Abstract
In recent years, national and international demand for mezcal has exponentially increased, promoting deforestation and the overexploitation of agave and firewood species from Mexican plant communities at a rate never seen. In this chapter, we present evidence that the impacts of such overexploitation, conducted mainly in Mexican arid ecosystems, will affect ecological interactions leading to the loss of biotic and cultural diversity. We do so by integrating the results of studies on cooperative interactions between plant species (facilitation), pollination, and seed dispersal studies, which explain how multiple ecosystems could collapse. Although interdependence of different ecological networks is indicative of ecosystem fragility and low resilience, these studies are central to closing the gap between ecology and conservation biology. Consequently, the goal of this chapter focuses on showing the importance of realistically modeling the interrelationship between species using a network approach that allows obtaining early indicators of deterioration where the loss of interactions usually precedes the loss of species.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Biotic interactions
- Co-extinction cascades
- Complex networks
- Extinction debts
- Nectar-feeding bats
- Mezcal boom
- Tehuacán-Cuicatlán Valley
1 Introduction
During the nineteenth century, ecology emerged as a scientific discipline, being Alexander von Humboldt one of the central figures who recognized the dominant role of climate in governing plant geography and vegetation zonation (Jackson 2009). Thus, histories of ecology and biogeography are indissolubly tied as they emerged at the same time with overlapping explanations for species richness patterns from local to global scales.
Humboldt’s expeditions, which occurred mainly within the American tropics, were decisive to relate the intrinsic relationships between nature and climate disturbances, as well as societal issues. In a century characterized by wars and worldwide colonialism, Humboldt noticed the profound environmental transformations in the colonized territories. Large-scale deforestation preceded monoculture plantations for exportation purposes, which replaced the typical food crops grown by local inhabitants (Norder 2019). Humboldt pointed out the disastrous effects of deforestation, not only on nature and on climate, but also on societal issues such as poverty and marginalization of the original inhabitants (Norder 2019).
In fact, he provided important evidence about the reciprocal effects of vegetation and the physical and chemical properties of the atmosphere with potential effects on climate. Consequently, he was one of the first worldwide to propose human-induced climate change, which, up to now, is one of the most important risks for humanity (Jackson et al. 2001; Norder 2019).
Surprisingly, and despite the prevalent mechanistic reductionistic paradigm by which phenomena is understood (through the analysis of the intrinsic properties of its components) (Levins and Lewontin 1985), Humboldt envisioned the world as an interconnected web of life (Norder 2019). Accordingly, animal and plant species depend on each other through their interactions for their survival (Norder 2019). Humboldt’s holistic view of nature that sets the beginning of ecology and biogeography contrasts with the reductionistic philosophical view coined during the sixteenth and seventeenth centuries during the named “Scientific Revolution,” which marked at the same time the beginning of the Industrial Revolution (Fritjof 1996). Under industrialism, “development” was based on accelerated exploitation of resources in the colonies, characterized by the generation of surpluses and the accumulation of capital. This form of “development” was accompanied and validated by the preponderant reductionistic science through technological proposals focused on specific species. Thus, Humboldt’s holistic view of nature did not echo in subsequent ecological research (Shiva 1988). On the contrary, further scientific research grew under an unlimited short-term desire for natural resources exploitation focusing on individual species and as an almost the unique way to understand the structure and dynamics of populations, communities, and ecosystems (Brown et al. 2001). In fact, more than 60% of studies published in the journal Ecology in the eighties, dealing with diversity and biotic interactions dealt at most with two species (Kareiva 1994).
However, since the eighties, ecology has slowly and differentially transited from the reductionistic approach to the study of systems ecology, in which the properties of the whole emerge from the interactions between the parts and therefore the whole is more than the sum of their parts (Levins and Lewontin 1985). This means that in an ecosystem, animals, plants, as well as microorganisms depend on each other for their maintenance through their interactions. This interdependence among species and their maintenance occurs within and across trophic levels not only locally, but also regionally, affecting species distribution, speciation, and extinction or persistence of species during past climate changes (Valiente-Banuet et al. 2006; Brooker et al. 2009; Wisz et al. 2013), as well as future ones (Davis et al. 1998). Consequently, any disturbance directed to one or a group of species may have concomitant effects on others, as well as on higher levels such as ecosystem processes.
At present, fast-paced rates of habitat loss and fragmentation, as well as species overexploitation, are some of the most important anthropogenic drivers of species extinctions and the present environmental crisis (Valiente-Banuet et al. 2015).
Therefore, and under the environmental crisis, forecasting plays a preponderant role to assess the effect of human activities on Earth and its ability to sustain biodiversity at local and global scales as a paramount for human subsistence (Barnosky et al. 2012). On this vein and under a systems ecology approach, different authors have demonstrated that critical transitions caused by threshold effects lead to state shifts producing unanticipated biotic effects (Scheffer et al. 2009; Wisz et al. 2013, and references therein). Thresholds leading to critical transitions are often crossed when local human impacts are amplified by the synergistic interactions of different ecological processes or through feedback loops (Barnosky et al. 2012). Thus, local extinction of species may produce drastic co-extinctions of their mutualistic partners through feedback processes that cascade across other ecological networks (Valiente-Banuet and Verdú 2013).
