Abstract
This chapter provides a comprehensive look at the etiology, diagnosis, and treatment of cervical myelopathy. The causes of cervical myelopathies are divided into extrinsic (i.e., cervical spondylosis, epidural abbesses, and disc herniations) and intrinsic (i.e., viral infections, multiple sclerosis, and motor neuron disease). The genetic and epidemiologic factors that predispose patients to cervical myelopathy are discussed in this chapter. Lastly, the chapter explores treatment options and prognosis for patients with cervical myelopathy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cervical myelopathy
- Cord compression
- Cervical stenosis
- Myelomalacia
- Cervical spondylosis
- Ossified posterior longitudinal ligament
Introduction
Cervical myelopathy, or symptomatic spinal cord compression [1], is defined as spinal cord dysfunction of insidious onset, more commonly diagnosed in the elderly and is caused by compression of the cervical spinal cord [2]. Compression of the cervical spinal cord results in neurological deficits that vary in presentation depending on the level of compression, etiology and mechanism of compression, and age and severity of the disease. Commonly reported symptoms include neck pain or stiffness, numbness, paresthesia, ataxic gait, and weakness in the upper and lower extremities and in later stages of the disease may present with additional bowel and bladder dysfunction [2,3,4,5].
“Degenerative Cervical Myelopathy’‘(DCM) is a term coined in 2015 to describe the nontraumatic, degenerative causes of cervical myelopathy that result in structural and functional abnormalities in the spinal cord through static and dynamic factors. It includes spondylosis, disc herniation, facet arthrosis, ligamentous hypertrophy, calcification, and ossification [5,6,7].The cervical column is particularly vulnerable to degenerative changes due to its mobility and these changes are more commonly seen with increasing age [4].
Cervical myelopathy is a disorder that encompasses multiple neurological conditions and can be categorized into extrinsic and intrinsic neural etiologies. Before discussing the different etiologies, it is important to clarify some nomenclature. In certain books and literature, “cervical spinal stenosis” is synonymously used with “cervical myelopathy”. However, while cervical myelopathy refers to the compression of the spinal cord, cervical spinal stenosis refers to the pathological (or congenital) narrowing of the spinal canal. Pathological narrowing of the spinal canal may cause compression of the spinal cord and potentially to symptomatic compression (cervical myelopathy). Cervical spinal stenosis can be congenital or acquired. Congenital stenosis could be structural secondary to short pedicles or in association with developmental disorders such as achondroplasia, trisomy 21, and others. Acquired cervical spinal stenosis is most commonly due to degenerative, hypertrophic or age-related changes but could also be due to other conditions such as ossification of the posterior longitudinal ligament (OPLL), atlantoaxial subluxation in rheumatoid arthritis or degenerative spondylolisthesis [1].
Myelomalacia
Myelomalacia has traditionally been defined as a radiographic finding on magnetic resonance imaging (MRI) visualized as a poorly defined region of spinal cord signal that appears hypointense on T1 and hyperintense on T2 weighted sequences [8]. Patients with cervical spondylotic myelopathy (CSM) can also demonstrate myelomalacia with the same characteristic MRI signal intensities [9]. Clinical correlation and prognostic factors utilizing these imaging findings, however, are more nuanced and not as clear. Increased signal intensity (SI) in T2 sequences can be classified into two grades, type 1 and type 2 [10, 11]. Type 1 or “faint, fuzzy, indistinct borders’‘correlate closely to reversible changes, while type 2 or “intense, well-defined borders” correlate with irreversible histological damage. These classifications were based on a histopathologic spinal cord study that showed severe changes (microcavitation, spongiform changes, and necrosis) have a higher water content, resulting in more intense borders. However, milder histological changes, such as edema, demyelination, and Wallerian degeneration, produce less well-defined borders. Both studies demonstrated improvements in SI in type 1 postsurgically, confirming that milder signal changes are reversible [10, 11]. A systematic review by Tetreault et al. provides a weak recommendation for using combined T1/T2 signal change, SI ratio, and a greater number of SI segments on a T2WI for post-surgical prognosis, as they were found to be negatively associated with outcome. Unfortunately, there currently are no reliable standardized classification methods to quantify the degree of signal change [12].
Etiologies
Various etiologies can lead to the symptomatic compression of the spinal cord (Table 6.1). Causes of cervical myelopathy can be divided into extrinsic and intrinsic neural etiologies. Extrinsic neural etiologies are conditions that are external to the spinal cord and cause mechanical compression of the cord, potentially leading to myelopathy. Intrinsic neural etiologies are primary pathologies that occur within the spinal cord itself causing the symptoms of myelopathy. The clinical significance of this categorization comes from intrinsic pathologies being used as a differential for mechanically compressive myelopathy etiologies.
Risk Factors
There are certain hereditary factors that have been shown to be correlated with the development of degenerative cervical myelopathy. Literature has shown MMP-2 and collagen IX genes to be associated with degenerative disc disease and collagen VI and XI to be associated with ossification of the posterior longitudinal ligament [6].
Degenerative changes of the bone, ligaments, and intervertebral discs cause a disruption of cervical kinetics. Patients with certain movement disorders such as Parkinson’s, cerebral palsy, and other neurodegenerative diseases of the cortical or basal ganglia may also develop progressive myelopathy due to dyskinesia and dystonia. Skeletal dysplasias, torticollis, Tourette syndrome, and psychogenic diseases may also increase the risk of degeneration due to static and dynamic injury mechanisms.
Certain medical conditions increase the risk of cervical degeneration. Patients who have hypoparathyroidism, poly-hypophosphatemic rickets, and short sleeping hours are also at increased risk of developing OPLL. Non-insulin dependent diabetes mellitus increases the general ossification of spinal ligaments. Individuals predisposed to atlantoaxial subluxation because of Rheumatoid Arthritis or Trisomy 21 also face an increased risk. Congenital stenosis is prevalent in disorders such as achondroplasia, Klippel-Feil syndrome, and Morquio syndrome [1].
Environmental factors, such as tobacco, use also increases the risk since smoking decreases bone mineral density, increases fracture risk as well as increases the incidence rates of degenerative disc disease [6]. Trauma, occupations requiring weight bearing on the head, tumors, metastatic disease, and abscess formation also increase the risk of cervical degeneration.
Demographics
Any demographic of patients with increased risk factors for cervical spine canal narrowing are more predisposed to compression of the cervical cord and, therefore, cervical myelopathy.
