Abstract
This chapter reviews recent work showing that vertebrate motoneurons can trigger spontaneous rhythmic activity in the developing spinal cord and can modulate the function of several different central pattern generators later in development. In both the embryonic chick and the fetal mouse spinal cords, antidromic activation of motoneurons can trigger bouts of rhythmic activity. In the neonatal mouse, optogenetic manipulation of motoneuron firing can modulate the frequency of fictive locomotion activated by a drug cocktail. In adult animals, motoneurons have been shown to regulate swimming in the zebrafish, and vocalization in fish and frogs. We discuss the significance of these findings and the degree to which motoneurons may be considered a part of these central pattern generators.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Motoneurons have been exhaustively investigated with intracellular recording methods since the early 1950s (Brock et al. 1952). Despite such intense study, work in the in the twenty-first century is providing new and exciting insights into their properties and functions. In this chapter, we will discuss these novel findings and consider their significance for motor function. We will first describe experiments that implicate motoneurons in the genesis of spontaneous activity in the developing spinal cord. We will then report the results of experiments in the mammalian spinal cord showing that motoneurons release an excitatory amino acid in addition to acetylcholine at their terminals with Renshaw cells and provide feedback to regulate the locomotor central pattern generator. This will be followed by a discussion of motoneuronal feedback to rhythmic circuits in several systems and we will conclude by considering the relevance of the new findings for motor network function.
2 The Role of Motoneurons in Initiating Spontaneous Bursting in the Developing Spinal Cord
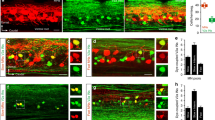
Early in development, vertebrate embryos exhibit spontaneous movements that are crucial for the proper functioning of joints, muscles, and neural networks (Toutant et al. 1979; Hall and Herring 1990; Borodinsky et al. 2004; Hanson and Landmesser 2004; Wenner 2014). These movements are generated by spontaneously active neural networks in the spinal cord (Provine et al. 1970; Landmesser and O’Donovan 1984). At embryonic day 4 (E4) in the chick embryo, spontaneous bursting can be recorded from lumbosacral motoneurons before their axons have innervated limb muscles (Milner and Landmesser 1999). At this stage, the neurotransmitter acetylcholine drives the spontaneous episodes and is thought to originate from motoneurons (Milner and Landmesser 1999) which are among the earliest spinal neurons to differentiate (Hamburger 1990). More direct evidence for the role of motoneurons in the initiation of spontaneous activity emerges later in development when motoneurons have innervated their respective limb muscles. At E10–11, calcium (Fig. 1b) and voltage sensitive dye (Fig. 1c, d) imaging reveals that an episode of spontaneous activity begins in the region of the motor nucleus and spreads to encompass the rest of the cord from there (O’Donovan et al. 1994; Arai et al. 2007) (Fig. 1). Voltage-sensitive dye imaging also demonstrated that the initial optical activity over the motor nucleus occurred at the onset of each cycle of activity in the episode, suggesting that motoneurons were critical for rhythmogenesis in this preparation (Fig. 1e–g). Consistent with motoneurons initiating a spontaneous episode, it was found that an episode could be triggered by a brief train of stimuli applied to motor axons in a deafferented muscle nerve or ventral root. Further work revealed that the connection between motoneurons and the spinal motor network was mediated by the projection of motoneurons to the avian equivalent of mammalian Renshaw cells – R-interneurons (Wenner and O’Donovan 1999).
Calcium and voltage imaging shows that activity begins ventro-laterally in the cut transverse face of the lumbosacral cord of the chick embryo at the onset of an episode of spontaneous bursting. (a) Spontaneous burst recorded from the femorotibialis (Fem) and the sartorius (Sart) muscle nerves in an E11 chick embryo. The red box at the beginning of the episode shows the approximate timing of the images shown in panel b. The expanded region indicated by the arrow shows the integrated femorotibialis neurogram corresponding to the frames shown in panel b. The first frame (−1) is the frame before the onset of the electrical activity and this and the subsequent frames are demarcated by the bars over the simultaneously recorded femorotibialis neurogram. Note that the data in the inset and the images of panel b are from a different embryo than that shown in a. (b) Calcium imaging of the onset of a spontaneous episode like the one shown in panel a. The panels are single frame difference images (active-control) averaged from 3 separate episodes all synchronized to the onset of the electrical activity recorded from the femorotibialis nerve. The color map below the image shows the color mapping of the fully expanded 8-bit images. (c) Voltage-sensitive dye imaging of a spontaneous episode of activity imaged with a 128-photodiode array. Each small square in the image corresponds to the output of one of the photodiodes. The top left-hand panel (Anti) shows the signals accompanying antidromic stimulation of the homonymous ventral root. The colored outlines identify the diodes whose signals were averaged together toFig. 1 (continued) provide the colored traces in panel d. The numbered panels correspond to the signals averaged from frames acquired during the temporal windows indicated in panel d. Panel 1 is the average of 105 fames (67 ms) before the onset of the main ventral root discharge indicated by the grey dotted line in d and amplified 4-fold compared to the remaining panels. Panels 2–8 were averaged from 45 frames (29 ms) and the data were acquired at 636 frames/s. The arrow shows that the earliest detectable activity begins over the motor nucleus from where it spreads contralaterally and ventro-dorsally. The data were combined from 2 E10 embryos and synchronized to the onset of the electrical activity. (d) Comparison between the timing of the optical signals and the low-passed ventral root activity (VR DC) and the integrated ventral root discharge (VR int.). (Modified from Arai et al. 2007). (e–h) Voltage-sensitive dye imaging reveals that the pattern of activity seen at the onset of a spontaneous burst recurs during each subsequent cycle of activity. (e) An episode of rhythmic activity initiated by a single stimulus to a dorsal root in an E10 embryo. The optical recording is from a single diode located over the motor nucleus ipsilateral to the ventral root recording. The dotted lines indicate the initiation of ventral root discharge. VR DC – ventral root activity filtered from DC-20Hz. VR Int – ventral root spiking. (f) Comparison of the timing of the ventral root electrical activity with the optical signals averaged from multiple diodes over three regions of the cord as shown in panel H. Note that with the exception of the first cycle, optical activity arises over the motor nucleus and propagates dorsally in each cycle. (g) Expansion of the rectangular region shown at the start of first cycle in f, showing that the dorsal root stimulus (s) initially triggers dorsal activity that subsequently propagates dorso-ventrally. (h) Transverse section of the cord showing the superimposed diode signals. The motoneurons (green) were labeled with DiO and the dorsal roots (red) with DiI. The diodes signals indicated in the different colors were averaged to generate the optical signals shown in (f). (Modified from Arai et al. 2007)
Although R-interneurons release the inhibitory neurotransmitter GABA (Wenner and O’Donovan 1999), their synaptic actions are depolarizing because the chloride equilibrium potential is elevated above rest potential in developing spinal neurons (Chub and O’Donovan 2001). To show that R-interneurons were the likely mediators communicating the motoneuronal activity to the rest of the network, we applied a calcium-sensitive dye to the ventrolateral funiculus to back-label the interneurons whose axons projected therein. The cut transverse face of the cord was then imaged during stimulation of motor axons sub-threshold for initiating an episode of bursting (Fig. 2a, panel labeled VR-stim). Intracellular recording from individual R-interneurons confirmed that the labeled region contained the neurons activated monosynaptically by ventral root stimulation of motoneurons (Wenner and O’Donovan 2001). Once this location was established, we then identified the first region to become active during a spontaneous episode and found it overlapped with the R-interneuron region (Fig. 2 panels 2 and 3). One limitation of these experiments is that only a subset of ventral interneurons is labeled with the calcium-sensitive dye applied to the ventro-lateral funiculus. Nevertheless, the observation that the first region to become active at the onset of a spontaneous episode contains neurons monosynaptically activated by motoneurons is consistent with their role in communicating the initial motoneuron activity to the rest of the network. However, we also found that the motoneuron-R-interneuron pathway was not obligatory for transmitting motoneuron activity to the rest of the network because when the recurrent connection between motoneurons and R-interneurons was depressed by cholinergic antagonists, spontaneous activity still occurred (Fig. 2b) but under this condition the interneuronal activity following motoneuronal bursting began medial to the motor column and not in the R-interneuron area (Wenner and O’Donovan 2001).