2 Problem Statement
This chapter is focused on the overexploitation of natural resources related to mezcal boom. Mezcal is an alcoholic beverage obtained from the distillation of plants known as agaves or magueyes, belonging to the genus Agave. Their center of origin is Mexico, where 159 of the 210 known species occur, of which 119 are endemic (García-Mendoza 2002, 2007, 2012; Colunga García Marín 2006). Mezcal production takes place in 27 states of the country (Colunga García Marín 2006) and is carried out by traditional producers who use more than 40 agave species and great amounts of firewood from different species obtained from nature. Mezcal’s production exponential growth rate has increased the overexploitation of agaves and firewood for cooking agaves and distillation which may affect a plethora of ecological interactions at local, regional, and probably geographic scales impacting biodiversity and eventually leading to collapse of ecosystems.
3 Sociological Setting
Mezcal is one of the most emblematic alcoholic beverages of Mexico whose production has been carried out by indigenous and peasant groups for centuries and constitutes the livelihood of thousands of families. Its traditional production, whether artisanal or ancestral, is characterized by a great organoleptic diversity. It is based on the extraction of agaves and firewood from natural vegetation, the former used for cooking piñas (i.e., agave stems whose leaves have been removed) and the latter as energy for distillation. Traditional production is the most widely practiced and constitutes the livelihood of at least 9000 peasant and indigenous families (Hernández-López 2018). Electric or fossil fuel-driven technologies are not involved in the traditional manufacturing process of mezcal. After cooking piñas with firewood, producers macerate them manually using wooden clubs (marros) or by means of rock wheels (tahona) which are moved by animals such as donkeys or horses. Fermentation is carried out in a variety of containers such as wood or animal skins, using yeasts associated with agaves. The resulting product is consumed locally and during festivities.
Mezcal’s recognized organoleptic diversity and richness is a result of variation in local environmental characteristics such as climate, soil, and microorganisms, as well as diverse techniques that vary among regions and among producers in the same region (Colunga-García Marín and Zizumbo-Villarreal 2007; Gutiérrez 2015). Mezcal holds centuries or even millennia of knowledge (Zizumbo-Villarreal et al. 2009; Serra and Lazcano 2015), which is backed and enriched by Mexico’s diverse traditional, cultural, and religious context.
In contrast, industrial production, focused on obtaining profits and the expansion of capital in the shortest possible time, is based on the modernization of production processes and the use of minimal labor (Pérez Hernández et al. 2016). To do this, they resort to planting large areas with agave monocultures, following the rules of the technified agriculture to achieve production. There is no cooking of piñas but rather a processing with diffusers. Industrial mills are used to obtain juices and few producers ferment naturally, accelerating the process using commercial yeasts and chemicals, contravening one of the basic laws of oenology: “the organoleptic richness of the final product is directly proportional to the fermentation time”. They use stainless-steel stills or even distillation towers, instead of copper stills (Gutiérrez 2015).
From the second half of the last century, the production of mezcal has undergone unusual industrialization, favoring international trade supported by the Mexican government as part of a neoliberal policy promoted since the eighties (Plascencia de la Torre and Peralta Gordon 2018). The creation of the Mezcal Regulatory Council (MRC) has played a central role through the regularization of production and the intellectual protection schemes with denominations of origin (DO). These regulations affect their organoleptic diversity, forcing traditional producers to homogenize their production under “quality standards,” which are difficult to reach. In addition, only 10 out of 27 mezcal producing states have DO. Therefore, 17 states are unable to officially name their product mezcal, hampering its commercialization. This is a paradoxical scenario given that international demand should increase the opportunities and contribute to improving the quality of life of all mezcal producers while culturally maintaining their traditions. This situation pushes the traditional production system to a disadvantaged position, with respect to the industrial one, since traditionally they move away from the capitalist mercantile criteria, with limited economic and technological capacity to comply with the regulations imposed by the State (Hernández-López 2018; Plascencia de la Torre and Peralta Gordon 2018). When produced outside the DO states, mezcal producers manufacture clandestinely in order to comply with regulations, including tax regulations. On the contrary, the demand for their high-quality mezcal by intermediaries encourages extractivism and the accelerated destruction of natural resources as a subsistence strategy. Paradoxically, the objective of the DOs was originally to protect socioeconomic, cultural, and environmental sustainability (Bowen 2015).
The Mezcal Regulatory Council (MRC) indicates that production follows an exponential increase rate that was 2.7 million liters in 2015 to 8 million in 2020. More than half of the mezcal volume was exported to 60 countries (CRM 2020). Nevertheless, these rates are underestimated since the MRC registers only industrial associate members.
