Abstract
Peer victimization (including physical, verbal, and relational forms of aggression) has a worldwide prevalence rate of nearly 13%, and has been implicated in prior literature and theory as a contributor to children’s behavioral and emotional problems, through stress-related pathways and mechanisms. However, little is known about the underlying molecular mechanisms that may account for these effects. In this chapter, we first briefly examine how the neuroendocrine system may be affected by peer victimization. Next, we describe the extent to which epigenetic mechanisms, especially DNA methylation, may be altered by life experiences and could jeopardize later development, a concept referred to as biological embedding. We conclude by outlining key methodological, biological, and statistical limitations constraining the generalization of emerging findings investigating epigenetic mechanisms in peer relationships research.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Epigenetics
- DNA methylation
- Peer victimization
- Early adversity
- Stress
- hypothalamic-pituitary-adrenocortical (HPA) axis
- Socioemotional problems
- Behavioral problems
- Biological embedding hypothesis
- Longitudinal studies
Introduction
Beyond the influence of the family environment, research shows that peer relationships contribute, positively and negatively, to the children’s trajectories of social, emotional, and behavioral development (Rubin et al., 2006). Adverse peer relationships include peer rejection, peer victimization, and affiliation with deviant peers (see chapters “Prologue: Introduction” and “Elementary School Social Experiences with Peers and Teachers: Manifestation and Development”, this volume). In this chapter, we will focus solely on peer victimization, for which the prevalence rate is estimated at 12.6% worldwide (Craig et al., 2009). A child is being victimized when he or she is exposed repeatedly and chronically to hurtful actions perpetrated by a peer or a group of peers between whom there is an imbalance of power (Olweus, 1994). Peer victimization occurs mostly in school settings, where children spend most of their time (Arseneault et al., 2010). Actions include physical aggression (e.g., hitting), verbal aggression (e.g., name-calling), and relational aggression (e.g., social exclusion) (Crick & Grotpeter, 1996). Longitudinal studies indicated that being victimized by peers in childhood may have serious consequences on health and functioning throughout the life course, including mental and physical health problems (Arseneault, 2018). A meta-analysis conducted by Reijntjes et al. (2010), comprising the data of 12,361 children, has indeed documented that children who were the target of peer victimization subsequently exhibited higher levels of loneliness, withdrawal behaviors, depression, and anxiety. Higher levels of physical aggression and attention problems have also been noted in a second meta-analysis totalizing the data of 5825 children (Reijntjes et al., 2011).
Longitudinal studies thus support the idea that peer victimization exerts a detrimental impact on several domains of functioning. Importantly, these studies have broadened our understanding of the consequences that may follow peer victimization while clarifying the temporal sequence of events, whereby peer victimization was associated with increased risk of adjustment difficulties later in life. However, genetic factors and other environmental factors could influence the magnitude of these effects. For example, the children’s genetic background could indirectly affect their sensitivity to stress or vulnerability to exhibit social, emotional, and behavioral problems when victimized by their peers (see chapter “How Peers and Teachers Shape Elementary School Children’s Academic and Socioemotional Development”, this volume). To this end, the discordant monozygotic (MZ) twin design is often regarded as a rigorous research design enabling a stronger control for a wider range of confounders. By contrasting genetically identical children—MZ twins who grew up together in the same families, but who have been exposed to distinct environments—researchers can estimate association between a specific environmental exposure (e.g., peer victimization) on functioning (e.g., aggressive behaviors), over and above the children’s genetic background and shared environmental influences (Vitaro et al., 2009). Using this design, Silberg et al. (2016) reported, in a sample of 145 MZ twin pairs discordant for peer victimization, that victimized twins had higher levels of anxiety, separation anxiety, as well as attention deficit hyperactivity disorder (ADHD) in childhood and higher rates of suicidal ideation in adulthood compared to their non-victimized co-twins. According to the same research design, Brendgen et al. (2013) showed that the higher levels of depressive symptoms and aggressive behaviors displayed by the victims were independent of (i.e., could not be explained by) their genetic risk for depression and aggression, once more supporting the hypothesized impact of peer victimization on emotional and behavioral problems.
Although numerous studies have suggested that peer victimization may detrimentally affect present and future functioning, as well as the well-being of the victims, little is known about the biological processes underlying these associations. Considering that peer victimization, unlike other adverse experiences that may occur during childhood (e.g., maltreatment), is often perceived by the victims as novel, unpredictable, uncontrollable, or threatening one’s physical and social self, researchers have hypothesized that such experiences may affect functioning because they jeopardize stress-related biological systems. Cumulative evidence generally supports this hypothesis. New lines of research are now pushing forward the frontier of knowledge to identify the molecular mechanisms by which peer victimization “gets under the skin and cells.”
In this chapter, we first briefly examine how the neuroendocrine system may be affected by peer victimization. Next, we describe the extent to which epigenetic mechanisms, especially DNA methylation, may be altered by life experiences and could, as such, jeopardize later development, a concept referred to as biological embedding (Hertzman, 2012). Finally, we outline key methodological, biological, and statistical limitations confining the generalization of the emerging findings investigating epigenetic mechanisms in peer relationships research.
