Abstract
Constructed wetland (CW), a decentralized wastewater treatment system is a potential solution to treat domestic and industrial effluents in an effective, efficient and economical way. In the present study, a lab-scale horizontal subsurface flow constructed wetland (HSSF-CW) planted with Canna indica plants was operated at a temperature below 13 °C to investigate its organics and nutrient removal efficiency in Hamirpur (H.P.), India. Test results indicated that the removal efficiency obtained in the experimental set-up for Chemical oxygen demand (COD), Ammonia nitrogen (\({\mathrm{NH}}_{4}^{+}\)-N), Total nitrogen (TN) and Total phosphorous (TP) is 90.58%, 88.04%, 64.87%, and 52.58%, respectively. Moreover, the modelling results presented a strong correlation between the observed and predicted values. The outcome of the present study implies that the HSSF-CW planted with Canna indica could be effectively and sustainably implemented in the cold climatic regions for treating effluents with low suspended impurities.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Industrial growth and rapid urbanization have resulted in unprecedented water demand and associated wastewater generation (Rashid et al. 2018; Qin et al. 2014). Due to the gap in the installed treatment capacity and the quantity of wastewater generated, a large quantity of untreated wastewater (domestic and industrial) is disposed of directly into water bodies deteriorating the surface water quality (Breida et al. 2020; Chang 2019). The situation is even more critical especially in developing countries where due to financial constraints, wastewater management and treatment are of less priority (Ilyas et al. 2019; Paul 2017; Wang et al. 2014).
The potential solution to the above-stated problem could be the use of sustainable technologies to treat wastewater. In this regard, constructed wetland (CW) is an engineered and ecological technology that simulates the processes of natural wetland for treating domestic and industrial wastewater (Kivaisi 2001; Wu et al. 2015a, b). The conventional wastewater treatment technologies are inefficient in removing heavy metals, nutrients, and emerging contaminants which ultimately enter surface waters contaminating those (Nourmohammadi et al. 2013; Petrović et al. 2003; Yang et al. 2015). Recent researches show that CWs have proven effective in treating effluents enriched with nutrients and other heavy metals (Gill et al. 2017; Jehawi et al. 2020; Tan et al. 2019). The mechanism for pollutant removal in CWs includes a series of physical, chemical, and biological processes (Kadlec and Wallace 2009). The aerobic and anaerobic degradation of organic matter by microbes is mainly accountable for removing organics in CWs, while the conventional nitrification and denitrification route is followed for nitrogen removal (Saeed and Sun 2017). Moreover, phosphorus is removed by getting adsorbed on the surface of substrate media and through plant uptake (Vymazal 2004). However, treatment efficiency in CWs depends upon several parameters and factors such as hydraulic loading rate (HLR), hydraulic retention time (HRT), mode of feeding (continuous or intermittent), pH, dissolved oxygen (DO), type of macrophytes, and substrate media (Saeed and Sun 2012; Wu et al. 2015a, b).
In addition to the factors influencing CWs, it has also been seen that low temperatures affect the removal efficiency of the CWs significantly (Kadlec and Reddy 2001; Zhu et al. 2018). In general, a feeble microbial activity prevails at lower temperatures resulting in the reduced removal efficiency in CWs (Werker et al. 2002). Moreover, the processes responsible for removing pollutants such as sedimentation, plant uptake, volatilization, filtration, precipitation, and adsorption are largely influenced by low temperatures (Stottmeister et al. 2003). Therefore, it is evident that despite showing satisfactory performance when applied in the warm climatic regions, the implementation of CWs in a cold climate is uncertain. Recent reviews by various researchers present a global view on the application of CWs in cold climatic regions (Ji et al. 2020a, b; Ji et al. 2020a, b; Wang et al. 2017). However, there is limited literature available to support its applicability in the remote and hilly areas in India having mild to cold climatic conditions.
The current study aims to investigate the potential of HSSF-CW to remove the organics and nutrients from secondary effluent under cold climatic conditions in Hamirpur, India. In a cold climate, the sub-surface flow CWs are effective in providing thermal insulation due to the presence of an unsaturated layer at the surface (Varma et al. 2021). To determine the pollutant removal efficiency in the prevailing climatic conditions, a lab-scale horizontal subsurface flow constructed wetland (HSSF-CW) model was designed and tested. Further, the performance of the experimental set-up obtained for chemical oxygen demand (COD), ammonia nitrogen (\({\mathrm{NH}}_{4}^{+}\)-N), total nitrogen (TN), and total phosphorus (TP) removal were modelled using the first-order Kinetic plug flow model.
