Abstract
Multidrug-resistant (MDR) bacteria, also called superbugs, pose serious threat to the human health and existence because of their ability to develop resistant mechanism against commercially available antibiotics. Besides increasing the morbidity and mortality rate of patients, MDR bacteria are also putting huge financial stress on health sectors across the globe. It is estimated that approximately 700,000 people are losing their life every year due to MDR bacteria. It has been projected that more than 10 million people around the world will be the victim of MDR bacteria by 2050, if the current trend continues, superseding cancer as the main cause of global mortality. Hence, the mankind is in dire need to find an effective and safe tool against MDR bacteria.
Several metallic nanoparticles including Au, Ag, ZnO, and GO (graphene oxide) have shown antibacterial propensity against a wide range of bacteria. However, gold nanoparticles (AuNPs) received wide attention because of their inertness for the human body, easy surface fabrication properties, and optical properties. AuNPs demonstrate antibacterial activity through direct interaction with bacterial cell wall, generating reactive oxygen species (ROS), passing through the cell membrane and interacting with cellular macromolecules (i.e., DNA and proteins). In this chapter, we shall discuss different types of AuNPs, their role as potent antibacterial agents against pathogenic as well as multidrug-resistant bacteria, mechanism of antibacterial activity, biocompatibility, and future prospects in healthcare system.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Gold (Au) is an inert metal with distinct electronic and optical properties. When Au nanoparticles (AuNPs) are excited with light of a particular wavelength, the incident photons interact strongly with the conduction band of electrons and cause them to oscillate with resonant frequency. The collective oscillation is known as localized surface plasmon resonance (LSPR) that creates strong and localized electromagnetic fields and allows sensitive detection of changes in dielectric environment surrounding the surface of nanoparticles. This unique property makes them highly useful in imaging, drug delivery, cancer theranostics, and tackling menace of multidrug-resistant bacteria (Shankar et al. 2004; Mukherjee et al. 2001; Li et al. 2014). Au nanoparticles induce hyperthermia (i.e., increased temperature to kill cancer cells) upon illumination with near-infrared (NIR) light (Dash and Bag 2014; Huang et al. 2011). Furthermore, AuNPs can also be successfully and selectively delivered to malignant as well as benign tumors and can act as carriers for chemotherapeutic drugs. They are used as imaging agents as well as biosensors because of their ability to emit photons upon illumination (Marangoni et al. 2013).
The misuse and overuse of antibiotics have resulted in the emergence of multidrug-resistant bacteria, which is the cause of an additional medical costs of up to billion dollars every year (Rossolini et al. 2014; Li et al. 2014). Hence, suitable antibacterial agents to deal with multidrug-resistant bacteria are urgently needed. The antibacterial activity demonstrated by various nanomaterials including Ag, Au, Cu, Ti, ZnO2, and MgO2 could be a suitable alternative to commercially available antibiotics (Vimbela et al. 2017; Hossain et al. 2019; Niloy et al. 2020; Polash et al. 2021; Ranjan Sarker et al. 2019). More specifically, gold nanoparticles (AuNPs) have distinctive properties including their adjustable shape, size, surface properties, optical properties, high stability, biocompatibility, and multiple functionalization potential that make them suitable for different applications in the field of nanomedicine (Ashraf et al. 2016).
Based on the unique physical and chemical properties of nanoparticles, they provide a common platform for therapeutic applications against drug-resistant bacteria (Li et al. 2014). For example, AuNPs have already been used in the treatment of gum disease and dental caries, diagnosis of cancer, and in tissue engineering. Since AuNPs have antifungal and antibacterial activity, they can be conjugated with biopolymers to improve their efficacy as bioactive materials (Bapat et al. 2020). In addition, AuNPs can also be used as carriers of antibacterial drugs. Antibacterial drugs are conjugated with AuNPs through chemical interactions so that drugs can be released at the desired site of action (Fan et al. 2019). Notably, AuNPs do not show toxic effects to normal cells at certain concentrations (Fang et al. 2019; Chatterjee et al. 2011). Therefore, it is possible to modify AuNPs that exhibit antibacterial activity against standard bacterial strains in general and have unique antibacterial activity against multidrug-resistant bacteria in particular (Su et al. 2020).
The recent development in nanoscience and nanotechnology has helped researchers to design and develop novel biomaterials including AuNPs with excellent bioactivity as well as biocompatibility. Many inorganic (i.e., metal) nanoparticles have been developed including AuNPs. The AuNPs show various colors based on their shape, size, and amount of aggregation of particles (Daniel and Astruc 2004). They are used in medical and pharmaceutical industries for various purposes: antibacterial agents, antibiofilm, diagnostic tools, drug delivery vehicles, personal care products, and for cosmetics development (Su et al. 2020; Abdalla et al. 2020). This chapter summarizes recent research works on the development of AuNPs and their application to tackle the menace of pathogenic and multidrug-resistant (MDR) bacteria.
9.2 Different Types of AuNPs
There are many different types of gold nanoparticles (AuNPs) depending on their size, shape, and physical properties (Fig. 9.1). Important AuNPs include Au nanospheres, nanorods, nanoshells, and nanocages. There is also another type of Au-based nanoparticles known as “SERS nanoparticles” with excellent surface-enhanced Raman scattering property. Most of the AuNPs are produced with well-defined size, shape, and monodispersity due to the continuous development of synthetic, and characterization techniques in the last two decades.
Different types of AuNPs according to their shape and morphology. (Adapted from Freitas de Freitas et al. (2018))
9.2.1 Au Nanospheres
The gold nanospheres, also known as gold colloids of 2 to 100 nm in diameter, can be synthesized through controlled reduction of an aqueous HAuCl4 solution using different reducing agents under varying conditions. Citrate is the most widely used reducing agent that can produce nearly monodisperse gold nanospheres (Turkevich et al. 1951; Frens 1973). The size of the nanospheres can be controlled by changing the citrate to gold ratio. Generally, less amount of citrate generates larger nanospheres. The two major limitations of this method are the low yield and the obligation of using water as the solvent. A two-phase method, introduced by Faraday in 1857, capable of producing stable (irrespective of temperature and air) gold nanospheres of reduced dispersity and defined size (i.e., 10 nm in diameter), was reported in 1993 (Giersig and Mulvaney 1993). The phase transfer reagent such as tetraoctylammonium bromide was used to improve this technique. Moreover, thiol/gold molar ratios can affect the average size of the nanospheres (Brust et al. 1994). Larger thiol-to-gold ratios and rapid addition of cold reductant solutions yield smaller and more monodispersed gold nanospheres. Many other methods have already been investigated for the synthesis of gold nanospheres using other reducing agents or ligands (Leff et al. 1996). On the other hand, dendrimers have been used as templates or stabilizers for the generation of Au nanospheres (Esumi et al. 1998). Biocompatible block copolymers have already been employed for the synthesis of sterically stabilized Au nanospheres in aqueous solution (Yuan et al. 2006). The shape and size of the Au nanospheres could be readily controlled by optimizing the synthesis parameters including block copolymer composition, relative/absolute concentrations of the block copolymer, and HAuCl4. It was also reported that Au nanospheres could be grown in human cells (Anshup et al. 2005). Furthermore, Au nanospheres display a single absorption peak in the visible range between 510 nm and 550 nm. The absorption peak shifts to a longer wavelength with the increasing particle size, and the width of the absorption spectra is related to the size distribution range. Many other types of AuNPs with different size/shape including nanorods, nanoshells, and nanocages have been explored to obtain optical properties suitable for biomedical applications.
9.2.2 Au Nanorods
The Au nanorods have received worldwide attention because of their inimitable shape-dependent optical properties (i.e., different plasmon bands) that make the Au nanorods exclusive materials for biological imaging, sensing, photothermal therapy, and drug delivery (Hainfeld et al. 2008; Kodiha et al. 2014; Nichols and Bae 2014; Choi et al. 2010). Their application is very precise because even a small change in the shape, size, and surface nature alter their properties which in turn affect their biological applications. The surface plasmon resonance (SPR) band of Au nanorods is in the NIR region that makes them suitable for photothermal therapy, biological sensing, and imaging (Martin 1994). The Au nanorods can be synthesized using the template method. The diameter of the template membrane pore can be used to determine the diameter of the Au nanorods, while the length of the nanorods can be controlled through the amount of gold deposited within the pores. The main disadvantage of this method is low yield since only one monolayer of nanorods is prepared. The formation of Au nanorods through electrochemical synthesis has been also reported (Reetz and Helbig 1994; Yu et al. 1997; Chang et al. 1999). According to this approach, many experimental parameters determine the length of the Au nanorods that influence their aspect ratio which is defined as the length divided by the width. On the other hand, the seed-mediated synthesis, the most established method for Au nanorod preparation, provides higher aspect ratio than that prepared by the other methods (Jana et al. 2001a; Busbee et al. 2003). Usually, Au seeds are prepared by chemical reduction of Au salt (i.e., HAuCl4.3H2O) with a strong reducing agent including sodium borohydride (i.e., NaBH4). These seeds, serving as the nucleation sites for Au nanorods, are then added to the growth solution containing Au salt, a weak reducing agent such as ascorbic acid, and hexadecyltrimethyl ammonium bromide. The aspect ratio of Au nanorods can be controlled by varying the amount of gold seeds with respect to the precursor. Furthermore, Au nanorods can be produced in quantitative yield upon addition of AgNO3 (Jana et al. 2001b, 2002). Besides, several other types of approaches have also been investigated for the fabrication of Au nanorods including bioreduction (Canizal et al. 2001), growth on mica surface (Mieszawska and Zamborini 2005), and photochemical synthesis (Kim et al. 2006).
9.2.3 Au Nanocages
Gold (Au) nanocages are characterized by their ultrathin porous walls and hollow interiors. They are synthesized using silver nanoparticles (AgNPs) as template that participate in the galvanic replacement (GR) reaction (Skrabalak et al. 2007, 2008; Lu et al. 2007). The controllable pores on the surface of Au nanocages have been synthesized via galvanic replacement reaction between silver nanocubes and aqueous HAuCl4 (Chen et al. 2006). The silver (Ag) nanostructures with controlled morphologies can be generated through polyol reduction where AgNO3 is reduced by ethylene glycol. Sequential addition of Ag atoms to the seeds produces the desired nanostructures by controlling Ag seed crystalline structures in the presence of poly(vinylpyrrolidone), a polymer capable of selectively binding to the surface. Ag nanostructures are used as supportive templates that can be transformed into Au nanostructures with hollow interiors via galvanic replacement (Chen et al. 2005, 2006). The wall thickness and dimension of the resultant Au nanocages could be readily controlled with high precision by adjusting the molar ratio of Ag to HAuCl4 (Cai et al. 2008). The light penetration ability in soft tissues can be maximized by restricting the light source to near infrared (NIR) region (i.e., 650 to 900 nm). This is because the light absorption ability of hemoglobin and water is negligible in the NIR region. The LSPR peaks can be concisely tuned throughout the visible and NIR region to make the Au nanocages suitable for this application (Kwon et al. 2012; Mahmoud et al. 2010; Au et al. 2008; Mahmoud and El-Sayed 2010). Au nanocages can also be functionalized with the bioactive molecules to target cancer cells for diagnosis and photothermal therapy at an early stage (Dreaden et al. 2012; Chen et al. 2012).
9.2.4 Au Nanoshells
The Au nanoshells are composed of silica core coated by a thin Au metallic shell. The most interesting property of Au nanoshells is their unique surface plasmon resonance property that can be finely tuned from visible to NIR region. There are multiple templates employed for the formation of hollow Au nanoshells including silica particles (Averitt et al. 1997), metal particles (Oldenburg et al. 1998, Oldenburg et al. 1999a, b; Tuersun and Han 2013), and so on. The Au nanoshells have been applied in various biomedical applications such as whole-blood immunoassays and photothermal cancer therapy (Loo et al. 2005; Park et al. 2008; Bickford et al. 2010). Cancer cells were successfully ablated in vitro by Au nanoshells as observed through magnetic resonance thermal imaging. Furthermore, the use of Au nanoshells for the photothermal ablation of tumors in mice showed complete regression of tumors while the mice remain healthy (Gobin et al. 2007, 2008; Hirsch et al. 2003; Lowery et al. 2006). Optical imaging techniques including those that use AuNPs as the contrast agents have limited applications in human studies. On the other hand, in the NIR region (i.e., 700–900 nm), the absorbance of all the bioactive molecules is negligible which provides clear window for optical imaging (Frangioni 2003). Au nanoshells can be designed and fabricated to control the location of surface plasmon resonance (SPR) peaks from visible to the NIR region of the electromagnetic spectrum by varying the composition and dimensions of the layers (Oldenburg et al. 1999a). The SPR peak can also be tuned by changing the ratio of the core size to its shell thickness for a given composition of Au nanoshells. Furthermore, Au nanoshells with SPR peaks in the NIR region can be prepared by coating Au shells with silica or polymer beads of variable thickness (Oldenburg et al. 1998; Caruso et al. 2001). The silica cores are grown according to the Stöber process, that is, the reduction of tetraethyl orthosilicate in basic ethanol. A seed-mediated growth technique is typically used to coat the silica nanoparticles with Au in an aqueous environment. The small Au nanospheres, such as 2–4 nm in diameter, can be attached to the silica core using an amine-terminated silane as a linear molecule that allows additional Au to be reduced until the seed particles coalesced into a complete shell (Oldenburg et al. 1999b). The diameter of the gold nanoshells is mainly determined by the diameter of the silica core, and the shell thickness can be controlled through the amount of gold deposited on the surface of the core. Gold nanoshells have also been synthesized via in situ gold nanoparticle formation using thermosensitive core-shell particles as the template (Suzuki and Kawaguchi 2005). The use of different microgels as the core offers significantly reduced particle aggregation. The thickness of Au nanoshells was also controlled through electroless deposition of Au. Recently, a virus scaffold has been used to assemble Au nanoshells which may potentially provide cores with a narrower size distribution and smaller diameters (i.e., 80 nm) than that of silica (Radloff et al. 2005).