Peer Victimization: A Stressful Experience?
The hypothalamic-pituitary-adrenocortical (HPA) axis is one of the main systems underlying the physiological response to stress (see chapter “The Impact of School Social Experiences on Socioemotional and Behavioral Problems: The Hypothesized Role of DNA Methylation ”, this volume). Both physical and psychological stressors, real or perceived, have thus the potential to induce the release of cortisol, a glucocorticoid hormone secreted by the HPA axis. Acute stress leads to a short-term activation of the HPA axis, resulting in temporary elevations of cortisol (Koss & Gunnar, 2018). Conversely, chronic stress leads to a prolonged exposure of the body and the brain to either enhanced or blunted secretion of cortisol later on, which are both potentially damaging to the organism (Koss & Gunnar, 2018).
Because of its repetitive and nature, peer victimization can be viewed as a form of chronic stress. Subjective reports of children victimized by their peers have indeed indicated that these experiences are perceived as highly stressful (Östberg et al., 2018). Accordingly, it has been hypothesized that peer victimization induces stable disruptions in cortisol secretion in basal and stressful contexts, which could eventually jeopardize other neurophysiological systems involved in emotional and behavioral regulation (Vaillancourt, 2018). Support for this hypothesis can be found in the knowledge that glucocorticoid receptors, to which the glucocorticoid stress hormone cortisol preferably binds, are found in several areas of the brain underlying emotional and behavioral regulation, such as the amygdala, the hippocampus, and the prefrontal cortex (de Kloet et al., 2005). A growing body of evidence suggests that children and adolescents who have been victimized by their peers have lower levels of cortisol secretion during the day (Knack et al., 2011; Östberg et al., 2018; Vaillancourt et al., 2008), in response to stress (Calhoun et al., 2014; Knack et al., 2011; Ouellet-Morin et al., 2011a, b), and lower and higher cortisol secretion over an extended period of time, as measured in hair (Ouellet-Morin et al., 2020). Nonetheless, inconsistent findings are reported, pointing either to higher (Chen et al., 2018) or to nonsignificant differences in cortisol responses between victims and non-victims (Hamilton et al., 2008; Katz et al., 2019; Rudolph et al., 2010, 2011). These findings echo other studies conducted with rodents and humans, which also reported dysregulated patterns of stress hormone secretion in basal and stressful contexts following early adversity, such as low maternal care in rodents (e.g., Liu, 1997) and child maltreatment in humans (e.g., Bernard et al., 2017; Bunea et al., 2017). In addition to understand why lower, and sometimes higher, cortisol secretion is noted in individuals with a history of peer victimization, researchers ought to identify possible molecular mechanisms that bring about these differences, as well as the consequences these “biological traces” may have on socioemotional and behavioral functioning and health over time.
Biological Embedding of Stress Through the Epigenome
In molecular biology, the term “epigenome” refers to the set of epigenetic modifications to the DNA, histone proteins, and chromatin structure (Feil & Fraga, 2012). Unlike genetic mutations, epigenetic modifications do not alter the DNA sequence and are thus potentially reversible. Furthermore, epigenetic modifications can be inherited and transmitted during cell divisions (Radford, 2018). The main function of the epigenome is to regulate the expression of genes, the process by which the DNA sequence of a gene is converted into a protein (Provençal & Binder, 2015). DNA methylation may interfere with gene expression. In other words, while genes provide the instructions to synthesize proteins, epigenetic modifications can influence the cell’s ability to read these instructions and to carry them out efficiently.
DNA methylation is currently the most studied epigenetic modification in humans because it is relatively stable over time and easily quantifiable (Jones et al., 2018). The DNA sequence consists of four bases, cytosine (C), guanine (G), adenine (A), and thymine (T). DNA methylation involves the addition of a methyl group (CH3) to a cytosine base paired with a guanine base (i.e., the reference to CpG sites). DNA methylation regulates the expression of genes in two ways. First, DNA methylation interferes with the binding of transcription factors to the DNA sequence, which are proteins that initiate gene expression (Bird, 2002). Second, DNA methylation attracts proteins that restrain even more gene expression (Bird, 2002). Depending on its location, DNA methylation may have different effects on gene expression. Gene expression can be either “turned on,” resulting in increased levels of protein synthesis, or “turned off,” resulting in decreased levels of protein synthesis. In general, when CpG sites are methylated in the promoter region of a gene, which is the region where gene transcription is initiated, the expression of that gene is “turned off” (Bird, 1986). However, when CpG sites are methylated in the body of a gene, which is the region that contains the DNA segment to be transcribed, gene expression could be either “turned on” or “turned off” (Jiang et al., 2013; Jjingo et al., 2012).
Researchers have investigated DNA methylation patterns according to two approaches. In early DNA methylation studies, researchers have adopted a candidate gene approach, involving the preselection of genes that are hypothesized to be associated with the variables of interest (e.g., depressive symptoms) or involved in neurobiological systems (e.g., emotion regulation) or in mechanisms of action of drugs (e.g., selective serotonin reuptake inhibitors) thought to affect these outcomes. Recently, epigenome-wide association studies (EWAS) or methylome-wide association studies (MWAS) have gained popularity as they allow researchers to examine the entire epigenome or methylome, thus providing the opportunity to discover novel epigenetic variations related to certain environments or phenotypes of interest.
