Abstract
Antimicrobial resistance (AMR) emergence has entangled the cure of health-related diseases with the existing medicines. Though several potent and novel antimicrobial agents have been identified in recent past, their safe and effective delivery is yet to be achieved fully. Nanotechnology has emerged as the continual and practical solution in the delivery of antimicrobial therapeutics using nanotechnology-based drug carriers (nanocarriers) and signifies the correlation between biological and physical sciences, by employing it in the variety of branches like nanomedicines and nanomaterial-based drug delivery approaches. Owing to their tiny size and large surface area, nanocarrier is the hotspot in the nanotechnology world. In the recent reports, biocompatibility, cost-effectiveness, controlled drug release, deep penetration, target specificity and sustainability of nanocarriers have revealed their ideal role in the drug delivery system. In this chapter, we discuss about the various nanomaterials and antimicrobial agents employed in the delivery of antimicrobials such as metals, peptides, drugs and plant resources to target drug determinants such as efflux pumps, cell membrane permeability, biofilms and quorum sensing in the drug-resistant bacteria with their applications in the clinical trials.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
Since earlier civilizations, natural products have been widely employed as potent therapeutics against numerous ailments. Based on historical learning, modern medications are thus mostly developed from medicinal plant resources (Veeresham 2012). Natural substances exhibiting various molecular backgrounds provide a starting point for the development of new medications. Various approaches such as natural product-based drug development and drug delivery have been developed to cure enormous diseases caused by drug-resistant bacteria (Atanasov et al. 2021). However, incompatibility issues, availability restrictions and tedious purification techniques (Siddiqui et al. 2014) somewhat restrict their full potential therapeutic usages, and thus newer technologies are required to address these and other issues for the development of efficient drug/antimicrobial delivery systems. Nanotechnology has been demonstrated to bridge the gap between the physical and the biological sciences by employing nanomaterials in a variety of sectors, including nanomedicines and nanomaterial-based drug delivery approaches (Patra et al. 2018). Employing nanocarriers as an effective drug delivery system has lately gotten a lot of press because of their capacity to identify and cure diseases caused by drug-resistant bacteria (Yeh et al. 2020).
Antimicrobial resistance (AMR) or antibiotic resistance (ABR) is a condition where microbes or bacteria show resistance against commonly used antimicrobial drugs, especially antibiotics. The AMR has become a major public health threat making it challenging to cure health-related diseases with existing medicines (Lee et al. 2019b). The World Health Organization (WHO) has recognized AMR and multidrug-resistant (MDR) bacteria as major global public health threats humanity is facing (WHO 2021) owing to their colonizing abilities both domestically and globally (Hall et al. 2020), though there are several successful attempts of identifying potent and novel antimicrobial agents in recent years. However, effective and safe delivery of potent antimicrobial agents has emerged a major hurdle. The use of nanomaterial- and nanotechnology-based drug carriers (nanocarriers) that can carry nano- or other antimicrobial therapeutics is emerging as a sustainable and practical solution (Krishnamoorthi et al. 2021). These approaches use nanoscale materials for the delivery of antimicrobials including natural products to their target tissues (Yeh et al. 2020). Various antimicrobial agents including metals, peptides, drugs and plant resources possessing different inhibitory mechanisms have been owned in nanocarriers. Nanocarriers or nanomaterial-based antimicrobial systems in MDR microorganisms have been shown to inhibit various drug resistance determinants such as efflux pumps (EPs), cell membrane, biofilms and quorum sensing (Baptista et al. 2018) showing their potential clinical applications.
In this chapter, we discuss the nanomaterials emerging as a nanocarrier for delivering wide-ranging antimicrobial agents. We describe the various types of nanomaterials and antimicrobial agents with their inhibitory mechanisms that act as the major components in the delivery of antimicrobials such as drugs, metals, peptides and plant resources. Further, we highlight the delivery of antimicrobial agents via nanomaterials to target the major bacterial drug resistance determinants (cell membrane, EPs, quorum sensing, biofilm formation). The potential applications of nanocarriers in clinical trials have been also discussed herein.
3.2 Nanocarriers as Emerging Drug Delivery Systems
The significance of nanocarriers as drug delivery systems was discovered around a century ago for the delivery of therapeutic drugs and other natural agents to the site of microbial infections (Patra et al. 2018). Nanocarriers are defined as the nanoparticles that can be employed to carry antimicrobial agents or other chemical agents to the target location for their effective treatment of infections caused by the pathogenic microbes including drug-resistant bacteria (Chamundeeswari et al. 2019). Nanocarriers mainly consist of many small-sized nanoparticles (1–100 nm range) such as nanomaterials, dendrimers, lipid-based nanoparticles and liposomes that effectively transport the antimicrobial agents to the target tissue (Lombardo et al. 2019). The property of enhanced stability, improved drug serum solubility, pharmacokinetics, sustainability, longer systemic circulation duration and reduced toxicity make them excellent choice as drug delivery systems (Zhang et al. 2010). Further, deep penetration abilities of nanomaterials into the host cells, controlled drug release and endocytosis for treating drug-resistant pathogens make them ideal drug carriers with their potential clinical applications against wide range of infectious diseases (Fatima et al. 2021). To enhance the pharmacokinetics and therapeutic effects of drugs, antimicrobial agents are loaded into nanomaterials via adsorption, chemical conjugation and physical encapsulation (Patra et al. 2018).
Nanocarriers are designed in a wide range of materials with different chemical compositions to transport diverse bioactive compounds in a regulated, systemic and targeted manner, making them highly effective drug delivery agents (Manju and Sreenivasan 2010). Various nanomaterials such as metal, non-metals, semiconductors, quantum dots, dendrimers, biopolymers and organic and inorganic nanomaterials have been successfully used as nanocarriers in medical applications. Interestingly, the organic nanomaterials including liposomes, ferritin and micelles have been reported to enhance the drug bioavailability and thus improved antimicrobial activity (Yetisgin et al. 2020). Besides, other metallic and non-metallic nanomaterials combined with drugs and other antimicrobial agents have also been widely used for the drug delivery applications (Mba and Nweze 2021). Recently, nanostructured lipid-based carriers (NLCs) have emerged as novel drug delivery systems for the delivery of chemotherapeutic agents because of their excellent physical stability, good drug-loading capacity and biocompatibility (Haider et al. 2020). The development of amoxicillin- and clarithromycin-loaded magnetic nanostructure lipid-based carriers (AMO-CLR-Fe3O4@NLCs) with enhanced and prolonged drug delivery with 3.13 μg/mL minimum inhibitory concentration (MIC) value against Staphylococcus aureus, Bacillus subtilis and Bordetella pertussis resulted in deterioration of bacterial cell morphology and ultimately led to cell death (Sharaf et al. 2021). Nano-drug carriers are also being explored in diagnosis and treatment of brain infections (Barani et al. 2021). Streptococcus pneumoniae, Staphylococcus aureus, Neisseria meningitidis, Haemophilus influenza and Listeria monocytogenes are found to invade the brain causing bacterial infections in the endothelial barrier and inflammation in meninges called meningitis (Al-Obaidi and Desa 2018). The intranasal route for delivering the drugs to the brain for overcoming the blood-brain barrier and central nervous system (CNS) is considered the most viable method. The nanocarriers for drug delivery are transferred to the brain via receptor-mediated transcytosis (Sharma et al. 2021). In vivo and in vitro studies on levofloxacin-/doxycycline-loaded solid lipid nanoparticles against bacterial meningitis (Abdel Hady et al. 2020), gentamicin-loaded polymeric nanoparticles against Pseudomonas aeruginosa (Abdelghany et al. 2012), ansamycin-loaded polymeric nanomaterials (Nair et al. 2020b) and ofloxacin-loaded nano-transfersomes against bacterial meningitis (Eid et al. 2019), recombinant protein OmpAVac-loaded chitosan-modified poly(lactic-co-glycolic acid) (PLGA) nanoparticles against E. coli K1 in neonatal meningitis-infected mice (Zhang et al. 2021) and bacitracin A and brain-targeting peptide (BTP)-loaded polymeric nanoparticles against Pneumococcal meningitis (Hong et al. 2018) have been investigated as drug delivery systems for their biocompatibility, controlled drug release and longer systemic circulation duration for treating brain bacterial infections.
Considering the phototoxicity and low tissue penetration, light-responsive nanomaterials are emerging with potential drug design and light-triggered controlled drug delivery systems mostly useful in photothermal therapy (PTT) and photodynamic therapy (PDT) (Zhao et al. 2019; Tang and Wang 2021). Liu et al. (2021a) developed rough carbon-iron oxide nanohybrids (RCF) for near-infrared (NIR) synergistic antibacterial therapy, resulting in increased RCF bacterial adhesion and PTT in methicillin-resistant Staphylococcus aureus (MRSA), proposing a facile strategy to construct antibacterial agents for designing drugs and medical applications. Further in vivo studies in MRSA rat wound models showed enhanced synergistic antibacterial effects revealing their potential role in treating drug-resistant bacterial infections (Liu et al. 2021b). Wang et al. (2018) developed Staphylococcus aureus-pre-treated macrophage-membrane-coated gold nanocage (Sa-M-GSNC) drug delivery system, where macrophage membrane receptors were used to achieve specific bacterial-targeted delivery under near-infrared (NIR) laser irradiation in infected mice, and this resulted in better bacterial adherence, effective delivery and retaining in infection site with prolonged blood circulation and system biocompatibility. Other than light-responsive nanomaterials, some of the alternative strategies such as pH-responsive nanomaterials, enzyme-responsive nanomaterials and redox-responsive nanomaterials are also used as drug delivery systems (Devnarain et al. 2021). Hassan et al. (2020) developed novel chitosan-based pH-responsive lipid polymer hybrid nanovesicles (OLA-LPHVs) as a vancomycin delivery system against MRSA biofilms leading to the easy release of vancomycin at pH 6.0 and inhibition of biofilms via damaging bacterial cell membrane and showing their potentials in treating bacterial infections. Enzyme-responsive nanogels developed from alginate/peptide ciprofloxacin conjugates with enhanced stability in dispersion and aqueous environment resulted in enzyme-triggered release of ciprofloxacin by degrading the peptide linkers against S. aureus (Bourgat et al. 2021). Similarly, Salamatipour et al. (2019) synthesized light-reduction-/oxidation-responsive alginate nano-hydrogels loaded with the folic acid drug by reverse emulsification-diffusion method and improved water retention capacity (WRC) under UV light that resulted in antibacterial activity against S. aureus and E. coli.
3.3 Types of Nanocarriers
3.3.1 Metal-Based
Metal nanoparticles usually have non-specific broad-spectrum bacterial toxicity mechanisms where they bind to outer membrane receptors (Yuan et al. 2018) that enhance their potencies. Metal-based nanoparticles have shown their efficacy in both Gram-positive and Gram-negative bacteria with multiple biomolecule target involved in the development of resistant strains (Slavin et al. 2017).
3.3.1.1 Silver Nanoparticles (AgNPs)
Chemical methods in the production of AgNPs include three components: a metal precursor, a reducing agent and a stabilizing agent (Singh et al. 2015). Appropriate size, shape and polydispersity of AgNPs can be achieved by monitoring experimental parameters such as precursors used in the reaction, reducing agents, reagent concentration, pH and temperature in the nucleation step during the synthesis process (Solomon et al. 2007; Dakal et al. 2016; Kumar et al. 2018b). Stabilization being the critical stage, chitosan, amine derivatives, thiols and gluconic acid have been recently used as stabilizers with polymeric compounds proven advantageous (Solomon et al. 2007). Beta-D glucose as the reducing agent has emerged as with special interest of researchers for the reduction of AgNO3 and green synthesis giving AgNO3 up to 10 nm mean size (Kumar et al. 2018b). Pal et al. (2019) studied antimicrobial peptide (AMP)-AgNP against MDR bacteria strains (Klebsiella pneumonia, Pseudomonas aeruginosa and Salmonella typhi) using combinations of AY1 (CAY1-AgNP and AY1C-AgNP) showing increased stability and antimicrobial activity. Recent investigations on tragacanth gum, N-isopropyl acrylamide and 2-(vinyloxy) ethanol-based stimuli-responsive silver nanocomposites (TGIAVE-Ag) resulted in controlled release of 5-fluorouracil against MDR bacteria (Nagaraja et al. 2021). Similarly, selective delivery of AgNP-responsive microparticles incorporated into dissolving microneedles against Staphylococcus aureus and Pseudomonas aeruginosa biofilms resulted in controlled release of AgNPs and eradication of biofilms with improved antibiofilm activities in ex vivo biofilm-infected rat skin model (Permana et al. 2021).
3.3.1.2 Gold Nanoparticles (AuNPs)
Gold nanoparticles are colloidal particles consisting of gold as a core substance with good biocompatible property. The synthetic versatility of these NPs allows them to control particle solubility, stability and interaction with the environment. Further, studies on gold nanospheres conjugated with gentamicin have shown great activity against S. aureus than gentamicin alone (Ahangari et al. 2013). Reduction of chloroauric acid followed by agglomeration in the presence of the stabilizing agent is the basic synthesis process of all chemical, biological and physical pathways (Newman and Blanchard 2006). Pathogen-specific antibodies or photosensitizing molecules for photothermal and PDT conjugated with AuNPs have also been proven to promote antimicrobial activity (Savas et al. 2018; ElZorkany et al. 2019). Flavonoid-coated AuNPs with enhanced antibacterial effects of chrysin, kaempferol and quercetin against Gram-negative E. coli bacteria resulted in bacterial cell membrane penetration and their ablation, hence making them good drug delivery candidates (Alhadrami et al. 2021). Similar to this, Punica granatum extract delivering chitosan-gold hybrid nanoparticles (CS-AuNPs) exhibited high synergistic effects against MRSA (Hussein et al. 2021).
3.3.1.3 Ceramic Nanoparticles
Ceramic nanoparticles constitute oxides, carbides, phosphates and carbonates of metals and metalloids such as calcium, titanium, silicon, etc. The favourable property of heat resistance and chemical inertness makes them suitable for their wide variety of applications in medicine where they are structured by heat and pressure (comprising of solid core and a combination of metal/non-metal, at least two non-metallic elemental solids, at least one metal and a non-metallic elemental solid or a non-metal) (Wu and Zreiqat 2010). Depending upon architectural differences, they are further categorized into ceramic nanoparticles, ceramic nano-scaffold and nano-clay and are made up of ceramic compounds such as silica-titania and alumina (Rawat et al. 2008). Nano-scaffolds are defined as a structure that allows interactions of cells and extracellular matrices with microporosity (pore size >50 nm), whereas nano-clay resembles thin layers having a thickness of few nanometres. These ceramic nanoparticles can be synthesized with microemulsion preparation, hydrothermal synthesis, sol-gel process, aerogel method, pechini-citrate gel method and low-temperature combustion (LCS) methods (Singh et al. 2016). In a recent study on biphasic calcium phosphate (BCP), a biocompatible and non-immune-responsive biphasic ceramic was used to synthesize silver-doped BCP/alginate (AgBA) microcluster stating their inhibitory action on S. aureus and E. coli (Nie et al. 2021). In one of the studies, the in vitro release profile of vancomycin-loaded hydroxyapatite compared with the pure vancomycin-HCl with increased antibiotic-loaded hydroxyapatite release rate and antibacterial activity (zone of inhibition 11.5 ± 0.5 mm and 15 ± 0.4 mm) in S. aureus and E. coli, respectively, when compared to antibiotic alone (Ain et al. 2020). Similarly, zirconia nanoparticle green synthesized using L. nobilis were found to be more effective against Gram-negative pathogenic bacteria (Chau et al. 2021). Chauhan (2021) reviewed the distinctive benefits of ceramic-based hybrid nanoparticle as a drug delivering system.