Overall, the growing mezcal demand has increased the overexploitation of wild agaves, and their seeds through their overcollection, which negatively affects the natural regeneration of populations. Added to this is the extraction of firewood from nature and large-scale deforestation in different areas of Mexico. This phenomenon faithfully reproduces the industrialized tequila expansion that began in the nineteenth century, which promoted the destruction of thousands of hectares of natural vegetation to convert them into monocultures (Huerta and Luna 2015). The overexploitation of agaves ended up extinguishing wild populations, so the production of agaves was carried out through clones obtained from the suckers and through tissue culture techniques under laboratory conditions. The selected clonal agave lines with the best performance were used to plant monocultures, which greatly reduced genetic diversity, affecting their ability to tolerate environmental changes and attack by pathogens. Over time, the depletion of soil fertility in monocultures and the attack by pests encouraged the use of agrochemicals (fertilizers, insecticides, and herbicides) polluting the soil and water, with a negative impact on pollinators.
4 The Agave Ecosystem
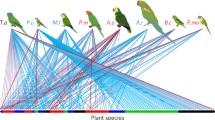
Most agave species are distributed in arid and semiarid environments with a marginal distribution in temperate areas of Mexico and a null presence in wet environments of Tabasco, Campeche, and Quintana Roo (García-Mendoza 2007). The highest diversity occurs in South-Central Mexico in the Tehuacán-Cuicatlán Valley (VTC) (García-Mendoza 2007). Two continuous distribution belts derive from South-Central Mexico: one along the Pacific coast and the other along the coast of the Gulf of Mexico. Both belts generally have fewer agave species. This distribution pattern matches with the distribution of other taxonomic groups with high endemicity such as columnar cacti (CC; tribe Pachycereeae) (Valiente-Banuet et al. 2002), Burseraceae (Becerra 2005), and animal groups such as nectar-feeding bats, among others (Valiente-Banuet et al. 1996, 2002; Fig. 14.1).
For example, in the Tehuacán-Cuicatlán Valley (VTC) agave and CC species are dominant elements of vegetation in 13 plant communities, 11 are columnar cactus forests, and the other ones are a rosetophyllous vegetation community (Valiente-Banuet et al. 2000) and the evergreen sclerophyllous vegetation or Mexical (Valiente-Banuet et al. 1998). CC form forests with densities between 1200–1900 ind ha−1, whereas Agave marmorata and A. potatorum, used for the production of mezcal, reach densities of 2620 and 1830 ind ha−1, the highest among agave species (Valiente-Banuet et al. 2000; Fig. 14.2).
5 Interdependence Among Species Through Biotic Interactions
Different studies show that CC exhibit obligate pollination by nectarivorous bats. Leptonycteris yerbabuenae, L. nivalis, and Choeronycteris mexicana are the most important pollinators. This is also the case for agave species that depend on bat pollination, such as A. marmorata, A. peacockii, A. salmiana, A. potatorum (Valiente-Banuet and Verdú 2013). Additionally, nectar-feeding bats are also the most important seed dispersers of various CC (Godínez-Alvarez et al. 2002; Castillo and Valiente-Banuet in prep), being able to remove most of the seeds and most of them are deposited under bushes and trees. Of all these bats species, L. yerbabuenae is the most effective pollinator and seed disperser and is resident throughout the year in the TCV. This annual residence depends on agave flower resources provided by Agave species and Ceiba spp. during almost seven months, and on flower and fruit resources provided by CC for 5 months, respectively (Rojas-Martínez et al. 1999; Valiente-Banuet and Verdú 2013).
Studies analyzing seedling establishment of all plant species at the community level (N = 5) indicate that on average 96% of the species recruit through the process of facilitation (Valiente-Banuet and Verdú 2013). Facilitation is a process by which one species generates the conditions for the establishment of another one under its canopy (Valiente-Banuet and Verdu 2007). This process is species-specific, since only phylogenetically distantly related species are able to enhance establishment (Verdú et al. 2010). Notably, legumes stand out for their ability to favor the regeneration of a great number of species, up to 95% in a community (Valiente-Banuet and Verdu 2007). The benefit of this cooperative interaction is maintained until distantly related species reach the adult stage (Verdú et al. 2010). Through the ontogeny of the interaction, the gradual arrival of seeds of other species results in the formation of patches of vegetation made up of several species (range 1–12) under the same one canopy. These patches are the structural components of all the plant communities constituting the arena in which plant regeneration occurs, indicating that regeneration niches of species depend on a complex context of multispecific interactions between plants (Castillo et al. 2010). In fact, microbial communities, mainly bacteria and arbuscular mycorrhizae inhabiting the rhizosphere of the different species, which also may be species-specific (Montesinos-Navarro et al. 2012), supply nutrients such as nitrogen and phosphorous, and the connection of roots through hyphae of different species, enabling a selective nutrient transference between them (Montesinos-Navarro et al. 2016, 2017). This indicates that in the facilitation process, besides the modification of the physical microenvironmental beneath plant canopies, microbiome–plant interaction networks play an outstanding role. This occurs through the acquisition of nutrients by plants, up to 80% of the phosphorus and 90% of the nitrogen used by plants, as well as their transference among plants through hyphae (Van Der Heijden et al. 2008; Montesinos-Navarro et al. 2017).