Although the use of brain tissues is often judged preferable to assess the impact that DNA methylation may have on behaviors, processes, and characteristics mediated by the brain, such as psychological functioning, DNA methylation cannot be measured directly in the brain of living humans. Researchers are thus forced to use peripheral tissues, such as blood or buccal cells, as surrogates for brain tissues in living individuals. The most common technique to measure DNA methylation levels from biological samples is the sodium bisulfite treatment, allowing to quantify DNA methylation as a percentage of unmethylated versus methylated sites (for an extended description of the laboratory techniques used to measure DNA methylation, see Jones et al., 2018).
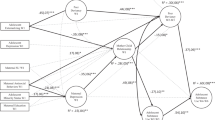
As illustrated in Fig. 1, DNA methylation patterns are primarily influenced by the genome (i.e., the DNA sequence). Nonetheless, DNA methylation patterns are not fixed. Accordingly, dynamic changes in DNA methylation patterns can occur during development in response to environmental signals emanating from inside (e.g., neurotransmitters, hormones) or outside (e.g., diet, pollutants) the organism, especially in utero and during the first years of life (Meaney, 2010). As displayed in Fig. 1, these sensitive periods of development are characterized by enhanced plasticity to environmental signals because the brain is still immature and undergoes rapid development and synaptic pruning, a natural process during which the brain eliminates extra synapses (Boyce & Kobor, 2015). In this manner, early social experiences could more readily influence DNA methylation than experiences occurring later in life. Altogether, these experiences could positively or negatively affect several neurophysiological systems supporting adaptation to the environment, including the HPA axis. DNA methylation thus represents a promising mechanism to better understand how peer victimization may have lasting consequences on socioemotional development, behaviors, and health.
Conceptual model of the interaction between the genome and the environment across development. The genome (blue box) includes all the genetic material (DNA) of an organism, providing all the information essential for functioning. The blue arrows represent the stability of the genome’s influence on DNA methylation patterns throughout development. The environment (green box) comprises various environmental influences, such as peer victimization. The green arrows illustrate the decline of the environment’s influence on DNA methylation patterns across development. Indeed, the epigenome is highly responsive to environmental exposures during sensitive periods of development, such as prenatal and early postnatal periods. The epigenome (red box) includes all the chemical modifications to the DNA and histone proteins of an organism. The red dots represent epigenetic modifications, such as DNA methylation, which may influence gene expression, and thus the activity of several neurophysiological stress-related systems, i.e., biological embedding (orange box). Together, these four factors contribute to the emergence of vulnerability to stress and individual’s propensity to experience socioemotional, behavioral, and health problems over the lifespan (purple box)
Early-Life Stress and DNA Methylation
The influence of social interactions on the epigenome was initially studied in rodents. Notably, it was shown that adult offspring exposed to less maternal care in the first weeks of life showed higher methylation levels at the exon 17 of the NR3C1 gene and a reduced expression of this gene (Weaver et al., 2004). The NR3C1 gene encodes glucocorticoid receptors (GRs), which help to regulate the activity and the effect of the HPA axis by binding to glucocorticoids such as cortisol (Kino & Chrousos, 2002). Based on the rodent maternal care model, McGowan et al. (2009) tested whether humans exposed to child maltreatment exhibited distinct DNA methylation patterns in hippocampal tissues of adults who committed suicide, as compared to adult suicide victims who did not have a history of child maltreatment. As expected, individuals who were maltreated as children showed higher levels of methylation of the exon 1F of the NR3C1 gene, the homolog region of the exon 17 in rodents. In contrast, however, the majority of studies conducted in humans have relied on peripheral tissues, such as blood, saliva, and buccal cells. In a systematic review, a majority of these studies (89%) reported similar findings, that is, higher levels of methylation of the exon 1F within the NR3C1 gene among individuals exposed to early-life adversity (Turecki & Meaney, 2016).
DNA methylation patterns were also investigated in other stress-related genes. For instance, Beach et al. (2010) found that adults who were physically and sexually abused as children had higher methylation levels within the promoter region of the SLC6A4 gene. Notably, the SLC6A4 gene is hypothesized to be involved in impulsivity and aggression behavior and is the target of many antidepressant medications (Coleman & Gouaux, 2018). Importantly, higher levels of methylation of the SLC6A4gene have been associated with reduced expression of the serotonin transporter (Philibert et al., 2007), albeit not consistently so (Duman & Canli, 2015). These findings thus partially support the putative impact that changes in DNA methylation may have on later socioemotional and behavioral difficulties following exposure to early adversity.
