Abstract
Plastic-based items are abundantly found on the globe because of their immense utility in daily lives. The poor biodegradability of plastics, particularly micro- and nanoplastics, has recently sparked environmental concerns around the world. These anthropogenic pollutants are either generated, particularly in the tiny size range, for diverse commercial applications or result from the environmental fragmentation of macropolymers. Micro- and nanoplastics are now found in large quantities in the oceans, freshwater bodies, and on land, as well as in food. Micro- and nanoplastics’ biological effects on aquatic creatures are extensively known, but their effects on human systems have not been thoroughly examined. The potential pathways of exposure to micro- and nanoplastics, the biological consequences of these particles in human cells, factors influencing toxicity, and the likely mechanisms of cytotoxicity are all discussed in this chapter. In general, cellular toxicity appears to be induced by oxidative stress, membrane damage, immunological response, and genotoxicity in micro/nanoplastics due to their tiny size, positive charge, high dose, and inclusion of hazardous chemicals or contaminants. A thorough understanding of these chemicals’ cellular destiny and toxicity may aid in extrapolating dangers to mammals.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
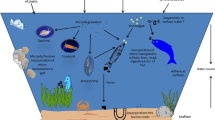
In our daily lives, plastic and plastic-based goods are extremely useful. From 15 million tonnes in 1964 to 359 million tonnes in 2018, global plastic manufacturing surged by around 24 times (Vidal, 2020; World Economic Forum, 2016). Plastic is now mostly used in packaging (26% of total Vidal, 2020; World Economic Forum, 2016 production), but it is also used in other industries such as electronics, construction, transportation, healthcare, and agriculture. The poor biodegradability of these polymers, on the other hand, poses a significant environmental risk. Every year, an estimated eight million tonnes of plastic waste enters the oceans, with 269,000 tonnes of plastic floating on the surface (National Oceanic and Atmospheric Administration, 2019). Plastics can break down into microplastics (0.1 m–5 mm diameter) or nanoplastics (0.1 m diameter) (NJDEP-Science Advisory Board, 2015), over time due to microbial degradation, extended ultraviolet radiation exposure, or physical wear. Microplastics (MPs)/Nanoplastics (NPs) are also produced for use in air blasting technologies, cleansers, cosmetics, medicine delivery formulations, paints, and toothpaste, adding to the MPs/NPs pool already present in the environment (Cole et al., 2011; Sharma & Chatterjee, 2017). Clothing, cigarette filters, automobile tires, and fishing equipment are also potential sources (Toussaint et al., 2019) (Fig. 12.1).
Sources and fate of micro and nanoplastics in the environment. Consumers and industries produce MPs/NPs from primary and secondary sources. Degradation of macroplastic materials that dissolve into micron-sized particles into nanoplastics. MPs/NPs found in both the aquatic and terrestrial environment, eventually entering the food chain and water supplies, resulting in their uptake and bioaccumulation in the human body
Plastics in the environment interact with terrestrial and marine biota, causing considerable worry about severe ecological consequences. Plastics consumed by organisms can bioaccumulate and make their way up the food chain to humans via trophic transfer (Carbery et al., 2018; Chae et al., 2018; Nelms et al., 2018). In addition, individuals can be exposed to plastic through the eating of plastic-contaminated food or the inhalation of plastic-polluted air (Prata et al., 2020). Seafood (fish, shrimp, mussels), home products (sea salt, honey, sugar, plastic tea bags), tap water, bottled water, beer, construction sites, factories, and agriculture have all been found to contain micronized plastics (Barbosa et al., 2020; de Souza Machado et al., 2018; EFSA Panel on Contaminants in the Food Chain, 2016; Hesler et al., 2019; Karami et al., 2017; Mason et al., 2018). The body’s excretory system is estimated to discharge >90% of micro-and nanoplastics in feces once consumed (Smith et al., 2018). The human stool has been found to contain 50–500 m sized PP and PET microplastics (20 particles/10 g stool) (Schwabl et al., 2019).
Due to ethical considerations, no meta-analysis clinical trial has been/can be undertaken to evaluate health risks in humans except from risk assessment data extracted from in vivo experiments (Yang et al., 2019a, b). As a result, the health effects of MPs/NPs on humans are unknown. It is unknown whether MPs/NPs can be absorbed and bioaccumulated by humans through ingestion/inhalation or other modes of exposure. The pharmacokinetics, pharmacodynamics, and variables that determine the pharmacological response to MPs/NPs are still unknown. In the absence of clinical evidence, in vitro investigations in human or other mammalian cells may be able to shed light on these issues. This chapter looks at recent research that looked at the biological effects of MPs/NPs in mammalian cells using various exposure methods. The need of addressing MPs/NPs toxicity about particle size, dose, charge, exposure period, additives/leachates, and/or other co-contaminants has been emphasized.
The cellular pathways that contribute to toxicity after MPs/NPs internalization are also discussed, as well as recommendations for future research. The information offered in this research will aid in a better understanding of the potential implications of human plastic exposure. Because in vitro research using weathered particles is essentially missing, the focus is mostly on studies utilizing MPs/NPs purposefully generated in the micro/nano-size range. Particle preparation techniques were notably noted in the review for experiments involving particles generated from technologies that mimicked the environmental degradation of bigger polymers.
2 Polymer Types of MPs/NPs
A variety of pathways could lead to breaking down plastics into macroplastics (>25 mm), mesoplastics (5–25 mm), microplastics (5 mm), and nanoplastics (0.1 μm). Microplastics (MPs) are plastic particles with a diameter of less than 5 mm that can be found in the environment in sizes ranging from a few microns to a few millimeters, and even nano-sized particles, which often have an unevenly mixed state (Boyle & Örmeci, 2020).
2.1 Primary Type
Primary (initially and consciously created for industrial and domestic uses within a microscopic size) and secondary (originally and purposefully manufactured for industrial and domestic applications within a microscopic size) origins are both present in microplastics (resulting from the continuous breakdown of large plastic debris). Primary microplastics (microbeads) are widely used in cosmetics formulations such as makeup, sunscreen, nail polish, hair coloring, eye shadow, shower gels, and personal care products containing scrubs and toothpaste, facial cleansers, and air-blasting.
2.2 Secondary Type
Secondary microplastics are formed by the breakdown and degradation of large plastic debris into small fragments when exposed to high solar UV radiation and mechanical abrasion as a result of a combination of physical (mechanical), chemical (photolytic), and biological processes and can be transported directly into marine environments from coastlines, rivers, and sewage pipes.
There are numerous different varieties of polymers, each of which can be classified as either natural or synthetic (Koelmans et al., 2015). PET (polyethylene terephthalate), HDPE (high-density polyethylene), PVC (polyvinyl chloride), LDPE (low-density polyethylene), PP (polypropylene), PS (polystyrene), and PU (polyurethane) are examples of synthetic polymers (PUR). Another important factor that affects the floating and sinking of MPs/NPs, as well as the removal rate, is chemical composition. There are currently around 30 different MPs/NPs types available. More than 30 different types of MPs/NPs polymers have indeed been discovered so far (Sun et al., 2019).
3 Detection of MPs/NPs
Understanding the behavior and bioavailability of microplastics requires precise knowledge of physical and chemical properties (i.e., form, size, polymer compositions, and functional groups) (Fu et al., 2020). Separation, identification, quantification, and characterization of plastics in terms of physicochemical attributes are all part of the detection process. MPs/NPs can be characterized in a variety of ways, including microscopic, chromatographic, and sophisticated spectroscopic techniques (Mintenig et al., 2018; Shim et al., 2017).
3.1 Separation
The initial and most important stage in the separation of MPs/NPs is usually accomplished using sieves with various mesh sizes. For MPs/NPs separation, these can be employed alone or in a sequence (Hollman et al., 2013). Filters with a fine mesh are commonly used to separate small MPs of size 5 μm (Löder & Gerdts, 2015). Furthermore, chromatographic techniques, both active and passive separation, are used to separate the majority of MPs/NPs with a size range of 1 μm that is of plastic origin (Mintenig et al., 2018). Field-Flow Fractionation (FFF) is used in active separation, while Hydrodynamic Chromatography (HDC) is used in passive separation (Mendoza & Jones, 2015). Both methods, when combined with sophisticated techniques such as GC-MS, size-exclusion chromatography, and plasma mass spectroscopy, have been demonstrated to be useful in quantifying and characterizing MPs/NPs of various chemical forms of PS, PE, and PACR with sizes ranging from 50 to 9900 nm and 90 to 106 m (Gigault et al., 2017; Correia & Loeschner, 2018; Philippe et al., 2014; Pirok et al., 2017). In the described study, MPs/NPs were extracted from tap water, surface water, and fish samples.
3.2 Visualization
A second phase in the identifying process is visualization. Large MPs are often recognized with a standard microscope, and their shape, color, and light transmittance can later be used to separate them from a combination of non-plastics (Hidalgo-Ruz et al., 2012). The hue of plastic litter may reflect its state of degradation and could be used as a proxy for environmental exposure duration (Marti et al., 2020). Polypropylene fibers, for example, were discovered to be typically hazy or red, whereas milky white color forms PS, and yellow and brown color generates PE, PP, PVC, PS, and PET (Eriksen et al., 2013; Brandon et al., 2016; Vianello et al., 2013). Though large microplastics with distinguishable colors or morphologies can be visually sorted and identified, particles without distinguishable color or form are difficult to sort with the naked eye. To identify confusing plastic-like particles, electron microscopy with magnified pictures is required (Song et al., 2015). SEM-EDS is a technique that combines scanning electron microscopy with energy-dispersive X-ray spectroscopy to characterize the shape of amazingly small materials and estimate their chemical constituents (Goldstein et al., 2017). SEM could produce high-resolution topographical images of objects, allowing microplastics to be distinguished from those other plastic-like particles more easily (Cooper & Corcoran, 2010). By identifying the characteristic X-rays released from the elements well within the specimen by the electron beam, EDS offers elemental information about the samples, allowing for certain characteristics of micro-plastic recognition in sample composites. The identification of several MPs/NPs (PP, PS, PE, PA) in seawater, shallow waterways, and beaches with sizes ranging from 1 to 5 mm was aided by these forms of microscope visualization (Fries et al., 2013). Fluorescence Microscopy has also proven to be a promising method for identifying plastic particles in seawater and studying their effects on marine assemblages and settling rates. For the first time, Qiu et al. (2015) used FCM to examine the presence and prevalence of microplastics (PET, PE, HDPE, and PS) with a size range smaller than 5 mm in China (Qiu et al., 2015). The tendency of zooplankton to consume microplastics was studied using fluorescence microscopy. This study found that marine microplastic debris (especially PS) with sizes ranging from 7.3 to 30.6 μm) can have a deleterious influence on zooplankton physiology and overall health (Cole et al., 2013). Similarly, fluorescence microscopy was employed in another work to discover the existence of fluorescent microplastic beads in copepods (PS with sizes ranging from 0.05 to 6 μm). The findings revealed that micro or nanoscale PS beads may reduce the survival rate and fertility of marine copepods. The colorful plastic fibers in the sample were observed using fluorescence microscopy, and this study successfully quantified the prevalence of MPs of uncertain origin with sizes ranging from 0.5 to 1 mm in Swedish west coast waters (Sweden, 2007). Fluorescence microscopy was utilized to confirm the integration of microspheres (Fluoresbrite carboxylate) with sizes ranging from 3.6 to 11 μm into planktons and to assist in microsphere quantification (Okubo et al., 2018).
