Abstract
Dissections are universal during the endovascular treatment of lower extremity peripheral arterial disease. High-grade dissections and high residual narrowing predict higher target lesion revascularization and reduced patency rates. Using intravascular ultrasound, the depth and width of dissections can be more accurately classified. Several devices are now available to reduce deeper dissections with a wide range of plaque removal from none to soft debulking (>50% residual) to aggressive debulking (<50% residual). The interaction between the degree of debulking, deep dissections, application of antiproliferative therapies, and dissection repair is discussed in this chapter. Further data are needed to determine the impact of dissections on long-term outcomes.
Modified from original publication “The Quest for Optimal Peripheral Angioplasty: Controlling Dissections” in the Journal of Arterial Venous and Lymphatic Interventions (https://javelinjournal.org/the-quest-for-optimal-peripheral-angioplasty-controlling-dissections/).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Intravascular ultrasound
- Angiographic dissections
- iDissection
- NHLBI classification
- Adventitial tears
- Plaque excision
- Dissection repair
Injury to the deeper layers of an infrainguinal artery is a potent trigger for restenosis, loss of patency, and the need for future target revascularization [1,2,3]. Dissections are an inevitable consequence of balloon angioplasty (PTA) and are the main mechanism to gain minimal luminal area (MLA) and prevent vessel recoil [4]. Recent research has focused on how to balance the occurrence of dissections and the gain in MLA without a detrimental disruption to the deeper layers of an artery. Also the interaction between residual narrowing, dissections’ extent, dissection repair, and the use of antiproliferative therapy needs to be better explored because paclitaxel-coated balloons and dissection repair have been shown to partially mitigate some of the negative consequences of dissections. In this chapter, I will explore this concept more based on some findings from clinical trials.
Traditionally, the NHLBI classification has been used to classify dissections based on angiographic findings [5] (Table 6.1). This classification was adopted from the coronary literature and has several limitations. It only considers the single worst dissection regardless of number of dissections and does not consider the length, depth, and extent of dissections. Deeper injury is also not well appreciated using angiography, and therefore, this type of injury is not adequately or accurately captured by the NHLBI classification. These deeper injuries may not be visible or may appear low grade on angiogram. Larger arcs of dissections can also be missed on angiography and may not be well represented by the NHLBI classification. Despite its shortcomings, the NHLBI classification has been able to predict acute vessel closure and loss of patency [6, 7]. NHLBI types C–F showed a significantly lower patency rate (p < 0.001) and higher clinically driven TLR (p < 0.001) compared to type A and B dissections.
Recently, a new angiographic-based classification was published which is dedicated to peripheral arteries. In the DISFORM study, Voute et al. [8] obtained expert consensus on features of dissections in the femoropopliteal artery that can potentially predict a poor outcome following intervention. An expert panel of 17 interventionalists ranked dissection features that have the potential to lead to acute technical failure and/or early restenosis and which combination of features would require repair of these dissections to improve outcome. Panelists recommended scaffolding in the presence of significant diameter reduction, spiral shape, flow impairment, or adverse morphology (Fig. 6.1). The Flow Impairment in Peripheral Intervention (FLIP) method was adopted (Table 6.2). The relationship of this classification to clinical outcome is yet unclear and needs to be determined in future studies.
Precise Imaging
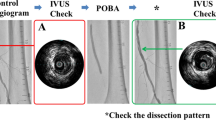
Precise imaging within the vessel wall is critical to evaluate the degree and extent of dissections. The iDissection grading system uses intravascular ultrasound (IVUS) to classify dissections in infrainguinal interventions. The depth of dissection is graded as A (intima), B (media), and C (adventitia). The arc of dissection is graded as 1 (less than 180°) or 2 (more or equal 180°). This six-grade classification (A1, B1, C1, A2, B2, C2) is reliable and can quickly be performed during the procedure (Fig. 6.2, Table 6.3) [9].