Emerging Evidence of Associations Between Peer Victimization and DNA Methylation
Building on the previously described evidence drawn from animal models of maternal care and studies conducted with humans in the context of child maltreatment, peer relationships researchers proposed that DNA methylation may also partly explain how peer victimization increases risk of socioemotional and behavioral problems later in life (Vaillancourt, 2018; Vaillancourt et al., 2013). So far, only a handful of studies have investigated the association between peer victimization and DNA methylation. These studies have adopted either a candidate gene or methylome-wide approach.
In a first study, Ouellet-Morin et al. (2013) studied DNA methylation patterns of the SLC6A4 gene from buccal cells in 28 MZ twin pairs discordant for peer victimization in elementary school. Both groups exhibited similar DNA methylation patterns prior to peer victimization, at 5 years of age. However, compared to their non-victim co-twins, twins who were victimized by their peers in elementary school had, on average, higher levels of DNA methylation at 10 years of age at a particular CpG site within the promoter region of the SLC6A4 gene. The difference was, however, small between the twins (i.e., 4% differences in methylation level). Nonetheless, the difference in DNA methylation noted between these groups was notable because it could not be attributed to children’s genetic makeup or shared family environments due to the discordant monozygotic twin design. Moreover, twins who exhibited higher levels of DNA methylation at this CpG site at 10 years had lower cortisol responses to stress 2 years later, at 12 years of age. This study was the first to provide support to the idea that peer victimization may induce changes in DNA methylation.
Conversely, Mulder et al. (2020) who studied the entire epigenome using peripheral blood collected before and after bullying victimization in two longitudinal population cohorts (totalizing 1352 children) found no evidence for association with methylation levels in the SLC6A4 or NR3C1 genes, which contrasted with the previous study, as well as with other reports of associations between early adversity and higher NR3C1 methylation in rodent and human studies (rodent: Weaver et al., 2004; human: McGowan et al., 2009; Turecki & Meaney, 2016). They, however, found that bullying exposure was associated with a small (between 0.12% and 0.21%) but significant decrease in DNA methylation over time in a CpG site annotated to RAB14 (e.g., important for cellular signaling) while methylation levels were increased at that CpG site in non-bullied children during the same period. This association remained significant after controlling for a wide range of potential confounders, including the exposure to stressful events other than bullying and alcohol use.
In addition, a population-based study including 2232 children did not either find evidence for associations between peer victimization in childhood and adolescence and methylome-wide profiles of DNA extracted from peripheral blood sampled at 18 years of age, once adequate control for confounders was applied (Marzi et al., 2018). Furthermore, additional associations were tested for six candidate genes, including the SLC6A4 gene. Only a few significant associations were detected, including with the SLC6A4 gene. While these mixed findings suggest that peer victimization may not have a pervasive effect on methylation profile, it should be noted that this inconsistency may also arise from the distinct approaches used in these studies. Indeed, the CpG sites examined in Ouellet-Morin et al.’ (2013) relied on a candidate gene approach conducted in childhood (age 10), while Mulder et al. (2020) and Marzi et al. (2018) investigated differential DNA methylation patterns across the epigenome in childhood (6 and 10 years) and early adulthood (age 18), respectively. These differences limit the direct comparison of the findings.
Peer Victimization and DNA Methylation: A Focus on Adjustment Difficulties
While informative, the studies we just described focused only on the association between peer victimization and differences in DNA methylation. We thus don’t know whether differences in DNA methylation explain higher levels of social, emotional, and behavioral problems noted in individuals who have been bullied. Efstathopoulos et al. (2018) first investigated these tripartite associations between peer victimization, methylation levels of the NR3C1 gene, and internalizing symptoms in saliva samples among 1149 adolescents aged between 13 and 14 years old. Peer victimization was associated with higher DNA methylation levels at one of the five CpG sites investigated within the exon 1F of the NR3C1 gene, with small but significant mean difference between the victims and non-victims (i.e., 0.37%). Furthermore, higher levels of DNA methylation at this CpG site, as well as two others, were associated with self-reported symptoms of anxiety and depression. Yet still, because peer victimization, DNA methylation levels, and internalizing symptoms were assessed simultaneously, the directionality of these associations remains unclear and the direct tests of the presumed mediation hypothesis were not conducted.
In addition, Buil et al. (submitted for publication) investigated associations between DNA methylation levels at birth, age 7, and ages 15–17 with chronic peer victimization throughout childhood and various forms of psychopathology at ages 7 and 15 in a population sample of 936 children followed up prospectively from birth to adolescence. The study showed that children who were persistently victimized by their peers throughout the elementary school period had higher levels of DNA methylation in both SLC6A4 and NR3C1 genes in adolescence as compared to non-victimized children. Notably, these differences were not present at birth. Similarly to the previous studies, the magnitude of these effects was small (i.e., ranging from 0.31% to 0.35% of differences between the victims and non-victims). Furthermore, chronic peer victimization was associated with increasing levels of generalized anxiety from childhood to adolescence, which was partially explained by the increasing levels of SLC6A4 methylation at ages 15–17. This indirect effect remained over and above a wide range of environmental risk factors (e.g., prenatal stressors, child maltreatment, and the participants’ substance use).