2 Materials and Methods
2.1 Study Area
The town Hamirpur (31.68°N 76.52°E) is located in the Hamirpur district of Himachal Pradesh, India. In Hamirpur, the temperature could fall to as low as 1 °C between mid-October and April. From the perspective of wastewater treatment and management, Hamirpur has 4 STPs: Hamirpur—Zone I, Zone II, Zone III, and STP at NIT Hamirpur, having the design capacity of 3.13, 1.35, 0.68, and 0.27 MLD respectively. (Ganguly and Dewan 2020) through his case study reported the overall treatment efficiency of the 4 STPs at Hamirpur as 80.67% (Zone I), 73.27% (Zone II), 76.08% (Zone III), and 57.29% (NIT Hamirpur). CW technology could be combined with the existing wastewater treatment plants (WWTPs) to enhance their overall treatment efficiency and can also be implemented independently with a pre-treatment facility to treat wastewater from small localities. Figure 14.1 shows the geographical location of Hamirpur.
2.2 Selection of Macrophytes
The plant species, namely Canna indica was selected for the study. The Canna indica plants having an average height of 20 cm were collected from the plant nursery in NIT Hamirpur (H.P.). The previous studies conducted by various researchers explaining the phytoremediation properties of the plant acted as a base for plant selection. These plants have proven effective in remediating heavy metals from the effluents (Solanki et al. 2018; Zhang et al. 2020). Moreover, their efficiency to treat organics and nutrients from wastewater (domestic and industrial) has been investigated by various researchers (Cui et al. 2010; Vankar and Srivastava 2018; Wang et al. 2016).
2.3 Selection of Substrate Material
The substrate media selected for the experimental set-up was composed of gravel and sand. Gravel and sand as a substrate have been used in the CWs in previous researches (Anh et al. 2020; Bulc and Ojstršek 2008). The volume of sand and gravel estimated to be used in the experimental set-up was 0.04 m3 and 0.12 m3, respectively. Physical properties of the substrate media were tested as per the IS 2386 Part-1 (1963) and were following the code.
2.4 Design and Construction of the Experimental Set-Up
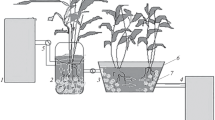
The experimental work was conducted in the Environmental Engineering laboratory at the Department of Civil Engineering, NIT Hamirpur (H.P.). The experimental set-up simulates a HSSF-CW under cold climatic conditions. The wetland model consisted of a glass tank having dimensions 100 cm × 40 cm × 60 cm (L × B × H). A tap was provided close to the base of the tank as an outlet to collect the treated effluent for analysis. The tank was filled with the substrate media up to a height of 30 cm; 10 cm thick gravel layer (Ø 5–10 mm) was laid at the bottom and 20 cm of sand layer (Ø 1–2 mm) above it (Wu et al. 2015a, b). Six Canna indica plants having an average height of 20 cm were planted spaced equally in the CW model after transplanting it from the NIT Hamirpur plant nursery. The secondary effluent received from the STP at NIT Hamirpur was fed into the experimental system for 2 months until the bio-layer formation and new plant growth were observed. Diagram and image of the experimental set-up along with detailed information is shown in Figs. 14.2 and 14.3, respectively.
2.5 Operation of the Experimental System
To avoid any disparity in the concentration of the influent, simulated secondary effluent was used. The synthetic wastewater was prepared by dissolving NH4Cl, CaCl2⋅2H2O, KH2PO4, CH3COONa, and MgSO4⋅7H2O in the tap water (Ge et al. 2018; Liu et al. 2017). This resulted in the COD, \({\mathrm{NH}}_{4}^{+}\)-N, TP and TN concentrations being approximately 105 mg/L, 19 mg/L, 2.5 mg/L, and 40 mg/L, respectively. The characteristics of the synthetic secondary effluent have been shown in Table 14.1. The experimental system received wastewater in the intermittent batch mode of 5 L/m2/day. During the experimental period (seeding and operating) the ambient temperature varied between 8 and 13 °C. The whole experiment lasted from 1 December 2020 to 8 February 2021. Plant growth was carefully monitored and measured in the seeding period, and relative growth rate (RGR) was calculated (Li and Guo 2017).