The iDissection grading system was used for the first time in a small study evaluating the number, depth, and extent of dissections following atherectomy [10]. In this study, Jetstream atherectomy (n = 13) and B-laser (n = 2) were used. De novo and non-stent restenotic lesions were included. Angiography and IVUS (Eagle Eye Platinum, Philips) were performed at baseline, post-atherectomy, and post-adjunctive balloon angioplasty. Core angiographic (Midwest Cardiovascular Research Foundation, Davenport, IA) and intravascular ultrasound (Midwest Cardiovascular Research Foundation, Davenport, IA, and St John Hospital, Detroit, Michigan) laboratories evaluated all images. In this study, critical limb ischemia was present in 26.7% of patients, and 60% of lesions had grade 3 and 4 PACCS grade calcification. Adjunctive balloon angioplasty was performed in all patients (Shockwave 33.3%, drug-coated balloons 100%). Mean balloon pressures and inflation times were 10.3 atmospheres and 310 s. Procedural success (<30% residual narrowing at the end of procedure) was accomplished in all patients, and residual narrowing post-angioplasty was 19.7%. Dissections were identified four to six times more on IVUS when compared to angiography (post-atherectomy and adjunctive angioplasty IVUS to angiographic dissection ratios were 5.75–1 and 3.55–1, respectively) (Fig. 6.1). Wider dissections >180° were also noted on IVUS in 13% and 31% post-atherectomy and adjunctive PTA, respectively. Furthermore, deeper dissections involving the media and adventitia occurred in 39.1% and 33.3% post-atherectomy and adjunctive PTA, respectively. Finally, IVUS identified intramural hematoma in 13.3% of vessels post-atherectomy. These results showed clearly that Jetstream atherectomy can create a damage to the inner layer of the arterial wall and potentially this may offset some of its benefit in reducing restenosis from plaque removal.
The iDissection classification also was tested with the Flex VP (VentureMed Group) atherotome and was found to have a low number of deeper dissections. The FLEX Vessel Prep System (VentureMed Group) is a one-size-fits-all device with three atherotomes mounted on a self-expanding treating element designed to create multiple longitudinal, controlled-depth, continuous micro-incisions across the entire lesion length. IVUS-based analysis post-FLEX VP was recently published and confirmed less deep dissections (media and adventitia) than seen with historical data from some atherectomy devices [11]. In 15 patients treated with the FLEX VP followed by adjunctive balloon angioplasty (Shockwave 33.3%, PTA 26.7%, drug-coated balloon 40%) for de novo or non-stent restenotic femoropopliteal disease, procedural success was 86.7% (<30% residual narrowing at the end of procedure). Minimal luminal area increased from a median of 5.2 to 15.0 mm2 (p < 0.001) with no change in reference lumen diameter or plaque burden area (p = 0.32). Of all new dissections (n = 37) post-FLEX VP and PTA, 18.9% were more than 180° in circumference, and 21.6% involved the media and adventitia. These numbers appear favorable compared to rotational and aspiration atherectomy, but head-to-head comparison data are not available. The low number of large flaps and deeper dissections may offer an explanation to the low provisional stenting seen with this device. The impact of these encouraging acute results on long-term outcomes is not yet known.
A recent study with the Auryon laser system has shown a very minimal number of C dissections based on the iDissection classification system [12]. In a prospective study of 29 patients, adventitial injury was assessed by IVUS following Auryon laser treatment and adjunctive balloon angioplasty. Core laboratory analysis was carried on all cases except for one patient (that crossed over to Jetstream atherectomy). Bailout stenting occurred in 21.4% patients (three for dissections, two for residual >30%, and one for both). By IVUS, there were 9 new dissections post-laser (1 adventitial; 3 ≥180°) and 21 new dissections post-laser and PTA (3 adventitial; 1 ≥180°). This small number of deep dissections can be attributed to the physics property of the long wavelength (355 nm) of the Auryon laser. The Auryon laser thermal injury dissipates quickly as it travels away from the tip of the catheter making deeper thermal injury less likely.