In sum, three candidate gene studies suggested that peer victimization is associated with changes in methylation of stress-related genes and that such changes may signal differences in stress reactivity or in the presence of emotional difficulties. Importantly, however, these findings have not been replicated in the epigenome-wide association studies and should, as such, be considered with caution.
Methodological, Biological, and Statistical Considerations
In this section, we present several challenges inherent to epigenetic studies and we propose recommendations to improve our understanding of the role of DNA methylation in the onset (or exacerbation) of adjustment difficulties following peer victimization.
Methodological Considerations
Important potential confounds are often overlooked in DNA methylation studies and could partly explain inconsistent findings, including genetic factors. Teh et al. (2014) and Czamara et al. (2019) showed that the majority of DNA methylation variation arises as a result of an interaction between genetic and environmental factors. In other words, the association between peer victimization and DNA methylation may vary according to the children’s genetic background. Future studies should thus consider genetic factors to ascertain with greater accuracy the role of DNA methylation in socioemotional and behavioral functioning following peer victimization. Second, studies should adjust for covariates, such as age, sex, the use of medication, and the use of alcohol, tobacco, or drugs (Jones et al., 2018). This echoes findings reported by Fraga et al. (2005) who showed that older MZ twin pairs exhibited larger differences in DNA methylation patterns in comparison with younger MZ twins, which was argued to arise randomly with time and as a function of exposure to distinct environments as the twins grow apart. Furthermore, Yousefi et al. (2015) found that newborn boys and girls exhibited distinct DNA methylation patterns at 3% of the 450,000 CpG sites analyzed, pointing to sex differences in methylation. A stringent control for a variety of potential confounders is thus warranted to properly estimate the magnitude of the association between peer victimization, DNA methylation, and socioemotional and behavioral problems.
Biological Considerations
Inconsistency in the previous findings may also be related to tissue specificity, because different types of tissues (e.g., whole saliva, blood, buccal epithelial cells) show distinct patterns of methylation. In fact, the type of tissues best predicts differences in DNA methylation patterns between and within individuals (Farré et al., 2015). While the brain is proposed to have effects on social, emotional, and behavioral functioning following peer victimization, DNA methylation cannot be measured in the living human brain for obvious reasons. Researchers must therefore use peripheral tissues, such as blood, saliva, and buccal cells, as surrogates for brain tissues in living individuals. Consequently, it is unclear whether the reported differences (or absence of differences) in DNA methylation patterns in peripheral tissues represent actual differences between the victims and non-victims in the brain (Jones et al., 2018). Smith et al. (2015) reported that DNA methylation patterns are more similar between the DNA extracted from whole saliva and several brain samples (i.e., cerebellum, frontal cortex, entorhinal cortex, and superior temporal gyrus), in comparison with those noted between blood or brain samples. The use of distinct types of tissues may thus, in theory, underline part of the inconsistent findings. Replication attempts should thus target DNA collected from the same tissues or be investigated systematically across several tissues.
Statistical Considerations
Existing studies suggest that peer victimization (but also child maltreatment) is associated with relatively small differences (or changes) in DNA methylation patterns, with differences ranging from less than 1% to 10% between exposed and unexposed participants (Breton et al., 2017). Therefore, researchers must design studies with adequate statistical power to be able to detect such small differences in DNA methylation, if they exist, particularly when studying epigenome-wide associations implying a larger number of tests (i.e., more than 450,000 sites tested; Jones et al., 2018). Furthermore, researchers should ascertain whether the small differences (or changes) noted in DNA methylation are biologically meaningful and yield distinct profiles of gene expression, for instance.
Altogether, these methodological, biological, and statistical considerations represent important challenges currently limiting the investigation of the presumed associations between peer victimization (or, more generally, social adversity), DNA methylation, and later difficulties. Nevertheless, they also represent promising avenues to improve the quality of the research conducted thus far to shed some light on the molecular mechanisms underlying the long-term effects peer victimization have on social, emotional and behavioral, and health problems.
Conclusion
Preliminary evidence suggests that peer victimization may be associated with distinct patterns of DNA methylation in stress-related candidate genes, although inconsistent findings have been reported and many limitations constrain these findings. Changes in DNA methylation patterns following early adverse experiences may bear long-lasting consequences on the stress-related biological systems, as well as brain neuronal development, activity, and connectivity (Heim & Binder, 2012). Moreover, only a few studies have formally tested whether individual differences in DNA methylation explain, at least partially, the onset or increasing levels of social, emotional, and behavioral problems following peer victimization. Future research should also consider a wider range of potential confounders, including differences present at the DNA levels, to capture more precisely the magnitude of the associations. Replication should be prioritized, along with the improvement of the measurement of peer victimization. We should also try to measure gene expression and protein synthesis, in addition to DNA methylation, to refine our understanding of the biological pathways involved in these associations and to determine whether the small differences reported in DNA methylation between victims and non-victims are biologically meaningful.
Notwithstanding these methodological, biological, and statistical challenges, the hypothesis that DNA methylation is a molecular pathway by which peer victimization increases vulnerability to stress and risk for social, emotional, and behavioral difficulties later in life still represents an avenue of research for which only time will tell whether it has hold a piece of the puzzle to better understand how peer victimization may jeopardize later well-being.
