Abstract
The concentrations of sex steroids modify the sensitivity to stressors in mammals: progesterone, reduce the stress response, while progesterone withdrawal increases it. Estrogens appear to increase the sensitivity to stressors, influencing the hypothalamic-pituitary-adrenal axis response. As the concentration of progesterone and estrogens differ according to the physiological status, such as pregnancy, anestrous or the phases of the estrous cycle, it may also modify the sensitivity to stressors. Therefore, it would be important to consider this information for various practices in farm animals as they are frequently subjected to stressful situations, including artificial weaning, shearing, isolation from the group, and transport. Moreover, pharmacological treatments with these hormones are commonly applied to cows, ewes, does, or buffaloes for using different biotechnologies, such as estrous synchronization, estrous induction, or synchronization of the ovulations. Accordingly, it is important to consider that those treatments might modify the sensitivity of females’ farm ruminants to human handling.
The chapter summarizes how sexual steroid profiles might change female ruminants’ stress response and welfare. The major points included are: a brief description of the natural variation in sex steroid profiles in female ruminants; the main hormones and administration pathways used in the application of reproductive biotechnologies; the different physiological indicators of stress and welfare considered in farm animals; and the main knowledge available on the effects of sex steroids on the stress responses of female ruminants to different farm practices. Lastly, we propose directions in which research is needed to understand better if stress, behavior, and welfare of female farm animals could be affected by the application of reproductive biotechnologies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Progesterone
- Progestagens
- Estrogen
- Progesterone withdrawal
- Oestrous cycle
- Hypothalamic-pituitary-adrenal axis
- Glucocorticoids
- Sheep
- Cattle
- Goat
1.1 Introduction
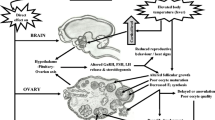
During the last years, hormonal treatments for reproductive biotechnologies in farm animals increased widely throughout the world. The development of fixed-timed artificial insemination treatments in cattle has a significant impact on the economy and employment of farm animal production (Mapletoft et al. 2018). Most of these treatments include administering sexual steroids, mainly progesterone or progestogens, and different estradiol formulas. Sex steroids are primarily related to reproductive functions, but these hormones also affect metabolism (Ashley et al. 2000; Kalkhoff 1982), immunological status (Bouman et al. 2005), functions in the central nervous system (Genazzani et al. 2002), and can also modulate the stress response (Sze and Brunton 2019). The effects of sex steroids on the stress response are of particular interest in farm animals as they might directly influence their welfare. However, these issues have been mainly studied in laboratory animals and humans, with a paucity of information on farm animals. In ewes, it was reported that the administration of progesterone reduces the stress response at weaning (Freitas-de-Melo et al. 2013), while progesterone withdrawal increases the secretion of cortisol after social isolation (Freitas-de-Melo et al. 2016). Ewes treated with estradiol benzoate respond to an ACTH challenge with greater cortisol concentrations than non-treated ewes (Van Lier et al. 2014).
The concentration of progesterone and estrogens differs according to the physiological status (pregnant, anestrous, or cycling females); thus, it might modify the sensitivity to stressors. Pregnant ewes have slighter behavioral responses than non-pregnant ewes to social isolation or a surprise effect (Viérin and Bouissou 2001) and shearing (Ungerfeld and Freitas-de-Melo 2019). Furthermore, the sensitivity to stressors is greater during the follicular than during the luteal phase (Pinto-Santini and Ungerfeld 2019; Freitas-de-Melo et al., 2022). The reactivity of cows also varies according to the reproductive status, with decreased reactions of pregnant cows to human handling (Freitas-de-Melo et al. 2019).
How female farm animals cope with stressful situations directly affects its’ productive, reproductive, and welfare outcomes; thus, the reproductive status and the concentration of different sex steroids should be considered when different managements are applied in these animals. For example, it has been widely known that stress affects cow fertility (see review: Dobson and Smith 2000). However, it is not well studied if the application of hormonal treatments for reproductive biotechnologies might modify the easiness of animal handling, productive efficiency, or animals’ welfare.
This chapter aimed to present a brief reminder on how profiles of sexual steroids vary according to the physiological status in female ruminants; the main hormones and administration pathways used in the application of reproductive biotechnologies; the main physiological indicators of stress and welfare; and the knowledge available on the effects of sex steroids during stressful situations in female ruminants. Lastly, we propose directions in which research is needed to understand better if the application of reproductive biotechnologies can affect the sensitivity to stressors, the behavioral responses, and the welfare of female farm animals.
1.2 Natural Variation in Sex Steroid Profiles in Female Ruminants
1.2.1 Estrous Cycle
Sexual steroid concentrations vary naturally throughout the reproductive lives of female ruminants. Before puberty, progesterone concentrations remain at basal levels, and estrogen concentrations may have some oscillations due to the physiological reproductive changes that determine puberty. However, estrogen concentration does not reach concentrations similar to those observed during regular estrous cycles. Since puberty, the estrous cycle is a set of reproductive events that repeats successively. While in sheep, it lasts an average of 17 days, in other ruminants, such as cow, goat, or buffalo, its’ length is approximately 21 days. The estrous cycle can be divided into a luteal phase, which in ewes extends from day 2–3 (heat = day 0) of the cycle, to approximately day 13–14; and a follicular phase that goes from luteolysis that occurs on day 13–14 until day 2. In species with estrous cycles of 21 days, the general pattern is the same, maintaining the proportion of the duration of these two phases (Fig. 1.1).
An illustration with the follicular phase on the right and left and the luteal phase in the middle. The follicular phase has an image of cows mating. Progesterone (blue line) and estrogen (red line) profiles during an estrous cycle in cows. The luteal phase is characterized by high concentrations of progesterone and low concentration of estrogens, and during the follicular phase, the opposite hormonal pattern is observed. The line for progesterone is at its peak in the luteal phase and descends in the follicular phase and the one for estrogen is at its peak during the follicular phase and lowest during the luteal phase
The follicular phase extends from the regression of the corpus luteum to ovulation. During the follicular phase, the growth of the ovulatory follicle ends with the luteinizing hormone surge, ovulation, and the beginning of the follicle luteinization. The ovulatory follicle(s) secrete substantial concentrations of estrogens, which are responsible for triggering the increase in luteinizing hormone secretion, reaching the luteinizing hormone peak during the second half of heat. In turn, these estrogens are responsible for determining the receptive behavior in the female. In summary, estrogen concentrations are high during the follicular phase, increasing from the beginning of this phase until ovulation, when a rather abrupt decrease in concentration begins. The preovulatory luteinizing hormone peak triggers the ovulation of the preovulatory follicle(s), and luteinization of the remaining structure(s), with the subsequent formation of the corpus luteum. As the corpus luteum develops, the amounts of progesterone secreted by it increase. After ovulation, the weight of the corpus luteum increases associated with an important angiogenesis process and an increase in the size of the luteal cells (Smith et al. 1993). The newly formed corpus luteum receives substantial amounts of blood flow in relation to its size. The production of progesterone increases more than ten times due to the increase of the enzymes that control the precursors of this steroidogenic pathway. In contrast, the production of androgens and estrogens decreases due to the enzymes’ loss involved in their synthesis.
1.2.2 Seasonality
According to the species, the estrous cycles are repeated successively in the non-pregnant animal throughout the year in non-seasonal polyestric species, like cows, or only during part of the year, in seasonal polyestric species, like sheep and goat (Fig. 1.2). Although there are variations according to the breed and the latitude where sheep and goats live, the breeding season comprises successive estrous cycles occurring during summer and autumn. The period of the year in which there are no estrous cycles or ovulations is the seasonal anestrus, when progesterone concentration remains at baseline, at subluteal concentrations. During this period, there are small oscillations in the estrogen concentration associated with follicular development, but without reaching the level of a follicular phase.
Model of the seasonality of reproduction in sheep. Blue lines show progesterone concentrations, with increases in each estrous cycle. The black arrows indicate when heat occurs, and the estrogen concentration is more remarkable. A graph with time on the x-axis and progesterone concentration on the y-axis. At the beginning and end are a series of continuous peaks for the breeding season with an image of ewes mating, and in between these two series of peaks is a flat line for seasonal anestrus. Arrows are pointing to the dips in the peaks
1.2.3 Gestation and Postpartum Anestrus
Progesterone is the main hormone responsible for the maintenance of pregnancy, so during this period, luteolysis does not occur, maintaining active the corpus luteum (Fig. 1.3). After conception, the embryo produces chemical signals that prevent luteolysis, which in ruminants is mainly interferon tau. The progesterone produced by the corpus luteum is essential to maintain pregnancy during the first half of it in all species. In some species, such as sheep, the placenta also produces progesterone in amounts enough to maintain pregnancy from the mid-gestation until its end. In other species, e.g., cow and goat, the placenta also produces progesterone, but the amount produced is below the threshold necessary to maintain pregnancy per se, so progesterone produced by the corpus luteum is essential to maintain the pregnancy. In any case, progesterone concentrations remain elevated throughout pregnancy.