Recruitment of interneurons retrogradely labeled with calcium green dextran at the onset of a spontaneous episode under control conditions and in the presence of cholinergic blockade. (a) The left most panel shows the cut transverse face of a single segment of the cord in which calcium green dextran was backfilled bilaterally from the ventrolateral funiculus (VLF backfill). The lateral motor columns (LMC) and the R-interneuron regions (R) are outlined. The next panel shows the optical signal averaged from 10 successive frames generated in response to a train of stimuli applied to the ventral root on that side of the cord. The other R-interneuron region was defined similarly. The remaining frames (1–6) show the interneuronal activity at the onset of a spontaneous episode. The activity commences in the R-interneuron region on that side of the cord (arrow) and propagates contralaterally from there. (b) The same cord section is shown during a spontaneous episode occurring in the presence of cholinergic antagonists. Under this condition the optical activity emerges bilaterally and medial to the lateral motor column (arrows) and intensifies to occupy most of the ventral cord. The frame rate was 30 Hz. The images in A were averaged from 4 episodes and those in B from 3 episodes. (Modified from Wenner and O’Donovan 2001)
We were concerned that activation of afferent fibers in the ventral roots (Coggeshall 1979) might contribute to the optical signals, so we performed experiments in the presence of CNQX and APV to block glutamatergic transmission. We found that neither the amplitude nor the location of the optical signals changed indicating that any contribution from afferent excitation was negligible (Wenner and O’Donovan 2001).
Similar findings have been made in the developing mouse spinal cord where a single stimulus to a ventral root can trigger a network burst (Hanson and Landmesser 2003). This is illustrated in Fig. 3b, which shows electrical recordings from the left and right sciatic nerve while stimulating the left sciatic nerve at increasing intensity in an E12.5 mouse embryo. At the lowest intensities (top traces) no bursts are evoked; with higher intensity a burst is evoked ipsilateral to the stimulated root (local burst) and at the highest intensity (lowest traces) bursts are evoked on both sides of the cord (propagated burst). The hypothesized circuitry responsible for the local and propagated bursts is shown in Fig. 3c. The local burst is presumed to be generated by reciprocal cholinergic connections between motoneurons and depolarizing responses from GABAergic neurons activated by motoneuron collaterals – presumably Renshaw cells. The propagated burst is hypothesized to be generated by motoneuronal connections with a glycinergic interneuronal population that projects to motoneurons and Renshaw cells in other segments and also contralaterally (Hanson and Landmesser 2003). It is not clear whether this glycinergic neuron is a novel cell class, because although Renshaw cells are known to project to each other, contralateral projections have not been demonstrated in the adult.
Stimulation of motoneuron axons in the sciatic nerve triggers network bursts in the embryonic mouse spinal cord. (a) Immunocytochemistry for the vesicular acetylcholine transporter (VAChT) in an E12.5 mouse embryo. Notice the limited extent of the VAChT positive neurites (asterisks) and the projections into the lateral (LF) and ventral (VF) funiculi. (b) Single stimuli applied at increasing current intensities to the left sciatic nerve. At the lowest intensity (top traces) no bursts are evoked in the left and right ventral roots. As the stimulus intensity is increased an ipsilateral burst is evoked and at the highest intensity bursting propagates and can be recorded contralaterally. (c) Schematic showing the hypothesized circuitry responsible for the local (Local Circuit) and the propagating (Entire Circuit) bursts. MN motoneuron, GLY N glycinergic neuron, Sen R sensitive receptor, Mec + dTC mecamylamine + d-tubocurarine, DHβE dihydro-β-erythroidine hydrobromide, Stryc strychnine. (Modified from Hanson and Landmesser 2003. Copyright (2003) Society for Neuroscience, U.S.A)
3 Excitatory Effects of Ventral Root Stimulation on Neonatal Mammalian Spinal Networks
The excitatory effects of ventral root stimulation on spinal networks persist into the neonatal period in both the mouse and rat. Stimulation of a ventral root can trigger bursting in an adjacent root (Nishimaru et al. 2005; Machacek and Hochman 2006) and entrain disinhibited bursting in both the neonatal rat (Machacek and Hochman 2006) and the mouse (Bonnot et al. 2009) spinal cords. However, unlike the situation earlier in development, these excitatory effects are labile, and they are not observed in every preparation. For example, in the neonatal mouse spinal cord, the ability to entrain bursting depends on the stimulated ventral root and varied from a minimum of ~15% for L2 stimulation to a maximum of ~50% for stimulation of the L6 root (Bonnot et al. 2009). In the neonatal rat spinal cord in vitro, entrainment of disinhibited bursting was observed in 4/11 experiments in P11–P14 rats (Machacek and Hochman 2006). In this study, other excitatory effects of ventral root stimulation, including ventral root evoked bursting in motoneurons and modulation of the frequency of the locomotor rhythm were seen in younger animals. Excitatory effects were observed in ~18% of the preparations although their frequency increased in the presence of bath-applied noradrenaline. The reason for this variability is unknown. In the neonatal rat, the latency of the VR-evoked bursts in the ventral roots was compatible with a disynaptic pathway, suggesting that motoneurons project to an excitatory interneuron that in turn projects back to motoneurons. Evidence for such a recurrent excitatory pathway in the neonatal mouse was found when it was demonstrated that stimulation of a ventral root produces monosynaptic EPSPs in glutamatergic V3 interneurons that in turn, project monosynaptically back to motoneurons (Chopek et al. 2018), and that motoneurons also make recurrent projections to glutamatergic spinocerebellar neurons (Chalif et al. 2022). However, the extent to which these connections mediate the excitatory effects of motoneurons is not clear, because there is no evidence that they are variable or labile.
