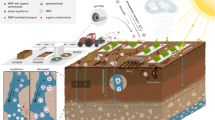
Abstract
Annual releases of plastic to the terrestrial environment are 4–23 times as high as releases to the marine environment. Microplastics can enter the soil in many routes, for example, compost and sewage sludge as fertilizer, plastic mulching, irrigation and flooding, and atmospheric deposition. The process of top-down irrigation into the soil causes MP/NPs to be transported downwards along with soil cavities and eventually possibly into groundwater. Contact of toxic and harmful metal pollutants with M&NPs will inevitably occur during the migration process in the environment. Various factors are considered in their transportation such as microplastic properties, pore water forms, and properties of packing materials to influence microplastic transport that can indicate the environmental chance of microplastics in soil conditions. Among the important roles in the environmental behavior of M&Ms are absorption and migration. Microplastics or nanoplastic particles as a carrier, adsorb contaminants and increase or decrease their transportation. The transfer of microplastics in the soil environment occurs in the form of vertical and horizontal migration and nonliving transport. Microplastics are known to adsorb toxic chemicals such as PCBs, PAHs, DDTs, PFASs, PPCPs, and heavy metals.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Microplastics soil pollution was first studied by Rillig (2012), and subsequent research has focused on this important issue. On World Environment Day 2018, the United Nations Environment Programme (UNEP) called for a more in-depth consideration of the impacts of microplastic pollution on the soil environment (Schnurr et al., 2018). The fact that soil is a more important sink for microplastics than marine environments has been a critical factor influencing UNEP’s decision. Annual releases of plastic to the terrestrial environment are 4–23 times as high as releases to the marine environment (Horton et al., 2017). Plastics are categorized into two groups: primary plastics produced in the size range, such as MPs/NPs in pharmaceutical and personal care products (PPCPs) (Rochman et al., 2015). Secondary plastics are generated by crushing major plastics, such as agricultural plastic mulch or car tires (Huang et al., 2020). Therefore, it is necessary to investigate the physical structure and surface properties of microplastics to facilitate a comprehensive understanding of the factors influencing the environmental fate of microplastics in soil. Inputs from agricultural practices, the influence of runoff and deposits, and degradation or fragmentation of plastic debris are the major sources of entry into the soil. The shape and composition of microplastics are almost identical to their sources. Microplastics can enter the soil in many routes, for example, compost and sewage sludge as fertilizer, plastic mulching, irrigation and flooding, and atmospheric deposition (Bläsing & Amelung, 2018). The degradation by ultraviolet (UV) radiation also may serve as a significant source of soil plastic pollution. Plastics can be degraded through various processes, such as hydrolysis, oxidation, photodegradation, mechanical corrosion, and biological degradation (Alimi et al., 2018). Usage of membranes in modern agriculture led to the discharge of MPs/NPs on the soil. The process of top-down irrigation into the soil causes MP/NPs to be transported downwards along with soil cavities and eventually possibly into groundwater (Zeng et al. 2020a, b). Microplastic accumulation may also occur in the soil, causing a number of adverse effects on the soil ecosystem, including alterations in the chemical, physical, and fertility properties of the soil, leading to disruption of the microbial population living in the soil (Zhang et al., 2017).
1.1 Micro- and Nanoplastic Transportation to Soil
Based on columnar experiments conducted in laboratory environments, the transfer of microplastics and nanoplastics has been investigated. Simulation is one of the main methods to analyze the transport behavior of pollutants in soil and groundwater. Glass beads and quartz sand are used as model porous media to simulate the soil environment. Polystyrene microspheres were used as research objects for the transport study of colloids (Bradford et al., 2002). Generally, to date investigations on the transfer of M&NPs have been conducted mainly on the basis of colloidal transfer, especially similar to that of engineered nanomaterials (Hüffer et al., 2017). Coexisting with other substances affected the stability of microplastics in a porous medium. Also, the interaction between M&NPs and collectors and thus changed transport and deposition in porous media are influenced. Other properties of porous media, solution environment, and the characteristics of M&NPs exhibit an effect on transport behavior. Quartz sands because of their homogeneous texture and negative charge are used as environment porous in transport investigations. Here, too, the simultaneous presence of other materials may occupy sedimentary sites in porous media for M&NPs. For example, the transportation of microplastics under positively charged surfactants was great than those under negatively charged surfactants due to competitive adsorption sites (Pelley & Tufenkji, 2008). Additional deposition sites could be provided by coexisting pollutants. The effect of dissolved black carbon (DBC) on the transport of polystyrene nanoplastics was explored with dissipation monitoring equipment (QCM-D) techniques (Gul et al., 2021). Two main items in the study of transport in porous media are natural and artificial colloids (Si et al., 2019). Another nanomaterial such as graphene oxide (GO) also supplied more sites to nanoplastic, especially at relatively high ionic power in CaCl2 (Xia et al., 2021). In another study by O’Connor et al. (2019), a sand column was used to study the penetration process of polypropylene (PP) and polyethylene (PE) microplastics. Polypropylene (PP) and polyethylene (PE) microplastics with various sizes and densities were used in the experiment, and they found that the microplastics all more moved downwards in the range of 1.5–7.5 cm (O’Connor et al., 2019). Although the simulation experiment helps us to better understand the mechanism of transmission of M&NPs in the soil, the results of laboratory studies are not generalizable to the actual soil environment. Generally, results showed that the existence of microplastics alters the bulk density, water holding capacity, structure, and hydrodynamics of the soil (de Souza Machado et al., 2018, 2019). Conversely, that may change the transfer of M&NPs in soil (Xia et al., 2021). M&NPs affect the soil media and, in turn, affect the movement of M&NPs. Since soil is a complex media with intricate pore systems and rich biological residents, therefore, the results of research in the actual natural environment are different from the laboratory.
1.1.1 Factors Involving the Transportation of M&NPs
Various factors are considered in laboratories such as microplastic properties, pore water forms, and properties of packing materials to influence microplastic transport that can indicate the environmental chance of microplastics in real soil conditions (Alimi et al., 2018). The co-occurrence of contaminants in porous media alters the transmission of M&NPs. Also, particle size and specific surface area characteristics are desirable for M&NPs to affect the fate of other contaminants such as metal and some organic contaminants (Bradford & Bettahar, 2006; Pelley & Tufenkji, 2008).
1.1.2 Micro- and Nanoplastic Movement Model in Soil
The transfer of microplastics in the soil environment occurs in the form of vertical and horizontal migration and nonliving transport. The transportation downwards of microplastics, especially nano-sheets, may pose a potential risk of groundwater contamination. The penetration of microplastics into the soil inevitably affects a series of biological processes that affect their carrier and fate (de Souza Machado et al., 2018).
1.1.3 Microplastic Transportation Through Porous Media
In previous studies, the PS microsphere transfer model (less than 5 mm) as a colloid has been examined due to its inertia and fluorescent traceability (Tong et al., 2005; Peng et al. 2017a, b, c). Generally, microplastics because of their similar properties and behaviors have a similar transmission. Compared to hydrophilic plastics, PS hydrophobic plastics had higher colloidal retention in unsaturated columns of sand (Wan & Wilson, 1994). Increasing the surface negative charge of PS nanoplastics by UV or O3 aging processes can lead to a significant increase in the movability of spherical PS nanoplastics (Zeng et al. 2020a, b). Factors such as flow velocity, water content, ionic strength, and natural organic matter significantly affect the transport of microplastics. This means that the microplastic transportation increases with the high velocity of water pores. The results showed that increasing the flow velocity reduces the shelf life of PS microplastics in quartz sand columns (Wu et al., 2012; Zhang et al., 2015). A significant correlation between substantiated and accelerated deposition and reduced mobility of PS microplastics with increasing ionic strength had been approved by most researchers (Elimelech & O’Melia, 1990; Kobayashi et al., 2009). Sedimented microplastics with lower ionic strength were more prone to re-entrain from glass surfaces into the bulk solutions. (Franchi & O’Melia, 2003). In addition, the wet-dry cycle affected the vertical migration of microplastics in sand columns (O’Connor et al., 2019). Besides mentioned factors, also, natural organic matter (NOM) contributes to the movability of microplastics. The existence of NOM not only led to the resistance of PS particles (Deshiikan et al., 1998) but also the larger the molecular size of NOM, the lower the particle size in absorbent media owing to the increase in spatial share. (Amirbahman & Olson, 1995).
