Abstract
The presence of Bisphenol A (BPA) as a component in products for human use represents a risk to public health because it is an endocrine disruptor. For this reason, it is necessary to detect the presence of this compound in an easy, fast, and economical way. To aid in the detection of this substance, this work presents an unmodified screen-printed electrode-based sensor for electrochemical detection, simple, low-cost and affordable to detect BPA. The electrochemical detection is based on methylene blue as a redox probe, a sample analysis is carried out using three different electrochemical techniques, cyclic voltammetry, linear-sweep voltammetry and square-wave voltammetry. The results show the sensor’s ability to detect and quantify BPA. In this sense, our unmodified screen-printed electrode-based sensor is an attractive, cost-effective and low-cost tool to determine the presence of BPA.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Bisphenol A (BPA) is a synthetic chemical that is often combined with other substances, such as polycarbonate or epoxy resins, to make containers, that store food and beverages, medical material, protective coatings for cans, or bottles, to mention only a few [1]. However, BPA is an endocrine disruptor with anti-androgen estrogenic properties, which can cause adverse health effects in humans such as reproductive, metabolic, cardiovascular and mental problems [2, 3]. Nowadays, BPA is considered an emerging contaminant due to its high toxicity to humans, being a public health risk, for this reason, detecting BPA is a fundamental key starting point to contain the human consumption of contaminated products [4].
Currently, there are different transduction mechanisms for detecting BPA one can find capillary electrophoresis-mass spectrometry devices [5], chromatography assays [6], electrochemical-based sensor [7], to mention only a few, which have proven to be very effective. However, these methods need complicated pretreatments, large volumes of samples, sophisticated fabrication process, and high operating costs, furthermore, they hardly ever can be used for in-situ measurements. On the other hand, electrochemical methods offer advantages such as simple operation, low energy consumption, short analysis time, low-cost, high sensitivity and selectivity [2]. Among the most widely used electrochemical techniques for BPA detection are voltammetric techniques, which employ a measuring current at various potential points, and they are the most sensitive to detect BPA [8]. Usually, voltammetric measurements are made with a three-electrode electrochemical cell, using a potentiostat circuit. However, conventional three-electrode electrochemical cells are pen-like electrodes, called reference (RE), counter (CE) and working (WE), these electrodes are bulky, expensive and fragile, which are problems to integrate within a single device for portable and in situ experiments. In addition, measurements with different manufacturing materials and modification of the electrodes have been proposed for the electrochemical detection of BPA. Though they achieve good sensing features, the modified electrodes are expensive, difficult to manufacture and single-use devices. A powerful and attractive tool is the screen-printed electrodes (SPE) system, which embed the three electrodes on a single planar structure. The SPE allows the possibility of performing selective and sensitive analyses, low-cost, designed to work with micro-volumetric samples and without electrode pretreatment or maintenance [9]. Nevertheless, the main challenge of this type of device is the analysis of complex samples at the point of care and in the field, and a very important factor that determines and limits the electrochemical properties of the SPE is the material of the working electrode [10]. For this reason, an important task is to improve the characteristics of SPE-based sensors without chemically or biologically modification, without losing specificity. Thus, to increase the sensibility of the sensor is common to blend the solution with a redox probe that increases the load transfer over the surface of the electrodes [11].
Therefore, in this work, we propose a cost-effective and affordable unmodified screen-printed electrode-based sensor for electrochemical detection of BPA blended with a redox probe. We assess three different voltammetric techniques to evaluate, which is the best, through their sensitivity and detection limit.
The rest of the paper is organized as follows. The Sect. 2 is devoted the experimental methodology. Then, in Sect. 3, we show the experimetal results to characterized our sensor. Finally, we present the conclusion in Sect. 4.
2 Materials and Methods
2.1 Chemicals
Bisphenol A (LVF, ACS reagent) and ethanol (99.8\(\%\), w/v) were purchased from Sigma-Aldrich (St. Louis, USA). Methylene blue (MB, 1\(\%\), w/v) and distilled water (DI) were obtained from Meyer (Mexico City, Mexico).