Changes in progesterone concentrations during the estrous cycle (dotted line) or early gestation in cows (continuous line). A graph with days after estrus on the x-axis and progesterone concentration on the y-axis. An image of a cow mating with estrus written below is presented at beginning of the graph. The line for the pregnant cow has an ascending curve that later flatlines and the line for non-pregnant cow has the same ascending curve but dips completely at 16
1.3 Exogenous Hormonal Control of Reproduction in Female Ruminants
The control of reproduction for the application of reproductive biotechnologies requires the exogenous administration of sexual steroids. Treatments for applying different biotechnologies, such as estrous synchronization, estrous induction, synchronization of the ovulations, or follicular development for superovulation, require controlling the estrous cycle and/or follicular development. There are several reviews on these topics (Bó et al. 2016; Bó and Baruselli 2014), so this section summarizes the different hormones and routes of administration used in farm ruminants. Table 1.1 summarizes information on the progestogens more frequently used in reproductive management in farm ruminants.
1.3.1 Progestagens and Progesterone Treatments
The use of treatments based on mimicking a luteal phase requires the administration of sustained amounts of progesterone or progestagens. Progestagens are synthetic molecules with similar reproductive effects to progesterone. In cattle, sheep, and goats progesterone is administered by intravaginal silicone devices impregnated with synthetic progesterone, identical to that natural molecule produced by the corpus luteum. These devices are inserted and remain in situ for 5–7 days, depending on the protocol used. After the insertion of these devices, progesterone concentration achieved in the blood increases sharply, remaining at luteal concentrations throughout the treatment (Fig. 1.4). These devices are frequently used more than once (Vilariño et al. 2011, 2013; Oliveira et al. 2001). Some authors autoclaved these devices before a second application, increasing progesterone concentration shortly after being inserted, achieving values greater than with used non-autoclaved devices (cattle: Zuluaga and Williams 2008; sheep: Ungerfeld et al. 2013; goats: Alvarez et al. 2013). However, in general, concentrations achieved with used devices are lower than those achieved with new devices (Fig. 1.5).
Patterns of fluorogestone, medroxyprogesterone, and progesterone blood concentrations in small ruminants after the insertion of commercial devices. The pattern is based on Gaston-Parry et al. (1988), Greyling et al. (1994), and Rubianes et al. (1998). There are three line graphs with time on the x-axis and progestogen concentration on the y-axis. The graph on top has a steep increase and later dips a little and flatlines. The second graph begins from the top and descends with a small peak in between. The last one has a steep peak which dips a little and flatlines, and finally dips completely
Variations in progesterone concentrations achieved in the blood of goats after the insertion of a new intravaginal silicone device impregnated with progesterone (continuous black line), a device used once before (grey line), or a device used twice before (black-spotted line) (Redrawn from Vilariño et al. 2011). A line graph with the time of the x-axis and progesterone concentration on the y-axis. There are three lines in the graph, and all three of them have a steep peak that descends slowly and finally dips. The peak value of the first line is 20, and the lowest is around 7
Similar devices are also used in sheep and goats, but traditional long treatments are frequently used, lasting 12–14 days in sheep and 14–16 days in goats. In this case, progesterone concentrations decrease to subluteal concentrations after 8–10 days of application (Fig. 1.4). During the last period of these treatments, progesterone concentrations are below to those observed during a normal late luteal phase. In these species, intravaginal sponges impregnated with synthetic progestagens, such as medroxyprogesterone and fluorogestone acetate, are more frequently used. There are commercial sponges with different quantities of progestagens, which in general exceed the amount needed for the desired reproductive effects (Ungerfeld et al. 2003). There is scarce information on how the concentrations of progestagens vary in blood after the insertion of the device. In all cases, progestagen concentration decreases throughout the treatment, but it is difficult to know its effects compared to progesterone, as progestogens have a longer half-life and potency. Concerning the main aim of this chapter, there is even less information about the possible impact of these hormones on the sensitivity to stressors in ewes and does.
Little is known on the effectiveness of injectable progesterone in oil-based formulas (cattle: Andrade et al. 2020; Morotti et al. 2018; Gimenes et al. 2009; goats: Álvarado-Espino et al. 2019a, b), which were developed to substitute the administration through devices. This strategy aims to reduce the hormonal residues in the environment, which can have deleterious effects on native fauna (Sauer et al. 2018; Liu et al. 2015). The effectiveness of injectable progesterone in oil-based is related to the formula used, determining when progesterone concentrations remain over luteal concentrations. The injectable progesterone might be impractical, as in most commercial products, the hormone should be administered several times. The scarce information available suggests that after a single administration of long-acting progesterone prepared in an oil solution decreases after 52 (Cavestany et al. 2008) to 96 h (Corréa Rocha et al. 2011) in cows, or 12–16 h in ewes (Ungerfeld and Freitas-de-Melo, unpublished data).
1.3.2 Estrogens
Although in some countries, the use of estrogens is not allowed, these hormones are widely used, mainly in treatments to control the follicular dynamics in cows. Estrogens are used in treatments to synchronize the ovulation associated with fixed-timed artificial insemination in cows. Although originally follicular wave was synchronized with estradiol-17β (Bó et al. 1994), currently the hormones more commonly used are estradiol benzoate and estradiol cypionate (Monteiro et al. 2015; Sales et al. 2012; Martínez et al. 2000). As it has low solubility in water, estradiol cypionate has a longer half-life than estradiol benzoate or estradiol-17β (Vynchier et al. 1990). In sheep and goats, there are scarce studies using estradiol esters. In some studies, estradiol-17β was initially used to determine if it might synchronize the follicular wave (Ungerfeld et al. 2004), as occurs in cattle. Later, it was used associated with progesterone treatments to synchronize the follicular dynamics for estrous induction (Ungerfeld 2008, 2009) or associated with superovulatory treatments (Souza-Fabjan et al. 2017). More recently, it has been used to synchronize the ovulation for timed artificial insemination in ewes and does (Cosentino et al. 2019). Estradiol benzoate has also been used to promote cervical relaxation in ewes before insemination or embryo collection (Hauschildt Dias et al. 2020).
1.4 Stress Response and Welfare
According to Broom (1986), the welfare of an animal is its state regarding its attempts to cope with its environment. Welfare includes the coordination of different responses, including physiological, behavioral, and immunological responses, and therefore, cannot be evaluated without including several aspects. In livestock cattle, sheep, and goats, females are repeatedly subjected to different challenges, which include human handlings, feeding management, social influences, and the occurrence of pathogens (Broom and Fraser 2015). All these challenges generate physiological, behavioral and immunological responses (Dobson and Smith 2000). As stress implies the modification of the homeostasis of the animal, the coordination of different systems is necessary to return the animal physiology to equilibrium. The responses to the same stressors – considered as any stimulus that triggers a stress response- might differ among different individuals, both in magnitude and duration, depending on their previous experience with that stressor and their temperament (Earley et al. 2010; McEwen and Wingfield 2003). However, other factors such as gender, age, or physiological reproductive status modulate the response to stressors (Freitas-de-Melo and Ungerfeld 2016a; Mormedè et al. 2007; Dallman et al. 2002).
Depending on the intensity of the stressor and the lapse during which it acts, the response can be classified as acute or chronic. When the stressor induces a short response enough to get back to the homeostatic status, it is classified as acute. However, if the stressor continues acting for longer or triggers an intense response with which the animal cannot cope, this is considered a chronic stress response.
1.4.1 Acute Stress Response
There are many different types of acute stressors, but in general, it is assumed that an acute stressor acts from minutes to hours, and the animal should cope with it, returning quickly to the status that it had before the action of the stressor. Acute stressors simultaneously trigger both responding pathways, including the sympathetic autonomic nervous system and the neuroendocrine system (Carrasco and Van de Kar 2003; O’Connor et al. 2021). The sympathetic system is activated immediately after a stressful situation, displaying the main responses a few seconds or even minutes after the perception of the stressor (Charmandari et al. 2005). However, its’ action is of short duration, ending quickly. On the other hand, although the neuroendocrine response takes longer to display the main effects, it is maintained during extended periods, from some minutes to hours. Both systems act simultaneously and synergistically, as glucocorticoids are synthesized and released into the blood; this pathway requires more time to be evidenced (Carrasco and Van de Kar 2003; Matteri et al. 2000). The activation of both responses leads to physiological and behavioral changes necessary to cope with the stressor (Mormedè et al. 2007).
