Abstract
Accurate calibration of the spectrometer output signal in terms of the content of elements under measurement is of great importance for the metrological assurance of high-precision inductively coupled plasma mass spectrometry (ICP-MS) and inductively coupled plasma optical emission spectrometry (ICP-OES). This paper aims to establish the certified values of a reference material for a multi-element solution of metals for use in measurements based on inductively coupled plasma spectrometry (ICP-CRM Multi 1). ICP-CRM Multi 1 is a solution with the certified values of the mass fraction of metals: barium, cadmium, cobalt, lithium, lead, and zinc. The solution was packed in high-density polyethylene bottles with a capacity of 4, 8, 15, 30, 60, and 125 cm3. The certified values of the mass fraction of metals in the solution was established by the gravimetric method of preparation and confirmed by the State Primary Standard of Unit of Mass Fraction and Unit of Mass (Molar) Concentration of Inorganic Components in Aqueous Solutions Based on Gravimetric and Spectral Methods GET 217–2018. The permissible certified values of the mass fraction of metals in the developed ICP-CRM are shown to range from 900 mg/kg to 1100 mg/kg. The authors have launched a study into the long-term stability of ICP-CRM Multi 1 with the purpose of establishing its expiration date. It is assumed that the expanded uncertainty of measurements of the certified value of the mass fraction of metals in the solution of ICP-CRM Multi 1 will not exceed 0.5%. ICP-CRM Multi 1 can be used for ensuring the metrological traceability of measurements to GET 217–2018 in inorganic analysis using ICP-MS and ICP-OES. The developed solution will also allow one of the main advantages of these methods to be applied in routine analysis, namely the ability to quickly and simultaneously measure several elements in samples.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Inductively coupled plasma mass spectrometry
- Inductively coupled plasma optical emission spectrometry
- Aqueous solution
- Reference material
- Water analysis
- Inorganic component
Introduction
Quality control of industrial products and monitoring of the state of environmental objects are important for both individual enterprises and the state as a whole. Various analytical methods can be used to analyze the chemical composition of substances and materials. The selection of the most appropriate method for the intended purpose is carried out depending on the number of elements to be measured and the number of samples to be analyzed.
As highly rapid and sensitive methods for qualitative and quantitative analysis of elements in various materials and substances, inductively coupled plasma optical emission spectroscopy (ICP-OES) and inductively coupled plasma mass spectrometry (ICP-MS) meet the performance requirements of many laboratories. These methods are increasingly being used in diverse fields of science and industry, including the food industry to control the content of various components in food and drinks [1,2,3]; environmental protection to analyze the metal content of various environmental objects [4,5,6]; forensic analysis to identify micro- and toxic elements in biomaterials (hair, nails, epithelial tissue, blood, urine, muscles, etc.) [7,8,9], etc. [10,11,12].
The establishment of a calibration dependence of the output signal of spectrometers on the content of elements under analysis is a necessary step for carrying out measurements using ICP-OES and ICP-MS. Such calibration is only appropriate when reliable reference materials (RMs) are used. RMs certified in the Russian Federation (CRM)—GSO—ensure the metrological traceability of measurements in all laboratories of the country [13, 14]. The calibration of spectrometers based on inductively coupled plasma is carried out using CRMs, which are solutions with the certified value of the mass fraction or mass concentration of a particular element of Mendeleev’s Periodic Table.
The methods of ICP-MS and ICP-OES allow several elements to be measured quickly and simultaneously. This goal can only be achieved when using multi-element RMs, since their use simplifies and accelerates the preparation of a series of working and calibration solutions.
In this research, we aimed to develop a CRM of a multi-element solution of metals (hereinafter ICP-CRM Multi 1), which would enable the calibration and measurement of several elements simultaneously. The CRM under development should be traceable to the State Primary Standard of Unit of Mass Fraction and Unit of Mass (Molar) Concentration of Inorganic Components in Aqueous Solutions Based on Gravimetric and Spectral Methods GET 217–2018 [15].
Materials and Methods
In order to develop a CRM of a multi-element solution of metals, the following metrological characteristics required normalization: the value of a CRM certified characteristic; error and/or uncertainty of a CRM certified value; the expiration date of a CRM.