4 Motoneuronal Regulation of Locomotion
The study of locomotion has been greatly facilitated using preparations in which the movements accompanying locomotion have been abrogated. In adult animals, this can be achieved by paralyzing muscles (Viala and Buser 1969; Grillner and Zangger 1979; Iles and Nicolopoulos-Stournaras 1996; Meehan et al. 2012) and in neonatal animals where the spinal cord is isolated and detached from the musculature (Grillner and Wallén 1980; Kudo and Yamada 1987; Smith and Feldman 1987; Whelan et al. 2000). Because no movements accompany the activity, it is referred to as fictive locomotion or locomotor-like activity. In such preparations, fictive locomotion is characterized by a rhythmic alternation between flexor and extensor muscle nerves and between bilateral flexor or extensor muscle nerves. A further simplification occurs in the isolated spinal cord preparation because the rostral lumbar ventral roots (L1 and L2) comprise predominantly flexor motoneurons and the caudal lumbar roots (L5 and L6) mostly extensor motoneurons (Cazalets et al. 1992; Kiehn et al. 1992). In the isolated spinal cord of the mouse, locomotor-like activity can be induced by a cocktail of drugs including NMDA (n-methyl-D-aspartate) and serotonin which can also be supplemented with dopamine (Jiang et al. 1999; Whelan et al. 2000). In addition, tonic low frequency (1–4 Hz) stimulation of sensory afferents in the dorsal roots or the locomotor centers in the brainstem can trigger locomotion in the neonatal mouse and rat spinal cords (Whelan et al. 2000; Zaporozhets et al. 2004)
The role of motoneurons in locomotion was first examined in the isolated lamprey spinal cord. This was done by antidromically stimulating a ventral root during fictive swimming induced by drugs (Wallen 1984). It was found that antidromic stimulation of 1–3 ventral roots failed to modify the ongoing locomotor pattern or frequency (Wallen 1984), suggesting that motoneuronal activity does not modulate the swimming central pattern generator. However, subsequent work in the fictively swimming Xenopus tadpole, challenged this idea by showing that the amplitude of the rhythmic drive potentials recorded from spinal interneurons was reduced by ~20% in the presence of bath-applied nicotinic antagonists (Perrins and Roberts 1995). Initially it was assumed that the source of acetylcholine was motoneurons, but later work revealed that spinal glutamatergic interneurons could also release acetylcholine (Li et al. 2004), raising doubts about this assumption.
In mammals, studies of the role of motoneurons in locomotion have been restricted to isolated preparations of neonatal mice (Mentis et al. 2005; Humphreys and Whelan 2012; Falgairolle et al. 2017) and rats (Machacek and Hochman 2006). In the de-afferented isolated cord of the neonatal mouse, low frequency (1–4 Hz) tonic stimulation of a ventral root or the deafferented sciatic nerve can trigger an episode of locomotor-like activity (Fig. 4) (Mentis et al. 2005).
Stimulation of the sciatic nerve with cut dorsal roots can evoke an episode of fictive locomotion in the isolated cord of the neonatal mouse. (a) Comparison between an episode of locomotor-like activity triggered by a train of stimuli (4 Hz for 10 s) applied to either the de-afferented sciatic nerve (left hand panels) or a dorsal root (right hand panels), under control conditions, in the presence of cholinergic blockers (50 μM mecamylamine, 50 μM dihydro-β-erythroidine, and 5 μM atropine), cholinergic blockers plus APV (100 μM) followed by washout of the drugs. The recordings are DC coupled from the left (L) and right (R) Lumbar(L) 1 ventral roots. (b and c)Fig. 4 (continued) Motoneurons release acetylcholine and an excitatory amino acid at their terminals with Renshaw cells. (b) Synaptic potentials recorded from a Renshaw cell in response to a single stimulus (arrow) applied to the ipsilateral ventral root. In the presence of cholinergic blockade (Chol. Block), a small potential persisted that had the same latency as the pre-drug potential (see inset). The smaller potential was abolished by glutamatergic antagonists (100 μM APV and 10 μM CNQX). (c) Voltage clamp recordings of a Renshaw cell following a single stimulus to the ipsilateral ventral root. After application of the nicotinic antagonist mecamylamine (50 μM Chol. Block) the evoked current was reduced and subsequently almost abolished when the broad-spectrum glutamatergic antagonist kynurenate (2 mM) was added. In the lower traces, the order of antagonists was reversed so that the glutamatergic antagonists (20 μM CNQX and 20 μM AP5) were applied first followed by the addition of 50 μM mecamylamine. (Panels a and b modified from (Mentis et al. 2005) and c from (Nishimaru et al. 2005) Copyright (2005) National Academy of Sciences, U.S.A)
The locomotor-like activity evoked by ventral root stimulation was blocked by ionotropic glutamate antagonists but not by cholinergic antagonists or a gap junction blocker (carbenoxolone). This was a very surprising result given that acetylcholine was assumed to be the only fast neurotransmitter released from motoneurons. However, it was shown that motoneurons release from their synaptic connections with Renshaw cells, an excitatory amino acid that binds to glutamatergic receptors (Fig. 4b, c) (Mentis et al. 2005; Nishimaru et al. 2005). Whether glutamate or aspartate is activating glutamatergic receptors has not yet be resolved (Richards et al. 2014). Nevertheless, if the excitatory effects of motoneurons were mediated by an excitatory interneuron then presumably both glutamatergic and cholinergic receptors would be activated when motoneurons were stimulated. The discovery that the excitatory projections of motoneurons to V3 interneurons were exclusively glutamatergic in the neonatal mouse spinal cord (Chopek et al. 2018) provided a possible explanation for this effect. Unfortunately, it is not clear that V3 interneurons mediate the locomotor actions of ventral root stimulation because optogenetic activation of V3 interneurons slows the locomotor rhythm (Danner et al. 2019) and silencing them leads to increased variability of the cycle length and flexor bursting (Zhang et al. 2008). A more likely candidate for mediating the effects of motoneuronal activity on the CPG are ventral spinocerebellar neurons that receive glutamatergic and cholinergic input from motoneurons in the neonatal mouse cord (Chalif et al. 2022). This is because optogenetic hyperpolarization of these neurons blocked the ability of motoneuron stimulation to trigger the locomotor rhythm in the neonatal mouse spinal cord, and these neurons were shown to be both necessary and sufficient for the generation of the locomotor rhythm in the neonatal mouse spinal cord (Chalif et al. 2022).
5 Ventral Root Afferents
One complicating factor in attributing the excitatory effects of ventral root stimulation to motor axons in the ventral roots is the possibility that they are mediated or complimented by the activation of sensory afferents that enter the cord through the ventral roots (Coggeshall 1979), or by excitation of sensory neurons in the ventral root itself (Windle 1931; Yamamoto et al. 1977). The existing data on this issue is somewhat contradictory. For example, direct injection of horseradish peroxidase into the spinal cord, labels cell bodies in the dorsal root ganglion when the dorsal roots have been cut (Maynard et al. 1977). Furthermore, a few studies using horseradish peroxidase applied to the ventral roots have revealed the existence of the occasional axon projecting to the dorsal horn and to preganglionic sympathetic neurons (Light and Metz 1978; Mawe et al. 1984; Beattie et al. 1987). Whether these projections are functional is unclear because activation of ventral root afferents could excite spinal neurons only if the dorsal roots remained intact (Clifton et al. 1976; Chung et al. 1983, 1985). Furthermore, more recent work has suggested that ventral root afferents end blindly, innervate the meninges or loop into the dorsal root (Shin et al. 1986; Hildebrand et al. 1997) contradicting the work showing dorsal root ganglion cells labeled from within the de-afferented cord. If ventral root afferents exist in the neonatal mouse is not known. However, it seems unlikely that such projections, even if they exist in the neonatal mouse, can account for the ability of ventral root stimulation to activate the locomotor CPG, because electrical stimulation of the dorsal root ganglion, with the dorsal roots cut, does not trigger locomotor activity (Pujala et al. 2016), although such stimulation would probably not activate neurons in the ventral root (Windle 1931; Yamamoto et al. 1977).