The activation of the sympathetic autonomic nervous system provokes the release of catecholamines into the sympathetic synapses, including epinephrine and norepinephrine. The chromaffin cells located in the medulla of the adrenal glands produce both catecholamines and release them into the bloodstream. In general, catecholamines increase the heart and respiratory rates, as well as the body temperature. Catecholamines also stimulate glycogenolysis and lipolysis, increasing glycemia and energy availability. The activation of the sympathetic autonomic nervous system also causes mydriasis, vasodilation in skeletal muscle and peripheral vasoconstriction (Matteri et al. 2000). Therefore, the activation of the sympathetic autonomic nervous system produces a blood redistribution to prioritize the perfusion of the brain, heart, and skeletal muscles. Catecholamines also modify the activity of the animal, increasing its’ alertness, vigilance, and animal excitement (Sabban 2010).
When the animal perceives the stressor, the hypothalamus releases corticotrophin-releasing hormone and vasopressin into the portal system. Corticotrophin-releasing hormone acts at the adenohypophysis, stimulating the release of the adrenocorticotrophic hormone into the bloodstream (Matteri et al. 2000). The adrenocorticotrophic hormone stimulates the secretion of glucocorticoids, mainly cortisol in domestic ruminants and corticosterone in rodents, in the cortex of the adrenal gland (Matteri et al. 2000). Glucocorticoids stimulate gluconeogenesis, lipolysis, and the catabolism of proteins, increasing glycemia and energy availability (Kudielka and Kirschbaum 2007). Glucocorticoids also promote an increase in cerebral perfusion, the use of glucose, an increase of blood pressure in most blood vessels, and stimulate heart frequency and cardiac output (Sapolsky et al. 2000). Glucocorticoids also act on the immune system, causing lymphopenia, eosinopenia, and neutrophilia (Griffin 1989). The glucocorticoids exert a negative feedback at the hypothalamus and the pituitary gland, inhibiting the secretion of corticotrophin-releasing hormone, vasopressin, and adrenocorticotrophic hormone (Sabban 2010; McEwen 2007). Therefore, even if the stressor continues acting longer, the high concentrations of glucocorticoids are not maintained, so the decrease in its concentration does not necessarily indicate that the stressful situation ended.
1.4.2 Chronic Stress Response
A chronic stress response is a consequence of a continuous or intermittent but repeated exposure of an animal to stressors during several days (Pacák and Palkovits 2001). Chronic stress response frequently occurs in farm animals, as the general allocating conditions, pathologies, or other types of stressors usually remain active for days or weeks. As it might be expected, a sustained action of glucocorticoids over several days has harmful consequences on the productive and reproductive results, affecting animal health and welfare. If the animal cannot cope with the chronic stressor, it might have several negative consequences, including the animal’s death. One consequence of repeated exposure to stressors is the recurrent increase in glucocorticoid concentrations, which negatively affect the normal function of the immune system. Therefore, a chronic stress response might cause immunosuppression, making the animal more susceptible to diseases (Griffin 1989). In this situation, animals lose the appetite, decrease the food conversion, and at the same time, prioritize the catabolic pathways, negatively affecting its’ general body condition. Frequently, chronically stressed animals are also more anxious, nervous, and restless, display stereotypic movements, and reduce their sexual behavior (Maniam and Morris 2012; Sapolsky et al. 2000).
Although it is assumed that the stress response is necessary for the animal to cope with stressors, facilitating its adaptation, if the stressor is maintained over time, or the intensity of its effect generates an intense or sustained response, the final result can be harmful to the animal. Therefore, if the reactions displayed by the animal are not enough to recover the homeostasis or the action of the stressor does not stop, the general status of the animal may continuously deteriorate until death. In many husbandry conditions, the stress response cannot modify the stressful situation (Moberg 2001), Therefore, the animal can reestablish its homeostasis only when the stressor disappears.
Some common chronic stressful situations in farm animals are early artificial weaning, transport, and shearing or mixing animals from different groups. Artificial weaning involves several stressors, as the mother-offspring bond is suddenly broken, the physical and social environment is new for the animal, and the animal has to adapt its feeding behavior and digestive processes to the replacement of milk by solid food (Freitas-de-Melo et al. 2022; Freitas-de-Melo and Ungerfeld 2016b). During transportation, the animals are allocated in trucks with high density, provoking injuries and subjecting them to prolonged fasting. Shearing is another stressful situation, as animals are moved from their paddocks, usually by dogs or unknown humans, and located in smaller pens close to the shearing shed. In this condition, they are exposed to high noise levels produced by the shearing machine. During the process, those animals entering to shearing are withdrawn from the group by humans, which implies taking out other group members, which also is a stressful situation for those animals. After shearing, mixing recently shorn animals with those still waiting to be shorn implies the introduction of stressed animals, who are also not easily recognized by non-shorn animals (Ungerfeld et al. 2018). After winter shearing, the stressful situation remains longer with the increase in thermoregulatory demands (Carcangiu et al. 2008; Hargreaves and Huston 1990a, b). Other examples are changes in the social environment, as happens after social grouping when the animals are forced to cohabit with previously unknown individuals (Giriboni et al. 2015), or after social isolation in gregarious species as farm ruminants (Freitas-de-Melo et al. 2016).
In summary, during a chronic stress response, the physiological and behavioral responses are costly with harmful consequences for the animals as the responses cannot modify the cause of the stress. Therefore, it is essential to reduce how the animal perceives the stressor and its response to improve animal welfare.
1.5 Influence Sex Steroids on Stress Response and Animal Welfare
Several studies demonstrate that the concentration of sexual steroids modifies how an animal perceives a stressor and the endocrine stress response itself. In general, it is assumed that progestogens reduce the stress response, inducing calm and relaxed states, while acute progesterone withdrawal and estrogens increase the responses to stressors (Freitas-de-Melo and Ungerfeld 2016a). There are two main metabolites derived from progesterone, allopregnanolone and pregnanolone, which are called neuroactive metabolites, and are responsible for reducing the stress response (Liang and Rasmusson 2018; Barbaccia et al. 2001). These neuroactive metabolites lessen the animal’s perception of the stressor, as they present an anxiolytic effect (Wang 2011), and they also act decreasing the response of the hypothalamic-pituitary-adrenal axis (Brunton et al. 2009; Patchev et al. 1996). All these mechanisms reduce the animal’s behavioral and physiological stress response (Bitran et al. 1993, 1995). The acute decrease of progesterone concentrations, as occurs immediately after luteolysis or after the withdrawal of progesterone intravaginal devices, increases anxiety and the sensitivity to stressors (Hantsoo and Epperson 2020; Smith et al. 2007). Furthermore, estrogens also increase anxiety and endocrine stress response in rodents (Figueiredo et al. 2007; Jasnow et al. 2006; Morgan and Pfaff 2002). Surprisingly, the effects of the physiological reproductive status and hormonal treatments on stress response are commonly not considered when female farm animals are subjected to stressful situations.
1.5.1 Mechanisms of Action of Progesterone, Progestagens, and Estrogens
Progestagens and estrogens act through genomic mechanisms, modifying the expression of specific genes, stimulating or inhibiting gene transcription, and synthesizing specific proteins (Schumacher et al. 1999). The effect of this pathway requires from minutes to days to be noted (McEwen 1991). Progesterone can also provoke short-term effects, acting through its’ neuroactive metabolites. Briefly, progesterone is metabolized mainly in the liver (Pluchino et al. 2009) to various compounds, including dihydro-progesterone, allopregnanolone, and pregnanolone (Corpechot et al. 1993; Seamark et al. 1969). These compounds can cross the blood-brain barrier (Pluchino et al. 2009). Furthermore, both progesterone and dihydro-progesterone can also be metabolized in the brain in allopregnanolone and pregnanolone (Compagnone and Mellon 2000). Therefore, these neuroactive metabolites can arrive from the blood to the central nervous system, or be produced in situ (Sze and Brunton 2019), achieving high concentrations in the brain (Paul and Purdy 1992).
Allopregnanolone and pregnanolone can bind to the aminobutyric acid type A (GABAA) and glycine (Gunn et al. 2011; Sarkar et al. 2011; Jiang et al. 2006), which are both brain’s inhibitory receptors. Allopregnanolone and pregnanolone can also inhibit excitatory pathways through the nicotine, serotonin, and glutamate receptors (Sedláèek et al. 2008; Kaura et al. 2007; Bullock et al. 1997). These neuroactive metabolites are GABAA positive allosteric modulators, reducing neuronal excitability (Paul et al. 2020; Lambert et al. 2009). The union of these neuroactive metabolites on GABAA receptor induces quickly anxiolytic, sedative, and analgesic effects (Akk et al. 2007; Wang 2011), exerting a stress-protective influence (Brunton et al. 2009; Ma et al. 2005). Allopregnanolone also decreases the synthesis of mRNA for corticotropin-releasing hormone and the secretion of both adrenocorticotrophic hormone and glucocorticoids in male rats (Brunton et al. 2009). The concentration of allopregnanolone increases along gestation in rats, significantly decreasing just before delivery (Concas et al. 1998). The increase in the concentration of allopregnanolone might be related to the availability of progesterone as a substrate and the increase in the concentration of enzymes that synthesize allopregnanolone (Brunton et al. 2005).