It is clear from precision imaging that various devices have the ability to remove a different amount of tissue from the treated vessels and lead to a range of deep damage to the inner layers of the artery. A balance between the extent of tissue excision and deep injury is likely to be of paramount interest when it comes to reducing long-term adverse events. The interaction of tissue removal, deep injury, dissection repair, and the application of antiproliferative therapy needs to be explored further.
The Relationship Between Deep Injury, Plaque Excision, Dissection Repair, and Antiproliferative Therapy
Several studies suggested an association between arterial dissections and high residual narrowing on poor outcomes in the treatment of infrainguinal arterial disease. However, the applications of antiproliferative therapy and dissections’ repair seem to mitigate at least partially the subsequent adverse outcomes of dissections and high residual narrowing. Below are some studies that may give some insights into this interaction.
Aggressive Debulking, High Rate of Deeper Dissections, and Antiproliferative Treatment
The Jetstream atherectomy device is a high-power device in plaque excision and leads to low residual narrowing on its own (typically less than 50%). However, it also causes its share of deeper dissections into the vessel wall by IVUS although angiographically this may not always be apparent [9]. In the JET Registry [13], a prospective study of Jetstream atherectomy with no drug-coated balloons (DCB), the freedom from TLR was 79–80% at 1 year. In the JET-SCE [14], a retrospective study of all comers treated with the Jetstream atherectomy device, freedom from TLR was reduced to 69.8% at 1 year, but the cohort of patients receiving Jetstream + DCB had a freedom from TLR of 95.2%. Recently, data from the JET-RANGER [15] study presented at TCT 2021 have shown that freedom from TLR was 100% at 1 year in the cohort of patients who received the Jetstream atherectomy and either the Ranger DCB or IN.PACT DCB balloons. In the JET-RANGER, there was no bailout stenting in the Jetstream + DCB cohort based on lack of angiographic presence of a flow-limiting dissection or the presence of more than 30% residual narrowing. These studies point to the following:
-
(a)
Aggressive debulking does not on its own lead to a better outcome.
-
(b)
The administration of antiproliferative therapy had a strong mitigating factor on reducing the poor outcomes of deeper dissections with (JET-SCE) or without (JET-RANGER) repair in the setting of aggressive debulking device.
No Debulking, Deeper Dissections, Dissection Repair, and/or Antiproliferative Treatment
Angioplasty (PTA) leads to high rate of deeper dissections particularly in complex arterial disease. Also PTA is not a debulking tool, and its mechanism of vessel expansion is mostly through dissections. Therefore, PTA leaves a high residual narrowing and leads to a high rate of deeper dissections. This combination is likely to be a major predictor of loss of patency and high TLR in most femoropopliteal or tibial interventions.
Multiple studies have shown that this poor outcome with PTA alone can be impacted positively by the application of DCB balloons [16,17,18] and repair of dissections [19,20,21,22,23] with limited metal left behind irrespective of the dissection severity (types A to F). With this wide application of focal repair of dissections with and without DCB, freedom from TLR seems to markedly improve when compared to historic control. Several randomized studies comparing DCB to PTA have shown a superiority in patency and freedom from TLR when compared to PTA alone. In the PTA + DCB arm of the JET-RANGER [15], a 92% freedom from TLR was noted at the expense of bailout stenting in almost half these patients. Lesions included in this study were complex with high rate of moderate to severe calcium, long lesions, and total occlusions which explain the need for high bailout stenting. In the TOBA II trial [21], an excellent outcome was seen with or without DCB after PTA when a strategy of focal repair was applied. Even mild type A and B NHLBI dissections were repaired in this study. This was also reproduced in the TOBA III study [22]. The application of wide focal repair or bailout stent to flow-limiting dissections along with DCB seems to have a strong mitigating factor on poor outcomes following treatment of femoropopliteal lesions. This was also seen in THUNDER trial [24] where DCB led to a significant reduction in TLR irrespective of the presence of dissections. These studies also point to the following:
-
(a)
PTA leads to a high rate of deeper dissections and leaves a large residual of plaque behind.