1.1.4 Microplastic Migration in Soil Media
During the tillage functions, plastic fibers and mulching parts were observed in the deeper layers of the soil (>20 cm), which is clear proof of the transportation of microplastic downwards in the soil environment (Huang et al., 2020). Besides, soil fauna is counted as a carrier of microplastics in soil. Following the migration of earthworms (L. terrestris) and providing a downwards path for the vertical transport of microplastics, the microplastics of polyethylene could be transported from layers near the ground to the deeper layer (>50 cm) (Huerta Lwanga et al., 2017). In addition to vertical transfer, digestion and adhesion to the exterior body of the earthworm were also involved in microplastic transfer (Rillig et al., 2017). In horizontal transport, microplastics are moved and distributed by collembolans. Interestingly, F. candida, due to its larger size, plays a larger role in the displacement of large particles than Proisotoma minuta (Maaß et al., 2017). The presence of feeding and nutrition relationships between various species such as collembola (F. candida) and mite (Hypoaspis aculeifer) in soil facilitated the movement of micro- and nanoplastics up to 40% compared with the presence of a homogenous species (Zhu et al., 2018). Totally, biogenic actions can elevate the forwarding of microplastics, particularly vertical transport, which led to a likely risk for groundwater and biotas’ existence in soil.
2 M&NPs as Carriers for Other Soil Pollutants
Understanding the interaction and simultaneous transmission of M&NPs with contaminants has been designed and popularized by many researchers. Because as a carrier, M&NPs are likely to carry certain dangerous pollutants over long distances and pose risks to the ecosystem and human health. During the interaction with micro- and nanoplastics, the transport of symbiotic materials may be facilitated or inhibited. In addition to their roles, microplastics are known to adsorb toxic chemicals such as PCBs, PAHs, DDTs, PFASs, PPCPs, and heavy metals (Peng et al. 2017a, b, c). The properties of plastics such as hydrophobicity of surfaces led to the condensation of PCBs and dichlorodiphenyldichloroethylene in PP pellets up to 105–106 times higher than concentrations in seawater (Mato et al., 2001). Microplastics and nanoplastics can attach to toxic emerging contaminants, such as hormones and pharmaceutical and personal care products, and bioaccumulate and would remain in the body of humans and animals, persistently (Zhou et al., 2022). Microplastics are able to carry bisphenol A and then release it as a source of environmental pollution (Zhou et al., 2022). Microplastics can also act as an effective sink for tetracycline by increasing deposition sites. The results of previous studies exhibited that polyester fibers (0.3% by weight) can influence the configuration of clay loam soils (Zhang et al., 2019). Besides, polyethylene film (1% w/w) can significantly raise the speed of water vaporization in clay (Wan et al., 2019). Polystyrene nanoplastics (PS) in different concentrations lead to modifications in the soil media, for example, polystyrene nanoplastics at 100 and 1000 ng g−1 which seriously reduced the soil microbial communities and raised basal aspiration, respectively (Awet et al., 2018). In extremely high modification rates, PP microplastics can increase soil basal aspiration rate about three times (Yang et al., 2018). PS nanoplastics (100 ng g−1) also reduced the activities of enzymes involved in C, N, and P cycles in soils with silt loam textures (Awet et al., 2018). PP microplastics greatly promoted the activity of fluorescein diacetate hydrolase in sandy loam soils and consequently improved the availability of nutrients for plants by enhancing microbial hydrolytic action on soil organic matter (SOM) (Yang et al., 2018). Plastic fragment remains (67.5 kg ha−1) can lead to considerable decreases in soil microbial biota type and reduce soil microbial C and N, plus decreased the activity of fluorescein diacetate hydrolase and dehydrogenase by 10% and 20%, respectively (Wang et al., 2016). The shape of microplastic (linear versus nonlinear), size, rate of growth, polymer design, and soil texture affect the enzyme activity and microbial biota of the soil. There is a possibility that the negative effects are probably related to concomitant phthalate contamination and not to the existence of plastic layers alone (Wang et al., 2016). Earthworms, one of the most well-known animals in the soil, have a layer of viscous body fluid on their surface that M&NPs may attach to, and the earthworms’ motion causes the spatial transfer of plastics (Rillig et al., 2017). Although their role is still unclear, Rong et al. lately recommended that the bacterial community could slow the transport of plastic particles, as biofilms narrow the pathway and increase the surface roughness, as well as the OH and NH groups at cellular levels. Rate probability of plastic particles forming hydrogen bonds was very high (He et al., 2020). Due to the large specific surface area and powerful hydrophobicity, micro- and nanoplastics can easily absorb contaminants and act as a carrier.
2.1 Adsorption and Migration
Among the important roles in the environmental behavior of M&Ms are absorption and migration. Microplastics or nanoplastic particles as a carrier adsorb contaminants and increase or decrease their transportation. The adsorption action of pollutants into M&NPs occurs with mechanisms such as hydrophobic action, electrostatic action, pore filling, van der Waals forces, hydrogen bond, and π-π interaction (Torres et al., 2021). Hydrophobic activity is the fundamental mechanism in describing the M&NP and pollutants. Polymer type, surface functional groups, and material structure of M&Ms define the relations between microplastics and related substances (Tourinho et al., 2019). Hydrophobic organic materials have a high tendency to adsorb on non-polar microplastics, due to their hydrophobic properties (Tourinho et al., 2019).
Owing to adsorption on the plastic, the transformation of organic contaminants from water to organisms could be increased. For example, by transferring polybrominated diphenyl ethers (PBDEs) on the surface, microplastics would persist in the body of the fish for many years (Wardrop et al., 2016). The type and size of microplastics impact the adsorption capacity. For example, the higher adsorption capacity of PS microplastics than PE and PP on tetracycline is mostly due to their polar properties (Xu et al., 2018). In addition to knowledge about the environmental manners of M&NPs as vectors for other pollutants, also discussion of the mutual effect available cotransport studies of M&NPs and coexisting pollutants is important, while there are rare investigations such as a number of academic reviews on cotransport of M&NPs and coexisting pollutants compared with adsorption studies. More researchers focused on individual transport behavior than cotransport of M&NPs which cannot explain the entire transport behavior completely (Alimi et al., 2018). The results of studies analyzing the interaction of M & NPs with hydrophobic organic matter have shown that they, especially certain persistent organic pollutantshave a great affinity for absorbing to surfaces of plastic particles. For example, microplastics and nanoplastics can absorb PCBs. The adsorption of PCBs to nano-PS was 1–2 times stronger than that to micro-PE due to higher aromaticity and surface-area-to-volume ratio (Velzeboer et al., 2014). Compared to adsorption examinations, investigations of the simultaneous transport of M&NPs with numerous pollutants in porous media is not adequate. According to previous studies, M&NPs as noteworthy carriers can improve or hinder the transfer of biocloids and non-biological colloids. Oppositely, the transport and storage of M&NPs may be altered by symbiotic substances.
