Abstract
This chapter reviews data on the pathways by which luminal, mainly duodenal, chemoreceptors modulate gastro-pyloro-duodenal motor function to control emptying of nutrients into the small intestine. The vagus mediates proximal gastric relaxation caused by nutrient stimulation of duodenal/jejunal mucosal chemoreceptors. Modulation of the spatial patterning and inhibition of antral contractions during duodenal chemoreceptor activation are somewhat conflicting: both vagal control and ascending intramural nerves appear to play a role. Intraduodenal nutrients stimulate the localized pyloric contractions that prevent transpyloric flow via ascending duodenal intramural nerve pathways. Though not yet formally investigated, patterns of activation of the duodenal brake motor mechanism suggest that duodenal loop mucosal chemoreceptors signal to a brake mechanism at the most aborad region of the duodenum via descending intramural duodenal nerves.
Intrinsic intramural pathways are important in the control of the first stages of digestion.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
3.1 Introduction
Understanding of the mechanisms of mucosal chemoreception [1] and how chemosensory receptors activate primary afferent nerves [2] have advanced substantially in recent years. By contrast, there is only limited information about the pathways via which mucosal chemoreceptors, mainly duodenal, signal to upper gastrointestinal motor components to vary the rate of movement of nutrients from the stomach and duodenum into the jejunum.
Demonstrations of gut-brain-gut vagal connections [3, 4] have led to the widely held view that the vagus is the primary modulator of normal gastric emptying. This chapter proposes that intrinsic duodenal intramural nerves are the dominant signaling pathways from duodenal chemoreceptors that cause variations of pyloric, duodenal, and upper jejunal motor function. These variations match the rate of gastric emptying to duodenal digestive function, an essential aspect of normal upper gastrointestinal nutrient digestion.
3.2 Spectrum of Duodenal Chemosensing
The duodenal mucosa is a very complex sensory organ [1, 2]. Chemoreceptor modulations of upper gastrointestinal motor function have been studied mainly by activating this system with “provocative” intraduodenal infusions of components of the postprandial duodenal luminal content. Components that have been studied include fatty emulsions and solutions of acid, isotonic and hypertonic dextrose, bile acids, and hypertonic saline.
3.3 Effects of Truncal Vagotomy on Duodenal Chemoreceptor Control of Gastric Emptying
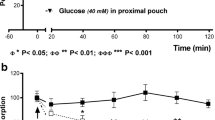
In chronic studies in five awake, trained dogs, emptying of 600 ml of water from the stomach was measured repeatedly during intraduodenal delivery of either water or three different provocative infusates (250 mM HCl, 10% dextrose and 5% “fat”) through an indwelling duodenal cannula (rate of delivery not stated), before and after bilateral transthoracic truncal vagotomy (75 and 95 experiments, respectively) [5]. Figure 3.1 shows that the hierarchy of increasing slowing of gastric emptying by the different intraduodenal infusions persisted after vagotomy, indicating that a major component of the signals from the duodenal chemoreceptors that caused the gradation of slowing of emptying with the provocative infusates, was not vagally transmitted. Compared to the control studies, vagotomy was however associated with slower gastric emptying for all of the 4 duodenal infusates (Fig. 3.1) (see below in Sect. 3.6, third paragraph). This study did not seek to assess gastric motor patterns.
Gastric emptying in five trained dogs before and after bilateral thoracic vagotomy during four types of intraduodenal infusion. The increasing level of stimulation of duodenal chemoreceptors by the 250 mM hydrochloric acid, 10% dextrose, and 5% fat infusates still slowed emptying progressively after vagotomy. The global slowing of emptying after vagotomy is discussed in Sect. 3.6, second paragraph. (Reproduced and redrawn with permission from Ref. [5])
3.4 Chemosensor Control of Proximal Gastric Motor Function
Tonic contraction of the proximal half of the stomach maintains a variable, nonpulsatile compression of the gastric content, which aids nonnutrient and nutrient emptying [4]. In six awake, trained dogs, a proximal jejunal infusion of a liquid nutrient supplement (osmolite) was shown to cause proximal gastric relaxation, which was prevented by reversible cervical or subdiaphragmatic vagal blockade by cooling [6]. This indicates that the vagus is the key control pathway for proximal jejunal chemoreceptor–induced upper gastric relaxation.