9.2.5 Au Nanostars
Gold (Au) nanostars belong to the anisotropic AuNPs category. Au nanostars are composed of a core and several branches with sharp tips. The unique characteristics of Au nanostars originate from these branches and their interaction with the core. The attractive features of Au nanostars are localized surface plasmon resonance (LSPR), surface-enhance Raman Scattering (SERS) activity, and catalytic activity. The Au nanostars are used for catalysis, nanosensing assays, thermal therapy, and drug delivery. The Au nanostars have multiple sharp branches that demonstrate significant electromagnetic field enhancement and have unique plasmon bands which can be tuned from visible to NIR region. The synthesis of Au nanostars has been driven by the interest on the localized surface plasmons (LSPs) response to the environment, especially on sharp tips and edges, where light can be highly concentrated (Hao et al. 2007; Rodríguez-Lorenzo et al. 2009; Hrelescu et al. 2009; Dondapati et al. 2010). They serve as effective tools in the field of nanomedicine due to their unique properties. The Au nanostars also display stronger surface-enhanced resonance activity than Au spheres or even rods (Mukherjee et al. 2001; Cai et al. 2008).
9.3 Antibacterial Activity of AuNPs
Although gold nanoparticles (AuNPs) are not strong antimicrobial agent as AgNPs, they have been reported to demonstrate antibacterial (Lima et al. 2013) as well as antifungal activity (Wani and Ahmad 2013; Zawrah et al. 2011). Furthermore, AuNPs have been used as an alternative tool to high-dose antibiotics against infectious diseases including antibiotic-resistant bacteria (Podsiadlo et al. 2008). The nanoparticle toxicity and antibacterial activity mainly depend on the intrinsic properties, surface modification, and tested bacterial species. The AuNPs of smaller diameter can penetrate the bacterial cells and cause cellular damage followed by death (Bindhu and Umadevi 2014a). Antibacterial properties of triangular-shaped AuNPs demonstrate better activity toward both gram-positive and gram-negative bacteria than that of spherical AuNPs (Smitha and Gopchandran 2013). The sharp-faced triangular NPs, irrespective of their surface chemistry, size, and composition, can pierce the endosomal membrane before translocating to the cytoplasm where they can be retained. This feature makes them preferable to round-shaped NPs for drug delivery, gene delivery, subcellular targeting, and long-term tracking (Chu et al. 2014). Recently, it has been reported that very small AuNPs (i.e., less than 2 nm) showed excellent antibacterial activity against gram-positive and gram-negative bacteria (Kundu 2017).
9.3.1 Antibacterial Activity Against Pathogenic Bacteria
The AuNPs synthesized using citrate, polyvinylpirrolidone (PVP), or other commonly used stabilizers usually do not show antibacterial activity (Amin et al. 2009). More specifically, the AuNPs (size: 20–30 nm) stabilized by PVP and/or sodium dodecyl sulfate (SDS) did not show antimicrobial activity against Staphylococcus aureus ATCC 6538, Escherichia coli K12 NCTC 10538, and fungi Candida albicans ATCC 10231 at a concentration of 0.0016 wt% (Mukha et al. 2010). Chatterjee et al. (2011) found that AuNPs showed no concentration-dependent antibacterial activity while stimulating the level of cell division. AuNPs are biologically inert and usually do not show antibacterial activity (Allahverdiyev et al. 2011) that can be convinced from their high MIC value and small zone of inhibition (ZOI) value when compared to AgNPs. Hernández-Sierra et al. (2008) compared the antibacterial activity of AgNPs (size: 25 nm) with that of AuNPs (size: 80 nm) against S. aureus ATCC 25923. The AgNPs had a MIC (minimum inhibitory concentration) value of 4.86 ± 2.71 μg/ml and MBC (minimum bactericidal concentration) value of 6.25 μg/ml. On the other hand, AuNPs showed antibacterial propensity at a very high concentration (197 μg/ml). Shankar et al. (2014) synthesized AuNPs, AgNPs, and Au-AgNPs having hydrodynamic size 140 ± 13, 74 ± 6, and 128 ± 15 nm, respectively. The growth of S. aureus and E. coli was inhibited at 16 μg/ml and 8 μg/ml of AgNPs, respectively. Similar pattern of inhibition was also observed for Au-AgNPs having MIC value 16 and 32 μg/ml against S. aureus and E. coli, respectively. On the other hand, AuNPs did not show any antibacterial activity at the tested concentration (i.e., 128 μg/ml). Sreelakshmi et al. (2011) compared the antibacterial activity of AuNPs and AgNPs (size: 10 nm) synthesized using natural honey as a source of stabilizing as well as reducing agent. Their MIC values confirmed that honey-capped AgNPs exhibit very good antibacterial activity, whereas AuNPs exhibit moderate activity against the tested strains. On the other hand, PVP-coated AuNPs have ~10 times higher MIC value than that of AgNPs synthesized using the same polymer (Mukha et al. 2010; Hernández-Sierra et al. 2008). Hence, AuNPs are not usually used as antibacterial agents. In addition, incorporation of AuNPs (2 nm), methylene blue (MB), and toluidine blue (TBO) with polyurethane polymer, a polymer used to prepare catheters, enhanced the killing of S. aureus suspension under white illumination with a hospital light source (Naik et al. 2011). However, many other studies have revealed that AuNPs synthesized under certain conditions showed efficient antibacterial activity and they can be functionalized with different biopolymers to make an effective antibacterial agent. The size, shape, surface modification (coating and capping agents), and purification methods of AuNPs are the key factors that determine their antibacterial activity. However, CTAB-coated AuNPs of 1~2 nm and 1~20 nm in size were reported to show similar zone of inhibition (~22 mm) against E. coli (ATCC 25922 strain) without any size dependence (Azam et al. 2009; Arshi et al. 2011).
Furthermore, Badwaik et al. (2012) investigated the antibacterial activity of dextrose-coated AuNPs having different hydrodynamic diameters such as 25, 60, and 120 ± 5 nm. AuNPs with hydrodynamic diameter of 120 and 60 nm inhibit the proliferation of E. coli in a concentration-dependent manner and the MIC values were 16 × 1010 and 16 × 1011 particles/ml, respectively. On the other hand, AuNPs having 25 nm hydrodynamic diameter did not show any significant effect against the proliferation of E. coli even at concentration as high as 128 × 1012 particles/ml. Hence, they concluded that the antibacterial activity of AuNPs increases as the particle size increases, for example, in the order of 25 < 60 < 120 < nm. On the other hand, Ahmad et al. reported that smaller AuNPs (i.e., 7 nm) showed excellent antifungal activity and greater biocidal effect against Candida species when compared to that of relatively large AuNPs (i.e., 15 nm) (Ahmad et al. 2013). The capping agent is also an important determinant of the antibacterial activity of AuNPs. Zhang et al. (2008) synthesized hyperbranched poly(amidoamine) having terminal dimethylamine groups (HPAMAM-N (CH3)2) to prepare AuNPs and they found that the cationic dimethylamine contributes to the antimicrobial activity through strong ionic interactions with bacteria. As shown in Table 9.1, AuNPs stabilized or modified by various coating agents have distinct antibacterial effects. Many small molecules were also used for the synthesis of AuNPs and were investigated for their antibacterial potential. For example, 4, 6-diamino-2-pyrimidinethiol (DAPT), an analogue of 2-pyrimidinethiol that is present in E. coli; two positively charged and amino-substituted pyrimidines 4-amino-2-pyrimidinethiol and 2,4-diamino-6- pyrimidine thiol (iDAPT) (Chatterjee et al. 2011); and one negatively charged pyrimidine 4,6-dihydroxyl-2- pyrimidine thiol (DHPT) (Zhao et al. 2010) were used to fabricate AuNPs. The MIC values of DAPT, APT, and iDAPT fabricated AuNPs against Pseudomonas aeruginosa were 16, 18, and 24 μg/ml, respectively. DHPT-coated AuNPs did not inhibit the growth of both E. coli and P. aeruginosa even at high concentration (80 μg/ml).
The methods used for the purification of AuNPs were neither mentioned nor adequately carried out before performing antibacterial assay. There could be debate on the antibacterial activity of AuNPs because of the presence of capping agents or other reagents used for the synthesis of nanoparticles that were not purified properly. It might be, therefore, the presence of Au(III) ions as well as unreacted reagents interfere with the antibacterial results, thereby producing false results. Several studies have reported the purification of chemically synthesized AuNPs prior to investigating their antimicrobial activity. For example, Daima et al. synthesized tyrosine-functionalized AuNPs and dialyzed to remove free ions and unbound tyrosine prior to investigating their antibacterial activity to avoid any interference on their toxicity bacteria (Daima et al. 2013). Furthermore, Zhou et al. (2012) prepared citrate-stabilized AuNPs followed by purification through centrifugation to test antibacterial activity against E. coli and Bacillus Calmette-Guérin. Nazari et al. also reported the synthesis and purification of AuNPs before testing their antibacterial activity against P. aeruginosa, S. aureus, and E. coli (Nazari et al. 2012).
On the other hand, AuNPs were also synthesized and fabricated using natural polymers extracted from either microorganisms or plants, or directly in the presence of microorganisms. They are summarized in Table 9.2. These processes are known as green or eco-friendly synthesis or biosynthesis. For instance, Nagaraj et al. (2012) synthesized spherical AuNPs (size: 10–50 nm) using Caesalpinia pulcherrima flower extract as the reducing agent that showed efficient antimicrobial activity against Aspergillus, E. coli, and Streptobacillus sp. Das et al. (2009) synthesized AuNPs (size: 10 nm) on the surface of Rhizopus oryzae MTCC 262 through in situ reduction of HAuCl4 that showed high antibacterial activity against several gram-positive and gram-negative pathogenic bacteria. Mishra et al. (2011) synthesized AuNPs (size: 50–70 nm) and AgNPs (size: 10–20 nm) via extracellular synthesis using yeast Candida guilliermondii and the highest antibacterial activity for both AuNPs and AgNPs was found against Staphylococcus aureus. AuNPs were also synthesized using various plant extracts including Mentha piperita (MubarakAli et al. 2011), root extract of Trianthema decandra, (33–65 nm) (Geethalakshmi and Sarada 2012), Helianthus annuus flower extracts (Geethalakshmi and Sarada 2012), and dried flower extract of Carthamus tinctorius (Liny et al. 2012). Interestingly, all the green synthesized AuNPs demonstrated efficient antibacterial activity against several bacteria strains that were unaffected in the presence of chemically synthesized AuNPs (Mishra et al. 2011). Finally, the question is what is responsible for the antibacterial activity of green-synthesized AuNPs. It may be due to the extracts alone, AuNPs or their combination with plant extracts, since several plant extracts including Euphorbia hirta plant alone demonstrated antibacterial activity (Annamalai et al. 2013). Hence, the antibacterial activity may also be due to the synergistic effect of the combination of AuNPs and extracts (Annamalai et al. 2013).
9.3.2 Antibacterial Activity Against Multidrug-Resistant (MDR) Bacteria
Metallic gold is inert and non-toxic that may change when shifts from metallic to oxidation states (0, I, and III) (Merchant 1998). The antibacterial mechanism of AuNPs is associated with (i) the collapse of membrane potential that inhibits the ATPase activity and causes deterioration of the cellular metabolism and (ii) inhibition of the binding subunit of ribosomes to tRNA (Cui et al. 2012). Also (iii) Shamaila and co-workers showed that AuNPs disrupt the bacterial respiratory chain by binding to the thiol group of enzymes including nicotinamide adenine (NADH) dehydrogenase and produce oxidative stress resulting in the cellular death (Shamaila et al. 2016). Since AuNPs are non-toxic to the host (Li et al. 2014; Conde et al. 2014; Rajchakit and Sarojini 2017), the possibility of fine-tuning their conjugation ability to act as carriers of antibiotics or other antibacterial moieties may enhance their bactericidal effect as well as potentiate the effect of antibiotics (Baptista et al. 2018). Functionalization of AuNPs with cationic and hydrophobic polymers was shown to be effective against both gram-negative and gram-positive uropathogens including MRSA. These AuNPs exhibited low toxicity to mammalian cells, and development of resistance to these nanoparticles was very low (Li et al. 2014). Vinoj et al. demonstrated that the conjugation of AuNPs with N-acylated homoserine lactonase proteins (AiiA AuNPs) resulted in a nano-composite with greater antibacterial activity against MDR species when compared to AiiA proteins alone (Vinoj et al. 2015).