6 Optogenetic Manipulation of Motoneuron Activity During Locomotion
One approach to circumvent the potential activation of ventral root afferents, is to manipulate the firing of motoneurons directly and to establish if this affects the function of the locomotor CPG. In the neonatal mouse, this was accomplished in a series of optogenetic experiments in which the light-sensitive opsin archaerhodopsin was introduced into cholinergic neurons expressing the enzyme responsible the synthesis of the neurotransmitter acetylcholine (choline acetyltransferase -ChAT) or neurons expressing the transcription factor islet-1, of which motoneurons are a subset (Falgairolle et al. 2017). Archaerhodopsin is a light-gated outward proton pump that hyperpolarizes neurons (Chow et al. 2010) and independently reduces synaptic transmission (El-Gaby et al. 2016) when illuminated with green light. During drug-induced locomotor-like activity, illumination of cords expressing archaerhodopsin in either ChAT-positive or Islet1 expressing neurons transiently abolished or slowed the locomotor rhythm and often made it less regular (Fig. 5).
Optogenetic hyperpolarization of motoneurons transiently abolishes and disrupts the locomotor rhythm during drug-induced fictive locomotion. (a) Z-stack projection of a 60μm transverse section of the second Lumbar (L2) segment of a P3 mouse spinal cord in which cholinergic (ChAT) neurons expressed the inhibitory opsin archaerhodopsin (Arch) coupled to enhanced green fluorescent protein (eGFP). (b) Green light (timing indicated by green bar) transiently inhibits and disrupts the locomotor rhythm induced by NMDA and serotonin in a ChAT/Arch animal. The lowest trace is an intracellular recording from an antidromically identified motoneuron. (c) Z-stack projection of a 60 μm transverse section of the L5 segment of a P2 mouse spinal cord in which Islet-1 positive neurons express archaerhodopsin coupled to eGFP. (d) Green light transiently suppresses and disrupts the locomotor rhythm induced by NMDA and serotonin in the Islet-1/Arch animal. The light grey lines superimposed on the neurograms in b and d are the slow potentials obtained by low pass filtering the raw ventral root signals (black traces). (Modified from Falgairolle et al. 2017)
To control for the intra- and extracellular changes in pH that accompany activation of archaerhodopsin (Chow et al. 2010), similar experiments were performed in ChAT+ neurons expressing another hyperpolarizing opsin – halorhodopsin. In contrast to archaerhodopsin, halorhodopsin is a light-gated chloride channel that hyperpolarizes the cell membrane by moving chloride ions into the cell (Zhang et al. 2007). Illumination of halorhodopsin during locomotor-like activity had similar results to the archaerhodopsin experiments indicating that changes in pH were not responsible for the slowing and disruption of the locomotor rhythm (Falgairolle et al. 2017). When the light was turned off in either experiment, the bursting of motoneurons was transiently enhanced and this was accompanied by a corresponding increase in the locomotor-like frequency. Confirmation that increased motoneuron firing could accelerate the locomotor-like frequency came from experiments in which the excitatory opsin channelrhodopsin was introduced into ChAT+ neurons. The pharmacology of the modulatory effects of motoneuron firing on the locomotor-like rhythm were similar to that of the locomotor-like activity evoked by ventral root stimulation. Specifically, it was not blocked by cholinergic antagonists but was abrogated by the AMPA receptor antagonist CNQX and persisted in the presence of carbenoxolone to block gap junctions. Ventral spinocerebellar neurons, which have reciprocal excitatory and electrical connections with motoneurons, are an obvious candidate to mediate the modulation of the CPG by motoneurons because their optogenetic excitation activates the locomotor rhythm and their hyperpolarization abolishes it (Chalif et al. 2022).
7 Motoneuronal Regulation of Central Pattern Generating Circuitry in Non-mammalian Vertebrates
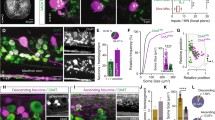
Motoneurons have been shown to regulate central pattern generator function for swimming and vocalization in fish and frogs. We will first consider the role of motoneurons in the regulation of fictive swimming in the adult zebrafish and then discuss the motoneuronal control of vocalization in toadfish and the frog Xenopus Laevis. The adult zebrafish can generate fictive swimming in response to tonic stimulation applied between the brainstem and the spinal cord (Gabriel et al. 2008). Research in this preparation had revealed that the rhythmic drive to motoneurons during fictive swimming is derived from a class of excitatory glutamatergic interneurons (V2a) that express the Chox-10 transcription factor (Eklof-Ljunggren et al. 2012). These interneurons express pacemaker properties and are organized into modules that are sequentially recruited as swimming speed increases (Ampatzis et al. 2014). They form hybrid chemical bidirectional electrical synapses with motoneurons (Song et al. 2016) so that motoneuron depolarization or hyperpolarization is transmitted electrotonically to the V2a synapses on motoneurons which increases or attenuates transmitter release from the V2a synapse (Fig. 6b). Furthermore, changes in motoneuronal membrane potential can regulate the membrane potential of the V2a interneurons and thereby affect their firing (Fig. 6c). When motoneurons expressing the inhibitory opsin halorhodopsin were illuminated by yellow light, swim episodes were shortened and occurred at a reduced frequency (Fig. 6d). This was accompanied by hyperpolarization of the V2a interneurons sufficient to block firing, thereby reducing the excitatory drive to motoneurons (Song et al. 2016). It seems unlikely that a similar mechanism accounts for the modulation of the locomotor rhythm in the neonatal mouse spinal cord because the phenomenon persists in the presence of the gap junction blocker carbenoxolone (Falgairolle et al. 2017) and motoneurons are not electrically coupled to V2a interneurons in the mouse spinal cord (Bhumbra and Beato 2018).