3.5 Duodenal Chemoreceptor Control of Antro-Pyloric Motor Function
The literature needs to be interpreted in the light of the significant methodological challenges associated with measurements of antral and pyloric motor functions.
3.5.1 Measurement of Antral and Pyloric Motor Functions
A manometric system that is suitably dampened to exclude rapidly changing contact pressures with the antral wall only detects an antral contraction when this causes active lumen-occlusion, which may only be in the very distal antrum [7]. Thus, though manometry underestimates the extent and number of antral contractions, it detects the lumen-occlusive antral contractions that usually lead to aborad pulsatile transpyloric flows, the major component of gastric emptying [8]. Therefore, manometry is informative about modulations of antral motor function relevant to changes of gastric emptying, especially when pyloric motor function is also reliably recorded. Fluoroscopy, especially when combined with manometry, is a sensitive method for evaluation of antro-pyloric motility but is subject to several major practical constraints, especially radiation exposure in humans [9].
The challenge of manometric recording of pyloric motor function was demonstrated in humans [10] during intraduodenal infusion of a high-calorie triglyceride emulsion; this stimulated highly localized or “isolated” phasic and tonic contractions of the pyloric ring (Fig. 3.2). These localized pressures were detected reliably by the sleeve sensor, as its 3.6 cm pressure-sensing length remained in contact with the less than 9-mm-wide region of isolated pyloric tonic and phasic contractions [10], despite unavoidable movements of the pylorus relative to the manometric assembly. Such movements confound recordings with single point pressure sensors, including high-resolution manometric catheters with their sensor spacings of no less than 10 mm (Fig. 3.2).
Manometric tracings in a healthy volunteer from the sleeve, positioned astride the pylorus (uppermost trace), and an array of side-holes 6 mm apart (SH 10–2) along a 24 mm segment of the sleeve. The left-hand panel shows a fasting phase II antro-pyloro-duodenal pressure event sweeping across the pylorus. The right-hand panel recording was made during inhibition of antral pressure events and stimulation of localized contractions of the pyloric ring with intraduodenal fat infusion. The isolated pyloric tonic and phasic contractions are only present over side-hole 8, documenting the very narrow zone of pyloric activity. The sleeve tracing also records the phasic and tonic pyloric motor activities captured by side-hole 8. (Reproduced with permission from Ref. [10])
The sleeve, coupled with transmucosal potential difference monitoring to validate its position astride the pylorus [10], remains the only valid method for monitoring of pyloric motor function. This technique was used in all of the studies of pyloric motility cited below.
3.5.2 Chemoreceptor Control of Antral and Pyloric Motor Functions in Humans
In humans, emptying of a small radiolabeled nutrient meal into the duodenum or direct intraduodenal infusion of substances that stimulate duodenal chemoreceptors causes inhibition of antral motility and stimulation of localized tonic and phasic pyloric contractions [10,11,12,13]. This pattern of response is maintained for at least 90 minutes by continuous provocative intraduodenal infusions [10, 14,15,16,17] and is blocked by atropine [15, 17].
Concurrent fluoroscopy and manometry in 11 healthy men has directly confirmed that a provocative duodenal infusion causes active closure of the pylorus during localized pyloric tonic and phasic contractions [9] (Fig. 3.3). This finding emphasizes the capability of the pylorus to seal off the exit from the stomach, while inhibition of the proximal and distal stomach contractions can only suspend gastric pumping and grinding. The “plug in the bottle” function of the pylorus might be a mechanism that becomes progressively more important, as the larger the size of the meal, the greater is the passive force that favors emptying across a relaxed pylorus. However, there is a dearth of data on gastro-pyloro-duodenal motor function after consumption of a normal-sized “main” meal.