Although it is not wholly well established in the literature, the reduction of stress response could be mediated by the action of progesterone on intracellular receptors. Some studies using mice knock-out for the intracellular receptors of progesterone reported a decline in the stress or anxiety responses, suggesting that progesterone also has a stress-protective effect throughout its receptors (Reddy et al. 2005). However, when rats were treated with progesterone and a progestin receptor antagonist before applying a stressor, the reduction in the stress response was still observed (Bitran et al. 1995). In female ovariectomized rats, the treatment with medroxyprogesterone, a progestin that does not produce the neuroactive metabolites, also reduces the stress response during restrainment (Hassell et al. 2011). It has been suggested that progestagens, progesterone, or dihydro-progesterone could directly join the intracellular receptors located in the amygdala and the bed nucleus of the stria terminalis (Brinton et al. 2008). These brain regions are related to the stress response, fear, or anxiety (Walker et al. 2003), modulating the stress response. Progesterone and medroxyprogesterone differ in their effects on the expression of a subunit of GABAA receptors in the hippocampus, which have important implications for the modulation of anxiety (Pazol et al. 2009), and in the effects of both hormones in cognition in rats (Frye et al. 2013). Overall, progestagens and the neuroactive metabolites of progesterone may reduce the perception of stressors, and thus, the endocrine stress response.
In contrast, the acute decreases in progesterone concentration and its neuroactive metabolites after chronic exposure can regulate the expression of a specific subunit of GABAA receptor (Smith et al. 1998, 2007). Progesterone withdrawal leads to an increase in the display of behaviors related to anxiety (Gulinello et al. 2002; Gallo and Smith 1993) and individual risk-taking in rats (Löfgren et al. 2006). In the same direction, the treatment with estradiol benzoate enhances anxiety behavior in three anxiogenic behavior tests in female mice (Morgan and Pfaff 2002). Furthermore, estrogens increase the expression of corticotrophin-releasing hormone mRNA in the central nucleus of the amygdala (Jasnow et al. 2006), and increase the glucocorticoid response to an acute stressor in rodents (Figueiredo et al. 2007; Burgess and Handa 1992).
1.5.2 Action of Sexual Steroids in the Stress Response in Ruminants
There are few studies in ruminants regarding the effects of sex steroids on the stress response, and even a few studies related to the possible mechanisms of action. The concentration of allopregnanolone in the cerebrospinal fluid increases during gestation, reaching a maximum concentration in the last month and decreasing during the first month of lactation (Misztal et al. 2020a). Misztal et al. (2020b) reported that administration of allopregnanolone reduces corticotrophin-releasing hormone and vasopressin mRNA expressions in the paraventricular nucleus of isolated and restrained ewes. Consequently, administration of allopregnanolone to those ewes also reduces the corticotrophin-releasing hormone, adrenocorticotrophic hormone, and cortisol concentrations (Misztal et al. 2020b). Furthermore, there is another possible pathway for the allopregnanolone anxiolytic and sedative effects in this species, as at least in sheep central nervous system-isolated tissue, in vitro allopregnanolone binds to the GABAA receptor (Crossley et al. 2000).
In rodents, the sensitivity to stressors decreases by the end of pregnancy according to the progesterone or allopregnanolone availability (Brunton 2010; Douglas et al. 2005). Although there are scarce studies, pregnant ewes also decrease their sensitivity to stressors. In effect, pregnant ewes have slighter behavioral responses than non-pregnant ewes to social isolation or a surprise test (Viérin and Bouissou 2001) and shearing (Ungerfeld and Freitas-de-Melo 2019). Pregnant ewes also respond with lower cortisol concentrations than non-pregnant ewes to the shearing handling (Ungerfeld and Freitas-de-Melo 2019). Progesterone concentration was negatively related to the intensity of behavioral stress responses (Viérin and Bouissou 2001), suggesting that the inhibitory effect is associated with the progesterone availability. The sensitivity of cows also varies according to the reproductive status, with a lower reaction of pregnant cows to human handling (Freitas-de-Melo et al. 2019).
Recently we observed that estrous ewes stayed more time standing up immobile and alert during social isolation than ewes in their luteal phase (Freitas-de-Melo et al. 2022). Pinto-Santini and Ungerfeld (2019) observed that ewes were probably more sensitive to stressors during the follicular than during the luteal phase, as while during the first there was a clear circadian pattern in cortisol secretion, with a rise during the early morning, but this pattern disappeared during the mid-luteal phase. Furthermore, the surface temperature, total serum protein, globulin, and plasma glucose concentrations were greater in ewes in the follicular phase than in ewes in the luteal phase (Freitas-de-Melo et al. 2022; Pinto-Santini and Ungerfeld 2019). Nevertheless, Kilgour and Szantar-Coddington (1997) did not find any differences between the behavioral response of estrous and non-estrous ewes during social isolation. Furthermore, Orihuela et al. (2002) did not observe differences in the cortisol concentration in diestrous or proestrus ewes after transportation.
The effect of progesterone administration to evaluate ruminants’ response to different stressors was studied in some experiments. In particular, long-term treatment with progesterone reduces ewes’ responses to weaning (Freitas-de-Melo et al. 2013). Ewes treated with intravaginal devices impregnated with progesterone displayed lesser behavioral changes after weaning. After artificial weaning, treated ewes also present lower globulins concentration than untreated ewes, suggesting a possible protective effect of the treatment. Therefore, to simplify the potential handling of animals, we administered an injection of oil-based progesterone to ewes immediately before weaning, and although it decreased the behavioral response (Freitas-de-Melo and Ungerfeld, unpublished data), the decrease was not as strong as that previously reported with longer treatments (Freitas-de-Melo et al. 2013). On the other hand, the acute withdrawn of progesterone concentrations increase the sensitivity to stressors. In effect, anestrous ewes secrete more cortisol after being isolated 24 h after ending the treatment with progesterone than remaining untreated (Freitas-de-Melo et al. 2016). Similarly, heifer response to human handling is greater after progesterone withdrawal than in untreated animals (Freitas-de-Melo et al. 2019). Although the effects of estrogens on stress response in ruminants were studied to a lesser degree, ewes treated with estradiol benzoate respond to an ACTH challenge with greater cortisol concentrations than untreated ewes (Van Lier et al. 2014).
In this context, although more research is required, there is room to consider treatments with progesterone as practical alternatives to reduce the stress responses to routine handlings in ruminants, thereby improving their well-being. The need for long-term treatments appears as a significant limitation, as it implies the use of intravaginal devices to ensure sustained progesterone concentrations during several days. However, new long-action progesterone formulas might be tested to avoid using devices, which are not allowed in some countries due to the residues in the environment. Table 1.2 summarizes the main effects of progesterone or the reproductive physiological state on the stress response in female ruminants.
1.6 Conclusions
According to our knowledge, there are no studies on how the sensitivity of the animals to stressors is affected by administering sexual steroids during standard reproductive practices. Moreover, although the effectiveness of administering different progestogens (Ungerfeld et al. 1999) or estradiol esters (Melo et al. 2016) on the reproductive responses have been compared, there are no studies relating the possible effects of these treatments with the stress responses. For example, in some studies, it has been reported that the pregnancy rate of ewes is greater when estrus is synchronized with progesterone than with medroxyprogesterone (Santos-Neto et al. 2015). The greater pregnancy rate was related to the characteristics of the intravaginal device or its’ effects on follicular dynamics. However, the insertion and withdrawal of the device imply repeatedly moving the animals, taking them to the farm facilities, contacting unknown technicians, and sometimes grouping with other unknown individuals, which are all stressful events. In this sense, as progestagens as medroxyprogesterone are not metabolized to the neuroactive steroids (Pluchino et al. 2009; Bernardi et al. 2006), it is possible that the effects of these hormones on the reduction of stress response also differ, and this might partially explain differences in fertility.
Therefore, considering that the devices are withdrawn close to the moment of artificial insemination, a period in which stress should be avoided to increase the pregnancy rates, the fertility might differ due to differences in the effects of different hormones in the sensitivity to practical handlings. Similarly, different estradiol esters have been used to modify the follicular growth pattern, and thus, the moment in which timed artificial insemination is performed in cattle. Estradiol benzoate is widely used instead of estradiol cypionate, although some studies were done expecting that the last would increase fertility due to its effects at the hypothalamus-pituitary-ovary axis, related to the longer half-life (Melo et al. 2016; Sales et al. 2012). However, the use of estradiol cypionate did not increase the pregnancy rates. Therefore, it should be studied if the sustained concentrations of estradiol during a longer time before the moment in which the cows are grouped, moved to the facilities, handled by the technicians, and inseminated, increase the sensitivity to these stressors, and this might partially explain the lower fertility rate.