Hyperpolarizing motoneurons expressing halorhodopsin lowers the swim frequency and shortens the duration of the swim bout in the adult zebrafish. (a) Micrograph of the spinal cord showing motoneurons (MNs) expressing halorhodopsin coupled with mCherry (NpHR-mCherry, red) and V2a interneurons (V2a Ins) expressing Chox10 coupled with GFP (Chx10-GFP, green). (b) Intracellular recordings showing that yellow light on the cord hyperpolarizes the motoneuron (MN, upper red trace) and also the V2a interneuron (V2a IN, lower green trace). (c) Intracellular recording from a V2a interneuron showing that light-induced hyperpolarization of motoneurons can block firing in the interneuron during a swim episode. (d) More frequently, however, motoneuron hyperpolarization reversibly slows the swim frequency and shortens the swim duration recorded in a V2a interneuron. Top trace control, middle trace light-induced hyperpolarization of motoneurons, lowest trace recovery. In all panels the duration of the light stimulus is shown by the yellow bar. (Modified from Song et al. 2016 with permission)
Another system in which gap junctions between motoneurons and interneurons may be involved in the regulation of a CPG, is the vocalization network of toadfish. Toadfish make two types of vocalization – grunts and boatwhistles (advertisement calls) – that are produced by superfast muscles attached to the swim bladder (Chagnaud and Bass 2014). The frequency of the rhythm is generated by the vocal pacemaker nucleus and the duration of the vocalization by the vocal pre-pacemaker nucleus located in the hindbrain (Chagnaud et al. 2011) (Fig. 7a). Vocal motoneurons are extensively connected through gap junctions to the premotor interneurons generating the vocal input to motoneurons including the neurons of the pacemaker nucleus (Bass et al. 1994), suggesting that they can directly influence pacemaker function. Moreover, antidromic stimulation of the vocal nerve reveals a short latency depolarization in intracellularly recorded motoneurons indicative of electrical coupling between motoneurons. As the stimulus intensity is increased a short latency hyperpolarization is also recorded. This is unlikely to be due to classic recurrent inhibition mediated by Renshaw cells because the vocal motoneurons lack recurrent collaterals (Chagnaud and Bass 2014), and appears to be the result of electrical coupling between motoneurons and a glycinergic interneuron. It is hypothesized that this antidromically driven inhibition is essential for repetitive firing of the vocal motoneurons because intracellular depolarization of individual motoneurons does not result in repetitive firing possibly because of weak repolarization after the action potential (Chagnaud et al. 2021). Thus, motoneuronal activity may contribute directly to the depolarization of the pacemaker neurons and to the synchronization of the motoneuronal bursts through gap junctional coupling (Chagnaud et al. 2021). Whether these gap junction connections are hybrid chemical/electrical synapses, as in the connection of spinal motoneurons to V2a neurons in the zebrafish, is not known.
Motoneurons regulate the vocalization circuitry in the toadfish and in Xenopus Laevis. (a) Schematic of the nuclei and connectivity in the circuit controlling vocalization in the toadfish. Motoneurons in the vocal motor nucleus (VMN) are gap-junction coupled to excitatory interneurons in the vocal pacemaker nucleus (VPN) and to a glycinergic inhibitory population. The VPN sets the frequency of the calls and is controlled by the vocal pre-pacemaker nucleus (VPP) that determines the call duration. It is hypothesized that motoneuronal activity feeds back to the VPN and glycinergic populations through gap junctions to regulate its function. (Modified from Fig. 9 in Chagnaud et al. 2021). (b) The organization of the vocal circuitry in Xenopus Laevis. b1. The vocal circuit comprises a premotor nucleus containing neurons that generate fast trills that project excitatory connections to motoneurons innervating the larynx. The hypothetical intracellular recordings from these premotor interneurons (shown on the right) illustrate how the proposed inhibitory feedback (grayed area) slows the frequency and synchronizes the population activity (lower panel). b2. The effect of interrupting the feedback from the motor nucleus to the premotor interneurons either by silencing motoneuron firing with QX-314 (an intracellular sodium channel blocker) or by sectioning the preparation between the premotor nucleus and the motor nucleus abolishes the feedback inhibition leading to an increased frequency of firing and population asynchrony of the fast trill neurons. (Modified from Lawton et al. 2017)
Xenopus Laevis is another species in which motoneuronal activity can modulate a vocal CPG (Lawton et al. 2017). In male frogs this CPG produces mating songs which can be activated in vitro by bath applying serotonin to the isolated brain (Zornik and Yamaguchi 2012). The evoked song comprises a brief ‘trill’ at 50–60 Hz and lasting ~500 ms which is similar to the naturally occurring vocalizations. The laryngeal motoneurons are driven by premotor neurons termed fast trill neurons that are located in the dorsal tegmental area of the medulla (DTAM). There is also an ascending inhibitory projection from the laryngeal motor nucleus to the DTAM (Fig. 7b1). When laryngeal motoneuron activity is silenced by retrograde loading of motoneuron axons with the sodium channel blocker QX-314, the fast trill neurons fire faster and asynchronously instead of synchronously (Fig. 7b2). This modulation appeared to be mediated by recurrently activated inhibitory neurons that synapse on the fast trill neurons. Whether or not these inhibitory neurons are the equivalent of mammalian Renshaw cells is not known. Spinal Renshaw cells are not known to project to locomotor CPG neurons and silencing them pharmacologically in the cat (Pratt and Jordan 1987) or genetically in the mouse (Enjin et al. 2017) has minimal effect on the locomotor pattern.
8 Concluding Remarks
The finding that motoneurons can regulate central pattern generator function and project to several different interneuronal types raises several fascinating questions. First, because motoneuronal excitation of the CPG increases the firing of motoneurons, this could lead to runaway excitation of the CPG by positive feedback. Since such runaway excitation does not occur, it is likely that the excitation is balanced by inhibition. Currently only inhibitory Renshaw cells receive input from motoneurons, and their activity is not believed to significantly affect locomotion (Pratt and Jordan 1987; Enjin et al. 2017). However, in the Vglut2 knockout mouse, stimulation of a ventral root can inhibit and slow rhythmogenesis (Talpalar et al. 2011), potentially providing inhibitory feedback from motoneurons to the CPG. Whether this pathway can inhibit the CPG in the intact cord is not clear because electrical stimulation of the ventral roots accelerates the drug-induced locomotor rhythm (Machacek and Hochman 2006). It is possible, therefore, that motoneurons synapse with another class of inhibitory interneuron in addition to Renshaw cells as hypothesized by Hanson and Landmesser in their work on the developing mouse lumbar cord (Hanson and Landmesser 2003) (Fig. 3c).
The presence of motoneuronal projections to spinal interneurons including V3 (Chopek et al. 2018) and spinocerebellar interneurons (Chalif et al. 2022) raises the question of additional functions of recurrent excitation within spinal and ascending circuits. One potential function of such projections could be efference copy. It is well established that sensory feedback from muscle proprioceptors and joint receptors provides information about the movements produced by muscle contraction. The existence of direct feedback from motoneurons provides the efferent signal driving muscle contraction. Thus, the intended action (motoneuronal activity) and the actual action (sensory feedback from muscles) can be compared both within spinal circuits and/or remotely in the cerebellum (Wolpert and Miall 1996; Popa and Ebner 2018). It might be argued that central command coming from the CPG or from descending control systems might be sufficient for efference copy. However, given the multiplicity of inputs to motoneurons and the non-linear behavior of the motoneuron membrane, such signals would provide a poor representation of the firing behavior of the motoneuron and the command signal to muscle.