Fluoroscopic appearances of the antrum (right side) pylorus (center) and proximal duodenum (left side) in a healthy human volunteer during stimulation of localized pyloric contractions by an intraduodenal fat infusion. The concurrent pyloric sleeve pressure recording displayed in the lower frame synchronized fluoroscopy with manometry. The narrow pyloric ring is closed over the sleeve assembly (the radio-opacity across the pyloric ring is due to a spring-wire sleeve stiffener), and during this motor pattern, no barium traversed the pylorus. (Reproduced with permission from Ref. [9])
3.5.3 Studies in Pigs and Dogs of Pathways via Which Duodenal Chemoreceptors Alter Antral and Pyloric Motor Function
In chronic studies in awake pigs with intact vagi, pyloric excision abolished the inhibition of antral pressure events normally induced by duodenal infusion of an isotonic dextrose/saline solution [18]. This suggests that the antral inhibition is signaled by intramural nerves (divided by the pyloric excision) that travel orad from the duodenum to the antrum, a conclusion that is at odds with studies of duodenal transection discussed in the next paragraph.
Duodenal transection with immediate reanastomosis (and no resection) restores normal gross anatomy but results in persisting interruption of intramural nervous pathways. This intervention has only been used in pigs to investigate pathways activated by duodenal distension that slow gastric emptying. In six unsedated, trained pigs, midduodenal balloon distension caused a dose-related stimulation of localized pyloric contractions, inhibition of antral pressure events, and retardation of gastric emptying [19]. In another six pigs whose duodenum had been transected just aborad of the pylorus, duodenal balloon distension failed to stimulate pyloric motor function, consistent with this being caused by ascending intramural nerves. Confusingly though, given the findings discussed in the paragraph immediately above [18], the duodenal balloon distension-induced antral motor inhibition persisted [19], suggesting these effects were vagally induced.
In a study of antral transection in five awake pigs, control measurements before transection confirmed that intraduodenal infusion of 25% dextrose stimulated localized pyloric contractions and inhibited antral pressure events [20]. The antrum was then transected 2 cm orad of the pylorus: as expected, the 25% dextrose-induced stimulation of localized pyloric contractions persisted, but the inhibition of antral motility was unaffected by the antral transection, consistent with it being mediated via the vagus.
An approach developed by E.E Daniel has been used to investigate pathways of duodenal control of pyloric motor function in acute studies on laparotomized, anesthetized dogs [21, 22] (Fig. 3.4). Bilateral cervical vagotomy had no consistent effect on sleeve-recorded localized basal pyloric motility. Electrical field stimulation of the proximal duodenum stimulated localized pyloric contractions, an effect blocked by the targeted intra-arterial delivery of atropine, hexamethonium, and tetrodotoxin to the proximal duodenal/pyloric region. Duodenal transection orad of the duodenal field stimulation electrodes also abolished their effect on pyloric motility, indicating that the pylorus was stimulated by an ascending intramural pathway from the duodenum to the pyloric ring [21].