3.3.1.4 Silica Nanoparticles
Because of large surface area, ease of functionalization and biocompatibility, silica nanoparticles are commonly used in drug delivery applications. The mesoporous silica nanoparticles (MSN) are the porous variant that confers amenities and have been recently demonstrated as a powerful drug delivery tool for combating bacterial infections (Şen et al. 2018; Martínez-Carmona et al. 2018; Bernardos et al. 2019; Selvarajan et al. 2020). Synthesis of silica nanoparticles is carried out by Stober’s method (Stober et al. 1968) and the microdilution method. Modifications in Stober’s process have been performed to suit user-specific requirements such as usage of low-cost precursor (sodium silicate solution instead of tetraethyl orthosilicate) (Zulfiqar et al. 2016a, b). Another method, the microdilution, involves the formation of oil-in-water (O/W) micelles and water-in-oil (W/O) reverse micelles (Arturo Lopez-Quintelá 2003) stabilized using surfactants (twins or pluronics) acting as nanoreactors to synthesize nanoparticles depending upon the nanoreactor volume (Selvarajan et al. 2020). As peptides can be loaded using silica, Kwon and his team used the tandem peptide cargo made of lactoferrin and a synthetic bacterial toxin D [KLAKLAK]2 for treating Pseudomonas aeruginosa infection in lungs (Kwon et al. 2017). Stewart et al. (2018) reported a lower drug release rate for a longer period compared to the initial burst release of the conventional drug formulation using co-assembly of an antimicrobial drug (octenidine dihydrochloride, OCT) and silica with the loading efficacy of 35%. Similarly, a nanoantibiotic system made of MSN loaded with levofloxacin (LEVO) was designed with anti-biofilm activity against S. aureus resulting in cell destruction (Pedraza et al. 2018). Further, effective penetration of LEVO-loaded MSN grafted with poly(propyleneimine) dendrimer of third generation (G3) in the cellular membrane of E. coli was reported with excellent anti-biofilm activity (González et al. 2018).
3.3.2 Liposome-Based
Liposomes are composed of lipids. Due to their similar structure and composition of the cell membrane, they are used for bacterial cell targeting that can carry both hydrophobic and hydrophilic antimicrobials and thus offering a wider choice of antimicrobial candidates to be loaded. Liposomes show fusogenicity property as they have a phospholipid bilayer structure which upon fusion with antimicrobials is directly available inside a bacterium. The most important factor of liposomes in in vivo investigations is the diameter, so to avoid rejection of liposomes by the reticuloendothelial system and allowing penetration through water channels in infectious biofilms, they should preferentially have a diameter in the range of 100–200 nm (Ferreira et al. 2021). Realization of biofilm targeting from the blood circulation, penetration and accumulation over the entire thickness of an infectious biofilm, associated with deep killing in the biofilm, are some of the challenges in the development of liposomal antimicrobial nanocarriers (Wang et al. 2020). Sanches et al. (2021) demonstrated the potential use of rhamnolipid-based liposomes as nanocarriers against E. coli and S. aureus with high haemolytic activity and negligible cytotoxicity (highest concentration of 1.3 mmol L−1) to HepG2 cells. Liposomes have also been identified as one of the major antimicrobial agent (meropenem, PEG, triclosan, benzyl penicillin, zinc citrate) delivery systems for treating bacterial biofilm-mediated infections (Wang 2021).
3.3.3 Quantum Dots (QDs)
The ultra-small size semiconductor nanocrystals, with the average size in the range of 1.5–10 nm, are defined as the quantum dots and are synthesized from group II–VI elements in the periodic table depending on their conductive properties and high surface to volume ratios. Due to their unique physical and chemical properties of QDs such as high stability, exceptionally narrow range of emission and high quantum yield, they are used in biosensors, real-tracking, multipolar labelling and imaging (Jahangir et al. 2019; Wang et al. 2019). Polymer-functionalized QDs give QD a promising feature with higher antibacterial activity. Based on structural dimensions (spherical, pentagonal and hexagonal) and size, QDs can be tuned with the ligands and polymer, and thus modified GQDs facilitate the attachment of GQDs to bacterial membrane. For example, PEGylated GQDs exhibited 100% growth inhibition for S. aureus and P. aeruginosa following 8 h of incubation (Habiba et al. 2015). Reports on antibiotics conjugated with QDs (ceftriaxone conjugated to CdTe QDs) with increased antibiotic efficiency have demonstrated the synergistic antimicrobial effect against E. coli (Luo et al. 2011). Recently, gentamicin (GEN)-loaded mesoporous silica nanoparticle sealed with acid-decomposable 3-mercaptopropionic acid capped-ZnS QDs (MPA-ZnS QDs) resulted in controlled release of GEN drug against E. coli (strain 0157:H7) and S. aureus (strain ATCC:25923) (Mandani et al. 2021).
3.3.4 Biopolymeric Nanomaterials
Polymers derived from living organisms are said to be biopolymers and are made up of several monomeric units forming macromolecular polymer structures with covalent bonds. Rational selection of biopolymers is the most important challenge in controlled drug delivery systems which necessitate a comprehensive understanding of surface and bulk characteristics of biopolymers to achieve optimum therapeutic efficacy. Chitosan (CS) is the most common linear polysaccharide derived from naturally occurring chitin and is mainly extracted from crustacean shellfish and certain fungi. Chemically, it consists of N-acetylglucosamine and glucosamine joining together with the beta-1-4 linkage, giving a positive charge under acidic pH (Kumar 2000; Rinaudo 2006). Chitosan is often chemically modified at amino or hydroxyl groups to make them more effective and widen their medical applications (Rabea et al. 2003; Verlee et al. 2017; Sahariah and Másson 2017). Antimicrobial chitosan is prepared mainly via quaternarization and carboxylation to improve its solubility and antimicrobial activity with the maintenance of its biodegradability and biosafety. Essential oils such as rosemary essential oil when nanoencapsulated on chitosan/polyglutamic acid nanoparticles resulted in a significant increase of the antibacterial activity against B. subtilis (Lee et al. 2019a). Bacterial cellulose combined with ZnO-NPs was analysed for the healing property (Mihai et al. 2019).
3.3.5 Dendrimers
Dendrimers are synthetic polymers with a large number of exposed anionic, neutral or cationic functionalities on the surfaces formed by the branched repeating units that emerge from a focal point (Lyu et al. 2019). Carbon, nitrogen and phosphorus as central atoms of dendrimer play an important role in determining the structure, branches and cavities (Elsabahy and Wooley 2012; Kulthe et al. 2012; Fox et al. 2018). Further, the structural specificity of dendrimers allows attachment of compounds and drug molecules to the outer surfaces of dendrimers with final inclusion inside the cavities, which helps in encapsulation and conjugation (Pandurangan et al. 2016; Kim et al. 2018).
Dendrimers can be used in combination with traditional drugs, besides their structures can be formulated based on the pharmacodynamics and pharmacokinetics of the drug (Authimoolam and Dziubla 2016). In an interesting study, poly(amidoamine) (PAMAM) dendrimers conjugated with fluoroquinolones (nadifloxacin and prulifloxacin) showed enhanced antimicrobial activity and water solubility (Kuwahara et al. 2005; Cheng et al. 2007). Further studies on nanodendrimers conjugated with erythromycin significantly showed delivery of erythromycin with four times lesser minimum inhibitory concentration (MBC) against P. aeruginosa, 2 times lower against S. aureus and 16 times lower against S. epidermis (Xue et al. 2013).
3.3.6 Photothermally Activated Nanomaterials (PANs)
PANs are the broad spectrum of nanoparticles that convert absorbed light into heat. Resonance oscillation of the surface electron (surface plasmon) or energy of band transition gives the thermal effect to the nanoparticles. These nanoparticles produce thermal relaxation which leads to temperature increase, and their effect depends on many factors such as irradiation intensity, wavelength, the concentration of nanoparticles and photothermal conversion efficacy (Borzenkov et al. 2019). In a recent study, a chitosan-based hydrogel with embedded gold nanorods under low-power diode laser irradiation showed antimicrobial activity against both Gram-positive and Gram-negative bacteria including MDR strains (Bermúdez-Jiménez et al. 2019). Another study on the photothermal effect of phospholipid-coated gold nanorods loaded into a poloxamer 407 hydrogel resulted delivery of poloxamer 407 in ≈4.5–5 log cycle reduction of P. aeruginosa biofilm (Al-Bakri and Mahmoud 2019).
3.3.7 Carbon-Based Nanomaterials
3.3.7.1 Graphene-Based Nanomaterials
Graphene is the thinnest two-dimensional crystal sheet of single-layer sp2 carbon (Goenka et al. 2014). Graphene nanomaterials are comprised of graphene oxide (GO), reduced graphene oxide, single layer, bi-layer graphene and multilayer graphene. Graphene-based nanostructures have wide applications including antimicrobial coatings, cellular targeting, biosensor, wound dressings, etc. The antimicrobial activity of GO increases after the reduction of sheet area. As GO is also a semiconductor material, hence it can be utilized for catalytic disinfection once exposed to UV-visible irradiation. Functionalization of GO with antibiotics, metallic compounds, immunoglobulins, chemotherapeutics, metallic nano-compounds and other organic/inorganic functionalities such as amine and carboxyl is comparatively easy because of its chemically reactive oxygen groups (carboxylic acid, hydroxyl and epoxy groups) (Sun et al. 2018a; Zarafu et al. 2018). Sharp edges of GO make it capable of killing bacteria through direct contact interactions. This mechanism of killing bacteria is called ‘trapping’ and ‘nanoknife’ mechanisms. DNA aptamer-conjugated magnetic graphene oxide (Apt@MGO) for rapid eradication of MRSA superbugs via generation of heat and cell death (~78%) under NIR laser irradiation considering them as biocompatible and light-activated photothermal agent for efficient ablation of MRSA (Ocsoy et al. 2021). Antibacterial activity of three-dimensional porous self-assembled graphene-based composite and VA-laden RGO-nHA composite scaffold (VA@RGO-nHA) against S. aureus with controlled release of vancomycin was reported using the S. aureus-infected bone by Weng et al. (2017).
3.3.7.2 Carbon Nanotubes (CNTs)
The size and surface area of carbon nanotubes are inversely proportional to each other which enhances the cell damage and subsequent cell death (Wang et al. 2016; Costa et al. 2020). Functionalization and modification help CNTs to improve their biocompatibility and dispersibility and to optimize their antimicrobial property (Rebelo et al. 2016). Enhanced antimicrobial activity of multiwall layer CNTs (MWCNTs) has been observed when functionalized with amino acids such as lysine and arginine. Antimicrobial activity of antibiotic ciprofloxacin can also be improved when coated with the single-wall CNTs (SW-CNTs) resulting in increased bactericidal activities against S. aureus and P. aeruginosa by 16-fold and E. coli by 8-fold, compared to ciprofloxacin alone (Assali et al. 2017).
3.3.7.3 Fullerenes
Fullerenes are ball-shaped molecules, C60 being the most common fullerene. Amphiphilic fullerenes are widely used as drug nanocarriers because of their biocompatibility and cage-like structure (Tan et al. 2017). Fullerene has also being used in PDT to treat drug-resistant bacteria, for instance, against P. aeruginosa in the form of its derivatives like fulleropyrrolidinium salts and sulfobutyl fullerene (Hamblin 2016). Photochemical activity and antimicrobial activity of fullerenes as drug carriers upon exposure to light via ROS have been studied in Gram-positive bacteria such as Streptococcus pyogenes (Kazemzadeh and Mozafari 2019).
3.3.7.4 Carbon-Based Nanodots
Carbon nanomaterials, such as graphene quantum dots and carbon nanodots with zero-dimensional, are celled as carbon-based nanodots (Manisha et al. 2019). Carbon quantum dots are electrically conductive materials and hence can be used with various antimicrobial materials (Miao et al. 2015). Carbon nanodots synthesized via top-down or bottom-up approaches with a diameter <10 nm have been investigated for loading ciprofloxacin hydrochloride for their antimicrobial activity. These ciprofloxacin hydrochloride-loaded carbon nanodots exhibited enhanced antimicrobial activity against both Gram-positive and Gram-negative bacteria (Thakur et al. 2014). Recent reports on enhanced antimicrobial activity of CDs via green synthesis medicinal turmeric leaves (Curcuma longa) against Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli and Klebsiella pneumoniae resulted in effective delivery of phytochemicals and reactive oxygen species (ROS) production leading to cell death (Nair et al. 2020a; Saravanan et al. 2021).
3.3.7.5 Carbon Nitride Nanomaterials
Graphite C3N4 (g-C3N4) is a metal-free photocatalyst. A study on strong mesoporous g-C3N4 which were manufactured with the cyanimide as raw material and silica as a template showed good inactivation of E. coli under visible irradiation (Huang et al. 2014). Modification of graphite ‘carbonitrides’ with other antimicrobial agents such as aerobic conditions (caused by photocatalytic oxidative inactivation) and under anaerobic circumstances (caused by photocatalytic reductive inactivation), co-rapping of g-C3N4 and reduced graphene oxide sheets have been reported to destroy bacteria (Wang et al. 2013). Graphitized carbonitride (g-C3N4) nanosheets with embedded AgNPs improved the generation of photoelectrons and thus proved to be effective antibacterial agents (Bing et al. 2015).
3.4 Antimicrobial Agents and Their Inhibitory Mechanisms
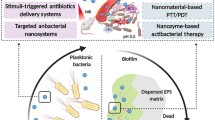
Antimicrobial agents destroy bacteria by interfering with their bacterial growth/survival/reproduction mechanisms. Various antimicrobial agents such as antibiotics/drugs, AMPs, phytochemicals and metal-based nanomaterials are used as or in delivery systems to treat microbial infections (Patra et al. 2018). These antimicrobial agents show specific inhibition mechanisms against bacteria as illustrated in Fig. 3.1 and Table 3.1.
3.4.1 Antibiotics
Antibiotics represent the most common antimicrobial agents that exert their effects by targeting major bacterial mechanisms such as cell wall synthesis, DNA synthesis, protein synthesis, DNA damage and mRNA synthesis and can be classified into various groups based on the mode of action (bacteriostatic or bactericidal) and their origin, route of administration, range of action (broad-spectrum or narrow-spectrum) and chemical structure (Table 3.2). β-Lactam antibiotics are the bactericidal agents that contain β-lactam ring in their molecular structures and interrupt bacterial cell wall formation by binding covalently to penicillin-binding protein (PBP) enzyme involving the terminal step of peptidoglycan cross-linking in both Gram-positive and Gram-negative bacteria (Bush and Bradford 2016) and include penicillins, cephalosporins, carbapenems and monobactams. Penicillins further can be broadly classified into four different groups: natural penicillins, aminopenicillins, extended-spectrum penicillins and penicillinase stable penicillins. Cephalosporins like penicillins are β-lactam antibiotics developed from cephalosporin C (a natural product of Cephalosporium acremonium). Successive modification of cephem ring structure has led to the ‘generations’ of cephalosporin to be divided into first, second, third, fourth and fifth generations. Carbapenems are derivatives of thienamycin from Streptomyces cattleya and differ from penicillins with replacement of sulphur by methylene group in a five-membered ring of β-lactams, further represented by meropenem, doripenem, ertapenem and imipenem. Monobactam is characterized by a non-fused β-lactam nucleus that differs from penicillins, cephalosporins and carbapenems including Aztreonam (Paris 2012). Aminoglycoside antibiotics are bactericidal agents structurally characterized by the presence of amino sugars attached to an aminocyclitol ring by glycosidic bond (Dasenaki and Thomaidis 2017) that include neomycin, amikacin, kanamycin, gentamicin and tobramycin (Shriram et al. 2018). Aminoglycosides inhibit protein synthesis in bacteria by irreversibly binding to the 30S ribosomal subunit, preventing the transfer of aminoacyl-tRNA to the peptidyl site, causing premature termination of the peptide chain and also increasing the frequency of mRNA misreading (Waller and Sampson 2018). Tetracyclines are usually considered as bacteriostatic antibiotics characterized chemically by a linear fused tetracyclic nucleus that inhibits bacterial protein synthesis by binding to 16S rRNA of 30S bacterial ribosomal subunit, arresting translation by interfering with the docking of incoming aminoacyl-transfer RNA (tRNA) at the acceptor site (A site) (Grossman 2016; Markley and Wencewicz 2018). Tetracycline antibiotics are broad spectrum in activity, spanning a wide range of Gram-positive and Gram-negative bacteria, obligate intracellular bacteria, protozoan parasites, chlamydia, mycoplasma, rickettsia and spirochetes and are represented by tetracycline, minocycline, demeclocycline and doxycycline. Streptogramins (pristinamycin, mikamycin, virginiamycin and quinupristin-dalfopristin) are composed of two structurally different components, A and B. A component (pristinamycin IIA, mikamycin A or dalfopristin, virginiamycin M) is polyunsaturated macrolactones, and B component (pristinamycin IB, mikamycin B or quinupristin, virginiamycin S) is a cyclic hexadepsipeptide (Schwarz et al. 2016). Component A interferes with polypeptide elongation by preventing binding of aminoacyl-tRNA to ribosome whereas component B destabilizes the peptidyl-tRNA resulting in enhanced bactericidal activity (Lee 2006).