3.3 Characterization
Physiological features of MPs/NPs can be examined at the third level of characterization. The hydrodynamic size as well as the surface charge of particles has been studied extensively using modern technologies such as Dynamic light scattering (DLS) (Bhattacharjee, 2016). In addition, the DLS approach can be integrated into other systems to provide quick and easy identification of microplastic deterioration. Gigault et al. used a photo-reactor in conjunction with DLS to study the photocatalytic degradation of ocean microplastic particles under various conditions without the need for sample or manipulation (15). In addition to hydrodynamic size measurement, nanoparticles tracking analysis (NTA) is an improved approach for measuring concentrations of poly-dispersed substances. With monochrome photography, NTA illuminates free diffusing particles with strong laser light to trace their Brownian motion (Gigault & Budzinski, 2016). The other sophisticated approach for testing the presence of organic substances or carbon in surface and ground waters is fluorescence spectrophotometry. The use of fluorescence spectroscopy to evaluate toxicity and explore the detrimental impacts of microplastics on microorganisms in soil and water, such as suppression of enzyme activity and energy metabolism, has been demonstrated (Henderson et al., 2009). Chen et al. (2018) mapped and described the microstructure of MPs (PS) with diameters of 20 mm, 6 mm, 500 nm, and 80 nm to analyze MPs’ environmental behavior. Similarly, detection of accumulated microplastics, particularly (PS) of size 5 μm in the gills, liver, and gut of crabs, detection of cadmium, lead, and bromine in MPs of uncertain origin with sizes 5–10 mm from beach waters, and measurement of NPs concentration (PS) of sizes 45 μm and 50 nm in zebrafish larvae and nematodes (Yu et al., 2018; Massos & Turner, 2017; Chen et al., 2017, 2018; Zhao et al., 2017).
Finally, advanced technology like Fourier transform infrared (FTIR) spectroscopy, can be used to analyze the chemical/functional group composition of MPs/NPs. It collects chemical data by sensing the modes of vibration of analyte at various infrared frequencies throughout a broad-spectrum range (Stuart, 2005). From sand samples collected in Sishili Bay, North Yellow Sea, FTIR was used to detect eight polymer kinds of MPs (Rayon, PE, PP, PA, PET, PS, PMMA, and PU) with diameters ranging from 34.97 to 4983.73 μm. These findings show that river and sewage discharge, as well as maritime activities, were the main sources of MP pollution (Zhang et al., 2019). Yang et al. (2019a, b) used FTIR to demonstrate the presence of MPs (PET, PS, PP) of varied sizes in municipal wastewater from China’s biggest water reclamation plant (Yang et al., 2019a, b). FTIR was also used to determine the existence of airborne MPs (PET, PES, PAN, RY, EVA, PAA, EP, ALK) in China’s atmosphere, as well as the polymer kinds of MPs (PET, PP, PS, Nylon) deposits in the Pacific Ocean (Liu et al., 2018a, b; Peng et al., 2020). Raman spectroscopy is another prominent biochemical characterization mapping technique that uses the Raman effect to extract the vibrational modes and identify analytes of samples by using the frequency response of inelastically scattered light from the samples (Araujo et al., 2018). Micro-Raman spectroscopy can detect MPs with a resolution of up to 10 μm, whereas Raman spectroscopy can detect MPs greater than roughly 1 μm (Imhof et al., 2016). Several other methods, such as matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) (Karas & Krüger, 2003), high-performance liquid chromatography (HPLC) (Siddiqui et al., 2017), and atomic force microscopy and its combinations with IR and Raman as a unique scanning probe technique, open up new opportunities in microplastics and nanoplastics characterization. AFM may be used to detect and quantify a variety of material physical and mechanical properties like elasticity, surface electric properties, and chemical properties (Akhatova et al., 2022). Several studies have also shown the presence of different micro and nanoplastics present in various food stuffs (Table 12.1).
4 Exposure of MPs/NPs to the Biological System
MPs/NPs can be ingested, inhaled, absorbed through the skin, or administered intravenously (NJDEP-Science Advisory Board, 2015). When particles are swallowed, they first come into contact with the gut mucosa, followed by the epithelia, which together provide a formidable barrier to xenobiotic uptake. Several investigations have revealed, however, that micro/nanoparticles can pass through the intestinal barrier and enter the bloodstream (Jenkins et al., 1994; Reineke et al., 2013; Walczak et al., 2015). Airborne MPs/NPs would come into direct contact with the mucus layer, periciliary layer, ciliated cells, non-ciliated secretory cells, and basal cells of the respiratory tract (Ganesan et al., 2013; Gasperi et al., 2018). When inhaled repeatedly, plastic fibers can infiltrate lung tissues, causing inflammation and subsequent genotoxicity (Gasperi et al., 2018). In the lungs of textile workers, granulomatous lesions harboring foreign substances (perhaps polyester, nylon, or acrylic dust) were discovered (Pimentel et al., 1975). Because the stratum corneum impedes the passage of molecules larger than 500 Da (1 nm) across the skin layers, transdermal absorption of MPs/NPs via intact skin is unlikely (Bos & Meinardi, 2000). MPs/NPs can also enter the bloodstream through plastic-based intravenous catheters, syringes, and other medication delivery methods (Stapleton, 2019). Because they are “inert and biocompatible,” polystyrene micro and nanoparticles are frequently utilized as vectors for medication delivery or to study bio-interactions (Loos et al., 2014a; Poon et al., 2016). Plastic absorption by cells, as well as the release of plastic additives or surface-adsorbed pollutants, can have a deleterious impact on cell function (Bouwmeester et al., 2015).
5 Factors That Influence Their Cytotoxicity
Plastic toxicity varies depending on the polymer type. Based on the hazard classification of monomers, polyurethane, polyacrylonitriles, PVC, epoxy resins, and styrene-based copolymers have been classified as the most dangerous (category 1A or 1B mutagen or carcinogen) (Lithner et al., 2011). Several factors can influence particle cytotoxicity within a plastic class.
5.1 Size of MPs/NPs and Dosage
Small particles are often internalized to a higher extent by cells than large particles (Florence et al., 1995). Smaller particles can be taken up via endocytic or passive absorption, but bigger particles require phagocytosis by specialized cells (Alberts et al., 2002). Internalization of 44 nm PS particles was an ATP-independent passive process in two primary mammalian cell lines – bovine oviductal epithelial cells and human colon fibroblasts (Fiorentino et al., 2015). In most cases, particle size and toxicity have an inverse relationship. Particles smaller than 10 nanometers are thought to act as a gaseous substance that can easily infiltrate tissues and cause broad damage (Bahadar et al., 2016). When evaluated using 100 L of 1 mg/mL particle solution in monomac-6 human monocytic cells, 64 nm PS particles induced a considerable rise in intracellular Ca2+ levels compared to bigger PS particles (202 and 535 nm) (Brown et al., 2001). The 64 nm particles produced higher IL-8 expression in human lung cancer A549 cells after 2 hours than the 202 and 535 nm particles (Brown et al., 2001).
Smaller particles can be more easily absorbed through the colon or lungs, affecting particle cellular fate and biodistribution. Particles larger than 150 nm can have local effects in the stomach, whereas smaller particles can cause toxicity in a variety of secondary organs and tissues (EFSA Panel on Contaminants in the Food Chain, 2016; Rubio et al., 2020). For particle intake and transport, however, a Goldilocks zone for size may exist. For example, 40 nm PS particles were found to have a greater absorption in 1321N1 human astrocytoma and A549 cells than 20 and 100 nm PS particles (Varela et al., 2012). The transfer of 200 nm PS particles through the cells in a microfluidic model of the blood-brain barrier (BBB) utilizing hCMEC/D3 cells was higher than that of 100 and 500 nm particles (Nowak et al., 2019).
High doses and long-term exposure can cause increased cellular absorption and toxicities. For example, at 20 and 50 g/mL doses, 20 nm plain PS particles did not cause toxicity in THP-1 monocytes in 24 hours, but at 200 g/mL, cellular viability was reduced to 12% (Mrakovcic et al., 2014). Cell number reduced considerably after 16 days of incubation with 50 g/mL of 20 nm PS particles, exhibiting dosage- and time-dependent toxicity. Similar findings were made with human umbilical vein EAhy 926 cells, where 20 nm plain PS particles displayed dose-dependent cytotoxicity in 24 hours, with an IC50 value of 120 g/mL (Mrakovcic et al., 2013). Cell number dropped by 50% after a 28-day incubation period with a 20 g/mL dosage of these particles.
5.2 Charge
Surface charge influences particle absorption, translocation, and toxicity. Positively charged PS particles transported 20 and 100–120 nm aminated or carboxylated PS particles 20–40 times faster than negatively charged PS particles across rat alveolar epithelial cell monolayers (Yacobi et al., 2010). Furthermore, cationic particles exhibit higher cytotoxicity in non-phagocytic cells than their anionic counterparts, owing to plasma membrane damage (Frohlich, 2012). Aminated PS particles (110 nm) decreased THP-1 cell proliferation, although carboxylated PS particles of similar size did not affect cell division (Loos et al., 2014a). The viability of THP-1 cells was similarly dramatically reduced after 72 hours of incubation with 10–100 g/mL aminated particles but not with carboxylated PS particles (Loos et al., 2014b).
5.3 Additives
Stabilizers, plasticizers, lubricants, dyes, and flame retardants are among the plastic additives/leachates that make up an average of 4% of microplastic content and can be harmful (Bouwmeester et al., 2015; Campanale et al., 2020; EFSA Panel on Contaminants in the Food Chain, 2016). Bisphenol A, phthalates, and brominated flame-retardants, all of which alter endocrine function, are of special concern (Campanale et al., 2020; De Toni et al., 2017; Legler & Brouwer, 2003; Rubin, 2011). At temperatures above 60 °C, commercially available PET water bottles leached Sb into the water; temperatures that could be reached if bottles were left inside cars and garages during the summer (Westerhoff et al., 2008). Surfactants can lyse cell membranes or affect the structure and function of cell surface receptors, glycoproteins, proteoglycans, signaling molecules, extracellular matrix components, and lipid rafts, to mention a few (Yong et al., 2020). However, the presence and release of additives do not always constitute a health risk, as toxicity is determined by the plastic composition and the velocity of leachate migration, or the amount and solubility of leachate in the surrounding environment. In fatty foods and when stored at high temperatures or for long periods, there is a higher migration of additives from plastics (Hahladakis et al., 2018).
5.4 Adsorbed Pollutants
MPs/NPs can absorb additional pollutants such as persistent organic pollutants (POP), heavy metals, and pathogens due to their small size and high surface to volume ratio (de Souza Machado et al., 2018; Yu et al., 2019). Persistent organic pollutants (polychlorinated biphenyls, polycyclic aromatic hydrocarbons, DDT), heavy metals (Cd, Cr, Cu, Zn, Sb, Al, Br, Hg, As, Sn, Ti, Co, Ba, Mn), and microorganisms (pathogenic vibrio spp.) can all be vectored by plastics (Brennecke et al., 2016; Campanale et al., 2020; Kirstein et al., 2016; Prinz & Korez, 2020; Rodrigues et al., 2019; Velzeboer et al., 2014). Pyrene and BDE-47 were carried by polystyrene nanoparticles (100 nm) in saturated soil (Liu et al., 2018a).
6 Toxicity Caused to Human Cells (In Vitro)/Potential Effects on Organ System
It’s crucial to look at MPs/NPs uptake and biological consequences in cells that are either immediately exposed to them or come into contact with them after systemic absorption. A list compiled of recent MPs/NPs toxicity studies was completed in mammalian cells from 2001 to 2020. MPs/NPs can be internalized by gastrointestinal, airway, immune, and other miscellaneous cell types and induce various cellular responses, the nature and extent of which may be governed by MPs/NPs size, dose, charge, exposure time, and the presence of additives/leachates/co-contaminants, as described in this section (Fig. 12.2).
