Abstract.
The production of high-carbon ferromanganese (HCFeMn) alloys is an energy-intensive process where manganese ores are smelted in a submerged arc furnace (SAF) using carbon reductants thereby generating CO2 emissions. In the prereduction zone of the SAF, higher manganese oxides in the ore are reduced to MnO through solid–gas exothermic reactions and at a temperature around 800 °C, the unwanted endothermic Boudouard reaction is also active. The Boudoaurd reaction consumes both carbon and energy. As such, the total coke and energy consumption are highly dependent on if the prereduction occurs by CO gas or solid C. Improvement of existing SAF ferromanganese process in resource and energy efficiency as well as reduction of CO2 emission through ore pretreatment in a separate unit is being explored in the EU H2020-funded PreMa project. A successful pretreatment limits the extent of Boudouard reaction thereby reducing the carbon and energy footprint of the process. Pilot-scale experiments have been conducted at SINTEF/NTNU in a 440 kVA AC electric furnace utilizing different feed mixtures of untreated manganese ore, manganese ore preheated in air, and manganese ore prereduced with solid carbon. The accounting material and energy balance calculations for the three pilot experiments were then calculated in HSC Chemistry software. In addition to a stable furnace operation, pretreatment of Comilog resulted in an increased energy consumption in the SAF mainly due to reduced exothermic reactions. Prereduction with solid carbon in separate unit was found not to be beneficial as there was insignificant difference in ore oxygen level compared to preheated ore.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords:
1 Introduction
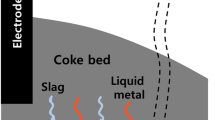
In the ferromanganese industry, several efforts to reduce the energy consumption and environmental CO2 footprint emanating from the direct process have received extensive attention. The ferromanganese furnace process consists of a prereduction zone and coke bed zone. The prereduction zone is characterized by a set of exothermic chemical reactions where high manganese oxides (MnO2, Mn2O3, Mn3O4) present in the ore are reduced in the solid state by CO gas generated from Mn metal formation reaction in the coke bed located at the bottom of the submerged arc furnace (SAF) [1]. These reactions generate CO2 which, if it occurs at temperatures above 800 °C, participates in the endothermic Boudouard reaction (C + CO2(g) = CO(g)), consuming energy and extra-carbon. Hence, the gas–solid reactions in the prereduction zone are to a large extent decisive for the variations of both coke and energy in ferromanganese production [2]. This will hence also affect the total CO2 emissions from the process.
One of the main focus areas in order to reduce direct process-related CO2 emissions and energy consumption is the optimization of the prereduction process and raw material’s usage. The usage of carbonated materials contributes to the CO2 emissions both through releasing the bound CO2 through an endothermic decomposition reaction and through the released CO2 reacting with solid carbon according to the Boudouard reaction. As such removal of carbonated fluxes is one potential way of reducing the CO2 emissions from the furnace. In a recent industrial trial on the elimination of carbonate fluxes in silicomanganese production, an 8.5% decrease in CO2 footprint was achieved in comparison to utilization of fluxes [3]. The presence of potassium in charge materials which recirculates and accumulates in the furnace has been reported to increase the kinetics of the Boudouard reaction and thus causing excessive carbon and energy consumption of the ferromanganese process [4, 5]. According to Swamy [5], the carbon consumption in an industrial furnace is between 305 and 380 kg per ton of alloy due to the Boudouard reaction. Typical energy requirements for the ferromanganese process range between 2.0 and 3.5 MWh per ton alloy [1, 6,7,8]. Several studies [8,9,10,11,12] have also been conducted on the prereduction behavior of various ores with the main goal of optimizing the prereduction reactions. The prereduction behavior differ and are significantly dependent on the ore type used. Prereduction of Comilog and Nchwaning ores in CO/CO2 atmospheres has been extensively studied by Larsen et al. [9, 10]. Investigated variables included temperature, particle size, and CO concentration in thermogravimetric experiments. It was reported that an increasing partial pressure of CO in CO/CO2 atmosphere promoted the reaction rate with an order of 0.7 and 1.5 for Comilog and Nchwaning ores, respectively.
In PreMa project [13], pretreatment of Mn-ores in a separate unit prior to feeding the SAF is an avenue to reduce overall energy consumption and CO2 emissions from the ferromanganese process currently under investigation. Several aspects of this process under investigation include design and optimization of the separate unit, use of sustainable energy sources, prereduction behavior of ores, and effect of pretreatment on SAF furnace performance through pilot investigations [14,15,16,17]. In this paper, energy and carbon consumption from utilization of pretreated and untreated ore are discussed on the basis of accounting mass and energy balances from pilot work.