-
(b)
DCB with bailout stenting mitigates the poor outcome of PTA.
-
(c)
The application of a wide range of repair of dissections (types A to F) with or without DCB seems to have a positive impact on outcome.
Low-Level Debulking, Minimal Rate of Deep Dissections, and Antiproliferative Therapy
From the iDissection Auryon study [12], IVUS showed that the Auryon laser has a low level of debulking with residual narrowing over 50% after laser only, but no to minimal deep dissections. This serves as an interesting platform to determine how this impacts TLR with or without DCB. Bailout stenting, when occurred, was driven by either the occurrence of dissections or high rate of residual narrowing after adjunctive PTA following the laser.
In the EX-PAD-03 IDE study [25], bailout stenting was very low, and the freedom from TLR was lower than 5% at 6 months in all comers including femoropopliteal artery disease or tibial disease and irrespective of the lesion being de novo or restenotic (stent or not). Also in this study, only 60% of treated limbs received a DCB. The limited damage to the deeper layers of the vessel could have played an important role in reducing the TLR rate. Patency was also very favorable when compared to historic control. The Auryon SCE (In print JIC 2022) was a retrospective study that evaluated the impact of the Auryon laser on outcome in a real-world cohort of patients. In this study, a total of 56 patients (66 procedures, 71 lesions) were enrolled. 48.2% were diabetics and 25% had limb ischemia. Baseline stenosis was 91.4 ± 9.7%, post-laser 56.0 ± 17.3%, and post-final treatment 11.6 ± 11.2%. Lesion length was 117.1 ± 100.4 mm and treated length 177.3 ± 115.5 mm. Bailout stenting occurred in 12/66 procedures (18.2%). Post-laser, there was no D dissection, and post-laser + PTA, there was one D dissection. DCB usage was the following: 46.5% Lutonix, 28.2% IN.PACT, and 1.4% both. The probability of freedom from TLR was 100.0% at 6 months.
Orbital atherectomy has likely the same mechanism of action with softer debulking. The TRUTH study [26] has shown a low rate of deep dissections by IVUS following orbital atherectomy. In the COMPLIANCE trial [27], residual narrowing was very low with a low rate of bailout stenting at 5.3%. Patency at 1 year was high at 81.2% despite the presence of severe calcium and complex disease enrolled.
These studies point to the following:
-
(a)
A soft debulking approach while preserving the deeper layer of the vessel has an excellent short-term and intermediate-term outcome despite a low rate of overall bailout stenting with or without drug-coated balloons.
-
(b)
Long-term outcomes need to be evaluated to ensure no late loss of patency and increase in TLR.
Other Technologies to Reduce Dissections
Several technologies besides atherectomy and the FLEX VP were developed to control dissections as seen on angiography. These include cutting (Boston Scientific Corp.) and scoring balloons (AngioSculpt® scoring balloon (Philips); UltraScore Focal Force (BD/Bard)), the Serranator (Cagent Vascular), and the lithotripsy shockwave balloon (Shockwave Medical).
Cutting balloons (CB) continue to show angiographic dissections post-treatment of femoropopliteal lesions, and more than half of these dissections are NHLBI type C or higher (54.8%). High-grade angiographic dissections have been shown to correlate with loss of patency on follow-up. CB was shown by IVUS to be more effective than scoring balloons [28, 29] in modifying calcified plaque with a higher acute luminal gain and better stent symmetry. In calcified lesions, the larger luminal gain occurs in lesions with evidence of dissections and without significant change in vessel expansion (external elastic lumen surface area remains unchanged) or plaque-media cross-sectional area. On the other hand, CB in non-calcified lesions yields larger lumen area mostly by larger plaque reduction and less vessel expansion compared to PTA [30]. The depth and extent of dissections seen by IVUS following CB have not been well defined. CB as a sole intervention has not been shown to yield better outcome than PTA in treating restenotic or femoropopliteal disease.