The integration of AuNPs on the shell of PVA-lysozyme microbubbles demonstrated better antibacterial potential against E. coil than that of only microbubbles (Mahalingam et al. 2015). Galic acid–capped AuNPs have also been found to be active against gram-negative and gram-positive bacteria (Kim et al. 2017). Recently, Ramasamy et al. reported one-pot synthesis of cinnamaldehyde-immobilized gold nanoparticles (CGNPs) having more than 80% effectivity against biofilm formation of gram-positive (methicillin-sensitive and -resistant strains of S. aureus, MSSA, and MRSA, respectively) and gram-negative (E. coli and P. aeruginosa) bacteria (Ramasamy et al. 2017a, b). The incorporation of AuNPs with ultrathin graphitic carbon nitride (g-C3N4) generates peroxidase activity and demonstrates excellent antibacterial potential against drug-resistant (DR) gram-positive and gram-negative bacteria. They also exhibit high efficiency in eliminating existing DR biofilms and preventing the formation of new biofilms in vitro (Wang et al. 2017a). The conjugation of antibiotics (e.g., vancomycin and methicillin) to AuNPs increases their intrinsic activity against MDR strains (Baptista et al. 2018). Recently, Payne et al. developed a single-step synthesis technique for kanamycin-capped AuNPs (Kan-AuNPs) that exhibit high antibacterial activity against both gram-positive and -negative bacteria including kanamycin-resistant bacteria. The authors observed a significant reduction in the MIC value against all the bacterial strains tested when compared to free drug. This higher efficacy was due to the disruption of the bacterial envelope that resulted in the leakage of cytoplasmic content and thereby cell death (Payne et al. 2016). Pradeepa et al. synthesized AuNPs using bacterial exopolysaccharide (EPS) and functionalized them with antibiotics (e.g., levofloxacin, cefotaxime, ceftriaxone, and ciprofloxacin). They observed that antibiotic-conjugated AuNPs exhibited excellent bactericidal activity against MDR gram-positive and -negative bacteria when compared to free drugs. E. coli was the most susceptible MDR bacteria followed by K. pneumoniae and S. aureus (Vidya et al. 2016). Recently, Yang et al. described the effect of small molecule (6-aminopenicillanic acid, APA) coated AuNPs to inhibit MDR bacteria (Yang et al. 2017). They conjugated AuNPs with electrospun fibers of poly(ε-caprolactone) (PCL)/gelatin to produce materials that inhibit wound infection by MDR bacteria and also demonstrated that APA-AuNPs reduce MDR bacterial infections (Yang et al. 2017). Shaker et al. evaluated the surface functionalization of AuNPs with carbapenems [i.e., imipenem (Ipm) and meropenem (Mem)] and investigated their antibacterial activity against carbapenem-resistant gram-negative bacteria isolated from an infected human. Both Ipm-AuNPs and Mem-AuNPs (size: 35 nm) showed significant increase in their antibacterial activity against all the tested isolates (Shaker and Shaaban 2017). Recently, Shaikh et al. described the synthesis and characterization of cefotaxime-conjugated AuNPs to target drug-resistant CTX-M-producing bacteria. The authors inverted resistance in cefotaxime-resistant bacterial strains (i.e., E. coli and K. pneumoniae) by using cefotaxime-AuNPs. Hence, the conjugation of unresponsive antibiotics with AuNPs can restore their antibacterial activity against drug-resistant bacterial strains (Shaikh et al. 2017).
One of the most important properties of AuNPs is their ability to generate heat upon illumination with laser (Mendes et al. 2017; Mocan et al. 2017). This property is very important because it can be exploited to develop photothermal nano-vectors to destroy MDR bacteria at the molecular level (Mocan et al. 2017). For example, Khan et al. showed that the combination of Concanavalin-A (ConA)-directed dextran capped AuNPs (GNPDEX-ConA) conjugated with methylene blue (MB) (MB@GNPDEX-ConA)-mediated photodynamic therapy (PDT) enhanced the efficacy and selectivity of MB-induced killing of MDR clinical isolates including E. coli, K. pneumoniae, and Enterobacter cloacae (Khan et al. 2017). Gil-Tomas et al. reported that the covalent conjugation of AuNPs with toluidine blue O–tiopronin forms an enhanced and exceptionally potent antimicrobial agent when activated by white light or 632 nm laser light (Gil-Tomás et al. 2007).
Hu et al. modified the surface of AuNPs with pH-responsive mixed charged zwitterionic self-assembled monolayers composed of weak electrolytic 11-mercaptoundecanoic acid (HS-C10-COOH) and strong electrolytic (10-mercaptodecyl) trimethylammonium bromide (HS-C10-N4) that exhibited an enhanced photothermal ablation of MRSA biofilm without any damage to the healthy tissues around the biofilm when illuminated with near infrared (NIR) laser (Hu et al. 2017). Furthermore, the antibacterial activity of glucosamine-gold nanoparticle-graphene oxide (GlcN-AuNP-GO) and UV-irradiated GlcN-AuNP-GO was evaluated against E. coli and E. faecalis. UV irradiation of GlcN-AuNP-GO demonstrated higher antibacterial activity than antibiotic kanamycin (Govindaraju et al. 2016).
Ocsoy et al. developed DNA aptamer-functionalized AuNPs (Apt@AuNPs) and gold nanorods (Apt@AuNRs) to kill methicillin-resistant Staphylococcus aureus (MRSA) through photothermal therapy (PTT) (Ocsoy et al. 2017). They found that both Apt@AuNPs and Apt@AuNRs attached to MRSA and inactivated cells by 5% and > 95%, respectively, through PTT. The difference in the induction of cell death was based on the relatively high longitudinal absorption of NIR radiation and strong photothermal conversion capability of the Apt@AuNRs compared to Apt@AuNPs. Recently, a new approach based on the conjugation of AuNPs with antimicrobial peptides (AMPs) has shown promising results (Rajchakit and Sarojini 2017). For example, Kuo et al. mixed synthetic peptides containing arginine, tryptophan, and cysteine termini [i.e., (DVFLG) 2REEW4C and (DVFLG) 2REEW2C] with aqueous tetrachloroauric acid to generate peptide-immobilized AuNPs [i.e., (DVFLG) 2REEW4C-AuNPs and (DVFLG) 2REEW2C-AuNPs] that were effective against Staphylococci, Enterococci, and other antibiotic-resistant bacterial strains (Kuo et al. 2016). Conjugation of AMPs with AuNPs usually involves the formation of Au-S coordinate covalent bond between the amine and thiol groups of peptides or conjugating linkers as well as terminal (N- or C-terminal) cysteine of AMPs which help in their conjugation with gold (Tielens and Santos 2010; Xue et al. 2014). However, there is one example when covalent conjugation of an AMP to AuNPs has been achieved via Au-O bond (Lai et al. 2015). Other approaches used a polyethylene glycol linker to covalently attach AMPs with AuNPs that showed significantly increased antibacterial and anti-biofilm activity against antibiotic-resistant gram-negative bacteria (Casciaro et al. 2017). Yeom and co-workers demonstrated the most advanced clinical application for AuNPs@AMP using infected mice in vivo that resulted in the inhibition of Salmonella typhi colonization in the organs of the animals (Yeom et al. 2016). The reason behind the increased antimicrobial activity of AuNPs@AMP over the free AMPs is that AuNPs can get a higher concentration of the peptides at the site of action. These NPs interact with lipopolysaccharides (LPS) and proteins of bacterial membrane and, in some cases, penetrate the bacterial membrane through the porin channel. Thus, the nanoparticles interact with the bacterial inner membrane making the AuNPs@AMP conjugate more efficient than the non-conjugated form (Baptista et al. 2018). Rai et al. demonstrated the conjugation of cecropin-melittin (CM-SH), a known peptide with inherent antibacterial propensity (Boman et al. 1989), with the surface of AuNPs through Au-S bond. The CM-SH-AuNPs showed greater antimicrobial activity in a systemic (i.e., animal) model than CM-SH (Rai et al. 2016) (Table 9.3).
9.4 Mechanism of Antibacterial Activity of Au Nanoparticles
Drug-resistant bacteria acquire genetic modification to exclude antimicrobial drugs and become less sensitive to drugs. To find an effective way to control the threat of bacterial drug resistance, a novel approach to enhance antimicrobial activity is urgently needed. AuNPs are the most studied metal nanoparticles (NPs) for antibacterial applications (Borzenkov et al. 2020). They are biologically inert and pure form of Au does not exert any antibacterial activity (Zhang et al. 2015). However, the surface area of AuNPs is suitable for conjugation with antibiotics and other drugs. A wide range of proteins, drugs, and even nucleotides have also been successfully delivered using AuNPs in the recent past (Perzanowska et al. 2021). Though the exact mechanism underlying the antimicrobial propensity of AuNPs remains unclear, several factors including size, shape, and surface functionalization significantly influence the activity of AuNP.
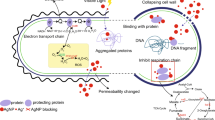
Major pathways through which AuNPs exert antimicrobial activity include (i) direct contact with bacteria (Shaikh et al. 2019), (ii) physical disruption of membrane (Piktel et al. 2021), (iii) generation of reactive oxygen species (ROS) (Yu et al. 2020), (iv) interaction with cellular proteins and genetic elements (Wang et al. 2015), and (v) trigger host-response immunity (Dykman and Khlebtsov 2017) (Fig. 9.2a). The antimicrobial efficacy of AuNP is largely dependent on their size and shape. The size of the AuNPs plays a critical role in their bactericidal activity, and functionalization with hydrophilic molecule enhances their interaction with bacterial membrane. Hayden et al. reported the size-dependent antimicrobial effect of cationic AuNPs (Hayden et al. 2012) (Fig. 9.2b). The larger particles (>100 nm) are unable to cross the bacterial membrane; however, smaller particles do and make pores in the membrane (Zheng et al. 2017; Xing et al. 2018).
Mechanism of antibacterial activity of AuNPs. (a) AuNPs cause irreversible membrane damage and, hence, particles get internalized in the cytosol. AuNPs then interfere with the cellular components and induce the generation of reactive oxygen species (ROS). ROS brings about different kinds of damage and finally promotes cells to apoptosis. Damaged electron transport chain (ETC) and efflux pump change the electron and proton homeostasis in a cell, respectively. (b) Size-dependent antimicrobial activity of gold (Au) NPs. TEM images confirmed B. subtilis membrane rupture by 2 nm AuNPs but not in case of 6 nm NPs. (Reproduced from Hayden et al. (2012) with permission. Copyright © 2012 American Chemical Society)
The large surface area of AuNPs allows the attachment of a wide range of functional elements to enhance the pristine antimicrobial effect. Hence, both AuNPs and their functionalized derivatives have been used to control infections caused by antimicrobial-resistant (AMR) pathogens (Li et al. 2014; Zhao et al. 2013). In addition to increased bactericidal effect, functionalization of the AuNPs’ surface also stabilizes the particle and provides prolonged effective and safe drug delivery (Tao 2018). Multivalent Au atom is conjugated with multiple ligands to enhance its antimicrobial activity (Zheng et al. 2017; Ortiz-Benítez et al. 2019). In addition, capping agents used in the synthesis of AuNPs also disrupt cell membrane via electrostatic interactions. AuNPs capped with a mixture of different small molecules can also enhance their antibacterial propensity. For example, AuNPs of 2 nm size capped with p-mercaptobenzoic acid (pMBA-Au) are not effective against bacteria. When pMBAs on the AuNPs’ surface are partially replaced with a mixture of 2-mercaptoethylamine and 3-mercaptopropylsulfonate, the resultant AuNPs show 99.9% growth inhibition against E. coli at 0.5 mM concentration (Bresee et al. 2011). Li et al. systematically modified the surface of AuNPs to combat multidrug-resistant (MDR) bacteria. Herein, they functionalized 2 nm size AuNPs using hydrophobic molecules having different chain length (Li et al. 2014). The hydrophobic interaction destroys the integrity of bacterial membrane and allows internalization of metal ions, loaded drugs, and protein inhibitors. On the contrary, it also allows the leakage of cytoplasmic content.
The interaction between cationic AuNPs and negatively charged membrane proteins (mostly teichoic acids) results in the aggregation, protrusion and, therefore, damage the membrane permanently (Hayden et al. 2012; Zhao et al. 2010). The positive charge of AuNPs is responsible for better interaction with negatively charged bacteria which leads to membrane rupture. However, it has been reported that interaction of AuNPs with bacteria changes the membrane permeability, interrupts the electrolyte balance, and deactivates protein function (Wang et al. 2017b). Payne et al. confirmed that the membrane-attached AuNPs penetrate the cell wall of bacteria using small-angle X-ray scattering (Payne et al. 2016). This phenomenon resulted in the disruption of cytosolic environment and leakage of cellular components (Fig. 9.3).
Transmission electron microscopic images of bacteria. Sequential images of gram-positive Staphylococcus epidermidis bacteria (a) and gram-negative Enterobacter aerogenes bacteria (b) treated with kanamycin-AuNPs after 0, 6, and 12 h of incubation. (Reproduced with permission Payne et al. (2016). Copyright © 2016)
Ortiz-Benítez et al. found that AuNPs reached the cytosolic environment of resistant S. pneumoniae and formed spherical cytoplasmic structures known as inclusion bodies (Ortiz-Benítez et al. 2019). They separated the proteins from the inclusion bodies that are potential candidates to facilitate the uptake of NPs in S. pneumoniae.