It is known that individual temperament affects the results of these treatments (Mello et al. 2020), and it is also known that cows are more responsive to tests used to evaluate temperament after progesterone withdrawal (Freitas-de-Melo et al. 2019), raising the hypothesis that the estradiol ester used might affect the cow perception of stressors, and therefore, their fertility. These examples demonstrate a big room to work in the link between these two types of research areas, which have scarce contribution between them until now. Understanding the animal as an indivisible individual, in which a hormone administrated to produce the desired reproductive effect and also influences other systems, might be the main direction to improve the animals’ welfare and results of the reproductive biotechnologies.
Abbreviations
- GABAA:
-
aminobutyric acid type A
References
Akk G, Covey DF, Evers AS, Steinbach JH, Zorumski CF, Mennerick S (2007) Mechanisms of neurosteroid interactions with GABAA receptors. Pharmacol Ther 116:35–57. https://doi.org/10.1016/j.pharmthera.2007.03.004
Alvarado-Espino AS, Menchaca A, Meza-Herrera CA, Carrillo-Moreno DI, Zúñiga-García S, Arellano-Rodríguez F, Mellado M, Véliz FG (2019a) Ovarian response is not affected by the stage of seasonal anestrus or breed of goats when using a progesterone injection plus human chorionic gonadotropin-based protocol. Anim Reprod Sci 204:60–65. https://doi.org/10.1016/j.anireprosci.2019.03.005
Alvarado-Espino AS, Menchaca A, Meza-Herrera CA, Mellado M, Arellano F, Véliz F (2019b) Use of injectable progesterone and hCG for fixed-time artificial insemination during the non-breeding season in goats. Theriogenology 127:21–25. https://doi.org/10.1016/j.theriogenology.2018.12.035
Alvarez L, Gamboa D, Zarco L, Ungerfeld R (2013) Response to the buck effect in goats primed with CIDRs, previously used CIDRs, or previously used autoclaved CIDRs during the non-breeding season. Livest Sci 155:459–462. https://doi.org/10.1016/j.livsci.2013.05.010
Andrade JPN, Gomez-León V, Andrade FS, Carvalho BP, Lacouth KL, Garcia FZ, Jacob JCF, Sales JNS, Wiltbank MC, Mello MRB (2020) Development of a novel 21-day reinsemination program, ReBreed21, in Bos indicus heifers. Theriogenology 155:125–131. https://doi.org/10.1016/j.theriogenology.2020.04.021
Ashley CD, Kramer ML, Bishop P (2000) Estrogen and substrate metabolism. Sports Med 29:221–227. https://doi.org/10.2165/00007256-200029040-00001
Barbaccia ML, Serra M, Purdy RH, Biggio G (2001) Stress and neuroactive steroids. Int Rev Neurobiol 46:243–272. https://doi.org/10.1016/S0074-7742(01)46065-X
Bernardi F, Pluchino N, Pieri M, Begliuomini S, Lenzi E, Puccetti S, Casarosa E, Luisi M, Genazzani AR (2006) Progesterone and medroxyprogesterone acetate effects on central and peripheral allopregnanolone and beta-endorphin levels. Neuroendocrinology 83:348–359. https://doi.org/10.1159/000095400
Bitran D, Purdy RH, Kellogg CK (1993) Anxiolytic effect of progesterone is associated with increases in cortical allopregnanolone and GABAA receptor function. Pharmacol Biochem Behav 45:423–428. https://doi.org/10.1016/0091-3057(93)90260-z
Bitran D, Shiekh M, McLeod M (1995) Anxiolytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABAA receptors. J Neuroendocrinol 3:171–177. https://doi.org/10.1111/j.1365-2826.1995.tb00744.x
Bó GA, Baruselli PS (2014) Synchronization of ovulation and fixed-time artificial insemination in beef cattle. Animal 8:144–150. https://doi.org/10.1017/S1751731114000822
Bó GA, Adams GP, Pierson RA, Tribulo HE, Caccia M, Mapletoft RJ (1994) Follicular wave dynamics after estradiol-17β treatment of heifers with or without a progestogen implant. Theriogenology 41:1555–1559. https://doi.org/10.1016/0093-691X(94)90821-Y
Bó GA, de la Mata JJ, Baruselli PS, Menchaca A (2016) Alternative programs for synchronizing and resynchronizing ovulation in beef cattle. Theriogenology 86:388–396. https://doi.org/10.1016/j.theriogenology.2016.04.053
Bouman A, Heineman MJ, Faas MM (2005) Sex hormones and the immune response in humans. Hum Reprod Update 11:411–423. https://doi.org/10.1093/humupd/dmi008
Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J (2008) Progesterone receptors: form and function in brain. Front Neuroendocrinol 29:313–339. https://doi.org/10.1016/j.yfrne.2008.02.001
Broom DM (1986) Indicators of poor welfare. Br Vet J 142:524–526. https://doi.org/10.1016/0007-1935(86)90109-0
Broom DM, Fraser AF (2015) Domestic animal behaviour and welfare, 5th edn. CABI Publishing, Wallingford
Brunton PJ (2010) Resetting the dynamic range of hypothalamo–pituitary–adrenal axis stress responses through pregnancy. J Neuroendocrinol 22:1198–1213. https://doi.org/10.1111/j.1365-2826.2010.02067.x
Brunton PJ, Meddle SL, Ma S, Ochedalski T, Douglas AJ, Russell JA (2005) Endogenous opioids and attenuated hypothalamic–pituitary–adrenal axis responses to immune challenge in pregnant rats. J Neurosci 25:5117–5126. https://doi.org/10.1523/JNEUROSCI.0866-05.2005
Brunton PJ, McKay AJ, Ochedalski T, Piastowska A, Rebas E, Lachowicz A, Russell JA (2009) Central opioid inhibition of neuroendocrine stress responses in pregnancy in the rat is induced by the neurosteroid allopregnanolone. J Neurosci 29:6449–6460. https://doi.org/10.1523/JNEUROSCI.0708-09.2009
Bullock AE, Clark AL, Grady SR, Robinson SF, Slobe BS, Marks MJ, Collins AC (1997) Neurosteroids modulate nicotinic receptor function in mouse striatal and thalamic synaptosomes. J Neurochem 68:2412–2423. https://doi.org/10.1046/j.1471-4159.1997.68062412.x
Burgess LH, Handa RJ (1992) Chronic estrogen-induced alterations in adrenocorticotropin and corticosterone secretion, andglucocorticoid receptor-mediated functions in female rats. Endocrinology 131:1261–1269. https://doi.org/10.1210/endo.131.3.1324155
Carcangiu V, Vacca GM, Parmeggiani A, Mura MC, Pazzola M, Dettori ML, Bini PP (2008) The effect of shearing procedures on blood levels of growth hormone, cortisol and other stress haematochemical parameters in Sarda sheep. Animal 2:606–612. https://doi.org/10.1017/S1751731108001651
Carrasco GA, Van de Kar LD (2003) Neuroendocrine pharmacology of stress. Eur J Pharmacol 463:235–272. https://doi.org/10.1016/S0014-2999(03)01285-8
Cavestany D, Fernández D, Salazar E, Sánchez A, Leyton L, Crespi D (2008) Determinación de niveles de progesterona en sangre luego de la administración parenteral de progesterona en vacas Holando ovariectomizadas o ciclando. In: Jornadas Uruguayas de Buiatría, XXXVI, Paysandú, Uruguay, pp 218–219
Charmandari E, Tsigos C, Chrousos G (2005) Endocrinology of the stress response. Annu Rev Physiol 67:259–284. https://doi.org/10.1146/annurev.physiol.67.040403.120816
Compagnone NA, Mellon SH (2000) Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol 21:1–56. https://doi.org/10.1006/frne.1999.0188
Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, Purdy RH, Grisenti P, Biggio G (1998) Role of brain allopregnanolone in the plasticity of g–aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci USA 95:13284–13289. https://doi.org/10.1073/pnas.95.22.13284
Corpechot C, Young J, Calvel M, Wehrey C, Veltz JN, Touyer G, Mouren M, Prasad VVK, Banner C, Sjijvall J, Baulieu EE, Robel P (1993) Neurosteroids: 3 alpha-hydroxy-5 alphapregnan-20-one and its precursors in the brain, plasma, and steroidogenic glands of male and female rats. Endocrinology 133:1003–1009. https://doi.org/10.1210/endo.133.3.8365352