6.1 Immune Cells
Immune cells serve as the body’s gatekeepers, assisting in the clearance of infections and xenobiotics. As a result, they are likely to interact with MPs/NPs found in food, water, and air, as well as those absorbed into the systemic circulation via various routes of exposure. Smaller particles can enter immune cells via clathrin/caveolae-mediated endocytosis, clathrin/caveolae-independent internalization, micropinocytosis, and phagocytosis, while microparticles can be ingested by immune cells via phagocytosis or micropinocytosis (Firdessa et al., 2014).
MPs/NPs can be quickly internalized by immune cells such as monocytes, macrophages, fibroblasts, and mast cells (Heinlaan et al., 2020). At dosages less than 100 μg/mL, nano- and sub-micron-sized particles did not appear to cause cytotoxicity in THP-1 macrophages. In most cases, the positive charge and tiny size exacerbated toxicity (Hwang et al., 2019). The viability of cells was harmed by particle leachates and additions. However, PS particles were used in the bulk of this research to explore biological effects in immune cells. To fully comprehend the influence of diverse plastic types on immune cells, more research employing different plastic polymers is required.
6.2 Gastrointestinal Cells
MPs/NPs toxicity in gastrointestinal cells has been intensively researched because ingestion of MPs/NPs contaminated food or water is the primary source of exposure to these particles. Enterocytes, mucus-producing goblet cells, and microfold or M cells make up the majority of the intestinal epithelia. Caco-2 cells, a commonly used in vitro model for enterocytes, were found to ingest 100-nm carboxylated PS particles largely by micropinocytosis-mediated uptake at the apical surface, followed by mostly storage or limited exocytosis at the basolateral membrane end (Reinholz et al., 2018). Regular epithelial cell shedding every 4–5 days could remove polystyrene particles retained in the cells (Reinholz et al., 2018). Diffusion across the cell membrane followed by basal exocytosis was a secondary, although limited, mechanism of uptake and excretion.
In vitro investigations of gastric cells show that nanoplastics can be taken up by stomach cells and that smaller particles are potentially more harmful than bigger particles. To better understand MPs/NPs toxicity in the stomach, more research is needed in other gastric cells such as SNU-1, SNU-5, and KATO III, utilizing particles of various sizes, charges, dosages, and exposure times (Liao & Yang, 2020). Numerous researches have looked into the toxicity of MPs/NPs in the intestine, however, no consensus has been reached on size/charge-dependent transport or toxicity (Baos et al., 2012; Chen et al., 2020; Collado-Gonzalez et al., 2019). To reconcile the contradictory data, a factorial study design assessing the effects of various plastic polymers, charges, sizes, doses, and exposure times in intestinal cells is required.
6.3 Airway Cells
Clothes, dried sludge, agriculture, tires, manufacturing operations, and sea salt aerosols are all reported sources of airborne microplastics (Wright & Kelly, 2017). Microplastics were found in air fallout in Paris, with a higher quantity in densely populated areas than in less densely populated areas (Gasperi et al., 2018; Wright & Kelly, 2017). When breathed in, plastic particles may pass through mucociliary clearance systems in the respiratory tract, especially if the particle size is more than 1 nm, or (ii) pass through the pleura and be absorbed by lung epithelial cells. As seen among workers in nylon flock, polyester, polyolefin, and polyamide fiber plants, inhaled plastic dust can cause respiratory distress such as irritation of the respiratory tract, dyspnea, decreased lung capacity, coughing, increased phlegm production, interstitial fibrosis, and granulomatous lesions (Wright & Kelly, 2017). Human lung tissues have also been discovered to contain plastic fibers (Pauly et al., 1998). Fibers made of polypropylene, polyethylene, and polycarbonate can last up to 6 months in extracellular lung fluid (Gasperi et al., 2018). The size, type, concentration, and duration of exposure to these particulate materials all influence the health risks they pose (occupational vs occasional). To assess if the airborne MPs/NPs constitute a health risk, researchers must first determine how they interact with respiratory cells (uptake, transport, cytotoxic potential, and metabolic effect).
MPs/NPs can be absorbed by several immortalized and primary airway epithelial (bronchial, alveolar) cells, according to in vitro investigations. Positively charged beads, a greater dose, and the inclusion of additives are all linked to increased cytotoxicity. The influence of nano- and sub-micron-sized PS particles on respiratory cells has been the focus of research. To model chronic exposure to varied MPs/NPs, however, the toxicity of different plastic polymers and at longer time points (72, 96, or more) are required. Furthermore, investigations involving airway cells at the air-lung interface are required for a more realistic understanding of the interaction between MPs/NPs exposure and lung damage.
6.4 Mammalian Cells
The toxicity of MPs/NPs has been investigated in a variety of mammalian cells, including blood, cerebral, endothelial, epithelial, hepatic, kidney, melanoma, ovarian, and placental cells, due to the possibility of systemic exposure or absorption. T98G glioma cells and HeLa epithelial cells were used to test the cytotoxicity of PE microspheres (0.1, 0.6, and 3–16) and PS particles (40–250 nm and 10) (Schirinzi et al., 2017). In 24 hours, the particles (0.05–10 mg/L) did not affect cellular viability in either cell line. In Madin Darby canine kidney cell II monolayers, charge-dependent quicker trafficking of PS particles was reported, with amidine-functionalized 20 and 120 nm PS beads translocating 500 times faster than 20 and 100 nm carboxyl-functionalized beads (Fazlollahi et al., 2011). In ovarian cancer cells SK-OV-3 and NIH-OVCAR3, 50 nm amine-functionalized PS particles were quickly taken up, accumulated in lysosomes, and caused cytotoxicity within 4–8 hours, whereas 30 nm carboxyl functionalized beads did not accumulate in lysosomes and were not cytotoxic even after 24 hours of treatment (Ekkapongpisit et al., 2012). The hemolytic potential of 100 nm PS particles isolated from commercial face washes was compared to 100 nm virgin PS particles in erythrocytes (Gopinath et al., 2019). Overnight incubation with isolated particles at 5 g/mL resulted in 40% hemolysis, but only 22% with virgin particles. The inclusion of additives or other toxic polymers on the surface of isolated particles was blamed for the greater toxicity of isolated PS compared to virgin PS.
These findings show that a variety of non-phagocytic cells can internalize MPs/NPs, with size and charge being the most important factors. When compared to large, negative, or non-functionalized particles, smaller, positively charged particles are more likely to be taken in and cause cellular damage. HeLa and T98 glioblastoma cells, on the other hand, demonstrate no size-dependent toxicity. Small (200 nm) particles can be internalized by red blood cells, whereas large (1000 nm) particles cannot. The toxic effects shown by different micro- and nanoplastics are mentioned below (Table 12.2).
6.5 Animals
Micro and nanoplastics have long been recognized as common contaminants in the environment. Their existence has been established in water bodies (fresh & marine), terrestrial systems, as well as the air we breathe (Rillig & Lehmann, 2020). MPs/NPs are taken up by animals, dispersed in their bodies, and deposited in several tissues, from which they were later transported widely through food chains, according to a growing body of evidence (Zhang et al., 2020) As a result, bioaccumulated micro and nanoplastics could endanger human health and the ecosystems (de Souza Machado et al., 2018) (Fig. 12.3).
The route of exposure of micro- and nanoplastics into the food chain. (Modified from Braden Wilkinson, 2019)
MPs/NPs have an impact on the growth, development, and reproduction of organisms and can even cause mortality in individuals. These tend to generate oxidative stress reactions in organisms, disrupt pigment formation or enzyme activities, create endocrine and metabolic abnormalities, and cause various degrees of genotoxicity, cytotoxicity, and neurotoxicity due to their small size. Simultaneously, as the food chain spreads, these consequences are aggregated and magnified step by step from people to populations to communities and finally to ecosystems, thereby worsening the fragile natural system (Ma et al., 2020).
Smaller microplastics can significantly affect algal growth, fertility, and even disturb photosynthesis (Chen et al., 2020). It was observed that HDPE (High-Density Polyethylene) plastic beads of 10–45 μm reduced the filtering rate of Dreissena bugensis (quagga mussel) (Pedersen et al., 2020). Many studies have shown that MPs/NPs can pile up in the intestines of earthworms and that the growth activity was significantly lowered and the rate of mortality increased at 28, 45, and 60% w/w microplastics (Gaylor et al., 2013; Wang et al., 2020; Huerta Lwanga et al., 2016). Earthworms’ immune systems and pathological responses were also reduced by microplastics (Rodriguez-Seijo et al., 2017). In soil contaminated with microplastics, the mobility of springtails (Lobella sokamensis) was reduced (Kim & An, 2019). As a result, microplastics may obstruct the movement of terrestrial animals by blocking gaps.
MPs/NPs can alter the early stages of development in aquatic animals. The embryonic stage is critical for aquatic animal development, and embryonic chorion serves as an effective barrier against exogenous contaminants. It was reported that zebrafish (Danio rerio) embryonic chorions had an effective barrier property against micro and nanoparticles. Though embryonic chorions can effectively prevent micro- and nanoplastics, they can however have an impact on aquatic species’ early development. MPs/NPs attaching to embryonic chorions may lead to an internal hypoxic microenvironment within embryos, as well as a delay in hatching. When embryos are exposed to polystyrene particles, specifically nanopolystyrene particles, the pathways of biosynthesis of unsaturated fatty acids, linoleic acid metabolism, alanine, and also glutamate, and aspartate metabolism, are significantly altered (Duan et al., 2020).
The absence of tools for characterization and measurement of these particles in complicated biological matrices has impeded research into micro and nanoplastic buildup in animal bodies. In microplastic research, ocular inspection (Sobhani et al., 2020), Fourier-transform infrared spectroscopy (González-Pleiter et al., 2019), and Raman spectroscopy (Gillibert et al., 2019) are being employed. However, these methods are often limited to particles with a diameter of 5 mm to 20 μm, with only a few studies focused on the sub-20 μm portion (Cole et al., 2013). Furthermore, organic debris on the micro and nanoplastics surfaces makes spectroscopic identification and quantification difficult. Pyrolysis combined with gas chromatography-mass spectrometry (Py-GC/MS) is a promising method for quantifying nanoplastics (Fischer & Scholz-Böttcher, 2017; Zhou et al., 2018). Even though this technique may be used to reliably identify and quantify micro and nanoplastics without regard to particle size, extracting and determining micro and nanoplastics from complicated biological matrices is a simple task (Mitrano et al., 2019).
7 Cellular and Molecular Interactions Caused by MPs and NPs
MPs/NPs toxicity is thought to be caused by membrane damage, oxidative stress, immunological response, and genotoxicity. Among these, MP/NP‘s cytotoxicity has been attributed mostly to membrane damage and oxidative stress (Bhattacharjee et al., 2014). Cationic particles, for example, have been known to damage the plasma membrane (Feng et al., 2019; Frohlich, 2012). Polyethylene nanoparticles were discovered to enter the plasma membrane bilayer’s hydrophobic milieu and cause structural alterations (Holloczki & Gehrke, 2020). Endocytosed particles can permeabilize the endosomal-lysosomal membrane, allowing them to interact with intracellular organelles (Wang et al., 2018a, b; Yong et al., 2020). ROS are produced during the polymerization and processing of plastic particles, and when they come into contact with the biological environment, they cause cellular stress (Rubio et al., 2020). While big particles can cause inflammation in the gastrointestinal and respiratory tracts, smaller particles can pass through the gut/lung barrier, causing intracellular oxidative stress and cytotoxicity in the organs where they collect (Rubio et al., 2020). Direct or indirect DNA damage caused by particle or ROS translocation into the nucleus, as well as disruption to the DNA replication/repair machinery, can all contribute to particle genotoxicity (Rubio et al., 2020) (Fig. 12.4).