However, conjugation of antibiotics to AuNPs provides enhanced bactericidal effect than pristine AuNPs (Rattanata et al. 2016). Bagga et al. reported that AuNPs-levofloxacin showed greater bactericidal effect against Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa (Payne et al. 2016; Bagga et al. 2017). XX et al. reported a one-pot, fast synthesis of vancomycin-conjugated AuNP which had 16-fold better antibacterial activity against vancomycin-resistant Enterococci when compared to free vancomycin (Wang et al. 2018). Other similar studies also support the codelivery of antibiotics with AuNP to reduce resistance development (Fuller et al. 2020; Justo and Bosso 2015; Sans-Serramitjana et al. 2016; Wang et al. 2020; Fan et al. 2019). Thus, the delivery of antibiotic-conjugated AuNPs not only improve antibacterial efficacy but also require low dose of free antibiotic. Moreover, they also retard bacteria from developing resistance to antibiotics (Chopra 2007). Antibiotic-conjugated AuNPs exert higher antibacterial activity with minimal toxicity (Huh and Kwon 2011). Though synergistic effect of AuNPs-drug conjugate is always discussed as responsible for enhanced antimicrobial activity, the exact mechanism is still unknown. It is hypothesized that this stable conjugation improves internalization of antibiotics to resistant cells (Gu et al. 2003). Only a stable conjugation offers better antibacterial effect. Major drawbacks of antibiotic adsorption on AuNPs include aggregation and poor dispersity of the nanoparticles leading to compound instability. On the other hand, the conjugation of amphiphilic antimicrobial peptides (AMPs) with AuNPs enhances the antibacterial activity of nanoparticles. The advantage of conjugating amphiphilic AMPs is that they facilitate the interaction of AMP-AuNP with bacterial membrane (Wimley and Hristova 2011; Craik et al. 2013; Wadhwani et al. 2017). For example, Lee et al. conjugated hexahistidine-tagged AMPs with aptamer-bound AuNP (Lee et al. 2017). The resultant conjugate was highly effective against infectious pathogens including Vibrio vulnificus.
The photothermal property of AuNPs is advantageous for their biomedical applications (Mahmoud et al. 2019). AuNPs produce heat upon illumination with laser, and the thermal energy damages the neighboring bacteria. It is important to make sure that the AuNPs and bacteria are in close proximity so that the photothermal energy generated in the local environment damages the target cell (Mahmoud et al. 2018). Photothermal therapy (PTT) and photodynamic therapy (PDT) are the two main approaches followed by AuNPs to kill bacteria upon laser irradiation. In case of PTT, AuNPs convert the light energy into heat and enhances local temperature to kill bacteria (Jo and Kim 2013; Zhu et al. 2014; Pallavicini et al. 2017). On the other hand, PDT relies on the irradiation of photosensitizers to generate more reactive oxygen species (ROS) that kill bacteria (Venditti 2019). TEM images confirmed the laser-induced bacterial death after treatment with AuNPs. Bermúdez-Jiménez et al. reported the antibacterial effect of chitosan hydrogel–embedded AuNPs against clinical MDR pathogens upon illumination with laser (Bermúdez-Jiménez et al. 2019) and the minimum inhibitory concentration (MIC) was <4 μg/ml. The low-power infrared diode laser ruptured the bacterial membrane through enhanced ROS production. The principle of PDT is to generate toxic singlet oxygen upon illumination of photosensitizers with visible light. The highly reactive singlet oxygen damages the neighboring bacterial membrane, interferes with the cellular metabolic pathways, and damages the DNA (Bertoloni et al. 2000; Narband et al. 2009). In recent studies, photosensitizer-embedded AuNPs showed effective antimicrobial activity against both gram-positive and gram-negative bacterial strains (Rossi et al. 2019; Jain et al. 2006; Pallares et al. 2016; Darby et al. 2016; Ni et al. 2008). PDT is very safe and effective; however, it is highly recommended to deliver therapeutic concentration of the photosensitizers.
Cui et al. investigated the molecular mechanism of bactericidal activity of AuNPs using transcriptomic and proteomic approaches. Two major ways of exerting antibacterial activities include decrease in the cellular ATP level that collapses the membrane potential and the inhibition of tRNA-binding ribosomal protein subunit (Cui et al. 2012). Thus, AuNPs interfere with the activities of ATPase and tRNA. Hence, decoding of mRNA responsible for functional protein enhances ROS-mediated chemotaxis in bacteria and leads to apoptosis (Cui et al. 2012). The most supported mechanism for antibacterial activity of the AuNP is the generation of reactive oxygen species (ROS). These highly reactive chemicals enhance oxidative stress and form vacuoles inside cells (Mohamed et al. 2017).
It is important to highlight that commercially available antibiotics do not follow the multidimensional mode of action like AuNPs to inhibit bacterial growth. Hence, AuNPs can be a good alternative tool to control MDR pathogens. The different mode of antibacterial action of AuNPs is due to the structural differences of gram-positive and gram-negative bacteria (Ranjan Sarker et al. 2019). Hence, AuNPs have a promising future in the field of drug-delivery system (DDS) because delivery of drugs using AuNP is not only effective but also less toxic.
In-depth studies are recommended to unravel the precise mode of action involved in AuNPs-bacteria interaction and their inhibition.
9.5 Biocompatibility of Au Nanoparticles
The field of nanomaterials is expanding rapidly with a wide range of applications (Khan et al. 2019). Besides having several medical applications, AuNPs have toxicities associated with them. Hence, it is very important to know the basic information about the nanomaterials, especially composition, variability of size, shape, surface charge, surface area, surrounding media, and aggregation tendency in biological fluid that influence the biocompatibility of NPs. In this section, properties of Au NPs will be discussed to understand their biocompatibility cell.
A range of techniques have been used to investigate the biocompatibility of AuNPs (Fig. 9.4). Table 9.4 summarizes the principle and benefits of each assay. MTT assay is considered as the “gold standard” to investigate the toxicity of AuNPs. It is a colorimetric assay to determine cellular metabolic activity (Stockert et al. 2018). Herein, enzymatic activity of mitochondrial reductase is monitored under a defined condition. In an oxidation reaction, the enzyme reduces a yellow-colored tetrazolium dye (known as MTT or 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) and produces insoluble purple-colored formazan. This irreversible reaction reflects the number of live cells at different time points. Cell number is quantified by measuring the absorbance of formazan at 570 nm. The degree of formazan production reflects the number of dead cells and is directly proportional to light absorption.
One of the pioneering reports on the biocompatibility assessment of AuNPs was reported by Shukla et al., where the biocompatibility and the uptake of AuNPs by RAW 264.7 macrophage cells were investigated (Shukla et al. 2005). Their findings suggest that AuNPs are highly biocompatible having antioxidation potential at higher doses besides suitable for prolonged treatment. In addition, they found that spherical AuNPs did not induce the secretion of proinflammatory cytokines (e.g., TNFα and interleukin β) by macrophage cells. Chithrani et al. studied the effect of AuNP’s shape, size, and toxicity (Chithrani et al. 2006). They concluded that citrate-capped spherical and rod-shaped AuNPs did not cause any significant toxicity to HeLa cells. This study was followed by other research groups and found negligible toxicity for spherical and rod-shaped AuNPs using different cell lines in vitro (Khan et al. 2007; Gu et al. 2009; Villiers et al. 2010). We demonstrated that functionalization of elongated tetrahexahedral (ETHH) AuNPs with α-lipoic acid, a natural antioxidant, increased their hemocompatibility as well as biocompatibility to human red blood cells (RBCs) and HeLa cells, respectively (Ranjan Sarker et al. 2019). Recently, we also reported that functionalization of concave cube AuNPs (CCAu) with α-lipoic acid and glutathione, a tripeptide with antioxidation potential, increased their hemocompatibility to human RBCs and biocompatibility to HeLa cells, L929 fibroblasts, and CHO-GFP cells (Pandala et al. 2021). On the contrary, Patra et al. synthesized spherical (33 nm in diameter) AuNPs and investigated their toxicity using three different cell lines (Patra et al. 2007). They found the particles were toxic to human lung carcinoma cells (A549), but compatible with baby hamster kidney cells (BHK21) and human liver carcinoma cells (HepG2).
The hydrodynamic size of AuNPs plays an important role in terms of their cellular uptake and biocompatibility. Earlier studies found that size variability of AuNPs demonstrates negligible toxicity (Khan et al. 2007; Gu et al. 2009; Villiers et al. 2010; Connor et al. 2005). Large AuNPs are generally stable, inactive, non-catalytic, and biocompatible (Khlebtsov and Dykman 2011). Sophisticated tools including inductively coupled plasma mass spectrometry (ICP-MS) are used to quantify AuNPs taken up inside cell (Merrifield et al. 2018). Moreover, electron microscopy confirmed the presence of Au particles in the treated cells (Shukla et al. 2005; García et al. 2013). Chithrani et al. and others studied the cellular uptake of a wide range of AuNPs and quantified the number of nanoparticles inside each cell using inductively coupled plasma atomic emission spectroscopy (Chithrani et al. 2006; Osaki et al. 2004; Huo et al. 2013; Wang et al. 2010; Heuskin et al. 2017). All these studies reported that the maximum uptake was observed in case of 50 nm AuNPs without any cytotoxic effect. Karakoçak et al. used two independent methods (i.e., electric cell-substrate impedance sensing and MTT assay) to investigate the cellular uptake and biocompatibility of spherical, rod-, and cube-shaped AuNPs (Karakoçak et al. 2016). They concluded that sphere-shaped AuNPs have better biocompatibility when compared to Au rods (Fig. 9.5). However, cube-shaped AuNPs neither entered into cells nor had any cytotoxic effect.
The mechanism of AuNPs-mediated cytotoxicity. (a) Exposure of AuNPs to ARPE-19 cell. (b) Dynamic bipolymer layer (thick and loose blue color coating) creates a superficial surface exposed to the cell membrane. (c,d) Uptake of AuNPs by endocytosis. (e) Fusion of endocytic vesicle with lysosome. (f) Complete internalization of AuNPs by lysosome. (g) AuNPs cross the single membrane of lysosome and penetrate the mitochondrial intermembrane space to initiate apoptosis signal. (h) AuNPs available in the cytoplasm cause cell death by activating subcellular signaling pathways for apoptosis, initiating cell shrinkage, decreasing cytoplasmic shrinkage, and beginning subcellular fragmentation. Here, L lysosome; M mitochondria; and N nucleus. (Reproduced with permission Karakoçak et al. (2016) Copyright © 2016 Elsevier Ltd.)
Most often rod-shaped AuNPs are synthesized using a cationic surfactant called CTAB. Wang et al. found that free CTAB up to 1 μM is toxic to mammalian cells (Wang et al. 2008). Therefore, several strategies including chemical exchange or surface functionalization have been chosen to make Au nanorods less toxic. Polyethylene glycol (PEG), a hydrophilic polymer, has been widely used to modify AuNPs’ surface. However, PEGylation of AuNPs interferes with the cellular uptake process through reduced endocytosis (Nel et al. 2009; Doak et al. 2009). Chen et al. evaluated intraperitoneally injected eight AuNPs of different size (3–100 nm) and found size dependent toxicity in mice (Chen et al. 2009).
9.6 Conclusions and Future Perspectives
Multidrug-resistance bacteria are exerting real threat to the existence of mankind because they have become resistant to almost all the commercially available antibiotics. Overuse and misuse of antibiotics are the main causes of the development of bacterial resistance mechanisms against antibiotics. Gold nanoparticles demonstrate antibacterial activity mainly through the oxidation of bacterial membrane resulting in the formation of pores on the membranes. As a result, AuNPs interact with the cellular DNA, proteins, and other macromolecules and cause bacterial death. Since AuNPs demonstrate antibacterial activity by damaging bacterial membrane and other subcellular organelles, they demonstrate antibacterial activity against a wide range of bacteria including pathogenic bacteria and multidrug-resistant bacteria. Furthermore, AuNPs are highly biocompatible in terms of their compatibility with various cell lines, red blood cells (RBCs), and mouse model (i.e., in vivo). Hence, in clinical trials, AuNPs can be recommended to be used as an alternative nanoweapon to commercially available antibiotics to tackle multidrug-resistant bacteria.