The values of CRM certified characteristics were normalized by establishing the interval across which the certified values of any RM of a given type must vary. The mass fraction of metals in the developed CRM should range from 900 mg/kg to 1100 mg/kg inclusive; the mass concentration of a metal should range from 900 mg/dm3 to 1100 mg/dm3 inclusive.
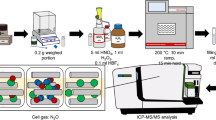
The selection of a manufacturing technology was conducted by analyzing possible procedures for preparing an ICP-CRM and approaches to establishing the ICP-CRM certified values. As a result, we decided to certify the developed ICP-CRM according to the gravimetric method of preparation, since the gravimetric preparation of RMs from high-purity starting materials is the most accurate method for reproducing and transferring the units of concentration of metals in a solution. Certification by the gravimetric preparation procedure is an approach to certifying an RM based on the known or specially investigated characteristics and quantitative ratios of the precursor components used for RM preparation by mixing them to obtain the calculated values of the RM metrological characteristics.
The material chosen for preparing the ICP-CRM is a solution of metals or their compounds in dilute nitric acid. The mass fraction of the main component in the starting materials and the solvent was established using GET 217–2018. The ICP-CRM was produced using the VNIIFTRI equipment. In order to carry out preliminary experiments, pure metals were chosen as a starting material for preparing a solution with a certified value of the mass fraction of zinc, cobalt, and cadmium; metal salts were chosen for a solution with a certified value of the mass fraction of lithium, barium, and lead. Bottles made of darkened high-density polyethylene with various capacities (30, 60, and 125 cm3) were selected as containers. They had been previously used in the development of single-element CRMs [16]. The solvent was 5% nitric acid by volume, which ensured complete dissolution of the starting material and stability of the ICP-CRM in accordance with the preparation procedure. Particular attention was paid to the selection and pre-cleaning of polymer ware intended for both laboratory research and further use as a container for the ICP-CRM.
In order to assess the purity of the starting materials, the “100% minus the amount of impurities” method was chosen. Thus, all detected impurities were subtracted from 100%, and elements with a concentration below the detection limit were taken into account by subtracting half of their detection limit from 100%. The mass fraction of the main component (\(\omega\)) was calculated by Eq. (1):
where wi is the mass fraction of detected impurities, %;
\(LOD_{j}\) is the detection limit of undetected impurities, %.
The impurity composition of the solvents after purification was assessed using GET 217–2018.
The mass fraction of a component in the ICP-CRM was estimated by equations [17]:
where \(\overline{{\mathop {A_{m} }\nolimits_{{_{i} }} }}\) is the weighted average estimate of the content of the i-th component in the mixture;
\(a_{{m_{ij} }}\) amis the content of the i-th component in the j-th component in the mixture;
\(\mathop w\nolimits_{j}\) are weight coefficients;
\(\mathop m\nolimits_{j}\) is the mass of the j-th component in the mixture.
A scheme of the ICP-CRM Multi 1 manufacturing technology from carrier materials is shown in Fig. 1. The mass fraction of the components of the carrier materials was preliminarily established according to the “100% minus the ∑amount of impurities” method using GET 217–2018.
The traceability of the certified values of the ICP-CRM to the measurement unit was carried out by establishing the value of the certified characteristics in the starting materials using GET 217–2018.
Results and Discussion
The developed ICP-CRM Multi 1 was packed in bottles with a capacity of 30, 60, and 125 cm3 (Fig. 2).
The reliability of the certified values of the mass fraction of metals in the developed ICP-CRM was repeatedly measured using GET 217–2018. The measurement results of the mass fraction of metals in the ICP-CRM obtained using both the gravimetric sample preparation and the measurement method using GET 217–2018 are in good agreement within the limits of their uncertainties, as well as with the results of comparative measurements carried out for the developed ICP-CRM and CRMs of other manufactures. The certified value of the developed ICP-CRM is taken to be that obtained by the gravimetric method of preparation.
The measurement uncertainty of the certified value of the mass fraction of a component in the solution was calculated in accordance with [18]. The uncertainty components for the gravimetric preparation of the solution of the mass fraction of a component are presented in Fig. 3 as a cause-and-effect diagram. The results of the gravimetric preparation and the measurement results using GET 217–2018 are presented in Fig. 4.