6 Mezcal Production and Extinction Debts
Exponential growth rate of mezcal production due to a growing national and international demand may be already generating extinction debts. Here, I expand the concept of extinction species debt (Tilman et al. 1994; Wearn Oliver et al. 2012) and biotic interactions debt (Valiente-Banuet et al. 2015) to cultural debt. Accordingly, an extinction debt is any loss of species, ecological interactions, and cultural aspects, including the potential disappearance of thousands of traditional producers, which occurs due to the different social and environmental dimensions of mezcal production.
A previous study (Valiente-Banuet and Verdú 2013) was designed to document how an ecosystem may collapse by the disruption of interaction networks. The study was conducted in the TCV in Los Reyes Metzontla, a town whose economic subsistence largely depends to ceramic pottery production. This context was considered by the authors as an ideal scenario because both firewood and agave overexploitation occur in this locality.
To alleviate poverty, since the 1960s pottery production was encouraged leading to an increase of firewood extraction (de la Vega Doria 2006). Annual wood extraction using for firing ceramics sums up 1.66 × 106 kg which has been increasing during the last two decades. Besides the overexploitation of species that are crucial in the networks of ecological interactions, the extraction scheme also has created large, degraded areas containing very few species with no evidence of recovery for more than 30 years after abandonment. Intermixed with these degraded areas, it is possible to find well-conserved areas protected by the inhabitants that represent the natural vegetation found in all the area, a columnar cacti forest dominated by Mitrocereus fulviceps and Neobuxbaumia macrocephala. Besides authors documented the requirements underlying overexploitation among the inhabitants, they obtained information about the extraction of agave species for mezcal production.
Under a realistic scenario, these authors documented quantitatively how human effects on a facilitation network may, through feedback loops, impact concomitant pollination and seed dispersal networks. By including feedback loops in mutualistic networks, it is assumed that extinction in one guild may produce co-extinctions in other guilds, which in turn may cause additional co-extinctions in the first guild and so on. Thus, many of the species exploited for firewood are nurse species that facilitate many species in the network (acting as hubs in the facilitation networks). Similarly, most of the species harvested for mezcal are facilitated species providing nectar and fruits for animals (acting as hubs in pollination and seed dispersal networks (Flores 2005; Estrella Ruiz 2008; Verdú et al. 2010).
By linking facilitation, pollination, and seed dispersal networks into a series, Valiente-Banuet and Verdú (2013), provided evidence for their hypothesis that human-induced extinction of a nurse plant will lead to co-extinction of its facilitated species, especially agaves and columnar cacti, which are primarily pollinated by bats.
The facilitation network was constructed as a matrix, linking the number of individuals of each facilitated species (<30 cm) growing associated with each nurse species and open space. Different experimental studies in the study area confirm that these spatial patterns of association between juvenile and larger plants are due to facilitation and not to mere spatial coincidence (Valiente-Banuet and Ezcurra 1991; Castillo and Valiente-Banuet 2010; Castillo et al. 2010). For the pollination and seed dispersal networks, they focused on the relationships established between bats and columnar cacti and agaves, the most abundant plant species in the area, whose flowers are strongly associated with pollination by animals and whose fleshy fruits (cacti only, since agave seeds are dispersed by wind) are dispersed by animals. Consequently, they worked with only a subset of species within the entire pollination and dispersal networks. The pollination network was based on experimental data and focal observations (Valiente-Banuet et al. 1996, 1997; Flores 2005; Estrella Ruiz 2008) and, for phenological reasons, was split into two stages: (1) early (February to April), the most nectar-limited time of the year, when most Agave species are in bloom and when columnar cacti start blooming, and (2) late (May to January), when most columnar cacti and A. potatorum are also blooming. Likewise, the seed dispersal network was constructed based on previous studies for dominant species in the TCV, Neobuxbaumia tetetzo and N. mezcalaensis (Godínez-Alvarez et al. 2002; Castillo and Valiente-Banuet in prep). For Cephalocereus columna-trajani, N. macrocephala, and Mitrocereus fulviceps, focal observations on fruits during day and night were performed, as well as the seed identification analyzing the frugivore feces captured using five mist nets (20 × 3 m), including that obtained from bat refugia as evidence of transport (Rojas-Martínez et al. 2012). Additionally, for N. macrocephala, the contribution of diurnal and nocturnal seed dispersers was sampled by placing 16 seed collectors of 0.25-m2 plastic net squares nailed to the ground under the canopy of eight different plant species and in open space in an area occupied by the species. All these observations indicate that nectar-feeding bats disperse most of the seeds of these columnar cacti.