Macrolides are a different group of compounds that has a lactone ring (14–16 atoms) bonded to one or more deoxy sugar and classified according to the number of carbon atoms in the lactone ring; 14 membered includes erythromycin, roxithromycin, troleandomycin, clarithromycin and dirithromycin, whereas 15 membered includes azithromycin and 16 membered includes spiramycin, josamycin, midecamycin and spiramycin (Kuruvilla 2018). Macrolide antibiotics inhibit protein synthesis by targeting bacterial ribosomes, further binding at nascent peptide exit tunnel and partially occluding it. Thus macrolides are viewed as ‘tunnel plugs’ that stop protein synthesis (Vázquez-Laslop and Mankin 2018). Another class of antimicrobial agents, the lincosamides, are derived from Streptomyces spp. Lincosamide structure consists of three components: an amino acid (L-proline substituted by a 4′-alkyl chain), a sugar (lincosamide) and an amide bond connecting these two moieties (Kwon 2017). Lincosamides inhibit protein synthesis by binding to 50S subunit at a site that overlaps both P and A sites on the bacterial ribosome, preventing charged tRNA docking and their movement through the peptidyl transferase centre (Sauberan and Bradley 2018). Lincomycin, clindamycin and pirlimycin are three antibiotics present in the lincosamide group.
Besides macrolides, antibiotics and lincosamides, the quinolones are another family of synthetic antimicrobial drugs that have been reported to be effective against various bacterial infections. The first quinolone reported, nalidixic acid, was introduced in 1964, and its further chemical manipulation and advancements resulted in the development of fluorinated quinolones (fluoroquinolones) that includes danofloxacin, difloxacin, marbofloxacin, orbifloxacin, enrofloxacin, ciprofloxacin, moxifloxacin and levofloxacin. The major mechanism involved in the inhibition of topoisomerase II (DNA gyrase and topoisomerase IV), regulates under-winding and over-winding of DNA. The binding of quinolones to enzyme-DNA complex results in the conformational changes of enzyme further inhibiting relegation of broken DNA strands leading to the bactericidal effect. Besides quinolones, sulfonamides are one of the oldest groups of antibacterial agents introduced into medical practice even before the discovery of penicillin and have broad-spectrum use concerning both Gram-negative and Gram-positive microorganisms. Sulfonamide drugs are the structural analogs of para-aminobenzoic acid (PABA), an essential component in the folic acid pathway. Sulfonamides inhibit the bacterial dihydropteroate synthetase (DPS) enzyme of the folic acid pathway, blocking bacterial nucleic acid synthesis. Sulfonamides also contribute in preventing the conversion of PABA to dihydrofolic acid by substituting competitively for PABA. Combinations with trimethoprim have also shown an excellent bactericidal effect. Trimethoprim inhibits dihydrofolic acid reductase thereby preventing the subsequent conversion of dihydrofolic acid to tetrahydrofolic acid thus blocking two successive steps in the folic acid pathway and exhibiting enhanced bactericidal effect (Ahern and Richardson 2012). Metronidazole and tinidazole are the main representatives of nitroimidazoles. Metronidazole is active against some anaerobic bacteria (e.g. Clostridium difficile), protozoan infections and microaerophilic bacteria (Gardenia vaginalis and helicobacter pylori). Metronidazole first diffuses across the membrane and then gets reduced by intracellular protein under anaerobic conditions, hence exerting its effect through cytotoxic intermediate and free radical’s formation that provoke DNA damage (Bury-Moné 2014).
Apart from the wide-spectrum activity and fast-action advantages of antibiotics, they face some disadvantages such as side effects, hypersensitivity, drug interaction and toxicity and negative effect on commensal microflora (Weledji et al. 2017). In addition to injudicious usage of conventional and commonly available antibiotics in human health, veterinary agriculture further adds to the evolution, persistence and spread of AMR with emergence of new drug-resistant bacterial strains at a frightening rate resulting in the inefficacy of existing drugs with very few or no solutions in sight. Therefore, to successfully combat the escalating problem of AMR, novel and effective antimicrobial agents are recommended such as phytochemicals, metal, metal-based complexes, metallic nanoparticles and AMPs.
3.4.2 Antimicrobial Peptides (AMPs)
AMPs are broadly defined as ‘naturally occurring polypeptide sequences of 12–15 residues comprising cationic and hydrophobic amino acid with direct antibacterial activity’ (Li et al. 2021). AMPs are produced by all organisms ranging from bacteria, plants, invertebrates and vertebrates and have a wide range of inhibitory effects against fungi, bacteria, viruses and parasites (Kumar et al. 2018a). AMPs have several advantages over conventional antibiotics showing the multifunctional mechanism of antibacterial action altering cell membrane and also attacking specific targets that take part in the development of different intracellular processes such as bacterial cell wall formation, transcription and translation that has antimicrobial activity against multidrug-resistant pathogens (León-Buitimea et al. 2020).
AMPs are found to be highly effective against Gram-negative bacteria which are more challenging to treat than their Gram-positive counterparts because of the outer membrane composition in the earlier that makes them impermeable to most of the conventional antibiotic drugs. AMPs are often introduced in literature as a ‘promising alternative to antibiotics’ and ‘potential to address the growing problem of antibiotic resistance’ and ‘hold promise to be developed as novel antibiotics’ (Li et al. 2021) because of a non-specific mechanism involving membrane target, oxidative damage, damage to intracellular molecules, potent microbicidal activity in the micromolar range and rapid drug action increasing difficulty in resistance development because of limited time for extensive mutation and growth (Koo and Seo 2019). In addition, AMPs are also known as host defence peptides (HDPs) as they can also enhance immune response highlighting the clinical potential of AMPs to stimulate innate immunity (Li et al. 2021). AMPs such as HPA3P (Helicobacter pylori-derived AMP) loaded onto a gold nanoparticle-DNA aptamer (AuNP-Apt) conjugate (AuNP-Apt-HPA3PHis) when utilized against Vibrio vulnificus resulted in HPA3PHis-induced bacterial cell death via disruption of membrane integrity and 100% survival rate in Vibrio vulnificus-infected mice resulting in complete inhibition of Vibrio vulnificus colonization, hence displaying effective drug delivery of AMPs (Lee et al. 2017).
AMPs are commonly known for non-receptor-mediated membrane-lytic bactericidal activity. Membrane-targeting mechanisms of AMPs can be described through pole and carpet models, barrel-stave models and toroidal pore models (Fig. 3.2). In the toroidal-pore model, the initial binding of the peptide to the membrane is followed by cascade aggregation of incoming monomer units, causing the lipid moieties of inner and outer membranes to fold inward, forming continuous channels lined by multiple peptide units and thus tightly associating lipid head groups of membrane phospholipids with peptides. A typical example of this model includes magainin 2, lacticin Q, arenicin and melittin (Huan et al. 2020). However, the barrel-stave model differs from the toroidal pore model by the peptide monomers inserted into the membrane arranged parallelly to phospholipid molecules of the membrane. Besides membrane penetration and pore formation, AMPs have another mechanism of action which includes inhibition of protein synthesis by affecting transcription, translation, protein folding and assembly of newly synthesized proteins. For example, PR-39, a proline, and arginine-rich AMP isolated from pigs’ small intestine were found primarily to penetrate rapidly into E. coli outer membrane that led to protein synthesis inhibition and degradation of the protein (Boman et al. 1993). Following penetration, inhibition of nucleic acid biosynthesis occurs by affecting the key enzymes of DNA synthesis or inducing degradation of the nucleic acid molecule. By inhibiting the DNA replication, DNA damage response (SOS response), causing chromosomal separation failure blocking cell cycle, and inhibiting cell division is the process of AMPs. Cruz et al. (2020) identified 40-amino acid residue MciZ as an effective inhibitor of bacterial cell division, Z-ring formation and localization. Histatin, eNAP-2 and indolicidin were also found to have strong protease inhibition mechanisms (Huan et al. 2020). Similarly, investigations on NP-6 from Sichuan pepper seeds showed inhibition of beta-galactosidase activity in E. coli (Hou et al. 2019). These multifunctional mechanisms of antibacterial action thus highlight the AMPs as a promising alternative to antibiotics.
3.4.3 Phytochemicals
Plants produce a wide array of phytochemicals that have been utilized for centuries in ethnomedicine or folk medicines. Phytochemicals are compounds that occur naturally in plants as secondary metabolites (Bai et al. 2011) and can be classified into many major classes depending upon the chemical structure (alkaloids, polyphenols(flavonoids and non-flavonoids), terpenoids, sulphur-containing phytochemicals), biosynthetic pathways, biological pathways and botanical origins (Górniak et al. 2019; Belščak-Cvitanović et al. 2018). Two major sub-classes of phenolic acid include hydroxybenzoic acid (e.g. gallic acid, vanillic acid, protocatechuic acid, salicylic acid, syringe) and hydroxycinnamic acid (e.g. chlorogenic acid, coumaric acid, caffeic acid, ferulic acid curcumin, caftaric acid, cinnamic acid) (Flamini and De Rosso 2018). Similar to phenolic acids, tannins are a group of structurally complex polyphenols comprising condensed (proanthocyanidins) and hydrolyzable tannins that can form complexes with proteins by non-specific interactions. Therefore, displaying antimicrobial activity may be associated with their potential to denature microbial transport protein, adhesins and microbial enzymes preventing microbial growth through deprivation of metal ions and substrates (Gupta and Pandey 2019). Bacterial cells can be affected by phytochemicals in several ways due to the greater diversity displayed by phytochemicals. The major mechanism of phytochemicals action includes membrane permeabilization, cell membrane disruption, EP inhibition, inhibition of biofilm formation and quorum sensing, targeting resistant plasmid, inhibition of cell division and DNA and protein synthesis (Table 3.1) (Navarro-Martínez et al. 2005; Gradišar et al. 2007; Domadia et al. 2008; Wu et al. 2008; Boulet et al. 2018). For instance, studies have shown enhanced bactericidal activity of thymol against S. aureus and E. coli by encapsulating thymol in hollow mesoporous silica sphere with cell membrane disruption as an inhibitory mechanism of action, thus highlighting enhanced resistance reversal potential antimicrobial agent when combined with nanocarriers (Liu et al. 2021a) that could speed up the successful application of antimicrobial agents in clinical settings.
Similarly, essential oils are known for their broad-spectrum antimicrobial potentials mainly attributable to their abilities of targeting major determinants of drug resistance, pathogenicity and spread, which include EPs, cell membrane, quorum sensing, resistant plasmids and biofilms. Recent reports confirm that essential oils show both direct killing (bactericidal) or re-sensitizing (or resistance-reversal) potentials providing effective solutions for tackling AMR and the potential to rejuvenate or replace otherwise fading antibiotic arsenal (Yu et al. 2020). Recent years have witnessed the use of nanomaterials as synergistic agents with essential oils as well as their carriers. Montmorillonite nanosheet-based (MMT-based) drug nanoplatform involving antibacterial metal copper ions, quaternized chitosan (QCS) and antibiotic 5-fluorocytosine (5-FC) [QCS/MMT/5-FCCu] strongly inhibited S. aureus, E. coli and Candida albicans with high drug-loading capacity, excellent wound healing and good biocompatibility in a mouse model infected with wound demonstrating enhanced killing effect against both bacteria (Sun et al. 2019). Similarly, cinnamaldehyde-loaded liposomes decorated with chitosan also showed strong antibacterial efficacy against S. aureus by damaging cell membrane integrity, causing cell death by leakage of intracellular components (Wang et al. 2021).
3.4.4 Metals, Metal-Based Complexes and Metallic Nanoparticles
Since ancient times, antimicrobial activities of metals such as silver (Ag), gold (Au), copper (Cu) titanium (Ti), mercury (Hg) and tellurium (Te) consisting of different properties defining the spectrum of activity and potencies are known that are used as antimicrobial agents because of their microbiocidal activity at extremely low concentration. Previous reports on E. coli and S. aureus treated with AgNO3 resulted in losing their replication ability and protein inactivation resulting in strong antibacterial activity of metals (Woo et al. 2008). The major mechanism of antibacterial action of metals includes production of ROS, impairing membrane function, interfering with nutrient assimilation, inducing genotoxicity, protein dysfunction and loss of enzyme activity (Lemire et al. 2013). For example, tellurite (TeO3 2−) toxicity in E. coli by treatment of K2TeO3 in E. coli leads to superoxide formation (Pérez et al. 2007). Similar results with loosening of cell walls, cytoplasmic aggregation and cell wall rupture were observed when Erwinia carotovora subsp. atroseptica was treated with aluminium chloride resulting in increased mortality (Yaganza et al. 2004). Further, as there is a chemical similarity between iron (Fe) and gallium (Ga), Ga can substitute Fe in a different biological system and inhibits Fe-dependent processes, for example, inhibition of growth, biofilm formation and death of P. aeruginosa by Ga-induced reduced uptake of Fe and reduced expression of genes involved in Fe uptake suggesting the importance of Ga in interference of nutrient assimilation. In addition, since Ga is FDA approved for intravenous (IV) administration suggesting Ga as potentially promising therapeutics in the dearth of new antibiotic development (Kaneko et al. 2007).
Treatment of E. coli (lacking copper homeotic system) with copper metal resulted in rapid inactivation of isopropyl malate dehydratase (an iron-sulphur cluster enzyme in the pathway of branched-chain amino acid synthesis) damaging essential enzymes of biosynthetic pathways (Macomber and Imlay 2009). In addition to this, metals when used in nanoformulations or complexed with other antimicrobial agents such as phytochemicals, antibiotics and synthetic metal complex show greater inhibitory effects against bacteria compared to their free ligand, exhibiting potent broad-spectrum antimicrobial activity, with low toxicity (Lemire et al. 2013). For example, when a metal complex of Ga and flavonoid quercetin (metal complex 1) and H2bbppd and Cu(II) (metal complex 2) were evaluated against Staphylococcus aureus (ATCC SP 25923), Escherichia coli (ATCC SP 11229), Enterococcus faecalis (ATCC SP 19433) and Pseudomonas fluorescens (ATCC SP 13525), both metal complex showed greater inhibitory effects as compared to their ligand with lower MIC ≤250 μg/ml, confirming broad-spectrum strong antibacterial activities.