A schematic illustration depicting possible cellular processes of MP/NP toxicity. Ingestion and inhalation are two ways to absorb MPs/NPs. These have the potential to disrupt the plasma membrane and compromise the gut barrier (left). These could also disrupt cell surface receptor signaling and change gene expression in the nucleus. Endocytosed MPs/NPs have the potential to disrupt the endocytic process and impair endosomal membranes. Endogenous and secreted damage-associated molecular patterns (DAMP) triggering the innate immunity-mediating toll-like receptors (TLRs) could activate the cellular innate immune system as a result of the aforesaid stresses. Stress may cause the NADP oxidases to produce reactive oxygen species (ROS) (NOXs). Mitochondrial dysfunction, whether caused by MPs/NPs from endosomes or as a result of stress, could result in an increase in ROS due to a decrease in the efficiency of electron transport chain (ETC) operations. If the gut–vascular barrier is breached, MPs/NPs gain access to the circulation, or transcytosis may occur, allowing them to reach other organs. The lung is more likely to have direct contact with airborne MPs/NPs (right). (Modified from Yong et al., 2020)
MPs/NPs can disrupt nuclear membranes, generate oxidative stress, release damage-associated molecular patterns, and activate inflammatory and apoptotic/necrotic pathways in mammalian cells (Hwang et al., 2020; Yong et al., 2020). Hepatocytes from 3-month-old mice have been treated with 50 nm PS particles for 24 hours, causing an increase in ROS (superoxide dismutase and malondialdehyde concentration) and DNA damage (Zheng et al., 2019). PS beads caused superoxide radical anion (O2·−) production in human hepatocyte-derived cancer Huh-7 cells, according to another study (Liu et al., 2018b). The toxicity of positively charged PS beads in RAW 264.7 and BEAS-2B cells, on the other hand, was found to be attributable to autophagy via the Akt/mTOR and AMPK pathways (Chiu et al., 2015). The majority of research points to an oxidative stress-mediated cellular response to MPs/NPs when taken together. Many of the aforementioned toxicity processes, however, are closely interrelated, and induction of one process might trigger a cascade of toxicological responses.
8 Regulatory Policies/International, National, and Regional Instruments
“Microplastics” (MPs) are a hot topic in the media and one of the most fiercely debated environmental issues among the general population. As a result, the public wants policymakers to address and handle the issue as quickly as possible (Sharma et al., 2021). In reality, policymakers are becoming more conscious of the problem. Some of the most powerful and influential international and intergovernmental organizations are debating the global effect of environmental plastics (e.g., G7, World Bank, United Nations, World Economic Forum, etc.) (Brennholt et al., 2018). Aside from that, the (micro)plastic/nanoplastics issue has been addressed in a few international and national rules and policy instruments. Because the majority of environmental MPs are caused by improper disposal and fragmented plastic litter, MP management is directly linked to a variety of policy areas (Coffin et al., 2021). Furthermore, regulatory responsibilities can shift throughout a single plastic product’s life cycle and include plastic production process layout, trade and consumer behavior, recycling, and waste management (Deme et al., 2022). It can also be called land-based policies, as well as sewage management and water protection, also called water-based policy (Freeman et al., 2020). As a result, plastics regulation is already addressed in several directives, recommendations, agreements, and other documents addressing the use of plastic products, beginning with restrictions on plastic monomer compositions and the addition of additives. Policy and regulatory instruments are now being established all over the globe at international/ regional/and national levels to handle the problem of (micro) plastics in the environment (Mitrano & Wohlleben, 2020). National policy instruments are limited to a single country, whereas regional policy instruments address specific issues within a geographical region, such as Europe. International accords and regional treaties, for example, are incorporated into national legislation. For the first time in January 2018, Europe enacted ESPCE (European Strategy for Plastics in a Circular Economy), which altered the way the European Union designs, manufactures, uses, and recycles plastic items (Campanale et al., 2020). Many instruments, such as the G7 Summit (2014), the G20 Summit (2017), and the United Nations Environment Assembly (UNEA) I, II, III in 2014, 2016, and 2017, have been vital in preventing marine litter from land-based sources and in reducing marine plastic litter and microplastics, as well as combating their spread. National instruments in countries such as the United States, the United Kingdom, Sweden, Canada, Italy, New Zealand, South Korea, Australia, Taiwan Province, and China primarily impose laws prohibiting the use of microbeads in rinse-off cosmetics, microbeads in toiletries, plastic cotton buds, microbead scrub particles in cosmetics, and the sale of microbead-containing products (Xu et al., 2021; Deng et al., 2020). Better plastic product design, increased waste plastic recycling rates, etc., and high-quality recycles will all assist to improve the demand for recycled plastics, protecting the environment, reducing marine debris, greenhouse gas emissions, and our dependence on external fossil fuels (Gago et al., 2020).
The Ministry of Ecology and Environment issued “Opinions on Further Strengthening the Control of Plastic Pollution” in January 2020, outlining three phases of action. Prohibiting and regulating the manufacture, sale, and use of certain plastic products, for example, shopping bags that are ultra-thin with 0.025 mm thickness. Simultaneously, we should encourage the use of non-plastic goods (such as paper bags and biodegradable shopping bags) and standardize plastic trash recycling and disposal. In addition, Japan, South Korea, Thailand, and other countries have also implemented harsher rules in recent years. The European Commission adopted new guidelines in May of the same year for ten typical throwaway plastics goods and fishing gear using plastics to minimize or limit the environmental effect of individual plastic products. In addition, Plastics Europe proposed the “Plastic 2030” voluntary commitment, which included “Zero Plastics to Landfill,” “Zero Pellet Loss,” and other initiatives aimed at preventing plastic leakage into the surroundings and trying to improve the resource productivity of plastic items and concentricity of plastic packaging. Some regions have seen positive results and increased public awareness of environmental preservation after China enacted the “Plastics Restriction Order” in 2008, however, this is far from enough (Wang et al., 2020). These policies, in contrast to previous rules and regulations, focus on the entire life cycle of plastic products, including the overall process and each link of manufacturing, circulation, use, reprocessing (such as mechanical and chemical recycling and energy recovery), and disposal, making it easier to establish a long-term mechanism for controlling plastic pollution. Preventing the flow of low-end plastic items from developed to developing countries, from places with high supervision and utilization competency to those with inadequate supervision and use competence, is critical. This will not only result in a circular economy but will also protect our planet and help us accomplish our sustainable development goals.
9 Conclusion/Future Directions
Although their cytotoxicity is mostly determined by their size, surface functionalization, dose, exposure period, and presence of co-contaminants, studies have revealed that micro/nanoplastics are not ‘inert’ materials. These pollutants have been found in our food, drinking water, and air, implying that we are constantly exposed to them. As has been found with occupational PVC dust exposure, chronic exposure to plastics can lead to bioaccumulation and subsequent biological consequences. Organic contaminants and heavy metals, for example, are plastic additives or compounds that hitchhike on plastic surfaces, posing additional toxicity concerns that require further exploration. Studies on the cytotoxicity of MPs/NPs in mammalian cells revealed that MPs/NPs with small size and positive charge, when delivered at high doses for long periods and including surfactants or other adsorbed contaminants, have higher toxicity. These conclusions, however, may not apply to all cells and particle kinds. Furthermore, the influence of certain physicochemical characteristics of particles in particular cell types has yet to be thoroughly understood. A multi-end-point toxicological investigation utilizing MPs/NPs of varied types and physicochemical features at environmentally realistic concentrations in human cells is required for a complete knowledge of the health impact of MPs/NPs.
Various physicochemical parameters of MPs/NPs have different toxicological implications in different cell types, and even within the same cell type, there are discrepancies. Several of these discrepancies can be attributed to changes in experimental settings, particle type/synthesis/source/extraction method, end-points studied, and the difficulties of regulating other parameters during a study. A multi-factorial study design that uses specific cell types, uniform experimental circumstances, and end-points could help to resolve the inconsistencies in cellular responses.
In many cell types, size has been demonstrated to govern cellular uptake and viability among the other particle properties. Gastric cells, hepatocytes, immune cells, RBCs, squamous carcinoma, melanoma, and umbilical cells, for example, have higher bioreactivity with small particles, but there is no clear consensus with airway, intestinal, or ovarian cancer cells. This could be because various cell types have an optimal size range for enthusiastic internalization. Positive charges on MPs/NPs increased toxicity in the airway, immune, ovarian cancer, MDCK-II kidney cells, adrenal medulla, mammary epithelial cells, and HEPA-1 hepatocytes at particular concentrations.
Positively charged particles, in general, can interact electrostatically with the negatively charged phospholipid cell membrane, resulting in greater internalization than negatively or neutrally charged particles (Foroozandeh & Aziz, 2018). High binding of positively charged particles to the plasma membrane, on the other hand, might raise surface tension and cause membrane portion or deformation (Li & Malmstadt, 2013). High doses boosted cellular responses in the airway, adrenal medulla, RBCs, immunological, intestinal, HEPA-1, and EAhy926 umbilical cells, among other characteristics. The cytotoxicity of BEAS-2B airway cells, on the other hand, increased with a longer incubation period. Internalization of particles can be increased by a high dose and a long exposure duration, resulting in greater toxicity. It should be emphasized that spherical polystyrene has been used as the model MPs/NPs in many of these cytotoxicity experiments, owing to the commercial availability of polystyrene micro/nanobeads. It’s also critical to assess the toxicity of various plastic polymers, particularly category 1 carcinogens and mutagens. Toxicity of fibrous or other shaped polymers at sizes that more closely resemble the majority of the MPs/NPs population in the natural environment is also required. Furthermore, several investigations have been conducted at extremely high particle doses, which may or may not be environmentally relevant.
The toxicity of these particles is not conclusive due to a mismatch between the concentration, size, shape, and type of microplastics examined in the laboratory and those found in nature (Burns & Boxall, 2018). The lack of standardized analytical techniques for detecting and quantifying plastics in various matrices, as well as the establishment of plastic contamination control protocols during the analysis of collected samples, are important hurdles to determining MPs/NPs toxicity (Barbosa et al., 2020). Plastics with a diameter of fewer than 20 μm are particularly difficult to identify and separate (Hale et al., 2020). The development of analytical procedures for MPs/NPs identification and quantitation would pave the way for the use of environmentally realistic dosages, sizes, forms, and kinds to better understand the health effects of these particles.
References
Akhatova, F., Ishmukhametov, I., Fakhrullina, G., & Fakhrullin, R. (2022). Nanomechanical atomic force microscopy to probe cellular microplastics uptake and distribution. International Journal of Molecular Sciences, 23(2), 806.
Akhbarizadeh, R., Moore, F., & Keshavarzi, B. (2019). Investigating microplastics bioaccumulation and biomagnification in seafood from the Persian Gulf: A threat to human health? Food Additives & Contaminants: Part A, 36(11), 1696–1708.
Alberts, B., Johnson, A., & Lewis, J. (2002). Transport into the cell from the plasma membrane: Endocytosis. In Molecular biology of the cell. Garland Science.
Araujo, C. F., Nolasco, M. M., Ribeiro, A. M., & Ribeiro-Claro, P. J. (2018). Identification of microplastics using Raman spectroscopy: Latest developments and future prospects. Water Research, 142, 426–440.