2.6 Sampling and Analytical Analysis
Water samples were collected at the inlet and outlet of the experimental set-up from February 1 to February 10. Sampling was done every second day up to the tenth day in the operating period, and collected samples were instantly stored at 4 °C for future analysis. For all water samples, COD, \({\mathrm{NH}}_{4}^{+}\)-N, TP, and TN were tested as per the APHA (1998), Standard Methods 20th Edition. The test methods adopted for the measurement of the above parameters were the Closed reflex method for COD, Nesslerisation method for \({\mathrm{NH}}_{4}^{+}\)-N, Stannous chloride method for TP and UV–Vis spectrophotometer method for TN. Other environmental parameters such as Total dissolved solids (TDS), pH, Temperature (T), and Electrical conductivity (EC) were measured in-situ using Multiparameter Analyzer (Agilent Technologies 3200 M Multi-parameter Analyzer). The DO concentration in the influent and effluent was measured using the DO meter (Agilent technologies 3200D Dissolved Oxygen Meter).
2.7 Plant Growth
Height of the plants and relative growth rate (RGR) values have been used to report the growth status of Canna indica plants during the seeding period. Equation (14.1) defines the relative growth rate in plants:
where H1 and H2 are the average plant heights recorded at time t1 and t2.
2.8 Modelling and Statistical Analyses
The treatment performance of the lab-scale HSSF-CW for organics and nutrient removal was modelled using the Ist order Kinetic plug flow model (KC*) (Eq. 14.2) to present a correlation between the experimental and simulated results (Kadlec and Wallace 2009).
where:
- \({\mathrm{C}}_{\mathrm{o}}\) :
-
Effluent concentration (mg/L)
- \({\mathrm{C}}_{\mathrm{i}}\) :
-
Influent concentration (mg/L)
- \({\mathrm{K}}_{\mathrm{t}}\) :
-
First order reaction coefficient (day−1)
- \(\uptau\) :
-
Theoretical HRT (day)
The R2, SSE, and RMSE values were also computed to indicate the goodness of fit between the observed and predicted values. All statistical analyses were performed by applying the software R2018a—MATLAB & Simulink—MathWorks.
3 Results and Discussion
3.1 Plant Growth
The variation in the plants’ heights and associated RGR values are shown in Table 14.2. Plants showed rapid growth during the first month of seeding and the RGR value varied between 0.0223 and 0.0357 day−1. This might be due to the excess availability of nutrients at the beginning of the experiment. Lesser plant growth was recorded in the second month with RGR values lying between 0.0131 and 0.0033 day−1. Figure 14.4 shows the growth and development of the Canna indica plants in the seeding period. At the end of the seeding period of 2 months, the plants’ height was 62 cm. Figure 14.5 shows the height variation recorded in the seeding period (2 months). The experimental set-up was now functional and was filled with synthetic wastewater for final testing.
3.2 Measurement of pH, DO, TDS and EC
The pH observed in the HSSF-CW system varied from 6.84 ± 0.26 in the influent to 7.45 ± 0.21 in the effluent. It has been observed that photosynthetically elevated pH affects the treatment performance of CWs (Yin et al. 2016). The influent DO concentration increased from 6.35 ± 0.11 mg/L to 10.84 ± 0.58 mg/L in the effluent. This might indicate the extent of degradation of contaminants that take place in the experimental system. However, DO concentration in the effluent from HSSF-CW could not be straightly related to the removal efficiency of the wetland system (Vymazal and Kröpfelová 2008). Figure 14.6 shows the increase in DO concentration and pH with increasing HRT.
The TDS and EC value of the synthetic effluent decreased from 1070.35 mg/L and 1835.428 µS/cm to 359.63 mg/L and 697.43 µS/cm, respectively after being retained for 10 days. In CWs, the main processes responsible for TDS removal are adsorption to the substrate, sedimentation, precipitation, microbial degradation, and plant uptake (Kadlec and Wallace 2009). This might be the reason for the low concentration of TDS in the effluent. Figure 14.7 shows the variation of TDS and EC with increasing HRT. This could be supported by the fact that TDS in water and its EC are correlated (Rusydi 2018).