2 Mass and Energy Balance for Pilot Experiments
The detailed description of the 440 kVA AC electric pilot furnace at SINTEF/NTNU and its operation have been previously presented [17]. The pilot-scale experiments were conducted with untreated and pretreated Comilog ore. The ore had been pretreated by Eramet Ideas [15] in a rotary kiln in two separate campaigns, one on preheating and another on prereduction with solid carbon. Therefore, three separate pilot experiments were conducted at SINTEF/NTNU with untreated (green ore) Comilog, preheated Comilog, and prereduced Comilog. Typically, the oxygen level in ore referred to as O/Mn ratio for Comilog is 1.98 and the pretreatment resulted in change in an O/Mn ratio to 1.6 and 1.5 for preheated and prereduced ore, respectively as shown in Fig. 1. The small difference in O/Mn ratio between preheated and prereduced ore indicates that there is not much change due to presence of solid carbon for the pretreatment used.
Table 1 shows the detailed chemical composition of the raw materials. Raw materials were chemically analyzed by SINTEF Norlab using X-ray fluorescence, while the oxygen content, given by the MnO2 amount, was obtained by titration for ore and sinter. The manganese ores were utilized in combination with other raw materials such as sinter, flux, and coke as charge mixtures in the pilot experiments. The charge mix was calculated aiming at 30% MnO in slag and the calculated charge mixture per pilot experiment is shown in Table 2. The pilot furnace was fed approximately a total of 22 batches weighing 30 kg per batch in all the pilot experiments. The tapped slag and alloy in the experiments were at a slag/alloy ratio equal to 0.6. During the pilot runs, the process off-gas was continuously measured throughout the experiment to quantify CO, CO2, H2, O2, N2, and CH4 contents. The accounting material and energy balance calculations for the three pilot experiments were then calculated in HSC Chemistry software [18] using the actual slag and alloy chemistry established from the products tapped during the pilot experiments and the input charge blend shown in Table 2. The composition of the tapped slag and alloy is as shown in Table 3.
In mass balance calculations, the MnO in tapped slag was fixed according to Table 3. The Mn in the alloy was obtained by the difference between the total Mn from the charge mixture and the one reported in the slag as MnO. The carbon content in the alloy was saturated and fixed at 7% in the alloy, while the total input of Fe was considered to report to the alloy phase. The unreducible oxides reported to the slag phase and SiO2 was calculated by balancing the amount of Si input from the charge mixture and Si reported in the alloy phase. In calculating the energy balance, raw material’s input temperatures were assumed to be 25 °C, whereas slag and alloy were considered to be tapped at 1500 °C and the off-gas was assumed to be 200 °C. The relationship between the specific carbon consumption, the CO2/(CO2 + CO) ratio, and energy consumption to produce 1 ton of HCFeMn alloy was established based on the calculations from 0 to 100 degree of prereduction. The degree of prereduction has been previously defined by Tangstad [19] as a measure of the extent of the gas reduction of higher manganese oxides, which is reflected in the CO2/(CO2 + CO) ratio of the off-gas and is inversely related to the coke and energy consumption. The degree of prereduction is defined to be 100% when all the Mn3O4 from the reduction of higher manganese oxides is reduced at low temperatures before the Boudouard reaction is activated. If all CO2 from the reduction of Mn3O4 is consumed by the Boudouard reaction, the degree of prereduction is 0%.
3 Results and Discussion
The accounting energy balances were calculated between 0% and 100% degree of prereduction, and the actual degree of prereduction for the three pilot experiments was calculated based on the CO/CO2 ratio from the off-gas measurements of the last stable period toward the end of the experiments. The CO/CO2 off-gas measured is summarized in Table 4. In addition, Table 5 shows, as an example, the mass balance at 0% degree of prereduction for untreated Comilog charge mixture per 100 kg input material, calculated in HSC Chemistry 10® [18].
As seen in Table 4, untreated Comilog charge mixture gave the highest CO2 concentrations during the experimental runs, followed by charge mixture with preheated Comilog and lastly, prereduced Comilog with the lowest observed CO2 concentration. The opposite trend will be observed for CO concentrations. The relationship between the specific carbon consumption, the off-gas CO2/(CO2 + CO) ratio, and energy consumption to produce 1 ton of HCFeMn alloy for the three different pilot-scale scenarios is shown in Figs. 2, 3, and 4 for untreated, preheated, and prereduced Comilog, respectively.
