronic properties of nanotubes, we have performed calculations for zigzag (8, 0) nanotube using transition metals. The band structure (BS) and density of state (DOS) of pure (8, 0) SWCNT is shown in Fig. 5. The energy band gap found from BS and DOS for pure CNT is 0.701 eV.
The binding energy for the most stable system is found for −0.906 eV for atomic position; −0.903 eV for axial position; −0.878 eV for chiral position; and −0.193 for hexagon position. The large negative value shows the higher stability of the system so from observation axial and atomic position is most stable system as compared to chiral and hexagon position for molybdenum atom with carbon nanotube. The negative value of binding energy shows the loss of energy of the system.
The DOS analysis show that the Fermi level is shifted towards the conduction band in atomic and axial position, because of this shift of Fermi level, the band gap reduces from 0.7 eV which is for pure CNT to 0.15 eV for atomic and axial positions as shown in Fig. 6. In hexagon and chiral position, extra energy state is created near Fermi level because of that band gap reduced from 0.7 to 0.381 eV for chiral position and to 0.191 eV for hexagon position as shown in Fig. 6. The same situation, we can see in band structure (see Fig. 6). The change in bandgap shows the significant change in conductivity of Mo-CNT system. We can conclude that for the system of molybdenum atom interacting with carbon nanotube, it is the same in all four positions.
Mulliken charge analysis was used to calculate the charge transfer between the CNTs. The metal interaction calculations were performed as shown in Table 3. Metal atoms gains electron from the surrounding carbon atoms, large amounts of electrons are gained when molybdenum atom interacts and atomic spin is present as shown in Table 3.
The analysis shows that molybdenum atom acquires positive charge of 0.591e for atomic and axial position with positive spin of 4.2e. The molybdenum atom in chiral acquires positive charge of 0.603e with positive spin of 4.19e and hexagon position acquires positive charge of 0.508e with no spin on system.
The isosurface charge density is shown in Fig. 7 from which it is clearly visible that the adsorption of molybdenum on CNT has forms sigma bond in all four positions.
3.3 Adsorption Properties
Figure 8 shows highest occupied molecular orbitals (HOMO) and lowest unoccupied molecular orbitals (LUMO) structures for all positions, in which area of molecular orbital which are blue in color shows gain of electron and which are yellow in color shows loss of electron in system. In HOMO system of all four positions, exchange of electrons is seen, but in LUMO system, we can see that exchange of electron is seen.
The frontier molecular orbitals are important in interpreting chemical reactivity. We thus exhibits the highest occupied molecular orbitals and lowest unoccupied molecular orbitals (see Fig. 8). From the figure, we found that the shape of orbital is dxy in all four positions (atomic, axial, chiral and hexagon). We found the HOMO energy (−3.65 eV) and LUMO energy (−3.422 eV) for the selected system. From the energies of HOMO and LUMO, we have calculated the HOMO–LUMO gap around −0.228 eV, a hardness of −0.114 eV, a chemical potential of −3.53 eV and 3.53 eV is electronegativity of system [17, 18]. The difference in energy gap between these two frontier orbitals is used to predict the strength and stability.
We also calculated the Fukui function for Mo-CNT for all four different positions. From Fukai function we can determine the nature of system whether electrophilic or nucleophilic in nature. We found that Mo-atom shows nucleophilic nature for atomic and axial cases; carbon atom is electrophilic in nature (see Fig. 9). Mo-CNT system in chiral and hexagon positions shows that Mo is electrophilic as well as nucleophilic in nature.