References
Abdalla SS, Katas H, Azmi F, Busra MFM (2020) Antibacterial and anti-biofilm biosynthesised silver and gold nanoparticles for medical applications: mechanism of action, toxicity and current status. Curr Drug Deliv 17(2):88–100
Adhikari MD, Goswami S, Panda BR, Chattopadhyay A, Ramesh A (2013) Membrane-directed high bactericidal activity of (gold nanoparticle)–polythiophene composite for niche applications against pathogenic bacteria. Adv Healthc Mater 2(4):599–606
Ahmad T, Wani IA, Lone IH, Ganguly A, Manzoor N, Ahmad A, Ahmed J, Al-Shihri AS (2013) Antifungal activity of gold nanoparticles prepared by solvothermal method. Mater Res Bull 48(1):12–20
Allahverdiyev AM, Kon KV, Abamor ES, Bagirova M, Rafailovich M (2011) Coping with antibiotic resistance: combining nanoparticles with antibiotics and other antimicrobial agents. Expert Rev Anti-Infect Ther 9(11):1035–1052
Amin RM, Mohamed MB, Ramadan MA, Verwanger T, Krammer B (2009) Rapid and sensitive microplate assay for screening the effect of silver and gold nanoparticles on bacteria. Nanomedicine 4(6):637–643
Annamalai A, Christina V, Sudha D, Kalpana M, Lakshmi P (2013) Green synthesis, characterization and antimicrobial activity of Au NPs using Euphorbia hirta L. leaf extract. Colloids Surf B 108:60–65
Anshup A, Venkataraman JS, Subramaniam C, Kumar RR, Priya S, Kumar TS, Omkumar R, John A, Pradeep T (2005) Growth of gold nanoparticles in human cells. Langmuir 21(25):11562–11567
Arshi N, Ahmed F, Kumar S, Anwar M, Lu J, Koo BH, Lee CG (2011) Microwave assisted synthesis of gold nanoparticles and their antibacterial activity against Escherichia coli (E. coli). Curr Appl Phys 11(1):S360–S363
Arunachalam KD, Annamalai SK, Hari S (2013) One-step green synthesis and characterization of leaf extract-mediated biocompatible silver and gold nanoparticles from Memecylon umbellatum. Int J Nanomedicine 8:1307
Ashraf S, Pelaz B, del Pino P, Carril M, Escudero A, Parak WJ, Soliman MG, Zhang Q, Carrillo-Carrion C (2016) Gold-based nanomaterials for applications in nanomedicine. In: Light-responsive nanostructured Systems for Applications in nanomedicine. Springer International Publishing, Cham, pp 169–202
Au L, Chen Y, Zhou F, Camargo PH, Lim B, Li Z-Y, Ginger DS, Xia Y (2008) Synthesis and optical properties of cubic gold nanoframes. Nano Res 1(6):441–449
Averitt R, Sarkar D, Halas N (1997) Plasmon resonance shifts of Au-coated Au 2 S nanoshells: insight into multicomponent nanoparticle growth. Phys Rev Lett 78(22):4217
Azam A, Ahmed F, Arshi N, Chaman M, Naqvi AJIJ (2009) One step synthesis and characterization of gold nanoparticles and their antibacterial activities against E. coli (ATCC 25922 strain). Nanomaterials (Basel) 1(2):1–4
Badwaik VD, Vangala LM, Pender DS, Willis CB, Aguilar ZP, Gonzalez MS, Paripelly R, Dakshinamurthy R (2012) Size-dependent antimicrobial properties of sugar-encapsulated gold nanoparticles synthesized by a green method. Nanoscale Res Lett 7(1):1–11
Bagga P, Siddiqui HH, Akhtar J, Mahmood T, Zahera M, Khan MS (2017) Gold nanoparticles conjugated levofloxacin: for improved antibacterial activity over levofloxacin alone. Curr Drug Deliv 14(8):1114–1119
Balagurunathan R, Radhakrishnan M, Rajendran RB, Velmurugan D (2011) Biosynthesis of gold nanoparticles by actinomycete Streptomyces viridogens strain HM10
Balasubramanian K (2014) Antibacterial application of polyvinylalcohol-nanogold composite membranes. Colloids Surf A 455:174–178
Bapat RA, Chaubal TV, Dharmadhikari S, Abdulla AM, Bapat P, Alexander A, Dubey SK, Kesharwani P (2020) Recent advances of gold nanoparticles as biomaterial in dentistry. Int J Pharm 586:119596
Baptista PV, McCusker MP, Carvalho A, Ferreira DA, Mohan NM, Martins M, Fernandes AR (2018) Nano-strategies to fight multidrug resistant bacteria—“a Battle of the titans”. Front Microbiol 9:1441
Basavegowda N, Sobczak-Kupiec A, Malina D, Yathirajan H, Keerthi V, Chandrashekar N, Salman D, Liny P (2013) Plant mediated synthesis of gold nanoparticles using fruit extracts of Ananas comosus (L.)(pineapple) and evaluation of biological activities. Adv Mater Lett 4:332–337
Bermúdez-Jiménez C, Romney MG, Roa-Flores SA, Martínez-Castañón G, Bach H (2019) Hydrogel-embedded gold nanorods activated by plasmonic photothermy with potent antimicrobial activity. Nanomedicine 22:102093
Bertoloni G, Lauro FM, Cortella G, Merchat M (2000) Photosensitizing activity of hematoporphyrin on Staphylococcus aureus cells. Biochim Biophys Acta 1475(2):169–174
Bickford LR, Agollah G, Drezek R, Yu T-K (2010) Silica-gold nanoshells as potential intraoperative molecular probes for HER2-overexpression in ex vivo breast tissue using near-infrared reflectance confocal microscopy. Breast Cancer Res Treat 120(3):547–555
Bindhu M, Umadevi M (2014a) Antibacterial activities of green synthesized gold nanoparticles. Mater Lett 120:122–125
Bindhu M, Umadevi M (2014b) Silver and gold nanoparticles for sensor and antibacterial applications. Spectrochim Acta A 128:37–45
Boman H, Wade D, Boman I, Wåhlin B, Merrifield R (1989) Antibacterial and antimalarial properties of peptides that are cecropin-melittin hybrids. FEBS Lett 259(1):103–106
Borah BJ, Yadav A, Dutta DK (2011) Au-nanoparticles: control size and morphology stabilized by tripodal phosphine based ligands and their antimicrobial activity. J Biomed Nanotechnol 7(1):152–153
Borzenkov M, Pallavicini P, Taglietti A, D’Alfonso L, Collini M, Chirico G (2020) Photothermally active nanoparticles as a promising tool for eliminating bacteria and biofilms. Beilstein J Nanotechnol 11:1134–1146
Bresee J, Maier KE, Boncella AE, Melander C, Feldheim DL (2011) Growth inhibition of Staphylococcus aureus by mixed monolayer gold nanoparticles. Small 7(14):2027–2031
Bresee J, Bond CM, Worthington RJ, Smith CA, Gifford JC, Simpson CA, Carter CJ, Wang G, Hartman J, Osbaugh NA (2014) Nanoscale structure–activity relationships, mode of action, and biocompatibility of gold nanoparticle antibiotics. J Am Chem Soc 136(14):5295–5300
Brown AN, Smith K, Samuels TA, Lu J, Obare SO, Scott ME (2012) Nanoparticles functionalized with ampicillin destroy multiple-antibiotic-resistant isolates of Pseudomonas aeruginosa and Enterobacter aerogenes and methicillin-resistant Staphylococcus aureus. Appl Environ Microbiol 78(8):2768–2774
Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman R (1994) Chemical communications, synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid–liquid system. J Chem Soc Chem Commun 7:801–802
Busbee BD, Obare SO, Murphy CJ (2003) An improved synthesis of high-aspect-ratio gold nanorods. Adv Mater 15(5):414–416
Cai W, Gao T, Hong H, Sun J (2008) Applications of gold nanoparticles in cancer nanotechnology. Nanotechnol Sci Appl 1:17
Cai L, Qin X, Xu Z, Song Y, Jiang H, Wu Y, Ruan H, Chen J (2019) Comparison of cytotoxicity evaluation of anticancer drugs between real-time cell analysis and CCK-8 method. ACS Omega 4(7):12036–12042
Canizal G, Ascencio J, Gardea-Torresday J, Yacamán MJ (2001) Multiple twinned gold nanorods grown by bio-reduction techniques. J Nanopart Res 3(5):475–481
Caruso F, Spasova M, Salgueiriño-Maceira V, Liz-Marzán L (2001) Multilayer assemblies of silica-encapsulated gold nanoparticles on decomposable colloid templates. Adv Mater 13(14):1090–1094
Casciaro B, Moros M, Rivera-Fernandez S, Bellelli A, Jesús M, Mangoni ML (2017) Gold-nanoparticles coated with the antimicrobial peptide esculentin-1a (1-21) NH2 as a reliable strategy for antipseudomonal drugs. Acta Biomater 47:170–181
Chang S-S, Shih C-W, Chen C-D, Lai W-C, Wang CC (1999) The shape transition of gold nanorods. Langmuir 15(3):701–709
Chatterjee S, Bandyopadhyay A, Sarkar K (2011) Effect of iron oxide and gold nanoparticles on bacterial growth leading towards biological application. J Nanobiotechnol 9(1):1–7
Chen J, Saeki F, Wiley BJ, Cang H, Cobb MJ, Li Z-Y, Au L, Zhang H, Kimmey MB, Li X (2005) Gold nanocages: bioconjugation and their potential use as optical imaging contrast agents. Nano Lett 5(3):473–477
Chen J, McLellan JM, Siekkinen A, Xiong Y, Li Z-Y, Xia Y (2006) Facile synthesis of gold− silver nanocages with controllable pores on the surface. J Am Chem Soc 128(46):14776–14777
Chen Y-S, Hung Y-C, Liau I, Huang GS (2009) Assessment of the in vivo toxicity of gold nanoparticles. Nanoscale Res Lett 4(8):858–864
Chen W-Y, Lin J-Y, Chen W-J, Luo L, Wei-Guang Diau E, Chen Y-C (2010) Functional gold nanoclusters as antimicrobial agents for antibiotic-resistant bacteria. Nanomedicine 5(5):755–764
Chen Y, Zhang Y, Liang W, Li X (2012) Gold nanocages as contrast agents for two-photon luminescence endomicroscopy imaging. Nanomedicine 8(8):1267–1270
Chithrani BD, Ghazani AA, Chan WC (2006) Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett 6(4):662–668
Choi CHJ, Alabi CA, Webster P, Davis ME (2010) Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles. Proc Natl Acad Sci 107(3):1235–1240
Chopra I (2007) The increasing use of silver-based products as antimicrobial agents: a useful development or a cause for concern? J Antimicrob Chemother 59(4):587–590
Chu Z, Zhang S, Zhang B, Zhang C, Fang C-Y, Rehor I, Cigler P, Chang H-C, Lin G, Liu R (2014) Unambiguous observation of shape effects on cellular fate of nanoparticles. Sci Rep 4(1):1–9
Conde J, Larguinho M, Cordeiro A, Raposo LR, Costa PM, Santos S, Diniz MS, Fernandes AR, Baptista PV (2014) Gold-nanobeacons for gene therapy: evaluation of genotoxicity, cell toxicity and proteome profiling analysis. Nanotoxicology 8(5):521–532
Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD (2005) Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 1(3):325–327
Craik DJ, Fairlie DP, Liras S, Price D (2013) The future of peptide-based drugs. Chem Biol Drug Des 81(1):136–147
Cui Y, Zhao Y, Tian Y, Zhang W, Lü X, Jiang X (2012) The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 33(7):2327–2333
Daima HK, Selvakannan P, Shukla R, Bhargava SK, Bansal V (2013) Fine-tuning the antimicrobial profile of biocompatible gold nanoparticles by sequential surface functionalization using polyoxometalates and lysine. PLoS One 8(10):e79676
Daniel M-C, Astruc D (2004) Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 104(1):293–346
Darby BL, Auguié B, Meyer M, Pantoja AE, Le Ru EC (2016) Modified optical absorption of molecules on metallic nanoparticles at sub-monolayer coverage. Nat Photonics 10(1):40–45
Das SK, Das AR, Guha AK (2009) Gold nanoparticles: microbial synthesis and application in water hygiene management. Langmuir 25(14):8192–8199
Dasari TS, Zhang Y, Yu H (2015) Antibacterial activity and cytotoxicity of gold (I) and (III) ions and gold nanoparticles. Biochem Pharmacol 4(6):199
Dash SS, Bag BG (2014) Synthesis of gold nanoparticles using renewable Punica granatum juice and study of its catalytic activity. Appl Nanosci 4(1):55–59
Doak SH, Griffiths SM, Manshian B, Singh N, Williams PM, Brown AP, Jenkins GJ (2009) Confounding experimental considerations in nanogenotoxicology. Mutagenesis 24(4):285–293
Dondapati SK, Sau TK, Hrelescu C, Klar TA, Stefani FD, Feldmann J (2010) Label-free biosensing based on single gold nanostars as plasmonic transducers. ACS Nano 4(11):6318–6322
Dreaden EC, Alkilany AM, Huang X, Murphy CJ, El-Sayed MA (2012) The golden age: gold nanoparticles for biomedicine. Chem Soc Rev 41(7):2740–2779
Dykman LA, Khlebtsov NG (2017) Immunological properties of gold nanoparticles. Chem Sci 8(3):1719–1735
El-Batal AI, Hashem A-AM, Abdelbaky NM (2013) Gamma radiation mediated green synthesis of gold nanoparticles using fermented soybean-garlic aqueous extract and their antimicrobial activity. Springerplus 2(1):1–10
Esumi K, Suzuki A, Aihara N, Usui K, Torigoe K (1998) Preparation of gold colloids with UV irradiation using dendrimers as stabilizer. Langmuir 14(12):3157–3159
