Abstract
As the number of studies investigating the phenomenon of mind wandering increases, so does the interest in manipulating this complex form of spontaneous cognition. Here, we aim to discuss how established and novel noninvasive brain stimulation techniques may provide a way to investigate the underlying mechanisms involved in mind wandering. We examine and compare the methodologies and stimulation parameters being used in recent studies, to try to ascertain the differences between those studies reporting an impact of transcranial direct current stimulation (tDCS) on mind wandering, and those that report an absence of an effect. A novel neuromodulatory approach, auditory beat stimulation, is considered as an alternative to conventional external stimulation methods, like tDCS. Furthermore, we briefly touch on the potential role of noninvasive brain stimulation in mind wandering, for application within the learning environment. Finally, we highlight the role of the hippocampus in both spontaneous and non-spontaneous cognition, and how it may serve as an important target for modulation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
As individuals throughout the course of any given day, we will spend almost half our time with our attention being diverted from the tasks that we engage in. This pervasive spontaneous process, commonly known as mind wandering or daydreaming, is notoriously difficult to control, often requiring the individual to recognize that they themselves are distracted by thoughts and feelings that are unrelated to the external environment or present task (for a review see Smallwood & Schooler, 2015). These lapses of attention or awareness often become frustrating when we are required to maintain our focus for a prolonged period of time, for example, within a classroom setting. When our attention becomes decoupled from the external environment, i.e., the learning environment, then integrating information successfully becomes increasingly difficult and poses a hindrance to the learning process itself.
Here, we aim to discuss the role of noninvasive brain stimulation in modulating mind wandering and meta-awareness, i.e., the awareness of thoughts having drifted away. The ability to safely and reversibly influence mind wandering, and therefore states of inattentiveness and distraction, would offer many useful applications – the ability to remain attentive to a learning task being just one of them (Smallwood et al., 2007). A short introduction to the wide variety of brain stimulation techniques is included. We review the few studies that examine the impact of transcranial direct current stimulation on mind wandering and discuss the contradictory outcomes which indicate that further investigation into the application of this type of neuromodulatory stimulation is indeed warranted. Furthermore, we briefly touch on the potential role for noninvasive brain stimulation as a tool for the learning environment and also highlight a novel brain stimulation technique, auditory beat stimulation, that may offer advantages over conventional neuromodulatory methods.
Mind Wandering Is a Spontaneous Cognitive Process
Mind wandering is a term used to describe a wide variety of thought processes, including task-unrelated thoughts (TUTS), daydreaming, unintentional thought, and stimulus-independent thought (Schooler et al., 2011; Seli et al., 2016; Shrimpton et al., 2017). Even though it is often hard to describe, this pervasive and ubiquitous mental phenomenon affects almost every individual on a daily basis, comprising of almost 20–50% of our waking hours (Killingsworth & Gilbert, 2010; Seli et al., 2018). Defined as a “shift of attention away from an ongoing task (the so-called task at hand) to thoughts and feelings un-associated with task performance” (for a review see Smallwood & Schooler, 2015), it can exert both positive and negative effects on mood states (Killingsworth & Gilbert, 2010), and in exacerbated cases lead to goal neglect (McVay & Kane, 2009). The mechanism understood to underlie mind wandering reflects the cyclic activity of two important core processes. The first process is the detachment of attention from external perception (perceptual decoupling). The second process involves the capacity to capture explicit knowledge of the current contents of consciousness, specifically of wandering thoughts (meta-awareness) (Schooler et al., 2011). Meta-awareness of mind wandering increases the ability to re-focus on the task at hand.
Further to gaining an understanding of the complex interplay of processes that underlie mind wandering, the need to identify the neural correlates of mind wandering and meta-awareness grows. Once this has been achieved, finding target brain regions and states for the modulation of mind wandering becomes much less complicated.
Over the last decade, studies investigating mind wandering have identified brain regions typically comprising the default mode network (Andrews-Hanna et al., 2014) and executive control network (Christoff et al., 2009). A meta-analysis of neuroimaging studies examining mind wandering identified a number of regions within the default mode network, including the medial prefrontal cortex, posterior cingulate cortex, medial temporal lobe, and the hippocampus (Fox et al., 2015). The frontoparietal areas comprising the executive control network, including the dorsolateral prefrontal cortex (DLPFC) and inferior parietal lobule (IPL), are also understood to be involved in mind wandering and spontaneous thought (Fox et al., 2015).
Findings from a recent fMRI study investigating the cortical areas associated with the generation of spontaneous thoughts indicate that the hippocampus is the primary region which is activated before spontaneous thoughts arose. The regions of the default mode network and executive control network were only subsequently activated (Ellamil et al., 2016). This study is one of a few that suggest an emerging role of the hippocampus in mind wandering (Andrews-Hanna et al., 2010). In a review addressing the dynamics of mind wandering, Christoff et al. (2016) suggest that the hippocampus may act as a kind of hub, whereby hippocampal-neocortical and neocortical-neocortical connections are reactivated prior to and during the generation of spontaneous thoughts (Christoff et al., 2016). New evidence from a study examining mind wandering in patients with bilateral hippocampal damage also indicates a role of the hippocampus in mind wandering, but rather for the contents of mind wandering and not for the propensity to mind wander (McCormick et al., 2018).
So far, the cortical regions involved in mind wandering have been identified using data from neuroimaging studies. Applying noninvasive brain stimulation may allow us to gain a deeper understanding of the causal role of these cortical regions in spontaneous cognitive processes.
The Role of Mind Wandering in Educational Contexts
While everyday occurrences of mind wandering may be simply distracting, but may not cause major inconveniences, attentional lapses in an educational environment can result in the failure to retain new information necessary for successful learning. Smallwood and Schooler (2006) state that “mind wandering represents a breakdown in the normal coupling between the internal and external environments” (Smallwood & Schooler, 2006). In that, when we are prone to mind wander, our focus of attention and awareness shifts away from the task at hand and does not encode elements of our external environment in a meaningful way. This underlines the need to prevent the occurrence of frequent episodes of mind wandering while learning, whether in a classroom setting or through online means.