3.3 Removal of Pollutants
3.3.1 COD Removal
Organics in the HSSF-CWs could primarily be degraded aerobically and anaerobically (Carballeira et al. 2017). The oxygen requirement for aerobic degradation is satisfied by atmospheric diffusion, convection, and plant root transfer. Anaerobic degradation of organic matter takes place in the substrate media where oxygen is deficient. In this study, the average COD concentration in the HSSF-CW declined from 104.45 mg/L to 9.84 mg/L indicating the overall removal efficiency to be 90.58%. This finding could be justified by the presence of high DO concentration in the effluent which was found to be 10.74 mg/L. As shown in Table 14.4, the COD concentration dropped to less than half of its value (<50 mg/L) in 2 to 4 days, signifying organic matter could be removed in a shorter period in the wetland system. COD reduced to about 20 mg/L when HRT reached 8 days and then remained nearly stable up to 10 days. The overall removal efficiency obtained for COD removal is 90.58%. The treatment efficiency obtained is in association with the previous studies in the cold climate using HSSF-CW (Paruch et al. 2016).
3.3.2 Nitrogen (NH4+ and TN) Removal
The mechanism for nitrogen removal in the sub-surface flow CWs majorly consists of classical nitrification and denitrification path which includes nitrification (NH4+ → NO2− → NO3−) followed by canonical denitrification (NO3− → NO2− → NO → N2O → N2) processes (Maharjan et al. 2020). In this study, the average concentration of NH4+ and TN in the treated effluent was found to be 2.23 mg/L and 39.48 mg/L, respectively.
A rapid decline was observed in the concentration of \({\mathrm{NH}}_{4}^{+}\)-N in the initial days where it dropped from 18.65 mg/L to 8.32 mg/L indicating a more than 50% removal of \({\mathrm{NH}}_{4}^{+}\)-N in 4 days. This was due to the adequate availability of oxygen in the beginning. However, the removal rate was significantly low at the later stage (8–10 days) of the experiment. The overall removal efficiency obtained for \({\mathrm{NH}}_{4}^{+}\)-N removal is 88.04%. The extent of \({\mathrm{NH}}_{4}^{+}\)-N removal obtained in this study is supported by the outcomes of the previously published literature (F. Wang et al. 2012).
The TN removal observed in the HSSF-CW decreased gradually. Though, at the initial stage (0–4 days), the concentration of TN reduced from 39.48 mg/L to 21.62 mg/L indicating an initial removal efficiency of less than 50%. However, beyond 4 days, the TN concentration reduced with a decreasing rate and was relatively stable between 8 and 10 days. The insufficient carbon source inhibits the denitrification rate resulting in a lower TN removal. The overall removal efficiency obtained for TN removal is 64.72%. Similar removal efficiency is obtained in other researches as well (Calheiros et al. 2012; Paruch et al. 2016).
The trend obtained in the COD removal justifies the above results. The availability of the carbon source was inadequate to support denitrification in the beginning. Moreover, due to the rapid degradation of organics at shorter HRTs by microbial action makes the carbon source scarcer unable to aid denitrification at longer HRT. A similar emphasis could be drawn from previous studies as well (Zhong et al. 2014; Zhou et al. 2017).
3.3.3 TP Removal
TP removal in the HSSF-CWs is primarily attributed to the processes such as adsorption, precipitation, and plant uptake (Vymazal 2004). In the present study, the treatment efficiency for TP in the lab-scale model was found to be low i.e. 52.58%. The major removal of phosphates in the wetland system takes place by getting adsorbed on the substrate media (Ballantine and Tanner 2010). However, in this case, the pH of the influent and effluent was found to be 6.89 and 7.45, respectively implying a relatively neutral environment in the experimental system. This neutrality of the influent and effluent could be associated with the oxidation state of the Fe (Fe2+) in the substrate media (Nandakumar et al. 2019). Therefore, the possibility of phosphate binding to the substrate material was negligible. The TP removal in the experimental system was primarily by plant uptake. The overall removal efficiency obtained for TP removal in the experimental system is 52.58%. The results obtained are supported by the findings of the previously published literature (Rai et al. 2015). Table 14.3 shows the reduction in concentration of organics (COD) and nutrients (\({\mathrm{NH}}_{4}^{+}\)-N, TN, and TP) with increasing HRT in the lab-scale HSSF-CW.