4 Conclusion
The effect of doping SWCNTs (8, 0) with molybdenum atom was investigated. Transition metal interaction caused a change in the structure of the nanotube and increased the system stability. After interaction the chosen system gained stability and the conductivity of the Mo-SWCNT system. We have studied the adsorption energies, stable geometries, density of states, band structure and mulliken charge of adatoms adsorbed on SWCNT (8, 0) using first-principles density-functional theory. We found reduction in band gap from 0.7 eV of pure CNT to 0.15 eV for atomic and axial position interaction of Mo. In hexagon and chiral position extra energy state is created near Fermi level because of that band gap reduced from 0.7 to 0.381 eV for chiral and 0.191 eV for hexagon position. The change in bandgap shows the significant change in conductivity of Mo-CNT system. The frontier molecular orbitals are important in interpreting chemical reactivity. We exhibits the highest occupied molecular orbitals (HOMO) and lowest unoccupied molecular orbitals (LUMO). The difference in energy gap between these two frontier orbitals shows good strength and stability of chosen Mo-SWCNT system. From Fukui function we found that Mo-atom shows nucleophilic nature for atomic and axial positions; carbon atom is electrophilic in nature. Mo-CNT system in chiral and hexagon position shows that Mo is electrophilic as well as nucleophilic in nature.
References
Coiffic J et al (2008) An application of carbon nanotubes for integrated circuit interconnects. International Society for Optics and Photonics, Carbon nanotubes and associated devices
Ali A, Parveen H (2006) Carbon nanotube interconnects for IC chips. Institute of Technology Massachusetts
Ebbesen T et al (1996) Electrical conductivity of individual carbon nanotubes. Nature 382(6586):54
Odom TW, Huang JL, Lieber CM (2002) Single-walled carbon nanotubes. Ann N-Y Acad Sci 960(1):203–215
Fuchs F et al (2015) Interaction between carbon nanotubes and metals: electronic properties, stability, and sensing. Micro Electron Eng 137:124–129
Zhuang H, Zheng G, Soh A (2008) Interactions between transition metals and defective carbon nanotubes. Comput Mater Sci 43(4):823–828
Kim H-S et al (2007) Controllable modification of transport properties of single-walled carbon nanotube field effect transistors with in situ Al decoration. Appl Phys Lett 91(15):153113
Wu X, Zeng XC (2006) Adsorption of transition-metal atoms on boron nitride nanotube: a density-functional study. J Chem Phys 125(4):044711
Zhang Y et al (2000) Metal coating on suspended carbon nanotubes and its implication to metal–tube interaction. Chem Phys Lett 331(1):35–41
Bezryadin A, Lau C, Tinkham M (2000) Quantum suppression of superconductivity in ultrathin nanowires. Nature 404(6781):971
Mananghaya MR, Santos GN, Yu D (2017) Nitrogen substitution and vacancy mediated scandium metal adsorption on carbon nanotubes. Adsorption 23(6):789–797. https://doi.org/10.1007/s10450-017-9901-6
Mananghaya M et al (2012) Theoretical investigation on single-wall carbon nanotubes doped with nitrogen, pyridine-like nitrogen defects, and transition metal atoms. J Nanomater 2012:62
Segall MD et al (2002) J Phys: Condens Matter 14:2717
Clark SJ et al (2005) Z Kristallogr 220:567
Engel E, Keller S, Dreizler RM (1996) Phys Rev A 53:1367
Perdew JP, Burke K, Ernzerhof M (1996) Phys Rev Lett 28:3865
Deb J, Paul D, Sarkar U, Ayers PW (2018) Characterizing the sensitivity of bonds to the curvature of carbon nanotubes. J Mol Model 24(9):1–11. https://doi.org/10.1007/s00894-018-3793-6
Tontapha S, Morkot N, Ruangpornvisuti V, Wanno B (2012) Geometries and stabilities of transition metals doped perfect and stone-wales defective armchair (5, 5) boron nitride nanotubes. Struct Chem 23:1819–1830
Acknowledgement
This research was carried out in Ismail Yusuf college laboratory.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Nirmal, S.A., Sonawane, M.R., Atram, R.G. (2023). Chemisorption of Molybdenum Atom on Carbon Nanotube Using Density Functional Theory. In: Parinov, I.A., Chang, SH., Soloviev, A.N. (eds) Physics and Mechanics of New Materials and Their Applications. Springer Proceedings in Materials, vol 20. Springer, Cham. https://doi.org/10.1007/978-3-031-21572-8_1
Download citation
DOI: https://doi.org/10.1007/978-3-031-21572-8_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-21571-1
Online ISBN: 978-3-031-21572-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)