Corréa Rocha D, Beskow A, Mc Manus Pimentel CM, Costa Mattos R, Macedo Gregory R (2011) Níveis séricos de progesterona em vacas ovariectomizadas tratadas com MAD4 com diferentes concentracões e vías de administracão. Acta Sci Vet 39:1–6
Cosentino IO, Balaro MFA, Arashiro EKN, Santos JDR, Carvalho ABDS, Pérez-Clariget R, Ungerfeld R, Brandão FZ (2019) Hormonal protocols for early resynchronization of ovulation in ewes: the use of progestagens, eCG, and inclusion of early pregnancy diagnosis with color Doppler ultrasound. Theriogenology 133:113–118. https://doi.org/10.1016/j.theriogenology.2019.04.033
Crossley KJ, Walker DW, Beart PM, Hirst JJ (2000) Characterization of GABAA receptors in fetal, neonatal and adult ovine brain: region and age related changes and the effects of allopregnanolone. Neuropharmacology 39:1514–1522. https://doi.org/10.1016/s0028-3908(99)00222-1
Dallman ME, Viau VG, Bhatnagar S, Gomez F, Laugero K, Bell ME (2002) Corticosteroids, stress, and sugar: energy balance, the brain, and behavior. In: Pfaff W et al (eds) Hormones, brain, and behaviour. Academic, San Diego, pp 571–632
Dobson H, Smith RF (2000) What is stress, and how does it affect reproduction? Anim Reprod Sci 60:743–752. https://doi.org/10.1016/s0378-4320(00)00080-4
Douglas AJ, Meddle SL, Toschi N, Bosch OJ, Neumann ID (2005) Reduced activity of the noradrenergic system in the paraventricular nucleus at the end of pregnancy: implications for stress hyporesponsiveness. J Neuroendocrinol 17:40–48. https://doi.org/10.1111/j.1365-2826.2005.01272.x
Earley B, Buckham-Sporer K, Gupta S, Pang W, Ting S (2010) Biologic response of animals to husbandry stress with implications for biomedical models. Anim Physiol 2:25–42. https://doi.org/10.2147/OAAP.S9354
Figueiredo HF, Ulrich YM, Choi DC, Herman JP (2007) Estrogen potentiates adrenocortical responses to stress in female rats. Am J Physiol Endocrinol Metab 292:E1173–E1182. https://doi.org/10.1152/ajpendo.00102.2006
Freitas-de-Melo A, Ungerfeld R (2016a) Progesterona y respuesta de estrés: mecanismos de acción y sus repercusiones en rumiantes domésticos. Revisión Rev Mex Cien Pec 7:185–199
Freitas-de-Melo A, Ungerfeld R (2016b) Destete artificial en ovinos: respuesta de estrés y bienestar animal. Revisión Rev Mex Cien Pec 7:361–375
Freitas-de-Melo A, Banchero G, Hötzel MJ, Damián JP, Ungerfeld R (2013) Progesterone administration reduces the behavioural and physiological responses of ewes to abrupt weaning of lambs. Animal 7:1367–1373. https://doi.org/10.1017/S1751731113000621
Freitas-de-Melo A, Damián JP, Hötzel MJ, Banchero G, Ungerfeld R (2016) Progesterone pretreatment increases the stress response to social isolation in ewes. Hormones 15:81–87. https://doi.org/10.14310/horm.2002.1625
Freitas-de-Melo A, Orihuela A, Magri G, Cruz BD, Rubio I, Corro M, Alonso MA, Ungerfeld R (2019) Physiological reproductive status and progesterone concentration affect the results of tests to measure temperament traits in female beef cattle. Livest Sci 221:39–43. https://doi.org/10.1016/j.livsci.2019.01.010
Freitas-de-Melo A, Garcia Kako Rodriguez M, Crosa C, Ungerfeld R (2022) Social Stress during the Estrus or Luteal Phase in Sheep. J Appl Anim Welf Sci 1–9. https://doi.org/10.1080/10888705.2021.2021408
Frye CA, Koonce CJ, Walf AA (2013) Progesterone, compared to medroxyprogesterone acetate, to C57BL/6, but not 5α-reductase mutant, mice enhances object recognition and placement memory and is associated with higher BDNF levels in the hippocampus and cortex. Neurosci Lett 551:53–57. https://doi.org/10.1016/j.neulet.2013.07.002
Gallo M, Smith S (1993) Progesterone withdrawal decreases latency to and increases duration of electrified prod burial: a possible rat model of PMS anxiety. Pharmacol Biochem Behav 46:897–904. https://doi.org/10.1016/0091-3057(93)90219-J
Gaston-Parry O, Heasman K, Nemorin JKE, Robinson TJ (1988) A radioimmunoassay for fluorogestone acetate (FGA) and its application to the measurement of plasma FGA and progesterone in ewes treated with FGA-impregnated intravaginal sponges. Austr J Biol Sci 41:57–67. https://doi.org/10.1071/BI9880057
Genazzani AR, Monteleone P, Gambacciani M (2002) Hormonal influence on the central nervous system. Maturitas 43:11–17. https://doi.org/10.1016/s0378-5122(02)00144-5
Gimenes LU, Fantinato Neto P, Arango JSP, Ayres H, Baruselli PS (2009) Follicular dynamics of Bos indicus, Bos taurus and Bubalus bubalis heifers treated with norgestomet ear implant associated or not to injectable progesterone. Anim Reprod 6:256 (abstract)
Giriboni J, Lacuesta L, Damián JP, Ungerfeld R (2015) Grouping previously unknown bucks is a stressor with negative effects on reproduction. Trop Anim Health Prod 47:317–322. https://doi.org/10.1007/s11250-014-0722-2
Greyling JPC, Kotzé WF, Taylor GJ, Hagendijk WJ (1994) Synchronization of oestrus in sheep: use of different doses of progestagen outside the normal breeding season. S Afr J Anim Sci 24:33–36
Griffin JFT (1989) Stress and immunity: a unifying concept. Vet Immunol Immunopathol 20:263–312. https://doi.org/10.1016/0165-2427(89)90005-6
Gulinello M, Gong QH, Smith SS (2002) Progesterone withdrawal increases the alpha4 subunit of the GABA(A) receptor in male rats in association with anxiety and altered pharmacology-a comparison with female rats. Neuropharmacology 43:701–714. https://doi.org/10.1016/s0028-3908(02)00171-5
Gunn BG, Brown AR, Lambert JJ, Belelli D (2011) Neurosteroids and GABA(A) receptor interactions: a focus on stress. Front Neurosci 5:article 131. https://doi.org/10.3389/fnins.2011.00131
Hantsoo L, Epperson CN (2020) Allopregnanolone in premenstrual dysphoric disorder (PMDD): evidence for dysregulated sensitivity to GABA-A receptor modulating neuroactive steroids across the menstrual cycle. Neurobiol Stress 12:100213. https://doi.org/10.1016/j.ynstr.2020.100213
Hargreaves AL, Hutson GD (1990a) The stress response in sheep during routine handling procedures. Appl Anim Behav Sci 26:83–90. https://doi.org/10.1016/0168-1591(90)90089-V
Hargreaves AL, Hutson GD (1990b) An evaluation of the contribution of isolation up-ending and wool removal to the stress response to shearing. Appl Anim Behav Sci 26:103–113. https://doi.org/10.1016/0168-1591(90)90091-Q
Hassell J, Miryala CSJ, Hiegel C, Uphouse L (2011) Mechanisms responsible for progesterone’s protection against lordosis-inhibiting effects of restraint: I. Role of progesterone receptors. Horm Behav 60:219–225. https://doi.org/10.1016/j.yhbeh.2011.05.006
Hauschildt Dias J, Pupin MA, Saloni Duarte G, Lopes Brair V, Carvalho de Paula CJ, Paula de Sousa MA, Tavares Pereira Batista RI, Souza-Fabjan JMG, Franco Oliveira ME, Fonseca JF (2020) Successful transcervical uterine flushing can be performed without or reduced dose of oestradiol benzoate in cervical relaxation protocol in Dorper ewes. Reprod Domest Anim 55:844–850. https://doi.org/10.1111/rda.13692
Jasnow AM, Schulkin J, Pfaff DW (2006) Estrogen facilitates fear conditioning and increases corticotropin-releasing hormone mRNA expression in the central amygdala in female mice. Horm Behav 49:197–205. https://doi.org/10.1016/j.yhbeh.2005.06.005
Jiang P, Yang CX, Wang YT, Xu TL (2006) Mechanisms of modulation of pregnanolone on glycinergic response in cultured spinal dorsal horn neurons of rat. Neuroscience 141:2041–2050. https://doi.org/10.1016/j.neuroscience.2006.05.009
Kalkhoff RK (1982) Metabolic effects of progesterone. Am J Obstet Gynecol 142:735–738. https://doi.org/10.1016/s0002-9378(16)32480-2
Kaura V, Ingram CD, Gartside SE, Young AH, Judge SJ (2007) The progesterone metabolite allopregnanolone potentiates GABAA receptor-mediated inhibition of 5-HT neuronal activity. Eur Neuropsychopharmacol 17:108–115. https://doi.org/10.1016/j.euroneuro.2006.02.006