3.4 Result of Modelling and Statistical Analysis
The Eq. (14.2) representing the first-order Kinetic plug flow model is of the form \(f\left(x\right)=a\times {e}^{(b\times x)}\), where f(x) is the function of the independent variable ‘x’ and, ‘a’ and ‘b’ are the constants. Modelling of the lab-scale HSSF-CW yielded reaction rate coefficients (Kt) for COD, \({\mathrm{NH}}_{4}^{+}\)-N, TN and TP as 0.31 day−1, 0.22 day−1, 0.12 day−1 and 0.09 day−1 respectively. The Kt value for COD illustrates that organics could be removed at a higher rate (30% per day) in the HSSF-CW under cold conditions. Moreover, the simulated first-order degradation coefficients (Kt) for \({\mathrm{NH}}_{4}^{+}\)-N and TN removal in the experimental system were 0.22 day−1 and 0.12 day−1, respectively which shows \({\mathrm{NH}}_{4}^{+}\)-N could be removed more effectively than TN at low temperatures. The least value of Kt was obtained for TP removal. To enhance the TP removal, various substrates such as blast furnace slags, activated carbon, marble, etc. could be used (Bachand and Bachand 2020). The coefficients of regression (R2) obtained are greater than 0.9 indicating the relationship between observed and predicted values to be strong (Dendukuri and Reinhold 2005). The results obtained from modelling and statistical analysis have been shown in Table 14.4. The dynamic transformations of COD, \({\mathrm{NH}}_{4}^{+}\)-N, TN, and TP in the HSSF-CW during the experimental period have been shown in Figs. 14.8, 14.9, 14.10, and 14.11 respectively.
4 Conclusion and Recommendations
-
As observed from the experimental and simulation results, the cold climatic conditions had a negligible impact on the efficiency of the HSSF-CW in terms of organics (COD) and ammonia (\({\mathrm{NH}}_{4}^{+}\)-N) removal. The results of the first order K-C* model indicated that organics and ammonia were removed quickly because of the presence of adequate DO level in the beginning.
-
It is evident from the results that the efficacy of the experimental system for TN removal was adversely affected under low temperatures. Due to the limited carbon supply, TN removal was hindered requiring a prolonged retention time for its removal.
-
The least efficiency was obtained for TP removal which indicates that the capacity of substrate adsorption and plant uptake was exhausted soon.
-
The effectiveness of other substrate materials such as activated carbon, steel slag, waste hollow bricks, ceramsite, etc. could be tested to enhance TP removal efficiency. The use of additional carbon sources and artificial aeration could improve nitrogen removal in the HSSF-CWs. Moreover, microbial activity in the HSSF-CW in a cold climate could be enhanced through bio-augmentation. Further, pilot-scale or full-scale studies are required to support the applicability of CWs in cold climates.
References
Anh BTK, Van Thanh N, Phuong NM, Ha NTH, Yen NH, Lap BQ, & Kim DD (2020) Selection of suitable filter materials for horizontal subsurface flow constructed wetland treating swine wastewater. Water Air Soil Pollut 231(2). https://doi.org/10.1007/s11270-020-4449-6
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, American Water Works Association and Water Environmental Federation, Washington DC
Bachand PAM, Bachand PAM (2020) Selecting substrates to enhance phosphorus removal in treatment wetlands: a review and engineering considerations regarding trophic state and Langmuir parameters. 1–35 (September 2019)
Ballantine DJ, Tanner CC (2010) Substrate and filter materials to enhance phosphorus removal in constructed wetlands treating diffuse farm runoff: a review. N Z J Agric Res 53(1):71–95. https://doi.org/10.1080/00288231003685843
Breida M, Alami Younssi S, Ouammou M, Bouhria M, Hafsi M (2020) Pollution of water sources from agricultural and industrial effluents: special attention to NO3−, Cr(VI), and Cu(II). Water Chem 3. https://doi.org/10.5772/intechopen.86921
Bulc TG, Ojstršek A (2008) The use of constructed wetland for dye-rich textile wastewater treatment. J Hazard Mater 155(1–2):76–82. https://doi.org/10.1016/j.jhazmat.2007.11.068