3 Adsorption of Various Toxic Chemicals into M&NPs
One of the most common types of microplastics that are examined by researchers is polyethylene, which is probably due to the high consumption in industry and the successive pollution levels of plastics (Andrady & Neal, 2009). The types of organic compounds that are adsorbed by micro- and nanoplastics have various adsorption amounts based on LogKMP values. Organic chemicals which supposed nonpolar and have a LogKow more than two important positive linear correlations were observed between LogKow and LogKMP for PE (p < .001), PP (p < .001), PS (p < .001), and PVC (p < .01) microplastics. This indicates that the hydrophobic property has a great role in the sorption of HOCs (LogKow > 2), i.e., the higher the hydrophobic property, the higher the sorption tendency; this result is consistent with the results of previous studies (Wang et al., 2018). The distribution coefficients (LogKMP) of HOCs (e.g., PAHs and HCHs) on PE, PP, and PS microplastics showed fit linear correlations with their LogKow values, with R2 values of 0.92, 0.94, and 0.84, respectively.
Hydrophobic distribution coefficients are considered a predominant sorption process for nonpolar organic compounds onto microplastics, compared with other processes such as electrostatic interchange and hydrogen bonding (Wang et al., 2015). When microplastic particles are exposed to organic compounds, non-polar organic compounds have stronger sorption due to their higher hydrophobic properties than polar compounds. The important correlation between distribution factor and hydrophobicity of HOCs shows that hydrophobic relations with microplastics are noteworthy (Seidensticker et al., 2018), in addition to the hydrophobicity properties of polar organic compounds, with LogKow < 2 and various acid dissociation constants (pKa), which are probably not the only adsorption regulators. Some types of polar organic compounds, under the influence of pH, can wildly change their adsorption to microplastics. The type of charge of the contaminants adsorbed on the micro- and nanoplastics also affects the adsorption power. Charged species are usually weaker adsorbed by microplastics than charge neutral species (Seidensticker et al., 2018). For example, due to the similar charge of tetracycline and microplastics such as PE, PP, and PS, the adsorption of tetracycline on the surface of microplastic particles is strongly hindered due to the incidence of electrostatic repulsion (Zhu et al., 2018). The adsorption of charged oxytetracycline on microplastics also had this fate (Wang et al., 2018). In contrast, because tylosin has a positive charge in acidic conditions that is opposite to the charge of microplastics, it is more easily absorbed by PS and PVC microplastics than neutral species. The extended role of organic matters is also proved in the sorption of organic compounds to microplastics. Fluorescence measurements confirmed a minor exchange between original microplastics and soluble organic matter (DOM; e.g., humic acid) (Seidensticker et al., 2018), which creates competition in the adsorption of HOCs between micro- and nanoplastics and DOM. For example, in the presence of DOM, the partitioning of phenanthrene and tonalide among microplastics and water was extremely altered (150–1000 mg L−1). In the attendance of DOM that easily affected polar organic compounds, even at little concentrations of DOM (e.g., 20 mg L−1), the adsorption of polar compounds was remarkably slow (Shen et al., 2018). According to Wang et al. (2018) the sorption of oxytetracycline on weathered PS microplastics was improved with the existence of DOM; it’s probably because of the complexation of humic acid with weathered PS (Wang et al., 2018). Also, when the size of origin microplastic is decreased to the nanometer size, these particles can interact with the aromatic structure of DOM among p-p conjugation (Chen et al., 2018).
3.1 Microplastic Properties
One of the effective parameters in the adsorption of organic compounds is the microplastic properties, plastic polymers usually have amorphous and crystalline regions. For example, PE and PP have crystalline and amorphous parts in their structure and are considered semi-crystalline polymers, their amorphous regions being desirable sites for the adsorption of organic chemicals (Endo & Koelmans, 2016). Study results of Guo and Wang (2019) demonstrate that the crystallinity of microplastics has an important impact on the adsorption of pollutants; they noted that the adsorption coefficients of phenanthrene, naphthalene, and lindane to PE declined with raising crystallinity of PE. Other researchers (Lu et al., 2019) report a positive relationship between the adsorption of 17b-estradiol and the crystallinity of microplastics (Velzeboer et al., 2014).
3.1.1 Transition Temperature
The glass transition temperature (Tg) of microplastics that can affect chemical sorption is different between species plastic polymers. Polymers that have Tg amounts more than ambient temperature are known as glassy polymers (e.g., PVC and PS), while Tg amounts lower than ambient temperature are called rubbery polymers (e.g., PE and PP). Following the pore-filling process of organic chemicals into glassy polymers, nonlinear isotherms are observed on glassy polymers, while linear sorption isotherms were usually observed on rubbery polymers (Endo & Koelmans, 2016; Seidensticker et al., 2018).
3.1.2 Size of Microplastics
Stronger adsorption occurs between micro- and nanoplastics and organic compounds (e.g., phenanthrene) as the size decreases (Chen et al., 2018). Nevertheless, for some microplastics, the size of the microplastics and the related surface area has no important role in the adsorption of organic compounds, while the properties of the microplastics (e.g., chemical structure and composition of the microplastic polymers) have a significant role (Hüffer & Hofmann, 2016).
3.1.3 Environmental Conditions
Typically, polymers of microplastic are exposed to abiotic and biological aging processes in environmental situation, which have the potential to change the nature of interactions with chemical contaminants. Result of studies showed the aging process can shift surface physical and chemical properties by raising the existence of oxygen-containing functional groups (e.g., carbonyl), reducing molecular weight, and producing a rough surfaces (Song et al., 2020). In laboratory conditions acceleration of the aging process led to the formation of polar functional groups on the surface of micro- and nanoplastics, which influences the sorption of organic compounds. Results of Huffer’s research, in which PS microplastics were treated with UV and 10% H2O2, showed that sorption coefficients of nonpolar organic compounds on older PS were one-time extent lower than those on origin PS (Hüffer & Hofmann, 2016).
Also, lower adsorption of benzene, toluene, ethylbenzene, and xylene on PS microplastics (MPs) after the aging process has been confirmed by researchers (Müller et al., 2018). On the contrary, after aging by UV, the sorption tendencies of ciprofloxacin to PS and PVC microplastics for hydrophilic organic compounds were increased by 123% and 20%, respectively, which may be the main reason for the formation of oxygen-containing functional groups (e.g., hydroxyl and carbonyl) on the surface of aged microplastics (Zhu et al., 2018). Of course, the confirmation of weathering in laboratory and field conditions requires further research; one of the factors that should be evaluated is the environmentally relevant concentration of organics and the aging process.
4 Co-transport of Microplastics with Colloids
In actual soil media, colloids and manmade products released certainly meet with micro nanoparticles and affect microplastic transportation. For instance, the co-presence of graphene oxide had a significant result on the movement of PS microplastics, and the effect depended on the ionic strength of the solution (Peng et al. 2017a, b, c). This interaction between graphene oxide and microplastics and nanoplastics can influence the transport and deposition of engineered nanoparticles. In one study microplastics raised the transport and lowered the deposition of nTiO2 in quartz sand at pH 7; also nanoplastics had a substantial effect on fullerene (C60) transportation (Dong et al., 2019).