In fact, the detrimental impact of mind wandering and related attentional failures on learning and education has been of concern for many years (Brown, 1927; Johnstone & Percival, 1976; Lloyd, 1968). Several approaches have been adopted to estimate the level of mind wandering that students engage in, and that ultimately exerts a significant impact on the retention of information. An early study investigating outward signs of mind wandering (e.g., gaze diversion, shifting of body position), reported that these physical signs of breaks in attention occur quite soon into a study period (10–18 minutes, after start), which increase in frequency toward the end of a lecture (every 3–4 minutes) (Johnstone & Percival, 1976). Other physical signals may also relate to mind wandering. A 2010 study examining the association between blinking and mind wandering during a reading task revealed that blinking often preceded moments of inattention (Smilek et al., 2010). This pattern of increasing frequency in attentional diversion, either intentional or unintentional, has been also observed in other studies using different approaches. For example, recent studies using experience sampling probes to directly access mind wandering while learning reveal that the most common attentional failures occurred while attending classes or lectures compared to carrying out everyday tasks (e.g., cooking or driving) or even while holding a conversation (Kane et al., 2007; Unsworth et al., 2012). A study by Unsworth et al. (2012) estimated that up to 76% of self-reported lapses in concentration and attention occurred either in the classroom or while studying in a classroom environment (Unsworth et al., 2012). A similar study by McVay et al. (2009) looked at episodes of mind wandering in the everyday lives of college students. Although students reported that they engaged in mind wandering on approximately only 30% of the experience sampling probes throughout the period in which they were measured, the frequency of mind wandering increased when students at the same time reported being tired or anxious or when the task that they were undertaking was stressful or boring (McVay et al., 2009).
Another avenue of research is to investigate techniques that mitigate the impact of mind wandering on students’ attentiveness. Although educational guidelines encourage the use of tasks such as short quizzes, group work, or live demonstrations to re-focus the attention of students (Middendorf & Kalish, 1996), very little research has been performed to help establish the efficacy of these methods. For example, Bunce et al. (2010) investigated the impact of these kinds of pedagogical practices on attention during chemistry lectures. The authors reported that after students had participated in the quizzes and observed the live demonstrations, bouts of mind wandering and lapses in attention decreased, and students were better able to retain information about the content of the lecture (Bunce et al., 2010).
Noninvasive Brain Stimulation Methods
Transcranial Noninvasive Brain Stimulation Methods
Over the last two decades, many forms of noninvasive brain stimulation (NIBS) have been developed. The most common can be divided into two main groups, either magnetic or electrical. The most frequently applied for both research and therapeutic purposes are transcranial magnetic stimulation (TMS), transcranial direct current stimulation (tDCS), and transcranial alternating current stimulation (tACS) (for a review, see Huang et al., 2017). The ease of application and reversible after-effects make these techniques an accessible and safe means of altering cortical excitability. These tools have different modes of action; TMS consists of high-intensity magnetic pulses created by passing current through a magnetic coil (Hallett, 2007). The magnetic pulses cause electric fields exciting or inhibiting a small volume of cortex under the stimulation coil. Such a technique is useful for cortical mapping and focal stimulation and has been used extensively as an adjunct treatment for depression (Chung et al., 2015) and some psychiatric disorders (Tremblay et al., 2019). Pulse train, frequency, and intensity determine the efficacy of TMS applications.
TDCS and tACS, however, are applied by placing two or more electrodes on the surface of the scalp, allowing current to flow between them and stimulating the brain underneath (Lefaucheur et al., 2017). TDCS is dependent upon directional current flow and intensity (Nitsche & Paulus, 2001). Early animal studies have demonstrated that tDCS induces cortical excitability changes via the modulation of neuronal resting membrane potentials (Bindman et al., 1962). Generally it is stated that current flow in an anodal direction causes depolarization, whereas cathodal stimulation induces a hyperpolarization of the resting membrane. TDCS itself cannot elicit action potentials; its application causes the spontaneous firing rate of neurons underneath the stimulating electrode to either increase or decrease depending on the direct of current flow (Bindman et al., 1962; Purpura & Mcmurtry, 1965). TACS is understood to induce alterations in cortical excitability via entrainment of ongoing cortical oscillations. This is due to the sinusoidal nature of the stimulation, as well as the ability to apply a wide range of stimulation frequencies (Antal & Herrmann, 2016). Each of these methods has been shown to induce plasticity-like after-effects that outlast the duration of stimulation (Antal et al., 2008; Nitsche & Paulus, 2001; Rossi et al., 2009).
Of the transcranial electrical techniques, tDCS is the most often used in studies seeking to modulate motor behaviors or cognitive processes. Due to its bipolar properties and long-lasting after-effects, it can be used to induce either excitation or inhibition in targeted cortical regions. It is important to note, however, that neural structures surrounding the targeted area may also be inadvertently affected by exposure to the stimulation (Filmer et al., 2014; Keeser et al., 2011). Therefore, care must be taken to apply tDCS with the most appropriate montage and optimal stimulation parameters, relative to the anticipated outcome. Current distribution modeling studies are increasingly useful for this purpose, as they give an accurate indication of current distribution in tissues and peak electric field under the stimulating electrodes (Opitz et al., 2015). Such approaches enable researchers to more precisely identify the neural impact of the stimulation accompanying the behavioral changes, for instance, modulations of the propensity to mind wander, or even the contents of mind wandering.
Auditory Beat Stimulation
Auditory beat stimulation (ABS) is emerging as a promising new method to safely and reversibly modulate cognitive processes. Recent studies have reported the effects of ABS on mood, anxiety, cognition, and pain perception (Chaieb et al., 2017; Ecsy et al., 2017; Garcia-Argibay et al., 2018). Auditory beat stimulation studies have focused on the application of two main types of auditory beats: binaural and monaural. These beats differ in application and how they exert their effects. Broadly speaking, monaural and binaural beats are generated when sine waves of nearby frequencies are presented to either one or both ears simultaneously (monaural) or to each ear separately (binaural). Monaural beats are physical, acoustic beats which are heard when two sine waves at neighboring frequencies are superposed and presented to one or both ears, resulting in an amplitude modulated signal. The beat itself corresponds to the difference between the two frequencies; for example, two nearby frequencies of 200 and 220 Hz would produce an acoustic beat of 20 Hz. The binaural percept, however, is created when sine waves of neighboring frequencies are presented to each ear separately. This beat, as opposed to those objectively heard during monaural beat stimulation, is subjective and feels like it is located “inside” the head. The beat itself, as in the case of monaural beats, corresponds to the frequency difference between the individual sine waves presented. The binaural beat percept was first described by Wilhelm Dove and can only be detected with carrier frequencies below 1000 (Licklider et al., 1950; Oster, 1973; Dove, 1839).