The AngioSculpt scoring balloon (Philips) has three nitinol spiral elements mounted on the surface of a semi-compliant balloon. This allows a homogenous transmission of pressure over the plaque, theoretically reducing dissections irrespective of calcification. AngioSculpt, when assessed by IVUS, increased minimal luminal area post-stenting [31] and had a low rate of angiographic dissections and stenting [32,33,34]. It also had no impact on target lesion revascularization. The UltraScore Focused Force PTA balloon (Bard/BD) has two longitudinal wires intended to concentrate the force against the plaque for a controlled fracture at low pressure. IVUS-based patterns of dissections with this balloon are lacking.
Lithotripsy using the Shockwave balloon [35] uses sound waves to disrupt calcium in the vessel wall. Optical coherence tomography (OCT) has shown that lithoplasty modifies calcium with fracture as the predominant mechanism leading to significant favorable luminal area gain and stent expansion [35]. Applications of lithoplasty to calcified stenotic common femoral artery also showed excellent acute success with no dissections needing stenting. There were only 5 NHLBI type B non-flow-limiting dissections (out of 21 patients treated) and no perforation, distal embolization, or abrupt closure [36]. Patterns of dissections using IVUS with Shockwave lithoplasty have not been defined.
Dissection Repair
Dissection repair has been recently introduced as a strategy to improve outcomes of infrainguinal interventions. Repair of dissections in the TOBA BKA study showed a freedom from CD-TLR of 93.5% and patency of 78.4% at 1 year, significantly better than historic control [23]. In the TOBA II study [21], 213 patients with 100% dissected vessels following plain old balloon angioplasty or drug-coated balloons underwent repair of their dissections using the Tack Endovascular System. Of all dissections identified, 92.1% were repaired. Freedom from TLR and patency at 1 year were 86.5% and 79.3%, respectively. Bailout stent rate was 0.5%.
In conclusion, high-grade dissections and high residual narrowing predict higher TLR rates and reduced patency. Using IVUS, the depth and width of dissections can be more accurately classified. Several devices are now available to reduce deeper dissections with a wide range of plaque removal from none to soft debulking (>50% residual) to aggressive debulking (<50% residual). The interaction between the degree of debulking, deep dissections, application of antiproliferative therapies, and dissection repair needs to be further explored to determine their impact on long-term outcomes.
References
Tarricone A, Ali Z, Rajamanickam A, et al. Histopathological evidence of adventitial or medial injury is a strong predictor of restenosis during directional atherectomy for peripheral artery disease. J Endovasc Ther. 2015;22:712–5.
Miki K, Fujii K, Kawasaki D, Shibuya M, Fukunaga M, Imanaka T, Tamaru H, Sumiyoshi A, Nishimura M, Horimatsu T, Saita T, Okada K, Kimura T, Honda Y, Fitzgerald PJ, Masuyama T, Ishihara M. Intravascular ultrasound-derived stent dimensions as predictors of angiographic restenosis following nitinol stent implantation in the superficial femoral artery. J Endovasc Ther. 2016;23(3):424–32. https://doi.org/10.1177/1526602816641669. Epub 2016 Apr 4.
Shammas NW, Radaideh Q, Shammas WJ, Daher GE, Rachwan RJ, Radaideh Y. The role of precise imaging with intravascular ultrasound in coronary and peripheral interventions. Vasc Health Risk Manag. 2019;15:283–90. https://doi.org/10.2147/VHRM.S210928. eCollection 2019.