The results of estimating the uncertainty due to the method for determining the certified value of the CRM of a multi-element solution of metals according to the gravimetric method of preparation are presented in Tables 1 and 2.
The uncertainty of a certified value includes the uncertainty due to instability and the uncertainty due to homogeneity, in addition to the uncertainty due to the method for determining the certified value. Therefore, future research should investigate the stability and homogeneity of the developed ICP-CRM.
At the current research stage, special attention was paid to the selection of both suitable packaging for storing the developed CRM and its stable composition. However, the mass fraction of any component in a solution is a time function and may change due to possible evaporation losses during storage after unpacking. In this regard, we are planning to conduct additional studies into the long-term stability of the certified value of ICP-CRM Multi 1 and its stability during storage after unpacking. Provided that positive results are obtained in terms of long-term stability, the expanded measurement uncertainty of the certified value of the metal mass fraction in the CRM aqueous solution will not exceed 0.5%.
Conclusion
In this research, we have developed a CRM of a multi-element solution of metals (ICP-CRM Multi 1), which can be used for ensuring the metrological traceability of measurements in inorganic analysis using ICP-MS and ICP-OES according to GET 217–2018. The developed solution will also allow one of the main advantages of these methods to be applied in routine analysis, namely the ability to quickly and simultaneously measure several elements in materials.
References
Tokalıoğlu Ş, Dokan FK, Köprü S (2019) ICP-MS multi-element analysis for determining the origin by multivariate analysis of red pepper flakes from three different regions of Turkey. LWT- Food Sci Technol 103:301–307. https://doi.org/10.1016/j.lwt.2019.01.015
Nguyen-Quang T, Do-Hoang G, Truong-Ngoc M (2021) Multielement analysis of pakchoi (Brassica rapa L. ssp. chinensis) by ICP-MS and their classification according to different small geographical origins. J Analy Methods Chem. https://doi.org/10.1155/2021/8860852
Gregorčič SH, Strojnik L, Potočnik D, Vogel-Mikuš K, Jagodic M, Camin F (2020) Can we discover truffle’s true identity? Molecules 25(9):2217. https://doi.org/10.3390/molecules25092217
Sader JA, Ryan S (2020) Advances in ICP-MS technology and the application of multi-element geochemistry to exploration. Geochem Expl Environ Anal 20(2):167–175. https://doi.org/10.1144/geochem2019-049
Carrier-Belleau C, Drolet D, McKindsey CW, Archambault P (2021) Environmental stressors, complex interactions and marine benthic communities’ responses. Scient Rep Feb;11(1):4194. https://doi.org/10.1038/s41598-021-83533-1
Noerpel M, Pribil M, Rutherford D et al (2020) Lead speciation, bioaccessibility and source attribution in Missouri’s Big River watershed. Appl Geochem J Intern Assoc Geochem Cosmochem 123. https://doi.org/10.1016/j.apgeochem.2020.104757
Vanini G, Souza MO, Carneiro MTWD, Filgueiras PR, Bruns RE, Romão W (2015) Multivariate optimisation of ICP OES instrumental parameters for Pb/Ba/Sb measurement in gunshot residues. Microchem J 120:58–63. https://doi.org/10.1016/j.microc.2015.01.003
Galay EP, Dorogin RV, Temerdashev AZ (2021) Quantification of cobalt and nickel in urine using inductively coupled plasma atomic emission spectroscopy. Heliyon 7(1):e06046. https://doi.org/10.1016/j.heliyon.2021.e06046
Wilk A, Romanowski M, Wiszniewska B (2021) Analysis of cadmium, mercury, and lead concentrations in erythrocytes of renal transplant Recipients from Northwestern Poland. Biology 10(1):62. https://doi.org/10.3390/biology10010062
Kreitals NM, Watling RJ (2014) Multi-element analysis using inductively coupled plasma mass spectrometry and inductively coupled plasma atomic emission spectroscopy for provenancing of animals at the continental scale. Forensic Sci Intern Synergy 244:116–121. https://doi.org/10.1016/j.forsciint.2014.08.016