2.2 Screen-Printed Electrodes
The sensitive electrodes are the element of recognition and they should be made of a specific material. For this task, we choose carbon as the sensitive material due to its advantages. The electrodes were fabricated using carbon paste, owing that is chemically inert, easy to electrochemically modify and economical [12]. Thus, we fabricated a screen-printed electrode system using low-cost technology over a polyethylene terephthalate (PET) substrate of 10 mm \(\times \) 4 mm dimensions. The working (WE) and counter (CE) electrodes were fabricated using carbon paste; meanwhile, the reference electrode (RE) was made of Ag/AgCl. Before using them, we washed the surface with 0.5 M sulphuric acid (H\(_2\)SO\(_4\)) and 0.1 M potassium chloride (KCl) solution with 10 cycles of cyclic voltammetry.
2.3 Instrumentation
In Fig. 1, we show the schematic diagram of the instrumentation system to characterize our screen-printed-based sensor. The system comprises a screen-printed electrodes (SPE), custom-designed data acquisition (DAQ) board and a potentiostat circuit [13]. Experimentally, in the SPE we placed a drop of 50 \(\mu \)L volume, in such a way that the drop covered the surface of the three electrodes. The DAQ provides the voltage signal, V\(_i\), for cyclic or square-wave voltammetry. The V\(_i\) is applied at the RE and CE with a control amplifier (CA) to compensate for the potential between the RE and the WE. The resultant current I is measured at the WE with a transimpedance amplifier (TIA) to convert the current I into an output voltage V\(_o\), which is proportional to the input current by a feedback resistor R\(_f\), such that V\(_o\) = −R\(_f\)I. Finally, the V\(_o\) is digitalized within the DAQ, which acquires the data and sends it to a personal computer (PC) to plot them with a custom-designed software, to obtain the voltammograms.
3 Results
To characterize the screen-printed electrodes as a BPA sensor, the redox probe was blended to the solutions, using six concentrations of BPA = \(\{\)0, 0.2, 0.4, 0.6, 0.8 and 1.0\(\}\) mM. To evaluate our sensor, we made three different voltammetric techniques: linear sweep voltammetry (LSV), cyclic voltammetry (CV), and square wave voltammetry (SWV). Then, we compared which technique performed best to measure BPA concentration through its sensitivity (\(\mathcal {S}\)) and detection limit (LoD).
3.1 Linear-Sweep Voltammetry Study
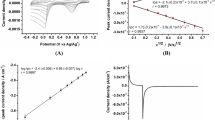
Using linear-sweep voltammetry (LSV), we characterized each SPE-based sensor in the range from 0 to −0.3 V and a scan rate of 50 mV/s. Figure 2 (a) shows the linear-sweep voltammograms obtained for six solutions of BPA to different concentration. Wherein, one can notice that anodic peak current increases when the concentration rises up. Consequently, the BPA concentration can be quantified based on the maximum value of the anodic current. Additionally, in Fig. 2 (b) we depict the calibration curve for the proposed sensor. This indicates that the relationship between BPA concentration and current is proportional. The sensitivity of the sensor is 2.32 \(\mu \)A/mM with a coefficient of determination r\(^2\) of 0.9846, which thus indicating a highly linear behavior, and a limit of detection (LoD) of 0.09 mM. This information shows that the unmodified SPE-based sensor is reliable to monitor BPA in the concentration range from 0 to 1 mM.
3.2 Cyclic Voltammetry Study
In this experiment, we performed measurements by cyclic voltammetry (CV) to evaluate the proposed sensor. CV was carried out from 1 to 2 V with a scan rate of 50 mV/s, for each of the BPA solutions. Figure 3(a) shows the cyclic voltammograms, wherein, one can notice that both, the anodic and cathodic peak changes as the concentration grows up. Thus, focusing on the anodic peak one can quantify the concentration of BPA, which is denoted with a black square. Following this idea, Fig. 3(b) shows the calibration curve of our sensor. Therein, we depict the experimental data (black dots), uncertainty (vertical lines), and the best fitted linear model (solid line). As expected, the current has a direct relationship with the concentration BPA. Thereby, the sensitivity of the sensor is 9.55 \(\mu \)A/mM with a determination coefficient r\(^2\) of 0.9942, which exhibits a highly linear behavior, and detection limit (LoD) of 0.06 mM. These findings indicate that the unmodified SPE-based sensor can be reliable for monitoring BPA in a range from 0 to 1 mM. However, we can also notice that the detection limit obtained by CV is lower than that obtained by LSV, but its sensitivity is higher.