Cervical myelopathy is the most common cause of spinal cord impairment in the elderly population. It is also the most common form of spinal cord injury in adults and makes up 54% of nontraumatic spinal cord injury in North America [13,14,15].
With age, many degenerative and hypertrophic changes progressively occur in the intervertebral discs, facet joints, ligamentum flavum, and uncovertebral joints (Joints of Luschka) that cause neural foraminal narrowing and thereby impinge the cervical spinal cord. Other degenerative changes such as spondylolisthesis, spondylosis, osteophytes, disc herniations, disc calcifications, and facet hypertrophy result in biomechanical changes that also obstruct the vertebral canal resulting in cervical myelopathy. These degenerative changes are present in 75%–85% of the population by the age of 65 [1]. Males present with a higher incidence of canal stenosis from spondylosis at a ratio of 3:2 and more severe stenosis at the primary level of pathology which later can develop into myelopathy [16].
Cervical myelopathy can overlap with many other neurological conditions and is often underdiagnosed [6]. The incidence and prevalence of degenerative cervical myelopathy is about 41 and 605 per million in North America. The incidence of hospitalizations due to cervical spondylotic myelopathy is 4.04/100,000 leading to an increase in surgical rates [6]. About 1.6 per 100,000 inhabitants underwent surgical intervention for cervical myelopathy [17, 18].
The Asian population is more predisposed to the ossification of the posterior longitudinal ligament and ligamenta flava and therefore are at a higher risk of developing myelopathy [19].
Degenerative Cervical Myelopathy
Degenerative Cervical Myelopathy (DCM) refers to nontraumatic, degenerative causes of cervical myelopathy that result in structural and functional abnormalities in the spinal cord through static and dynamic factors. It includes spondylosis, disc herniation, facet arthrosis, ligamentous hypertrophy, calcification, and ossification [5,6,7]. The cervical column is particularly vulnerable to degenerative changes due to its mobility and these changes are more commonly seen with increasing age [4].
DCM is more commonly diagnosed in the elderly above the age of 50, in men more than women with a 3:1 male to female ratio of patients [3]. Exact incidence of DCM is difficult to determine for multiple reasons:
-
Differences in terminology:
The term “Degenerative Cervical Myelopathy” was introduced in 2015 and was previously diagnosed depending on its etiology which caused much ambiguity when referring to this collective set of diseases.
-
Many patients go undiagnosed:
A small study showed 18% of 66 patients with hip fractures were found to have undiagnosed cervical myelopathy [18]. DCM diagnosis could be missed because:
-
Radiological findings can be present in asymptomatic patients:
If you randomly select people between the ages 40 and 80, around 59% of them will have incidental cervical cord compression detected on MRI [20]. 8% of these patients will develop DCM after 1 year and around 22% in total will develop DCM later in life [21].
Cervical Spondylotic Myelopathy
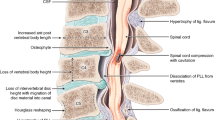
Cervical spondylotic myelopathy (CSM) is the most common cause of compressive cervical myelopathy [2]. Cervical spondylosis is the degeneration of the intervertebral discs of the cervical spine that develop spontaneously with age, repetitive daily use, occasional trauma, and excessive use, with nutritional or environmental factors, or with a combination of these factors. As the intervertebral disc degenerates, a cascade of altered weight-bearing biomechanics causes uneven pressure placement on the vertebra resulting in adaptive remodeling of the spine through osteophyte formation or “spurs.” Chronically, this pathologic process may be further complicated by acute disc herniation. The bulging of the annulus fibrosus is referred to as “soft” disc herniation. In cervical spondylosis, the enlarging calcification of a posterior marginal osteophyte is referred to as a “hard” disc herniation and may co-occur with a soft disc herniation [6, 22].
Cervical spondylosis as a degenerative process is a common finding in the asymptomatic elderly and is of no clinical concern unless it correlates with corresponding neurological symptoms on history and physical exam. Depending on where the osteophytic remodeling and “hard” herniation occurs (to the side and towards the nerve root or posteriorly towards the spinal cord itself), a specific neurological symptomatology will develop. Radiculopathy occurs when the nerve root is impinged resulting in neurological deficits corresponding to that specific nerve root. Cervical spondylotic myelopathy occurs when spondylosis results in narrowing of the vertebral foramen with the compression of the spinal cord. It is easily differentiated from radiculopathy as cervical spinal cord damage will result in neurological deficits observed in all four extremities [6, 16, 22].
Cervical Disc Herniation
Cervical Disc Herniation (CDH) refers to isolated “soft” disc herniation. As previously mentioned, “soft” disc herniation is the bulging of the intervertebral disc while “hard” disc herniation is the posterior enlargement of osteophytic spurs into the spinal canal. Posterolateral herniations cause nerve root impingement and result in radiculopathy while posteromedial herniations that bulge centrally and compress the spinal cord result in cervical myelopathy [2, 22].
Soft disc herniation can be an isolated etiology for cervical myelopathy in severe cases of disc degeneration that causes significant bulging of the intervertebral disc into the spinal cord. However, soft disc herniations more commonly present as mild cases of disc bulging accompanied by progressive degenerative and osteophytic changes leading to superimposed “hard” disc herniations presenting in the elderly as cervical spondylotic myelopathy [2, 22, 23].
Given their interrelated pathophysiologies, CSM and CDH are sometimes grouped together under the term “Degenerative Disc Disease” (DDD) as one of the osteoarthritic etiologies of DCM.
Isolated soft disc herniations causing cervical myelopathy are more commonly seen in younger patients with a history of cervical spinal trauma being a common predisposing factor [24]. The clinical presentation of cervical myelopathy in the setting of isolated soft disc herniation would be more acute with rapidly progressing neurological deficits compared to cervical myelopathy in soft with superimposed hard disc herniations. The latter mixed pathophysiology would more likely present as a CSM clinical picture [6, 23].
Ossification of the Posterior Longitudinal Ligament
The posterior longitudinal ligament (PLL) is a paravertebral ligament that originates from the dorsum of C2 vertebra and courses distally towards the sacrum and functions to resist hyperflexion and distraction [25, 26]. The Ossification of the Posterior Longitudinal Ligament (OPLL) accounts for approximately 10% of cervical myelopathy patients [27] and is defined as a hyperostotic pathologic process of the PLL of uncertain pathophysiology that results in the replacement of the PLL’s ligamentous tissue with lamellar bone [2, 25, 27].