Calheiros CSC, Quitério PVB, Silva G, Crispim LFC, Brix H, Moura SC, Castro PML (2012) Use of constructed wetland systems with Arundo and Sarcocornia for polishing high salinity tannery wastewater. J Environ Manage 95(1):66–71. https://doi.org/10.1016/j.jenvman.2011.10.003
Carballeira T, Ruiz I, Soto M (2017) Aerobic and anaerobic biodegradability of accumulated solids in horizontal subsurface flow constructed wetlands. Int Biodeterior Biodegradation 119:396–404. https://doi.org/10.1016/j.ibiod.2016.10.048
Chang H (2019) Water and climate change. In: International encyclopedia of geography. https://doi.org/10.1002/9781118786352.wbieg0793.pub2
Cui L, Ouyang Y, Lou Q, Yang F, Chen Y, Zhu W, Luo S (2010) Removal of nutrients from wastewater with Canna indica L. under different vertical-flow constructed wetland conditions. Ecol Eng 36(8):1083–1088. https://doi.org/10.1016/j.ecoleng.2010.04.026
Dendukuri N, Reinhold C (2005) Correlation and regression. Am J Roentgenol 185(1):3–18. https://doi.org/10.2214/ajr.185.1.01850003
Ganguly R, Dewan H (2020) Application of decision making tool to determine effluent quality index of existing sewage treatment plants. J Inst Eng (India) Ser A 101(1):207–219. https://doi.org/10.1007/s40030-019-00416-5
Ge S, Qiu S, Tremblay D, Viner K, Champagne P, Jessop PG (2018) Centrate wastewater treatment with Chlorella vulgaris: simultaneous enhancement of nutrient removal, biomass and lipid production. Chem Eng J 342:310–320. https://doi.org/10.1016/j.cej.2018.02.058
Gill LW, Ring P, Casey B, Higgins NMP, Johnston PM (2017) Long term heavy metal removal by a constructed wetland treating rainfall runoff from a motorway. Sci Total Environ 601–602:32–44. https://doi.org/10.1016/j.scitotenv.2017.05.182
Hassan Rashid MAU, Manzoor MM, Mukhtar S (2018) Urbanization and its effects on water resources: an exploratory analysis. Asian J Water Environ Pollut 15(1):67–74. https://doi.org/10.3233/AJW-180007
Ilyas M, Ahmad W, Khan H, Yousaf S, Yasir M, Khan A (2019) Environmental and health impacts of industrial wastewater effluents in Pakistan: a review. Rev Environ Health 34(2):171–186. https://doi.org/10.1515/reveh-2018-0078
IS 2386–1 (1963) Methods of Test for aggregates for concrete, Part I: particle size and shape. Bureau of Indian Standards
Jehawi OH, Abdullah SRS, Kurniawan SB, Ismail N ‘Izzati, Idris M, Al Sbani NH, Muhamad MH, Hasan HA (2020) Performance of pilot hybrid reed bed constructed wetland with aeration system on nutrient removal for domestic wastewater treatment. Environ Technol Innov 19:100891. https://doi.org/10.1016/j.eti.2020.100891
Ji B, Zhao Y, Vymazal J, Qiao S, Wei T, Li J, Mander Ü (2020a) Can subsurface flow constructed wetlands be applied in cold climate regions? A review of the current knowledge. Ecol Eng 157. https://doi.org/10.1016/j.ecoleng.2020.105992
Ji M, Hu Z, Hou C, Liu H, Ngo HH, Guo W, Lu S, Zhang J (2020b) New insights for enhancing the performance of constructed wetlands at low temperatures. Biores Technol 301(72):122722. https://doi.org/10.1016/j.biortech.2019.122722
Kadlec RH, Reddy KR (2001) Temperature effects in treatment wetlands. Water Environ Res 73(5):543–557. https://doi.org/10.2175/106143001x139614
Kadlec RH, Wallace S (2009) Treatment wetlands, 2nd edn. Taylor & Francis, Boca Raton. https://doi.org/10.1201/9781420012514
Kivaisi AK (2001) The potential for constructed wetlands for wastewater treatment and reuse in developing countries: a review. Ecol Eng 16(4):545–560. https://doi.org/10.1016/S0925-8574(00)00113-0
Li X, Guo RC (2017) Comparison of nitrogen removal in floating treatment wetlands constructed with Phragmites australis and Acorus calamus in a cold temperate zone. Water Air Soil Pollut 228(4). https://doi.org/10.1007/s11270-017-3266-z
Liu X, Wang H, Yang Q, Li J, Zhang Y, Peng Y (2017) Online control of biofilm and reducing carbon dosage in denitrifying biofilter: pilot and full-scale application. Front Environ Sci Eng 11(1):1–8. https://doi.org/10.1007/s11783-017-0895-9