It can be seen that as the CO content of the off-gas increases, the energy consumption due to the Boudouard reaction increases. The main conclusion is that for a fixed charge the lower CO2 to (CO + CO2) ratio in the off-gas indicates higher CO2 consumed in the Boudouard reaction and consequently higher carbon and energy consumption as has been observed previously [5, 20, 21]. The minimum and maximum carbon consumption for the three pilots shown by Figs. 2, 3, and 4 are within the same range mainly because chemistry of the slags and alloys tapped presented in Table 3 is quite similar with insignificant variations. However, based on the composition of the measured furnace off-gas, differences in carbon consumption are observed. Untreated Comilog has a wide range of carbon consumed and this decreases for preheated and prereduced Comilog. The narrowing of the range of carbon consumption as is reflected in off-gas composition shows that the process was more stable for pretreated Comilog in comparison to untreated. The energy consumption when utilizing untreated Comilog ranging from 1970 to 2060 kWh is lower compared to preheated Comilog (2140–2210 kWh) and prereduced Comilog (2170–2210 kWh). If the prereduction reactions commences in the prereduction zone of the SAF, higher manganese oxides will reduce to lower manganese oxides from MnO2 to Mn2O3 to MnO3O4 to MnO in addition to reduction of iron oxides. The reduction of these reactions is exothermic. Pretreatment of Comilog ore through preheating and prereduction lowers the O/Mn ratio of the ores and as such, this will eliminate a part of exothermic reactions resulting in increased energy consumption in the SAF.
The measured off-gas was used to calculate the degree of prereduction for the different charge mixtures in the pilot scenarios and these are summarized together with energy consumption and carbon consumption in Table 6.
To evaluate the benefit of charging hot pretreated Comilog ore, calculations were done assuming that the ores are pretreated in a separate unit and fed into the furnace at 600 °C. The calculation was made using the highest degree of prereduction summarized in Table 6, for each ore type. The off-gas was assumed to exit the furnace at 700 °C, while the slag and alloy are tapped at 1500 °C. The energy comparison of the scenarios is as shown in Fig. 5. It can be seen that pretreatment of Comilog ore and charging it hot into the furnace will significantly decrease the energy consumption by about 300 kWh in the SAF. The observed trend is in close agreement with the previous reported calculations of different pretreatment scenarios [22]. However, sustainable energy sources should be utilized in preheating the ore in a separate unit in order to achieve reduced overall energy consumption for the ferromanganese process.
4 Concluding Remarks
Pilot-scale experiments have been conducted at SINTEF/NTNU in a 440 kVA AC electric furnace utilizing different feed mixtures of untreated manganese ore, manganese ore preheated in air, and manganese ore prereduced with solid carbon. Energy and carbon consumption of a HCFeMn furnace were performed for untreated and pretreated Comilog based on pilot experimental data. The main conclusions can be summarized as follows:
-
The minimum carbon consumption is fixed for a given metal composition and was calculated at 100% degree of prereduction for the reduction of MnO and SiO2 to Mn and Si in metal, respectively, and carbon dissolution in the metal. This was found to be within similar values due to the closeness of the composition of the tapped measured alloy and slag from the pilot runs.
-
The off-gas measurements show that the use of untreated Comilog ore leads to considerably lower CO/CO2 off-gas composition compared to pretreated Comilog ores, largely related to the high oxygen level of Comilog ore. Hence, the use of pretreated ore will keep the CO2 emissions low.
-
Pretreatment of Comilog will lead to an increased energy consumption in the SAF. In addition, prereduced Comilog did not have any significant difference from preheated Comilog with regard to energy and carbon consumption owing to insignificant change in oxygen level when Comilog is prereduced with solid carbon.
-
Within the variations of the experiments, the degree of prereduction is the same for the three experiments. Hence, the CO2 content will be higher for untreated ore and the energy consumption of untreated ore will be lower due to more exothermic reactions. However, charging hot pretreated ore will result in lowering of the energy consumption in SAF by 300 kWh/ton alloy.
References
Olsen SE, Tangstad M, Lindstad T. Production of manganese ferroalloys. Trondheim: Tapir Academic Press; 2007.
Ishak R, Tangstad M. Degree of prereduction without coke consumption in industrial furnaces. In: 11th international ferroalloys congress. New Dehli, India; 2007. p. 268–80.
Davidsen J. Reducing the CO2 Footprint from SiMn production by optimization of fluxes. In: 16th international ferroalloys congress; 2021. p. 27–9. https://doi.org/10.2139/ssrn.3926019.
Lindstad T, Syvertsen M, Ishak RJ, Arntzen HB, Grøntvedt PO. The influence of alkalis on the boudouard reaction. In: 10th international ferroalloys congress; 2004. p. 261–71.
Swamy KN, Robertson DGC, Calvert P, Kozak D. Factors affecting carbon consumption in the production of high carbon ferromanganese. In: 9th international ferroalloys congress. Quebec City, Canada; 2001. p. 293–301.
Eissa M, El-Faramawy H, Ahmed A, Nabil S, Halfa H. Parameters affecting the production of high carbon ferromanganese in closed submerged arc furnace. J Miner Mater Charact Eng. 2012;11:1–20. https://doi.org/10.4236/jmmce.2012.111001.