OPLL is more commonly diagnosed in men (2:1 male to female ratio) and in older individuals (ages 40–60) [28]. OPLL is traditionally known to be a disease associated with the Japanese and other East Asian populations; however, more recent research is challenging this long held view of the disease epidemiology. Recent data has shown the sporadic distribution of the disease and the prevalence across various ethnicities [29,30,31,32]. The overall prevalence of cervical OPLL has been found to be consistently around 1.9–2.5%. OPLL has been found in 1.9–4.3% of the Japanese, 0.8–3% of Southeast Asian, and 0.01–1.7% in North American and European populations [29, 31, 33,34,35]. A cross-sectional study in 2015, San Francisco of 3161 patients revealed prevalence of 1.3% in Caucasian Americans, 4.8% in Asian Americans, 1.9% in Hispanic Americans, 2.1% in African Americans, and 3.2% in Native Americans [32].
Cervical OPLL is more common than thoracic OPPL and presents with a variable neurological sequelae of myelopathy and radiculopathy depending on the affected structures [33, 36]. Primary (idiopathic) OPLL has shown to be associated primarily with Diffuse Idiopathic Skeletal Hyperostosis (DISH) and Ossification of the Ligamentum Flavum but also with other comorbidities and factors such as diabetes mellitus, obesity, vitamin A rich diet, exercise, and mechanical stress to the head. Secondary OPLL is associated with hypophosphatemic rickets and endocrine disorders such as hypoparathyroidism and acromegaly. OPLL has also been linked to multiple genes including BMP2, BMP4, COL6A1, COL11A2, NPPS, and TGFβ [25, 33].
OPLL has been classified into four types based on morphology:
-
Localized: Ossification confined to the disc space.
-
Segmental: Ossification is fragmented posterior to the vertebral body throughout the cervical spine.
-
Continuous: Ossification extends across multiple consecutive vertebra.
-
Mixed: Combination of segmental and continuous picture of ossification [37].
Ossification and Calcification of the Ligamentum Flavum
Limited research has been done on Calcification of the Ligamentum Flavum (CLF). CLF is known to occur more commonly in females and is associated with pseudogout (calcium pyrophosphate dihydrate deposition disease) [6, 38]. It is also potentially associated with hypercalcemia, hyperparathyroidism, hemochromatosis, and renal failure. CLF more commonly affects the cervical spine as compared to Ossification of the Ligamentum Flavum (OLF) which affects the thoracic spine and also differs from CLF in histopathology. OLF is a metaplastic process more common in older men with similar pathology to OPLL in that it results in lamellar bone formation from endochondral ossification that bridges the upper and lower edges of two adjacent laminae. OPLL and OLF have differing natural histories; however, they share similar pathologies which could possibly be attributed to common associations with certain genetic variants and mutations such as in NPPS, COL6A1, and RUNX2 [6]. In contrast to OLF, CLF does not affect the superficial and deep layers of the ligamentum flavum and it occurs in degenerated and thickened ligaments with the calcified regions having no continuity with the lamina. These disease entities are best seen and diagnosed with computed tomography (CT) scans [6, 38, 39].
Rheumatoid Arthritis
Rheumatoid arthritis (RA) is an inflammatory disorder that commonly affects the cervical spine and is associated with females more than males (3:1 female-to-male ratio); however, men tend to have more severe cervical involvement in RA than women [40]. The prevalence of cervical spine abnormalities and cervical myelopathy in RA varies greatly and different ranges have been reported in the literature with cervical involvement ranging from 17% to 88% in RA patients and neurological complications ranging from 7% to 70% [40, 41].
The most common form of cervical involvement in RA is atlantoaxial instability and specifically anterior atlantoaxial subluxation (AAS) which occurs due to the laxity of primary and secondary ligamentous restraints, inflammation at the ligamentous insertion sites of the atlas and erosion of the odontoid process resulting in decreased space available for cord (SAC). Further damage occurs when a rheumatoid pannus forms from granulation tissue within the synovium destroying other spinal structures and further decreasing the SAC. As the SAC decreases in subluxation, the potential for cord compression and subsequent cervical myelopathy increases [41].
Neck pain is the most common complaint presenting in 40–80% of patients with RA involvement in the cervical spine [41]. MRI is the best modality at monitoring and diagnosing the severity of the RA cervical spine. Patients are neurologically evaluated using the Ranawat grading system for the rheumatoid spine (Table 6.2).
Spinal Tumors
Intradural extramedullary (IDEM) tumors or metastatic tumors to the cervical cord may cause external compression of the spinal cord and lead to cervical myelopathy. Primary IDEM tumors include meningiomas, neurofibromas, neurilemmomas, and schwannomas and are best diagnosed with Gadolinium-enhanced MRI. Primary IDEM tumors clinically present as Brown-Sequard type myelopathy as they typically compress the cord eccentrically. Metastasis to the cervical spine is less common compared to metastasis to other regions of the spinal cord as is commonly seen from primary tumors of the breast, prostate and lung [2].
Destructive Spondyloarthropathy
Destructive Spondyloarthropathy (DSA) or Dialysis-related Spondyloarthropathy (DRSA) is another cause of cervical myelopathy observed in patients receiving long term hemodialysis (10 or more years) and more commonly affects the cervical spine [43]. MRI of DSA patients shows amyloid deposition affecting the ligaments as well as atlantoaxial subluxation and odontoid destruction similar to the pathology in rheumatoid arthritis [44]. Possible pathophysiologic processes contributing to DSA include secondary hyperparathyroidism, microcrystal deposition, β2-microglobulin-associated amyloidosis, and aluminum intoxication [45].
Cervical Spine Anomalies of Congenital Disorders
Cervical spine anomalies associated with certain diseases can potentially lead to the compression of the spinal cord. There are numerous diseases associated with cervical spine anomalies including: Klippel-Feil Syndrome, Down Syndrome causing atlantoaxial instability, Morquio syndrome, Kniest syndrome, Goldenhar syndrome, Fibrodysplasia Ossificans, and others. Discussed below are some congenital disorders associated with cervical myelopathy [6].
Down Syndrome (DS) or trisomy 21 has been associated with cervical spine instability and can occur at the atlantoaxial and/or at the occiput-C1 levels with atlantoaxial instability occurring in 10–20% of DS patients. Cervical spine instability in Down syndrome patients rarely leads to symptoms with 1–2% of patients presenting with cervical myelopathy [46].