It is not known if motoneurons regulate locomotion in the adult rodent. This has not been addressed experimentally because of the challenges of studying the issue in the mature spinal cord. However, with the development of isolated sacral cord (Manuel et al. 2012) and decerebrate preparations of the adult mouse that can generate fictive locomotion (Nakanishi and Whelan 2012), it should be possible to answer the questions using opto- or chemo-genetics. In addition, we do not know if all classes of motoneuron are capable of modulating locomotor activity or whether it is restricted to a subset. Previous work has suggested that type S motoneurons or even gamma motoneurons might mediate the effects (Pujala et al. 2016), but definitive resolution of this issue must await the ability to selectively activate the different classes of motoneuron.
Finally, we must ask if motoneurons could be part of the central pattern generator for locomotion. If the CPG is a dedicated class of interneuron, such as ventral spinocerebellar neurons, obviously the answer is no. Current evidence suggests that several different neuronal classes, in addition to spinocerebellar neurons, contribute to locomotor rhythmogenesis in the neonatal mouse cord, including HB9 interneurons (Hinckley et al. 2005; Wilson et al. 2005; Kwan et al. 2009), Shox2 glutamatergic interneurons (Dougherty et al. 2013) and motoneurons (Falgairolle et al. 2017). Here, we hypothesize that many neuron classes participate in rhythmogenesis and the membership varies according to motor task and the state of the spinal networks. This is most likely to be true for drug-induced fictive locomotion because the drug cocktail used to induce locomotion contains NMDA which induces membrane potential oscillations in many spinal neurons including motoneurons (MacLean et al. 1997; Wilson et al. 2005). Consistent with this thinking, when the vesicular glutamate transporter VGluT2 is knocked out, rendering the great majority of glutamatergic neurons non-functional, a drug cocktail containing NMDA can still generate fictive locomotion. It has been suggested that NMDA-induced oscillations in 1a inhibitory interneurons, Renshaw cell and motoneurons may support the rhythm under these conditions – a very different neuronal cohort than when glutamatergic neurons are also present (Talpalar et al. 2011). Another piece of evidence in support of the idea of a fluid CPG comes from calcium imaging of neurons in the cut transverse face of the cord during fictive locomotion induced by NMDA and serotonin at different frequencies. As locomotor speed increases interneuronal recruitment in the ventral part of the cord shifted from lateral to medial with few neurons co-active at the different speeds (Rancic et al. 2020). Variability in the recruitment of interneurons during fictive locomotion is not restricted to the neonatal mouse and has also been observed in the adult mouse during successive bouts of treadmill locomotion (Pham et al. 2020). Using the FosTRAP mouse that allows a comparison between the neurons activated on two separate occasions it was shown that only 20% of the spinal neurons active in an episode of treadmill locomotion are also active in a second bout 2 weeks later (Pham et al. 2020). Whether this reflects a change in the membership of the CPG or is an artefact of the indirect measure of neuronal activity provided by Fos signaling is not clear at present and must await chronic single unit recordings or 2-photon imaging of single neurons over extended periods. Nevertheless, if the neonatal mouse CPG is organized with a varying cellular membership it may account for the apparent variability in the participation of motoneurons in rhythmogenesis.
The idea that motoneurons might participate directly in locomotor rhythmogenesis is suggested by a number of observations. First, optical hyperpolarization of motoneurons in either islet-1 or Chat mice expressing archaerhodopsin slows the locomotor rhythm and in some cases abolishes the rhythmic locomotor drive potentials recorded intracellularly from motoneurons (Fig. 8). Second, neonatal lumbar and adult sacral motoneurons exhibit TTX-resistant NMDA-induced membrane potential oscillations that are likely activated during locomotion. In the adult mouse sacral cord preparation, where motoneurons exhibit NMDA induced oscillations (Manuel et al. 2012), it was argued that these are unlikely to contribute to locomotion because motoneurons are not coupled by electrical or chemical synapses in the adult cord. As a result, in the absence of interneuronal drive, the activity of different motoneurons would not be synchronized. However, later work in both the neonate and the adult mouse spinal cord revealed that motoneurons are coupled by chemical excitatory synapses (Bhumbra and Beato 2018), rendering this objection moot.
Intracellular recording from motoneurons in 4 different cords in which ChAT+ neurons expressed archaerhodopsin, showing a transient cessation of rhythmic drive potentials during illumination with green light. The motoneurons are separated into flexor and extensor motoneurons according to the phase relation of their bursting with simultaneous ventral root recordings (not shown). The arrows indicate periods when the rhythmic drive potentials could not be detected. The calibration bars to the right of the records are 40 mV
References
Ampatzis K, Song J, Ausborn J, El Manira A (2014) Separate microcircuit modules of distinct v2a interneurons and motoneurons control the speed of locomotion. Neuron 83(4):934–943
Arai Y, Mentis GZ, Wu JY, O’Donovan MJ (2007) Ventrolateral origin of each cycle of rhythmic activity generated by the spinal cord of the chick embryo. PLoS One 2(5):e417
Bass AH, Marchaterre MA, Baker R (1994) Vocal-acoustic pathways in a teleost fish. J Neurosci 14(7):4025–4039
Beattie MS, Bresnahan JC, Mawe GM, Finn S (1987) Distribution and ultrastructure of ventral root afferents to lamina I of the cat sacral spinal cord. Neurosci Lett 76(1):1–6
Bhumbra GS, Beato M (2018) Recurrent excitation between motoneurones propagates across segments and is purely glutamatergic. PLoS Biol 16(3):e2003586
Bonnot A, Chub N, Pujala A, O’Donovan MJ (2009) Excitatory actions of ventral root stimulation during network activity generated by the disinhibited neonatal mouse spinal cord. J Neurophysiol 101(6):2995–3011
Borodinsky LN, Root CM, Cronin JA, Sann SB, Gu X, Spitzer NC (2004) Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature 429(6991):523–530
Brock LG, Coombs JS, Eccles JC (1952) The recording of potentials from motoneurones with an intracellular electrode. J Physiol 117(4):431–460