Schematic of the technique developed by EE Daniel, which was used by Allescher et al. [22] to investigate pyloric motor function in the anesthetized dog. A multilumen sleeve/side-hole catheter was passed via a gastrotomy so that the 3 cm sleeve (S) was astride the pylorus and side-holes (SH) were in the antrum and duodenum at each end of the sleeve. Strain gauges (SG) were sutured to the antrum and proximal duodenum. Silver wire electrical field stimulation electrodes (E) were embedded in the proximal duodenum. Two cannulae enabled close intra-arterial injections of drugs to the pylorus and antrum. The perfusion territory covered by these cannulae was confirmed initially by observing blanching following an injection of a bolus of Krebs solution and at the end of each study by injection of black ink. (Reproduced with permission from Ref. [21])
A further study in anesthetized dogs [22] documented stimulation of phasic and tonic localized pyloric and proximal duodenal pressure events within several seconds of the start of an intraduodenal infusion (0.92 ml/minute for 2 minute) of 0.1 normal hydrochloric acid. This response was mimicked by intraduodenal infusion of the sensory nerve-end stimulant, phenyl-biguanide. In contrast, these acid-induced pyloric phasic and tonic pressure events were blocked by an intraduodenal infusion of 2% xylocaine and intravenous or close intra-arterial injection of atropine or hexamethonium. Bilateral cervical vagotomy had no effect on these pyloric responses.
3.6 Synthesis: Paths of Duodenal Chemoreceptor Control of Antral and Pyloric Motor Function During Normal Nutrient Processing
Though the data summarized above on the pathways of duodenal chemoreceptor control of antral motor function are conflicting, the weight of the evidence suggests that the vagi are the main signaling pathway through which duodenal chemoreceptors modulate antral motility via a vago-vagal pathway.
Data on the pylorus point overwhelmingly to duodenal chemoreceptors stimulating the pylorus via ascending duodenal intramural nerves. Pharmacological data, mainly from anesthetized dogs, are consistent with this pathway being a chain of orally projecting cholinergic nerves.
The existence of a rich extrinsic innervation in the stomach and pylorus [4] is a reminder that the vagus can influence gastric motor function as well as secretion. The retardation of emptying by truncal vagotomy [5] mentioned in Sect. 3.3 (Fig. 3.1) was probably due to interruption of descending vagal nerves that modulate the spatial patterning of gastric motor function. These extrinsic nerves enter the wall of the stomach and then project intramurally along the antrum. Studies in pigs have shown that division of these nerves by antral transection with reanastomosis retards gastric emptying by causing earlier-than-normal closure of the pyloric ring during antro-pyloric contractions, with reduction of the volume of flow pulses delivered into the duodenum [20].
The modulation of motor mechanisms by duodenal chemoreceptors that occurs within seconds, and the data from physical and pharmacological interventions on duodenal intramural nervous pathways indicate that neural pathways are of paramount importance for driving duodenal chemoreceptor–induced modulations of antro-pyloric motor function. This does not exclude the possibility that hormone release can, in certain circumstances, also play a modulatory role.
3.7 Duodenal Chemoreceptors and the Duodenal Brake Mechanism
The term “duodenal brake” appears to have first been used by Shadidullah et al. in 1975 [5], but this term was then used to refer to the ability of duodenal luminal receptors to slow gastric emptying, through undefined motor mechanisms. Rao et al. have revived and extended the concept of a “duodenal brake” with limited fluoroscopic data that indicate that there is a motor mechanism, which can prevent outflow of duodenal content into the proximal jejunum [23].
In a recent manometric study summarized below, we have described a specialized zone of motor function, which extends from the very distal duodenum into the proximal jejunum; we have proposed that this is the brake mechanism. Furthermore, we suggest that this mechanism plays a significant role in the control of normal nutrient emptying.
3.7.1 Fluoroscopic Demonstration of the Duodenal Brake
Fluoroscopic observations in healthy subjects by Rao et al. (1996) have revealed a motor mechanism at the very distal duodenum of healthy subjects, which causes closure of the extreme distal duodenal lumen [23]. This occlusion was triggered by an intraduodenal infusion of the lipid-rich sodium oleate solution made radio-opaque with a barium suspension. The duodenal closure developed within about 15 seconds of starting the 1 minute 0.33 ml/second infusion (Fig. 3.5). Control normal saline/barium duodenal infusions passed freely into the jejunum.