Klippel-Feil Syndrome (KFS) is a bone disorder that presents with a clinical triad of short neck, low posterior hairline, and restricted neck mobility and is diagnosed by radiographic imaging showing congenital fusion of cervical vertebrae. KFS is commonly sporadic but has been reported with other inheritance patterns and is associated with mutations in MEOX1 and GDF6 genes. KFS has been associated with cervical joint degeneration due to its direct vertebral involvement [6].
Morquio Disease (Mucopolysaccharidoses—MPS IV) is an autosomal recessive disease that causes glycosaminoglycans accumulation posterior to the dens, odontoid hypoplasia and atlantoaxial instability leading to severe myelopathy.
Larsen Syndrome is a rare autosomal dominant or autosomal recessive disease of the filamin B gene. Mutations in filamin B affect connective tissue which often causes developmental abnormalities of the spine such as cervical kyphosis and instability which increases the risk of spinal cord compression and cervical myelopathy.
Goldenhar Syndrome is caused by abnormal development of the first and second branchial arches and can result in vertebral anomalies with cervical involvement due to odontoid hypoplasia or basilar impression [47].
Pathophysiology
Cervical myelopathy develops over time as degenerative changes of the spine result in encroachment on the spinal cord and nearby structures. In adults, the cervical canal diameter is about 17–18 mm and the spinal cord diameter is about 10 mm [48]. With degeneration, the diameter of the canal reduces over time causing definite myelopathy at less than 6 mm disc cord space. The C5-C7 discal levels have increased mobility and, as a result, are the most affected levels of cord compression in the anterior-posterior axis [1]. Due to laxity of cervical vertebrae, anterolisthesis or retrolisthesis can also develop further implicating the myelopathy [49]. Compression of the spinal cord can result in demyelination, gliosis, myelomalacia, atrophy, exiting nerve root compression, and ischemia if the anterior spinal artery is involved [48, 49]. Many of the white matter tracts are also compressed which include the lateral corticospinal tracts, spinocerebellar tracts, spinothalamic tracts, posterior columns, as well as the dorsal nerve root itself. Clinical symptoms develop as a sequelae from the tracts affected. Voluntary skeletal muscle control is impaired from the lateral corticospinal tracts, proprioception is affected from the spinocerebellar tracts, pain and temperature is impaired by the spinothalamic tracts, position and vibration sense is impaired from the posterior columns, and dermatomal sensation is affected from the dorsal nerve root.
Signs and Symptoms
Symptoms of cervical myelopathy vary depending on the cervical levels involved, the etiology of the myelopathy, the anatomic structures involved, and the pathophysiological processes of disease. Neck pain or stiffness may manifest in structural disease caused by degeneration of discs and zygapophyseal joint arthritis. Symptoms of cervical stenosis from foraminal narrowing involve radiating arm pain, paresthesias, dysesthesias, numbness, and weakness of the upper extremities [1]. Cervical central canal stenosis can present with symptoms of the upper and lower extremities, neurogenic bladder or bowel, and unsteady gait along with weakness, paresthesias, or numbness of the lower extremities [1].
Since spinal cord compression can affect many of the white matter tracts, cervical myelopathy can present with both upper and lower motor neuron symptoms. Upper motor neuron involvement affects the lower extremities more than the upper extremities and causes hypertonic muscles, hyperreflexia, spastic paralysis, pronator drift, Babinski’s sign, and Hoffman’s sign [48,49,50]. Lower motor neuron affects the upper extremities more than the lower extremities causing hypotonic muscles, hyporeflexia, fasciculations, fibrillations, and flaccid paralysis [50]. Spinocerebellar tract involvement causes symptoms of ataxia and gait dysfunction due to proprioception disturbances. Posterior column involvement causes dysfunctions in deep touch, sensation, and vibration.
Clinically, patients will have upper extremity involvement more than lower extremity. Symptoms include numbness or tingling in the arms, fingers, and hands as well as weakness. Patients can have symptoms unilaterally or bilaterally [4]. Patients or their family members will notice them having difficulty grasping onto things and dropping items more frequently. Patients will experience difficulty with writing, buttoning shirts, and even eating. Patients can also present with falling more frequently due to balance problems and difficulty walking because of a wide based gait [50]. Some patients may lose the ability to walk at all. Some patients may also develop severe myelopathy that may lead to tetraplegia (Davies). Patients may also develop autonomic symptoms like urinary or bowel incontinence, urinary retention, or erectile dysfunction [4].
History
Patient’s history is helpful for understanding what the possible cause of cervical myelopathy might be as well the anatomical structures involved by the symptoms presented. Onset of pain, any radiation of pain, recent trauma, associated symptoms provide the background to understanding etiology as well as appropriate treatment options.
Physical Exam
Neurological examinations are vital to assessing cervical myelopathy. Cerebellar involvement affecting regulation of balance, muscle tone, and coordination of voluntary movements should be assessed. The JOA Scale is a questionnaire that can be used to assess overall patient debilitation with six sections evaluating upper extremity motor, lower extremity motor, upper extremity sensory, lower extremity sensory, truncal sensory, and bladder function [2, 51]. The lower the score is, the more severe the disability. The Japanese Orthopedic Association Scale (Table 6.3) assesses the severity of cervical myelopathy with mild severity for a score > 13, moderate for scores of 9–13 and severe for scores <9 [52].
The Nurick scale is used to assess gait dysfunction (Table 6.4). Patient’s ambulation should be observed for signs of ataxia as well as a wide based and staggering gait. Patients may have difficulty maintaining balance and can be seen holding onto nearby objects for support especially when asked to tandem walk.
Upper and lower motor symptoms should be evaluated. Lower motor findings will be found at the level of the compressive lesion and upper motor findings will be observed below the lesion [2]. Adduction and extension of the ulnar two or three fingers may become very weak known as “myelopathy hand.” [2] Rapid alternating movements can also be impaired such as flipping one hand back and forth or opening and closing the fist. It takes a healthy person about 10 s to rapidly close and open a fist and 20 times which cannot be done in that time frame in patients with cervical myelopathy [48, 53].
Upper extremities should be evaluated for lower motor neuron symptoms of hyporeflexia, fasciculations, flaccid paralysis, and hypotonicity. The upper extremities should be examined for weakness due to atrophy of muscles and lack of coordination. Patients will have difficulty conducting motor tasks involving buttoning shirts or picking up small items due to muscle atrophy of the intrinsic and extrinsic hand muscles. Patients may complain of numbness of the hands causing them to drop things more frequently and limiting them from conducting their daily activities. Patients may complain of weakness making it difficult to carry objects. Patient’s strength should be tested and evaluated. Reflex examinations of the biceps, brachioradialis, patellar, and Achilles will be hyperreflexic. If the upper and mid cervical spinal cord is involved, patients may have upper motor neuron symptoms like positive Hoffman sign and Romberg test [50]. Another reflex known as the “Scapulohumeral Reflex” is also seen in 95% of patients with lesions to the C3 vertebral body level in which tapping at the spine of the scapula or at the acromion in a caudal direction causes the scapula to elevate or the humerus to abduct [2].