Cazalets JR, Sqalli-Houssaini Y, Clarac F (1992) Activation of the central pattern generators for locomotion by serotonin and excitatory amino acids in neonatal rat. J Physiol 455:187–204
Chagnaud BP, Baker R, Bass AH (2011) Vocalization frequency and duration are coded in separate hindbrain nuclei. Nat Commun 2(1):346
Chagnaud BP, Bass AH (2014) Vocal behavior and vocal central pattern generator organization diverge among toadfishes. Brain Behav Evol 84(1):51–65
Chagnaud BP, Perelmuter JT, Forlano PM, Bass AH (2021) Gap junction-mediated glycinergic inhibition ensures precise temporal patterning in vocal behavior. elife 10
Chalif JI, Martínez-Silva MDL, Pagiazitis JG, Murray AJ, Mentis GZ (2022) Control of mammalian locomotion by ventral spinocerebellar tract neurons. Cell 185(2):328–344.e326
Chopek JW, Nascimento F, Beato M, Brownstone RM, Zhang Y (2018) Sub-populations of spinal V3 interneurons form focal modules of layered pre-motor microcircuits. Cell Rep 25(1):146–156 e143
Chow BY, Han X, Dobry AS, Qian X, Chuong AS, Li M, Henninger MA, Belfort GM, Lin Y, Monahan PE, Boyden ES (2010) High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature 463(7277):98–102
Chub N, O’Donovan MJ (2001) Post-episode depression of GABAergic transmission in spinal neurons of the chick embryo. J Neurophysiol 85(5):2166–2176
Chung JM, Lee KH, Endo K, Coggeshall RE (1983) Activation of central neurons by ventral root afferents. Science 222(4626):934–935
Chung JM, Lee KH, Kim J, Coggeshall RE (1985) Activation of dorsal horn cells by ventral root stimulation in the cat. J Neurophysiol 54(2):261–272
Clifton GL, Coggeshall RE, Vance WH, Willis WD (1976) Receptive fields of unmyelinated ventral root afferent fibres in the cat. J Physiol 256(3):573–600
Coggeshall R (1979) Afferent fibers in the ventral root. Neurosurgery 4(5):443–448
Danner SM, Zhang H, Shevtsova NA, Borowska-Fielding J, Deska-Gauthier D, Rybak IA, Zhang Y (2019) Spinal V3 interneurons and left-right coordination in mammalian locomotion. Front Cell Neurosci 13:516
Dougherty KJ, Zagoraiou L, Satoh D, Rozani I, Doobar S, Arber S, Jessell TM, Kiehn O (2013) Locomotor rhythm generation linked to the output of spinal shox2 excitatory interneurons. Neuron 80(4):920–933
Eklof-Ljunggren E, Haupt S, Ausborn J, Dehnisch I, Uhlen P, Higashijima S, El Manira A (2012) Origin of excitation underlying locomotion in the spinal circuit of zebrafish. Proc Natl Acad Sci U S A 109(14):5511–5516
El-Gaby M, Zhang Y, Wolf K, Schwiening J, Christof OP, Shipton A, Olivia (2016) Archaerhodopsin selectively and reversibly silences synaptic transmission through altered pH. Cell Rep 16(8):2259–2268
Enjin A, Perry S, Hilscher MM, Nagaraja C, Larhammar M, Gezelius H, Eriksson A, Leao KE, Kullander K (2017) Developmental disruption of recurrent inhibitory feedback results in compensatory adaptation in the Renshaw cell-motor neuron circuit. J Neurosci 37(23):5634–5647
Falgairolle M, Puhl JG, Pujala A, Liu W, O’Donovan MJ (2017) Motoneurons regulate the central pattern generator during drug-induced locomotor-like activity in the neonatal mouse. elife 6
Gabriel JP, Mahmood R, Walter AM, Kyriakatos A, Hauptmann G, Calabrese RL, El Manira A (2008) Locomotor pattern in the adult zebrafish spinal cord in vitro. J Neurophysiol 99(1):37–48
Grillner S, Wallén P (1980) Does the central pattern generation for locomotion in lamprey depend on glycine inhibition? Acta Physiol Scand 110(1):103–105
Grillner S, Zangger P (1979) On the central generation of locomotion in the low spinal cat. Exp Brain Res 34(2)
Hall BK, Herring SW (1990) Paralysis and growth of the musculoskeletal system in the embryonic chick. J Morphol 206(1):45–56
Hamburger V (1990) The developmental history of the motor neuron. Neuroembryology 15:1–37
Hanson MG, Landmesser LT (2003) Characterization of the circuits that generate spontaneous episodes of activity in the early embryonic mouse spinal cord. J Neurosci 23(2):587–600
Hanson MG, Landmesser LT (2004) Normal patterns of spontaneous activity are required for correct motor axon guidance and the expression of specific guidance molecules. Neuron 43(5):687–701
Hildebrand C, Karlsson M, Risling M (1997) Ganglionic axons in motor roots and PIA mater. Prog Neurobiol 51(2):89–128
Hinckley CA, Hartley R, Wu L, Todd A, Ziskind-Conhaim, L (2005) Locomotor-Like Rhythms in a Genetically Distinct Cluster of Interneurons in the Mammalian Spinal Cord. J Neurophysiol 93(3):1439–1449. https://doi.org/10.1152/jn.00647.2004
Humphreys JM, Whelan PJ (2012) Dopamine exerts activation-dependent modulation of spinal locomotor circuits in the neonatal mouse. J Neurophysiol 108(12):3370–3381
Iles JF, Nicolopoulos-Stournaras S (1996) Fictive locomotion in the adult decerebrate rat. Exp Brain Res 109(3):393–398
Jiang Z, Carlin KP, Brownstone RM (1999) An in vitro functionally mature mouse spinal cord preparation for the study of spinal motor networks. Brain Res 816(2):493–499
Kiehn O, Iizuka M, Kudo N (1992) Resetting from low threshold afferents of N-methyl-D-aspartate-induced locomotor rhythm in the isolated spinal cord-hindlimb preparation from newborn rats. Neurosci Lett 148(1-2):43–46
Kudo N, Yamada T (1987) locomotor activity in a spinal cord-indlimb muscles preparation of the newborn rat studied in vitro. Neurosci Lett 75(1):43–48
Kwan AC, Dietz SB, Webb WW, Harris-Warrick RM (2009) Activity of Hb9 interneurons during fictive locomotion in mouse spinal cord. J Neurosci 29(37):11601–11613
Landmesser LT, O’Donovan MJ (1984) Activation patterns of embryonic chick hind limb muscles recorded in ovo and in an isolated spinal cord preparation. J Physiol 347(1):189–204
Lawton KJ, Perry WM, Yamaguchi A, Zornik E (2017) Motor neurons tune premotor activity in a vertebrate central pattern generator. J Neurosci 37(12):3264–3275
Li WC, Soffe SR, Roberts A (2004) Glutamate and acetylcholine corelease at developing synapses. Proc Natl Acad Sci U S A 101(43):15488–15493
Light AR, Metz CB (1978) The morphology of the spinal cord efferent and afferent neurons contributing to the ventral roots of the cat. J Comp Neurol 179(3):501–515
Machacek DW, Hochman S (2006) Noradrenaline unmasks novel self-reinforcing motor circuits within the mammalian spinal cord. J Neurosci 26(22):5920–5928
MacLean JN, Schmidt BJ, Hochman S (1997) NMDA receptor activation triggers voltage oscillations, plateau potentials and bursting in neonatal rat lumbar motoneurons in vitro. Eur J Neurosci 9(12):2702–2711
Manuel M, Li Y, Elbasiouny SM, Murray K, Griener A, Heckman CJ, Bennett DJ (2012) NMDA induces persistent inward and outward currents that cause rhythmic bursting in adult rodent motoneurons. J Neurophysiol 108(11):2991–2998