This shows simultaneous fluoroscopic imaging and manometry of the duodenum. The brake was stimulated with sodium oleate mixed with a 20% barium suspension. Fluoroscopy showed occlusion of the most distal part of the duodenum for the entire 52 seconds of fluoroscopic observation: the infusate remained within the duodenal loop. The right-hand panel displays concurrently recorded duodenal loop pressures, but the most aborad sensor was not within the occluded distal duodenum/proximal jejunum. (Reproduced with permission from Ref. [23])
The above duodenal infusates were probably supraphysiological and so, activation of the duodenal brake could have been either due to a mechanism normally active during gastric emptying of nutrient or to a duodenal “alarm” response to a pathologically intense stimulus.
3.7.2 Manometric Definition of the Duodenal Brake
The study of Rao et al. [23] included measurements from several duodenal manometric sensors, but all of these were positioned well orad of the duodenal brake zone defined on fluoroscopy. In a study of healthy subjects, we have used a high-resolution, fiber-optic manometric catheter incorporating 72 sensors, each spaced at 1 cm intervals [24]. This had been modified especially for upper gastrointestinal intubation [25] and enabled sensors to be placed into the region of the duodenal brake, allowing for the first detailed manometric recordings of fed-state pressure patterns within the region. After a period of fasting recordings, fed-state pressure patterns were recorded for at least an hour after ingestion of a 200 ml, 480 kcal nutrient drink.
Fed-state recordings
In all 15 subjects, the most aborad pressure sensor was beyond the duodeno-jejunal flexure, and in 12, it was at least 15 cm beyond this landmark. Thus, the zone of the duodenal brake identified by Rao et al. [24] was well encompassed. After ingestion of the nutrient drink, each subject showed development of a sharp transition of pressure patterns at a mean of 18.8 ± 3.7 cm (range 13–28 cm) aborad from the pylorus [25] (Fig. 3.6). Beyond this transition point, the dominant motor pattern was regular, nonpropagating pressure events at 11.5 ± 0.5 cycles per minute, which extended to the end of the recording sensors in all subjects, in some cases for more than 20 cm aborad from the transition point. We named this motor pattern the duodeno-jejunal complex (DJC).
The upper panel (a) shows a 22-minute period of very intense fed-state duodeno-jejunal complex (DJC) activity, which emphasizes the existence of the transition point between the differing motor patterns in the duodenal loop and duodeno-jejunal regions. The lower panel (b) shows a very time-expanded small section of panel A. This shows the nonpropagated obstructive pattern of DJC activity, which extends well into the proximal jejunum. (Reproduced with permission from Ref. [25])
Orad of the transition point, in the duodenal loop, pressure events were less frequent and dominated by aborad-propagated events at a rate of 4–6/minute, more than half of which travelled more than 10 cm along the duodenal loop region (Figs. 3.6 and 3.7). These data show that the duodenum and upper jejunum have two major zones from the perspective of fed-state motor function: the zone between the pylorus and transition point has been named the duodenal loop (DL) region and that aborad from the transition the duodeno-jejunal (DJ) region [25].
The top panel (a) shows a 26-minute period of fed-state clustered DJC activity. The two panels below are time-expanded tracings (see time-base at the bottom of each panel). The left panel (b) mainly shows pressures aborad of the transition point. The right panel (c) is entirely from the duodenal loop region. Most of the very long segment, relatively slowly propagated antro-duodenal pressure events travel to the transition point. (Reproduced with permission from Ref. [25])
Typically, vigorous DJC activity started within 90 seconds after complete ingestion of the nutrient drink. In some subjects, DJC activity occurred in clusters, the onsets of which were usually associated with pressure events that propagated from the antrum across the pylorus deep into the DL region (Fig. 3.7), a pattern previously shown to propel a pulse of gastric content into the duodenum [8]. In all subjects, DJC activity remained prominent for the hour of fed-state recordings.