4.1 Transfer of Microplastics Attached to Contaminants
Because of the size exclusion effect which is important in screening larger particles from small pores, the movement of colloids or nanoparticles could be quicker than that of the pore water. PS nanoplastics at low concentrations greatly increase the transfer of non-polar and weakly polar pollutants but have little effect on the transfer of polar pollutants (Liu et al., 2018). And also, the aging process increased the dynamism of PS nanoplastics, thereby greatly enhancing the ability to transport contaminants (e.g., non-polar pyrene and polar 4-nonylphenol) through slow-release kinetics and immutable adsorption of pollutants (Wang et al. 2019a, b, c). The chemical configurations and compounds of plastics definitely influence the sorption of organic pollutants and consequently affect transport by nanoplastics and microplastics (Guo et al., 2012).
4.2 Soil Fauna’s Role in Pollutant Transport by Microplastics
After devouring microplastics by present organisms in the soil media and then transferring them to humans along the food chain, various toxic chemicals are able to enter the human body.
4.3 Organic Pollutants
Results of previous studies displayed that microplastics may transport HOCs to the aquatic amphipod Allorchestes compressa (Chua et al., 2014), while current analyses exhibited that in real soil media, microplastics have a narrow role in the expansion of HOCs in sea organisms such as the deposit-dwelling lugworm (Endo & Koelmans, 2016). In the soil which was rich with organic compounds (e.g., PAHs and PCBs) and despite the ingestion of microplastics by earthworms (E. fetida), a minor impact of microplastics on the bioaccumulation of HOCs in E. fetida with 10% (w/w) microplastics in agrarian soil was observed (Wang et al. 2019a, b, c). This contrast results of studies indicate that microplastics can hardly be the carrier of HOCs to earthworms, or facilitate the bioaccumulation of HOCs in earthworms, so further investigations are needed to clarify the role of microplastics as carriers of HOCs in other soil organisms.
4.4 Inorganic Contaminants
There is no conclusive evidence that microplastics increase or decrease the risk associated with trace elements (e.g., As and Zn) for earthworms. Although zinc is an element with high bioavailability on microplastics (greater desorption) than soil, no detectable effect of zinc-contaminated microplastics on zinc accumulation, fatality, and increase or decrease in weight of earthworms (L. terrestris) faced with earthworms to zinc-contaminated microplastics in arable soil was observed (Hodson et al., 2017). Nevertheless, one study stated that the presence of microplastics reduced As accumulation and prevented As (V) accumulation in earthworms (Metaphire californica), resulting in less toxicity to M. californica (Wang et al. 2019a, b, c).
4.5 Antibiotics
Combination of microplastics in soil with antibiotics can contribute to increased biological resistance. The presence of tetracycline and microplastic significantly disturbs the microbial residents in soil (Ma et al., 2020). On a laboratory scale, disturbance between the microbial communities by microplastics combined with tetracycline has been proved. Moreover, the diversity of antibiotic resistance genes (ARGs) was increased (Ma et al., 2020). As we know ARGs is recognized as one of the most important emerging pollutants which is a severe threat to the ecosystem (Sanderson et al., 2016). Recent research has confirmed that microplastics can act as a vector for ARGs in landfill leachate. As mentioned above antibiotics exhibited intense disorder to ecology and include disrupting the endocrine system and chronic toxicity (Ma et al., 2020).
The interaction between microplastics and antibiotics remains to be explored because antibiotics are among the widely used pharmaceutical and personal care products that are resistant to biodegradation, and their adsorption on microplastics played a significant function during cotransport (Li et al., 2021).
For example, the results of studies showed that the sorption of oxytetracycline on the surface of polyamide (PA) microplastics takes place weaker than sorption on soil. The aging process of PP and PE microplastic is a factor that improved enrichment for ARGs due to changes in MP surface properties and oxygen-containing active groups. Interaction between microplastic and PPCPs is intrinsically related to characteristics of the sorbate and environmental factors such as solution pH. This is because PPCPS are usually hydrophilic. Inverse hydrophobic substances and other mechanisms also control the adsorption of PPCPs on M&NPs including electrostatic interaction, not merely hydrophobicity. In current studies, the sorption of three different nonsteroidal anti-inflammatory drugs (NSAIDs) to microplastics is examined. They found an apparent pH dependency. This mechanism could be defined by changes in the surface charge of drugs and microplastics (Elizalde-Velazquez et al., 2020).
4.6 Heavy Metals
Contact of toxic and harmful metal pollutants with M&NPs will inevitably occur during the migration process in the environment. The adsorbed pollutants on M&NPs undoubtedly make complex situations that interfere with the growth and survival of organisms (Wang et al. 2019a, b, c). For example, metals accumulated on the microplastics may pose a higher risk to aquatic organisms. Mixed contaminants related to heavy metals can enter the food chain and affect the human body indirectly (Dobaradaran et al., 2018). A previous study demonstrated that micro- and nanoplastics acting as vectors then could transport metal into organisms. Higher desorption of Zn is enriched in fragmented plastic bags (particle size was approximately 1.32 ± 0.72 mm and 0.71 ± 0.43 mm) than in soil. The results confirm the carrier role of microplastics and intensify their effect when exposed to metals (Hodson et al., 2017). The surface of the microplastics when exposed to UV forms more holes making it easier to absorb metals. Typically environmental factors such as the aging process showed dominant effects on the sorption capacity of heavy metals on M&NPs. Sorption of heavy metal on microplastic increased with increasing aging treatment time (Mao et al., 2020). Solution chemistry also influences the aging process. Adsorption capacity of cadmium onto the MPs first increased and then decreased when solution pH increased from 2.0 to 9.0, reaching highest at pH 6 (Zhou et al., 2020). The ionic strength of the solution also affects the adsorption behavior strongly (Ren et al., 2021). For example, along with the raising NaCl concentration, lead (II) adsorption to aged nylon microplastics is reduced likely due to competing for adsorption areas and decreased electrostatic potential of microplastic. Seidensticker et al. (2018) reported that the partition coefficient (KMP) of Cr on aged PE microplastics was one degree of extent higher than that on original microplastic particles, which was also comparable with or higher than that of some PPCPs in a similar concentration range (Seidensticker et al., 2018). By other studies by Turner and Holmes (Turner & Holmes, 2015), the improved sorption of heavy metals on aged microplastics has been documented. A recent study also indicated that nanoplastics had a high sorption capacity for Pb (II), with a removal rate of up to 79–97% (Wang et al., 2019a). Generally, microplastics with aged surfaces and smaller sizes have the highest chance, to carry heavy metals. Nevertheless, the role of organic matter in this sorption process needs more elucidation, i.e., it is ambiguous whether sorption to microplastics and organic matter will be synergistic or competitive in nature.
5 Competition Microplastics and Soils in Sorption of Toxic Chemicals
If the amount of LogKow of organic compounds is more than two, there is a powerful linear relevance between LogKow and LogKoc. Doucette et al. (Doucette, 2003) suggested that LogKow is the suitable predictor for the sorption of neutral HOCs onto soils media and deposits but is not suitable for highly polar or ionizable organic chemicals. This might explain the relatively low linear correlation (R2 ¼ 0.425) and the scattered points with LogKow < 2, because many organic compounds investigated are polar and ionizable (e.g., some PPCPs).