Finet G, Abrysch F, Rioufol G, Ohayon J, Lagache M. Qualitative and quantitative descriptions of the mechanisms of action of the angioplasty balloon on coronary stenosis. An endovascular ultrasonic study. Arch Mal Coeur Vaiss. 2000;93:1109–17.
Rogers JH, Lasala JM. Coronary artery dissection and perforation complicating percutaneous coronary intervention. J Invasive Cardiol. 2004;16(9):493–9.
Fujihara M, Takahara M, Sasaki S, Nanto K, Utsunomiya M, Iida O, Yokoi Y. Angiographic dissection patterns and patency outcomes after balloon angioplasty for superficial femoral artery disease. J Endovasc Ther. 2017;24(3):367–75. https://doi.org/10.1177/1526602817698634. Epub 2017 Mar 20.
Kobayashi N, Hirano K, Yamawaki M, Araki M, Sakai T, Sakamoto Y, Mori S, Tsutsumi M, Honda Y, Ito Y. Simple classification and clinical outcomes of angiographic dissection after balloon angioplasty for femoropopliteal disease. J Vasc Surg. 2018;67(4):1151–8. https://doi.org/10.1016/j.jvs.2017.08.092. Epub 2017 Dec 11.
Voûte MT, Stathis A, Schneider PA, Thomas SD, Brodmann M, Armstrong EJ, Holden A, Varcoe RL. Delphi consensus study toward a comprehensive classification system for angioplasty-induced femoropopliteal dissection: the DISFORM study. JACC Cardiovasc Interv. 2021;14(21):2391–401.
Shammas NW, Torey JT, Shammas WJ. Dissections in peripheral vascular interventions: a proposed classification using intravascular ultrasound. J Invasive Cardiol. 2018;30(4):145–6.
Shammas NW, Torey JT, Shammas WJ, Jones-Miller S, Shammas GA. Intravascular ultrasound assessment and correlation with angiographic findings demonstrating femoropopliteal arterial dissections post atherectomy: results from the iDissection study. J Invasive Cardiol. 2018;30(7):240–4.
Shammas NW, Shammas WJ, Jones-Miller S, Radaideh Q, Shammas GA. Femoropopliteal arterial dissections post flex vessel prep and adjunctive angioplasty: results of the flex iDissection study. J Invasive Cardiol. 2019;31(5):121–6.
Shammas NW, Torey JT, Shammas WJ, Jones-Miller S, Shammas GA. Intravascular ultrasound assessment and correlation with angiographic findings of arterial dissections following Auryon laser Atherectomy and adjunctive balloon angioplasty: results of the iDissection Auryon laser study. J Endovasc Ther. 2022;29(1):23–31. https://doi.org/10.1177/15266028211028200. Online ahead of print.
Gray WA, Garcia LA, Amin A, Shammas NW, JET Registry Investigators. Jetstream atherectomy system treatment of femoropopliteal arteries: results of the post-market JET registry. Cardiovasc Revasc Med. 2018;19(5 Pt A):506–11. https://doi.org/10.1016/j.carrev.2017.12.015. Epub 2017 Dec 27.
Shammas NW, Shammas GA, Jones-Miller S, Shammas WJ, Bou-Dargham B, Shammas AN, Banerjee S, Rachwan RJ, Daher GE. Long-term outcomes with Jetstream atherectomy with or without drug coated balloons in treating femoropopliteal arteries: a single center experience (JET-SCE). Cardiovasc Revasc Med. 2018;19(7 Pt A):771–7. https://doi.org/10.1016/j.carrev.2018.02.003. Epub 2018 Feb 9.
Shammas NW, Purushottam B, Shammas WJ, et al. TCT-17 Jetstream atherectomy followed by paclitaxel-coated balloons versus balloon angioplasty followed by paclitaxel-coated balloons: twelve-month results of the prospective randomized JET-RANGER study. J Am Coll Cardiol. 2021;78(19):B8. https://doi.org/10.1016/j.jacc.2021.09.876.