Of importance to note is how monaural and binaural beats are processed differently in the brain. Monaural beats are detected by the ears and then relayed via the auditory pathway, interacting at the level of the cochlear, where sound information is further relayed to the brainstem and inferior colliculus and processed in the auditory cortex. Binaural beats, however, are perceived when brainstem neurons in the superior olivary nuclei, phase-sensitive to intra-aural shifts, fire action potentials at a rate corresponding to the phase difference between both ears. This interaction produces the binaural beat percept (Kuwada et al., 1979). As a result, monaural and binaural beats are often termed “peripheral” and “central,” respectively (Draganova et al., 2008). Although ABS is a relatively novel neuromodulatory tool, recent studies have demonstrated its ability to induce electrophysiological effects in medial temporal lobe regions associated with memory processes (Becher et al., 2015; Derner et al., 2018). Based on intracranial EEG (iEEG) data acquired from presurgical epilepsy patients, Becher et al. (2015) reported changes in iEEG power and phase synchronization after monaural and binaural beat stimulation, in medial temporal lobe structures, including the hippocampus.
Studies examining the impact of ABS on cognition, mood, and pain have often yielded contrasting results, in particular concerning the effects of binaural beats. Monaural beats, on the other hand, have been somewhat overlooked with regard to cognition, mood effects, and other targets of stimulation. Such studies often report weak effects that do not persist much longer than the stimulation duration itself and do not implement measurement techniques like EEG in order to quantify electrophysiological effects (for a review see Chaieb et al., 2015).
Modulation of Mind Wandering by TDCS and ABS
We know we are mind wandering when our attention becomes decoupled from an ongoing task and instead becomes associated with thoughts and feelings unrelated to the current task at hand (for a review see Smallwood & Schooler, 2015). While this can be mentally refreshing, and can sometimes promote creative thinking (Baird et al., 2012; Leszczynski et al., 2017), persistent mind wandering can often lead to a decline in mood states (Killingsworth & Gilbert, 2010) and in extreme cases rumination (Stawarczyk et al., 2013). This negative aspect of mind wandering lends itself as a target for NIBS and ABS. Up to now, tDCS and ABS have been used to investigate their potential to modulate mind wandering.
Studies Using tDCS to Alter Mind Wandering
Even though much research has been dedicated to mind wandering, to date, very few studies have examined the effects of noninvasive brain stimulation on this cognitive process, and even fewer have looked at its effects on meta-awareness. Altogether only nine studies, all utilizing transcranial direct current stimulation, have investigated the impact of NIBS on mind wandering (see also Chaieb et al., 2019). As we will see, these studies report inconsistent or absent effects of tDCS on mind wandering. This may be, in part, due to a number of methodological differences, which will be discussed in more detail further on in this section.
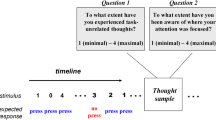
Axelrod and colleagues conducted the first study investigating the effects of tDCS on mind wandering (Axelrod et al., 2015). They applied anodal tDCS at 1 mA over the left dorsolateral prefrontal cortex (DLPFC), with the return electrode (the cathode) over the right supraorbital ridge, for 20 minutes. To control for unspecific tDCS effects, they applied sham stimulation conditions comprising of anodal tDCS over occipital lobe, and also using the DLPFC montage, with stimulation lasting only 2 minutes. During stimulation, participants were asked to perform a variant of the sustained attention to response task (SART), widely used as a measure of mind wandering (Christoff et al., 2009; Robertson et al., 1997). Episodes of mind wandering were assessed using experience sampling probes, which were intermittently and randomly presented during the SART task. In this study, Axelrod and colleagues reported an increased propensity to mind wander during anodal tDCS over the DLPFC, compared to the control conditions. However, tDCS had no impact on the performance of the task (Axelrod et al., 2015). In a subsequent study, Axelrod et al. (2018) aimed to replicate their earlier findings in addition to assessing the effect of tDCS on meta-awareness of mind wandering. Here, in addition to the mind wandering probe, they also asked the participants to assess their level of meta-awareness during the task (“To what extent have you been aware of where your attention was focused?”). The authors reported findings that were in line with their previous study: that anodal tDCS over the DLPFC increased the propensity to mind wander, compared to the sham stimulation conditions. Again, anodal tDCS did not impact upon task performance. They also noted that meta-awareness was unaffected by the stimulation and that similar to an earlier study by Christoff et al. (2009), high levels of meta-awareness were associated with a decline in mind wandering (Axelrod et al., 2018; Christoff et al., 2009). Taken together, these studies suggest a role for tDCS in the modulation of mind wandering. In another attempt to replicate the findings reported by Axelrod et al. (2015, 2018), by an independent group, Boayue et al. (2019) published the results of a preregistered, multicenter study. Here, the authors utilized the same stimulation parameters and experimental procedure, within a much larger cohort of 192 participants. In this study, no effect of anodal stimulation of the DLPFC was found, either on mind wandering or task performance. The authors reported, instead, evidence of absence of any stimulation-related effects, based on analyses derived from Bayesian statistics (Boayue et al., 2019). The initial study by Axelrod and colleagues was the first of three to apply tDCS over the DLPFC. The six remaining studies applied tDCS in similar montages, but over heterogeneous regions associated with the default mode and executive control networks.