Lower extremities should be evaluated for upper motor neuron symptoms of hyperreflexia, weakness, muscle atrophy, spasticity, increased muscle tone, clonus, and Babinski sign. Assessment of the dorsal column involvement can be assessed with altered position and vibration sense. Patient’s strength should be tested and evaluated.
Patients may complain of neck pain which can be assessed by testing range of motion. Patients will usually present with restricted motion in flexion and extension. Lhermitte sign can also be tested for by flexing the patient’s neck which will produce electric-like sensation radiating down the torso when positive. Spurling sign can be assessed by extending the head, rotating, and applying pressure on inciting radicular pain [1].
Hypesthesia, paresthesia, or anesthesia should also be evaluated by sensory exams using dull and sharp objects as well as objects of different temperatures.
Diagnosis
What is the diagnostic approach and evaluation for cervical myelopathy?
Normally, the anterior-posterior measurement of the C3 through C7 spinal canal is 16–18 mm. With flexion, this diameter decreases by 2–3 mm and with extension may decrease it up to 3.5 mm [54, 55]. Absolute stenosis of the spinal canal is defined as a canal space less than 10 mm and relative spinal stenosis is 10–13 mm. The Torg ratio is measured to predict significant spinal stenosis to rule out any inherent radiographic measurement errors. This is calculated by dividing the sagittal diameter of the spinal canal by the sagittal diameter of the respective vertebral body and a ratio 0.8 or less is significant.
Cervical spine radiographs (X-rays) in AP and Lateral views are useful for evaluating the anatomy of the cervical spine and the progression of degenerative disease. These views portray neuroforaminal narrowing, spinal stenosis, loss of intervertebral disc space, facet abnormalities, uncovertebral joint arthropathy, and the degree of spondylolisthesis. Lateral views are useful to evaluate the posterior longitudinal ligament. Flexion-extension views are useful for evaluating ligamentous instability and can show anterolisthesis on flexion and retrolisthesis on extension. The Torg-Pavlov ratio of 0.8 or less is used by dividing the anteroposterior diameter of the vertebral body by the anteroposterior diameter of the spinal canal to diagnose congenital spinal stenosis [56].
Magnetic resonance imaging (MRI) is useful for evaluating the extent of spinal cord and nerve root compression as well as any soft tissue or osseous disease [57]. T1 and T2 weighted images are helpful to see compression and signal changes which are indicative of myelopathy. T1-weighted images show superior spatial resolution and T2-weighted images are helpful for evaluating the central canal and thecal sac [2].
Sagittal and axial cuts are used to evaluate discs, thickened ligamenta flava, cord anomalies, and severity of cord compression. Increased cord signal on T2 weighted images is helpful to determine the presence of edema, demyelination, myelomalacia, or gliosis [48].
Diffusion tensor imaging can portray the severity of cord injury before it can be seen on T2 images by detecting early damage to the myelin sheath. This scan has shown high sensitivity for detection of early cervical spondylotic myelopathy and intramedullary lesions [58, 59].
Computed tomographic myelography is used before surgery to depict the severity and location of neural compression. Use of CT is superior to MRI when distinguishing bone from soft tissue intrusion into the cervical foramina for stenotic pathologic changes [60].
Nerve conduction tests which include somatosensory evoked potentials are used to confirm myelopathy and electromyography is used to differentiate between peripheral and central nerve root involvement. Motor evoked potentials help differentiate between spinal cord compression and neurodegenerative disorders. Both SEPs and MEPs can also be used as markers of sensory and motor function during surgical treatment and rehabilitation [61].
Differential Diagnosis
What is the differential diagnosis for compressive cervical myelopathy?
The symptomatology of cervical myelopathy may present similarly in extrinsic vs. intrinsic causes as mentioned earlier in the chapter. The clinical significance of this classification is due to the importance of differentiating compressive cervical myelopathy from conditions of the nerve tissue itself. The differentials of compressive myelopathy have different treatments and prognosis and are important to consider when a patient presents with symptoms of cervical myelopathy. The differential diagnoses for compressive cervical myelopathy are listed in Table 6.5 [2].
Treatment and Management
What is the management and treatment for cervical myelopathy?
There are different modalities of treatment depending on the progress of disease. Patient’s course can be unpredictable at times and follow a slow stepwise decline.
In the absence of clinical evidence of cervical myelopathy, patients are managed with conservative treatment and followed closely for biannual neurologic examinations and annual MRI tests. Patients should be instructed to make daily habit changes such as drinking from a straw to avoid extending the neck because hyperextending the neck can further compress the spinal cord. Patients should also avoid any overhead activities for this reason [48, 62]. High risk activities such as horseback riding, contact sports, ladder climbing, and breaststroke swimming should be avoided [1]. In general, any hyperextension or hyperflexion activities should be avoided.
Physical activity is encouraged such as walking, stationary bicycling, and stretching. Static neck exercises are encouraged as well as strengthening the upper and lower extremities with resistance techniques. Patients are encouraged to feel steady and can be prescribed any assistive devices such as a cane or walker for walking. Pain can be managed with over the counter anti-inflammatory medications if needed. All fall precautions should be taken to prevent further injuries.
If patients display clinical evidence of cervical myelopathy, patients should be referred to a spine surgical specialist for an evaluation [1]. The cross-sectional area of the cord as well as the cord signal help determine whether surgery is indicated. If patients complain of pain, but not myelopathic symptoms, patients are advised to decrease physical activity for 2–3 days. If patients experience severe cervical or radicular pain, a soft neck collar is encouraged to be used for a few days [63]. Applying heat or ice packs can be therapeutic for some patients who experience radicular symptoms. Conservative pain management with acetaminophen and NSAIDs taken at regular intervals is encouraged. Increasing the pain regimen would require more than one family of NSAIDs to be completely ineffective or contraindicated. If pain is severe, opioids and muscle relaxants can be prescribed. Patients with severe and functionally limiting radicular symptoms may benefit from a taper dose of oral steroids for 7–10 days. None-remitting radicular pain can also be treated with gabapentin, pregabalin, tricyclic antidepressants, duloxetine, and others. Relieving depression and anxiety can also help relieve symptoms by medication, cognitive-behavioral therapy, biofeedback, self- hypnosis, and relaxation techniques.