Steenkamp JD. FactSage-based design calculations for the production of high-carbon ferromanganese on pilot-scale. In: 11th international symposium on high-temperature metallurgical processing. San Diego, California, USA; 2020. p. 757–71. https://doi.org/10.1007/978-3-030-36540-0_67.
Ringdalen E, Gjøvik JE, Larssen TA, Tangstad M. Pretreatment of manganese ores in different gas-atmospheres- a method to reduce energy consumption and CO2 emissions in Mn-alloy production. In: 16th international ferroalloys congress; 2021. p. 27–9. https://doi.org/10.2139/ssrn.3930059.
Larssen TA, Senk D, Tangstad M. Reaction rate analysis of manganese ore prereduction in CO-CO2 atmosphere. Metall Mater Trans B Process Metall Mater Process Sci. 2021;52:2087–100. https://doi.org/10.1007/s11663-021-02162-1.
Larssen TA, Senk D, Tangstad M. Reduction of manganese ores in CO-CO2 atmospheres. Metall Mater Trans B Process Metall Mater Process Sci. 2021;52:363–81. https://doi.org/10.1007/s11663-020-02018-0.
Larssen TA, Tangstad M. Off-gas characteristics for varying conditions in the prereduction zone of a ferromanganese furnace –a basis for energy recovery. In: 16th international ferroalloys congress; 2021. p. 27–9. https://doi.org/10.2139/ssrn.3926228.
Turkova K, Slizovskiy D, Tangstad M. CO reactivity and porosity of manganese materials. ISIJ Int. 2014;54:1204–8. https://doi.org/10.2355/isijinternational.54.1204.
PreMa project. n.d. https://www.spire2030.eu/prema. Accessed 15 Aug 2021.
Kazdal T, Haas-Wittmuess R, Richter S, Lang S, Binder C, Reuter M. Process design for the pre-treatment of manganese ores. In: 16th international ferroalloys congress; 2021. p. 27–9. https://doi.org/10.2139/ssrn.3926619.
Julia N, Hecquet A, Nussbaum G, Blancher S, Amalric A. Pre-heating manganese ore in a pilot-scale rotary kiln. In: 16th international ferroalloys congress; 2021. p. 27–9. https://doi.org/10.2139/ssrn.3926242.
Hamuyuni J, Saarenmaa J, Mäkelä P, Pekkala O, Binder C, Rannantie S, Lindgren M. Pretreatment of manganese ore for improved energy efficiency and smelting furnace stability. In: 16th international ferroalloys congress; 2021. p. 27–9. https://doi.org/10.2139/ssrn.3926120.
Mukono T, Gjøvik JE, Gærtner H, Wallin M, Ringdalen E, Tangstad M. Extent of ore prereduction in pilot-scale production of high carbon ferromanganese. In: 16th international ferroalloys congress. Trondheim; 2021. p. 1–14. https://doi.org/10.2139/ssrn.3926275.
Roine A. HSC Chemistry Software. In: HSC Chem. Software, v 10.0.4.3. Pori: Metso Outotec; 2021. www.mogroup.com/hsc.
Tangstad, M. Ph.D. thesis. Trondheim, Norway: The Norwegian Institute of Technology; 1996.
Tangstad M, Olsen SE. The ferromanganese process – material and energy balance. In: 7th international ferroalloys congress INFACON VII. p. 621–630; 1995. https://www.pyrometallurgy.co.za/InfaconVII/621-Tangstad.pdf.
Ahmed A, Haifa H, El-Fawakhry MK, El-Faramawy H, Eissa M. Parameters affecting energy consumption for producing high carbon ferromanganese in a closed submerged arc furnace. J Iron Steel Res Int. 2014;21:666–72. https://doi.org/10.1016/S1006-706X(14)60103-5.
Tangstad M, Ichihara K, Ringdalen E. Pretreatment unit in ferromanganese production. In: 14th international ferroalloys congress: energy efficiency and environmental friendliness are the future of the global ferroalloy industry. Kiev, Ukraine; 2015. p. 99–106.
Acknowledgments
This work is part of the PreMa project, which has received funding from the European Union’s Horizon 2020 Research and Innovation Programme under Grant Agreement No 820561 and industry partners: Eramet, Ferrogroble, Transalloys, OFZ, and Outotec.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Mukono, T. et al. (2023). Utilization of Pretreated Mn-Ore in a Pilot-Scale Ferromanganese Furnace: Effect of Ore Pretreatment on Carbon and Energy Consumption. In: Proceedings of the 61st Conference of Metallurgists, COM 2022. COM 2022. Springer, Cham. https://doi.org/10.1007/978-3-031-17425-4_25
Download citation
DOI: https://doi.org/10.1007/978-3-031-17425-4_25
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-17424-7
Online ISBN: 978-3-031-17425-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)