5.1 Biodegradable Plastics
In addition to conventional plastics, the application of biodegradable plastics has also increased environmental concerns. With the advent of biodegradable plastics, their entry into the environment, especially the soil system, is inevitable (Liao & Yang, 2020). In various studies, considering the interaction of degradable microplastics with contaminants, the ability of biodegradable plastics to absorb certain organic compounds compared to ordinary plastics has been proven. For example, the role of biodegradable plastics in the absorption of antibiotics and drugs was greater than that of traditional plastics (Fan et al., 2021). However, investigation on the environmental behavior of degradable plastics, particularly its transport behavior in soil and groundwater, is limited and requires further research. The interaction of M&NPs and pollutants in the meningeal environment coincides with contamination. The transfer of auxiliary pollutants generally depends on the characteristics of the M&NPs and external environmental factors. Compared to microplastics, the simultaneous transport of nanoplastics deserves more attention. Because, as mentioned earlier, due to the size of the nano and the large specific surface area of the nanoplastics, especially in porous media, it may be a threat to human safety and, in addition, they are easier to transport and move. To reverse the migration of symbiotic substances. The impact of M&NPs on the transmission of organic pollutants, natural or synthetic colloids, and bacteria still needs to be investigated.
Different adsorption tendencies played a key part. Polar compounds are just absorbed on the surface of polystyrene, whereas nonpolar compounds were entrapped in the inner matrices due to the glassy polymeric structure. A similar study showed the cotransport behavior of polystyrene nanoplastics and naphthalene in different ionic strengths. As a nonpolar organic pollutant, naphthalene quickly contacted the sorption sites. Transport of nonpolar naphthalene was enhanced by nanoplastics since the binding strength of naphthalene to PSNP was stronger than that of porous media (Liu et al., 2018).
5.2 M&NPs and Natural Colloid
As soil pollution intensifies, the impact of natural soil minerals on the fate of pollutants becomes more important and complicates interactions between M&NPs and natural colloids. According to a column experiment, researchers have found interactions between natural colloids and M&NPs. Their results showed that smaller plastic particles (0.02 and 0.2 μm) improved the transfer of goethite and hematite, while larger plastic particles (2 μm) did not. Competitive deposition and spatial repulsion help increase the transfer of iron oxides, while the adsorption of 0.2 μm megapixels on iron oxides had a great function in increasing the transfer of iron oxides. In the case of a 2 μm transfer reduction, this was because iron oxides, due to their attractive interaction, preferred to be deposited on quartz sand rather than plastic particles. Consequence, the interaction between microplastics and iron oxides showed low impact on the transfer of iron oxides. At the same time, the surface properties of M&NPs affect equity. Carboxyl microplastic modification can increase the transport of clay particles due to the microplastic rival adsorption sites, while amino-modified microplastics did not show an important influence on kaolinite transport behavior (Li et al., 2021). However, following the electrostatic attraction, the exchange between positively charged amino microplastics and negatively charged quartz sand was very strong. The mobility of kaolinite was limited by the formation of aggregates with amino microplastics.
5.3 Engineered Nanomaterials
As we know natural or synthesized fabrics are continually utilized to adsorb toxic and harmful substances to reach the objective of treating contaminated soil. Nanomaterials are commonly well-known as adsorbents to coexisting pollutants in the soil. When exposed to microplastics, both of their surface characteristics may change, thus changing their transport and sediment in porous media. Graphene oxide (GO) is widely used as a soil remediation. Studies were tested cotransport of difference-sized microplastic and GO (Peng et al. 2017a, b, c). The results recommended that cotransport of both two components was mostly controlled by GO, due to the adsorption of microplastic on GO. Parallel analysis on cotransport of GO, reduced-GO (RGO), and polystyrene nanoplastic delivered that the presence of nanoplastics declined transport of (R)GO due to further retention sites supplied by plastic particles. The sediment of nanoplastics on the sand surface restricted pore throats and hindered transport of (R) GO (Xia et al., 2021). Examination of metal nanomaterials and microplastics revealed that the surface charge of titanium dioxide (nTiO2) in acidic solution was positive. Though positively charged nTiO2 easily attracted negatively charged microplastics forming aggregates, transport of nTiO2 did not change owing to attractive electrostatic interaction between nTiO2-microplastic cluster and porous media, whereas microplastic enhanced transport of nanomaterial at pH 7. The plastic particles preferred to be adsorbed on sand rather than on nTiO2. This competition on the deposition site of quartz sand led to increased transport of nTiO2. The effect of soluble chemical conditions on joint transport cannot be underestimated. Weathering processes also affect the interaction between microplastics and metal nanomaterials. According to stated researchers, weathered XPs adsorbed CeO2 nanoparticles more easily than pristine ones (Singh et al., 2021).
6 Conclusions
Indeed, co-contamination in the actual environment is not a simple binary system. The transmission route and fate of combined pollutants containing multiple pollutants has always been a concern (Zhang et al., 2017). Zhao et al. investigated the effect of simultaneous transfer of metal composite contaminants and antibiotics with graphene oxide (Yu et al., 2020). Taken together, existing research on transport of M&NPs and coexisting pollutants focus more on such binary compound system as mentioned above, and there are still few studies on the cotransport of M&NPs with a diversity of contaminants. Microorganisms are everywhere in the realistic conditions and can perform self-migrate. Microplastics were potential carriers for colonizing or forming biofilms in marine environments and then may take microorganisms such as bacteria transport to faraway areas (Deshiikan et al., 1998). He et al. found that the low ionic strength of the solution had little effect on the transfer of M&NPs, whereas increased bacterial transfer occurs under conditions of high ionic strength. The adsorption mechanisms of M&NPs under experimental conditions varied with different particle sizes during common transport (He et al., 2018). Research on interaction and cotransport of microorganisms and M&NPs is far from adequate. Because the laboratory environment is a simple environment compared to real soil, more research is needed to better analyze the interaction of M&NPs and microorganisms in the porous medium. Laboratory research on M&NPs verified their great transportability, but field studies or pilot-scale experiments of M&NPs are still necessary to more comprehend the true state of transport in soil and groundwater.
7 Perspective
Generally, the transfer of pollutants by micro-nanoplastics is influenced by factors such as the existence of natural organic environments, heterogeneous porosity, and type variety of microplastics and the impact of coupling environmental factors on the transfer of chemicals and requires further investigation. The interchange of microplastics or nanoplastics with other symbiotic materials must be carefully considered. The properties of M&NPs, including surface properties and density, may change with the coexistence of other materials, resulting in different environmental behaviors. Nonetheless, there is yet a gap between the experimental environment and the actual conditions. Biofilms are always present on the surface of M&NPs in the real environment, whereas this effect has always been mostly forgotten in previous laboratory studies. The physical and chemical properties of the micro- and nanoplastics will change by coating with biofilm (Rummel et al., 2017). The difference in properties such as surface roughness, zeta potential, hydrophobicity, and surface energy will impact adsorption capacity and then affect their interaction with other pollutants in the cotransport system. For example, mass recovery of polystyrene nanoplastic (PSNP) declined from 77.60% to 62.48% because of naphthalene. This mechanism could be explained by the charge shielding effect. Because naphthalene is non-polar, it may trap some negative charges on the PSNP surface and increase neoplastic retention on the sand (Hu et al., 2020). Differences in electrostatic repulsion force and hydrophobicity would well clarify the simultaneous transport behavior (Yu et al., 2020). Also, the existence of NOM (natural organic matter) in the environment has a significant function in the transport of M&NPs (Pelley & Tufenkji, 2008). The adsorption of pollutants and aggregation of microplastics are greatly affected by the existence of NOM (Rong et al., 2021). Although organic matter is everywhere in the background, it should be regarded as an important element in the simultaneous transfer of micro- and nanoplastic with other materials. According to the present examinations on cotransport of M&NPs with contaminants, solution chemical conditions such as ionic strength and ion species were the principal influencing parts. The effect of some coexisting substances on microplastics migration cannot be considered from a single perspective mentioned above. For example, two kinds of bacteria, E. coli (Gram (−)) and B. subtilis (Gram (+)), decreased transport of negatively charged MPs (carboxylate-modified, CMPs), yet raised positively charged microplastic (amine-modified, AMPs). For example, in 5 mM NaCl, 73.1% CMPs, and 0.94% AMPs without bacteria passed through the columns. Meantime, the bulk recovery of CMPs and AMPs with E. coli is 58.6% and 18.8%, and the corresponding percentage with B. subtilis was 38.9% and 30%. Changing surface charge, extra deposition sites, and aggregation all contributed to such results (He et al., 2021).