Maharjan AK, Mori K, Toyama T (2020) Nitrogen removal ability and characteristics of the laboratory-scale tidal flow constructed wetlands for treating ammonium-nitrogen contaminated groundwater. Water (Switz) 12(5). https://doi.org/10.3390/W12051326
Nandakumar S, Pipil H, Ray S, Haritash AK (2019) Removal of phosphorous and nitrogen from wastewater in Brachiaria-based constructed wetland. Chemosphere 233:216–222. https://doi.org/10.1016/j.chemosphere.2019.05.240
Nourmohammadi D, Esmaeeli MB, Akbarian H, Ghasemian M (2013) Nitrogen removal in a full-scale domestic wastewater treatment plant with activated sludge and trickling filter. J Environ Public Health. https://doi.org/10.1155/2013/504705
Paruch AM, Mæhlum T, Haarstad K, Blankenberg A-GB, Hensel G (2016) Natural and constructed wetlands. Nat Constructed Wetlands 1431:41–55. https://doi.org/10.1007/978-3-319-38927-1
Paul D (2017) Research on heavy metal pollution of river Ganga: a review. Ann Agrarian Sci 15(2):278–286. https://doi.org/10.1016/j.aasci.2017.04.001
Petrović M, Gonzalez S, Barceló D (2003) Analysis and removal of emerging contaminants in wastewater and drinking water. TrAC Trends Anal Chem 22(10):685–696. https://doi.org/10.1016/S0165-9936(03)01105-1
Qin HP, Su Q, Khu ST, Tang N (2014) Water quality changes during rapid urbanization in the Shenzhen river catchment: an integrated view of socio-economic and infrastructure development. Sustain (switz) 6(10):7433–7451. https://doi.org/10.3390/su6107433
Rai UN, Upadhyay AK, Singh NK, Dwivedi S, Tripathi RD (2015) Seasonal applicability of horizontal sub-surface flow constructed wetland for trace elements and nutrient removal from urban wastes to conserve Ganga River water quality at Haridwar, India. Ecol Eng 81:115–122. https://doi.org/10.1016/j.ecoleng.2015.04.039
Rusydi AF (2018) Correlation between conductivity and total dissolved solid in various type of water: a review. IOP Conf Ser Earth Environ Sci 118(1). https://doi.org/10.1088/1755-1315/118/1/012019
Saeed T, Sun G (2012) A review on nitrogen and organics removal mechanisms in subsurface flow constructed wetlands: dependency on environmental parameters, operating conditions and supporting media. J Environ Manage 112:429–448. https://doi.org/10.1016/j.jenvman.2012.08.011
Saeed T, Sun G (2017) A comprehensive review on nutrients and organics removal from different wastewaters employing subsurface flow constructed wetlands. Crit Rev Environ Sci Technol 47(4):203–288. https://doi.org/10.1080/10643389.2017.1318615
Solanki P, Narayan M, Rabha AK, Srivastava RK (2018) Assessment of cadmium scavenging potential of Canna indica L. Bull Environ Contam Toxicol 101(4):446–450. https://doi.org/10.1007/s00128-018-2416-3
Stottmeister U, Wießner A, Kuschk P, Kappelmeyer U, Kästner M, Bederski O, Müller RA, Moormann H (2003) Effects of plants and microorganisms in constructed wetlands for wastewater treatment. Biotechnol Adv 22(1–2):93–117. https://doi.org/10.1016/j.biotechadv.2003.08.010
Tan X, Yang Y, Liu Y, Li X, Fan X, Zhou Z, Liu C, Yin W (2019) Enhanced simultaneous organics and nutrients removal in tidal flow constructed wetland using activated alumina as substrate treating domestic wastewater. Biores Technol 280(100):441–446. https://doi.org/10.1016/j.biortech.2019.02.036
Vankar PS, Srivastava J (2018). A review-canna the wonder plant. J Text Eng Fashion Technol 4(2):158–162. https://doi.org/10.15406/jteft.2018.04.00134
Varma M, Gupta AK, Ghosal PS, Majumder A (2021) A review on performance of constructed wetlands in tropical and cold climate: insights of mechanism, role of influencing factors, and system modification in low temperature. Sci Total Environ 755:142540. https://doi.org/10.1016/j.scitotenv.2020.142540
Vymazal J (2004) Removal of phosphorus in constructed wetlands with horizontal sub. Water Air Soil Pollut Focus 4:657–670