Based in the fact that the study system depicts a cyclical dynamics governing facilitation processes in which species x acts as a nurse for the recruitment of species y, species y acts as a nurse for species z, and species z acts as a nurse for species x (see Fig. 1 in Verdu et al. 2009), they simulated co-extinction cascades across these ecological networks quantitatively in order to incorporate metrics reflecting the dependence of facilitated plant species on nurse species and to relate this dependence not only to species abundance but also to the specificity of each particular interaction (Verdú et al. 2010). In addition, it was considered that the concomitant pollination and seed dispersal networks collapse when all the plant species supplying nectar and pollen resources to the bats went co-extinct with their nurse species. Thus, the consequence of removing a particular species from the network is most important in analyzing mutualists, which are more strongly dependent on it.
To simulate a quantitative scenario in which nurse species extinction produces coextinction of their facilitated species, they calculated the dependence dij of facilitated plant species i on nurse species j (i.e., the proportion of the total number of individuals of species i recruiting under nurse species j) (Bascompte et al. 2006). Because dij measures the importance of nurse species for each facilitated plant species, they considered a facilitated plant species to become co-extinct when the sum of its dependencies across nurse species (di) was lower than a particular threshold. They also considered open ground (as possible sites of plant recruitment) as an element in the network. Several extinction thresholds ranging from 0 to 1 were simulated. A threshold equaling 0 indicates that a facilitated species is removed from the network only when the sum of its dependencies is zero (that is, when all of its nurse species have disappeared). When the threshold is 0.5, a facilitated species is removed from the network when the sum of its dependencies is less than 0.5. This threshold can be achieved by removing a very important nurse species for the facilitated species or by removing several less important nurse species.
Valiente-Banuet and Verdú (2013) found that their co-extinction simulations of a facilitation network populated by 50 nurse species and 90 facilitated species triggered by the removal of only 16% of species show that extinctions are dramatically accelerated. In other words, a reduction in the abundance of nurse plants, but not their total extinction, contributes significantly to the co-extinction of species. This means that the extinction of interactions precedes the extinction of species (Valiente-Banuet et al. 2015).
A distinct threshold appears at dij = 0.24, indicating that the ecosystem collapses when the nurse species habitat availability is reduced to below 76% of its original extent (Fig. 14.3). The presence of complex interdependent networks of species and their interactions emphasizes the inherent fragility of ecosystems governed by facilitation and their reduced capacity for resilience. In other words, the disruption of the multi-network structure contributes greatly to ecosystem collapse, consequently affecting ecosystem services.
Co-extinction simulation in facilitation networks. Thresholds indicate the dependence dij of facilitated plant species i on nurse species j (proportion of individuals of a species recruiting under a given nurse species). A facilitated plant species becomes co-extinct when the sum of its dependencies across nurse species (di) was lower than a particular threshold. The horizontal line shows the number of species surviving in the degraded community. The maximum similarity between predicted and observed extinctions occurred when the threshold was equal to 0.24 (Sorensen index S = 0.91). The asterisk denotes that all thresholds ≥0.24 in the cyclical scenario revealed a significant association between observed and predicted extinctions (χ2 test). The arrow indicates collapses in pollination and dispersal networks. (Modified from Valiente-Banuet and Verdú 2013. Copyright license number 5134920539265)
In addition, simulations correctly predicted 75 out 77 extinctions and 8 out of 22 survivals observed in the degraded area (scientific names in blue text in Fig. 14.4). The model also predicted 14 extinctions that were not observed in the degraded area (scientific names in red text in Fig. 14.4) which correspond to species able to resprout (Acacia constricta) or peasant managed species (Lippia graveolens, Stenocereus stellatus). Others are able to recolonize through bird-mediated dispersal (Cordia curassavica, Opuntia spp, Lantana spp) or abiotic dispersal (Ipomoea arborescens, Viguiera grammatoglossa, Aeschynomene compacta, Ayenia fruticosa, Cardiospermum halicacabum, Croton ciliato-glanduliferus).