References
Arseneault, L. (2018). Annual research review: The persistent and pervasive impact of being bullied in childhood and adolescence: Implications for policy and practice. Journal of Child Psychology and Psychiatry, 59(4), 405–421.
Arseneault, L., Bowes, L., & Shakoor, S. (2010). Bullying victimization in youths and mental health problems: ‘Much ado about nothing’? Psychological Medicine, 40(5), 717–729.
Beach, S. R. H., Brody, G. H., Todorov, A. A., Gunter, T. D., & Philibert, R. A. (2010). Methylation at SLC6A4 is linked to family history of child abuse: An examination of the Iowa adoptee sample. Journal of Medical Genetics, 1(2), 710–713.
Bernard, K., Frost, A., Bennett, C. B., & Lindhiem, O. (2017). Maltreatment and diurnal cortisol regulation: A meta-analysis. Psychoneuroendocrinology, 78, 57–67.
Bird, A. P. (1986). CpG-rich islands and the function of DNA methylation. Nature, 321(6067), 209–213.
Bird, A. (2002). DNA methylation patterns and epigenetic memory. Genes & Development, 16(1), 6–21.
Boyce, W. T., & Kobor, M. S. (2015). Development and the epigenome: The ‘synapse’ of gene-environment interplay. Developmental Science, 18(1), 1–23.
Brendgen, M., Vitaro, F., Barker, E. D., Girard, A., Dionne, G., Tremblay, R. E., & Boivin, M. (2013). Do other people’s plights matter? A genetically informed twin study of the role of social context in the link between peer victimization and children’s aggression and depression symptoms. Developmental Psychology, 49(2), 327–340.
Breton, C. V., Marsit, C. J., Faustman, E., Nadeau, K., Goodrich, J. M., Dolinoy, D. C., et al. (2017). Small-magnitude effect sizes in epigenetic end points are important in children’s environmental health studies: The Children’s environmental health and disease prevention research center’s Epigenetics Working Group. Environmental Health Perspectives, 125(4), 511–526.
Buil, J. M., Cecil, C. M., van Lier, P. A. C., Relton, C. L., & Barker, E. D. (unpublished findings). Relational peer-victimization, DNA methylation and mental health problems: A longitudinal study spanning birth to adolescence.
Bunea, I. M., Szentágotai-Tătar, A., & Miu, A. C. (2017). Early-life adversity and cortisol response to social stress: A meta-analysis. Translational Psychiatry, 7(12), 1274.
Calhoun, C. D., Helms, S. W., Heilbron, N., Rudolph, K. D., Hastings, P. D., & Prinstein, M. J. (2014). Relational victimization, friendship, and adolescents’ hypothalamic–pituitary–adrenal axis responses to an in vivo social stressor. Development and Psychopathology, 26(3), 605–618.
Chen, G., Kong, Y., Deater-Deckard, K., & Zhang, W. (2018). Bullying victimization heightens cortisol response to psychosocial stress in Chinese children. Journal of Abnormal Child Psychology, 46(5), 1051–1059.
Coleman, J. A., & Gouaux, E. (2018). Structural basis for recognition of diverse antidepressants by the human serotonin transporter. Nature Structural & Molecular Biology, 25(2), 170–175.
Craig, W., Harel-Fisch, Y., Fogel-Grinvald, H., Dostaler, S., Hetland, J., Simons-Morton, B., et al. (2009). A cross-national profile of bullying and victimization among adolescents in 40 countries. International Journal of Public Health, 54(Suppl 2), 216–224.
Crick, N. R., & Grotpeter, J. K. (1996). Children’s treatment by peers: Victims of relational and overt aggression. Development and Psychopathology, 8(2), 367–380.
Czamara, D., Eraslan, G., Page, C. M., Lahti, J., Lahti-Pulkkinen, M., Hämäläinen, E., et al. (2019). Integrated analysis of environmental and genetic influences on cord blood DNA methylation in new-borns. Nature Communications, 10(1), 1–18.
de Kloet, E. R., Sibug, R. M., Helmerhorst, F. M., & Schmidt, M. (2005). Stress, genes and the mechanism of programming the brain for later life. Neuroscience and Biobehavioral Reviews, 29, 271–281.
Duman, E. A., & Canli, T. (2015). Influence of life stress, 5-HTTLPR genotype, and SLC6A4 methylation on gene expression and stress response in healthy Caucasian males. Biology of Mood & Anxiety Disorders, 5(1), 2.
Efstathopoulos, P., Andersson, F., Melas, P. A., Yang, L. L., Villaescusa, J. C., Rȕegg, J., et al. (2018). NR3C1 hypermethylation in depressed and bullied adolescents. Translational Psychiatry, 8(1), 121.
Farré, P., Jones, M. J., Meaney, M. J., Emberly, E., Turecki, G., & Kobor, M. S. (2015). Concordant and discordant DNA methylation signatures of aging in human blood and brain. Epigenetics & Chromatin, 8(1), 19.