Fairbairn DW, Olive PL, O’Neill KL (1995) The comet assay: a comprehensive review. Mutat Res 339(1):37–59
Fan Y, Pauer AC, Gonzales AA, Fenniri H (2019) Enhanced antibiotic activity of ampicillin conjugated to gold nanoparticles on PEGylated rosette nanotubes. Int J Nanomed 14:7281–7289
Fang L, Fan H, Guo C, Cui L, Zhang P, Mu H, Xu H, Zhao F, Chen D (2019) Novel mitochondrial targeting multifunctional surface charge-reversal polymeric nanoparticles for cancer treatment. J Biomed Nanotechnol 15(11):2151–2163
Frangioni JV (2003) In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol 7(5):626–634
Freitas de Freitas L, Varca GHC, dos Santos Batista JG, Benévolo Lugão A (2018) An overview of the synthesis of gold nanoparticles using radiation technologies. Nano 8(11):939
Frens G (1973) Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat Phys Sci 241(105):20–22
Fuller M, Whiley H, Köper I (2020) Antibiotic delivery using gold nanoparticles. SN Appl Sci 2(6):1–7
Galanzha EI, Shashkov E, Sarimollaoglu M, Beenken KE, Basnakian AG, Shirtliff ME, Kim J-W, Smeltzer MS, Zharov VP (2012) In vivo magnetic enrichment, photoacoustic diagnosis, and photothermal purging of infected blood using multifunctional gold and magnetic nanoparticles. PLoS One 7:e45557
García CP, Sumbayev V, Gilliland D, Yasinska IM, Gibbs BF, Mehn D, Calzolai L, Rossi F (2013) Microscopic analysis of the interaction of gold nanoparticles with cells of the innate immune system. Sci Rep 3(1):1–7
Geethalakshmi R, Sarada D (2012) Gold and silver nanoparticles from Trianthema decandra: synthesis, characterization, and antimicrobial properties. Int J Nanomedicine 7:5375
Geethalakshmi R, Sarada D (2013) Characterization and antimicrobial activity of gold and silver nanoparticles synthesized using saponin isolated from Trianthema decandra L. Ind Crop Prod 51:107–115
Giersig M, Mulvaney P (1993) Preparation of ordered colloid monolayers by electrophoretic deposition. Langmuir 9(12):3408–3413
Gil-Tomás J, Tubby S, Parkin IP, Narband N, Dekker L, Nair SP, Wilson M, Street CJ (2007) Lethal photosensitisation of Staphylococcus aureus using a toluidine blue O–tiopronin–gold nanoparticle conjugate. J Mater Chem 17(35):3739–3746
Gobin AM, Lee MH, Halas NJ, James WD, Drezek RA, West JL (2007) Near-infrared resonant nanoshells for combined optical imaging and photothermal cancer therapy. Nano Lett 7(7):1929–1934
Gobin AM, Moon JJ, West JL (2008) EphrinAl-targeted nanoshells for photothermal ablation of prostate cancer cells. Int J Nanomedicine 3(3):351
Govindaraju S, Samal M, Yun K (2016) Superior antibacterial activity of GlcN-AuNP-GO by ultraviolet irradiation. Mater Sci Eng 69:366–372
Gu H, Ho P, Tong E, Wang L, Xu B (2003) Presenting vancomycin on nanoparticles to enhance antimicrobial activities. Nano Lett 3(9):1261–1263
Gu YJ, Cheng J, Lin CC, Lam YW, Cheng SH, Wong WT (2009) Nuclear penetration of surface functionalized gold nanoparticles. Toxicol Appl Pharmacol 237(2):196–204
Hainfeld JF, Dilmanian FA, Slatkin DN, Smilowitz HM (2008) Radiotherapy enhancement with gold nanoparticles. J Pharm Pharmacol 60(8):977–985
Hao F, Nehl CL, Hafner JH, Nordlander P (2007) Plasmon resonances of a gold nanostar. Nano Lett 7(3):729–732
Hayden SC, Zhao G, Saha K, Phillips RL, Li X, Miranda OR, Rotello VM, El-Sayed MA, Schmidt-Krey I, Bunz UH (2012) Aggregation and interaction of cationic nanoparticles on bacterial surfaces. J Am Chem Soc 134(16):6920–6923
Hernández-Sierra JF, Ruiz F, Pena DCC, Martínez-Gutiérrez F, Martínez AE, Guillén ADJP, Tapia-Pérez H, Castañón GM (2008) Biology; medicine, the antimicrobial sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold. Nanomedicine 4(3):237–240
Heuskin AC, Gallez B, Feron O, Martinive P, Michiels C, Lucas S (2017) Metallic nanoparticles irradiated by low-energy protons for radiation therapy: are there significant physical effects to enhance the dose delivery? Med Phys 44(8):4299–4312
Hirsch LR, Stafford RJ, Bankson J, Sershen SR, Rivera B, Price R, Hazle JD, Halas NJ, West JL (2003) Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci 100(23):13549–13554
Hossain M, Polash SA, Takikawa M, Shubhra RD, Saha T, Islam Z, Hossain M, Hasan M, Takeoka S, Sarker SR (2019) Investigation of the antibacterial activity and in vivo cytotoxicity of biogenic silver nanoparticles as potent therapeutics. Front Bioeng Biotechnol 7:239
Hrelescu C, Sau TK, Rogach AL, Jäckel F, Feldmann J (2009) Single gold nanostars enhance Raman scattering. Appl Phys Lett 94(15):153113
Hu D, Li H, Wang B, Ye Z, Lei W, Jia F, Jin Q, Ren K-F, Ji J (2017) Surface-adaptive gold nanoparticles with effective adherence and enhanced photothermal ablation of methicillin-resistant Staphylococcus aureus biofilm. ACS Nano 11(9):9330–9339
Huang P, Bao L, Zhang C, Lin J, Luo T, Yang D, He M, Li Z, Gao G, Gao B (2011). Biomaterials 32(36):9796–9809
Huh AJ, Kwon YJ (2011) “Nanoantibiotics”: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J Control Release 156(2):128–145
Huo S, Ma H, Huang K, Liu J, Wei T, Jin S, Zhang J, He S, Liang XJ (2013) Superior penetration and retention behavior of 50 nm gold nanoparticles in tumors. Cancer Res 73(1):319–330
Jain PK, Lee KS, El-Sayed IH, El-Sayed MA (2006) Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: applications in biological imaging and biomedicine. J Phys Chem B 110(14):7238–7248
Jana NR, Gearheart L, Murphy CJ (2001a) Wet chemical synthesis of high aspect ratio cylindrical gold nanorods. J Phys Chem B 105(19):4065–4067
Jana NR, Gearheart L, Murphy CJ (2001b) Seed-mediated growth approach for shape-controlled synthesis of spheroidal and rod-like gold nanoparticles using a surfactant template. Adv Mater 13(18):1389–1393
Jana NR, Gearheart L, Obare SO, Murphy CJ (2002) Anisotropic chemical reactivity of gold spheroids and nanorods. Langmuir 18(3):922–927
Jo W, Kim MJ (2013) Influence of the photothermal effect of a gold nanorod cluster on biofilm disinfection. Nanotechnology 24(19):195104
Justo JA, Bosso JA (2015) Adverse reactions associated with systemic polymyxin therapy. Pharmacotherapy 35(1):28–33
Karakoçak BB, Raliya R, Davis JT, Chavalmane S, Wang WN, Ravi N, Biswas P (2016) Biocompatibility of gold nanoparticles in retinal pigment epithelial cell line. Toxicol In Vitro 37:61–69
Khan JA, Pillai B, Das TK, Singh Y, Maiti S (2007) Molecular effects of uptake of gold nanoparticles in HeLa cells. Chembiochem 8(11):1237–1240
Khan S, Khan SN, Meena R, Dar AM, Pal R, Khan AU (2017) Photoinactivation of multidrug resistant bacteria by monomeric methylene blue conjugated gold nanoparticles. J Photochem Photobiol B 174:150–161
Khan I, Saeed K, Khan I (2019) Nanoparticles: properties, applications and toxicities. Arab J Chem 12(7):908–931
Khlebtsov N, Dykman L (2011) Biodistribution and toxicity of engineered gold nanoparticles: a review of in vitro and in vivo studies. Chem Soc Rev 40(3):1647–1671
Kim Y-P, Oh E, Hong M-Y, Lee D, Han M-K, Shon HK, Moon DW, Kim H-S, Lee TG (2006) Gold nanoparticle-enhanced secondary ion mass spectrometry imaging of peptides on self-assembled monolayers. Anal Chem 78(6):1913–1920
Kim D-Y, Kim M, Shinde S, Sung J-S, Ghodake G (2017) Cytotoxicity and antibacterial assessment of gallic acid capped gold nanoparticles. Colloids Surf B 149:162–167
Kodiha M, Hutter E, Boridy S, Juhas M, Maysinger D, Stochaj U (2014) Gold nanoparticles induce nuclear damage in breast cancer cells, which is further amplified by hyperthermia. Cell Mol Life Sci 71(21):4259–4273
Kumar P, Nagarajan A, Uchil PD (2018) Analysis of cell viability by the lactate dehydrogenase assay. Cold Spring Harb Protoc 2018(6):pdb-prot095497
Kundu S (2017) Gold nanoparticles: their application as antimicrobial agents and vehicles of gene delivery. Adv Biotechnol Microbiol 4:556–558
Kuo W-S, Chang C-N, Chang Y-T, Yeh C-S (2009) Antimicrobial gold nanorods with dual-modality photodynamic inactivation and hyperthermia. Chem Commun 32:4853–4855
Kuo Y-L, Wang S-G, Wu C-Y, Lee K-C, Jao C-J, Chou S-H, Chen Y-C (2016) Functional gold nanoparticle-based antibacterial agents for nosocomial and antibiotic-resistant bacteria. Nanomedicine 11(19):2497–2510
Kwon MJ, Lee J, Wark AW, Lee HJ (2012) Nanoparticle-enhanced surface plasmon resonance detection of proteins at attomolar concentrations: comparing different nanoparticle shapes and sizes. Anal Chem 84(3):1702–1707
Lai H-Z, Chen W-Y, Wu C-Y, Chen Y-C (2015) Potent antibacterial nanoparticles for pathogenic bacteria. ACS Appl Mater Interfaces 7(3):2046–2054
Lee B, Park J, Ryu M, Kim S, Joo M, Yeom JH, Kim S, Park Y, Lee K, Bae J (2017) Antimicrobial peptide-loaded gold nanoparticle-DNA aptamer conjugates as highly effective antibacterial therapeutics against Vibrio vulnificus. Sci Rep 7(1):13572
Leff DV, Brandt L, Heath JR (1996) Synthesis and characterization of hydrophobic, organically-soluble gold nanocrystals functionalized with primary amines. Langmuir 12(20):4723–4730
Li X, Robinson SM, Gupta A, Saha K, Jiang Z, Moyano DF, Sahar A, Riley MA, Rotello VM (2014) Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS Nano 8(10):10682–10686
Lima E, Guerra R, Lara V, Guzmán AJCCJ (2013) Gold nanoparticles as efficient antimicrobial agents for Escherichia coli and salmonella typhi. Chem Cent J 7(1):1–7
Lin Y, Hamme AT II (2015) Gold nanoparticle labeling based ICP-MS detection/measurement of bacteria, and their quantitative photothermal destruction. J Mater Chem B 3(17):3573–3582
Liny P, Divya T, Malakar B, Nagaraj B, Krishnamurthy N, Dinesh R (2012) Preparation of gold nanoparticles from Helianthus annuus (sun flower) flowers and evaluation of their antimicrobial activities. Int J Pharm Bio Sci 3(1):439–446
Lokina S, Suresh R, Giribabu K, Stephen A, Sundaram RL, Narayanan V (2014) Spectroscopic investigations, antimicrobial, and cytotoxic activity of green synthesized gold nanoparticles. Spectrochim Acta A 129:484–490
Loo C, Hirsch L, Lee M-H, Chang E, West J, Halas N, Drezek R (2005) Gold nanoshell bioconjugates for molecular imaging in living cells. Opt Lett 30(9):1012–1014
Lowery AR, Gobin AM, Day ES, Halas NJ, West JL (2006) Immunonanoshells for targeted photothermal ablation of tumor cells. Int J Nanomedicine 1(2):149
Lu X, Chen J, Skrabalak S, Xia Y (2007) Nanosystems, galvanic replacement reaction: a simple and powerful route to hollow and porous metal nanostructures. Proc Inst Mech Eng, Part N 221(1):1–16
Mahalingam S, Xu Z, Edirisinghe M (2015) Antibacterial activity and biosensing of PVA-lysozyme microbubbles formed by pressurized gyration. Langmuir 31(36):9771–9780
Mahitha B, Raju BDP, Madhavi T, Lakshmi CNDM, Sushma NJ (2013) Evaluation of antibacterial efficacy of phyto fabricated gold nanoparticles using bacope monniera plant extract. Ind J Adv Chem Sci 1(2):94–98
Mahmoud MA, El-Sayed MA (2010) Gold nanoframes: very high surface plasmon fields and excellent near-infrared sensors. J Am Chem Soc 132(36):12704–12710
Mahmoud M, Snyder B, El-Sayed M (2010) Surface plasmon fields and coupling in the hollow gold nanoparticles and surface-enhanced Raman spectroscopy. Theory and experiment. J Phys Chem C 114(16):7436–7443
Mahmoud NN, Alkilany AM, Khalil EA, Al-Bakri AG (2018) Nano-photothermal ablation effect of hydrophilic and hydrophobic functionalized gold nanorods on Staphylococcus aureus and Propionibacterium acnes. Sci Rep 8(1):6881
Mahmoud NN, Alhusban AA, Ali JI, Al-Bakri AG, Hamed R, Khalil EA (2019) Preferential accumulation of phospholipid-PEG and cholesterol-PEG decorated gold nanorods into human skin layers and their photothermal-based antibacterial activity. Sci Rep 9(1):5796
Marangoni VS, Paino IM, Zucolotto V (2013) Synthesis and characterization of jacalin-gold nanoparticles conjugates as specific markers for cancer cells. Colloids Surf B 112:380–386