Co-extinction cascades produced by the overexploitation of a few nurse species in the facilitation network propagate toward pollination and seed dispersal networks. The quantitative facilitation network in the conserved vegetation connects nurse species (left) with facilitated species (right). Open ground was also considered as a site for recruitment. The size of each rectangle is proportional to the number of interactions. Black rectangles indicate the overexploited nurse species. Scientific names in green text indicate species in which extinction was predicted and observed; in black text, that extinction was neither predicted nor observed; in blue text, that extinction was observed but not predicted; and in red text, that extinction was predicted but not observed. Stenocereus stellatus extinction would have occurred in the degraded community but the species is artificially maintained. (Taken from Valiente-Banuet and Verdú (2013). Copyright license number 5134920539265)
Authors noticed also that none of the incorrectly predicted extinctions due to strong recolonization ability corresponded with bat-dispersed plants (e.g., columnar cacti), and the simulations correctly predicted their extinction. Recruitment of bat-dispersed plants in the degraded area is completely absent despite the presence of protected sites provided by nurse species in the community and the opportunity for seeds to potentially establish below them. To test this hypothesis, the authors performed seed sowing experiments with three species of columnar cacti (Neobuxbaumia macrocephala, N. tetetzo, and Mitrocereus fulviceps) (see methods in Valiente-Banuet and Verdú 2013), showing that seedling establishment is not limited by the availability of safe sites, but by dispersal limitation. This result is consistent with the impact that co-extinctions in the facilitation network have in the concomitant pollination and dispersal networks at local scale (Fig. 14.5). Without the nectar and pollen of agaves and columnar cacti, nectar-feeding bats must migrate out of this part of the valley and consequently are no longer available as pollinators and seed dispersers. Pollination and dispersal services performed by bats may abruptly disappear when a critical abundance threshold is crossed (McConkey and Drake 2006). The feeding behavior of the nectar-feeding bat Leptonycteris yerbabuenae, the most important pollinator of Agave and CC, is highly affected by floral availability (Estrella Ruiz 2008). This author found that visitation rates and pollination of the nectar-feeding bat to A. potatorum, one of the most important species for mezcal production, is the highest in localities with the highest overall floral density, at medium floral densities bat pollination is inefficient, whereas at the lowest floral density pollination is totally absent (Estrella Ruiz 2008).
(a) Pristine scenario of a community of the TCV depicting the multiple positive ecological relationships that conform the network of interactions that allows the presence and maintenance of a high biological diversity. (b,c) Nurse species and agaves have gone locally extinct due to intensive extraction. By this point, most interaction networks have undergone co-extinction processes. Without nurse plants, seedlings are not able to establish, impeding regeneration. Bats can be considered functionally extinct, stopping fruit production for columnar cacti and agaves. (d) Collapsed ecosystem after man intervention. The remaining vegetation is composed by adult columnar cacti that had established long before the elimination of regeneration sites. Due to the low density of floral and fruit resources, bat populations no longer visit this community. Regeneration becomes virtually impossible due to the lack of safe sites provided by nurse plants
Clearly, mismatches expected through the network simulations and observed ones may be a consequence of working with a limited number of interaction networks. In addition, this limitation precludes to have a complete real evaluation of the effects of species overextraction. For example, columnar cacti can produce between 815 kg ha−1 to 1100 kg ha−1 fruits (Rojas-Martínez et al. 2012). According to our preliminary observations, fruits, pulp, or seeds are consumed by at least 119 species, among invertebrates (ants which mostly are granivores) and vertebrates, (mainly birds, rodents, and some carnivores such as Canis latrans and Urocyon cinereoargenteus), which means that a reduction in the number of fruits, as a consequence of bat-abundance reduction may affect the maintenance of these species. Moreover, and considering that the TCV vegetation is a mosaic of plant communities dominated by columnar cacti and agave species below 2000 m of altitude, it is possible that mezcal boom may collapse different ecosystems at a regional level. In fact, the mezcal boom has the potential to scale up considering that the distribution of columnar cacti and agaves follows a similar pattern in Mexico. As long as the national and international demand for mezcal increase at an exponential rate, different regions of the country’s 27 producing states will be strongly affected by overexploitation, as well as by deforestation to establish agave monocultures.
This will have great impacts, especially the co-extinction of species and eventually the collapse of ecosystems mainly in arid environments and possibly with variants, depending on the degree of interdependence between species to those already described.
The arid ecosystems of Mexico, which are those that maintain the highest percentage of the country’s endemic flora (Rzedowski 1962) and which are ecosystems governed by facilitation processes (Valiente-Banuet and Verdu 2007), and inhabited by at least 30 ethnic groups (Casas et al. 2010) and unquantified number of peasant communities, are in danger because they depend on their ecosystems’ services for survival.