References
Alimi, O. S., Farner Budarz, J., Hernandez, L. M., & Tufenkji, N. (2018). Microplastics and nanoplastics in aquatic environments: Aggregation, deposition, and enhanced contaminant transport. Environmental Science & Technology, 52(4), 1704–1724.
Amirbahman, A., & Olson, T. M. (1995). Deposition kinetics of humic matter-coated hematite in porous media in the presence of Ca2+. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 99(1), 1–10.
Andrady, A. L., & Neal, M. A. (2009). Applications and societal benefits of plastics. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1526), 1977–1984.
Awet, T., Kohl, Y., Meier, F., Straskraba, S., Grün, A.-L., Ruf, T., Jost, C., Drexel, R., Tunc, E., & Emmerling, C. (2018). Effects of polystyrene nanoparticles on the microbiota and functional diversity of enzymes in soil. Environmental Sciences Europe, 30(1), 1–10.
Bläsing, M., & Amelung, W. (2018). Plastics in soil: Analytical methods and possible sources. Science of the Total Environment, 612, 422–435.
Bradford, S. A., & Bettahar, M. (2006). Concentration dependent transport of colloids in saturated porous media. Journal of Contaminant Hydrology, 82(1–2), 99–117.
Bradford, S. A., Yates, S. R., Bettahar, M., & Simunek, J. (2002). Physical factors affecting the transport and fate of colloids in saturated porous media. Water Resources Research, 38(12), 63-61-63-12.
Chen, W., Ouyang, Z.-Y., Qian, C., & Yu, H.-Q. (2018). Induced structural changes of humic acid by exposure of polystyrene microplastics: A spectroscopic insight. Environmental Pollution, 233, 1–7.
Chua, E. M., Shimeta, J., Nugegoda, D., Morrison, P. D., & Clarke, B. O. (2014). Assimilation of polybrominated diphenyl ethers from microplastics by the marine amphipod, Allorchestes compressa. Environmental Science & Technology, 48(14), 8127–8134.
de Souza Machado, A. A., Lau, C. W., Till, J., Kloas, W., Lehmann, A., Becker, R., & Rillig, M. C. (2018). Impacts of microplastics on the soil biophysical environment. Environmental Science & Technology, 52(17), 9656–9665.
de Souza Machado, A. A., Lau, C. W., Kloas, W., Bergmann, J., Bachelier, J. B., Faltin, E., Becker, R., Görlich, A. S., & Rillig, M. C. (2019). Microplastics can change soil properties and affect plant performance. Environmental Science & Technology, 53(10), 6044–6052.
Deshiikan, S. R., Eschenazi, E., & Papadopoulos, K. D. (1998). Transport of colloids through porous beds in the presence of natural organic matter. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 145(1–3), 93–100.
Dobaradaran, S., Schmidt, T. C., Nabipour, I., Khajeahmadi, N., Tajbakhsh, S., Saeedi, R., Mohammadi, M. J., Keshtkar, M., Khorsand, M., & Ghasemi, F. F. (2018). Characterization of plastic debris and association of metals with microplastics in coastline sediment along the Persian Gulf. Waste Management, 78, 649–658.
Dong, Z., Zhang, W., Qiu, Y., Yang, Z., Wang, J., & Zhang, Y. (2019). Cotransport of nanoplastics (NPs) with fullerene (C60) in saturated sand: Effect of NPs/C60 ratio and seawater salinity. Water Research, 148, 469–478.
Doucette, W. J. (2003). Quantitative structure-activity relationships for predicting soil-sediment sorption coefficients for organic chemicals. Environmental Toxicology and Chemistry: An International Journal, 22(8), 1771–1788.
Elimelech, M., & O’Melia, C. R. (1990). Kinetics of deposition of colloidal particles in porous media. Environmental Science & Technology, 24(10), 1528–1536.
Elizalde-Velazquez, A., Subbiah, S., Anderson, T. A., Green, M. J., Zhao, X., & Cañas-Carrell, J. E. (2020). Sorption of three common nonsteroidal anti-inflammatory drugs (NSAIDs) to microplastics. Science of the Total Environment, 715, 136974.
Endo, S., & Koelmans, A. A. (2016). Sorption of hydrophobic organic compounds to plastics in the marine environment: Equilibrium. Hazardous chemicals associated with plastics in the marine environment (pp. 185–204). Springer.
Fan, X., Zou, Y., Geng, N., Liu, J., Hou, J., Li, D., Yang, C., & Li, Y. (2021). Investigation on the adsorption and desorption behaviors of antibiotics by degradable MPs with or without UV ageing process. Journal of Hazardous Materials, 401, 123363.
Franchi, A., & O’Melia, C. R. (2003). Effects of natural organic matter and solution chemistry on the deposition and reentrainment of colloids in porous media. Environmental Science & Technology, 37(6), 1122–1129.
Gul, C., Mahapatra, P. S., Kang, S., Singh, P. K., Wu, X., He, C., Kumar, R., Rai, M., Xu, Y., & Puppala, S. P. (2021). Black carbon concentration in the central Himalayas: Impact on glacier melt and potential source contribution. Environmental Pollution, 275, 116544.
Guo, X., & Wang, J. (2019). The chemical behaviors of microplastics in marine environment: A review. Marine Pollution Bulletin, 142, 1–14.
Guo, X., Wang, X., Zhou, X., Kong, X., Tao, S., & Xing, B. (2012). Sorption of four hydrophobic organic compounds by three chemically distinct polymers: Role of chemical and physical composition. Environmental Science & Technology, 46(13), 7252–7259.
He, L., Wu, D., Rong, H., Li, M., Tong, M., & Kim, H. (2018). Influence of nano-and microplastic particles on the transport and deposition behaviors of bacteria in quartz sand. Environmental Science & Technology, 52(20), 11555–11563.
He, L., Rong, H., Wu, D., Li, M., Wang, C., & Tong, M. (2020). Influence of biofilm on the transport and deposition behaviors of nano-and micro-plastic particles in quartz sand. Water Research, 178, 115808.
He, L., Rong, H., Li, M., Zhang, M., Liu, S., Yang, M., & Tong, M. (2021). Bacteria have different effects on the transport behaviors of positively and negatively charged microplastics in porous media. Journal of Hazardous Materials, 415, 125550.
Hodson, M. E., Duffus-Hodson, C. A., Clark, A., Prendergast-Miller, M. T., & Thorpe, K. L. (2017). Plastic bag derived-microplastics as a vector for metal exposure in terrestrial invertebrates. Environmental Science & Technology, 51(8), 4714–4721.
Horton, A. A., Walton, A., Spurgeon, D. J., Lahive, E., & Svendsen, C. (2017). Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Science of the Total Environment, 586, 127–141.
Hu, D., Zhang, Y., & Shen, M. (2020). Investigation on microplastic pollution of Dongting Lake and its affiliated rivers. Marine Pollution Bulletin, 160, 111555.