In the first of two studies applying tDCS over the left prefrontal cortex (LPFC: site of active anodal stimulation) and right inferior parietal lobule (rIPL: site of reference electrode), Kajimura and Nomura (2015) reported that the propensity of participants to mind wander, compared to sham stimulation, significantly increased. In the reverse montage, however (cathode over LPFC and anode over rIPL), the authors reported the opposite effect, in that the propensity to mind wander declined (Kajimura & Nomura, 2015). The authors also observed an effect of tDCS on a flanker task that participants were asked to perform post-stimulation and during which the mind wandering probes were collected; the load dependence of target detection accuracy was reversed for the stimulation conditions, compared to the sham condition. In a further study, using the same stimulation conditions (tDCS at 1.5 mA for 20 minutes) and montages, Kajimura et al. (2016) investigated this increase/decrease in propensity to mind wander using fMRI. Analyses of data derived from this experiment indicated that anodal stimulation of the rIPL resulted in diminished afferent functional connections of the posterior cingulate cortex (PCC) from the rIPL and medial prefrontal cortex (mPFC). Further examination of the data using mediation analysis showed that connections from the rIPL to the PCC suppressed mind wandering, while those originating in the mPFC to the PCC facilitated it (Kajimura et al., 2016). In another fMRI study, and using a different stimulation montage, Kajimura et al. (2019) aimed to explore the impact of functional asymmetry between the IPLs, on mind wandering. They did this by applying anodal tDCS to the right and left IPL (using the contralateral cheek as the return electrode) alternately. The experience sampling probes in this study were similar to those implemented by Christoff et al. (2009), in that levels of meta-awareness were also assessed. The authors reported a decrease in the propensity to mind wander for stimulation over the rIPL versus sham condition, but not for the lIPL. However, stimulation of the lIPL resulted in a decrease in reaction times during the execution of the SART task. Analysis of the blood-oxygen level-dependent signals during resting state revealed that only stimulation of the rIPL modulated default mode network connectivity, compared to sham stimulation. No effects of the tDCS stimulation were reported on meta-awareness (Kajimura et al., 2019).
In a recent study, Coulborn et al. (2020) aimed to investigate whether stimulating the default mode network using tDCS could alter the propensity to mind wander in a double-blind, counterbalanced study. The authors applied anodal, cathodal, and sham tDCS (1.5 mA, 20 minutes) to the right IPL of 23 healthy participants prior to and after completing a SART with intermittent experience sampling probes. By targeting the rIPL (the return electrode was placed over the left cheek), the authors aimed to elucidate whether the default mode network was primarily responsible for the modulatory effects on the propensity to mind wander reported in previous studies that targeted both the default mode and executive control networks (Coulborn et al., 2020; Kajimura et al., 2016). Similar to Boayue et al. (2019), the authors found no evidence that tDCS over the rIPL was able to modulate the propensity to mind wander. In fact, the two groups found evidence to the contrary, in that using Bayesian (Boayue et al., 2019) and Frequentists (Boayue et al., 2019; Coulborn et al., 2020) analyses they found strong indications supporting the lack of an effect of stimulation in both behavioral and subjective measures of mind wandering.
Using another approach with an alternative brain region and stimulation parameters, Bertossi et al. (2017) examined the role of the medial prefrontal cortex (mPFC) in mind wandering. The authors applied cathodal tDCS over the mPFC for 15 mins and at an intensity of 2 mA. The return electrode was placed over the right deltoid. They asked participants to perform a variant of the choice reaction time task, where subjects were presented with a number stream consisting of digits shown in two colors, one often and the other infrequently. Subjects were required to report whether the infrequently presented color was an even or odd digit. The task was interspersed with experience sampling probes. In this study, the CRT was performed both prior to and post-stimulation. The authors reported stimulation-induced alterations in the propensity to mind wander (post-stimulation vs. pre-stimulation) that occurred in different directions for cathodal mPFC stimulation, when compared to the control conditions. This effect, however, was only observed in male participants and not in the female cohort. In addition, male participants also showed changes in the self-relatedness of mind wandering for all stimulation conditions (occipital control and active mPFC) (Bertossi et al., 2017).
In a final, preregistered study, Filmer et al. (2019) investigated the effect of stimulation polarity and intensity on mind wandering, by applying anodal and cathodal stimulation to the left prefrontal cortex. In a large sample of 150 participants, the authors applied tDCS in both anodal and cathodal polarities to the lPFC (position F3, reference electrode placed over the right contralateral orbit), and cathodal stimulation is applied at 1, 1.5, and 2 mA. Anodal stimulation was applied only at 1 mA. Participants performed the SART with periodically presented thought probes (Filmer et al., 2019). Filmer et al. (2019) found that cathodal tDCS modulated mind wandering, in that the propensity to mind wander increased, in contrast to findings previously reported by Kajimura and colleagues (2016) and Kajimura and Nomura (2015). The authors also reported that the effect of cathodal tDCS was dose dependent, showing a linear trend with strongest effects being apparent at higher stimulation intensities (in this case, 2 mA). The increase in the propensity to mind wander was quite significant; participants in the tDCS group showed a 31% higher number of task-unrelated thoughts compared to those receiving sham stimulation. Similar to earlier studies, the authors reported no effect of stimulation on task performance (Axelrod et al., 2015, 2018; Filmer et al., 2019). A slightly puzzling aspect of the findings reported by Filmer et al. (2019), however, is that anodal compared to sham stimulation showed changes in mind wandering propensity in the same direction as cathodal stimulation, albeit not statistically significant (p = 0.111).
Taken together, as these studies demonstrate, it is increasingly difficult to ascertain which approaches are most suitable when looking to induce long-lasting modulations with regard to mind wandering, using tDCS. The variation in heterogeneous montages and brain regions targeted with stimulation, the widely varying sample sizes, and, most importantly, the inconsistent and partly contradictory results may call into question the efficacy of this kind of brain stimulation in influencing spontaneous cognitive processes.
Mind Wandering as a Target of ABS
Binaural and monaural beat stimulation have been shown to alter brain activity in regions involved in mind wandering, for instance, mediotemporal regions (Becher et al., 2015; Derner et al., 2018). Until now, only one study has investigated the effects of these kinds of auditory beat stimulation on mind wandering. In this study, 40 healthy participants (20 male and 20 female) were asked to perform a variant of the SART, with experience sampling probes embedded intermittently throughout the task. Simultaneously, they listened to binaural and monaural beats at 5 Hz and 40 Hz for a duration of approximately 30 minutes. Sixty experience sampling probes per experimental run were used to subjectively assess the participants’ level of mind wandering (whether the participant was “on” or “off” the task) and meta-awareness (“were you aware that your attention was off-task?”). An overall analysis of the entire cohort, across stimulation conditions, revealed no significant modulation of mind wandering by auditory stimulation. However, a median split of the data into two subgroups – those with a high propensity to mind wander versus those with a low propensity to mind wander during silence – showed an effect of stimulation on levels of mind wandering in the high mind wandering subgroup. The authors reported that 5 Hz monaural beat stimulation reduced the propensity to mind wander in participants who have a greater tendency to do so during silence (Chaieb et al., 2020). The participants’ levels of meta-awareness remained unchanged by exposure to the beat stimulation at these frequencies, and the propensity to mind wander was negatively correlated with levels of meta-awareness. Christoff et al. (2009), in an earlier study, showed that meta-awareness during mind wandering reflects decreased activity in default mode network and executive control regions, suggesting a reductive effect of meta-awareness on mind wandering itself. This study highlights a pertinent and important aspect of manipulating mind wandering processes: that brain stimulation techniques may be most efficacious in individuals who exhibit a greater tendency toward mind wandering.