Tepe G, Laird J, Schneider P, Brodmann M, Krishnan P, Micari A, Metzger C, Scheinert D, Zeller T, Cohen DJ, Snead DB, Alexander B, Landini M, Jaff MR, IN.PACT SFA Trial Investigators. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation. 2015;131:495–502.
Krishnan P, Faries P, Niazi K, Jain A, Sachar R, Bachinsky WB, Cardenas J, Werner M, Brodmann M, Mustapha JA, Mena-Hurtado C, Jaff MR, Holden AH, Lyden SP. Stellarex drug-coated balloon for treatment of femoropopliteal disease: twelve-month outcomes from the randomized ILLUMENATE pivotal and pharmacokinetic studies. Circulation. 2017;136:1102–13.
Rosenfield K, Jaff MR, White CJ, Rocha-Singh K, Mena-Hurtado C, Metzger DC, Brodmann M, Pilger E, Zeller T, Krishnan P, Gammon R, Müller-Hülsbeck S, Nehler MR, Benenati JF, Scheinert D, LEVANT 2 Investigators. Trial of a paclitaxel-coated balloon for femoropopliteal artery disease. N Engl J Med. 2015;373:145–53.
Bosiers M, Scheinert D, Hendriks JM, et al. Results from the tack optimized balloon angioplasty (TOBA) study demonstrate the benefits of minimal metal implants for dissection repair after angioplasty. J Vasc Surg. 2016;64:109–16.
Geraghty P, Adams GL, Schmidt A, Lichtenberg M, Wissgott C, Armstrong EJ, Hertting K, on behalf of the TOBA II BTK Investigators. Twelve-month results of tack-optimized balloon angioplasty using the tack endovascular system in below-the-knee arteries (TOBA II BTK). J Endovasc Ther. 2020;27(4):626–36.
Gray WA, Cardenas JA, Brodmann M, et al. Treating post-angioplasty dissection in the femoropopliteal arteries using the tack endovascular system: 12-month results from the TOBA II study. JACC Cardiovasc Interv. 2019;12:2375–84.
Brodmann M, Wissgott C, Brechtel K, et al. Optimized drug-coated balloon angioplasty of the superficial femoral and proximal popliteal arteries using the tack endovascular system: TOBA III 12-month results. J Vasc Surg. 2020;72(5):1636–1647.e1. https://doi.org/10.1016/j.jvs.2020.01.078.
Brodmann M, Wissgott C, Holden A, et al. Treatment of infrapopliteal post-PTA dissection with tack implants: 12-month results from the TOBA-BTK study. Catheter Cardiovasc Interv. 2018;92:96–105.
Tepe G, Zeller T, Schnorr B, Claussen CD, Beschorner U, Brechtel K, Scheller B, Speck U. High-grade, non-flow-limiting dissections do not negatively impact long-term outcome after paclitaxel-coated balloon angioplasty: an additional analysis from the THUNDER study. J Endovasc Ther. 2013;20(6):792–800. https://doi.org/10.1583/13-4392R.1.
Shammas NW, Chandra P, Brodmann M, Weinstock B, Sedillo G, Cawich I, Micari A, Lee A, Metzger C, Palena LM, Rundback J, EX-PAD-03 Investigators. Acute and 30-day safety and effectiveness evaluation of Eximo Medical’s B-laser™, a novel Atherectomy device, in subjects affected with infrainguinal peripheral arterial disease: results of the EX-PAD-03 trial. Cardiovasc Revasc Med. 2020;21(1):86–92. https://doi.org/10.1016/j.carrev.2018.11.022. Epub 2018 Nov 29.
Babaev A, Zavlunova S, Attubato MJ, Martinsen BJ, Mintz GS, Maehara A. Orbital atherectomy plaque modification assessment of the femoropopliteal artery via intravascular ultrasound (TRUTH study). Vasc Endovasc Surg. 2015;49(7):188–94. https://doi.org/10.1177/1538574415607361. Epub 2015 Oct 20.