Haraguchi H et al (1994) Multielement profiling analysis of geochemical and environmental samples by inductively coupled plasma-atomic emission spectroscopy (ICP-AES) and inductively coupled plasma-mass spectrometry (ICP-MS). In: Conference Proceedings. 10th Anniversary. IMTC/94. Advanced Technologies in I & M. 1994 IEEE Instrumentation and Measurement Technolgy Conference (Cat. No.94CH3424–9). Hamamatsu, Japan, pp 1–6. https://doi.org/10.1109/IMTC.1994.351792
Michalak I, Chojnacka K (2009) Multielemental analysis of macroalgae from the Baltic sea by ICP-OES to monitor environmental pollution and assess their potential uses. Int J Environ Anal Chem 89(8–12):583–596. https://doi.org/10.1080/03067310802627213
De Bièvre P, Dybkaer R, Fajgelj A, Hibbert DB (2011) Metrological traceability of measurement results in chemistry: concepts and implementation (IUPAC Technical Report). Pure Appl Chem 83(10):1873–1935. https://doi.org/10.1351/PAC-REP-07-09-39
Fed Federal Law (2008) № 102-FZ of 26/06/2008 “On Ensuring the Uniformity of Measurements” (as amended and supplemented). https://normativ.kontur.ru/document?moduleId=1&documentId=398044 (In Russ)
GET 217–2018 National primary standard for the units of mass fraction and mass (molar) concentration of inorganic components in aqueous solutions based on gravimetric and spectral methods. In: Federal information fund for ensuring the uniformity of measurements https://fgis.gost.ru/fundmetrology/registry/12/items/397906 (In Russ)
Stakheev AA, Stolboushkina TP (2019) Developing and testing a certified reference material of the lead mass fraction in solutions for measurements using inductively coupled plasma methods. Reference Materials 15(4):25–31. https://doi.org/10.20915/2077-1177-2019-15-4-25-31 (In Russ)
Migal PV, Medvedevskikh SV, Firsanov VA (2020) A method for estimating the certified value uncertainty of a multicomponent reference material. In: Medvedevskikh S, Kremleva O, Vasil’eva I, Sobina E (eds) Reference Materials in Measurement and Technology Springer, Cham. pp 175–185. https://doi.org/10.1007/978-3-030-32534-3_17
JCGM 100:2008. Evaluation of measurement data—Guide to the expression of uncertainty in measurement (GUM). Joint Committee for Guides in Metrology
MI 3560–2016 Recommendation (2016) GSE. Estimation of uncertainty in measurements of the mass fraction of the main component in inorganic substances. UNIIM, Ekaterinburg
Migal PV, Gorbunova EM, Sobina EP, Tabatchikova TN (2013) Development and testing of a reference material of nickel (II) mass fraction in solution. Ref Mater 3:39–44 (In Russ)
Acknowledgements
All measurements were carried out using the equipment of VNIIFTRI.
Author information
Authors and Affiliations
Contributions
Stolboushkina T. P.: conducting experiments, collection of experimental data, development of preparation and measurement methods, literature review.
Stakheev A. A.: development of the research and article concept, critical analysis and revision of the text.
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
Conflict of Interest
The article was prepared on the basis of a report presented at the IV International Scientific Conference “Reference Materials in Measurement and Technology” (St. Petersburg, December 1–3, 2020). The article was admitted for publication after the abstract was revised, the article was formalized and the review procedure was carried out.
The version in the Russian language is published in the journal “Measurement Standards. Reference Materials” 2021;17(2):49–57. https://doi.org/10.20915/2687-0886-2021-17-2-49-57.
Rights and permissions
Copyright information
© 2022 D. I. Mendeleyev Institute for Metrology
About this paper
Cite this paper
Stolboushkina, T.P., Stakheev, A.A. (2022). Development and Testing of a Multi-Element Reference Material for Methods Based on Inductively-Coupled Plasma. In: Medvedevskikh, S.V., Sobina, E.P., Kremleva, O.N., Okrepilov, M.V. (eds) Reference Materials in Measurement and Technology . RMMT 2020. Springer, Cham. https://doi.org/10.1007/978-3-031-06285-8_14
Download citation
DOI: https://doi.org/10.1007/978-3-031-06285-8_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-06284-1
Online ISBN: 978-3-031-06285-8
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)