Abstract.
The Canadian economy relies largely on natural resources, yet processing of these resources and/or associated by-products is well known to negatively affect the environment. Reusing slag, a common waste stream of pyrometallurgical extraction processes, as construction materials serves to reduce the impact on the natural resources currently used in construction industry such as sand, gravel, and crushed stone. Every year 12–16 million tonnes of ferrochromium slag is produced, a majority of which is dumped in landfills. The mechanical properties of this slag make it a potential material to be used as an inexpensive construction material, the utilization of which can reduce the use of natural resources. Despite its potential use, ferrochromium slag is treated as a waste due to environmental and health concerns regarding the leaching of its heavy metal content, the most concerning of which is carcinogenic chromium (VI). Research has shown that the spinel phase in ferrochromium slag stabilizes chromium by trapping it in the spinel structure and preventing its leaching to the environment. This study examined slag samples of the MgO-Al2O3-SiO2-CaO-FeO-Cr2O3 synthesized at 1650 °C. The slag was synthesized to mimic the possible composition of the Ring of Fire’s ferrochromium slag. Samples were heat treated before quenching. Leaching tests on heat-treated slag samples showed that samples held at 1400 °C have the lowest chromium leachability. Higher Cr leaching is observed from samples as the Al2O3 content increases. An increase in basicity in the range of 0.3–0.7 increases the amount of Cr released from the samples during leaching experiments.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords:
1 Introduction
Ferrochrome production involves the production of slag as the by-product at a rate of 1.1–1.5 times that of the metal itself. This results in a global ferrochromium slag production of around 12–16 million tonnes annually. Although most of this slag is discarded as waste in landfills, it is an attractive construction material due to its suitable mechanical properties. Ferrochromium producers from Finland, Sweden, and Russia have utilized slag in road making and building materials [1]. However, the use of ferrochromium slag as a construction material is still in its infancy mainly due to environmental concerns about the content and leachability of heavy metals, especially chromium. Environmentally safe utilization of this slag would offset the cost of production and reduce the impact on the natural resources used as these aggregates.
The leaching of carcinogenic Cr(VI) from ferrochromium slag is a major environmental concern for its application in the construction industry. The slag composition is expected to have a strong effect on the leachability of Cr from the slag.
This study examines the chemical composition of a synthetic ferrochromium slag for its utilization as construction materials. The main concern with the utilization of ferrochromium slag as a construction material is the leachability of Cr and its oxidation to carcinogenic and environmentally hazardous Cr(VI). Canadian and Ontario criteria require a maximum 2.77 mg/L of total chromium in aqueous waste leachate using TCLP (Toxicity Characteristic Leaching Procedure) leaching method [2].
2 Methodology
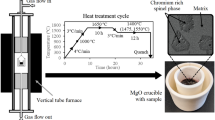
The chemical composition of the synthesized samples with variations in Al2O3 content and basicity (CaO/SiO2) is shown in Table 1. Reagent-grade oxides were mixed in an agate mortar before being pressed inside a MgO crucible. The sample was then hung inside a vertical tube furnace using Molybdenum (Mo) wire. Following melting, the temperature was reduced to 1400 °C and held for 12 h. After the holding time, the sample is dropped in a bucket of cold tap water for quenching, by cutting the Mo wire. Quenched slag samples were extracted, milled, and sieved to <106 μm for leaching and chemical analysis.
Previous studies have examined the leachability of Cr from EAF using various leaching tests such as DIN 38414-S4 [3, 4]. However, in the present study, the leaching experiments were conducted using AV002.1 availability test [5] on slag samples with a particle size of <106 μm using the extraction liquid of 50 mM EDTA. The pH of the solution was adjusted to 7.5 before leaching by using 2 N nitric acid (HNO3) solution and 1 N potassium hydroxide (KOH) solution. One gram of <106 μm slag with 100 ml of EDTA extraction liquid was placed in 150 ml high-density polypropylene (HDPP) containers. The extraction containers were tightly sealed and set to rotate in an end-over-end fashion for 48 h at 35 rpm. The leachate was then filtered to remove the solid residue. The leachate was analyzed with ICP-AES [6].
3 Results and Discussion
Slag samples A1-A7 were prepared with Al2O3 content varying from 0% to 20%, to study the effect of Al2O3 content on the Cr leachability of FeCr slag. The Cr concentration of the leachate obtained from slag samples A1–A7 with particle size <106 μm is shown in Fig. 1. The Cr concentration increases from 0.10 to 0.18 ppm as the Al2O3 content increased from 0% to 20%. The increase in Al2O3 content of the slag promotes Cr leaching out of it. It is proposed that Al3+ ions replace the Cr3+ ions in the stable spinel phase that resists Cr dissolution in water. The Cr present in silicate phases (Åkermanite, olivine, quartz) of the matrix would not be able to completely resist water dissolution and thus leach out of the slag.
Slag samples B1-B5 with basicity ranging from 0.3 to 0.7 were made to determine its effect on Cr leachability of FeCr slag. A noticeable Cr concentration difference in the leachates is observed by increasing basicity. The leachate of slag B1 with basicity of 0.3 has 0.15 ppm Cr. The Cr concentration increases to 0.25 ppm for the leachate of sample B5 with the basicity of 0.7 (Fig. 2).
The Cr content of the leachate of all the slag samples examined in this work are below the Canadian and Ontario criteria of maximum 2.77 mg/L of Cr in waste leachate using TCLP test. The Cr content of the leachates is also lower than the non-potable ground water limit of 0.81 ppm total Cr but higher than Ontario’s total Cr content standard limit of 0.05 ppm for drinking water [7].
References
Ndlovu S, Simate GS, Matinde E. Waste production and utilization in the metal extraction industry. CRC Press, Taylor and Francis; 2017.
Ontario Environmental Protection Act R.R.O 1990, Regulation 347, General Waste Management. p. 65–145.
Kuhn M, Mudersbach D, Baena Liberato JM, De Angelis V, Capodilupo C, de Fries U. Chrome immobilisation in EAF slags from high-alloy steelmaking: development of a slag treatment processes. In: European Commission, technical steel research, steel-making processes; 2006.
Romera Q, Capodilupo, Behmenburg, Kuhn. Decreasing the scorification of chrome. In: European Commission, technical steel research, primary steel-making; 2000.
Kosson DS, van der Sloot HA, Sanchez F, Garrabrants AC. An integrated framework for evaluating leaching in waste management and utilization of secondary materials. Environ Eng Sci. 2002;19(3):159–204.
Bin Tasnim, T., Tafaghodi Khajavi, L. Chromium Stabilization in Ferrochromium Slag for its Utilization as Aggregate Material. J. Sustain. Metall. 2022;8:1041–1052.
Soil, Ground Water and Sediment Standards for use under Part XV.1 of the Environmental Protection Act. Ontario Ministry of Environment, Canada, 15 April 2011.
Acknowledgments
The authors would like to thank Dr. Dogan Paktunc for his valuable insight. We would also like to acknowledge NRCan’s financial support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Tasnim, T.B., Tafaghodi, L. (2023). Stabilizing Chromium in Ferrochromium Slag for Utilization as Aggregate Material. In: Proceedings of the 61st Conference of Metallurgists, COM 2022. COM 2022. Springer, Cham. https://doi.org/10.1007/978-3-031-17425-4_29
Download citation
DOI: https://doi.org/10.1007/978-3-031-17425-4_29
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-17424-7
Online ISBN: 978-3-031-17425-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)