Vymazal J, Kröpfelová L (2008) Is concentration of dissolved oxygen a good indicator of processes in filtration beds of horizontal-flow constructed wetlands? Wastewater Treat Plant Dyn Manag Constructed Nat Wetlands 2:311–317. https://doi.org/10.1007/978-1-4020-8235-1_27
Wang F, Liu Y, Ma Y, Wu X, Yang H (2012) Characterization of nitrification and microbial community in a shallow moss constructed wetland at cold temperatures. Ecol Eng 42:124–129. https://doi.org/10.1016/j.ecoleng.2012.01.006
Wang H, Wang T, Zhang B, Li F, Toure B, Omosa IB, Chiramba T, Abdel-Monem M, Pradhan M (2014) water and wastewater treatment in Africa—current practices and challenges. Clean: Soil, Air, Water 42(8):1029–1035. https://doi.org/10.1002/clen.201300208
Wang M, Zhang DQ, Dong JW, Tan SK (2017) Constructed wetlands for wastewater treatment in cold climate—a review. J Environ Sci (china) 57:293–311. https://doi.org/10.1016/j.jes.2016.12.019
Wang W, Ding Y, Ullman JL, Ambrose RF, Wang Y, Song X, Zhao Z (2016) Nitrogen removal performance in planted and unplanted horizontal subsurface flow constructed wetlands treating different influent COD/N ratios. Environ Sci Pollut Res 23(9):9012–9018. https://doi.org/10.1007/s11356-016-6115-5
Werker AG, Dougherty JM, McHenry JL, Van Loon WA (2002) Treatment variability for wetland wastewater treatment design in cold climates. Ecol Eng 19(1):1–11. https://doi.org/10.1016/S0925-8574(02)00016-2
Wu H, Zhang J, Ngo HH, Guo W, Hu Z, Liang S, Fan J, Liu H (2015a) A review on the sustainability of constructed wetlands for wastewater treatment: design and operation. Biores Technol 175:594–601. https://doi.org/10.1016/j.biortech.2014.10.068
Wu S, Wallace S, Brix H, Kuschk P, Kirui WK, Masi F, Dong R (2015b) Treatment of industrial effluents in constructed wetlands: challenges, operational strategies and overall performance. Environ Pollut 201:107–120. https://doi.org/10.1016/j.envpol.2015.03.006
Yang J, Gao D, Chen TB, Lei M, Zheng, G.D, Zhou XY (2015) Comparison of heavy metal removal efficiencies in four activated sludge processes. J Central South University 22(10):3788–3794. https://doi.org/10.1007/s11771-015-2923-x
Yin X, Zhang J, Hu Z, Xie H, Guo W, Wang Q, Ngo HH, Liang S, Lu S, Wu W (2016) Effect of photosynthetically elevated pH on performance of surface flow-constructed wetland planted with Phragmites australis. Environ Sci Pollut Res 23(15):15524–15531. https://doi.org/10.1007/s11356-016-6730-1
Zhang X, Wang T, Xu Z, Zhang L, Dai Y, Tang X, Tao R, Li R, Yang Y, Tai Y (2020) Effect of heavy metals in mixed domestic-industrial wastewater on performance of recirculating standing hybrid constructed wetlands (RSHCWs) and their removal. Chem Eng J 379:122363. https://doi.org/10.1016/j.cej.2019.122363
Zhong F, Wu J, Dai Y, Cheng S, Zhang Z, Ji H (2014) Effects of front aeration on the purification process in horizontal subsurface flow constructed wetlands shown with 2D contour plots. Ecol Eng 73:699–704. https://doi.org/10.1016/j.ecoleng.2014.09.119
Zhou X, Wang X, Zhang H, Wu H (2017) Enhanced nitrogen removal of low C/N domestic wastewater using a biochar-amended aerated vertical flow constructed wetland. Biores Technol 241:269–275. https://doi.org/10.1016/j.biortech.2017.05.072
Zhu H, Zhou QW, Yan BX, Liang YX, Yu XF, Gerchman Y, Cheng XW (2018) Influence of vegetation type and temperature on the performance of constructed wetlands for nutrient removal. Water Sci Technol 77(3):829–837. https://doi.org/10.2166/wst.2017.556
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Singh, A., Rawat, A., Katoch, S.S., Bajpai, M. (2022). Performance Analysis of Constructed Wetland Treating Secondary Effluent Under Cold Climatic Conditions in Hamirpur (H.P.), India. In: Yadav, B., Mohanty, M.P., Pandey, A., Singh, V.P., Singh, R.D. (eds) Sustainability of Water Resources. Water Science and Technology Library, vol 116. Springer, Cham. https://doi.org/10.1007/978-3-031-13467-8_14
Download citation
DOI: https://doi.org/10.1007/978-3-031-13467-8_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-13466-1
Online ISBN: 978-3-031-13467-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)