Fasting recordings
Review of the period of fasting recording immediately before ingestion of the nutrient drink also revealed a less obvious transition point of motor function in the same position as in the fed state (Fig. 3.8). Aborad of the transition, there was short-extent, low-amplitude DJC activity, which usually occurred in brief clusters, associated with just-prior groups of vigorous, propagated phase II antro-duodenal pressure events, most of which extended more than 10 cm into the duodenal loop region (Fig. 3.8). These events are known to empty pulses of acid into and along the duodenum [26], suggesting that fasting DJC activity is driven at least in part by episodic entry of presumably small volumes of acid into the duodenum during the highly expulsive Phase II interdigestive motor activity. The right-hand panel of Fig. 3.8 shows a period of fed-state recording in the same subject for comparison.
The two panels show fasting and fed-state recordings from the same subject. The left-hand panel shows three main clusters of fasting DJC activity, following a series of phase II antro-duodenal pressure events. Following the nutrient drink (right-hand panel), DJC activity is much more sustained and vigorous. (Reproduced with permission from Ref. [25])
3.8 Synthesis: Interpretation of Duodeno-Jejunal Complex Activity
3.8.1 Spatial Correlation of DJC Activity
An abdominal X-ray at the end of recordings described above defined the positions of the radio-opaque fiber-optic sensors, so the position of the transition point of motor function at the orad margin of DJC activity could be accurately located in each subject. This position was consistent with the localization of the duodenal brake by Rao et al. [23]
3.8.2 The Mechanical Outcome of DJC Activity
The repetitive, nonperistaltic, and high-frequency pattern of DJC pressure events is consistent with it impeding luminal flow (Fig. 3.6). The pattern of DJC activity resembles phase III of the migrating motor complex, except it is somewhat “ragged,” originates close to the duodeno-jejunal flexure, and does not propagate aborad along the duodenum and jejunum in a stereotyped pattern. Correlation of fluoroscopy with manometry by Schemann and Ehrlein (1986) has shown that the segment of small intestine involved in phase III activity of the migrating motor complex is occluded [27].
3.8.3 Entry of Chyme into the Duodenum Stimulates DJC Activity
The intensity, prevalence, and distal extent of DJC activity were strikingly greater in the fed state compared to fasting, consistent with their stimulation by chyme. As described above, clusters of DJC activity were usually closely preceded by expulsive antro-duodenal pressure events both during fasting and after the nutrient drink was consumed (Figs. 3.7 and 3.8).
3.8.4 Physiological Significance of DJC Activity
The considerable DJC activity that occurred during emptying of a very modest volume of highly nutrient liquid indicates that DJC activity is a physiological mechanism, active during normal gastric emptying. An obvious next step in researching DJC activity would be to determine directly whether and when this motor activity prevents duodenal outflow and whether this is stimulated in a dose-related fashion by graded rates of delivery of provocative solutions into the duodenum. Such studies could be done in animals.
We have proposed [25] that the retention of content within the duodenum, along with the mixing duodenal loop contractions [23], ensures that the normal gastric, duodenal mucosal, hepatic, and pancreatic secretions that all come together in the duodenal loop (the most complex digestive region of the gut) are well mixed with chyme. We further propose that once the duodenal chemoreceptors detect adequate chemical processing of the duodenal content, the stimulus for DJC activity subsides, allowing emptying into the jejunum.
3.8.5 Pathways of Stimulation of DJC Activity
Assuming that DJC activity is a basic physiological mechanism present in animals other than humans, the pathways that control it could be studied directly in acute experiments with techniques similar to those used by Allescher et al. in dogs [21] (Fig. 3.4). The clustered patterning of DJC activity in close temporal association with propagated expulsive antro-duodenal pressure events is consistent with aborad signaling from duodenal chemoreceptors to the duodenal brake region on a second-by-second basis. Neural signaling is the only feasible mechanism.
Given the data that support signaling from duodenal chemoreceptors via ascending intramural nerve pathways to the pylorus discussed in Sect. 3.6, by far the most likely neural signaling pathway would seem to be descending intramural nerves to the region of the duodenal brake.