Discussion
The studies investigating the impact of tDCS on mind wandering discussed in this review are increasingly difficult to compare and contrast due to the differences in their methodological approaches, sample sizes, experimental design, and choice of task including experience sampling probes. Those studies seeking to replicate previous findings often report no effect at all of tDCS on mind wandering, even finding evidence suggesting the lack of an effect (Boayue et al., 2019; Coulborn et al., 2020). For instance, the main difference between the initial study reporting an increase in the propensity to mind wander after anodal tDCS of the DLPFC (Axelrod et al., 2015) and the replication study conducted by Boayue et al. (2019) was the larger sample size in the latter study. The authors found no effect of stimulation either online (during stimulation) or offline (post-stimulation) on mind wandering or task performance. In fact, using Bayesian statistics they calculated that a null effect was ten times more probable than an increase in the propensity to mind wander resulting from anodal tDCS over the DLPFC (Boayue et al., 2019).
As another example, a recent study (Coulborn et al., 2020) sought to re-examine the findings reported by Kajimura et al. (2019). Although this was not an exact replication of the earlier study, Coulborn et al. (2020) investigated the effect of tDCS of the right IPL. Kajimura et al. (2019) previously found that anodal tDCS over the right IPL (with the return electrode placed over the contralateral cheek) decreased the frequency of mind wandering compared to a sham condition. The later study by Coulborn and colleagues sought to address the limitations of the earlier study by also implementing a cathodal tDCS condition and measuring levels of mind wandering not only after but also before stimulation. In contrast to Kajimura et al. (2019) and Coulborn et al. (2020) reported no effect of either anodal or cathodal tDCS of the right IPL on mind wandering or task performance. One possible explanation for this discrepancy is the difference in experience sampling probes used in the latter study: that they were not binary responses (“on task” or “off task”) but were graded (Likert scale from 1 to 4: 1 = maximum, 4 = minimum) and may influence the interpretation of the experience of the mind wandering itself. Additionally, the earlier study was conducted in an fMRI scanner, whereas the study by Coulborn et al. (2020) was not. Also important to note is that the findings from the initial study were based on a sample size of 13, whereas the latter study included data measured from 23 participants (Coulborn et al., 2020; Kajimura et al., 2019).
In general, the disparity between stimulation montages and the impact of tDCS on the sites of cortical stimulation may also make the interpretation of the outcomes of these studies much harder to disentangle. For example, the stimulation montage used by Kajimura et al. (2016) and Kajimura and Nomura (2015) in two prior studies targeted the LPFC and IPL concurrently. It therefore, remains inconclusive whether the reported decline in propensity to mind wander can be attributed to the effect of cathodal tDCS over the LPFC or anodal stimulation over IPL, or a complex interplay between the two structures, which are part of the default mode network and executive control network, respectively.
Another important point of note is the timing of the cognitive or motor task relative to the application of stimulation. Studies from the motor cortex indicate that performance of a motor task is enhanced when paired with tDCS (an online effect), rather than when executed after the stimulation (Lefebvre et al., 2012; Marquez et al., 2015; Nitsche et al., 2003). Although the study designs by Axelrod et al. (2015, 2018) and Boayue et al. (2019) both included an online stimulation sequence, their outcomes were contradictory.
As we see, targeting mind wandering with tDCS is not a trivial challenge, and even small alterations in stimulation parameters, montages, and study designs can yield contrasting results. Regarding electric and magnetic stimulation, so far, only tDCS has been utilized in this function; a recent study employing a novel neuromodulatory technique, auditory beat stimulation, reported an effect of monaural beats at 5 Hz on the propensity to mind wander in individuals who exhibited a greater tendency to do so (Chaieb et al., 2020). This interesting finding may help unravel some of the effects observed from previous studies and may enable researchers to make more informed choices with regard to study design. For example, a mind wandering questionnaire may be implemented initially (e.g., Mrazek et al., 2013), in order to screen for “high” mind wandering candidates, i.e., individuals who show a greater tendency to mind wander.
In addition, since the likelihood of mind wandering within a classroom setting or while attending a lecture is not negligible, tools like tDCS or ABS, which are able to modulate this process safely and reversibly, would be desirable. The high cost of attentional failures during learning can directly impact upon how and whether we are able to retain important information. This, for instance, may translate to our educational performance and ultimately to our job prospects and life accomplishments. Of course, the benefits of brain stimulation techniques allowing modulation of mind wandering have to be carefully weighed against ethical concerns, and possible side effects, and potential overuse risks have to be precisely assessed. Combining useful and effective pedagogical practices like implementing quizzes, group work, or live demonstrations may also aid in mitigating the negative effects of mind wandering, but more evidence is needed to understand why these methods alleviate the urge for distraction (for a review see Szpunar et al., 2013).
In summary, it is still too early to say whether auditory beat stimulation is a superior method to tDCS to modulate mind wandering. However, there are some aspects which do speak in favor of auditory beat stimulation: the easy applicability, the wide availability, the reduced risk of side effects, as well as the appeal to younger participants. Future studies have to show whether the effects of auditory beat stimulation on mind wandering are replicable and robust or not.