Mawe GM, Bresnahan JC, Beattie MS (1984) Primary afferent projections from dorsal and ventral roots to autonomic preganglionic neurons in the cat sacral spinal cord: light and electron microscopic observations. Brain Res 290(1):152–157
Maynard CW, Leonard RB, Dan Coulter J, Coggeshall RE (1977) Central connections of ventral root afferents as demonstrated by the HRP method. J Comp Neurol 172(4):601–608
Meehan CF, Grondahl L, Nielsen JB, Hultborn H (2012) Fictive locomotion in the adult decerebrate and spinal mouse in vivo. J Physiol 590(2):289–300
Mentis GZ, Alvarez FJ, Bonnot A, Richards DS, Gonzalez-Forero D, Zerda R, O’Donovan MJ (2005) Noncholinergic excitatory actions of motoneurons in the neonatal mammalian spinal cord. Proc Natl Acad Sci U S A 102(20):7344–7349
Milner LD, Landmesser LT (1999) Cholinergic and GABAergic inputs drive patterned spontaneous motoneuron activity before target contact. J Neurosci 19(8):3007–3022
Nakanishi ST, Whelan PJ (2012) A decerebrate adult mouse model for examining the sensorimotor control of locomotion. J Neurophysiol 107(1):500–515
Nishimaru H, Restrepo CE, Ryge J, Yanagawa Y, Kiehn O (2005) Mammalian motor neurons corelease glutamate and acetylcholine at central synapses. Proc Natl Acad Sci U S A 102(14):5245–5249
O’Donovan M, Ho S, Yee W (1994) Calcium imaging of rhythmic network activity in the developing spinal cord of the chick embryo. J Neurosci 14(11 Pt 1):6354–6369
Perrins R, Roberts A (1995) Cholinergic contribution to excitation in a spinal locomotor central pattern generator in Xenopus embryos. J Neurophysiol 73(3):1013–1019
Pham BN, Luo J, Anand H, Kola O, Salcedo P, Nguyen C, Gaunt S, Zhong H, Garfinkel A, Tillakaratne N, Edgerton VR (2020) Redundancy and multifunctionality among spinal locomotor networks. J Neurophysiol 124(5):1469–1479
Popa LS, Ebner TJ (2018) Cerebellum, predictions and errors. Front Cell Neurosci 12:524
Pratt CA, Jordan LM (1987) Ia inhibitory interneurons and Renshaw cells as contributors to the spinal mechanisms of fictive locomotion. J Neurophysiol 57(1):56–71
Provine RR, Sharma SC, Sandel TT, Hamburger V (1970) Electrical activity in the spinal cord of the chick embryo, in situ. Proc Natl Acad Sci 65(3):508–515
Pujala A, Blivis D, O’Donovan MJ (2016) Interactions between dorsal and ventral root stimulation on the generation of locomotor-like activity in the neonatal mouse spinal cord. eneuro 3(3):ENEURO.0101-0116
Rancic V, Ballanyi K, Gosgnach S (2020) Mapping the dynamic recruitment of spinal neurons during fictive locomotion. J Neurosci 40(50):9692–9700
Richards DS, Griffith RW, Romer SH, Alvarez FJ (2014) Motor axon synapses on Renshaw cells contain higher levels of aspartate than glutamate. PLoS One 9(5):e97240
Shin HK, Kim J, Nam SC, Paik KS, Chung JM (1986) Spinal entry route for ventral root afferent fibers in the cat. Exp Neurol 94(3):714–725
Smith JC, Feldman JL (1987) In vitro brainstem-spinal cord preparations for study of motor systems for mammalian respiration and locomotion. J Neurosci Methods 21(2-4):321–333
Song J, Ampatzis K, Bjornfors ER, El Manira A (2016) Motor neurons control locomotor circuit function retrogradely via gap junctions. Nature 529(7586):399–402
Talpalar AE, Endo T, Low P, Borgius L, Hagglund M, Dougherty KJ, Ryge J, Hnasko TS, Kiehn O (2011) Identification of minimal neuronal networks involved in flexor-extensor alternation in the mammalian spinal cord. Neuron 71(6):1071–1084
Toutant JP, Toutant MN, Renaud D, Le Douarin GH (1979) Enzymatic differentiation of muscle fibre types in embryonic Latissimus dorsii of the chick: effects of spinal cord stimulation. Cell Diff 8(5):375–382
Viala D, Buser P (1969) The effects of DOPA and 5-HTP on rhythmic efferent discharges in hind limb nerves in the rabbit. Brain Res 12(2):437–443
Wallen PLA (1984) Do the motoneurones constitute a part of the spinal network generating the Swimming rhythm in the lamprey? J Exp Biol 113(November):493–497
Wenner P (2014) Homeostatic synaptic plasticity in developing spinal networks driven by excitatory GABAergic currents. Neuropharmacology 78:55–62
Wenner P, O’Donovan MJ (1999) Identification of an interneuronal population that mediates recurrent inhibition of motoneurons in the developing chick spinal cord. J Neurosci 19(17):7557–7567
Wenner P, O’Donovan MJ (2001) Mechanisms that initiate spontaneous network activity in the developing chick spinal cord. J Neurophysiol 86(3):1481–1498
Whelan P, Bonnot A, O’Donovan MJ (2000) Properties of rhythmic activity generated by the isolated spinal cord of the neonatal mouse. J Neurophysiol 84(6):2821–2833
Wilson JM, Hartley R, Maxwell DJ, Todd AJ, Lieberam I, Kaltschmidt JA, Yoshida Y, Jessell TM, Brownstone RM (2005) Conditional rhythmicity of ventral spinal interneurons defined by expression of the Hb9 homeodomain protein. J Neurosci 25(24):5710–5719
Windle WF (1931) Neurons of the sensory type in the ventral roots of man and of other mammals. Arch Neurol Psychiatr 26(4):791
Wolpert DM, Miall RC (1996) Forward models for physiological motor control. Neural Netw 9(8):1265–1279
Yamamoto T, Takahashi K, Satomi H, Ise H (1977) Origins of primary afferent fibers in the spinal ventral roots in the cat as demonstrated by the horseradish peroxidase method. Brain Res 126(2):350–354
Zaporozhets E, Cowley KC, Schmidt BJ (2004) A reliable technique for the induction of locomotor-like activity in the in vitro neonatal rat spinal cord using brainstem electrical stimulation. J Neurosci Methods 139(1):33–41
Zhang F, Wang L-P, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K (2007) Multimodal fast optical interrogation of neural circuitry. Nature 446(7136):633–639
Zhang Y, Narayan S, Geiman E, Lanuza GM, Velasquez T, Shanks B, Akay T, Dyck J, Pearson K, Gosgnach S, Fan CM, Goulding M (2008) V3 spinal neurons establish a robust and balanced locomotor rhythm during walking. Neuron 60(1):84–96
Zornik E, Yamaguchi A (2012) Coding rate and duration of vocalizations of the frog, Xenopus laevis. J Neurosci 32(35):12102–12114
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Falgairolle, M., O’Donovan, M.J. (2022). Motoneuronal Regulation of Central Pattern Generator and Network Function. In: O'Donovan, M.J., Falgairolle, M. (eds) Vertebrate Motoneurons. Advances in Neurobiology, vol 28. Springer, Cham. https://doi.org/10.1007/978-3-031-07167-6_11
Download citation
DOI: https://doi.org/10.1007/978-3-031-07167-6_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-07166-9
Online ISBN: 978-3-031-07167-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)