3.9 Potential Clinical Significance of the Duodenal Brake
This can only be a “blue sky” commentary, given the limited data available. An abnormally overactive duodenal brake has the potential to hold the normal upstream motor mechanisms that control gastric emptying to ransom. Duodenal dilatation activates localized pyloric contractions and inhibition of antral contractions, with attendant restriction of gastric outflow [19]. Abnormal retention of content in the duodenum could also cause persistent stimulation of duodenal chemoreceptors. The other side of the coin, a “lazy” duodenal brake mechanism, could allow premature release of the duodenal content into the jejunum, with attendant symptoms due to jejunal maldigestion.
By determining the rate of emptying of carbohydrates into the duodenum, duodenal and probably jejunal chemoreceptors also influence glucose tolerance. A high sugar and fat diet causes hypertrophy of the duodenal mucosa in rats with an unusually high density of enterochromaffin cells, in association with development/worsening of diabetes [28]. Similar duodenal mucosal and small intestinal mucosal hypertrophy has also been reported in diabetic patients compared to healthy controls [28]. Furthermore, when the duodenum (and its chemosensors) is bypassed surgically in patients with type 2 diabetes and nonalcoholic liver disease, blood glucose control and measures of liver disease activity have improved [28].
Van Baar et al. (2020) [29] report that endoscopic hydrothermal ablation of all of the duodenal mucosa aborad of the sphincter of Oddi in 37 poorly-controlled type 2 diabetic patients was associated with clinically valuable improvements of measures of blood glucose control and blood levels of markers of nonalcoholic fatty liver disease (p < 0.001). These effects persisted at 12 months. Unfortunately, this study did not include any measurements of motor function or detailed reporting of duodenal mucosal histology. These remarkable results emphasize the importance of working toward better understanding of duodenal chemoreceptor signaling pathways.
References
Iwasaki M, Akiba Y, Kaunitz JD (2018) Duodenal chemosensing. Curr Opin Gastroenterol 34:422–427
Bellono NW, Bayrer JR, Leitch DB et al (2017) Enterochromaffin cells are gut Chemosensors that couple to sensory neural pathways. Cell 170(185–198):e16
Iggo A (1957) Gastric mucosal chemoreceptors with vagal afferent fibres in the cat. Q J Exp Physiol Cogn Med Sci 42:398–409
Goyal RK, Guo Y, Mashimo H (2019) Advances in the physiology of gastric emptying. Neurogastroenterol Motil 31:e13546
Shahidullah M, Kennedy TL, Parks TG (1975) The vagus, the duodenal brake, and gastric emptying. Gut 16:331–336
Azpiroz F, Malagelada JR (1986) Vagally mediated gastric relaxation induced by intestinal nutrients in the dog. Am J Phys 251:G727–G735
Fone DR, Akkermans LM, Dent J et al (1990) Evaluation of patterns of human antral and pyloric motility with an antral wall motion detector. Am J Phys 258:G616–G623
Anvari M, Dent J, Malbert C et al (1995) Mechanics of pulsatile transpyloric flow in the pig. J Physiol 488(Pt 1):193–202
Tougas G, Anvari M, Dent J et al (1992) Relation of pyloric motility to pyloric opening and closure in healthy subjects. Gut 33:466–471
Heddle R, Dent J, Toouli J et al (1988) Topography and measurement of pyloric pressure waves and tone in humans. Am J Phys 255:G490–G497
Houghton LA, Read NW, Heddle R et al (1988) Motor activity of the gastric antrum, pylorus, and duodenum under fasted conditions and after a liquid meal. Gastroenterology 94:1276–1284
Houghton LA, Read NW, Heddle R et al (1988) Relationship of the motor activity of the antrum, pylorus, and duodenum to gastric emptying of a solid-liquid mixed meal. Gastroenterology 94:1285–1291