Bahadar, H., Maqbool, F., Niaz, K., & Abdollahi, M. (2016). Toxicity of nanoparticles and an overview of current experimental models. Iranian Biomedical Journal., 20, 1–11. Pii-IBJ A-10-1853-1.
Baos, S. C., Phillips, D. B., Wildling, L., McMaster, T. J., & Berry, M. (2012). Distribution of sialic acids on mucins and gels: A defense mechanism. Biophysical Journal, 102, 176–184. https://doi.org/10.1016/j.bpj.2011.08.058
Barbosa, F., Adeyemi, J. A., Bocato, M. Z., Comas, A., & Campiglia, A. (2020). A critical viewpoint on current issues, limitations, and future research needs on micro- and nanoplastic studies: From the detection to the toxicological assessment. Environmental Research, 182, 109089. https://doi.org/10.1016/j.envres.2019.109089
Bhattacharjee, S. (2016). DLS and zeta potential–what they are and what they are not? Journal of Controlled Release, 235, 337–351.
Bhattacharjee, S., Ershov, D., Islam, M. A., Kämpfer, A. M., Maslowska, K. A., van der Gucht, J., Alink, G. M., Marcelis, A. T., Zuilhof, H., & Rietjens, I. M. (2014). Role of membrane disturbance and oxidative stress in the mode of action underlying the toxicity of differently charged polystyrene nanoparticles. RSC Advances, 4, 19321–19330. https://doi.org/10.1039/C3RA46869K
Bos, J. D., & Meinardi, M. M. (2000). The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Experimental Dermatology, 9, 165–169.
Bouwmeester, H., Hollman, P. C., & Peters, R. J. (2015). Potential health impact of environmentally released micro- and Nanoplastics in the human food production chain: experiences from nanotoxicology. Environmental Science & Technology, 49, 8932–8947. https://doi.org/10.1021/acs.est.5b01090
Boyle, K., & Örmeci, B. (2020). Microplastics and nanoplastics in the freshwater and terrestrial environment: A review. Water, 12(9), 2633.
Brandon, J., Goldstein, M., & Ohman, M. D. (2016). Long-term aging and degradation of microplastic particles: Comparing in situ oceanic and experimental weathering patterns. Marine Pollution Bulletin, 110(1), 299–308.
Brennecke, D., Duarte, B., Paiva, F., Caçador, I., & Canning-Clode, J. (2016). Microplastics as vector for heavy metal contamination from the marine environment. Estuarine, Coastal and Shelf Science, 178, 189–195. https://doi.org/10.1016/j.ecss.2015.12.003
Brennholt, N., Heß, M., & Reifferscheid, G. (2018). Freshwater microplastics: Challenges for regulation and management. In Freshwater microplastics (pp. 239–272). Springer.
Brown, D. M., Wilson, M. R., MacNee, W., Stone, V., & Donaldson, K. (2001). Size-dependent proinflammatory effects of ultrafine polystyrene particles: A role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicology and Applied Pharmacology, 175, 191–199. https://doi.org/10.1006/taap.2001.9240
Burns, E. E., & Boxall, A. B. A. (2018). Microplastics in the aquatic environment: Evidence for or against adverse impacts and major knowledge gaps. Environmental Toxicology and Chemistry, 37, 2776–2796. https://doi.org/10.1002/etc.4268
Campanale, C., Massarelli, C., Savino, I., Locaputo, V., & Uricchio, V. F. (2020). A detailed review study on potential effects of microplastics and additives of concern on human health. International Journal of Environmental Research and Public Health, 17(4), 1212. https://doi.org/10.3390/ijerph17041212. E1212 [pii].
Carbery, M., O'Connor, W., & Palanisami, T. (2018). Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environment International, 115, 400–409.
Chae, Y., Kim, D., Kim, S. W., & An, Y. J. (2018). Trophic transfer and individual impact of nano- sized polystyrene in a four-species freshwater food chain. Scientific Reports., 8, 284. https://doi.org/10.1038/s41598,017-18849-y
Chen, Q., Gundlach, M., Yang, S., Jiang, J., Velki, M., Yin, D., & Hollert, H. (2017). Quantitative investigation of the mechanisms of microplastics and nanoplastics toward zebrafish larvae locomotor activity. Science of the Total Environment, 584, 1022–1031.
Chen, W., Ouyang, Z. Y., Qian, C., & Yu, H. Q. (2018). Induced structural changes of humic acid by exposure of polystyrene microplastics: A spectroscopic insight. Environmental Pollution, 233, 1–7.
Chen, Y., Ling, Y., Li, X., Hu, J., Cao, C., & He, D. (2020). Size-dependent cellular internalization and effects of polystyrene microplastics in microalgae P. helgolandica var. tsingtaoensis and S. quadricauda. Journal of Hazardous Materials, 399, 123092.
Chiu, H. W., Xia, T., Lee, Y. H., Chen, C. W., Tsai, J. C., & Wang, Y. J. (2015). Cationic polystyrene nanospheres induce autophagic cell death through the induction of endoplasmic reticulum stress. Nanoscale., 7, 736–746. https://doi.org/10.1039/c4nr05509h
Coffin, S., Wyer, H., & Leapman, J. C. (2021). Addressing the environmental and health impacts of microplastics requires open collaboration between diverse sectors. PLoS Biology, 19(3), e3000932.
Cole, M., Lindeque, P., Halsband, C., & Galloway, T. S. (2011). Microplastics as contaminants in the marine environment: A review. Marine Pollution Bulletin, 62, 2588–2597. https://doi.org/10.1016/j.marpolbul.2011.09.025
Cole, M., Lindeque, P., Fileman, E., Halsband, C., Goodhead, R., Moger, J., & Galloway, T. S. (2013). Microplastic ingestion by zooplankton. Environmental Science & Technology, 47(12), 6646–6655.
Collado-Gonzalez, M., Gonzalez Espinosa, Y., & Goycoolea, F. M. (2019). Interaction between chitosan and mucin: Fundamentals and applications. Biomimetics (Basel), 4. https://doi.org/10.3390/biomimetics4020032. E32 [pii].
Conti, G. O., Ferrante, M., Banni, M., Favara, C., Nicolosi, I., Cristaldi, A., et al. (2020). Micro-and nano-plastics in edible fruit and vegetables. The first diet risks assessment for the general population. Environmental Research, 187, 109677.
Cooper, D. A., & Corcoran, P. L. (2010). Effects of mechanical and chemical processes on the degradation of plastic beach debris on the island of Kauai, Hawaii. Marine Pollution Bulletin, 60(5), 650–654.
Correia, M., & Loeschner, K. (2018). Detection of nanoplastics in food by asymmetric flow field-flow fractionation coupled to multi-angle light scattering: Possibilities, challenges and analytical limitations. Analytical and Bioanalytical Chemistry, 410(22), 5603–5615.
De Souza Machado, A. A., Kloas, W., Zarfl, C., Hempel, S., & Rillig, M. C. (2018). Microplastics as an emerging threat to terrestrial ecosystems. Global Change Biology, 24, 1405–1416. https://doi.org/10.1111/gcb.14020
De Toni, L., Tisato, F., Seraglia, R., Roverso, M., Gandin, V., Marzano, C., Padrini, R., & Foresta, C. (2017). Phthalates and heavy metals as endocrine disruptors in food: A study on pre-packed coffee products. Toxicology Reports, 4, 234–239. https://doi.org/10.1016/j.toxrep.2017.05.004
Deme, G. G., Ewusi-Mensah, D., Olagbaju, O. A., Okeke, E. S., Okoye, C. O., Odii, E. C., et al. (2022). Macro problems from microplastics: Toward a sustainable policy framework for managing microplastic waste in Africa. Science of the Total Environment, 804, 150170.
Deng, Y., Zhang, Y., Lemos, B., & Ren, H. (2017). Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Scientific Reports, 7(1), 1–10.
Deng, Y., Zhang, J., Zhang, C., Ding, Z., Hao, C., & An, L. (2020). Countermeasures on plastic and microplastic garbage management. In Microplastics in terrestrial environments: Emerging contaminants and major challenges (pp. 447–469). Springer.
Duan, Z., Duan, X., Zhao, S., Wang, X., Wang, J., Liu, Y., et al. (2020). Barrier function of zebrafish embryonic chorions against microplastics and nanoplastics and its impact on embryo development. Journal of Hazardous Materials, 395, 122621.
EFSA Panel on Contaminants in the Food Chain. (2016). Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA Journal, 14, 1–30. https://doi.org/10.2903/j.efsa.2016.4501
Ekkapongpisit, M., Giovia, A., Follo, C., Caputo, G., & Isidoro, C. (2012). Biocompatibility, endocytosis, and intracellular trafficking of mesoporous silica and polystyrene nanoparticles in ovarian cancer cells: Effects of size and surface charge groups. International Journal of Nanomedicine, 7, 4147–4158. https://doi.org/10.2147/IJN.S33803
Eriksen, M., Mason, S., Wilson, S., Box, C., Zellers, A., Edwards, W., et al. (2013). Microplastic pollution in the surface waters of the Laurentian Great Lakes. Marine Pollution Bulletin, 77(1–2), 177–182.
Fazlollahi, F., Angelow, S., Yacobi, N. R., Marchelletta, R., Yu, A. S. L., Hamm-Alvarez, S. F., Borok, Z., Kim, K., & Crandall, E. D. (2011). Polystyrene nanoparticle trafficking across MDCK-II. Nanomedicine: Nanotechnology, Biology and Medicine, 7, 588–594. https://doi.org/10.1016/j.nano.2011.01.008
Feng, L., Li, J., Xu, E. G., Sun, X., Zhu, F., Ding, Z., Tian, H., Dong, S., Xiaa, P., & Yuan, X. (2019). Short-term exposure to positively charged polystyrene nanoparticles causes oxidative stress and membrane destruction in cyanobacteria. Environmental Science: Nano, 6, 3072–3079. https://doi.org/10.1039/C9EN00807A
Fiorentino, I., Gualtieri, R., Barbato, V., Mollo, V., Braun, S., Angrisani, A., Turano, M., Furia, M., Netti, P. A., Guarnieri, D., Fusco, S., & Talevi, R. (2015). Energy independent uptake and release of polystyrene nanoparticles in primary mammalian cell cultures. Experimental Cell Research, 330, 240–247. https://doi.org/10.1016/j.yexcr.2014.09.017
Firdessa, R., Oelschlaeger, T. A., & Moll, H. (2014). Identification of multiple cellular uptake pathways of polystyrene nanoparticles and factors affecting the uptake: Relevance for drug delivery systems. European Journal of Cell Biology, 93, 323–337. https://doi.org/10.1016/j.ejcb.2014.08.001
Fischer, M., & Scholz-Böttcher, B. M. (2017). Simultaneous trace identification and quantification of common types of microplastics in environmental samples by pyrolysis-gas chromatography–mass spectrometry. Environmental Science & Technology, 51(9), 5052–5060.
Florence, A. T., Hillery, A. M., Hussain, N., & Jani, P. U. (1995). Factors affecting the oral uptake and translocation of polystyrene nanoparticles: Histological and analytical evidence. Journal of drug targeting, 3, 65–70. https://doi.org/10.3109/10611869509015936
Foroozandeh, P., & Aziz, A. A. (2018). Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Research Letters, 13, 339. https://doi.org/10.1186/s11671-0182728-6
Forte, M., Iachetta, G., Tussellino, M., Carotenuto, R., Prisco, M., De Falco, M., et al. (2016). Polystyrene nanoparticles internalization in human gastric adenocarcinoma cells. Toxicology In Vitro, 31, 126–136.