3.5 Nanomaterial-Based Antimicrobial Delivery Targeting Drug-Resistant Determinants
3.5.1 Bacterial Cell Membrane
The first line of defence in bacteria is the cell membrane that maintains the necessary osmotic balance between the outer environment and the cytoplasm (Yeh et al. 2020). Various nanomaterials have been found interacting with the bacterial cell membrane to increase the membrane permeability via the generation of ROS and production of radicals [singlet oxygen (1O2), electrons (e−), hydroxyl radicals (●OH) and superoxide radicals (O2●−)] (Wang et al. 2017). As an alternative to traditional antibiotics, photothermally active nanomaterials have emerged as a potential drug delivery system to target bacterial drug-resistant determinants (Borzenkov et al. 2020; Kaur et al. 2021). Multifunctional drug delivery nanoparticle (MDD-NP) and crystalline ruthenium polypyridine nanoparticles (Sph-Ru-MMT@PZ) consisting of adhesive and surface-anchoring properties, under 670 nm red irradiation therapy (R-IT), resulted in bacterial destruction and cell lysis of E. coli via ROS production (Yin et al. 2021). Further in vivo studies in mice revealed synergistic anti-infective effects of nanoparticles, hence promoting wound healing. Vancomycin-encapsulated, pH-responsive, surface charge-switching poly(D,l-lactic-co-glycolic acid)-b-poly(l-histidine)-b-poly(ethylene glycol) (PLGA-PLH-PEG) nanocarriers demonstrated pH-sensitive NP binding to bacteria (pH 6.0) and drug delivery to bacterial cell membrane of S. aureus causing cystic fibrosis with an 1.3-fold increase in MIC (Radovic-Moreno et al. 2012). A study on controlled release of drug at the injection site was conducted with kanamycin-loaded TiO2 nanotubes (NTs) under NIR irradiation via disrupting the bacterial cell membrane integrity by damaging bacterial cell wall and radical-induced inflammation and cytotoxicity resulting in ≥99.9% reduction in E. coli (Xu et al. 2021). Similar results were observed in eco-friendly chitosan-based nanoantibiotic system (LD@CN/DA) for potential delivery of linezolid (LD) with 3,5-dinitrosalicylic acid (DA) as antimicrobial agents with 98.4% drug release efficiency against MRSA, E. coli and E. faecalis resulting in the formation of ROS and enhancing pathogen-specific activity (Teaima et al. 2020).
3.5.2 Biofilms
Human infections can be caused by bacteria that are in the form of biofilms, planktonic cultures and intracellular residence depending on their surroundings and growth parameters (Yeh et al. 2020). Biofilms are well-organized community of bacteria that adhere to the host cells to protect themselves from the harsh environmental, physiological conditions and action of antibiotics (Sharma et al. 2019). Recent reports on worldwide human infections caused by biofilms have crossed 60% making them the primary cause of various treatment failures in medicine (Huang et al. 2021). Therefore, biofilms have emerged as one of the major resistance mechanisms and spreading AMR. Recent years have witnessed the successful applications of nanomaterials in eradicating biofilms as well as in carrying effective anti-biofilm agents.
Endophthalmitis is defined as the bacterial infections caused by various microorganisms inside the eye vitreous and aqueous humour (Durand 2013). Chen et al. (2019) studied the eradication of E. coli, S. aureus and MRSA biofilms causing endophthalmitis using ammonium methylbenzene blue-loaded pH-responsive zeolitic imidazolate framework-8-polyacrylic acid (ZIF-8-PAA) modified with AgNO3 and secondary modification of vancomycin/NH2-polyethylene glycol (Van/NH2-PEG) composite nanomaterial (ZIF-8-PAA-MB@AgNPs@Van-PEG). Further in vitro retinal pigment epithelium cellular experiments and in vivo mice endophthalmitis models resulted in effective drug release, biocompatibility and antibacterial efficiency of composite nanomaterial against biofilm-causing bacteria (Chen et al. 2019). Pseudomonas aeruginosa, another pathogen found in adult patients infected with cystic fibrosis (CF), is the major biofilm-forming bacteria (Davies 2002). The development of novel aerosolized ciprofloxacin-loaded poly(lactic-co-glycolic (PLGA) acid) nanocarriers onto the in vitro model of Pseudomonas aeruginosa biofilm-infected human bronchial epithelial cells resulted in the eradication of planktonic bacteria and reduced biofilm fraction by log 6 revealing their potential avenues in preclinical studies (Juntke et al. 2021).
Nitric oxide has emerged as a promising agent for disrupting biofilms and promoting wound healing (Englande and Friedman 2010). Hasan et al. (2019) developed polyethyleneimine/diazeniumdiolate (PEI/NONOate)-doped PLGA nanoparticles (PLGA-PEI/NO NPs) against MRSA biofilm of diabetic wounds resulting in binding of NPs to biofilm matrix facilitating NO delivery and enhanced anti-biofilm activity. Further in vivo studies in MRSA biofilm-infected wounds in diabetic mice accelerated healing via biofilm binding NO release from NPs (Hasan et al. 2019). Amikacin and ciprofloxacin drugs encapsulated in liposomes have shown their effective penetration abilities in P. aeruginosa biofilms (Zhang et al. 2018; Chalmers et al. 2021). Besides liposomes, AMP-based nanocarriers have greatly enhanced their medicinal benefits by improving stability, solubility and in vivo half-life in various pulmonary, gastrointestinal and wound infections (Song et al. 2021) (Table 3.3).
3.5.3 Efflux Pumps (EPs)
Extrusion of therapeutically relevant antimicrobial agents/drugs from inside cells to the extracellular environment via EPs has been frequently involved in microbial antibiotic resistance and spreading AMR (Alav et al. 2018). Investigations have identified several EP genes in chromosomes and plasmids of different bacterial species that mediate drug resistance (Li and Nikaido 2009). EPs are also found to play key roles in biofilm formation by extruding quorum sensing molecules and quorum quenchers that mediate the formation of biofilm matrix, thus promoting surface adhesion (Ugwuanyi et al. 2021). EPs have been characterized as one of the major drug-resistant determinants. Numerous nanomaterials for delivering antimicrobials to EP target sites have been investigated using in vivo and in vitro models as a potential tool for treating bacterial infection (Prasher et al. 2021). A recent study has reported on the synergistic effects of ciprofloxacin with embelin-loaded chitosan-gold nanoparticles against environmental MDR P. aeruginosa and E. coli strains by inhibiting EPs by interacting with PA-r (MexA, MexB and OprM) and EC-r (AcrA, AcrB and TolC) active sites (Khare et al. 2021). Further advancements in the microfluidic assembly of pomegranate-like hierarchical microspheres and meropenem-loaded porous silica (MCM-48), for efflux regulation in oral drug delivery against S. aureus and P. aeruginosa, demonstrated reduced efflux of MER back into the gastrointestinal lumen (Raza et al. 2021). One of the recent innovative strategies includes the application of combinations of different antibiotics on nanomaterials to combat MDR bacteria. Khameneh et al. (2015) investigated the antibacterial activity of co-loaded piperine and gentamicin nanoliposomes in MRSA resulting in EP inhibition with MIC of 32 and 100 μg/mL, respectively. Similarly, liposome-encapsulated phenylalanine-arginine β-naphthylamide (PAβN), an EP inhibitor (EPI), has been proven a cost-effective and worthwhile delivery system against MDR P. aeruginosa in lung infections (Ray et al. 2021). However, deeper studies are much further required in this field.
3.5.4 Quorum Sensing
The communication mechanism between the bacteria cells with each other that entails the synthesis, detection and autoinducer extracellular signalling molecules is defined as quorum sensing (QS) (Rutherford and Bassler 2012). Molecular mechanisms involving acyl-homoserine lactones, peptide autoinducers and autoinducer 2 are the major QS systems present in bacteria involved in intercellular signalling during human bacterial infections (Irie and Parsek 2008). As a result, there is an increasing demand for viable, non-toxic/anti-QS agents exhibiting dual actin modes addressing both biofilm formation and QS in bacterial infections. In recent years, nanomaterials as antimicrobial agents/drug delivery systems have been reported as an effective tool for QS elimination and treating microbial infections. Bueloni et al. (2020) developed vanadium-nalidixic acid complex (V-NA) nanoencapsulated into myristyl myristate nanostructured lipid carriers (NLCs), and polymeric nanoparticles of Eudragit NE 30D (EuNPs) with enhanced antibacterial and anti-quorum sensing properties against P. aeruginosa and Chromobacterium violaceum resulted in controlled release of V-NA (30–40% for 3 days) with 59.3 and 129.9 μM MIC values, respectively. Similar results were observed in chitosan-gum acacia gold nanocomposite (CS-GA-AuNC) against MDR P. aeruginosa with a greater reduction in Las-R gene expression levels majorly involved as a virulence factor in biofilm formation and QS (Raja Namasivayam et al. 2020). Further in vivo studies on murine macrophage cell line revealed their excellent biocompatibility, an excellent property for drug delivery systems. Recently, the formulations of AMP dendrimers and QSIs (anti-MvfR compounds) for treating burn wound infections caused by P. aeruginosa were developed that inhibited the MvfR virulence pathway in the QS system of the bacteria (Jafari et al. 2021). Similar results in tobramycin antibiotic and alkylquinolone quorum sensing inhibitor (QSI)-loaded squalenyl hydrogen sulphate nanoparticles (SqNPs) in in vitro models of pulmonary P. aeruginosa infections were observed with improved biofilm penetration and enhanced antimicrobial efficiency (Ho et al. 2020).
3.6 Conclusion and Future Perspectives
Biocompatibility, cost-effectiveness, controlled drug release, deep penetration, target specificity and sustainability properties of nanocarriers make them ideal drug carriers, for delivering wide-ranging antimicrobial agents. However, despite the seemingly large corpus of research and development of a nanomaterial-based delivery system of antimicrobial agents, numerous hurdles need to be overcome before nanomaterial-based approaches for the optimum treatment of drug-resistant bacterial infections may be successfully translated to clinical settings. Silver-oxide and zinc-oxide nanomaterials being approved by the FDA have increased the likelihood of clinical settings among the current leads. Antimicrobial agents such as phytochemicals, AMPs, antibiotics and metallic complexes comprising great biocompatibility and enhanced antimicrobial activity in conjugation with nanocarriers such as liposomes, nanoparticles, nanocomposites and dendrimers are the emerging promising tools for prolonged and regulated release of drugs/antimicrobial agents against microbial infections. These nanomaterial-based drug delivery systems are proven to be targeting key drug-resistant determinants (cell membrane, EPs, biofilm formation, QS) in pathogenic and threatening bacteria. Nanoliposomes are been already employed in clinical settings for delivering antimicrobials to biofilm-forming bacterial infections. PLGA NPs and GO-NPs have the broadest drug delivery range including AMPs that are found to target biofilms and QS systems. However, deeper research is still required in the field of nanomaterial-based delivery of antimicrobials targeting specific EPs, drug release kinetics, biodegradation, pharmacokinetics and their clearance. For their development, research necessitates multidisciplinary clinical and industrial collaborations for fighting these human microbial infections and making them available from bench to bedside.
References
Abdel Hady M, Sayed OM, Akl MA (2020) Brain uptake and accumulation of new levofloxacin-doxycycline combination through the use of solid lipid nanoparticles: formulation; Optimization and in-vivo evaluation. Colloids Surf B Biointerfaces 139. https://doi.org/10.1016/j.colsurfb.2020.111076
Abdelghany SM, Quinn DJ, Ingram RJ et al (2012) Gentamicin-loaded nanoparticles show improved antimicrobial effects towards Pseudomonas aeruginosa infection. Int J Nanomedicine 7(518):4053–4063. https://doi.org/10.2147/IJN.S34341
Abdi-Ali A, Mohammadi-Mehr M, Agha Alaei Y (2006) Bactericidal activity of various antibiotics against biofilm-producing Pseudomonas aeruginosa. Int J Antimicrob Agents 27:196–200. https://doi.org/10.1016/j.ijantimicag.2005.10.007
Aghayan SS, Mogadam HK, Fazli M et al (2017) The effects of Berberine and palmatine on efflux pumps inhibition with different gene patterns in Pseudomonas aeruginosa isolated from burn infections. Avicenna J Med Biotechnol 9:1–7
Ahangari A, Salouti M, Heidari Z et al (2013) Development of gentamicin-gold nanospheres for antimicrobial drug delivery to Staphylococcal infected foci. Drug Deliv 20(1):34–39. https://doi.org/10.3109/10717544.2012.746402
Ahern BJ, Richardson DW (2012) Surgical site infection and the use of antimicrobials. 4th, Elsevier Inc
Ain Q, Munir H, Jelani F et al (2020) Antibacterial potential of biomaterial derived nanoparticles for drug delivery application. Mater Res Express 6(2):125426. https://doi.org/10.1088/2053-1591/ab715d
Alav I, Sutton JM, Rahman KM (2018) Role of bacterial efflux pumps in biofilm formation. J Antimicrob Chemother 73(8):2003–2020. https://doi.org/10.1093/jac/dky042
Al-Bakri AG, Mahmoud NN (2019) Photothermal-induced antibacterial activity of gold nanorods loaded into polymeric hydrogel against pseudomonas aeruginosa biofilm. Molecules 24(14):2661. https://doi.org/10.3390/molecules24142661
Alhadrami HA, Orfali R, Hamed AA et al (2021) Flavonoid-coated gold nanoparticles as efficient antibiotics against gram-negative bacteria-evidence from in silico-supported in vitro studies. Antibiotics 10(8):968. https://doi.org/10.3390/antibiotics10080968
Almaaytah A, Mohammed GK, Abualhaijaa A, Al-Balas Q (2017) Development of novel ultrashort antimicrobial peptide nanoparticles with potent antimicrobial and antibiofilm activities against multidrug-resistant bacteria. Drug Des Devel Ther 3(11):3159–3170. https://doi.org/10.2147/DDDT.S147450
Al-Obaidi MMJ, Desa MNM (2018) Mechanisms of blood brain barrier disruption by different types of bacteria, and bacterial–host interactions facilitate the bacterial pathogen invading the brain. Cell Mol Neurobiol 38(7):1349–1368. https://doi.org/10.1007/s10571-018-0609-2