Martin CRS (1994) Nanomaterials: a membrane-based synthetic approach. Science 266(5193):1961–1966
Mendes R, Pedrosa P, Lima JC, Fernandes AR, Baptista PV (2017) Photothermal enhancement of chemotherapy in breast cancer by visible irradiation of gold nanoparticles. Sci Rep 7(1):1–9
Merchant B (1998) Gold, the noble metal and the paradoxes of its toxicology. Biologicals 26(1):49–59
Merrifield RC, Stephan C, Lead JR (2018) Quantification of Au nanoparticle biouptake and distribution to freshwater algae using single cell - ICP-MS. Environ Sci Technol 52(4):2271–2277
Mieszawska AJ, Zamborini FP (2005) Gold nanorods grown directly on surfaces from microscale patterns of gold seeds. Chem Mater 17(13):3415–3420
Millenbaugh NJ, Baskin JB, DeSilva MN, Elliott WR, Glickman RD (2015) Photothermal killing of Staphylococcus aureus using antibody-targeted gold nanoparticles. Int J Nanomed 10:1953
Mishra A, Tripathy SK, Yun S-I (2011) Bio-synthesis of gold and silver nanoparticles from Candida guilliermondii and their antimicrobial effect against pathogenic bacteria. J Nanosci Nanotechnol 11(1):243–248
Mishra A, Kumari M, Pandey S, Chaudhry V, Gupta K, Nautiyal C (2014) Biocatalytic and antimicrobial activities of gold nanoparticles synthesized by Trichoderma sp. Bioresour Technol 166:235–242
Mitra P, Chakraborty PK, Saha P, Ray P, Basu S (2014) Antibacterial efficacy of acridine derivatives conjugated with gold nanoparticles. Int J Pharm 473(1–2):636–643
Mocan L, Matea C, Tabaran FA, Mosteanu O, Pop T, Puia C, Agoston-Coldea L, Gonciar D, Kalman E, Zaharie G (2016) Selective in vitro photothermal nano-therapy of MRSA infections mediated by IgG conjugated gold nanoparticles. Sci Rep 6(1):1–9
Mocan L, Tabaran FA, Mocan T, Pop T, Mosteanu O, Agoston-Coldea L, Matea CT, Gonciar D, Zdrehus C, Iancu C (2017) Laser thermal ablation of multidrug-resistant bacteria using functionalized gold nanoparticles. Int J Nanomedicine 12:2255
Mohamed MM, Fouad SA, Elshoky HA, Mohammed GM, Salaheldin TA (2017) Antibacterial effect of gold nanoparticles against Corynebacterium pseudotuberculosis. Int J Vet Sci Med 5(1):23–29
Mollick MMR, Bhowmick B, Mondal D, Maity D, Rana D, Dash SK, Chattopadhyay S, Roy S, Sarkar J, Acharya K (2014) Anticancer (in vitro) and antimicrobial effect of gold nanoparticles synthesized using Abelmoschus esculentus (L.) pulp extract via a green route. RSC Adv 4(71):37838–37848
Moreno-Álvarez S, Martínez-Castañón G, Niño-Martínez N, Reyes-Macías J, Patiño-Marín N, Loyola-Rodríguez J, Ruiz F (2010) Preparation and bactericide activity of gallic acid stabilized gold nanoparticles. J Nanopart Res 12(8):2741–2746
MubarakAli D, Thajuddin N, Jeganathan K, Gunasekaran M (2011) Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Colloids Surf B 85(2):360–365
Mukha I, Eremenko А, Korchak G, Michienkova А (2010) Antibacterial action and physicochemical properties of stabilized silver and gold nanostructures on the surface of disperse silica. J Water Resour Prot 2:131–136
Mukherjee P, Ahmad A, Mandal D, Senapati S, Sainkar SR, Khan MI, Ramani R, Parischa R, Ajayakumar P, Alam M, Sastry M (2001) Bioreduction of AuCl4− ions by the fungus, Verticillium sp. and surface trapping of the gold nanoparticles formed. Angew Chem Int Ed 40(19):3585–3588
Nagajyothi P, Sreekanth T, Prasad T, Lee KD (2012) Harvesting Au nanoparticles from Carthamus tinctorius flower extract and evaluation of their antimicrobial activity. Adv Sci Lett 5(1):124–130
Nagaraj B, Divya T, Krishnamurthy N, Dinesh R, Negrila C, Predoi D (2012) Phytosynthesis of gold nanoparticles using caesalpinia pulcherrima (peacock flower) flower extract and evaluation of their antimicrobial activities. J Optoelectron Adv Mater 7(3)
Naik AJ, Ismail S, Kay C, Wilson M, Parkin IP (2011) Antimicrobial activity of polyurethane embedded with methylene blue, toluidene blue and gold nanoparticles against Staphylococcus aureus; illuminated with white light. Mater Chem Phys 129(1–2):446–450
Narband N, Uppal M, Dunnill CW, Hyett G, Wilson M, Parkin IP (2009) The interaction between gold nanoparticles and cationic and anionic dyes: enhanced UV-visible absorption. Phys Chem Chem Phys 11(44):10513–10518
Naveena BE, Prakash S (2013) Biological synthesis of gold nanoparticles using marine algae Gracilaria corticata and its application as a potent antimicrobial and antioxidant agent. Asian J Pharm Clin 6(2):179–182
Nazari ZE, Banoee M, Sepahi AA, Rafii F, Shahverdi AR (2012) The combination effects of trivalent gold ions and gold nanoparticles with different antibiotics against resistant Pseudomonas aeruginosa. Gold Bull 45(2):53–59
Nel AE, Mädler L, Velegol D, Xia T, Hoek EM, Somasundaran P, Klaessig F, Castranova V, Thompson M (2009) Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater 8(7):543–557
Ni W, Yang Z, Chen H, Li L, Wang J (2008) Coupling between molecular and plasmonic resonances in freestanding dye-gold nanorod hybrid nanostructures. J Am Chem Soc 130(21):6692–6693
Nichols JW, Bae YH (2014) EPR: evidence and fallacy. J Control Release 190:451–464
Niloy MS, Hossain MM, Takikawa M, Shakil MS, Polash SA, Mahmud KM, Uddin MF, Alam M, Shubhra RD, Shawan MMAK, Saha T (2020) Synthesis of biogenic silver nanoparticles using caesalpinia digyna and investigation of their antimicrobial activity and in vivo biocompatibility. ACS Appl Biol Mater 3:7722–7733
Ocsoy I, Yusufbeyoglu S, Yılmaz V, McLamore ES, Ildız N, Ülgen A (2017) DNA aptamer functionalized gold nanostructures for molecular recognition and photothermal inactivation of methicillin-resistant Staphylococcus aureus. Colloids Surf B 159:16–22
Oldenburg S, Averitt R, Westcott S, Halas NJ (1998) Nanoengineering of optical resonances. Chem Phys Lett 288(2–4):243–247
Oldenburg SJ, Westcott SL, Averitt RD, Halas NJ (1999a) Surface enhanced Raman scattering in the near infrared using metal nanoshell substrates. J Chem Phys 111(10):4729–4735
Oldenburg SJ, Jackson JB, Westcott SL, Halas NJ (1999b) Infrared extinction properties of gold nanoshells. Appl Phys Lett 75(19):2897–2899
Ortiz-Benítez EA, Velázquez-Guadarrama N, Durán Figueroa NV, Quezada H, Olivares-Trejo JJ (2019) Antibacterial mechanism of gold nanoparticles on Streptococcus pneumoniae. Metallomics 11(7):1265–1276
Osaki F, Kanamori T, Sando S, Sera T, Aoyama Y (2004) A quantum dot conjugated sugar ball and its cellular uptake. On the size effects of endocytosis in the subviral region. J Am Chem Soc 126(21):6520–6521
Pal S, Mitra K, Azmi S, Ghosh JK, Chakraborty TK (2011) Towards the synthesis of sugar amino acid containing antimicrobial noncytotoxic CAP conjugates with gold nanoparticles and a mechanistic study of cell disruption. Org Biomol Chem 9(13):4806–4810
Pallares RM, Su X, Lim SH, Thanh NT (2016) Fine-tuning of gold nanorod dimensions and plasmonic properties using the Hofmeister effects. J Mater Chem C 4(1):53–61
Pallavicini P, Dona A, Taglietti A, Minzioni P, Patrini M, Dacarro G, Chirico G, Sironi L, Bloise N, Visai L (2014) Self-assembled monolayers of gold nanostars: a convenient tool for near-IR photothermal biofilm eradication. Chem Commun 50(16):1969–1971
Pallavicini P, Bassi B, Chirico G, Collini M, Dacarro G, Fratini E, Grisoli P, Patrini M, Sironi L, Taglietti A, Moritz M, Sorzabal-Bellido I, Susarrey-Arce A, Latter E, Beckett AJ, Prior IA, Raval R, Diaz Fernandez YA (2017) Modular approach for bimodal antibacterial surfaces combining photo-switchable activity and sustained biocidal release. Sci Rep 7(1):5259
Pandala N, LaScola MA, Tang Y, Bieberich M, Korley LT, Lavik E (2021) Screen printing tissue models using chemically cross-linked hydrogel systems: a simple approach to efficiently make highly tunable matrices. ACS Biomater Sci Eng 7(11):5007–5013
Park J, Estrada A, Sharp K, Sang K, Schwartz JA, Smith DK, Coleman C, Payne JD, Korgel BA, Dunn AK (2008) Two-photon-induced photoluminescence imaging of tumors using near-infrared excited gold nanoshells. Opt Express 16(3):1590–1599
Patra HK, Banerjee S, Chaudhuri U, Lahiri P, Dasgupta AK (2007) Cell selective response to gold nanoparticles. Nanomedicine 3(2):111–119
Payne JN, Waghwani HK, Connor MG, Hamilton W, Tockstein S, Moolani H, Chavda F, Badwaik V, Lawrenz MB, Dakshinamurthy R (2016) Novel synthesis of kanamycin conjugated gold nanoparticles with potent antibacterial activity. Front Microbiol 7:607
Perzanowska O, Majewski M, Strenkowska M, Głowala P, Czarnocki-Cieciura M, Mazur M, Kowalska J, Jemielity J (2021) Nucleotide-decorated AuNPs as probes for nucleotide-binding proteins. Sci Rep 11(1):15741
Piktel E, Suprewicz Ł, Depciuch J, Chmielewska S, Skłodowski K, Daniluk T, Król G, Kołat-Brodecka P, Bijak P, Pajor-Świerzy A, Fiedoruk K, Parlinska-Wojtan M, Bucki R (2021) Varied-shaped gold nanoparticles with nanogram killing efficiency as potential antimicrobial surface coatings for the medical devices. Sci Rep 11(1):12546
Podsiadlo P, Sinani VA, Bahng JH, Kam NWS, Lee J, Kotov NA (2008) Gold nanoparticles enhance the anti-leukemia action of a 6-mercaptopurine chemotherapeutic agent. Langmuir 24(2):568–574
Polash SA, Hossain M, Saha T, Sarker SR (2021) Biogenic silver nanoparticles: a potent therapeutic agent. In: Emerging trends in nanomedicine. Springer, Berlin, pp 81–127
Radloff C, Vaia RA, Brunton J, Bouwer GT, Ward VK (2005) Metal nanoshell assembly on a virus bioscaffold. Nano Lett 5(6):1187–1191
Rai A, Pinto S, Velho TR, Ferreira AF, Moita C, Trivedi U, Evangelista M, Comune M, Rumbaugh KP, Simões PN (2016) One-step synthesis of high-density peptide-conjugated gold nanoparticles with antimicrobial efficacy in a systemic infection model. Biomaterials 85:99–110
Rai M, Ingle AP, Pandit R, Paralikar P, Gupta I, Chaud MV, Dos Santos CA (2017) Broadening the spectrum of small-molecule antibacterials by metallic nanoparticles to overcome microbial resistance. Int J Pharm 532(1):139–148
Rajchakit U, Sarojini V (2017) Recent developments in antimicrobial-peptide-conjugated gold nanoparticles. Bioconjug Chem 28(11):2673–2686
Ramamurthy C, Padma M, Mareeswaran R, Suyavaran A, Kumar MS, Premkumar K, Thirunavukkarasu C (2013) The extra cellular synthesis of gold and silver nanoparticles and their free radical scavenging and antibacterial properties. Colloids Surf B 102:808–815
Ramasamy M, Lee J-H, Lee J (2017a) Direct one-pot synthesis of cinnamaldehyde immobilized on gold nanoparticles and their antibiofilm properties. Colloids Surf B 160:639–648
Ramasamy M, Lee J-H, Lee J (2017b) Development of gold nanoparticles coated with silica containing the antibiofilm drug cinnamaldehyde and their effects on pathogenic bacteria. Int J Nanomed 12:2813
Ranjan Sarker S, Polash SA, Boath J, Kandjani AE, Poddar A, Dekiwadia C, Shukla R, Sabri Y, Bhargava SK (2019) Functionalization of elongated tetrahexahedral Au nanoparticles and their antimicrobial activity assay. ACS Appl Mater Interfaces 11(14):13450–13459
Rattanata N, Klaynongsruang S, Leelayuwat C, Limpaiboon T, Lulitanond A, Boonsiri P, Chio-Srichan S, Soontaranon S, Rugmai S, Daduang J (2016) Gallic acid conjugated with gold nanoparticles: antibacterial activity and mechanism of action on foodborne pathogens. Int J Nanomedicine 11:3347–3356
Reetz MT, Helbig W (1994) Size-selective synthesis of nanostructured transition metal clusters. J Am Chem Soc 116(16):7401–7402
Repetto G, del Peso A, Zurita JL (2008) Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc 3(7):1125–1131
Rodríguez-Lorenzo L, Alvarez-Puebla RA, Pastoriza-Santos I, Mazzucco S, Stéphan O, Kociak M, Liz-Marzán LM, García de Abajo FJ (2009) Zeptomol detection through controlled ultrasensitive surface-enhanced Raman scattering. J Am Chem Soc 131(13):4616–4618