References
Andrews-Hanna, J. R., Reidler, J. S., Huang, C., & Buckner, R. L. (2010). Evidence for the default network’s role in spontaneous cognition. Journal of Neurophysiology, 104(1), 322–335. https://doi.org/10.1152/jn.00830.2009
Andrews-Hanna, J. R., Smallwood, J., & Spreng, R. N. (2014). The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences, 1316, 29–52. https://doi.org/10.1111/nyas.12360
Antal, A., & Herrmann, C. S. (2016). Transcranial alternating current and random noise stimulation: Possible mechanisms. Neural Plasticity, 2016, 3616807. https://doi.org/10.1155/2016/3616807
Antal, A., Boros, K., Poreisz, C., Chaieb, L., Terney, D., & Paulus, W. (2008). Comparatively weak after-effects of transcranial alternating current stimulation (tACS) on cortical excitability in humans. Brain Stimulation, 1(2), 97–105. https://doi.org/10.1016/j.brs.2007.10.001
Axelrod, V., Rees, G., Lavidor, M., & Bar, M. (2015). Increasing propensity to mind-wander with transcranial direct current stimulation. Proceedings of the National Academy of Sciences of the United States of America, 112(11), 3314–3319. https://doi.org/10.1073/pnas.1421435112
Axelrod, V., Zhu, X., & Qiu, J. (2018). Transcranial stimulation of the frontal lobes increases propensity of mind-wandering without changing meta-awareness. Scientific Reports, 8(1), 15975. https://doi.org/10.1038/s41598-018-34098-z
Baird, B., Smallwood, J., Mrazek, M. D., Kam, J. W. Y., Franklin, M. S., & Schooler, J. W. (2012). Inspired by distraction: Mind wandering facilitates creative incubation. Psychological Science, 23(10), 1117–1122. https://doi.org/10.1177/0956797612446024
Becher, A.-K., Höhne, M., Axmacher, N., Chaieb, L., Elger, C. E., & Fell, J. (2015). Intracranial electroencephalography power and phase synchronization changes during monaural and binaural beat stimulation. The European Journal of Neuroscience, 41(2), 254–263. https://doi.org/10.1111/ejn.12760
Bertossi, E., Peccenini, L., Solmi, A., Avenanti, A., & Ciaramelli, E. (2017). Transcranial direct current stimulation of the medial prefrontal cortex dampens mind-wandering in men. Scientific Reports, 7(1), 16962. https://doi.org/10.1038/s41598-017-17267-4
Bindman, L. J., Lippold, O. C., & Redfearn, J. W. (1962). Long-lasting changes in the level of the electrical activity of the cerebral cortex produced by polarizing currents. Nature, 196, 584–585.
Boayue, N. M., Csifcsák, G., Aslaksen, P., Turi, Z., Antal, A., Groot, J., Hawkins, G. E., Forstmann, B., Opitz, A., Thielscher, A., & Mittner, M. (2019). Increasing propensity to mind-wander by transcranial direct current stimulation? A registered report. The European Journal of Neuroscience. https://doi.org/10.1111/ejn.14347
Brown, G. L. (1927). Daydreams: A cause of mind wandering and inferior scholarship. The Journal of Educational Research, 15(4), 276–279. https://doi.org/10.1080/00220671.1927.10879744
Bunce, D. M., Flens, E. A., & Neiles, K. Y. (2010). How long can students pay attention in class? A study of student attention decline using clickers. Journal of Chemical Education, 87(12), 1438–1443. https://doi.org/10.1021/ed100409p
Chaieb, L., Wilpert, E. C., Reber, T. P., & Fell, J. (2015). Auditory beat stimulation and its effects on cognition and mood states. Frontiers in Psychiatry, 6, 70. https://doi.org/10.3389/fpsyt.2015.00070
Chaieb, L., Wilpert, E. C., Hoppe, C., Axmacher, N., & Fell, J. (2017). The impact of monaural beat stimulation on anxiety and cognition. Frontiers in Human Neuroscience, 11, 251. https://doi.org/10.3389/fnhum.2017.00251
Chaieb, L., Antal, A., Derner, M., Leszczyński, M., & Fell, J. (2019). New perspectives for the modulation of mind-wandering using transcranial electric brain stimulation. Neuroscience, 409, 69–80. https://doi.org/10.1016/j.neuroscience.2019.04.032
Chaieb, L., Derner, M., Leszczyński, M., & Fell, J. (2020). Modulation of mind wandering using auditory beat stimulation: A pilot study. Journal of Cognitive Enhancement, 4(1), 40–48. https://doi.org/10.1007/s41465-019-00137-4
Christoff, K., Gordon, A. M., Smallwood, J., Smith, R., & Schooler, J. W. (2009). Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences of the United States of America, 106(21), 8719–8724. https://doi.org/10.1073/pnas.0900234106
Christoff, K., Irving, Z. C., Fox, K. C. R., Spreng, R. N., & Andrews-Hanna, J. R. (2016). Mind-wandering as spontaneous thought: A dynamic framework. Nature Reviews Neuroscience, 17(11), 718–731. https://doi.org/10.1038/nrn.2016.113
Chung, S. W., Hoy, K. E., & Fitzgerald, P. B. (2015). Theta-burst stimulation: A new form of TMS treatment for depression? Depression and Anxiety, 32(3), 182–192. https://doi.org/10.1002/da.22335
Coulborn, S., Bowman, H., Miall, R. C., & Fernández-Espejo, D. (2020). Effect of tDCS over the right inferior parietal lobule on mind-wandering propensity. Frontiers in Human Neuroscience, 14, 230. https://doi.org/10.3389/fnhum.2020.00230
Derner, M., Chaieb, L., Surges, R., Staresina, B. P., & Fell, J. (2018). Modulation of item and source memory by auditory beat stimulation: A pilot study with intracranial EEG. Frontiers in Human Neuroscience, 12, 500. https://doi.org/10.3389/fnhum.2018.00500
Dove, H. W., u. a. (1839). Akustik, Theoretische Optik, Meteorologie. In: Repertorium der Physik. Band 3, S. 404.