Huang, Y., Liu, Q., Jia, W., Yan, C., & Wang, J. (2020). Agricultural plastic mulching as a source of microplastics in the terrestrial environment. Environmental Pollution, 260, 114096.
Huerta Lwanga, E., Mendoza Vega, J., Ku Quej, V., Chi, J. D. L. A., Sanchez del Cid, L., Chi, C., Escalona Segura, G., Gertsen, H., Salánki, T., & van der Ploeg, M. (2017). Field evidence for transfer of plastic debris along a terrestrial food chain. Scientific Reports, 7(1), 1–7.
Hüffer, T., & Hofmann, T. (2016). Sorption of non-polar organic compounds by micro-sized plastic particles in aqueous solution. Environmental Pollution, 214, 194–201.
Hüffer, T., Praetorius, A., Wagner, S., Von der Kammer, F., & Hofmann, T. (2017). Microplastic exposure assessment in aquatic environments: Learning from similarities and differences to engineered nanoparticles. ACS Publications.
Kobayashi, M., Nanaumi, H., & Muto, Y. (2009). Initial deposition rate of latex particles in the packed bed of zirconia beads. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 347(1–3), 2–7.
Li, J., Guo, K., Cao, Y., Wang, S., Song, Y., & Zhang, H. (2021). Enhance in mobility of oxytetracycline in a sandy loamy soil caused by the presence of microplastics. Environmental Pollution, 269, 116151.
Liao, Y.-L., & Yang, J.-Y. (2020). Microplastic serves as a potential vector for Cr in an in-vitro human digestive model. Science of the Total Environment, 703, 134805.
Liu, M., Lu, S., Song, Y., Lei, L., Hu, J., Lv, W., Zhou, W., Cao, C., Shi, H., & Yang, X. (2018). Microplastic and mesoplastic pollution in farmland soils in suburbs of Shanghai, China. Environmental Pollution, 242, 855–862.
Lu, L., Luo, T., Zhao, Y., Cai, C., Fu, Z., & Jin, Y. (2019). Interaction between microplastics and microorganism as well as gut microbiota: A consideration on environmental animal and human health. Science of the Total Environment, 667, 94–100.
Ma, H., Pu, S., Liu, S., Bai, Y., Mandal, S., & Xing, B. (2020). Microplastics in aquatic environments: Toxicity to trigger ecological consequences. Environmental Pollution, 261, 114089.
Maaß, S., Daphi, D., Lehmann, A., & Rillig, M. C. (2017). Transport of microplastics by two collembolan species. Environmental Pollution, 225, 456–459.
Mao, S., Gu, W., Bai, J., Dong, B., Huang, Q., Zhao, J., Zhuang, X., Zhang, C., Yuan, W., & Wang, J. (2020). Migration of heavy metal in electronic waste plastics during simulated recycling on a laboratory scale. Chemosphere, 245, 125645.
Mato, Y., Isobe, T., Takada, H., Kanehiro, H., Ohtake, C., & Kaminuma, T. (2001). Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environmental Science & Technology, 35(2), 318–324.
Müller, A., Becker, R., Dorgerloh, U., Simon, F.-G., & Braun, U. (2018). The effect of polymer aging on the uptake of fuel aromatics and ethers by microplastics. Environmental Pollution, 240, 639–646.
O’Connor, D., Pan, S., Shen, Z., Song, Y., Jin, Y., Wu, W.-M., & Hou, D. (2019). Microplastics undergo accelerated vertical migration in sand soil due to small size and wet-dry cycles. Environmental Pollution, 249, 527–534.
Pelley, A. J., & Tufenkji, N. (2008). Effect of particle size and natural organic matter on the migration of nano-and microscale latex particles in saturated porous media. Journal of Colloid and Interface Science, 321(1), 74–83.
Peng, G., Zhu, B., Yang, D., Su, L., Shi, H., & Li, D. (2017a). Microplastics in sediments of the Changjiang Estuary, China. Environmental Pollution, 225, 283–290.
Peng, J., Wang, J., & Cai, L. (2017b). Current understanding of microplastics in the environment: Occurrence, fate, risks, and what we should do. Integrated Environmental Assessment and Management, 13(3), 476–482.
Peng, S., Wu, D., Ge, Z., Tong, M., & Kim, H. (2017c). Influence of graphene oxide on the transport and deposition behaviors of colloids in saturated porous media. Environmental Pollution, 225, 141–149.
Ren, Z., Gui, X., Wei, Y., Chen, X., Xu, X., Zhao, L., Qiu, H., & Cao, X. (2021). Chemical and photo-initiated aging enhances transport risk of microplastics in saturated soils: Key factors, mechanisms, and modeling. Water Research, 202, 117407.
Rillig, M. C. (2012). Microplastic in terrestrial ecosystems and the soil? ACS Publications.
Rillig, M. C., Ziersch, L., & Hempel, S. (2017). Microplastic transport in soil by earthworms. Scientific Reports, 7(1), 1–6.
Rochman, C. M., Tahir, A., Williams, S. L., Baxa, D. V., Lam, R., Miller, J. T., Teh, F.-C., Werorilangi, S., & Teh, S. J. (2015). Anthropogenic debris in seafood: Plastic debris and fibers from textiles in fish and bivalves sold for human consumption. Scientific Reports, 5(1), 1–10.
Rong, L., Zhao, L., Zhao, L., Cheng, Z., Yao, Y., Yuan, C., Wang, L., & Sun, H. (2021). LDPE microplastics affect soil microbial communities and nitrogen cycling. Science of the Total Environment, 773, 145640.
Rummel, C. D., Jahnke, A., Gorokhova, E., Kühnel, D., & Schmitt-Jansen, M. (2017). Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environmental Science & Technology Letters, 4(7), 258–267.
Sanderson, H., Fricker, C., Brown, R. S., Majury, A., & Liss, S. N. (2016). Antibiotic resistance genes as an emerging environmental contaminant. Environmental Reviews, 24(2), 205–218.
Schnurr, R. E., Alboiu, V., Chaudhary, M., Corbett, R. A., Quanz, M. E., Sankar, K., Srain, H. S., Thavarajah, V., Xanthos, D., & Walker, T. R. (2018). Reducing marine pollution from single-use plastics (SUPs): A review. Marine Pollution Bulletin, 137, 157–171.
Seidensticker, S., Grathwohl, P., Lamprecht, J., & Zarfl, C. (2018). A combined experimental and modeling study to evaluate pH-dependent sorption of polar and non-polar compounds to polyethylene and polystyrene microplastics. Environmental Sciences Europe, 30(1), 1–12.
Shen, X.-C., Li, D.-C., Sima, X.-F., Cheng, H.-Y., & Jiang, H. (2018). The effects of environmental conditions on the enrichment of antibiotics on microplastics in simulated natural water column. Environmental Research, 166, 377–383.
Si, W., Cai, Y., Liu, J., Shen, J., Chen, Q., Chen, C., & Ning, L. (2019). Investigating the role of colloids on the distribution of bisphenol analogues in surface water from an ecological demonstration area, China. Science of the Total Environment, 673, 699–707.
Singh, N., Khandelwal, N., Tiwari, E., Naskar, N., Lahiri, S., Lützenkirchen, J., & Darbha, G. K. (2021). Interaction of metal oxide nanoparticles with microplastics: Impact of weathering under riverine conditions. Water Research, 189, 116622.
Song, C., Liu, Z., Wang, C., Li, S., & Kitamura, Y. (2020). Different interaction performance between microplastics and microalgae: The bio-elimination potential of Chlorella sp. L38 and Phaeodactylum tricornutum MASCC-0025. Science of the Total Environment, 723, 138146.