3.3 Square-Wave Voltammetry Study
The last method used was square-wave voltammetry (SWV). The measuments were performed at a scan rate of 50 mV in the range from 0 to 1, for each BPA dilution. Figure 4 (a) shows the voltammograms obtained for the same six BPA solutions. From there, one can notice that, as with CV and LSV, the peak anodic current increases with higher concentration. Likewise, the concentration of Bisphenol A can be quantified based on the maximum anodic current, which is also indicated by a black square. Besides, Fig. 4 (b) shows the calibration curve of the sensor wich indicates that the relationship between BPA concentration and current is straightforward. Hence, the sensitivity of the sensor is 30.46 \(\mu \)A/mM with a coefficient of determination r\(^2\) of 0.9976, which presents a highly linear behavior, and a limit of detection (LoD) of 0.04 mM. This information indicates that the unmodified SPE-based sensoris reliable to monitor BPA in a range from 0 to 1 mM. In addition, we can also observe that the detection limit obtained by SWV is lower than those obtained by LSV and CV, and its sensitivity is higher than those obtained by the other two techniques.
3.4 Comparison Between Electrochemical Methods
Finally, once all the measurements and calibration curves were obtained, we evaluated the parameters of the proposed sensor for linear-sweep (LSV), cyclic (CV) and square-wave (SWV) voltammetry. To determinate the best electrochemical method, we compared each technique trough its sensitivity (\(\mathcal {S}\)), detection limit (LoD) and determination coefficient (r\(^2\)). As shown in the results of the Table 1, the SWV has the best parameters as a BPA concentration sensor due to its \(\mathcal {S}\) of 30.46 \(\mu \)A/mM is higher than CV and SWV. Moreover, the LoD using SWV is the lowest, thus meaning that SWV can quantify highly diluted solutions. Also, SWV exhibit the best linear behavior in the fitting model due to the r\(^2\) is greater than 99 \(\%\). This information indicates that, the technique with the lowest detection limit and the highest sensitivity is SWV. Hence, our findings indicate that SWV could be the most sensitive and reliable method to detect BPA using SPE-based sensors. Based on the experimental results, our sensor provides high sensitivity and low detection limit, in the same order of magnitude as previous reports with similar detection techniques [14, 15].
In further studies, it would be also useful to assess other electrochemical techniques such as conductimetric and amperometric, to devise the best option for quantifying BPA.
4 Conclusions
In this work, the performance of screen-printed electrodes as a low-cost, affordable and accurate electrochemical sensor for the sensitive detection of Bisphenol A was developed and evaluated by analysis with three different electrochemical techniques, linear-sweep voltammetry (LSV), cyclic voltammetry (CV) and square-wave voltammetry (SWV). The results demonstrated the ability of the sensor to perform BPA measurements at different concentrations, and the difference in detection limits using the different techniques. The proposed sensor was mainly dedicated to detect the presence of BPA by voltammetric measurements with a detection limit of 0.06 mM, 0.09 mM and 0.04 mM in CV, LSV and SWV, respectively, indicating a better performance of the square-wave voltammetry technique. In addition, it was also possible to determine the sensitivity of the sensing device obtaining S = 9.55 \(\mu \)A/mM for CV, S = 2.32 \(\mu \)A/mM for LSV, and S = 30.46 \(\mu \)A/mM for SWV. The versatility and performance characteristics of screen-printed electrodes together with the electrochemical analysis techniques make them an attractive alternative to detect the presence of BPA in liquid samples.