Treatment
When conservative management fails, interlaminar or transforaminal epidural steroid injections can provide pain relief up to several months. If patients still do not respond, diagnostic medial branch nerve blocks and radiofrequency nerve ablation can provide relief. Some patients without significant central spinal canal stenosis are also candidates for spinal cord stimulator trial and implantation.
Symptom control of cervical myelopathy provides temporary relief and when the severity progresses, surgery should be considered. Surgery is not curative but can stop the progression of symptoms by increasing the canal space and lessening the cord compression. Although surgery comes with risks and can have serious complications, it can be very beneficial depending on the cause of the cervical myelopathy.
The Japanese Orthopaedic Association (JOA) classification system which evaluates the severity of spondylotic myelopathy as well as the Nurick scale which evaluates ambulatory function can be useful for determining the need for surgery. A JOA score of less than 13 with clinical symptoms and evidence of spinal cord compression on imaging is recommended for surgery [64]. Surgical options include decompressive single-level or multilevel laminectomy, laminoplasty, discectomy, foraminotomy, and cervical fusion by use of bone graft. Criteria for immediate surgical intervention include progressive weakness, bladder or bowel incontinence, unsteady gait, and upper motor neuron findings.
Patients who suffer from moderate to severe progressive CSM with significant cord compression or cord signal changes benefit from decompressive surgery which can be done from an anterior or posterior approach [48]. Patients with myelopathy that affects up to three spinal levels and patients with cervical kyphotic deformity benefit from the anterior approach which targets pathologic changes anterior to the spinal cord [64]. These anterior structures include the soft disc, hard disc, vertebral body spurs, and ossified posterior longitudinal ligament which can be removed without operating on the spinal cord [48]. Bone grafting and instrumentation ensure stabilization and fusion after the anterior cervical decompression and fusion.
The posterior approach consists of a laminectomy and laminoplasty giving access to hypertrophied laminae and ligamenta flava which is considered posterior disease. Laminectomies are less demanding than anterior corpectomies but can destabilize the cervical spine by pulling back the paraspinal muscles and resulting in loss of lordosis. Laminoplasty preserves the cervical facets and the laminae by increasing the sagittal canal diameter by lifting the laminae away from the degenerative site [65]. Unilateral or bilateral hinges allow symmetric expansion of the spinal canal which allows for the spinal cord to move and be decompressed.
Prognosis
Classifying the severity of cervical myelopathy is important to assess the efficacy of interventions. A variety of scales have been established and used in studies. More commonly used are the Nurick grading system and the Japanese Orthopedic Association system discussed previously in this chapter [66].
Early surgical intervention in cervical myelopathy patients, especially moderate and severe cases, has been associated with better clinical and neurological outcomes. Despite the poorer outcomes associated with delayed diagnosis and treatment of cervical myelopathy, studies have shown the diagnosis of Cervical Spondylotic Myelopathy frequently missed by primary care physicians who encounter the majority of patients initially presenting with cervical myelopathy symptoms [17].
More studies are needed to determine the effect of non-operative management on the clinical outcomes of cervical myelopathy. A systematic review showed that conservative management yielded poorer outcomes in moderate or severe cervical myelopathy patients. It showed that if non-operative management could have any beneficial effects in cervical myelopathy patients, it would be in mild forms of the disease. However, more studies are required to validate the effect of conservative management in mild cervical myelopathy with emphasis on the type on conservative management (e.g.: physical therapy, medications, injections, orthoses, traction, or a combination of treatments) [67].
Cervical myelopathy patients with underlying OPLL have also been noted to be at higher risk of worsening myelopathy with trauma and patients should be counseled on such a possibility [67].
It has also been shown that patients with a greater preoperative Nurick grade and symptoms for more than 12 months may have significantly lower odds of experiencing gait improvement or gait recovery after surgery for cervical myelopathy [68].
Conclusion
Cervical myelopathy is a compression of the spinal cord in the setting of either extrinsic factors, most commonly cervical stenosis or a cervical disc bulge, or intrinsic factors, which include viral infections, motor neuron disease or multiple sclerosis. When diagnosing cervical myelopathy a thorough neurological exam must be performed to detect any upper or lower motor neuron signs and to assess for gait dysfunction or impaired coordination. The treatment of cervical myelopathy is focused on symptomatic relief and strategies to arrest the progression of symptoms. Included among treatment these strategies are lifestyle modifications (avoiding activities that require hyperextension), oral analgesics, spinal injections and, if neurological deficits are present, surgery.
References
Meleger AL, Egyhazi R. Essentials of physical medicine and rehabilitation: musculoskeletal disorders, pain, and rehabilitation, chapter 7. Elsevier; 2015.
Kawaguchi Y. Interventional spine: an algorithmic approach, chapter 50: specific disorders. Saunders; 2007.
Kane SF, Abadie KV, Willson A. Degenerative cervical myelopathy: recognition and management. Am Fam Physician. 2020;102(12):740–50.
Davies BM, Mowforth OD, Smith EK, Kotter MR. Degenerative cervical myelopathy. BMJ. 2018:360.
Choi SH, Kang CN. Degenerative cervical myelopathy: pathophysiology and current treatment strategies. Asian Spine J. 2020;14(5):710.
Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG. Degenerative cervical myelopathy: epidemiology, genetics, and pathogenesis. Spine. 2015;40(12):E675–93.
Badhiwala JH, Ahuja CS, Akbar MA, Witiw CD, Nassiri F, Furlan JC, Curt A, Wilson JR, Fehlings MG. Degenerative cervical myelopathy—update and future directions. Nat Rev Neurol. 2020;16(2):108–24.
Potter K, Saifuddin A. MRI of chronic spinal cord injury. Br J Radiol. 2003;76(905):347–52.
Al-Mefty O, Harkey LH, Middleton TH, Smith RR, Fox JL. Myelopathic cervical spondylotic lesions demonstrated by magnetic resonance imaging. J Neurosurg. 1988;68(2):217–22.
Chen CJ, Lyu RK, Lee ST, Wong YC, Wang LJ. Intramedullary high signal intensity on T2-weighted MR images in cervical spondylotic myelopathy: prediction of prognosis with type of intensity. Radiology. 2001;221(3):789–94.
Vedantam A, Jonathan A, Rajshekhar V. Association of magnetic resonance imaging signal changes and outcome prediction after surgery for cervical spondylotic myelopathy. J Neurosurg Spine. 2011;15(6):660–6.