References
Barnosky AD, Hadly E, Bascompte J et al (2012) Approaching a state shift in Earth’s biosphere. Nature 486:52–58
Bascompte J, Jordano P, Olesen JM (2006) Asymmetric coevolutionary networks facilitate biodiversity maintenance. Science 312:431–433
Becerra JX (2005) Timing the origin and expansion of the Mexican tropical dry forest. PNAS 102:10919–10923
Bowen S (2015) Divided spirits: Tequila, Mezcal and the politics of production. University of California Press, California
Brooker RW, Callaway RM, Cavieres LA et al (2009) Don’t Diss integration: a comment on Ricklefs’s disintegrating communities. Am Nat 174:919–927
Brown JH, Whitham TG, Ernest SKM et al (2001) Complex species interactions and the dynamics of ecological systems: long-term experiments. Science 293:643–650
Casas A, Valiente-Banuet A, Solis-Rojas L et al (2010) El manejo de la biodiversidad en el desierto: el Valle de Tehuacán-Cuicatlán. In: Toledo VM (ed) La biodiversidad de México: Inventarios, manejos, usos, informática, conservación e importancia cultutal. CONACULTA, Ciudad de México
Castillo JP, Valiente-Banuet A (2010) Species-specificity of nurse plants for the establishment, survivorship, and growth of a columnar cactus. Am J Bot 97:1289–1295
Castillo JP, Valiente-Banuet A (in prep.) Specialization through coevolution in a vertebrate cactus seed dispersal system. Ecol Monogr
Castillo JP, Verdú M, Valiente-Banuet A (2010) Neighborhood phylodiversity affects plant performance. Ecology 91:3656–3663
Colunga García Marín SP (2006) Base de datos de nombres técnicos o de uso común en el aprovechamiento de los agaves en México. Centro de Investigación Científica de Yucatán AC. SNIB-CONABIO Proyecto No. CS007. Ciudad de México
Colunga-García Marín P, Zizumbo-Villarreal D (2007) Tequila and other Agave spirits from west-central Mexico: current germplasm diversity, conservation and origin. In: Hawksworth DL, Bull AT (eds) Plant conservation and biodiversity. Springer, Dordrecht
CRM (2020) Informe estadístico 2020. El mezcal, la cultura líquida de México. Consejo Regulador del Mezcal. México
Davis AJ, Jenkinson LS, Lawton JH et al (1998) Making mistakes when predicting shifts in species range in response to global warming. Nature 391:783–786
de la Vega Doria S (2006) La Alfarería en Los Reyes Motzontla: pasado, presente y futuro, Instituto Nacional de Antropología e Historia, México
Estrella Ruiz JP (2008) Efecto de la explotación humana en la biología de la polinización de Agave salmiana y Agave potatorum en el Valle de Tehuacán-Cuicatlán. MSc Thesis. UNAM, Ciudad de México
Flores A (2005) Competencia por polinización en dos especies de agave con floración traslapada del Valle de Tehucán, México. MSc Thesis. UNAM, Ciudad de México
Fritjof C (1996) Web of life: a new scientific understanding of living systems. Bantam Doubleday Dell Publishing Group, New York
García-Mendoza A (2002) Distribution of agave (Agavaceae) in México. Cact Succ J 74:177–188
García-Mendoza A (2007) Los agaves de México. Ciencias 87:14–23
García-Mendoza A (2012) México, país de magueyes. La Jornada del campo. 18/02/2018
Godínez-Alvarez H, Valiente-Banuet A, Rojas-Martínez A (2002) The role of seed dispersers in the population dynamics of the columnar cactus Neobuxbaumia tetetzo. Ecology 83:2617–2629
Gutiérrez GS (2015) La riqueza organoléptica. In: Vera CJ, Fernández R (eds) Agua de las Verdes Matas. Tequila y Mezcal. CONACULTA-INAH- -Artes de México, Caballito Cerrero, México
Hernández-López JJ (2018) El mezcal como patrimonio social: de indicaciones geográficas genéricas a denominaciones de origen regionales. Em Questão 24:404–433
Huerta RR, Luna ZR (2015) Los caminos del mezcal. In: Vera CJL, Fernández R (eds) Agua de las Verdes Matas. Tequila y Mezcal. México: CONACULTA, INAH, Artes de México, Caballito Cerrero
Jackson ST (2009) Alexander von Humboldt and the general physics of the earth. Science 324:596–597
Jackson JBC, Kirby MX, Berger WH et al (2001) Historical overfishing and the recent collapse of coastal ecosystems. Science 293:629–637
Kareiva P (1994) Special feature: higher order interactions as a foil to reductionist ecology. Ecology 75:1527–1528
Levins R, Lewontin R (1985) The dialectical biologist. Harvard University Press
McConkey KR, Drake DR (2006) Flying foxes cease to function as seed dispersers long before they become rare. Ecology 87:271–276
Montesinos-Navarro A, Segarra-Moragues JG et al (2012) The network structure of plant–arbuscular mycorrhizal fungi. New Phytol 194:536–547
Montesinos-Navarro A, Verdú M, Querejeta JI et al (2016) Soil fungi promote nitrogen transfer among plants involved in long-lasting facilitative interactions. Perspect Plant Ecol Evol Syst 18:45–51
Montesinos-Navarro A, Verdú M, Querejeta JI et al (2017) Nurse plants transfer more nitrogen to distantly related species. Ecology 98:1300–1310
Norder SJ (2019) Alexander von Humboldt (1769–1859): Connecting geodiversity, biodiversity and society. J Biogeogr 46:1627–1630