Freeman, S., Booth, A. M., Sabbah, I., Tiller, R., Dierking, J., Klun, K., et al. (2020). Between source and sea: The role of wastewater treatment in reducing marine microplastics. Journal of Environmental Management, 266, 110642.
Fries, E., Dekiff, J. H., Willmeyer, J., Nuelle, M. T., Ebert, M., & Remy, D. (2013). Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environmental Science: Processes & Impacts, 15(10), 1949–1956.
Frohlich, E. (2012). The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. International Journal of Nanomedicine, 7, 5577–5591. https://doi.org/10.2147/IJN.S36111
Fu, W., Min, J., Jiang, W., Li, Y., & Zhang, W. (2020). Separation, characterization and identification of microplastics and nanoplastics in the environment. Science of the Total Environment, 721, 137561.
Fuchs, A. K., Syrovets, T., Haas, K. A., Loos, C., Musyanovych, A., Mailänder, V., et al. (2016). Carboxyl-and amino-functionalized polystyrene nanoparticles differentially affect the polarization profile of M1 and M2 macrophage subsets. Biomaterials, 85, 78–87.
Gago, J., Booth, A. M., Tiller, R., Maes, T., & Larreta, J. (2020). Microplastics pollution and regulation. In Handbook of microplastics in the environment (pp. 1–27). Springer.
Ganesan, S., Comstock, A. T., & Sajjan, U. S. (2013). Barrier function of airway tract epithelium. Tissue Barriers, 1, e24997. https://doi.org/10.4161/tisb.24997
Gasperi, J., Wright, S. L., Dris, R., Collard, F., Mandin, C., Guerrouache, M., Langlois, V., Kelly, F. J., & Tassin, B. (2018). Microplastics in air: Are we breathing it in? Current Opinion in Environmental Science & Health, 1, 1–5. https://doi.org/10.1016/j.coesh.2017.10.002
Gaylor, M. O., Harvey, E., & Hale, R. C. (2013). Polybrominated diphenyl ether (PBDE) accumulation by earthworms (Eisenia fetida) exposed to biosolids-, polyurethane foam microparticle-, and penta-BDE-amended soils. Environmental Science & Technology, 47(23), 13831–13839.
Gigault, J., & Budzinski, H. (2016). Selection of an appropriate aqueous nano-fullerene (nC60) preparation protocol for studying its environmental fate and behavior. TrAC Trends in Analytical Chemistry, 80, 1–11.
Gigault, J., El Hadri, H., Reynaud, S., Deniau, E., & Grassl, B. (2017). Asymmetrical flow field flow fractionation methods to characterize submicron particles: Application to carbon-based aggregates and nanoplastics. Analytical and Bioanalytical Chemistry, 409(29), 6761–6769.
Gillibert, R., Balakrishnan, G., Deshoules, Q., Tardivel, M., Magazzù, A., Donato, M. G., et al. (2019). Raman tweezers for small microplastics and nanoplastics identification in seawater. Environmental Science & Technology, 53(15), 9003–9013.
Goldstein, J. I., Newbury, D. E., Michael, J. R., Ritchie, N. W., Scott, J. H. J., & Joy, D. C. (2017). Scanning electron microscopy and X-ray microanalysis. Springer.
González-Pleiter, M., Tamayo-Belda, M., Pulido-Reyes, G., Amariei, G., Leganés, F., Rosal, R., & Fernández-Piñas, F. (2019). Secondary nanoplastics released from a biodegradable microplastic severely impact freshwater environments. Environmental Science: Nano, 6(5), 1382–1392.
Gopinath, P. M., Saranya, V., Vijayakumar, S., Mythili Meera, M., Ruprekha, S., Kunal, R., Pranay, A., Thomas, J., Mukherjee, A., & Chandrasekaran, N. (2019). Assessment on interactive prospectives of nanoplastics with plasma proteins and the toxicological impacts of virgin, coronated and environmentally released-nanoplastics. Scientific Reports, 9, 8860, 019-45139-6. https://doi.org/10.1038/s41598-019-45139-6
Green, T. R., Fisher, J., Stone, M., Wroblewski, B. M., & Ingham, E. (1998). Polyethylene particles of a ‘critical size’are necessary for the induction of cytokines by macrophages in vitro. Biomaterials, 19(24), 2297–2302.
Hahladakis, J. N., Velis, C. A., Weber, R., Iacovidou, E., & Purnell, P. (2018). An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. Journal of Hazardous Materials., 344, 179–199. https://doi.org/10.1016/j.jhazmat.2017.10.014
Hale, R. C., Seeley, M. E., La Guardia, M. J., Mai, L., & Zeng, E. Y. (2020). A global perspective on microplastics. Journal of Geophysical Research: Oceans, 125, 1–40. https://doi.org/10.1029/2018JC014719
Heinlaan, M., Kasemets, K., Aruoja, V., Blinova, I., Bondarenko, O., Lukjanova, A., Khosrovyan, A., Kurvet, I., Pullerits, M., Sihtmae, M., Vasiliev, G., Vija, H., & Kahru, A. (2020). Hazard evaluation of polystyrene nanoplastic with nine bioassays did not show particle-specific acute toxicity. Science of the Total Environment, 707, 136073. S0048 9697(19)36069-3 [pii].
Henderson, R. K., Baker, A., Murphy, K. R., Hambly, A., Stuetz, R. M., & Khan, S. J. (2009). Fluorescence as a potential monitoring tool for recycled water systems: A review. Water Research, 43(4), 863–881.
Hesler, M., Aengenheister, L., Ellinger, B., Drexel, R., Straskraba, S., Jost, C., Wagner, S., Meier, F., von Briesen, H., Buchel, C., Wick, P., Buerki-Thurnherr, T., & Kohl, Y. (2019). Multi- endpoint toxicological assessment of polystyrene nano- and microparticles in different biological models in vitro. Toxicology In Vitro, 61, 104610. S0887-2333(19)30260-7 [pii].
Hidalgo-Ruz, V., Gutow, L., Thompson, R. C., & Thiel, M. (2012). Microplastics in the marine environment: A review of the methods used for identification and quantification. Environmental Science & Technology, 46(6), 3060–3075.
Hollman, P. C., Bouwmeester, H., & Peters, R. J. B. (2013). Microplastics in aquatic food chain: Sources, measurement, occurrence and potential health risks (No. 2013.003). Rikilt-Institute of Food Safety.
Holloczki, O., & Gehrke, S. (2020). Can nanoplastics alter cell membranes? ChemPhysChem, 21, 9–12. https://doi.org/10.1002/cphc.201900481
Huerta Lwanga, E., Gertsen, H., Gooren, H., Peters, P., Salánki, T., Van Der Ploeg, M., et al. (2016). Microplastics in the terrestrial ecosystem: Implications for Lumbricus terrestris (Oligochaeta, Lumbricidae). Environmental Science & Technology, 50(5), 2685–2691.
Hwang, J., Choi, D., Han, S., Choi, J., & Hong, J. (2019). An assessment of the toxicity of polypropylene microplastics in human derived cells. Science of the Total Environment, 684, 657–669. https://doi.org/10.1016/j.scitotenv.2019.05.071
Hwang, J., Choi, D., Han, S., Jung, S. Y., Choi, J., & Hong, J. (2020). Potential toxicity of polystyrene micro plastic particles. Scientific Reports, 10, 7391. https://doi.org/10.1038/s41598-02064464-9
Imhof, H. K., Laforsch, C., Wiesheu, A. C., Schmid, J., Anger, P. M., Niessner, R., & Ivleva, N. P. (2016). Pigments and plastic in limnetic ecosystems: A qualitative and quantitative study on microparticles of different size classes. Water Research, 98, 64–74.
Inkielewicz-Stepniak, I., Tajber, L., Behan, G., Zhang, H., Radomski, M. W., Medina, C., & Santos-Martinez, M. J. (2018). The role of mucin in the toxicological impact of polystyrene nanoparticles. Materials, 11(5), 724.
Jenkins, P. G., Howard, K. A., Blackhall, N. W., Thomas, N. W., Davis, S. S., & O’Hagan, D. T. (1994). The quantitation of the absorption of microparticles into the intestinal lymph of Wistar rats. International Journal of Pharmaceutics, 102, 261–266. https://doi.org/10.1016/0378-5173(94)90064-7
Jin, Y., Lu, L., Tu, W., Luo, T., & Fu, Z. (2019). Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Science of the Total Environment, 649, 308–317.
Karami, A., Golieskardi, A., Keong Choo, C., Larat, V., Galloway, T. S., & Salamatinia, B. (2017). The presence of microplastics in commercial salts from different countries. Scientific Reports, 7, 46173. https://doi.org/10.1038/srep46173
Karas, M., & Krüger, R. (2003). Ion formation in MALDI: The cluster ionization mechanism. Chemical Reviews, 103(2), 427–440.
Kedzierski, M., Lechat, B., Sire, O., Le Maguer, G., Le Tilly, V., & Bruzaud, S. (2020). Microplastic contamination of packaged meat: Occurrence and associated risks. Food Packaging and Shelf Life, 24, 100489.
Kim, S. W., & An, Y. J. (2019). Soil microplastics inhibit the movement of springtail species. Environment International, 126, 699–706.
Kirstein, I. V., Kirmizi, S., Wichels, A., Garin-Fernandez, A., Erler, R., Loder, M., & Gerdts, G. (2016). Dangerous hitchhikers? Evidence for potentially pathogenic Vibrio spp. on microplastic particles. Marine Environmental Research, 120, 1–8. https://doi.org/10.1016/j.marenvres.2016.07.004
Koelmans, A. A., Besseling, E., & Shim, W. J. (2015). Nanoplastics in the aquatic environment. Critical review. Marine Anthropogenic Litter, 325–340.
Kosuth, M., Mason, S. A., & Wattenberg, E. V. (2018). Anthropogenic contamination of tap water, beer, and sea salt. PLoS One, 13(4), e0194970.
Legler, J., & Brouwer, A. (2003). Are brominated flame retardants endocrine disruptors? Environment International, 29, 879–885. S0160-4120(03)00104-1 [pii].
Li, S., & Malmstadt, N. (2013). Deformation and poration of lipid bilayer membranes by cationic nanoparticles. Soft Matter, 9, 4969–4976. https://doi.org/10.1039/C3SM27578G
Liao, Y., & Yang, J. (2020). Microplastic serves as a potential vector for Cr in an in-vitro human digestive model. Science of the Total Environment, 703, 134805. https://doi.org/10.1016/j.scitotenv.2019.134805
Lithner, D., Larsson, Å., & Dave, G. (2011). Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Science of the Total Environment, 409, 3309–3324. https://doi.org/10.1016/j.scitotenv.2011.04.038
Liu, X., Tian, X., Xu, X., & Lu, J. (2018a). Design of a phosphinate-based bioluminescent probe for superoxide radical anion imaging in living cells. Luminescence, 33(6), 1101–1106.
Liu, J., Ma, Y., Zhu, D., Xia, T., Qi, Y., Yao, Y., Guo, X., Ji, R., & Chen, W. (2018b). Polystyrene nanoplastics-enhanced contaminant transport: Role of irreversible adsorption in glassy polymeric domain. Environmental Science & Technology, 52, 2677–2685. https://doi.org/10.1021/acs.est.7b05211
Llorca, M., & Farré, M. (2021). Current insights into potential effects of micro-nanoplastics on human health by in-vitro tests. Frontiers in Toxicology, 39.
Löder, M. G., & Gerdts, G. (2015). Methodology used for the detection and identification of microplastics—A critical appraisal. Marine Anthropogenic Litter, 201–227.