Dattilo R, Himmelstein SI, Cuff RF. The COMPLIANCE 360° trial: a randomized, prospective, multicenter, pilot study comparing acute and long-term results of orbital atherectomy to balloon angioplasty for calcified femoropopliteal disease. J Invasive Cardiol. 2014;26(8):355–60.
Miyazaki T, Latib A, Ruparelia N, Kawamoto H, Sato K, Figini F, Colombo A. The use of a scoring balloon for optimal lesion preparation prior to bioresorbable scaffold implantation: a comparison with conventional balloon predilatation. EuroIntervention. 2016;11(14):e1580–8. https://doi.org/10.4244/EIJV11I14A308.
Matsukawa R, Kozai T, Tokutome M, Nakashima R, Nishimura R, Matsumoto S, Katsuki M, Masuda S, Meno H. Plaque modification using a cutting balloon is more effective for stenting of heavily calcified lesion than other scoring balloons. Cardiovasc Interv Ther. 2019;34(4):325–34. https://doi.org/10.1007/s12928-019-00578-w. [Epub ahead of print].
Okura H, Hayase M, Shimodozono S, Kobayashi T, Sano K, Matsushita T, Kondo T, Kijima M, Nishikawa H, Kurogane H, Aizawa T, Hosokawa H, Suzuki T, Yamaguchi T, Bonneau HN, Yock PG, Fitzgerald PJ, REDUCE Investigators. Restenosis reduction by cutting balloon evaluation. Mechanisms of acute lumen gain following cutting balloon angioplasty in calcified and noncalcified lesions: an intravascular ultrasound study. Catheter Cardiovasc Interv. 2002;57(4):429–36.
Fonseca A, Costa JR, Abizaid A, et al. Intravascular ultrasound assessment of the novel AngioSculpt scoring balloon catheter for the treatment of complex coronary lesions. J Invasive Cardiol. 2008;20:21–7.
Kiesz RS, Scheinert D, Peeters PJ, et al. Results from the international registry of the AngioSculpt scoring balloon catheter for the treatment of infrapopliteal disease. J Am Coll Cardiol. 2008;51(10 (suppl B)):75.
Scheinert D, Peeters P, Bosiers M, et al. Results of the multicenter first-in-man study of a novel scoring balloon catheter for the treatment of infra-popliteal peripheral arterial disease. Catheter Cardiovasc Interv. 2007;70:1034–9.
Bosiers M, et al. Use of the AngioSculpt scoring balloon for infrapopliteal lesions in patients with critical limb ischemia: 1-year outcome. Vascular. 2009;17(1):29–35.
Ali ZA, Brinton TJ, Hill JM, Maehara A, Matsumura M, Karimi Galougahi K, Illindala U, Götberg M, Whitbourn R, Van Mieghem N, Meredith IT, Di Mario C, Fajadet J. Optical coherence tomography characterization of coronary lithoplasty for treatment of calcified lesions: first description. JACC Cardiovasc Imaging. 2017;10(8):897–906. https://doi.org/10.1016/j.jcmg.2017.05.012.
Brodmann M, Schwindt A, Argyriou A, Gammon R. Safety and feasibility of intravascular lithotripsy for treatment of common femoral artery stenoses. J Endovasc Ther. 2019;26(3):283–7.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Shammas, N.W. (2022). Controlling Dissections in Peripheral Arterial Interventions. In: Shammas, N.W. (eds) Peripheral Arterial Interventions. Contemporary Cardiology. Springer, Cham. https://doi.org/10.1007/978-3-031-09741-6_6
Download citation
DOI: https://doi.org/10.1007/978-3-031-09741-6_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-09740-9
Online ISBN: 978-3-031-09741-6
eBook Packages: MedicineMedicine (R0)