Pérez Hernández E, Chávez Parga MC, González Hernández JC (2016) Revisión del agave y el mezcal. Rev Col Biotecnol 18:148–164
Plascencia de la Torre MF, Peralta Gordon LM (2018) Análisis histórico de los mezcales y su situación actual, desde una perspectiva ecomarxista. Eutopía 14:23–42
Rojas-Martínez A, Valiente-Banuet A, Del Coro Arizmendi M et al (1999) Seasonal distribution of the long-nosed bat (Leptonycteris curasoae) in North America: does a generalized migration pattern really exist? J Biogeogr 26:1065–1077
Rojas-Martínez A, Godínez-Alvarez H, Valiente-Banuet A et al (2012) Frugivory diet of the lesser long-nosed bat (Leptonycteris yerbabuenae), in the Tehuacán Valley of central Mexico. Therya 3:371–380
Rzedowski J (1962) Contribuciones a la fitogeografía florística e histórica de México. I. Algunas consideraciones acerca del elemento endémico en la flora mexicana. Bot Sci:52–65
Scheffer M, Bascompte J, Brock WA et al (2009) Early-warning signals for critical transitions. Nature 461:53–59
Serra MC, Lazcano AJC (2015) Etnoarqueología del Mezcal: su origen y uso en Mesoamérica. In: Vera CL, Fernández R (eds) Agua de las verdes matas. Tequila y mezcal. CONACULTA-INAH-Artes de México, El Caballito Cerrero, México
Shiva B (1988) Abrazar la vida. Mujer, ecología y supervivencia. Horas y Horas, España
Tilman D, May RM, Lehman CL et al (1994) Habitat destruction and the extinction debt. Nature 371:65–66
Valiente-Banuet A, Ezcurra E (1991) Shade as a cause of the association between the cactus Neobuxbaumia tetetzo and the nurse plant Mimosa luisana in the Tehuacan Valley, Mexico. J Ecol:961–971
Valiente-Banuet A, Verdu M (2007) Facilitation can increase the phylogenetic diversity of plant communities. Ecol Lett 10:1029–1036
Valiente-Banuet A, Verdú M (2013) Human impacts on multiple ecological networks act synergistically to drive ecosystem collapse. Front Ecol Environ 11:408–413
Valiente-Banuet A, Arizmendi MDC, Rojas-Martínez A et al (1996) Ecological relationships between columnar cacti and nectar-feeding bats in Mexico. J Trop Ecol 12:103–119
Valiente-Banuet A, Rojas-Martinez A, Casas A et al (1997) Pollination biology of two winter-blooming giant columnar cacti in the Tehuacan Valley, central Mexico. J Arid Environ 37:331–341
Valiente-Banuet A, Flores-Hernández N, Verdú M et al (1998) The chaparral vegetation in Mexico under nonmediterranean climate: the convergence and Madrean-Tethyan hypotheses reconsidered. Am J Bot 85:1398–1408
Valiente-Banuet A, Casas A, Alcántara-Eguren A et al (2000) La vegetación del Valle de Tehuacán-Cuicatlán. Bol Soc Bot 67:17–74
Valiente-Banuet A, Arizmendi MC, Rojas-Martínez A et al (2002) Biotic Interactions and population dynamics of columnar cacti. In: Fleming TH, Valiente-Vanuet A (eds) Evolution, ecology and conservation of Columnar Cacti and their mutualists. University of Arizona Press, USA
Valiente-Banuet A, Rumebe AV, Verdú M (2006) Modern Quaternary plant lineages promote diversity through facilitation of ancient Tertiary lineages. PNAS 103(16812–16):817
Valiente-Banuet A, Aizen MA, Alcántara JM et al (2015) Beyond species loss: the extinction of ecological interactions in a changing world. Funct Ecol 29:299–307
Van Der Heijden MGA, Bardgett RD, Van Straalen NM (2008) The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11:296–310
Verdu M, Rey PJ, Alcantara JM et al (2009) Phylogenetic signatures of facilitation and competition in successional communities. J Ecol 97:1171–1180
Verdú M, Jordano P, Valiente-Banuet A (2010) The phylogenetic structure of plant facilitation networks changes with competition. J Ecol 98:1454–1461
Wearn Oliver R, Reuman DC, Ewers RM (2012) Extinction debt and windows of conservation opportunity in the Brazilian Amazon. Science 337:228–232
Wisz MS, Pottier J, Kissling WD et al (2013) The role of biotic interactions in shaping distributions and realized assemblages of species: implications for species distribution modelling. Biol Rev 88:15–30
Zizumbo-Villarreal D, González-Zozaya F, Olay-Barrientos A et al (2009) Distillation in Western Mesoamerica before European Contact. Econ Bot 63:413
Acknowledgments
Special thanks to Tania Sánchez Ortiz for her valuable revision to the manuscript which improved it considerably and for her help with the figures’ elaboration. Thanks to Regina Lira who provided important suggestions to the manuscript. Financial support was obtained from PAPIIT-DGAPA-UNAM (IN-214020) and Consejo Nacional de Ciencia y Tecnología (CONACyT-FOP07-2021-04-319061).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Valiente-Banuet, A. (2023). Mezcal Boom and Extinction Debts. In: Jones, R.W., Ornelas-García, C.P., Pineda-López, R., Álvarez, F. (eds) Mexican Fauna in the Anthropocene. Springer, Cham. https://doi.org/10.1007/978-3-031-17277-9_14
Download citation
DOI: https://doi.org/10.1007/978-3-031-17277-9_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-17276-2
Online ISBN: 978-3-031-17277-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)