Arturo Lopez-Quintelá M (2003) Synthesis of nanomaterials in microemulsions: formation mechanisms and growth control. Curr Opin Colloid Interface Sci 8(2):137–144. https://doi.org/10.1016/S1359-0294Ž03.00019-0
Assali M, Zaid AN, Abdallah F et al (2017) Single-walled carbon nanotubes-ciprofloxacin nanoantibiotic: strategy to improve ciprofloxacin antibacterial activity. Int J Nanomedicine 12:6647–6659. https://doi.org/10.2147/IJN.S140625
Atanasov AG, Zotchev SB, Dirsch VM et al (2021) Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov 20:200–216. https://doi.org/10.1038/s41573-020-00114-z
Authimoolam SP, Dziubla TD (2016) Biopolymeric mucin and synthetic polymer analogs: their structure, function and role in biomedical applications. Polymers (Basel) 8(3):71. https://doi.org/10.3390/polym8030071
Bai FW, Zhao XQ, Xu J (2011) Immobilization technology: cells. Compr Biotechnol Second Ed 2:478–489. https://doi.org/10.1016/B978-0-08-088504-9.00115-X
Banerjee M, Parai D, Chattopadhyay S, Mukherjee SK (2017) Andrographolide: antibacterial activity against common bacteria of human health concern and possible mechanism of action. Folia Microbiol (Praha) 62:237–244. https://doi.org/10.1007/s12223-017-0496-9
Baptista PV, McCusker MP, Carvalho A et al (2018) Nano-strategies to fight multidrug resistant bacteria – “A Battle of the Titans”. Front Microbiol 9:1441. https://doi.org/10.3389/fmicb.2018.01441
Barani M, Mukhtar M, Rahdar A et al (2021) Progress in the application of nanoparticles and graphene as drug carriers and on the diagnosis of brain infections. Molecules 26(1):186. https://doi.org/10.3390/molecules26010186
Barber KE, Smith JR, Raut A, Rybak MJ (2016) Evaluation of tedizolid against Staphylococcus aureus and enterococci with reduced susceptibility to vancomycin, daptomycin or linezolid. J Antimicrob Chemother 71:152–155. https://doi.org/10.1093/jac/dkv302
Barnes AC, Amyes SGB, Hastings TS, Lewin CS (1991) Fluoroquinolones display rapid bactericidal activity and low mutation frequencies against Aeromonas salmonicida. J Fish Dis 14:661–667. https://doi.org/10.1111/j.1365-2761.1991.tb00624.x
Bartoloni A, Colao MG, Orsi A, Dep R, Giganti E, Parent F, Infettive M, Firenze U (1990) In-vitro activity of vancomycin, teicoplanin, daptomycin, ramoplanin, MDL 62873 and other agents against staphylococci, enterococci and Clostridium difficile. J Antimicrob Chemother 26:627–633
Belščak-Cvitanović A, Durgo K, Huđek A et al (2018) Overview of polyphenols and their properties. In: Polyphenols: properties, recovery, and applications, pp 3–44. https://doi.org/10.1016/B978-0-12-813572-3.00001-4
Bermúdez-Jiménez C, Romney MG, Roa-Flores SA et al (2019) Hydrogel-embedded gold nanorods activated by plasmonic photothermy with potent antimicrobial activity. Nanomed Nanotechnol Biol Med 22:1549–9634. https://doi.org/10.1016/j.nano.2019.102093
Bernardos A, Piacenza E, Sancenón F et al (2019) Mesoporous silica-based materials with bactericidal properties. Small 15(24). https://doi.org/10.1002/smll.201900669
Betriu C, Gómez M, Palau ML, Sánchez A, Picazo JJ (1999) Activities of new antimicrobial agents (trovafloxacin, moxifloxacin, sanfetrinem, and quinupristin-dalfopristin) against Bacteroides fragilis group: comparison with the activities of 14 other agents. Antimicrob Agents Chemother 43:2320–2322
Biavasco F, Manso E, Varaldo PE (1991) In vitro activities of ramoplanin and four glycopeptide antibiotics against clinical isolates of Clostridium difficile. Antimicrob Agents Chemother 35:195–197. https://doi.org/10.1128/AAC.35.1.195
Bielenica A, Drzewiecka-Antonik A, Rejmak P et al (2018) Synthesis, structural and antimicrobial studies of type II topoisomerase-targeted copper(II) complexes of 1,3-disubstituted thiourea ligands. J Inorg Biochem 182:61–70. https://doi.org/10.1016/j.jinorgbio.2018.01.005
Bing W, Chen Z, Sun H et al (2015) Visible-light-driven enhanced antibacterial and biofilm elimination activity of graphitic carbon nitride by embedded Ag nanoparticles. Nano Res 8:1648–1658. https://doi.org/10.1007/s12274-014-0654-1
Boman HG, Agerberth B, Boman A (1993) Mechanisms of action on Escherichia coli of cecropin P1 and PR-39, two antibacterial peptides from pig intestine. Infect Immun 61:2978–2984. https://doi.org/10.1128/iai.61.7.2978-2984.1993
Borzenkov M, Pallavicini P, Chirico G (2019) Photothermally active inorganic nanoparticles: from colloidal solutions to photothermally active printed surfaces and polymeric nanocomposite materials. Eur J Inorg Chem 2019(41):4397–4404. https://doi.org/10.1002/ejic.201900836
Borzenkov M, Pallavicini P, Taglietti A et al (2020) Photothermally active nanoparticles as a promising tool for eliminating bacteria and biofilms. Beilstein J Nanotechnol 11(1):1134–1146. https://doi.org/10.3762/BJNANO.11.98
Boulet ML, Isabelle C, Guay I et al (2018) Tomatidine is a lead antibiotic molecule that targets staphylococcus aureus ATP Synthase subunit C. Antimicrob Agents Chemother 62(6):e02197–e02117. https://doi.org/10.1128/AAC.02197-17
Bourgat Y, Mikolai C, Stiesch M et al (2021) Enzyme-responsive nanoparticles and coatings made from alginate/peptide ciprofloxacin conjugates as drug release system. Antibiotics 10(6):653. https://doi.org/10.3390/antibiotics10060653
Brown-Elliott BA, Wallace RJ (2021) In vitro susceptibility testing of omadacycline against nontuberculous mycobacteria. Antimicrob Agents Chemother 65. https://doi.org/10.1128/AAC.01947-20
Bueloni B, Sanna D, Garribba E et al (2020) Design of nalidixic acid-vanadium complex loaded into chitosan hybrid nanoparticles as smart strategy to inhibit bacterial growth and quorum sensing. Int J Biol Macromol 161:1568–1580. https://doi.org/10.1016/j.ijbiomac.2020.07.304
Bury-Moné S (2014) Antibacterial therapeutic agents. Ref Modul Biomed Sci:1–13. https://doi.org/10.1016/b978-0-12-801238-3.00244-0
Bush K, Bradford PA (2016) β-lactams and β-lactamase inhibitors an overview. Cold Spring Harb Perspect Med 6(8):a025247
Carvalhaes CG (2019) Antimicrobial activity of omadacycline tested against clinical bacterial isolates from hospitals in mainland China, Hong Kong, and Taiwan: results from the SENTRY Antimicrobial Surveillance Program (2013 to 2016). Antimicrob Agents Chemother:1–9
Castanheira M, Jones RN, Livermore DM (2009) Antimicrobial activities of doripenem and other carbapenems against Pseudomonas aeruginosa, other nonfermentative bacilli, and Aeromonas spp. Diagn Microbiol Infect Dis 63:426–433. https://doi.org/10.1016/j.diagmicrobio.2009.01.026
Castanheira M, Davis AP, Mendes RE, Serio AW, Krause KM, Flamm K (2018) In vitro activity of plazomicin against gram-negative and gram-positive isolates collected from U.S. hospitals and comparative activities of aminoglycosides against carbapenem-resistant enterobacteriaceae and isolates carrying carbapenemase genes. Antimicrob Agents Chemother 62(8):e00313-18
Cha R, Brown WJ, Rybak MJ (2003) Bactericidal activities of daptomycin, quinupristin-dalfopristin, and linezolid against vancomycin-resistant Staphylococcus aureus in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob Agents Chemother 47:3960–3963. https://doi.org/10.1128/AAC.47.12.3960-3963.2003
Chalmers JD, van Ingen J, van der Laan R, Herrmann JL (2021) Liposomal drug delivery to manage nontuberculous mycobacterial pulmonary disease and other chronic lung infections. Eur Respir Rev 30(161). https://doi.org/10.1183/16000617.0010-202
Champney WS, Burdine R (1998) Macrolide antibiotic inhibition of translation and 50S ribosomal subunit assembly in methicillin-resistant Staphylococcus aureus cells. Microb Drug Resist 4:169–174. https://doi.org/10.1089/mdr.1998.4.169
Chamundeeswari M, Jeslin J, Verma ML (2019) Nanocarriers for drug delivery applications. Environ Chem Lett 17(2):849–865. https://doi.org/10.1007/s10311-018-00841-1
Chau TP, Kandasamy S, Chinnathambi A et al (2021) Synthesis of zirconia nanoparticles using Laurus nobilis for use as an antimicrobial agent. Appl Nanosci:1–8. https://doi.org/10.1007/s13204-021-02041-w
Chauhan NPS (2021) Ceramic-based hybrid nanoparticles in drug delivery, pp 109–131. https://doi.org/10.1007/978-981-16-2119-2_5
Chen H, Yang J, Sun L et al (2019) Synergistic chemotherapy and photodynamic therapy of endophthalmitis mediated by zeolitic imidazolate framework-based drug delivery systems. Small 15(47):1903880. https://doi.org/10.1002/smll.201903880
Cheng Y, Qu H, Ma M et al (2007) Polyamidoamine (PAMAM) dendrimers as biocompatible carriers of quinolone antimicrobials: an in vitro study. Eur J Med Chem 42(7):1032–1038. https://doi.org/10.1016/j.ejmech.2006.12.035
Cherubin CE, Stratton CW (1994) Assessment of the bactericidal activity of sparfloxacin, ofloxacin, levofloxacin, and other fluoroquinolones compared with selected agents of proven efficacy against Listeria monocytogenes. Diagn Microbiol Infect Dis 20:21–25. https://doi.org/10.1016/0732-8893(94)90014-0
Cho HS, Lee JH, Ryu SY et al (2013) Inhibition of Pseudomonas aeruginosa and Escherichia coli O157:H7 biofilm formation by plant metabolite ε-viniferin. J Agric Food Chem 61(29):7120–7126. https://doi.org/10.1021/jf4009313
Costa E, Piazza V, Lavorano S et al (2020) Trophic transfer of microplastics from copepods to jellyfish in the marine environment. Front Environ Sci 8:158. https://doi.org/10.3389/fenvs.2020.571732
Cremades R, Rodríguez JC, García-Pachón E, Galiana A, Ruiz-garcía M, López P, Royo G (2011) Comparison of the bactericidal activity of various fluoroquinolones against Mycobacterium tuberculosis in an in vitro experimental model. J Antimicrob Chemother 66:2281–2283. https://doi.org/10.1093/jac/dkr281
Cruz GF, de Araujo I, Torres MDT et al (2020) Photochemically-generated silver chloride nanoparticles stabilized by a peptide inhibitor of cell division and its antimicrobial properties. J Inorg Organomet Polym Mater 30(7):2464–2474. https://doi.org/10.1007/s10904-019-01427-2
Dakal TC, Kumar A, Majumdar RS, Yadav V (2016) Mechanistic basis of antimicrobial actions of silver nanoparticles. Front Microbiol 7:1831. https://doi.org/10.3389/fmicb.2016.01831
Dasenaki ME, Thomaidis NS (2017) Meat safety: II residues and contaminants. Elsevier Ltd, pp 553–583. https://doi.org/10.1016/B978-0-08-100694-8.00018-2
Davies JC (2002) Pseudomonas aeruginosa in cystic fibrosis: pathogenesis and persistence. Paediatr Respir Rev 3(2):128–134. https://doi.org/10.1016/S1526-0550(02)00003-3
Davies TA, Shang W, Bush K, Flamm RK (2008) Affinity of doripenem and comparators to penicillin-binding proteins in Escherichia coli and Pseudomonas aeruginosa. Antimicrob Agents Chemother 52:1510–1512. https://doi.org/10.1128/AAC.01529-07
Devnarain N, Osman N, Fasiku VO et al (2021) Intrinsic stimuli-responsive nanocarriers for smart drug delivery of antibacterial agents—an in-depth review of the last two decades. Wiley Interdiscip Rev Nanomed Nanobiotechnol 13(1):e1664. https://doi.org/10.1002/wnan.1664
Domadia PN, Bhunia A, Sivaraman J et al (2008) Berberine targets assembly of Escherichia coli cell division protein FtsZ. Biochemistry 47(10):3225–3234. https://doi.org/10.1021/bi7018546
Doucet-Populaire F (1998) Molecular basis of clarithromycin activity against Mycobacterium avium and mycobacterium smegmatis. J Antimicrob Chemother 41:179–187. https://doi.org/10.1093/jac/41.2.179
Dowzicky M, Nadler HL, Feger C, Talbot G, Bompart F, Pease M (1998) Evaluation of in vitro activity of quinupristin/dalfopristin and comparator antimicrobial agents against worldwide clinical trial and other laboratory isolates. Pneumologie 52:640
Durand ML (2013) Endophthalmitis. Clin Microbiol Infect 19(3):227–234
Eid HM, Elkomy MH, El Menshawe SF, Salem HF (2019) Transfersomal nanovesicles for nose-to-brain delivery of ofloxacin for better management of bacterial meningitis: formulation, optimization by Box-Behnken design, characterization and in vivo pharmacokinetic study. J Drug Deliv Sci Technol 54:101304. https://doi.org/10.1016/j.jddst.2019.101304
Elsabahy M, Wooley KL (2012) Design of polymeric nanoparticles for biomedical delivery applications. Chem Soc Rev 41(7):2545–2561. https://doi.org/10.1039/c2cs15327k
ElZorkany HES, Youssef T, Mohamed MB, Amin RM (2019) Photothermal versus photodynamic treatment for the inactivation of the bacteria Escherichia coli and Bacillus cereus: an in vitro study. Photodiagn Photodyn Ther 27:317–326. https://doi.org/10.1016/j.pdpdt.2019.06.020
Englande L, Friedman A (2010) Nitric oxide nanoparticle technology: a novel antimicrobial agent in the context of current treatment of skin and soft tissue infection. J Clin Aesthet Dermatol 3(6):45–50
Farrell DJ, Morrissey I, Bakker S, Buckridge S, Felmingham D (2004) In vitro activities of telithromycin, linezolid, and quinupristin- dalfopristin against Streptococcus pneumoniae with macrolide resistance due to ribosomal mutations. Antimicrob Agents Chemother 48:3169–3171. https://doi.org/10.1128/AAC.48.8.3169-3171.2004
Fathima A, Rao JR (2018) Is Cr(III) toxic to bacteria: toxicity studies using Bacillus subtilis and Escherichia coli as model organism. Arch Microbiol 200(3):453–462. https://doi.org/10.1007/s00203-017-1444-4
Fatima F, Siddiqui S, Khan WA (2021) Nanoparticles as novel emerging therapeutic antibacterial agents in the antibiotics resistant era. Biol Trace Elem Res 199(7):2552–2564. https://doi.org/10.1007/s12011-020-02394-3
Ferreira M, Ogren M, Dias JNR et al (2021) Liposomes as antibiotic delivery systems: a promising nanotechnological strategy against antimicrobial resistance. Molecules 26(7):2047. https://doi.org/10.3390/molecules26072047
Flamini R, De Rosso M (2018) High-resolution mass spectrometry and biological properties of grapevine and wine stilbenoids, vol 61, 1st edn. Elsevier B.V., pp 175–210
Fox LJ, Richardson RM, Briscoe WH (2018) PAMAM dendrimer – cell membrane interactions. Adv Colloid Interf Sci 257:1–18
Goenka S, Sant V, Sant S (2014) Graphene-based nanomaterials for drug delivery and tissue engineering. J Control Release 173:75–88
González B, Colilla M, Díez J et al (2018) Mesoporous silica nanoparticles decorated with polycationic dendrimers for infection treatment. Acta Biomater 68:261–271. https://doi.org/10.1016/j.actbio.2017.12.041