Loos, C., Syrovets, T., Musyanovych, A., Mailander, V., Landfester, K., Nienhaus, G. U., & Simmet, T. (2014a). Functionalized polystyrene nanoparticles as a platform for studying bio-nano interactions. Beilstein Journal of Nanotechnology, 5, 2403–2412. https://doi.org/10.3762/bjnano.5.250
Loos, C., Syrovets, T., Musyanovych, A., Mailänder, V., Landfester, K., & Simmet, T. (2014b). Amino-functionalized nanoparticles as inhibitors of mTOR and inducers of cell cycle arrest in leukemia cells. Biomaterials, 35, 1944–1953. https://doi.org/10.1016/j.biomaterials.2013.11.056
Lu, L., Wan, Z., Luo, T., Fu, Z., & Jin, Y. (2018). Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Science of the Total Environment, 631, 449–458.
Luo, T., Zhang, Y., Wang, C., Wang, X., Zhou, J., Shen, M., et al. (2019). Maternal exposure to different sizes of polystyrene microplastics during gestation causes metabolic disorders in their offspring. Environmental Pollution, 255, 113122.
Ma, H., Pu, S., Liu, S., Bai, Y., Mandal, S., & Xing, B. (2020). Microplastics in aquatic environments: Toxicity to trigger ecological consequences. Environmental Pollution, 261, 114089.
Mahadevan, G., & Valiyaveettil, S. (2021). Understanding the interactions of poly (methyl methacrylate) and poly (vinyl chloride) nanoparticles with BHK-21 cell line. Scientific Reports, 11(1), 1–15.
Mahler, G. J., Esch, M. B., Tako, E., Southard, T. L., Archer, S. D., Glahn, R. P., & Shuler, M. L. (2012). Oral exposure to polystyrene nanoparticles affects iron absorption. Nature Nanotechnology, 7(4), 264–271.
Marti, E., Martin, C., Galli, M., Echevarría, F., Duarte, C. M., & Cózar, A. (2020). The colors of the ocean plastics. Environmental Science & Technology, 54(11), 6594–6601.
Mason, S. A., Welch, V. G., & Neratko, J. (2018). Synthetic polymer contamination in bottled water. Frontiers in Chemistry., 6, 407. https://doi.org/10.3389/fchem.2018.00407
Massos, A., & Turner, A. (2017). Cadmium, lead and bromine in beached microplastics. Environmental Pollution, 227, 139–145.
McCarthy, J., Gong, X., Nahirney, D., Duszyk, M., & Radomski, M. W. (2011). Polystyrene nanoparticles activate ion transport in human airway epithelial cells. International Journal of Nanomedicine, 6, 1343.
Mendoza, L. M. R., & Jones, P. R. (2015). Characterisation of microplastics and toxic chemicals extracted from microplastic samples from the North Pacific Gyre. Environmental Chemistry, 12(5), 611–617.
Mintenig, S. M., Bäuerlein, P. S., Koelmans, A. A., Dekker, S. C., & Van Wezel, A. P. (2018). Closing the gap between small and smaller: Towards a framework to analyse nano-and microplastics in aqueous environmental samples. Environmental Science: Nano, 5(7), 1640–1649.
Mintenig, S. M., Löder, M. G. J., Primpke, S., & Gerdts, G. (2019). Low numbers of microplastics detected in drinking water from ground water sources. Science of the Total Environment, 648, 631–635.
Mitrano, D. M., & Wohlleben, W. (2020). Microplastic regulation should be more precise to incentivize both innovation and environmental safety. Nature Communications, 11(1), 1–12.
Mitrano, D. M., Beltzung, A., Frehland, S., Schmiedgruber, M., Cingolani, A., & Schmidt, F. (2019). Synthesis of metal-doped nanoplastics and their utility to investigate fate and behaviour in complex environmental systems. Nature Nanotechnology, 14(4), 362–368.
Mrakovcic, M., Absenger, M., Riedl, R., Smole, C., Roblegg, E., Fröhlich, L. F., & Fröhlich, E. (2013). Assessment of long-term effects of nanoparticles in a microcarrier cell culture system. Plos One, 8, e56791. https://doi.org/10.1371/journal.pone.0056791
Mrakovcic, M., Meindl, C., Roblegg, E., & Fröhlich, E. (2014). Reaction of monocytes to polystyrene and silica nanoparticles in short-term and long-term exposures. Toxicology Research, 3, 86–97. https://doi.org/10.1039/C3TX50112D
Naji, A., Nuri, M., & Vethaak, A. D. (2018). Microplastics contamination in molluscs from the northern part of the Persian Gulf. Environmental Pollution, 235, 113–120.
Nalbone, L., Cincotta, F., Giarratana, F., Ziino, G., & Panebianco, A. (2021). Microplastics in fresh and processed mussels sampled from fish shops and large retail chains in Italy. Food Control, 125, 108003.
National Oceanic and Atmospheric Administration. (2019). A guide to plastic in the ocean. https://oceanservice.noaa.gov/hazards/marinedebris/plastics-in-the-ocean.html. Accessed 9 Mar 2020.
Nelms, S. E., Galloway, T. S., Godley, B. J., Jarvis, D. S., & Lindeque, P. K. (2018). Investigating microplastic trophic transfer in marine top predators. Environmental Pollution, 238, 999–1007. https://doi.org/10.1016/j.envpol.2018.02.016
Nich, C., & Goodman, S. B. (2014). Role of macrophages in the biological reaction to wear debris from joint replacements. Journal of Long-Term Effects of Medical Implants, 24(4), 259.
NJDEP-Science Advisory Board. (2015). Human health impacts of microplastics and nano plastics.
Nowak, M., Brown, T. D., Graham, A., Helgeson, M. E., & Mitragotri, S. (2019). Size, shape, and flexibility influence nanoparticle transport across brain endothelium under flow. Bioengineering & Translational Medicine, 1–11. https://doi.org/10.1002/btm2.10153
Okubo, N., Takahashi, S., & Nakano, Y. (2018). Microplastics disturb the anthozoan-algae symbiotic relationship. Marine Pollution Bulletin, 135, 83–89.
Paget, V., Dekali, S., Kortulewski, T., Grall, R., Gamez, C., Blazy, K., et al. (2015). Specific uptake and genotoxicity induced by polystyrene nanobeads with distinct surface chemistry on human lung epithelial cells and macrophages. PLoS One, 10(4), e0123297.
Pauly, J. L., Stegmeier, S. J., Allaart, H. A., Cheney, R. T., Zhang, P. J., Mayer, A. G., & Streck, R. J. (1998). Inhaled cellulosic and plastic fibers found in human lung tissue. Cancer Epidemiology, Biomarkers & Prevention, 7, 419–428.
Pedersen, A. F., Gopalakrishnan, K., Boegehold, A. G., Peraino, N. J., Westrick, J. A., & Kashian, D. R. (2020). Microplastic ingestion by quagga mussels, Dreissena bugensis, and its effects on physiological processes. Environmental Pollution, 260, 113964.
Peng, G., Bellerby, R., Zhang, F., Sun, X., & Li, D. (2020). The ocean’s ultimate trashcan: Hadal trenches as major depositories for plastic pollution. Water Research, 168, 115121.
Philippe, A., Gangloff, M., Rakcheev, D., & Schaumann, G. E. (2014). Evaluation of hydrodynamic chromatography coupled with inductively coupled plasma mass spectrometry detector for analysis of colloids in environmental media–effects of colloid composition, coating and shape. Analytical Methods, 6(21), 8722–8728.
Pimentel, J. C., Avila, R., & Lourenço, A. G. (1975). Respiratory disease caused by synthetic fibers: a new occupational disease. Thorax, 30, 204–219. https://doi.org/10.1136/thx.30.2.204
Pirok, B. W., Abdulhussain, N., Aalbers, T., Wouters, B., Peters, R. A., & Schoenmakers, P. J. (2017). Nanoparticle analysis by online comprehensive two-dimensional liquid chromatography combining hydrodynamic chromatography and size-exclusion chromatography with intermediate sample transformation. Analytical Chemistry, 89(17), 9167–9174.
Poon, C. K., Tang, O., Chen, X. M., Pham, B. T., Gody, G., Pollock, C. A., Hawkett, B. S., & Perrier, S. (2016). Preparation of inert polystyrene latex particles as MicroRNA delivery vectors by surfactant-free RAFT emulsion polymerization. Biomacromolecules, 17, 965–973. https://doi.org/10.1021/acs.biomac.5b01633
Prata, J. C., da Costa, J. P., Lopes, I., Duarte, A. C., & Rocha-Santos, T. (2020). Environmental exposure to microplastics: An overview on possible human health effects. Science of the Total Environment, 702, 134455. https://doi.org/10.1016/j.scitotenv.2019.134455
Prietl, B., Meindl, C., Roblegg, E., Pieber, T. R., Lanzer, G., & Fröhlich, E. (2014). Nano-sized and micro-sized polystyrene particles affect phagocyte function. Cell Biology and Toxicology, 30(1), 1–16.
Prinz, N., & Korez, Š. (2020). Understanding how microplastics affect marine biota on the cellular level is important for assessing ecosystem function: A review. In S. Jungblut, V. Liebich, & M. Bode-Dalby (Eds.), YOUMARES 9 – The oceans: Our research, our future (pp. 101–120). Springer. https://doi.org/10.1007/978-3-030-20389-4_6
Qiu, Q., Peng, J., Yu, X., Chen, F., Wang, J., & Dong, F. (2015). Occurrence of microplastics in the coastal marine environment: First observation on sediment of China. Marine Pollution Bulletin, 98(1–2), 274–280.
Reineke, J. J., Cho, D. Y., Dingle, Y. T., Morello, A. P., 3rd, Jacob, J., Thanos, C. G., & Mathiowitz, E. (2013). Unique insights into the intestinal absorption, transit, and subsequent biodistribution of polymer-derived microspheres. Proceedings of the National Academy of Sciences, 110, 13803–13808. https://doi.org/10.1073/pnas.1305882110
Reinholz, J., Diesler, C., Schöttler, S., Kokkinopoulou, M., Ritz, S., Landfester, K., & Mailänder, V. (2018). Protein machineries defining pathways of nanocarrier exocytosis and transcytosis. Acta Biomaterialia, 71, 432–443. https://doi.org/10.1016/j.actbio.2018.03.006
Rillig, M. C., & Lehmann, A. (2020). Microplastic in terrestrial ecosystems. Science, 368(6498), 1430–1431.
Rodrigues, J. P., Duarte, A. C., Santos-Echeandía, J., & Rocha-Santos, T. (2019). Significance of interactions between microplastics and POPs in the marine environment: A critical overview. TrAC Trends in Analytical Chemistry, 111, 252–260. https://doi.org/10.1016/j.trac.2018.11.038
Rodriguez-Seijo, A., Lourenço, J., Rocha-Santos, T. A. P., Da Costa, J., Duarte, A. C., Vala, H., & Pereira, R. (2017). Histopathological and molecular effects of microplastics in Eisenia andrei Bouché. Environmental Pollution, 220, 495–503.
Rubin, B. S. (2011). Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. The Journal of Steroid Biochemistry and Molecular Biology, 127, 27–34. https://doi.org/10.1016/j.jsbmb.2011.05.002
Rubio, L., Marcos, R., & Hernandez, A. (2020). Potential adverse health effects of ingested micro- and nanoplastics on humans. Lessons learned from in vivo and in vitro mammalian models. Journal of Toxicology and Environmental Health: Part B, Critical Reviews, 23, 51–68. https://doi.org/10.1080/10937404.2019.1700598
Schirinzi, G. F., Perez-Pomeda, I., Sanchis, J., Rossini, C., Farre, M., & Barcelo, D. (2017). Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environmental Research, 159, 579–587. S0013-9351(17)31077-0 [pii].