Kilgour RJ, Szantar-Coddington MR (1997) The arena test and cortisol response of sheep as indirect selection criteria for the improvement of lamb survival. Anim Reprod Sci 46:97–108. https://doi.org/10.1016/s0378-4320(96)01591-6
Kudielka BM, Kirschbaum C (2007) Sex differences in HPA axis responses to stress: a review. Biol Psychol 69:113–132. https://doi.org/10.1016/j.biopsycho.2004.11.009
Lambert JJ, Cooper MA, Simmons RDJ, Weir CJ, Belelli D (2009) Neurosteroids: endogenous allosteric modulatorsof GABAA receptors. Psychoneuroendocrinology 34:48–58. https://doi.org/10.1016/j.psyneuen.2009.08.009
Liang JJ, Rasmusson AM (2018) Overview of the molecular steps in steroidogenesis of the GABAergic neurosteroids allopregnanolone and pregnanolone. Chronic Stress 2:2470547018818555. https://doi.org/10.1177/2470547018818555
Liu SS, Ying GG, Liu YS, Yang YY, He LY, Chen J, Liu WR, Zhao JL (2015) Occurrence and removal of progestagens in two representative swine farms: effectiveness of lagoon and digester treatment. Water Res 77:146–154. https://doi.org/10.1016/j.watres.2015.03.022
Löfgren M, Johansson IM, Meyerson B, Lundgren P, Bäckström T (2006) Progesterone withdrawal effects in the open field test can be predicted by elevated plus maze performance. Horm Behav 50:208–215. https://doi.org/10.1016/j.yhbeh.2006.03.002
Ma S, Shipston MJ, Morilak D, Russell JA (2005) Reduced hypothalamic vasopressin secretion underlies attenuated adrenocorticotropin stress responses in pregnant rats. Endocrinology 146:1626–1637. https://doi.org/10.1210/en.2004-1368
Maniam J, Morris MJ (2012) The link between stress and feeding behaviour. Neuropharmacology 63:97–110. https://doi.org/10.1016/j.neuropharm.2012.04.017
Mapletoft RJ, Bó GA, Baruselli PS, Menchaca A, Sartori R (2018) Evolution of knowledge on ovarian physiology and its contribution to the widespread application of reproductive biotechnologies in South American cattle. Anim Reprod 15:1003–1014. https://doi.org/10.21451/1984-3143-AR2018-0007
Martínez MF, Kastelic JP, Adams GP, Janzen E, McCartney DH, Mapletoft RJ (2000) Estrus synchronization and pregnancy rates in beef cattle given CIDR-B, prostaglandin and estradiol, or GnRH. Can Vet J 41:786–790
Matteri RL, Carroll JA, Dyer CJ (2000) Neuroendocrine responses to stress. In: Moberg GP, Mench JA (eds) The biology of animal stress: basic principles and implications for animal welfare. CABI Publishing, Cambridge, pp 43–76
McEwen BS (1991) Non-genomic and genomic effects of steroids on neural activity. Trends Pharmacol Sci 12:141–147. https://doi.org/10.1016/0165-6147(91)90531-v
McEwen BS (2007) Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 87:873e904. https://doi.org/10.1152/physrev.00041.2006
McEwen BS, Wingfield JC (2003) The concept of allostasis in biology and biomedicine. Horm Behav 43:2–15. https://doi.org/10.1016/s0018-506x(02)00024-7
Mello BP, Maturana Filho M, Lemes KM, Gonçalvez RL, Mendes Lollatod JP, Zanella AJ, Vasconcelos Ferreira TF, Pugliesif G, Madureira EH, Gonella-Diaza A, Membrive CMB (2020) Importance of temperament in the pregnancy by timed insemination in bovine females Bos taurus indicus. Livest Sci 240:104104. https://doi.org/10.1016/j.livsci.2020.104104
Melo LF, Monteiro PLJ, Surjus RS, Drum JN, Wiltbank MC, Sartori R (2016) Progesterone-based fixed-time artificial insemination protocols for dairy cows: gonadotropin-releasing hormone versus estradiol benzoate at initiation and estradiol cypionate versus estradiol benzoate at the end. J Dairy Sci 99:9227–9237. https://doi.org/10.3168/jds.2016-11220
Misztal T, Czauderna MR, Młotkowska P, Misztal A, Marciniak E (2020a) Temporal changes in the cerebrospinal fluid allopregnanolone concentration and hypothalamic-pituitary-adrenal axis activity in sheep during pregnancy and early lactation. Livest Sci 231:103871. https://doi.org/10.1016/j.livsci.2019.103871
Misztal T, Młotkowska P, Marciniak E, Misztal A (2020b) Allopregnanolone reduces neuroendocrine response to acute stressful stimuli in sheep. J Endocrinol 244:201–211. https://doi.org/10.1530/JOE-19-0376
Moberg GP (2001) Biological response to stress: implications for animal welfare. In: Moberg GP, Mench JA (eds) The biology of animal stress: basic principles and implications for animal welfare. CABI Publishing, Wallingford, pp 1–21
Monteiro PLJ Jr, Borsato M, Silva FLM, Prata AB, Wiltbank MC, Sartori R (2015) Increasing estradiol benzoate, pretreatment with gonadotropin-releasing hormone, and impediments for successful estradiol-based fixed-time artificial insemination protocols in dairy cattle. J Dairy Sci 98:3826–3839. https://doi.org/10.3168/jds.2014-9040
Morgan MA, Pfaff DW (2002) Estrogen’s effects on activity, anxiety, and fear in two mouse strains. Behav Brain Res 132:85–93. https://doi.org/10.1016/S0166-4328(01)00398-9
Mormedè P, Andanson S, Aupérin B, Beerda B, Guémené D, Malmkvist J, Manteca X, Manteuffel G, Prunet P, van Reenen CG, Richard S, Veissier I (2007) Exploration of the hypothalamic-pituitary-adrenal function as a tool to evaluate animal welfare. Physiol Behav 92:317–339. https://doi.org/10.1016/j.physbeh.2006.12.003
Morotti F, de Campos JT, Lunardelli PA, Costa CB, Bergamo LZ, Rigo Barreiros TR, dos Santos GMG, Seneda MM (2018) Injectable progesterone in timed artificial insemination programs in beef cows. Anim Reprod 15:17–22. https://doi.org/10.21451/1984-3143-2017-AR928
O’Connor DB, Thayer JF, Vedhara K (2021) Stress and health: a review of psychobiological processes. Annu Rev Psychol 72:663–688. https://doi.org/10.1146/annurev-psych-062520-122331
Oliveira MAL, Guido SI, Lima PF (2001) Comparison of different protocols used to induce and synchronize estrus cycle of Saanen goats. Small Rumin Res 40:149–153. https://doi.org/10.1016/S0921-4488(00)00222-4
Orihuela A, Sanchez-Mejorada H, Toledo M (2002) Effect of short transport during di-oestrus and pro-oestrus on cortisol levels and oestrous behaviour of sheep. J Agric Sci 138:93–96. https://doi.org/10.1017/S0021859601001733
Pacák K, Palkovits M (2001) Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr Rev 22:502–548. https://doi.org/10.1210/edrv.22.4.0436
Patchev VK, Hassan AH, Holsboer DF, Almeida OF (1996) The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology 5:533–540. https://doi.org/10.1016/S0893-133X(96)00096-6
Paul SM, Purdy RH (1992) Neuroactive steroids. FASEB J 6:2311–2322. https://doi.org/10.1096/fasebj.6.6.1347506
Paul SM, Pinna G, Guidotti A (2020) Allopregnanolone: from molecular pathophysiology to therapeutics. A historical perspective. Neurobiol Stress 12:100215. https://doi.org/10.1016/j.ynstr.2020.100215
Pazol K, Northcutt KV, Patisaul HB, Wallen K, Wilson ME (2009) Progesterone and medroxyprogesterone acetate differentially regulate α4 subunit expression of GABAA receptors in the CA1 hippocampus of female rats. Physiol Behav 97:58–61. https://doi.org/10.1016/j.physbeh.2009.01.021
Pinto-Santini L, Ungerfeld R (2019) The phase of the estrous cycle modifies the endocrine, metabolic and behavior rhythms in ewes. Physiol Behav 204:324–335. https://doi.org/10.1016/j.physbeh.2019.03.011
Pluchino N, Cubeddu A, Giannini A, Merlini S, Cela V, Angioni S, Genazzani AR (2009) Progestogens and brain: an update. Maturitas 62:349–355. https://doi.org/10.1016/j.maturitas.2008.11.023
Reddy DS, O’Malley BW, Rogawski MA (2005) Anxiolytic activity of progesterone in progesterone receptor knock-out mice. Neuropharmacology 48:14–24. https://doi.org/10.1016/j.neuropharm.2004.09.002