Feil, R., & Fraga, M. F. (2012). Epigenetics and the environment: Emerging patterns and implications. Nature Reviews Genetics, 13(2), 97–109.
Fraga, M. F., Ballestar, E., Paz, M. F., Ropero, S., Setien, F., Ballestar, M. L., et al. (2005). Epigenetic differences arise during the lifetime of monozygotic twins. Proceedings of the National Academy of Sciences of the United States of America, 102(30), 10604–10609.
Hamilton, L. D., Newman, M. L., Delville, C. L., & Delville, Y. (2008). Physiological stress response of young adults exposed to bullying during adolescence. Physiology & Behavior, 95(5), 617–624.
Heim, C., & Binder, E. B. (2012). Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene–environment interactions, and epigenetics. Experimental Neurology, 233(1), 102–111.
Hertzman, C. (2012). Putting the concept of biological embedding in historical perspective. Proceedings of the National Academy of Sciences, 109(Supplement_2), 17160–17167.
Jiang, Y., Liu, S., Chen, X., Cao, Y., & Tao, Y. (2013). Genome-wide distribution of DNA methylation and DNA demethylation and related chromatin regulators in cancer. Biochimica et Biophysica Acta (BBA) – Reviews on Cancer, 1835(2), 155–163.
Jjingo, D., Conley, A. B., Yi, S. V., Lunyak, V. V., & Jordan, I. K. (2012). On the presence and role of human gene-body DNA methylation. Oncotarget, 3(4), 462–474.
Jones, M. J., Moore, S. R., & Kobor, M. S. (2018). Principles and challenges of applying epigenetic epidemiology to psychology. Annual Review of Psychology, 69(1), 459–485.
Katz, D. A., Peckins, M. K., & Lyon, C. C. (2019). Adolescent stress reactivity: Examining physiological, psychological and peer relationship measures with a group stress protocol in a school setting. Journal of Adolescence, 74, 45–62.
Kino, T., & Chrousos, G. P. (2002). Tissue-specific glucocorticoid resistance-hypersensitivity syndromes: Multifactorial states of clinical importance. Journal of Allergy and Clinical Immunology, 109(4), 609–613.
Knack, J. M., Jensen-Campbell, L. A., & Baum, A. (2011). Worse than sticks and stones? Bullying is associated with altered HPA axis functioning and poorer health. Brain and Cognition, 77(2), 183–190.
Koss, K. J., & Gunnar, M. R. (2018). Annual research review: Early adversity, the hypothalamic–pituitary–adrenocortical axis, and child psychopathology. Journal of Child Psychology and Psychiatry, 59(4), 327–346.
Liu, D. (1997). Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science, 277(5332), 1659–1662.
Marzi, S. J., Sugden, K., Arseneault, L., Belsky, D. W., Burrage, J., Corcoran, D. L., et al. (2018). Analysis of DNA methylation in young people: Limited evidence for an association between victimization stress and epigenetic variation in blood. American Journal of Psychiatry, 175(6), 517–529.
McGowan, P. O., Sasaki, A., D’alessio, A. C., Dymov, S., Labonté, B., Szyf, M., et al. (2009). Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neuroscience, 12(3), 342.
Meaney, M. J. (2010). Epigenetics and the biological definition of gene × environment interactions. Child Development, 81(1), 41–79.
Mulder, R. H., Walton, E., Neumann, A., Houtepen, L. C., Felix, J. F., Bakermans-Kranenburg, M. J., et al. (2020). Epigenomics of being bullied: Changes in DNA methylation following bullying exposure. Epigenetics, 15(6-7), 750–764.
Olweus, D. (1994). Bullying at school. In L. R. Huesmann (Ed.), Aggressive behavior: Current perspectives (pp. 97–130). Springer US.
Östberg, V., Låftman, S. B., Modin, B., & Lindfors, P. (2018). Bullying as a stressor in mid-adolescent girls and boys–associations with perceived stress, recurrent pain, and salivary cortisol. International Journal of Environmental Research and Public Health, 15(2), 364.
Ouellet-Morin, I., Danese, A., Bowes, L., Shakoor, S., Ambler, A., Pariante, C. M., et al. (2011a). A discordant monozygotic twin design shows blunted cortisol reactivity among bullied children. Journal of the American Academy of Child & Adolescent Psychiatry, 50(6), 574–582.e573.
Ouellet-Morin, I., Odgers, C. L., Danese, A., Bowes, L., Shakoor, S., Papadopoulos, A. S., et al. (2011b). Blunted cortisol responses to stress signal social and behavioral problems among maltreated/bullied 12-year-old children. Biological Psychiatry, 70(11), 1016–1023.
Ouellet-Morin, I., Wong, C., Danese, A., Pariante, C., Papadopoulos, A., Mill, J., & Arseneault, L. (2013). Increased serotonin transporter gene (SERT) DNA methylation is associated with bullying victimization and blunted cortisol response to stress in childhood: A longitudinal study of discordant monozygotic twins. Psychological Medicine, 43(9), 1813–1823.