Górniak I, Bartoszewski R, Króliczewski J (2019) Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem Rev 18(1):241–272. https://doi.org/10.1007/s11101-018-9591-z
Graber H, Arr M, Csiba A (1981) Azlocillin and mezlocillin: evaluation of new ureido penicillins. Int J Clin Pharmacol Ther Toxicol 19:539–546
Gradišar H, Pristovšek P, Plaper A, Jerala R (2007) Green tea catechins inhibit bacterial DNA gyrase by interaction with its ATP binding site. J Med Chem 50(2):264–271. https://doi.org/10.1021/jm060817o
Grossman TH (2016) Tetracycline antibiotics and resistance. Cold Spring Harb Perspect Med 6(4):1–24. https://doi.org/10.1101/cshperspect.a025387
Gupta A, Pandey AK (2019) Antibacterial lead compounds and their targets for drug development. Elsevier Inc, pp 275–292. https://doi.org/10.1016/B978-0-12-817890-4.00018-4
Haas W, Pillar CM, Hesje CK, Sanfilippo CM, Morris TW (2010) Bactericidal activity of besifloxacin against staphylococci, Streptococcus pneumoniae and Haemophilus influenzae. J Antimicrob Chemother 65:1441–1447. https://doi.org/10.1093/jac/dkq127
Habiba K, Bracho-Rincon DP, Gonzalez-Feliciano JA et al (2015) Synergistic antibacterial activity of PEGylated silver-graphene quantum dots nanocomposites. Appl Mater Today 1(2):80–87. https://doi.org/10.1016/j.apmt.2015.10.001
Haider M, Abdin SM, Kamal L, Orive G (2020) Nanostructured lipid carriers for delivery of chemotherapeutics: a review. Pharmaceutics 12(3):288. https://doi.org/10.3390/pharmaceutics12030288
Hall K, Shoemaker-Hunt S, Hoffman L et al (2020) Making healthcare safer III: a critical analysis of existing and emerging patient safety practices
Hamblin MR (2016) Antimicrobial photodynamic inactivation: a bright new technique to kill resistant microbes. Curr Opin Microbiol 33:67–73. https://doi.org/10.1016/j.mib.2016.06.008
Hasan N, Cao J, Lee J et al (2019) PEI/NONOates-doped PLGA nanoparticles for eradicating methicillin-resistant Staphylococcus aureus biofilm in diabetic wounds via binding to the biofilm matrix. Mater Sci Eng C 103. https://doi.org/10.1016/j.msec.2019.109741
Hassan D, Omolo CA, Fasiku VO et al (2020) Novel chitosan-based pH-responsive lipid-polymer hybrid nanovesicles (OLA-LPHVs) for delivery of vancomycin against methicillin-resistant Staphylococcus aureus infections. Int J Biol Macromol 147:385–398. https://doi.org/10.1016/j.ijbiomac.2020.01.019
Ho DK, Murgia X, De Rossi C et al (2020) Squalenyl hydrogen sulfate nanoparticles for simultaneous delivery of tobramycin and an alkylquinolone quorum sensing inhibitor enable the eradication of P. aeruginosa biofilm infections. Angew Chemie Int Ed 59(26):10292–10296. https://doi.org/10.1002/anie.202001407
Hong R, Kang TY, Michels CA, Gadura N (2012) Membrane lipid peroxidation in copper alloy-mediated contact killing of Escherichia coli. Appl Environ Microbiol 78(6):1776–1784. https://doi.org/10.1128/AEM.07068-11
Hong W, Zhang Z, Liu L et al (2018) Brain-targeted delivery of PEGylated nano-bacitracin A against Penicillin-sensitive and-resistant Pneumococcal meningitis: formulated with RVG29 and Pluronic® P85 unimers. Drug Deliv 25(1):1886–1897. https://doi.org/10.1080/10717544.2018.1486473
Hou X, Feng C, Li S et al (2019) Mechanism of antimicrobial peptide NP-6 from Sichuan pepper seeds against E. coli and effects of different environmental factors on its activity. Appl Microbiol Biotechnol 103(16):6593–6604. https://doi.org/10.1007/s00253-019-09981-y
Hu Y, Liu A, Ortega-Muro F, Alameda-Martin L, Mitchison D, Coates A (2015) High-dose rifampicin kills persisters, shortens treatment duration, and reduces relapse rate in vitro and in vivo. Front Microbiol 6:1–10. https://doi.org/10.3389/fmicb.2015.00641
Huan Y, Kong Q, Mou H, Yi H (2020) Antimicrobial peptides: classification, design, application and research progress in multiple fields. Front Microbiol 11:1–21. https://doi.org/10.3389/fmicb.2020.582779
Huang J, Ho W, Wang X (2014) Metal-free disinfection effects induced by graphitic carbon nitride polymers under visible light illumination. Chem Commun 50(33):4338–4340. https://doi.org/10.1039/c3cc48374f
Huang Z, Kłodzińska SN, Wan F, Nielsen HM (2021) Nanoparticle-mediated pulmonary drug delivery: state of the art towards efficient treatment of recalcitrant respiratory tract bacterial infections. Drug Deliv Transl Res 10:1–21. https://doi.org/10.1007/s13346-021-00954-1
Hussein MAM, Grinholc M, Dena ASA et al (2021) Boosting the antibacterial activity of chitosan–gold nanoparticles against antibiotic–resistant bacteria by Punicagranatum L. extract. Carbohydr Polym 256:117498. https://doi.org/10.1016/j.carbpol.2020.117498
Ibrahim UH, Devnarain N, Omolo CA et al (2021) Biomimetic pH/lipase dual responsive vitamin-based solid lipid nanoparticles for on-demand delivery of vancomycin. Int J Pharm 607:120960. https://doi.org/10.1016/j.ijpharm.2021.120960
Irie Y, Parsek MR (2008) Quorum sensing and microbial biofilms. Curr Top Microbiol Immunol 322:67–84. https://doi.org/10.1007/978-3-540-75418-3_4
Jacobs MR (1991) Evaluation of the bactericidal activity of temafloxacin. Am J Med 91:31–34. https://doi.org/10.1016/0002-9343(91)90307-J
Jafari P, Luscher A, Siriwardena T et al (2021) Antimicrobial peptide dendrimers and quorum-sensing inhibitors in formulating next-generation anti-infection cell therapy dressings for burns. Molecules 26(13):3839. https://doi.org/10.3390/molecules26133839
Jahangir MA, Gilani SJ, Muheem A et al (2019) Quantum dots: next generation of smart nano-systems. Pharm Nanotechnol 7(3):234–245. https://doi.org/10.2174/2211738507666190429113906
Jasim NA, Al-Gasha’a FA, Al-Marjani MF et al (2020) ZnO nanoparticles inhibit growth and biofilm formation of vancomycin-resistant S. aureus (VRSA). Biocatal Agric Biotechnol 29:101745. https://doi.org/10.1016/j.bcab.2020.101745
Jiale Z, Jian J, Xinyi T et al (2021) Design of a novel antimicrobial peptide 1018M targeted ppGpp to inhibit MRSA biofilm formation. AMB Express 11(1):1–4. https://doi.org/10.1186/s13568-021-01208-6
Jokipii AM, Jokipii L (1977) Bactericidal activity of tinidazole. An in vitro comparison of the effects of tinidazole and metronidazole against Bacteroides fragilis and other Anaerobic bacteria
Jokipii AMM, Jokipii L (1987) Comparative activity of metronidazole and tinidazole against Clostridium difficile and Peptostreptococcus anaerobius. Antimicrob Agents Chemother 31:183–186. https://doi.org/10.1128/AAC.31.2.183
Jones ME, Visser MR, Klootwijk M, Heisig P, Verhoef J, Schmitz FJ (1999) Comparative activities of clinafloxacin, grepafloxacin, levofloxacin, moxifloxacin, ofloxacin, sparfloxacin, and trovafloxacin and nonquinolones linezolid, quinupristin-dalfopristin, gentamicin, and vancomycin against clinical isolates of ciprofloxacin-re. Antimicrob Agents Chemother 43:421–423. https://doi.org/10.1128/aac.43.2.421
Jones RN, Sader HS, Fritsche TR (2005) Comparative activity of doripenem and three other carbapenems tested against Gram-negative bacilli with various β-lactamase resistance mechanisms. Diagn Microbiol Infect Dis 52:71–74. https://doi.org/10.1016/j.diagmicrobio.2004.12.008
Jones RN, Sader HS, Fritsche TR, Pottumarthy S (2007) Comparisons of parenteral broad-spectrum cephalosporins tested against bacterial isolates from pediatric patients: report from the SENTRY Antimicrobial Surveillance Program (1998–2004). Diagn Microbiol Infect Dis 57:109–116. https://doi.org/10.1016/j.diagmicrobio.2006.06.011
Joshi JR, Khazanov N, Senderowitz H et al (2016) Plant phenolic volatiles inhibit quorum sensing in pectobacteria and reduce their virulence by potential binding to ExpI and ExpR proteins. Sci Rep 6(1):1–5. https://doi.org/10.1038/srep38126
Juntke J, Murgia X, Günday Türeli N et al (2021) Testing of aerosolized ciprofloxacin nanocarriers on cystic fibrosis airway cells infected with P. aeruginosa biofilms. Drug Deliv Transl Res 28:1–4. https://doi.org/10.1007/s13346-021-01002-8
Kaneko Y, Thoendel M, Olakanmi O et al (2007) The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J Clin Invest 117(4):877–888. https://doi.org/10.1172/JCI30783
Kaur K, Reddy S, Barathe P et al (2021) Combating drug-resistant bacteria using photothermally active nanomaterials: a perspective review. Front Microbiol 12:3405
Kazemzadeh H, Mozafari M (2019) Fullerene-based delivery systems. Drug Discov Today. https://doi.org/10.1016/j.drudis.2019.01.013
Keel RA, Tessier PR, Crandon JL, Nicolau DP (2012) Comparative efficacies of human simulated exposures of tedizolid and linezolid against Staphylococcus aureus in the murine thigh infection model. Antimicrob Agents Chemother 56:4403–4407. https://doi.org/10.1128/AAC.00122-12
Khameneh B, Iranshahy M, Ghandadi M et al (2015) Investigation of the antibacterial activity and efflux pump inhibitory effect of co-loaded piperine and gentamicin nanoliposomes in methicillin-resistant Staphylococcus aureus. Drug Dev Ind Pharm 41(6):989–994. https://doi.org/10.3109/03639045.2014.920025
Khare T, Mahalunkar S, Shriram V et al (2021) Embelin-loaded chitosan gold nanoparticles interact synergistically with ciprofloxacin by inhibiting efflux pumps in multidrug-resistant Pseudomonas aeruginosa and Escherichia coli. Environ Res 199:111321. https://doi.org/10.1016/j.envres.2021.111321
Kim Y, Park EJ, Na DH (2018) Recent progress in dendrimer-based nanomedicine development. Arch Pharm Res 41(6):571–582. https://doi.org/10.1007/s12272-018-1008-4
Koga T, Masuda N, Kakuta M, Namba E, Sugihara C, Fukuoka T (2008) Potent in vitro activity of tomopenem (CS-023) against methicillin- resistant Staphylococcus aureus and Pseudomonas aeruginosa. Antimicrob Agents Chemother 52:2849–2854. https://doi.org/10.1128/AAC.00413-08
Kondo M, Oishi T, Tsuchiya K, Goto S, Kuwahara S (1973) Maridomycin, a new macrolide antibiotic. In vitro antibacterial activity of 9-propionylmaridomycin. Antimicrob Agents Chemother 4:149–155. https://doi.org/10.1128/AAC.4.2.149
Koo HB, Seo J (2019) Antimicrobial peptides under clinical investigation. Pept Sci 111(5):e24122. https://doi.org/10.1002/pep2.24122
Krishnamoorthi R, Bharathakumar S, Malaikozhundan B, Mahalingam PU (2021) Mycofabrication of gold nanoparticles: optimization, characterization, stabilization and evaluation of its antimicrobial potential on selected human pathogens. Biocatal Agric Biotechnol 35:102107. https://doi.org/10.1016/j.bcab.2021.102107
Kulthe SS, Choudhari YM, Inamdar NN, Mourya V (2012) Polymeric micelles: authoritative aspects for drug delivery. Des Monomers Polym 15(5):465–521. https://doi.org/10.1080/1385772X.2012.688328
Kumar MNVR (2000) A review of chitin and chitosan applications. React Funct Polym 46(1):1–27. https://doi.org/10.1016/S1381-5148(00)00038-9
Kumar P, Kizhakkedathu JN, Straus SK (2018a) Antimicrobial peptides: diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomol Ther 8(1):4. https://doi.org/10.3390/biom8010004
Kumar SV, Bafana AP, Pawar P et al (2018b) High conversion synthesis of <10 nm starch-stabilized silver nanoparticles using microwave technology. Sci Rep 8(1):1–10. https://doi.org/10.1038/s41598-018-23480-6
Kuruvilla M (2018) Macrolide allergy. Elsevier Inc.
Kuwahara K, Kitazawa T, Kitagaki H et al (2005) Nadifloxacin, an antiacne quinolone antimicrobial, inhibits the production of proinflammatory cytokines by human peripheral blood mononuclear cells and normal human keratinocytes. J Dermatol Sci 38(1):47–55. https://doi.org/10.1016/j.jdermsci.2005.01.002
Kwon JH (2017) Macrolides, ketolides, lincosamides and streptogramins, 4th edn. Elsevier Ltd, pp 1217–1229
Kwon EJ, Skalak M, Bertucci A et al (2017) Porous silicon nanoparticle delivery of tandem peptide anti-infectives for the treatment of Pseudomonas aeruginosa lung infections. Adv Mater 29(35):1701527. https://doi.org/10.1002/adma.201701527
Lee VJ (2006) Anti-gram positive agents of natural product origins. Compr Med Chem II 7:653–671. https://doi.org/10.1016/b0-08-045044-x/00222-4
Lee H, Hwang JS, Lee J et al (2015) Scolopendin 2, a cationic antimicrobial peptide from centipede, and its membrane-active mechanism. Biochim Biophys Acta Biomembr 1848(2):634–642. https://doi.org/10.1016/j.bbamem.2014.11.016
Lee B, Park J, Ryu M et al (2017) Antimicrobial peptide-loaded gold nanoparticle-DNA aptamer conjugates as highly effective antibacterial therapeutics against Vibrio vulnificus. Sci Rep 7(1):1–10. https://doi.org/10.1038/s41598-017-14127-z
Lee KH, Lee JS, Kim ES, Lee HG (2019a) Preparation, characterization, and food application of rosemary extract-loaded antimicrobial nanoparticle dispersions. LWT 101:138–144. https://doi.org/10.1016/j.lwt.2018.10.072
Lee NY, Ko WC, Hsueh PR (2019b) Nanoparticles in the treatment of infections caused by multidrug-resistant organisms. Front Pharmacol 10:1153. https://doi.org/10.3389/fphar.2019.01153
Lemire JA, Harrison JJ, Turner RJ (2013) Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat Rev Microbiol 11(6):371–384. https://doi.org/10.1038/nrmicro3028
León-Buitimea A, Garza-Cárdenas CR, Garza-Cervantes JA et al (2020) The demand for new antibiotics: antimicrobial peptides, nanoparticles, and combinatorial therapies as future strategies in antibacterial agent design. Front Microbiol 11:1–10. https://doi.org/10.3389/fmicb.2020.01669
Li XZ, Nikaido H (2009) Efflux-mediated drug resistance in bacteria: an update. Drugs 69(12):1555–1623. https://doi.org/10.2165/11317030-000000000-00000
Li L, Shi Y, Cheng X et al (2015) A cell-penetrating peptide analogue, P7, exerts antimicrobial activity against Escherichia coli ATCC25922 via penetrating cell membrane and targeting intracellular DNA. Food Chem 166:231–239. https://doi.org/10.1016/j.foodchem.2014.05.113
Li W, Separovic F, O’Brien-Simpson NM, Wade JD (2021) Chemically modified and conjugated antimicrobial peptides against superbugs. Chem Soc Rev 50:4932–4973. https://doi.org/10.1039/d0cs01026j
Liu Y, Jin L, Wang C et al (2021a) Thymol-functionalized hollow mesoporous silica spheres nanoparticles: preparation, characterization and bactericidal activity. Bull Mater Sci 44(2):1–7. https://doi.org/10.1007/s12034-021-02425-2
Liu Z, Zhao X, Yu B et al (2021b) Rough carbon-iron oxide nanohybrids for near-infrared-II light-responsive synergistic antibacterial therapy. ACS Nano 15(4):7482–7490. https://doi.org/10.1021/acsnano.1c00894
Lyu Z, Ding L, Huang AT, Kao CL, Peng L (2019) Poly (amidoamine) dendrimers: Covalent and supramolecular synthesis. Materials Today Chemistry 13:34–48. https://doi.org/10.1016/j.mtchem.2019.04.004