Draganova, R., Ross, B., Wollbrink, A., & Pantev, C. (2008). Cortical steady-state responses to central and peripheral auditory beats. Cerebral Cortex (New York, N.Y.: 1991), 18(5), 1193–1200. https://doi.org/10.1093/cercor/bhm153
Ecsy, K., Jones, A. K. P., & Brown, C. A. (2017). Alpha-range visual and auditory stimulation reduces the perception of pain. European Journal of Pain (London, England), 21(3), 562–572. https://doi.org/10.1002/ejp.960
Ellamil, M., Fox, K. C. R., Dixon, M. L., Pritchard, S., Todd, R. M., Thompson, E., & Christoff, K. (2016). Dynamics of neural recruitment surrounding the spontaneous arising of thoughts in experienced mindfulness practitioners. NeuroImage, 136, 186–196. https://doi.org/10.1016/j.neuroimage.2016.04.034
Filmer, H. L., Dux, P. E., & Mattingley, J. B. (2014). Applications of transcranial direct current stimulation for understanding brain function. Trends in Neurosciences, 37(12), 742–753. https://doi.org/10.1016/j.tins.2014.08.003
Filmer, H. L., Griffin, A., & Dux, P. E. (2019). For a minute there, I lost myself … dosage dependent increases in mind wandering via prefrontal tDCS. Neuropsychologia, 129, 379–384. https://doi.org/10.1016/j.neuropsychologia.2019.04.013
Fox, K. C. R., Spreng, R. N., Ellamil, M., Andrews-Hanna, J. R., & Christoff, K. (2015). The wandering brain: Meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. NeuroImage, 111, 611–621. https://doi.org/10.1016/j.neuroimage.2015.02.039
Garcia-Argibay, M., Santed, M. A., & Reales, J. M. (2018). Efficacy of binaural auditory beats in cognition, anxiety, and pain perception: A meta-analysis. Psychological Research Psychologische Forschung. https://doi.org/10.1007/s00426-018-1066-8
Hallett, M. (2007). Transcranial magnetic stimulation: A primer. Neuron, 55(2), 187–199. https://doi.org/10.1016/j.neuron.2007.06.026
Huang, Y.-Z., Lu, M.-K., Antal, A., Classen, J., Nitsche, M., Ziemann, U., Ridding, M., Hamada, M., Ugawa, Y., Jaberzadeh, S., Suppa, A., Paulus, W., & Rothwell, J. (2017). Plasticity induced by non-invasive transcranial brain stimulation: A position paper. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 128(11), 2318–2329. https://doi.org/10.1016/j.clinph.2017.09.007
Johnstone, A. H., & Percival, F. (1976). Attention breaks in lectures. Education in Chemistry, 13, 2, 49–50.
Kajimura, S., & Nomura, M. (2015). Decreasing propensity to mind-wander with transcranial direct current stimulation. Neuropsychologia, 75, 533–537. https://doi.org/10.1016/j.neuropsychologia.2015.07.013
Kajimura, S., Kochiyama, T., Nakai, R., Abe, N., & Nomura, M. (2016). Causal relationship between effective connectivity within the default mode network and mind-wandering regulation and facilitation. NeuroImage, 133, 21–30. https://doi.org/10.1016/j.neuroimage.2016.03.009
Kajimura, S., Kochiyama, T., Abe, N., & Nomura, M. (2019). Challenge to Unity: Relationship between hemispheric asymmetry of the default mode network and mind wandering. Cerebral Cortex (New York, N.Y.: 1991), 29(5), 2061–2071. https://doi.org/10.1093/cercor/bhy086
Kane, M. J., Brown, L. H., McVay, J. C., Silvia, P. J., Myin-Germeys, I., & Kwapil, T. R. (2007). For whom the mind wanders, and when: An experience-sampling study of working memory and executive control in daily life. Psychological Science, 18(7), 614–621. https://doi.org/10.1111/j.1467-9280.2007.01948.x
Keeser, D., Meindl, T., Bor, J., Palm, U., Pogarell, O., Mulert, C., Brunelin, J., Möller, H.-J., Reiser, M., & Padberg, F. (2011). Prefrontal transcranial direct current stimulation changes connectivity of resting-state networks during fMRI. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 31(43), 15284–15293. https://doi.org/10.1523/JNEUROSCI.0542-11.2011
Killingsworth, M. A., & Gilbert, D. T. (2010). A wandering mind is an unhappy mind. Science (New York, N.Y.), 330(6006), 932. https://doi.org/10.1126/science.1192439
Kuwada, S., Yin, T. C., & Wickesberg, R. E. (1979). Response of cat inferior colliculus neurons to binaural beat stimuli: Possible mechanisms for sound localization. Science (New York, N.Y.), 206(4418), 586–588.
Lefaucheur, J.-P., Antal, A., Ayache, S. S., Benninger, D. H., Brunelin, J., Cogiamanian, F., Cotelli, M., De Ridder, D., Ferrucci, R., Langguth, B., Marangolo, P., Mylius, V., Nitsche, M. A., Padberg, F., Palm, U., Poulet, E., Priori, A., Rossi, S., Schecklmann, M., … Paulus, W. (2017). Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 128(1), 56–92. https://doi.org/10.1016/j.clinph.2016.10.087
Lefebvre, S., Laloux, P., Peeters, A., Desfontaines, P., Jamart, J., & Vandermeeren, Y. (2012). Dual-tDCS enhances online motor skill learning and long-term retention in chronic stroke patients. Frontiers in Human Neuroscience, 6, 343. https://doi.org/10.3389/fnhum.2012.00343
Leszczynski, M., Chaieb, L., Reber, T. P., Derner, M., Axmacher, N., & Fell, J. (2017). Mind wandering simultaneously prolongs reactions and promotes creative incubation. Scientific Reports, 7(1), 10197. https://doi.org/10.1038/s41598-017-10616-3
Licklider, J. C. R., Webster, J. C., & Hedlun, J. M. (1950). On the frequency limits of binaural beats. The Journal of the Acoustical Society of America, 22(4), 468–473. https://doi.org/10.1121/1.1906629
Lloyd, D. H. (1968). A concept of improvement of learning response in the taught lesson. https://scholar.google.com/scholar
Marquez, J., van Vliet, P., McElduff, P., Lagopoulos, J., & Parsons, M. (2015). Transcranial direct current stimulation (tDCS): Does it have merit in stroke rehabilitation? A systematic review. International Journal of Stroke: Official Journal of the International Stroke Society, 10(3), 306–316. https://doi.org/10.1111/ijs.12169
McCormick, C., Rosenthal, C. R., Miller, T. D., & Maguire, E. A. (2018). Mind-wandering in people with hippocampal damage. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 38(11), 2745–2754. https://doi.org/10.1523/JNEUROSCI.1812-17.2018
McVay, J. C., & Kane, M. J. (2009). Conducting the train of thought: Working memory capacity, goal neglect, and mind wandering in an executive-control task. Journal of Experimental Psychology: Learning, Memory, and Cognition, 35(1), 196–204. https://doi.org/10.1037/a0014104
McVay, J. C., Kane, M. J., & Kwapil, T. R. (2009). Tracking the train of thought from the laboratory into everyday life: An experience-sampling study of mind wandering across controlled and ecological contexts. Psychonomic Bulletin & Review, 16(5), 857–863. https://doi.org/10.3758/PBR.16.5.857
Middendorf, J., & Kalish, A. (1996). The “change-up” in lectures. National Teaching and Learning Forum, 5(2), 1–5.