Ouellet-Morin, I., Cantave, C., Paquin, S., Geoffroy, M.-C., Brendgen, M., Vitaro, F., et al. (2020). Associations between developmental trajectories of peer victimization, hair cortisol and depressive symptoms: A longitudinal study. Journal of Child Psychology and Psychiatry, 62(1), 19–27.
Philibert, R., Madan, A., Andersen, A., Cadoret, R., Packer, H., & Sandhu, H. (2007). Serotonin transporter mRNA levels are associated with the methylation of an upstream CpG island. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 144B(1), 101–105.
Provençal, N., & Binder, E. B. (2015). The effects of early life stress on the epigenome: From the womb to adulthood and even before. Experimental Neurology, 268, 10–20.
Radford, E. J. (2018). Exploring the extent and scope of epigenetic inheritance. Nature Reviews Endocrinology, 14(6), 345.
Reijntjes, A., Kamphuis, J. H., Prinzie, P., & Telch, M. J. (2010). Peer victimization and internalizing problems in children: A meta-analysis of longitudinal studies. Child Abuse & Neglect, 34(4), 244–252.
Reijntjes, A., Kamphuis, J. H., Prinzie, P., Boelen, P. A., van der Schoot, M., & Telch, M. J. (2011). Prospective linkages between peer victimization and externalizing problems in children: A meta-analysis. Aggressive Behavior, 37(3), 215–222.
Rubin, K. H., Bukowski, W. M., & Parker, J. G. (2006). Peer interactions, relationships, and groups. In Handbook of child psychology: Social, emotional, and personality development (Vol. Vol. 3, 6th ed., pp. 571–645). Wiley.
Rudolph, K. D., Troop-Gordon, W., & Granger, D. A. (2010). Peer victimization and aggression: Moderation by individual differences in salivary Cortiol and alpha-amylase. Journal of Abnormal Child Psychology, 38(6), 843–856.
Rudolph, K. D., Troop-Gordon, W., & Granger, D. A. (2011). Individual differences in biological stress responses moderate the contribution of early peer victimization to subsequent depressive symptoms. Psychopharmacology, 214(1), 209–219.
Silberg, J. L., Copeland, W., Linker, J., Moore, A. A., Roberson-Nay, R., & York, T. P. (2016). Psychiatric outcomes of bullying victimization: A study of discordant monozygotic twins. Psychological Medicine, 46(9), 1875–1883.
Smith, A. K., Kilaru, V., Klengel, T., Mercer, K. B., Bradley, B., Conneely, K. N., et al. (2015). DNA extracted from saliva for methylation studies of psychiatric traits: Evidence tissue specificity and relatedness to brain. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics, 168B(1), 36–44.
Teh, A. L., Pan, H., Chen, L., Ong, M.-L., Dogra, S., Wong, J., et al. (2014). The effect of genotype and in utero environment on interindividual variation in neonate DNA methylomes. Genome Research, 24(7), 1064–1074.
Turecki, G., & Meaney, M. J. (2016). Effects of the social environment and stress on glucocorticoid receptor gene methylation: A systematic review. Biological Psychiatry, 79(2), 87–96.
Vaillancourt, T. (2018). Introduction to the special issue: The neurobiology of peer victimization. Merrill-Palmer Quarterly, 64(1), 1–11.
Vaillancourt, T., Duku, E., Decatanzaro, D., Macmillan, H., Muir, C., & Schmidt, L. A. (2008). Variation in hypothalamic–pituitary–adrenal axis activity among bullied and non-bullied children. Aggressive Behavior: Official Journal of the International Society for Research on Aggression, 34(3), 294–305.
Vaillancourt, T., Hymel, S., & McDougall, P. (2013). The biological underpinnings of peer victimization: Understanding why and how the effects of bullying can last a lifetime. Theory Into Practice, 52(4), 241–248.
Vitaro, F., Brendgen, M., & Arseneault, L. (2009). The discordant MZ-twin method: One step closer to the holy grail of causality. International Journal of Behavioral Development, 33(4), 376–382.
Weaver, I. C. G., Cervoni, N., Champagne, F. A., D’Alessio, A. C., Sharma, S., Seckl, J. R., et al. (2004). Epigenetic programming by maternal behavior. Nature Neuroscience, 7(8), 847–854.
Yousefi, P., Huen, K., Davé, V., Barcellos, L., Eskenazi, B., & Holland, N. (2015). Sex differences in DNA methylation assessed by 450 K BeadChip in newborns. BMC Genomics, 16(1), 911.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Comtois-Cabana, M., Buil, J.M., Provençal, N., Ouellet-Morin, I. (2022). The Impact of School Social Experiences on Socioemotional and Behavioral Problems: The Hypothesized Role of DNA Methylation. In: van Lier, P.A., Deater-Deckard, K. (eds) Biosocial Interplay During Elementary School. Springer, Cham. https://doi.org/10.1007/978-3-031-07109-6_5
Download citation
DOI: https://doi.org/10.1007/978-3-031-07109-6_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-07108-9
Online ISBN: 978-3-031-07109-6
eBook Packages: Behavioral Science and PsychologyBehavioral Science and Psychology (R0)