Roshmi T, Soumya K, Jyothis M, Radhakrishnan E (2015) Effect of biofabricated gold nanoparticle-based antibiotic conjugates on minimum inhibitory concentration of bacterial isolates of clinical origin. Gold Bull 48(1):63–71
Rossi F, Thanh NTK, Su XD (2019) Gold nanorods embedded in polymeric film for killing bacteria by generating reactive oxygen species with light. ACS Appl Biol Mater 2(7):3059–3067
Rossolini GM, Arena F, Pecile P, Pollini S (2014) Update on the antibiotic resistance crisis. Curr Opin Pharmacol 18:56–60
Sans-Serramitjana E, Fusté E, Martínez-Garriga B, Merlos A, Pastor M, Pedraz JL, Esquisabel A, Bachiller D, Vinuesa T, Viñas M (2016) Killing effect of nanoencapsulated colistin sulfate on Pseudomonas aeruginosa from cystic fibrosis patients. J Cyst Fibros 15(5):611–618
Selvaraj V, Grace AN, Alagar M, Hamerton I (2010) Antimicrobial and anticancer efficacy of antineoplastic agent capped gold nanoparticles. J Biomed Nanotechnol 6(2):129–137
Shaikh S, Rizvi SMD, Shakil S, Hussain T, Alshammari TM, Ahmad W, Tabrez S, Al-Qahtani MH, Abuzenadah AM (2017) Synthesis and characterization of cefotaxime conjugated gold nanoparticles and their use to target drug-resistant CTX-M-producing bacterial pathogens. J Cell Biochem 118(9):2802–2808
Shaikh S, Nazam N, Rizvi SMD, Ahmad K, Baig MH, Lee EJ, Choi I (2019) Mechanistic insights into the antimicrobial actions of metallic nanoparticles and their implications for multidrug resistance. Int J Mol Sci 20(10):2468
Shaker MA, Shaaban MI (2017) Formulation of carbapenems loaded gold nanoparticles to combat multi-antibiotic bacterial resistance: in vitro antibacterial study. Int J Pharm 525(1):71–84
Shamaila S, Zafar N, Riaz S, Sharif R, Nazir J, Naseem S (2016) Gold nanoparticles: an efficient antimicrobial agent against enteric bacterial human pathogen. J Nano 6(4):71
Shankar SS, Rai A, Ahmad A, Sastry MJ (2004) Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J Colloid Interface Sci 275(2):496–502
Shankar S, Jaiswal L, Aparna R, Prasad R (2014) Synthesis, characterization, in vitro biocompatibility, and antimicrobial activity of gold, silver and gold silver alloy nanoparticles prepared from Lansium domesticum fruit peel extract. Mater Lett 137:75–78
Shukla R, Bansal V, Chaudhary M, Basu A, Bhonde RR, Sastry M (2005) Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: a microscopic overview. Langmuir 21(23):10644–10654
Skrabalak SE, Au L, Li X, Xia Y (2007) Facile synthesis of ag nanocubes and Au nanocages. Nat Protoc 2(9):2182–2190
Skrabalak SE, Chen J, Sun Y, Lu X, Au L, Cobley CM, Xia Y (2008) Gold nanocages: synthesis, properties, and applications. Acc Chem Res 41(12):1587–1595
Smitha S, Gopchandran KG (2013) Surface enhanced Raman scattering, antibacterial and antifungal active triangular gold nanoparticles. Spectrochim Acta A 102:114–119
Sreekanth T, Nagajyothi P, Lee KD (2012) Biosynthesis of gold nanoparticles and their antimicrobial activity and cytotoxicity. Adv Sci Lett 6(1):63–69
Sreekanth T, Nagajyothi P, Supraja N, Prasad T (2015) Evaluation of the antimicrobial activity and cytotoxicity of phytogenic gold nanoparticles. Appl Nanosci 5(5):595–602
Sreelakshmi C, Datta K, Yadav J, Reddy B (2011) Honey derivatized Au and Ag nanoparticles and evaluation of its antimicrobial activity. J Nanosci Nanotechnol 11(8):6995–7000
Stockert JC, Horobin RW, Colombo LL, Blázquez-Castro A (2018) Tetrazolium salts and formazan products in cell biology: viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem 120(3):159–167
Strober W (2001) Trypan blue exclusion test of cell viability. Curr Protoc Immunol, Appendix 3, Appendix 3B
Su C, Huang K, Li H-H, Lu Y-G, Zheng D-L (2020) Antibacterial properties of functionalized gold nanoparticles and their application in oral biology. J Nanomater 2020:1–13
Suresh AK, Pelletier DA, Wang W, Broich ML, Moon J-W, Gu B, Allison DP, Joy DC, Phelps TJ, Doktycz MJ (2011) Biofabrication of discrete spherical gold nanoparticles using the metal-reducing bacterium Shewanella oneidensis. Acta Biomater 7(5):2148–2152
Suzuki D, Kawaguchi H (2005) Gold nanoparticle localization at the core surface by using thermosensitive core−shell particles as a template. Langmuir 21(25):12016–12024
Tao C (2018) Antimicrobial activity and toxicity of gold nanoparticles: research progress, challenges and prospects. Lett Appl Microbiol 67(6):537–543
Tielens F, Santos E (2010) AuS and SH bond formation/breaking during the formation of alkanethiol SAMs on Au (111): a theoretical study. J Phys Chem C 114(20):9444–9452
Tuersun P, Han X (2013) Optical absorption analysis and optimization of gold nanoshells. Appl Opt 52(6):1325–1329
Turkevich J, Stevenson PC, Hillier J (1951) A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc 11:55–75
Venditti I (2019) Engineered gold-based nanomaterials: morphologies and functionalities in biomedical applications. A mini review. Bioengineering (Basel) 6(2):53
Vidya S, Mutalik S, Bhat KU, Huilgol P, Avadhani K (2016) Preparation of gold nanoparticles by novel bacterial exopolysaccharide for antibiotic delivery. Life Sci 153:171–179
Villiers C, Freitas H, Couderc R, Villiers MB, Marche P (2010) Analysis of the toxicity of gold nano particles on the immune system: effect on dendritic cell functions. J Nanopart Res 12(1):55–60
Vimbela GV, Ngo SM, Fraze C, Yang L, Stout DA (2017) Antibacterial properties and toxicity from metallic nanomaterials. Int J Nanomed 12:3941
Vinoj G, Pati R, Sonawane A, Vaseeharan B (2015) In vitro cytotoxic effects of gold nanoparticles coated with functional acyl homoserine lactone lactonase protein from Bacillus licheniformis and their antibiofilm activity against Proteus species. Antimicrob Agents Chemother 59(2):763–771
Vistica DT, Skehan P, Scudiero D, Monks A, Pittman A, Boyd MR (1991) Tetrazolium-based assays for cellular viability: a critical examination of selected parameters affecting formazan production. Cancer Res 51(10):2515–2520
Wadhwani P, Heidenreich N, Podeyn B, Bürck J, Ulrich AS (2017) Antibiotic gold: tethering of antimicrobial peptides to gold nanoparticles maintains conformational flexibility of peptides and improves trypsin susceptibility. Biomater Sci 5(4):817–827
Walker T, Dumadag S, Lee CJ, Lee SH, Bender JM, Cupo Abbott J, She RC (2016) Clinical impact of laboratory implementation of Verigene BC-GN microarray-based assay for detection of gram-negative bacteria in positive blood cultures. J Clin Microbiol 54(7):1789–1796
Wan W, Yeow JT (2012) Antibacterial properties of poly (quaternary ammonium) modified gold and titanium dioxide nanoparticles. J Nanosci Nanotechnol 12(6):4601–4606
Wang S, Lu W, Tovmachenko O, Rai US, Yu H, Ray PC (2008) Challenge in understanding size and shape dependent toxicity of gold nanomaterials in human skin keratinocytes. Chem Phys Lett 463(1–3):145–149
Wang SH, Lee CW, Chiou A, Wei PK (2010) Size-dependent endocytosis of gold nanoparticles studied by three-dimensional mapping of plasmonic scattering images. J Nanobiotechnol 8:33
Wang P, Wang X, Wang L, Hou X, Liu W, Chen C (2015) Interaction of gold nanoparticles with proteins and cells. Sci Technol Adv Mater 16(3):034610
Wang Z, Dong K, Liu Z, Zhang Y, Chen Z, Sun H, Ren J, Qu X (2017a) Activation of biologically relevant levels of reactive oxygen species by Au/g-C3N4 hybrid nanozyme for bacteria killing and wound disinfection. Biomaterials 113:145–157
Wang L, Hu C, Shao L (2017b) The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int J Nanomed 12:1227
Wang SG, Chen YC, Chen YC (2018) Antibacterial gold nanoparticle-based photothermal killing of vancomycin-resistant bacteria. Nanomedicine (Lond) 13(12):1405–1416
Wang J, Zhang J, Liu K, He J, Zhang Y, Chen S, Ma G, Cui Y, Wang L, Gao D (2020) Synthesis of gold nanoflowers stabilized with amphiphilic daptomycin for enhanced photothermal antitumor and antibacterial effects. Int J Pharm 580:119231
Wani IA, Ahmad T (2013) Size and shape dependant antifungal activity of gold nanoparticles: a case study of Candida. Colloids Surf B 101:162–170
Wimley WC, Hristova K (2011) Antimicrobial peptides: successes, challenges and unanswered questions. J Membr Biol 239(1–2):27–34
Xing X, Ma W, Zhao X, Wang J, Yao L, Jiang X, Wu Z (2018) Interaction between surface charge-modified gold nanoparticles and phospholipid membranes. Langmuir 34(42):12583–12589
Xue Y, Li X, Li H, Zhang W (2014) Quantifying thiol–gold interactions towards the efficient strength control. Nat Commun 5(1):1–9
Yang X, Yang J, Wang L, Ran B, Jia Y, Zhang L, Yang G, Shao H, Jiang X (2017) Pharmaceutical intermediate-modified gold nanoparticles: against multidrug-resistant bacteria and wound-healing application via an electrospun scaffold. ACS Nano 11(6):5737–5745
Yeom J-H, Lee B, Kim D, Lee J-K, Kim S, Bae J, Park Y, Lee K (2016) Gold nanoparticle-DNA aptamer conjugate-assisted delivery of antimicrobial peptide effectively eliminates intracellular salmonella enterica serovar typhimurium. Biomaterials 104:43–51
Yu Y-Y, Chang S-S, Lee C-L, Wang CC (1997) Gold nanorods: electrochemical synthesis and optical properties. J Phys Chem B 101(34):6661–6664
Yu Q, Li J, Zhang Y, Wang Y, Liu L, Li M (2016) Inhibition of gold nanoparticles (AuNPs) on pathogenic biofilm formation and invasion to host cells. Sci Rep 6:26667
Yu Z, Li Q, Wang J, Yu Y, Wang Y, Zhou Q, Li P (2020) Reactive oxygen species-related nanoparticle toxicity in the biomedical field. Nanoscale Res Lett 15(1):115
Yuan J-J, Schmid A, Armes SP, Lewis AL (2006) Facile synthesis of highly biocompatible poly (2-(methacryloyloxy) ethyl phosphorylcholine)-coated gold nanoparticles in aqueous solution. Langmuir 22(26):11022–11027
Zawrah M, El-Moez S, Center D (2011) Antimicrobial activities of gold nanoparticles against major foodborne pathogens. Life Sci J 8(4):37–44
Zhang Y, Peng H, Huang W, Zhou Y, Yan D (2008) Facile preparation and characterization of highly antimicrobial colloid Ag or Au nanoparticles. J Colloid Interface Sci 325(2):371–376
Zhang W, Li Y, Niu J, Chen Y (2013) Photogeneration of reactive oxygen species on uncoated silver, gold, nickel, and silicon nanoparticles and their antibacterial effects. Langmuir 29(15):4647–4651
Zhang Y, Shareena Dasari TP, Deng H, Yu H (2015) Antimicrobial activity of gold nanoparticles and ionic gold. J Environ Sci Health C 33(3):286–327
Zhao Y, Tian Y, Cui Y, Liu W, Ma W, Jiang X (2010) Small molecule-capped gold nanoparticles as potent antibacterial agents that target gram-negative bacteria. J Am Chem Soc 132(35):12349–12356
Zhao Y, Chen Z, Chen Y, Xu J, Li J, Jiang X (2013) Synergy of non-antibiotic drugs and pyrimidinethiol on gold nanoparticles against superbugs. J Am Chem Soc 135(35):12940–12943
Zheng K, Setyawati MI, Lim TP, Leong DT, Xie J (2016) Antimicrobial cluster bombs: silver nanoclusters packed with daptomycin. ACS Nano 10(8):7934–7942
Zheng K, Setyawati MI, Leong DT, Xie J (2017) Antimicrobial gold nanoclusters. ACS Nano 11(7):6904–6910
Zhou Y, Kong Y, Kundu S, Cirillo JD, Liang H (2012) Antibacterial activities of gold and silver nanoparticles against Escherichia coli and bacillus Calmette-Guérin. J Nanobiotechnol 10(1):1–9
Zhu Y, Ramasamy M, Yi DK (2014) Antibacterial activity of ordered gold nanorod arrays. ACS Appl Mater Interfaces 6(17):15078–15085
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Hossain, M.M., Polash, S.A., Saha, T., Sarker, S.R. (2022). Gold Nanoparticles: A Lethal Nanoweapon Against Multidrug-Resistant Bacteria. In: Kumar, V., Shriram, V., Shukla, R., Gosavi, S. (eds) Nano-Strategies for Addressing Antimicrobial Resistance. Nanotechnology in the Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-031-10220-2_9
Download citation
DOI: https://doi.org/10.1007/978-3-031-10220-2_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-10219-6
Online ISBN: 978-3-031-10220-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)