Heddle R, Collins PJ, Dent J et al (1989) Motor mechanisms associated with slowing of the gastric emptying of a solid meal by an intraduodenal lipid infusion. J Gastroenterol Hepatol 4:437–447
Heddle R, Dent J, Read NW et al (1988) Antropyloroduodenal motor responses to intraduodenal lipid infusion in healthy volunteers. Am J Phys 254:G671–G679
Fraser R, Fone D, Heddle R et al (1992) Stimulation of pyloric contractions by intraduodenal triglyceride is persistent and sensitive to atropine. J Gastroenterol Hepatol 7:563–568
Heddle R, Fone D, Dent J et al (1988) Stimulation of pyloric motility by intraduodenal dextrose in normal subjects. Gut 29:1349–1357
Fone DR, Horowitz M, Dent J et al (1989) Pyloric motor response to intraduodenal dextrose involves muscarinic mechanisms. Gastroenterology 97:83–90
Treacy PJ, Jamieson GG, Dent J (1994) Pyloric motility and liquid gastric emptying during barostatic control of gastric pressure in pigs. J Physiol 474:361–366
Treacy PJ, Jamieson GG, Dent J (1996) The effect of duodenal distention upon antro-pyloric motility and liquid gastric emptying in pigs. ANZ J Surg 66:37–40
Anvari M, Yu P, Dent J et al (1995) Role of antral intramural neural pathways in control of gastric emptying in the pig. J Physiol 488(Pt 1):203–209
Allescher HD, Daniel EE, Dent J et al (1988) Extrinsic and intrinsic neural control of pyloric sphincter pressure in the dog. J Physiol 401:17–38
Allescher HD, Daniel EE, Dent J et al (1989) Neural reflex of the canine pylorus to intraduodenal acid infusion. Gastroenterology 96:18–28
Rao SS, Lu C, Schulze-Delrieu K (1996) Duodenum as a immediate brake to gastric outflow: a videofluoroscopic and manometric assessment. Gastroenterology 110:740–747
Dinning PG, Wiklendt L, Maslen L et al (2014) Quantification of in vivo colonic motor patterns in healthy humans before and after a meal revealed by high-resolution fiber-optic manometry. Neurogastroenterol Motil 26:1443–1457
Dent J, Deloose E, Dinning P et al (2020) Manometric demonstration of duodenal/jejunal motor function consistent with the duodenal brake mechanism. Neurogastroenterol Motil 32:e13835
Dent J, Chir B (1976) A new technique for continuous sphincter pressure measurement. Gastroenterology 71:263–267
Schemann M, Ehrlein H (1986) Mechanical characteristics of phase II and phase III of the interdigestive migrating complex in dogs. Gastroenterology 91:117–123
Cherrington AD, Rajagopalan H, Maggs D et al (2017) Hydrothermal duodenal mucosal resurfacing: role in the treatment of metabolic disease. Gastrointest Endosc Clin N Am 27:299–311
van Baar ACG, Holleman F, Crenier L et al (2020) Endoscopic duodenal mucosal resurfacing for the treatment of type 2 diabetes mellitus: one year results from the first international, open-label, prospective, multicentre study. Gut 69:295–303
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Additional information
Dedication
JD dedicates this chapter to Edwin (“Ed”) E Daniel, the most scientifically omnivorous and inquisitive person he has ever had the good fortune to work with.
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Dent, J., Dinning, P.G. (2022). Luminal Chemoreceptors and Intrinsic Nerves: Key Modulators of Digestive Motor Function. In: Spencer, N.J., Costa, M., Brierley, S.M. (eds) The Enteric Nervous System II. Advances in Experimental Medicine and Biology, vol 1383. Springer, Cham. https://doi.org/10.1007/978-3-031-05843-1_3
Download citation
DOI: https://doi.org/10.1007/978-3-031-05843-1_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-05842-4
Online ISBN: 978-3-031-05843-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)