Lombardo D, Kiselev MA, Caccamo MT (2019) Smart nanoparticles for drug delivery application: development of versatile Nanocarrier platforms in biotechnology and nanomedicine. J Nanomater. https://doi.org/10.1155/2019/3702518
Lotha R, Sundaramoorthy NS, Shamprasad BR et al (2018) Plant nutraceuticals (Quercetrin and Afzelin) capped silver nanoparticles exert potent antibiofilm effect against food borne pathogen salmonella enterica serovar Typhi and curtail planktonic growth in zebrafish infection model. Microb Pathog 120:109–118. https://doi.org/10.1016/j.micpath.2018.04.044
Luo Z, Wu Q, Zhang M et al (2011) Cooperative antimicrobial activity of CdTe quantum dots with rocephin and fluorescence monitoring for Escherichia coli. J Colloid Interface Sci 362(1):100–106. https://doi.org/10.1016/j.jcis.2011.06.039
Macomber L, Imlay JA (2009) The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc Natl Acad Sci U S A 106(20):8344–8349. https://doi.org/10.1073/pnas.0812808106
Macomber L, Elsey SP, Hausinger RP (2011) Fructose-1,6-bisphosphate aldolase (class II) is the primary site of nickel toxicity in Escherichia coli. Mol Microbiol 82(5):1291–1300. https://doi.org/10.1111/j.1365-2958.2011.07891.x
Mandani S, Rezaei B, Ensafi AA, Sabzalian MR (2021) Development of a new simple spectroscopic determination coupled acid-motivated delivery system based on fluorescence turn-off MSNs@MPA-ZnS QDs for infection. Microporous Mesoporous Mater 317:110971. https://doi.org/10.1016/j.micromeso.2021.110971
Manisha H, Priya Swetha PD, Shim YB, Prasad KS (2019) Revisiting fluorescent carbon nanodots for environmental, biomedical applications and puzzle about fluorophore impurities. Nano-Struct Nano-Objects 20(20):100391. https://doi.org/10.1016/j.nanoso.2019.100391
Manju S, Sreenivasan K (2010) Functionalised nanoparticles for targeted drug delivery. In: Biointegration of medical implant materials: science and design, pp 267–297. https://doi.org/10.1533/9781845699802.2.267
Mardirossian M, Sola R, Beckert B et al (2019) Proline-rich peptides with improved antimicrobial activity against E. coli, K. pneumoniae, and A. baumannii. ChemMedChem 14(24):2025–2033. https://doi.org/10.1002/cmdc.201900465
Markley JL, Wencewicz TA (2018) Tetracycline-inactivating enzymes. Front Microbiol 9:1–22. https://doi.org/10.3389/fmicb.2018.01058
Martínez-Carmona M, Gun’ko YK, Vallet-Regí M (2018) Mesoporous silica materials as drug delivery: “the nightmare” of bacterial infection. Pharmaceutics 10(4):279. https://doi.org/10.3390/pharmaceutics10040279
Mba IE, Nweze EI (2021) Nanoparticles as therapeutic options for treating multidrug-resistant bacteria: research progress, challenges, and prospects. World J Microbiol Biotechnol 37(6):1–30. https://doi.org/10.1007/s11274-021-03070-x
McGowan JE, Terry PM, Nahmias AJ (1976) Susceptibility of Haemophilus influenzae isolates from blood and cerebrospinal fluid to ampicillin, chloramphenicol, and trimethoprim sulfamethoxazole. Antimicrob Agents Chemother 9:137–139. https://doi.org/10.1128/AAC.9.1.137
Miao P, Han K, Tang Y et al (2015) Recent advances in carbon nanodots: synthesis, properties and biomedical applications. Nanoscale 7(5):1586–1595. https://doi.org/10.1039/C4NR05712K
Mihai MM, Dima MB, Dima B, Holban AM (2019) Infect Control 12(13):2176. https://doi.org/10.3390/ma12132176
Miyake Y, Tsuruda K, Okuda K, Widowati, Iwamoto Y, Suginaka H (1995) In vitro activity of tetracyclines, macrolides, quinolones, clindamycin and metronidazole against periodontopathic bacteria. J Periodontal Res 30:290–293. https://doi.org/10.1111/j.1600-0765.1995.tb02136.x
Mizunaga S, Kamiyama T, Fukuda Y, Takahata M, Mitsuyama J (2005) Influence of inoculum size of Staphylococcus aureus and Pseudomonas aeruginosa on in vitro activities and in vivo efficacy of fluoroquinolones and carbapenems. J Antimicrob Chemother 56:91–96. https://doi.org/10.1093/jac/dki163
Morrissey I, Ge Y, Janes R (2009) Activity of the new cephalosporin ceftaroline against bacteraemia isolates from patients with community-acquired pneumonia. Int J Antimicrob Agents 33:515–519. https://doi.org/10.1016/j.ijantimicag.2008.12.005
Mosselhy DA, He W, Hynönen U et al (2018) Silica–gentamicin nanohybrids: combating antibiotic resistance, bacterial biofilms, and in vivo toxicity. Int J Nanomedicine. https://doi.org/10.2147/IJN.S182611
Nagaraja K, Rao KM, Reddy GV, Rao KSVK (2021) Tragacanth gum-based multifunctional hydrogels and green synthesis of their silver nanocomposites for drug delivery and inactivation of multidrug resistant bacteria. Int J Biol Macromol 174:502–511. https://doi.org/10.1016/j.ijbiomac.2021.01.203
Nair A, Haponiuk JT, Thomas S, Gopi S (2020a) Natural carbon-based quantum dots and their applications in drug delivery: a review. Biomed Pharmacother 132:110834. https://doi.org/10.1016/j.biopha.2020.110834
Nair KGS, Velmurugan R, Sukumaran SK (2020b) Formulation and optimization of ansamycin-loaded polymeric nanoparticles using response surface methodology for bacterial meningitis. Bionanoscience 8:1–3. https://doi.org/10.1007/s12668-019-00713-0
Nakagawa S, Hillebrand GG, Nunez G (2020) Rosmarinus officinalis l. (rosemary) extracts containing carnosic acid and carnosol are potent quorum sensing inhibitors of staphylococcus aureus virulence. Antibiotics 9(4):149. https://doi.org/10.3390/antibiotics9040149
Navarro-Martínez MD, Navarro-Perán E, Cabezas-Herrera J et al (2005) Antifolate activity of epigallocatechin gallate against Stenotrophomonas maltophilia. Antimicrob Agents Chemother 49(7):2914–2920. https://doi.org/10.1128/AAC.49.7.2914-2920.2005
Nepka M, Perivolioti E, Kraniotaki E, Politi L, Tsakris A, Pournaras S (2016) In vitro bactericidal activity of trimethoprim-sulfamethoxazole alone and in combination with colistin against carbapenem-resistant Acinetobacter baumannii clinical isolates. Antimicrob Agents Chemother 60:6903–6906. https://doi.org/10.1128/AAC.01082-16
Neuharold C, Feldman HA (1973) Effects of trimethoprim and sulfisoxazole alone and in combination on murine toxoplasmosis. J Infect Dis 128:S774–S777. https://doi.org/10.1093/infdis/128.Supplement_3.S774
Newman JDS, Blanchard GJ (2006) Formation of gold nanoparticles using amine reducing agents. Langmuir 22(13):5882–5887. https://doi.org/10.1021/la060045z
Nie L, Deng Y, Zhang Y et al (2021) Silver-doped biphasic calcium phosphate/alginate microclusters with antibacterial property and controlled doxorubicin delivery. J Appl Polym Sci 138(19):50433. https://doi.org/10.1002/app.50433
Ocsoy MA, Yusufbeyoglu S, Ildiz N et al (2021) DNA aptamer-conjugated magnetic graphene oxide for pathogenic bacteria aggregation: selective and enhanced photothermal therapy for effective and rapid killing. ACS Omega 6(31):20637–20643. https://doi.org/10.1021/acsomega.1c02832
Pal I, Bhattacharyya D, Kar RK et al (2019) A peptide-nanoparticle system with improved efficacy against multidrug resistant bacteria. Sci Rep 9(1):1–1. https://doi.org/10.1038/s41598-019-41005-7
Pandurangan AK, Kanagesan S, Narayanaswamy R et al (2016) Nanobiomaterial-based delivery of drugs in various cancer therapies: classifying the mechanisms of action (using biochemical and molecular biomarkers). In: Nanobiomaterials in cancer therapy: applications of nanobiomaterials. Elsevier Inc, pp 331–365
Paris JM (2012) 1.3 chirality in antibacterial agents. Elsevier Ltd
Patra JK, Das G, Fraceto LF et al (2018) Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol 16(1):1–33. https://doi.org/10.1186/s12951-018-0392-8
Pedraza D, Díez J, Barba II et al (2018) Amine-functionalized mesoporous silica nanoparticles: a new nanoantibiotic for bone infection treatment. Biomed Glasses 4(1):1–12. https://doi.org/10.1515/bglass-2018-0001
Pérez JM, Calderón IL, Arenas FA et al (2007) Bacterial toxicity of potassium tellurite: unveiling an ancient enigma. PLoS One 2(2):e211. https://doi.org/10.1371/journal.pone.0000211
Permana AD, Anjani QK, Utomo E et al (2021) Selective delivery of silver nanoparticles for improved treatment of biofilm skin infection using bacteria-responsive microparticles loaded into dissolving microneedles. Mater Sci Eng C 120:111786. https://doi.org/10.1016/j.msec.2020.111786
Pfaller MA, Flamm RK, Jones RN, Farrell DJ, Mendes RE (2016) Activities of tedizolid and linezolid determined by the reference broth microdilution method against 3,032 Gram-positive bacterial isolates collected in Asia-Pacific, Eastern Europe, and Latin American countries in 2014. Antimicrob Agents Chemother 60:5393–5399. https://doi.org/10.1128/AAC.00881-16
Prasher P, Sharma M, Singh SP (2021) Drug encapsulating polysaccharide-loaded metal nanoparticles: a perspective drug delivery system. Drug Dev Res 82(2):145–148. https://doi.org/10.1002/ddr.21754
Qian W, Sun Z, Wang T et al (2020) Antimicrobial activity of eugenol against carbapenem-resistant Klebsiella pneumoniae and its effect on biofilms. Microb Pathog 139:103924. https://doi.org/10.1016/j.micpath.2019.103924
Rabea EI, Badawy MET, Stevens CV et al (2003) Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules 4(6):1457–1465. https://doi.org/10.1021/bm034130m
Radovic-Moreno AF, Lu TK, Puscasu VA et al (2012) Surface charge-switching polymeric nanoparticles for bacterial cell wall-targeted delivery of antibiotics. ACS Nano 6(5):4279–4287. https://doi.org/10.1021/nn3008383
Rahal JJ, Simberkoff MS (1979) Bactericidal and bacteriostatic action of chloramphenicol against meningeal pathogens. Antimicrob Agents Chemother 16:13–18. https://doi.org/10.1128/AAC.16.1.13
Raja Namasivayam SK, Venkatachalam G, Arvind Bharani RS (2020) Immuno biocompatibility and anti-quorum sensing activities of chitosan-gum acacia gold nanocomposite (CS-GA-AuNC) against Pseudomonas aeruginosa drug-resistant pathogen. Sustain Chem Pharm 17:100300. https://doi.org/10.1016/j.scp.2020.100300
Ramalingam B, Parandhaman T, Das SK (2016) Antibacterial effects of biosynthesized silver nanoparticles on surface ultrastructure and Nanomechanical properties of gram-negative bacteria viz. Escherichia coli and Pseudomonas aeruginosa. ACS Appl Mater Interfaces 8(7):4963–4976. https://doi.org/10.1021/acsami.6b00161
Rawat M, Singh D, Saraf S, Saraf S (2008) Development and in vitro evaluation of alginate gel-encapsulated, chitosan-coated ceramic nanocores for oral delivery of enzyme. Drug Dev Ind Pharm 34(2):181–188. https://doi.org/10.1080/03639040701539479
Ray P, Nguyen K, Nguyen T, Sachdev S (2021) Resurrecting the dead: mitigating efflux-pump inhibitor toxicity using a liposomal delivery system to recover efficacy of antimicrobial drugs. Undergrad Res Nat Clin Sci Technol J. https://doi.org/10.26685/urncst.278
Raza A, Alavi SE, Sime FB et al (2021) Microfluidic assembly of pomegranate-like hierarchical microspheres for efflux regulation in oral drug delivery. Acta Biomater 126:277–290. https://doi.org/10.1016/j.actbio.2021.03.042
Rebelo SLH, Guedes A, Szefczyk ME et al (2016) Progress in the Raman spectra analysis of covalently functionalized multiwalled carbon nanotubes: unraveling disorder in graphitic materials. Phys Chem Phys 18(18):12784–12796. https://doi.org/10.1039/c5cp06519d
Rinaudo M (2006) Chitin and chitosan: properties and applications. Prog Polym Sci 31(1):603–632. https://doi.org/10.1016/j.progpolymsci.2006.06.001
Rutherford ST, Bassler BL (2012) Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb Perspect Med. 2(11):a012427. https://doi.org/10.1101/cshperspect.a012427
Rybak MJ, Hershberger E, Moldovan T, Grucz RG (2000) In vitro activities of daptomycin, vancomycin, linezolid, and quinupristin-dalfopristin against staphylococci and enterococci, including vancomycin-intermediate and -resistant strains. Antimicrob Agents Chemother 44:1062–1066. https://doi.org/10.1128/AAC.44.4.1062-1066.2000
Sader HS, Mendes RE, Farrell DJ, Flamm RK, Jones RN (2014) Ceftaroline activity tested against bacterial isolates from pediatric patients results from the assessing worldwide antimicrobial resistance and evaluation program for the United States (2011–2012). Pediatr Infect Dis J 33:837–842. https://doi.org/10.1097/INF.0000000000000307
Sader HS, Farrell DJ, Mendes RE, Flamm RK, Castanheira M, Jones RN (2015) Antimicrobial activity of ceftaroline tested against bacterial isolates causing respiratory tract and skin and skin structure infections in US medical centers in 2013. Diagn Microbiol Infect Dis 82:78–84. https://doi.org/10.1016/j.diagmicrobio.2015.01.015
Sahariah P, Másson M (2017) Antimicrobial chitosan and chitosan derivatives: a review of the structure-activity relationship. Biomacromolecules 18(11):3846–3868. https://doi.org/10.1021/acs.biomac.7b01058
Salamatipour N, Hemmatinejad N, Bashari A (2019) Synthesis of redox-light responsive alginate nano hydrogel to produce smart textile. Fibers Polym 20(4):690–697. https://doi.org/10.1007/s12221-019-8905-0
Sanches BCP, Rocha CA, Bedoya JGM et al (2021) Rhamnolipid-based liposomes as promising nano-carriers for enhancing the antibacterial activity of peptides derived from bacterial toxin-antitoxin systems. Int J Nanomed 16:925. https://doi.org/10.2147/IJN.S283400
Saravanan A, Maruthapandi M, Das P et al (2021) Green synthesis of multifunctional carbon dots with antibacterial activities. Nanomaterials 11(2):369. https://doi.org/10.3390/nano11020369
Sareena C, Vasu ST (2020) Identification of 4-diphenylamino 3-iodo coumarin as a potent inhibitor of DNA gyrase B of S. aureus. Microb Pathog 147:104387. https://doi.org/10.1016/j.micpath.2020.104387
Sauberan JB, Bradley JS (2018) Antimicrobial agents. In: Principles and practice of pediatric infectious diseases, 5th edn. Elsevier Inc. https://doi.org/10.1016/B978-0-323-40181-4.00292-9
Savas S, Ersoy A, Gulmez Y et al (2018) Nanoparticle enhanced antibody and DNA biosensors for sensitive detection of salmonella. Materials (Basel) 11(9):1541. https://doi.org/10.3390/ma11091541
Schwarz S, Shen J, Kadlec K et al (2016) Lincosamides, streptogramins, phenicols, and pleuromutilins: mode of action and mechanisms of resistance. Cold Spring Harb Perspect Med:1–30
Selvarajan V, Obuobi S, Ee PLR (2020) Silica nanoparticles—a versatile tool for the treatment of bacterial infections. Front Chem 8:602. https://doi.org/10.3389/fchem.2020.00602