Rubianes E, de Castro T, Kmaid S (1998) Estrous response after a short progesterone priming in seasonally anestrous goats. Theriogenology 49:356 (abstract)
Sabban EL (2010) Catecholamines and stress. In: Soreq H et al (eds) Stress: from molecules to behavior: a comprehensive analysis of the neurobiology of stress responses. Wiley Blackweel, Weinheim, pp 19–36
Sales JNS, Carvalho JBP, Crepaldi GA, Cipriano RS, Jacomini JO, Maio JRG, Souza JC, Nogueira GP, Baruselli PS (2012) Effects of two estradiol esters (benzoate and cypionate) on the induction of synchronized ovulations in Bos indicus cows submitted to a timed artificial insemination protocol. Theriogenology 78:510–516. https://doi.org/10.1016/j.theriogenology.2012.02.031
Santos-Neto PCD, García-Pintos C, Pinczak A, Menchaca A (2015) Fertility obtained with different progestogen intravaginal devices using Short-term protocol for fixed-time artificial insemination (FTAI) in sheep. Livest Sci 182:125–128. https://doi.org/10.1016/j.livsci.2015.11.005
Sapolsky RM, Romero LM, Munck AU (2000) How do glucocorticoids influence stress response? Integrationing, permissive, suppressive stimulatory and preparative actions. Endocr Rev 2:55–89. https://doi.org/10.1210/edrv.21.1.0389
Sarkar J, Wakefield S, MacKenzie G, Moss SJ, Maguire J (2011) Neurosteroidogenesis is required for the physiological response to stress: role of neurosteroidsensitive GABAA receptors. J Neurosci 31:18198e210. https://doi.org/10.1523/JNEUROSCI.2560-11.2011
Sauer P, Stará A, Golovko O, Valentová O, Bořík A, Grabic R, Kroupová HK (2018) Two synthetic progestins and natural progesterone are responsible for most of the progestagenic activities in municipal wastewater treatment plant effluents in the Czech and Slovak republics. Water Res 137:64–71. https://doi.org/10.1016/j.watres.2018.02.065
Schumacher M, Coirini H, Robert F, Guennoun R, El-Etr M (1999) Genomic and membrane actions of progesterone: implications for reproductive physiology and behavior. Behav Brain Res 105:37–52. https://doi.org/10.1016/s0166-4328(99)00081-9
Seamark RF, Nancarrow CD, Gardiner J (1969) Progesterone metabolism in ovine blood: the formation of 3α-hydroxypregn-4-en-20-one and other substances. Steroids 15:589–604. https://doi.org/10.1016/S0039-128X(70)80086-1
Sedláèek M, Koøínek M, Petroviè M, Cais O, Adamusová E, Chodounská H, Vyklický L Jr (2008) Neurosteroid modulation of ionotropic glutamate receptors and excitatory synaptic transmission. Physiol Res 97:49–57
Smith MF, McIntush A, Smith GW (1993) Mechanisms associated with corpus luteum development. J Anim Sci 72:1857–1872. https://doi.org/10.2527/1994.7271857x
Smith S, Gong Q, Hsu F, Markowitz R, Ffrench-Mullen J, Li X (1998) GABA(A) receptor alpha4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature 392:926–930. https://doi.org/10.1038/31948
Smith SS, Shen H, Gong QH, Zhou X (2007) Neurosteroid regulation of GABAA receptors: focus on the 4 and subunits. Pharmacol Therap 116:58–76. https://doi.org/10.1016/j.pharmthera.2007.03.008
Souza-Fabjan JMG, da Rosa RM, Balaro MFA, Pinto PHN, dos Santos GB, Arashiro EKN, da Fonseca JF, Ungerfeld R, Brandão FZ (2017) Effect of different hormonal combinations on follicular wave emergence and superovulatory response in sheep. Theriogenology 103:24–29. https://doi.org/10.1016/j.theriogenology.2017.07.036
Sze Y, Brunton PJ (2019) Sex, stress and steroids. Eur J Neurosci 52:2487–2515. https://doi.org/10.1111/ejn.14615
Ungerfeld R (2008) Response of anestrous ewes pretreated with a single dose of oestradiol-17beta or progesterone and oestradiol-17beta to the introduction of rams and ewes in oestrus. N Z Vet J 56:36–39. https://doi.org/10.1080/00480169.2008.36802
Ungerfeld R (2009) The induction of oestrus in ewes during the non-breeding season using pre-used CIDRs and oestradiol-17beta treatment. Small Rumin Res 84:129–131. https://doi.org/10.1016/j.smallrumres.2009.06.011
Ungerfeld R, Freitas-de-Melo A (2019) Stress and behavioural responses to winter shearing differ between pregnant and non-pregnant ewes. Physiol Behav 210:112653. https://doi.org/10.1016/j.physbeh.2019.112653
Ungerfeld R, Pinczak A, Forsberg M, Rubianes E (1999) Response of Corriedale ewes to the ram effect after primings with medroxyprogesterone, fluorogestone, or progesterone in the non-breeding season. Acta Vet Scand 40:299–305. https://doi.org/10.1186/BF03547009
Ungerfeld R, Suárez G, Carbajal B, Silva L, Laca M, Forsberg M, Rubianes E (2003) Medroxyprogesterone primings and response to the ram effect in Corriedale ewes during the non-breeding season. Theriogenology 60:35–45. https://doi.org/10.1016/s0093-691x(02)01302-x
Ungerfeld R, Dago AL, Rubianes E, Forsberg M (2004) Response of anestrous ewes to the ram effect after follicular wave synchronization with a single dose of estradiol-17beta. Reprod Nutr Dev 44:89–98. https://doi.org/10.1051/rnd:2004010
Ungerfeld R, Gamboa D, Alvarez L (2013) Response of ewes primed with CIDRs, previously used CIDRs, or previously used and autoclaved CIDRs to the ram effect during the non-breeding season. Anim Reprod 10:704–707
Ungerfeld R, Freitas-de-Melo A, Damián JP, Fernández-Werner A, Kremer R (2018) Behavioural and physiological changes in pregnant ewes after winter shearing. Small Rumin Res 169:57–61
Van Lier E, Carriquiry M, Meikle A (2014) Sex steroid modulation of cortisol secretion in sheep. Animal 8:960–967. https://doi.org/10.1017/S1751731114000780
Viérin M, Bouissou MF (2001) Pregnancy is associated with low fear reactions in ewes. Physiol Behav 72:579–587. https://doi.org/10.1016/S0031-9384(01)00416-4
Vilariño M, Rubianes E, Menchaca A (2011) Re-use of intravaginal progesterone devices associated with the short-term protocol for timed artificial insemination in goats. Theriogenology 75:1195–1200. https://doi.org/10.1016/j.theriogenology.2010.11.030
Vilariño M, Rubianes E, Menchaca A (2013) Ovarian responses and pregnancy rate with previously used intravaginal progesterone releasing devices for fixed-time artificial insemination in sheep. Theriogenology 79:206–210. https://doi.org/10.1016/j.theriogenology.2012.10.007
Vynchier L, Debackere M, De Kruif A, Coryn M (1990) Plasma estradiol-17b concentrations in the cow during induced estrus and after injection of estradiol-17b benzoate and estradiol-17b cypionate-a preliminary study. J Vet Pharmacol Therap 13:36–42. https://doi.org/10.1111/j.1365-2885.1990.tb00745.x
Walker DL, Toufexis DJ, Davis M (2003) Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol 463:199–216. https://doi.org/10.1016/s0014-2999(03)01282-2
Wang M (2011) Neurosteroids and GABAA receptor function. Front Endocrinol 2:1–23. https://doi.org/10.3389/fendo.2011.00044
Zuluaga JF, Williams GL (2008) High-pressure steam sterilization of previously used CIDR inserts enhances the magnitude of the acute increase in circulating progesterone after insertion in cows. Anim Reprod Sci 10:30–35. https://doi.org/10.1016/j.anireprosci.2007.06.006
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Freitas-de-Melo, A., Ungerfeld, R. (2022). Impact of Sex Steroids on the Stress Response and Welfare in Female Farm Ruminants. In: Yata, V.K., Mohanty, A.K., Lichtfouse, E. (eds) Sustainable Agriculture Reviews 57. Sustainable Agriculture Reviews, vol 57. Springer, Cham. https://doi.org/10.1007/978-3-031-07496-7_1
Download citation
DOI: https://doi.org/10.1007/978-3-031-07496-7_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-07495-0
Online ISBN: 978-3-031-07496-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)