Tetreault LA, Dettori JR, Wilson JR, Singh A, Nouri A, Fehlings MG, Brodt ED, Jacobs WB. Systematic review of magnetic resonance imaging characteristics that affect treatment decision making and predict clinical outcome in patients with cervical spondylotic myelopathy. Spine. 2013;38(22S):S89–110.
Bakhsheshian J, Mehta VA, Liu JC. Current diagnosis and management of cervical spondylotic myelopathy. Global Spine J. 2017;7(6):572–86.
Iyer A, Azad TD, Tharin S. Cervical spondylotic myelopathy. Clin Spine Surg. 2016;29(10):408–14.
New PW, Cripps RA, Bonne LB. Global maps of non-traumatic spinal cord injury epidemiology: towards a living data repository. Spinal Cord. 2014;52(2):97–109.
Toledano M, Bartleson JD. Cervical spondylotic myelopathy. Neurol Clin. 2013;31(1):287–305.
Behrbalk E, Salame K, Regev GJ, Keynan O, Boszczyk B, Lidar Z. Delayed diagnosis of cervical spondylotic myelopathy by primary care physicians. Neurosurg Focus. 2013;35(1):E1.
Boogaarts HD, Bartels RH. Prevalence of cervical spondylotic myelopathy. Eur Spine J. 2015;24(2):139–41.
Rahimizadeh A, Asgari N, Soufiani H, Rahimizadeh S. Ossification of the cervical ligamentum flavum and case report with myelopathy. Surgical. Neurol Int. 2018;9
Kovalova I, Kerkovsky M, Kadanka Z, Kadanka Z Jr, Nemec M, Jurova B, Dusek L, Jarkovsky J, Bednarik J. Prevalence and imaging characteristics of nonmyelopathic and myelopathic spondylotic cervical cord compression. Spine. 2016;41(24):1908–16.
Bednarik J, Kadanka Z, Dusek L, Kerkovsky M, Vohanka S, Novotny O, Urbanek I, Kratochvilova D. Presymptomatic spondylotic cervical myelopathy: an updated predictive model. Eur Spine J. 2008;17(3):421–31.
Voorhies RM. Cervical spondylosis: recognition, differential diagnosis, and management. Ochsner J. 2001;3(2):78–84.
Park SJ, Kim SB, Kim MK, Lee SH, Oh IH. Clinical features and surgical results of cervical myelopathy caused by soft disc herniation. Korean J Spine. 2013;10(3):138.
O'Laoire SA, Thomas DG. Spinal cord compression due to prolapse of cervical intervertebral disc (herniation of nucleus pulposus): treatment in 26 cases by discectomy without interbody bone graft. J Neurosurg. 1983;59(5):847–53.
Boody BS, Lendner M, Vaccaro AR. Ossification of the posterior longitudinal ligament in the cervical spine: a review. Int Orthop. 2019;43(4):797–805.
Ehara S, Shimamura T, Nakamura R, Yamazaki K. Paravertebral ligamentous ossification: DISH, OPLL and OLF. Eur J Radiol. 1998;27(3):196–205.
Nouri A, Martin AR, Tetreault L, Nater A, Kato S, Nakashima H, Nagoshi N, Reihani-Kermani H, Fehlings MG. MRI analysis of the combined prospectively collected AOSpine North America and International Data: the prevalence and spectrum of pathologies in a global cohort of patients with degenerative cervical myelopathy. Spine. 2017;42(14):1058–67.
Maeda S, Koga H, Matsunaga S, Numasawa T, Ikari K, Furushima K, Harata S, Takeda J, Sakou T, Komiya S, Inoue I. Gender-specific haplotype association of collagen α2 (XI) gene in ossification of the posterior longitudinal ligament of the spine. J Hum Genet. 2001;46(1):1–4.
Yan L, Gao R, Liu Y, He B, Lv S, Hao D. The pathogenesis of ossification of the posterior longitudinal ligament. Aging Dis. 2017;8(5):570.
Abiola R, Rubery P, Mesfin A. Ossification of the posterior longitudinal ligament: etiology, diagnosis, and outcomes of nonoperative and operative management. Global spine J. 2016;6(2):195–204.
Bakhsh W, Saleh A, Yokogawa N, Gruber J, Rubery PT, Mesfin A. Cervical ossification of the posterior longitudinal ligament: a computed tomography–based epidemiological study of 2917 patients. Global Spine J 2019;9(8):820–825.
Fujimori T, Le H, Hu SS, Chin C, Pekmezci M, Schairer W, Tay BK, Hamasaki T, Yoshikawa H, Iwasaki M. Ossification of the posterior longitudinal ligament of the cervical spine in 3161 patients: a CT-based study. Spine. 2015;40(7):E394–403.
Stapleton CJ, Pham MH, Attenello FJ, Hsieh PC. Ossification of the posterior longitudinal ligament: genetics and pathophysiology. Neurosurg Focus. 2011;30(3):E6.
Matsunaga S, Sakou T. OPLL: disease entity, incidence, literature search, and prognosis. InOPLL 2006 (pp. 11-17). Springer, Tokyo.
Yoshimura N, Nagata K, Muraki S, Oka H, Yoshida M, Enyo Y, Kagotani R, Hashizume H, Yamada H, Ishimoto Y, Teraguchi M. Prevalence and progression of radiographic ossification of the posterior longitudinal ligament and associated factors in the Japanese population: a 3-year follow-up of the ROAD study. Osteoporos Int. 2014;25(3):1089–98.
Hollenberg AM, Mesfin A. Ossification of the posterior longitudinal ligament in north American patients: does presentation with spinal cord injury matter? World Neurosurg. 2020;143:e581–9.
Tsuyama NA. Ossification of the posterior longitudinal ligament of the spine. Clin Orthop Relat Res. 1984;1(184):71–84.
Miyasaka K, Kaneda K, Sato S, Iwasaki Y, Abe S, Takei H, Tsuru M, Tashiro K, Abe H, Fujioka Y. Myelopathy due to ossification or calcification of the ligamentum flavum: radiologic and histologic evaluations. Am J Neuroradiol. 1983;4(3):629–32.
Meyer CA, Vagal AS, Seaman D. Put your back into it: pathologic conditions of the spine at chest CT. Radiographics. 2011;31(5):1425–41.
Nguyen HV, Ludwig SC, Silber J, Gelb DE, Anderson PA, Frank L, Vaccaro AR. Rheumatoid arthritis of the cervical spine. Spine J. 2004;4(3):329–34.