Mrazek, M. D., Phillips, D. T., Franklin, M. S., Broadway, J. M., & Schooler, J. W. (2013). Young and restless: Validation of the Mind-Wandering Questionnaire (MWQ) reveals disruptive impact of mind-wandering for youth. Frontiers in Psychology, 4, 560. https://doi.org/10.3389/fpsyg.2013.00560
Nitsche, M. A., & Paulus, W. (2001). Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology, 57(10), 1899–1901.
Nitsche, M. A., Schauenburg, A., Lang, N., Liebetanz, D., Exner, C., Paulus, W., & Tergau, F. (2003). Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. Journal of Cognitive Neuroscience, 15(4), 619–626. https://doi.org/10.1162/089892903321662994
Opitz, A., Paulus, W., Will, S., Antunes, A., & Thielscher, A. (2015). Determinants of the electric field during transcranial direct current stimulation. NeuroImage, 109, 140–150. https://doi.org/10.1016/j.neuroimage.2015.01.033
Oster, G. (1973). Auditory beats in the brain. Scientific American, 229(4), 94–102.
Purpura, D. P., & Mcmurtry, J. G. (1965). Intracellular activities and evoked potential changes during polarization of motor cortex. Journal of Neurophysiology, 28, 166–185. https://doi.org/10.1152/jn.1965.28.1.166
Robertson, I. H., Manly, T., Andrade, J., Baddeley, B. T., & Yiend, J. (1997). “Oops!”: Performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia, 35(6), 747–758.
Rossi, S., Hallett, M., Rossini, P. M., Pascual-Leone, A., & Safety of TMS Consensus Group. (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 120(12), 2008–2039. https://doi.org/10.1016/j.clinph.2009.08.016
Schooler, J. W., Smallwood, J., Christoff, K., Handy, T. C., Reichle, E. D., & Sayette, M. A. (2011). Meta-awareness, perceptual decoupling and the wandering mind. Trends in Cognitive Sciences, 15(7), 319–326. https://doi.org/10.1016/j.tics.2011.05.006
Seli, P., Risko, E. F., Smilek, D., & Schacter, D. L. (2016). Mind-wandering with and without intention. Trends in Cognitive Sciences, 20(8), 605–617. https://doi.org/10.1016/j.tics.2016.05.010
Seli, P., Beaty, R. E., Cheyne, J. A., Smilek, D., Oakman, J., & Schacter, D. L. (2018). How pervasive is mind wandering, really? Consciousness and Cognition, 66, 74–78. https://doi.org/10.1016/j.concog.2018.10.002
Shrimpton, D., McGann, D., & Riby, L. M. (2017). Daydream believer: Rumination, self-reflection and the temporal focus of mind wandering content. Europe’s Journal of Psychology, 13(4), 794–809. https://doi.org/10.5964/ejop.v13i4.1425
Smallwood, J., & Schooler, J. W. (2006). The restless mind. Psychological Bulletin, 132(6), 946–958. https://doi.org/10.1037/0033-2909.132.6.946
Smallwood, J., & Schooler, J. W. (2015). The science of mind wandering: Empirically navigating the stream of consciousness. Annual Review of Psychology, 66, 487–518. https://doi.org/10.1146/annurev-psych-010814-015331
Smallwood, J., Fishman, D. J., & Schooler, J. W. (2007). Counting the cost of an absent mind: Mind wandering as an underrecognized influence on educational performance. Psychonomic Bulletin & Review, 14(2), 230–236. https://doi.org/10.3758/bf03194057
Smilek, D., Carriere, J. S. A., & Cheyne, J. A. (2010). Out of mind, out of sight: Eye blinking as indicator and embodiment of mind wandering. Psychological Science, 21(6), 786–789. https://doi.org/10.1177/0956797610368063
Stawarczyk, D., Majerus, S., & D’Argembeau, A. (2013). Concern-induced negative affect is associated with the occurrence and content of mind-wandering. Consciousness and Cognition, 22(2), 442–448. https://doi.org/10.1016/j.concog.2013.01.012
Szpunar, K. K., Moulton, S. T., & Schacter, D. L. (2013). Mind wandering and education: From the classroom to online learning. Frontiers in Psychology, 4, 495. https://doi.org/10.3389/fpsyg.2013.00495
Tremblay, S., Rogasch, N. C., Premoli, I., Blumberger, D. M., Casarotto, S., Chen, R., Di Lazzaro, V., Farzan, F., Ferrarelli, F., Fitzgerald, P. B., Hui, J., Ilmoniemi, R. J., Kimiskidis, V. K., Kugiumtzis, D., Lioumis, P., Pascual-Leone, A., Pellicciari, M. C., Rajji, T., Thut, G., … Daskalakis, Z. J. (2019). Clinical utility and prospective of TMS-EEG. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 130(5), 802–844. https://doi.org/10.1016/j.clinph.2019.01.001
Unsworth, N., McMillan, B. D., Brewer, G. A., & Spillers, G. J. (2012). Everyday attention failures: An individual differences investigation. Journal of Experimental Psychology: Learning, Memory, and Cognition, 38(6), 1765–1772. https://doi.org/10.1037/a0028075
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Chaieb, L., Reber, T.P., Krakau, S., Fell, J. (2022). Noninvasive Brain Stimulation for the Modulation of Mind Wandering. In: Dario, N., Tateo, L. (eds) New Perspectives on Mind-Wandering. Springer, Cham. https://doi.org/10.1007/978-3-031-06955-0_8
Download citation
DOI: https://doi.org/10.1007/978-3-031-06955-0_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-06954-3
Online ISBN: 978-3-031-06955-0
eBook Packages: Behavioral Science and PsychologyBehavioral Science and Psychology (R0)