Tong, M., Li, X., Brow, C. N., & Johnson, W. P. (2005). Detachment-influenced transport of an adhesion-deficient bacterial strain within water-reactive porous media. Environmental Science & Technology, 39(8), 2500–2508.
Torres, F. G., Dioses-Salinas, D. C., Pizarro-Ortega, C. I., & De-la-Torre, G. E. (2021). Sorption of chemical contaminants on degradable and non-degradable microplastics: Recent progress and research trends. Science of the Total Environment, 757, 143875.
Tourinho, P. S., Kočí, V., Loureiro, S., & van Gestel, C. A. (2019). Partitioning of chemical contaminants to microplastics: Sorption mechanisms, environmental distribution and effects on toxicity and bioaccumulation. Environmental Pollution, 252, 1246–1256.
Turner, A., & Holmes, L. A. (2015). Adsorption of trace metals by microplastic pellets in fresh water. Environmental Chemistry, 12(5), 600–610.
Velzeboer, I., Kwadijk, C., & Koelmans, A. (2014). Strong sorption of PCBs to nanoplastics, microplastics, carbon nanotubes, and fullerenes. Environmental Science & Technology, 48(9), 4869–4876.
Wan, J., & Wilson, J. L. (1994). Colloid transport in unsaturated porous media. Water Resources Research, 30(4), 857–864.
Wan, Y., Wu, C., Xue, Q., & Hui, X. (2019). Effects of plastic contamination on water evaporation and desiccation cracking in soil. Science of the Total Environment, 654, 576–582.
Wang, F., Shih, K. M., & Li, X. Y. (2015). The partition behavior of perfluorooctanesulfonate (PFOS) and perfluorooctanesulfonamide (FOSA) on microplastics. Chemosphere, 119, 841–847.
Wang, J., Lv, S., Zhang, M., Chen, G., Zhu, T., Zhang, S., Teng, Y., Christie, P., & Luo, Y. (2016). Effects of plastic film residues on occurrence of phthalates and microbial activity in soils. Chemosphere, 151, 171–177.
Wang, F., Wong, C. S., Chen, D., Lu, X., Wang, F., & Zeng, E. Y. (2018). Interaction of toxic chemicals with microplastics: A critical review. Water Research, 139, 208–219.
Wang, H.-T., Ding, J., Xiong, C., Zhu, D., Li, G., Jia, X.-Y., Zhu, Y.-G., & Xue, X.-M. (2019a). Exposure to microplastics lowers arsenic accumulation and alters gut bacterial communities of earthworm Metaphire californica. Environmental Pollution, 251, 110–116.
Wang, J., Coffin, S., Sun, C., Schlenk, D., & Gan, J. (2019b). Negligible effects of microplastics on animal fitness and HOC bioaccumulation in earthworm Eisenia fetida in soil. Environmental Pollution, 249, 776–784.
Wang, J., Liu, X., Li, Y., Powell, T., Wang, X., Wang, G., & Zhang, P. (2019c). Microplastics as contaminants in the soil environment: A mini-review. Science of the Total Environment, 691, 848–857.
Wardrop, P., Shimeta, J., Nugegoda, D., Morrison, P. D., Miranda, A., Tang, M., & Clarke, B. O. (2016). Chemical pollutants sorbed to ingested microbeads from personal care products accumulate in fish. Environmental Science & Technology, 50(7), 4037–4044.
Wu, L., Gao, B., Muñoz-Carpena, R., & Pachepsky, Y. A. (2012). Single collector attachment efficiency of colloid capture by a cylindrical collector in laminar overland flow. Environmental Science & Technology, 46(16), 8878–8886.
Xia, T., Lin, Y., Li, S., Yan, N., Xie, Y., He, M., Guo, X., & Zhu, L. (2021). Co-transport of negatively charged nanoparticles in saturated porous media: Impacts of hydrophobicity and surface O-functional groups. Journal of Hazardous Materials, 409, 124477.
Xu, B., Liu, F., Brookes, P. C., & Xu, J. (2018). Microplastics play a minor role in tetracycline sorption in the presence of dissolved organic matter. Environmental Pollution, 240, 87–94.
Yang, X., Bento, C. P., Chen, H., Zhang, H., Xue, S., Lwanga, E. H., Zomer, P., Ritsema, C. J., & Geissen, V. (2018). Influence of microplastic addition on glyphosate decay and soil microbial activities in Chinese loess soil. Environmental Pollution, 242, 338–347.
Yu, F., Li, Y., Huang, G., Yang, C., Chen, C., Zhou, T., Zhao, Y., & Ma, J. (2020). Adsorption behavior of the antibiotic levofloxacin on microplastics in the presence of different heavy metals in an aqueous solution. Chemosphere, 260, 127650.
Zeng, L.-J., Huang, Y.-H., Chen, X.-T., Chen, X.-H., Mo, C.-H., Feng, Y.-X., Lü, H., Xiang, L., Li, Y.-W., & Li, H. (2020a). Prevalent phthalates in air-soil-vegetable systems of plastic greenhouses in a subtropical city and health risk assessments. Science of the Total Environment, 743, 140755.
Zeng, Q.-L., Yu, Z.-J., Gou, J.-J., Li, G.-M., Ma, S.-H., Zhang, G.-F., Xu, J.-H., Lin, W.-B., Cui, G.-L., & Zhang, M.-M. (2020b). Effect of convalescent plasma therapy on viral shedding and survival in patients with coronavirus disease 2019. The Journal of Infectious Diseases, 222(1), 38–43.
Zhang, Q., Raoof, A., & Hassanizadeh, S. (2015). Pore-scale study of flow rate on colloid attachment and remobilization in a saturated micromodel. Journal of Environmental Quality, 44(5), 1376–1383.
Zhang, K., Xiong, X., Hu, H., Wu, C., Bi, Y., Wu, Y., Zhou, B., Lam, P. K., & Liu, J. (2017). Occurrence and characteristics of microplastic pollution in Xiangxi Bay of Three Gorges Reservoir, China. Environmental Science & Technology, 51(7), 3794–3801.
Zhang, Y., Xu, X., Li, Z., Liu, M., Xu, C., Zhang, R., & Luo, W. (2019). Effects of vegetation restoration on soil quality in degraded karst landscapes of southwest China. Science of the Total Environment, 650, 2657–2665.
Zhou, Y., Wang, J., Zou, M., Jia, Z., Zhou, S., & Li, Y. (2020). Microplastics in soils: A review of methods, occurrence, fate, transport, ecological and environmental risks. Science of the Total Environment, 748, 141368.
Zhou, D., Cai, Y., & Yang, Z. (2022). Key factors controlling transport of micro-and nanoplastic in porous media and its effect on coexisting pollutants. Environmental Pollution, 293, 118503.
Zhu, D., Bi, Q.-F., Xiang, Q., Chen, Q.-L., Christie, P., Ke, X., Wu, L.-H., & Zhu, Y.-G. (2018). Trophic predator-prey relationships promote transport of microplastics compared with the single Hypoaspis aculeifer and Folsomia candida. Environmental Pollution, 235, 150–154.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Khoshnamvand, N. (2023). Micro- and Nanoplastics as Carriers for Other Soil Pollutants. In: Maddela, N.R., Reddy, K.V., Ranjit, P. (eds) Micro and Nanoplastics in Soil. Springer, Cham. https://doi.org/10.1007/978-3-031-21195-9_6
Download citation
DOI: https://doi.org/10.1007/978-3-031-21195-9_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-21194-2
Online ISBN: 978-3-031-21195-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)