Wasserman BR, Moskovicich R, Razi AE. Rheumatoid arthritis of the cervical spine. Bull NYU Hosp Joint Dis. 2011;69:136–48.
Ranawat CS, O'Leary PA, Pellicci PA, Tsairis PE, Marchisello PE, Dorr LA. Cervical spine fusion in rheumatoid arthritis. J Bone Joint Surg Am. 1979;61(7):1003–10.
Kuntz D, Naveau B, Bardin T, Drueke T, Treves R, Dryll A. Destructive spondylarthropathy in hemodialyzed patients. Arthritis Rheum. 1984;27(4):369–75.
Shiota E, Naito M, Tsuchiya K. Surgical therapy for dialysis-related spondyloarthropathy: review of 30 cases. Clin Spine Surg. 2001;14(2):165–71.
Bindi P, Chanard J. Destructive spondyloarthropathy in dialysis patients: an overview. Nephron. 1990;55(2):104–9.
Ali FE, Al-Bustan MA, Al-Busairi WA, Al-Mulla FA, Esbaita EY. Cervical spine abnormalities associated with Down syndrome. Int Orthop. 2006;30(4):284–9.
McKay SD, Al-Omari A, Tomlinson LA, Dormans JP. Review of cervical spine anomalies in genetic syndromes. Spine. 2012;37(5):E269–77.
Fast A, Dudkiewicz I. Essentials of physical medicine and rehabilitation: musculoskeletal disorders, pain, and rehabilitation, chapter 1, cervical Spondylotic myelopathy. Elsevier; 2015.
Rao R. Neck pain, cervical radiculopathy, and cervical myelopathy: pathophysiology, natural history, and clinical evaluation. JBJS. 2002;84(10):1872–81.
McCartney S, Baskerville R, Blagg S, McCartney D. Cervical radiculopathy and cervical myelopathy: diagnosis and management in primary care. Br J Gen Pract. 2018;68(666):44–6.
Donnally CJ III, Butler AJ, Rush AJ III, Bondar KJ, Wang MY, Eismont FJ. The most influential publications in cervical myelopathy. J Spine Surg. 2018;4(4):770.
Yonenobu K, Abumi K, Nagata K, Taketomi E, Ueyama K. Interobserver and intraobserver reliability of the Japanese Orthopaedic Association scoring system for evaluation of cervical compression myelopathy. Spine. 2001;26(17):1890–4.
Ono K, Ebara S, Fuji TA, Yonenobu KA, Fujiwara KE, Yamashita KA. Myelopathy hand. New clinical signs of cervical cord damage. J Bone Joint Surg. 1987;69(2):215–9.
Penning L. Functional radiographic examination. Functional pathology of the cervical spine. Penning, pp. 1–27.
Gu R, Zhu Q, Lin Y, Yang X, Gao Z, Tanaka Y. Dynamic canal encroachment of ligamentum flavum: an in vitro study of cadaveric specimens. Clin Spine Surg. 2006;19(3):187–90.
Cantu RC. The cervical spinal stenosis controversy. Clin Sports Med. 1998;17(1):121–6.
Nouri A, Martin AR, Mikulis D, Fehlings MG. Magnetic resonance imaging assessment of degenerative cervical myelopathy: a review of structural changes and measurement techniques. Neurosurg Focus. 2016;40(6):E5.
Rajasekaran S, Kanna RM, Shetty AP. Diffusion tensor imaging of the spinal cord and its clinical applications. J Bone Joint Surg Br. 2012;94(8):1024–31.
Song T, Chen WJ, Yang B, Zhao HP, Huang JW, Cai MJ, Dong TF, Li TS. Diffusion tensor imaging in the cervical spinal cord. Eur Spine J. 2011;20(3):422–8.
Del Grande F, Maus TP, Carrino JA. Imaging the intervertebral disk: age-related changes, herniations, and radicular pain. Radiol Clin. 2012;50(4):629–49.
Nardone R, Höller Y, Brigo F, Frey VN, Lochner P, Leis S, Golaszewski S, Trinka E. The contribution of neurophysiology in the diagnosis and management of cervical spondylotic myelopathy: a review. Spinal Cord. 2016;54(10):756–66.
Lauryssen C, Riew KD, Wang JC. Severe cervical stenosis: operative treatment of continued conservative care. Spine Line. 2006;8(1):21–5.
Persson LC, Carlsson CA, Carlsson JY. Long-lasting cervical radicular pain managed with surgery, physiotherapy, or a cervical collar: a prospective, randomized study. Spine. 1997;22(7):751–8.
Bono CM, Fisher CG, editors. Prove it! Evidence-based analysis of common spine practice. Lippincott Williams & Wilkins; 2010.
Hirabayashi K, Watanabe K, Wakano K, Suzuki N, Satomi K, Ishii Y. Expansive open-door laminoplasty for cervical spinal stenotic myelopathy. Spine. 1983;8(7):693–9.
Kim DH, Vaccaro AR. Interventional spine: an algorithmic approach, chapter 70: surgical treatment of cervical myelopathy. Saunders; 2007.
Rhee JM, Shamji MF, Erwin WM, Bransford RJ, Yoon ST, Smith JS, Kim HJ, Ely CG, Dettori JR, Patel AA, Kalsi-Ryan S. Nonoperative management of cervical myelopathy: a systematic review. Spine. 2013;38(22S):S55–67.
De la Garza-Ramos R, Ramhmdani S, Kosztowski T, Xu R, Yassari R, Witham TF, Bydon A. Prognostic value of preoperative nurick grade and time with symptoms in patients with cervical myelopathy and gait impairment. World Neurosurg. 2017;105:314–20.
Benzel EC, Lancon J, Kesterson L, Hadden T. Cervical laminectomy and dentate ligament section for cervical spondy- lotic myelopathy. J Spinal Disord. 1991;4(3):286–95.
Nurick S. The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95:87–100.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Afifi, T., Zektser, K., Raghunandan, A. (2022). Cervical Myelopathy. In: Harbus, M., Cooper, G., Herrera, J.E., Meyler, Z., Funiciello, M. (eds) A Case-Based Approach to Neck Pain. Springer, Cham. https://doi.org/10.1007/978-3-031-17308-0_6
Download citation
DOI: https://doi.org/10.1007/978-3-031-17308-0_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-17307-3
Online ISBN: 978-3-031-17308-0
eBook Packages: MedicineMedicine (R0)