Şen KD, Manner S, Rosenholm JM (2018) Mesoporous silica nanoparticles as diagnostic and therapeutic tools: how can they combat bacterial infection? Ther Deliv 9:241–244
Sharaf M, Hamouda HI, Shabana S et al (2021) Design of lipid-based nanocarrier for drug delivery has a double therapy for six common pathogens eradication. Colloids Surf A Physicochem Eng Asp. https://doi.org/10.1016/j.colsurfa.2021.126662
Sharma D, Misba L, Khan AU (2019) Antibiotics versus biofilm: an emerging battleground in microbial communities. Antimicrob Resist Infect Control 8(1):1–10. https://doi.org/10.1186/s13756-019-0533-3
Sharma N, Zahoor I, Sachdeva M et al (2021) Deciphering the role of nanoparticles for management of bacterial meningitis: an update on recent studies. Environ Sci Pollut Res:1–8
Shibl AM (1989) Comparative in vitro antibacterial activity of aztreonam against clinical isolates of gram-negative bacteria. Chemotherapy 35(Suppl 1):72–76
Shriram V, Jahagirdar S, Latha C et al (2008) A potential plasmid-curing agent, 8-epidiosbulbin E acetate, from Dioscorea bulbifera L. against multidrug-resistant bacteria. Int J Antimicrob Agents 32(5):405–410. https://doi.org/10.1016/j.ijantimicag.2008.05.013
Shriram V, Khare T, Bhagwat R et al (2018) Inhibiting bacterial drug efflux pumps via phyto-therapeutics to combat threatening antimicrobial resistance. Front Microbiol 9:2990. https://doi.org/10.3389/fmicb.2018.02990
Siddiqui AA, Iram F, Siddiqui S, Sahu K (2014) Role of natural products in drug discovery process. Int J Drug Dev Res 6(2):172–204
Simmons G, Jones N, Calder L (2000) Equivalence of ceftriaxone and rifampicin in eliminating nasopharyngeal carriage of serogroup B Neisseria meningitidis. J Antimicrob Chemother 45:909–911. https://doi.org/10.1093/jac/45.6.909
Singh J, Kaurav N, Choudhary KK, Okram GS (2015) Synthesis and optical properties of silver nanoparticles. AIP Conf Proc. American Institute of Physics Inc 1601(1):030034. https://doi.org/10.1063/1.4926718
Singh D, Singh S, Sahu J et al (2016) Ceramic nanoparticles: recompense, cellular uptake and toxicity concerns. Artif Cells Nanomed Biotechnol 44(1):401–409. https://doi.org/10.3109/21691401.2014.955106
Siriyong T, Srimanote P, Chusri S et al (2017) Conessine as a novel inhibitor of multidrug efflux pump systems in Pseudomonas aeruginosa. BMC Complement Altern Med 17(1):1–7. https://doi.org/10.1186/s12906-017-1913-y
Slavin YN, Asnis J, Häfeli UO, Bach H (2017) Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J Nanobiotechnol 15(1):1–20. https://doi.org/10.1186/s12951-017-0308-z
Solomon S, Bahadori M, Jeyarajasingam AV et al (2007) Synthesis and study of silver nanoparticles. J Chem Educ 84(2):322. https://doi.org/10.1021/ed084p322
Song X, Liu P, Liu X et al (2021) Dealing with MDR bacteria and biofilm in the post-antibiotic era: application of antimicrobial peptides-based nano-formulation. Mater Sci Eng C:112318. https://doi.org/10.1016/j.msec.2021.112318
Stein GE, Schooley SL, Nicolau DP (2008) Urinary bactericidal activity of single doses (250, 500, 750 and 1000 mg) of levofloxacin against fluoroquinolone-resistant strains of Escherichia coli. Int J Antimicrob Agents 32:320–325. https://doi.org/10.1016/j.ijantimicag.2008.04.025
Stewart CA, Finer Y, Hatton BD (2018) Drug self-assembly for synthesis of highly-loaded antimicrobial drug-silica particles. Sci Rep 8(1):1–2. https://doi.org/10.1038/s41598-018-19166-8
Stober W, Fink A, Ernst Bohn D (1968) Controlled growth of monodisperse silica spheres in the micron size range. J Colloid Interface Sci 26(1):62–69. https://doi.org/10.1016/0021-9797(68)90272-5
Sun H, Zhou Y, Ren J, Qu X (2018a) Carbon nanozymes: enzymatic properties, catalytic mechanism, and applications. Angew Chemie Int Ed 57(30):9224–9237. https://doi.org/10.1002/anie.201712469
Sun P, Zhang Y, Ran X et al (2018b) Phytochemical-encapsulated nanoplatform for “on-demand” synergistic treatment of multidrug-resistant bacteria. Nano Res 11(7):3762–3770. https://doi.org/10.1007/s12274-017-1947-y
Sun B, Xi Z, Wu F et al (2019) Quaternized chitosan-coated montmorillonite interior antimicrobial metal-antibiotic in situ coordination complexation for mixed infections of wounds. Langmuir 35(47):15275–15286. https://doi.org/10.1021/acs.langmuir.9b02821
Sun X, Zhang B, Xu G, Chen J, Shang Y, Lin Z, Yu Z, Zheng J, Bai B (2021) In vitro activity of the novel tetracyclines, tigecycline, eravacycline, and omadacycline, against moraxella catarrhalis. Ann Lab Med 41:293–301. https://doi.org/10.3343/ALM.2021.41.3.293
Tan Q, Chu Y, Bie M et al (2017) Preparation and investigation of amphiphilic block copolymers/fullerene nanocomposites as nanocarriers for hydrophobic drug. Materials (Basel) 10(2):192. https://doi.org/10.3390/ma10020192
Tang Y, Wang G (2021) NIR light-responsive nanocarriers for controlled release. J Photochem Photobiol C Photochem Rev:100420. https://doi.org/10.1016/j.jphotochemrev.2021.100420
Teaima MH, Elasaly MK, Omar SA et al (2020) Eco-friendly synthesis of functionalized chitosan-based nanoantibiotic system for potential delivery of linezolid as antimicrobial agents. Saudi Pharm J 28(7):859–868. https://doi.org/10.1016/j.jsps.2020.06.005
Thakur M, Pandey S, Mewada A et al (2014) Antibiotic conjugated fluorescent carbon dots as a theranostic agent for controlled drug release, bioimaging, and enhanced antimicrobial activity. J Drug Deliv 2014:1–9. https://doi.org/10.1155/2014/282193
Thornsberry C, Hill BC, Swenson JM, McDougal LK (1983) Rifampin: Spectrum of antibacterial activity. Rev Infect Dis 5:S412–S417. https://doi.org/10.1093/clinids/5.Supplement_3.S412
Ugwuanyi FC, Ajayi A, Ojo DA et al (2021) Evaluation of efflux pump activity and biofilm formation in multidrug resistant clinical isolates of Pseudomonas aeruginosa isolated from a Federal Medical Center in Nigeria. Ann Clin Microbiol Antimicrob 20(1):1–7. https://doi.org/10.1186/s12941-021-00417-y
Van Laethem Y, Husson M, Klastersky J (1984) Serum bactericidal activity of aztreonam, cefoperazone, and amikacin, alone or in combination, against Escherichia coli, Klebsiella pneumoniae, Serratia marcescens, and Pseudomonas aeruginosa. Antimicrob Agents Chemother 26:224–227. https://doi.org/10.1128/AAC.26.2.224
Vázquez-Laslop N, Mankin AS (2018) How macrolide antibiotics work. Trends Biochem Sci 43(9):668–684. https://doi.org/10.1016/j.tibs.2018.06.011
Veeresham C (2012) Natural products derived from plants as a source of drugs. J Adv Pharm Technol Res 3(4):200–201. https://doi.org/10.4103/2231-4040.104709
Verlee A, Mincke S, Stevens CV (2017) Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr Polym 164:268–283. https://doi.org/10.1016/j.carbpol.2017.02.001
Waller DG, Sampson AP (2018) Chemotherapy of infections. Med Pharmacol Ther:581–629. https://doi.org/10.1016/b978-0-7020-7167-6.00051-8
Wang Y (2021) Liposome as a delivery system for the treatment of biofilm-mediated infections. J Appl Microbiol. https://doi.org/10.1111/jam.15053
Wang W, Yu JC, Xia D et al (2013) Graphene and g-C3N4 nanosheets cowrapped elemental α-sulfur as a novel metal-free heterojunction photocatalyst for bacterial inactivation under visible-light. Environ Sci Technol 47(15):8724–8732. https://doi.org/10.1021/es4013504
Wang X, Mansukhani ND, Guiney LM et al (2016) Toxicological profiling of highly purified metallic and semiconducting single-walled carbon nanotubes in the rodent lung and E. coli. ACS Nano 10(6):6008–6019. https://doi.org/10.1021/acsnano.6b01560
Wang L, Hu C, Shao L (2017) The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int. J Nanomed 12(1227):10.2147%2FIJN.S121956
Wang C, Wang Y, Zhang L et al (2018) Pretreated macrophage-membrane-coated gold nanocages for precise drug delivery for treatment of bacterial infections. Adv Mater 30(46):1804023. https://doi.org/10.1002/adma.201804023
Wang X, Yang P, Feng Q et al (2019) Green preparation of fluorescent carbon quantum dots from cyanobacteria for biological imaging. Polymers (Basel) 11(4):616. https://doi.org/10.3390/polym11040616
Wang DY, van der Mei HC, Ren Y et al (2020) Lipid-based antimicrobial delivery-systems for the treatment of bacterial infections. Front Chem 7:872. https://doi.org/10.3389/fchem.2019.00872
Wang X, Cheng F, Wang X et al (2021) Chitosan decoration improves the rapid and long-term antibacterial activities of cinnamaldehyde-loaded liposomes. Int J Biol Macromol 168:59–66. https://doi.org/10.1016/j.ijbiomac.2020.12.003
Weledji EP, Weledji EK, Assob JC, Nsagha DS (2017) Pros, cons and future of antibiotics. New Horiz Transl Med 4(1–4):9–14. https://doi.org/10.1016/j.nhtm.2017.08.001
Weng W, Nie W, Zhou Q et al (2017) Controlled release of vancomycin from 3D porous graphene-based composites for dual-purpose treatment of infected bone defects. RSC Adv 7:2753–2765. https://doi.org/10.1039/c6ra26062d
White GW, Malow JB, Zimelis VM (1979) Comparative in vitro activity of azlocillin, ampicillin, mezlocillin, piperacillin, and ticarcillin, alone and in combination with an aminoglycoside. Antimicrob Agents Chemother 15:540–543. https://doi.org/10.1128/AAC.15.4.540
WHO (2021) https://www.who.int/ar/news-room/fact-sheets/detail/antimicrobial-resistance
Wilmes M, Stockem M, Bierbaum G et al (2014) Killing of staphylococci by θ-defensins involves membrane impairment and activation of autolytic enzymes. Antibiotics 3(4):617–631. https://doi.org/10.3390/antibiotics3040617
Wise R, Andrews JM (1982) Activity of mezlocillin against gram negative and gram positive organisms comparison with other penicillins. J Antimicrob Chemother 9:1–9. https://doi.org/10.1093/jac/9.suppl_A.1
Woo KJ, Hye CK, Ki WK et al (2008) Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl Environ Microbiol 74:2171–2178. https://doi.org/10.1128/AEM.02001-07
Wu C, Zreiqat H (2010) Porous bioactive diopside (CaMgSi2O6) ceramic microspheres for drug delivery. Acta Biomater 6(3):820–829. https://doi.org/10.1016/j.actbio.2009.09.025
Wu D, Kong Y, Han C et al (2008) d-Alanine: d-alanine ligase as a new target for the flavonoids quercetin and apigenin. Int J Antimicrob Agents 32(5):421–426. https://doi.org/10.1016/j.ijantimicag.2008.06.010
Xu Y, Zhao C, Zhang X et al (2021) Engineering tailorable TiO2 nanotubes for NIR-controlled drug delivery. Nano Res. https://doi.org/10.1007/s12274-021-3338-7
Xue X, Chen X, Mao X et al (2013) Amino-terminated generation 2 poly(amidoamine) dendrimer as a potential broad-spectrum, nonresistance-inducing antibacterial agent. AAPS J 15(1):132–142. https://doi.org/10.1208/s12248-012-9416-8
Yaganza E, Rioux D, Simard M et al (2004) Caused by treatment with aluminum chloride and sodium metabisulfite. Society 70(11):6800–6808. https://doi.org/10.1128/AEM.70.11.6800
Yeh YC, Huang TH, Yang SC et al (2020) Nano-based drug delivery or targeting to eradicate bacteria for infection mitigation: a review of recent advances. Front Chem 8:286. https://doi.org/10.3389/fchem.2020.00286
Yetisgin AA, Cetinel S, Zuvin M et al (2020) Therapeutic nanoparticles and their targeted delivery applications. Molecules 25(9):2193. https://doi.org/10.3390/molecules25092193
Yin C, Wang Z, Ding X et al (2021) Crystalline ruthenium polypyridine nanoparticles: a targeted treatment of bacterial infection with multifunctional antibacterial, adhesion and surface-anchoring photosensitizer properties. J Mater Chem B 9(18):3808–3825. https://doi.org/10.1039/d1tb00103e
Yu Z, Tang J, Khare T, Kumar V (2020) The alarming antimicrobial resistance in ESKAPEE pathogens: can essential oils come to the rescue? Fitoterapia 140:104433. https://doi.org/10.1016/j.fitote.2019.104433
Yu H, Ma Z, Meng S et al (2021a) A novel nanohybrid antimicrobial based on chitosan nanoparticles and antimicrobial peptide microcin J25 with low toxicity. Carbohydr Polym 253:11309. https://doi.org/10.1016/j.carbpol.2020.117309
Yu M, Zhang G, Li P et al (2021b) Acid-activated ROS generator with folic acid targeting for bacterial biofilm elimination. Mater Sci Eng C. https://doi.org/10.1016/j.msec.2021.112225
Yuan P, Ding X, Yang YY, Xu QH (2018) Metal nanoparticles for diagnosis and therapy of bacterial infection. Adv Healthc Mater 7(13):1701392. https://doi.org/10.1002/adhm.201701392
Zarafu I, Turcu I, Culită DC et al (2018) Antimicrobial features of organic functionalized Graphene-oxide with selected amines. Materials (Basel) 11(9):1704. https://doi.org/10.3390/ma11091704
Zhang L, Pornpattananangkul D, Hu C-M, Huang C-M (2010) Development of nanoparticles for antimicrobial drug delivery. Curr Med Chem 17(6):585–594. https://doi.org/10.2174/092986710790416290
Zhang J, Leifer F, Rose S et al (2018) Amikacin liposome inhalation suspension (ALIS) penetrates non-tuberculous mycobacterial biofilms and enhances amikacin uptake into macrophages. Front Microbiol 9:915. https://doi.org/10.3389/fmicb.2018.00915
Zhang J, Sun H, Gao C et al (2021) Development of a chitosan-modified PLGA nanoparticle vaccine for protection against Escherichia coli K1 caused meningitis in mice. J Nanobiotechnol 19(1):1–5. https://doi.org/10.1186/s12951-021-00812-9
Zhao W, Zhao Y, Wang Q et al (2019) Remote light-responsive nanocarriers for controlled drug delivery: advances and perspectives. Small 15(45):1903060. https://doi.org/10.1002/smll.201903060
Zulfiqar U, Subhani T, Husain SW (2016a) Synthesis and characterization of silica nanoparticles from clay. J Asian Ceramic Soc 4(1):91–96. https://doi.org/10.1016/j.jascer.2015.12.001
Zulfiqar U, Subhani T, Wilayat Husain S (2016b) Synthesis of silica nanoparticles from sodium silicate under alkaline conditions. J Sol-Gel Sci Technol 77(3):753–758. https://doi.org/10.1007/s10971-015-3950-7
Acknowledgements
VK acknowledge the use of facilities created under DST-FIST (SR/FST/COLLEGE-/19/568), DBT Star Status (BT/HRD/11/030/2012) and DBT-BUILDER Schemes implemented at the Modern College of Arts, Science and Commerce, Ganeshkhind, Pune, India.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Barathe, P. et al. (2022). Nanomaterial-Mediated Delivery of Antimicrobial Agents: ‘The Nanocarriers’. In: Kumar, V., Shriram, V., Shukla, R., Gosavi, S. (eds) Nano-Strategies for Addressing Antimicrobial Resistance. Nanotechnology in the Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-031-10220-2_3
Download citation
DOI: https://doi.org/10.1007/978-3-031-10220-2_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-10219-6
Online ISBN: 978-3-031-10220-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)