Schwabl, P., Koppel, S., Konigshofer, P., Bucsics, T., Trauner, M., Reiberger, T., & Liebmann, B. (2019). Detection of various microplastics in human stool: A prospective case series. Annals of Internal Medicine. https://doi.org/10.7326/M19-0618
Schymanski, D., Goldbeck, C., Humpf, H. U., & Fürst, P. (2018). Analysis of microplastics in water by micro-Raman spectroscopy: Release of plastic particles from different packaging into mineral water. Water Research, 129, 154–162.
Sharma, S., & Chatterjee, S. (2017). Microplastic pollution, a threat to marine ecosystem and human health: A short review. Environmental Science and Pollution Research International, 24, 21530–21547. https://doi.org/10.1007/s11356-017-9910-8
Sharma, S., Sharma, V., & Chatterjee, S. (2021). Microplastics in the Mediterranean Sea: Sources, pollution intensity, sea health, and regulatory policies. Frontiers in Marine Science, 8, 634934.
Shim, W. J., Hong, S. H., & Eo, S. E. (2017). Identification methods in microplastic analysis: A review. Analytical Methods, 9(9), 1384–1391.
Siddiqui, M. R., AlOthman, Z. A., & Rahman, N. (2017). Analytical techniques in pharmaceutical analysis: A review. Arabian Journal of Chemistry, 10, S1409–S1421.
Smith, M., Love, D. C., Rochman, C. M., & Neff, R. A. (2018). Microplastics in seafood and the implications for human health. Current Environmental Health Reports, 5, 375–386. https://doi.org/10.1007/s40572-018-0206-z
Sobhani, Z., Zhang, X., Gibson, C., Naidu, R., Megharaj, M., & Fang, C. (2020). Identification and visualisation of microplastics/nanoplastics by Raman imaging (i): Down to 100 nm. Water Research, 174, 115658.
Song, Y. K., Hong, S. H., Jang, M., Han, G. M., Rani, M., Lee, J., & Shim, W. J. (2015). A comparison of microscopic and spectroscopic identification methods for analysis of microplastics in environmental samples. Marine Pollution Bulletin, 93(1–2), 202–209.
Stapleton, P. A. (2019). Toxicological considerations of nano-sized plastics. AIMS Environmental Science, 6, 367–378. https://doi.org/10.3934/environsci.2019.5.367
Stuart, B. (2005). Infrared spectroscopy. Kirk-Othmer encyclopedia of chemical technology. Wiley.
Su, L., Deng, H., Li, B., Chen, Q., Pettigrove, V., Wu, C., & Shi, H. (2019). The occurrence of microplastic in specific organs in commercially caught fishes from coast and estuary area of east China. Journal of Hazardous Materials, 365, 716–724.
Sun, J., Dai, X., Wang, Q., van Loosdrecht, M. C., & Ni, B. J. (2019). Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Research, 152, 21–37.
Sweden, K. I. M. O. (2007). Small plastic particles in Coastal Swedish waters. N-Research Report.
Teng, J., Wang, Q., Ran, W., Wu, D., Liu, Y., Sun, S., et al. (2019). Microplastic in cultured oysters from different coastal areas of China. Science of the Total Environment, 653, 1282–1292.
Toussaint, B., Raffael, B., Angers-Loustau, A., Gilliland, D., Kestens, V., Petrillo, M., Rio-Echevarria, I. M., & Van den Eede, G. (2019). Review of micro- and nanoplastic contamination in the food chain. Food Additives & Contaminants: Part A Chemistry, Analysis, Control, Exposure & Risk Assessment., 36, 639–673. https://doi.org/10.1080/19440049.2019.1583381
Varela, J. A., Bexiga, M. G., Aberg, C., Simpson, J. C., & Dawson, K. A. (2012). Quantifying size- dependent interactions between fluorescently labeled polystyrene nanoparticles and mammalian cells. Journal of Nanobiotechnology, 10, 39, 3155-10-39. https://doi.org/10.1186/1477-3155-10-39
Velzeboer, I., Kwadijk, C. J., & Koelmans, A. A. (2014). Strong sorption of PCBs to nanoplastics, microplastics, carbon nanotubes, and fullerenes. Environmental Science & Technology, 48, 4869–4876. https://doi.org/10.1021/es405721v
Vianello, A., Boldrin, A., Guerriero, P., Moschino, V., Rella, R., Sturaro, A., & Da Ros, L. (2013). Microplastic particles in sediments of Lagoon of Venice, Italy: First observations on occurrence, spatial patterns and identification. Estuarine, Coastal and Shelf Science, 130, 54–61.
Vidal, J. (2020). The plastic polluters won 2019 – and we’re running out of time to stop them. https://www.theguardian.com/environment/2020/jan/02/year-plastic-pollution-clean-beaches-seas. Accessed 9 Mar 2020.
Walczak, A. P., Hendriksen, P. J., Woutersen, R. A., van der Zande, M., Undas, A. K., Helsdingen, R., van den Berg, H. H., Rietjens, I. M., & Bouwmeester, H. (2015). Bioavailability and biodistribution of differently charged polystyrene nanoparticles upon oral exposure in rats. Journal of Nanoparticle Research, 17, 231015-3029-y. Epub 2015 May 22. https://doi.org/10.1007/s11051-015-3029-y
Wang, F., Salvati, A., & Boya, P. (2018a). Lysosome-dependent cell death and deregulated autophagy induced by amine-modified polystyrene nanoparticles. Open Biology, 8. https://doi.org/10.1098/rsob.170271. 170271 [pii].
Wang, J., Zheng, L., & Li, J. (2018b). A critical review on the sources and instruments of marine microplastics and prospects on the relevant management in China. Waste Management & Research, 36(10), 898–911.
Wang, J., Qin, X., Guo, J., Jia, W., Wang, Q., Zhang, M., & Huang, Y. (2020). Evidence of selective enrichment of bacterial assemblages and antibiotic resistant genes by microplastics in urban rivers. Water Research, 183, 116113.
Westerhoff, P., Prapaipong, P., Shock, E., & Hillaireau, A. (2008). Antimony leaching from polyethylene terephthalate (PET) plastic used for bottled drinking water. Water Research, 42, 551–556. https://doi.org/10.1016/j.watres.2007.07.048
Wilkinson, B. (2019, July 12). Conservation captured microplastics- are they in your food? Two Oceans Aquarium. https://www.aquarium.co.za/blog/entry/microplastics-are-they-in-your-food
World Economic Forum. (2016). The new plastics economy: Rethinking the future of plastics. http://www3.weforum.org/docs/WEF_The_New_Plastics_Economy.pdf. Accessed 25 Mar 2020.
Wright, S. L., & Kelly, F. J. (2017). Plastic and human health: A micro issue? Environmental Science & Technology, 51, 6634–6647. https://doi.org/10.1021/acs.est.7b00423
Xia, T., Kovochich, M., Liong, M., Zink, J. I., & Nel, A. E. (2008). Cationic polystyrene nanosphere toxicity depends on cell-specific endocytic and mitochondrial injury pathways. ACS Nano, 2(1), 85–96.
Xia, L., Gu, W., Zhang, M., Chang, Y. N., Chen, K., Bai, X., et al. (2016). Endocytosed nanoparticles hold endosomes and stimulate binucleated cells formation. Particle and Fibre Toxicology, 13(1), 1–12.
Xu, Y., Chan, F. K. S., He, J., Johnson, M., Gibbins, C., Kay, P., et al. (2021). A critical review of microplastic pollution in urban freshwater environments and legislative progress in China: Recommendations and insights. Critical Reviews in Environmental Science and Technology, 51(22), 2637–2680.
Yacobi, N. R., Malmstadt, N., Fazlollahi, F., DeMaio, L., Marchelletta, R., Hamm-Alvarez, S. F., Borok, Z., Kim, K. J., & Crandall, E. D. (2010). Mechanisms of alveolar epithelial translocation of a defined population of nanoparticles. American Journal of Respiratory Cell and Molecular Biology, 42, 604–614. https://doi.org/10.1165/rcmb.2009-0138OC
Yang, Y. F., Chen, C. Y., Lu, T. H., & Liao, C. M. (2019a). Toxicity-based toxicokinetic/toxicodynamic assessment for bioaccumulation of polystyrene microplastics in mice. Journal of Hazardous Materials, 366, 703–713. S0304-3894(18)31188-9 [pii].
Yang, L., Li, K., Cui, S., Kang, Y., An, L., & Lei, K. (2019b). Removal of microplastics in municipal sewage from China’s largest water reclamation plant. Water Research, 155, 175–181.
Yee, M. S. L., Hii, L. W., Looi, C. K., Lim, W. M., Wong, S. F., Kok, Y. Y., et al. (2021). Impact of microplastics and nanoplastics on human health. Nanomaterials, 11(2), 496.
Yong, C. Q. Y., Valiyaveetill, S., & Tang, B. L. (2020). Toxicity of microplastics and nanoplastics in mammalian systems. International Journal of Environmental Research and Public Health, 17. https://doi.org/10.3390/ijerph17051509. E1509 [pii].
Yu, P., Liu, Z., Wu, D., Chen, M., Lv, W., & Zhao, Y. (2018). Accumulation of polystyrene microplastics in juvenile Eriocheir sinensis and oxidative stress effects in the liver. Aquatic Toxicology, 200, 28–36.
Yu, F., Yang, C., Zhu, Z., Bai, X., & Ma, J. (2019). Adsorption behavior of organic pollutants and metals on micro/nanoplastics in the aquatic environment. Science of the Total Environment, 694, 133643. https://doi.org/10.1016/j.scitotenv.2019.133643
Zhang, B., Wu, D., Yang, X., Teng, J., Liu, Y., Zhang, C., et al. (2019). Microplastic pollution in the surface sediments collected from Sishili Bay, North Yellow Sea, China. Marine Pollution Bulletin, 141, 9–15.
Zhang, C., Wang, S., Pan, Z., Sun, D., Xie, S., Zhou, A., et al. (2020). Occurrence and distribution of microplastics in commercial fishes from estuarine areas of Guangdong, South China. Chemosphere, 260, 127656.
Zhao, L., Qu, M., Wong, G., & Wang, D. (2017). Transgenerational toxicity of nanopolystyrene particles in the range of μg L− 1 in the nematode Caenorhabditis elegans. Environmental Science: Nano, 4(12), 2356–2366.
Zheng, T., Yuan, D., & Liu, C. (2019). Molecular toxicity of nanoplastics involving in oxidative stress and desoxyribonucleic acid damage. Journal of Molecular Recognition, 32, e2804. https://doi.org/10.1002/jmr.2804
Zhou, X. X., Hao, L. T., Wang, H. Y. Z., Li, Y. J., & Liu, J. F. (2018). Cloud-point extraction combined with thermal degradation for nanoplastic analysis using pyrolysis gas chromatography–mass spectrometry. Analytical Chemistry, 91(3), 1785–1790.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
D, M., Tulasi, C.D.S.L.N., Chepuri, K. (2023). Cellular and Animal Toxicities of Micro- and Nanoplastics. In: Maddela, N.R., Reddy, K.V., Ranjit, P. (eds) Micro and Nanoplastics in Soil. Springer, Cham. https://doi.org/10.1007/978-3-031-21195-9_12
Download citation
DOI: https://doi.org/10.1007/978-3-031-21195-9_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-21194-2
Online ISBN: 978-3-031-21195-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)