References
Antony, S., et al.: Bioremediation of endocrine disrupting chemicals-advancements and challenges. Environ. Res. 213, 113509 (2022)
Zhang, Y., et al.: Electrochemical detection of bisphenols in food: a review. Food Chem. 346, 128895 (2021)
Tarafdar, A., et al.: The hazardous threat of Bisphenol A: toxicity, detection and remediation. J. Hazard. Mater. 423, 127097 (2022)
Tang, Y., et al.: Removal of emerging contaminants (bisphenol A and antibiotics) from kitchen wastewater by alkali-modified biochar. Sci. Total Environ. 805, 150158 (2022)
Yahaya, N., Za, H., Yan, B., Chen, D.D.: Capillary electrophoresis–mass spectrometry analysis of bisphenol A and its analogues in bottled tea beverages with dynamic pH focusing. Food Chem. 372, 131220 (2022)
Moscoso-Ruiz, I., Gálvez-Ontiveros, Y., Cantarero-Malagón, S., Rivas, A., Zafra-Gómez, A.: Optimization of an ultrasound-assisted extraction method for the determination of parabens and bisphenol homologues in human saliva by liquid chromatography-tandem mass spectrometry. Microchem. J. 175, 107122 (2022)
Jebril, S., Cubillana-Aguilera, L., Palacios-Santander, J.M., Dridi, C.: A novel electrochemical sensor modified with green gold sononanoparticles and carbon black nanocomposite for bisphenol A detection. Mater. Sci. Eng., B 264, 114951 (2021)
Tajik, S., et al.: Recent advances in electrochemical sensors and biosensors for detecting bisphenol A. Sensors 20(12), 3364 (2020)
Mincu, N.B., Lazar, V., Stan, D., Mihailescu, C.M., Iosub, R., Mateescu, A.L.: Screen-Printed Electrodes (SPE) for in vitro diagnostic purpose. Diagnostics 10(8), 517 (2020)
Musa, A.M., Kiely, J., Luxton, R., Honeychurch, K.C.: Recent progress in screen-printed electrochemical sensors and biosensors for the detection of estrogens. TrAC Trends Analyt. Chem. 139, 116254 (2021)
Moro, G., De Wael, K., Moretto, L.M.: Challenges in the electrochemical (bio) sensing of nonelectroactive food and environmental contaminants. Curr. Opin. Electrochem. 16, 57–65 (2019)
Suresh, R.R., et al.: Fabrication of screen-printed electrodes: opportunities and challenges. J. Mater. Sci. 56(15), 8951–9006 (2021). https://doi.org/10.1007/s10853-020-05499-1
Ramírez-Chavarría, R.G., Alvarez-Serna, B.E., Schoukens, M., Alvarez-Icaza, L.: Data-driven modeling of impedance biosensors: a subspace approach. Meas. Sci. Technol. 32(10), 104009 (2021)
Zheng, A.L.T., Andou, Y.: Detection and remediation of bisphenol A (BPA) using graphene-based materials: mini-review. Int. J. Environ. Sci. Technol. 19, 6869–6888 (2022). https://doi.org/10.1007/s13762-021-03512-x
Ragavan, K.V., Rastogi, N.K., Thakur, M.S.: Sensors and biosensors for analysis of bisphenol-A. TrAC Trends Analyt. Chem. 52, 248–260 (2013)
Acknowledgments
This work was supported by the grants DGAPA-UNAM PAPIIT TA100221 and PAPIME PE101222. Bryan E. Alvarez-Serna acknowledges CONACYT for the Ph.D. studies grant (CVU 1004078).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Hernández-Gordillo, M.J., Alvarez-Serna, B.E., Ramírez-Chavarría, R.G. (2023). Unmodified Screen-Printed Electrodes-Based Sensor for Electrochemical Detection of Bisphenol A. In: Trujillo-Romero, C.J., et al. XLV Mexican Conference on Biomedical Engineering. CNIB 2022. IFMBE Proceedings, vol 86. Springer, Cham. https://doi.org/10.1007/978-3-031-18256-3_63
Download citation
DOI: https://doi.org/10.1007/978-3-031-18256-3_63
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-18255-6
Online ISBN: 978-3-031-18256-3
eBook Packages: EngineeringEngineering (R0)