Abstract
Patients with locally advanced or metastatic disease account for the majority of those with newly diagnosed pancreatic cancer. Treatment of both is palliative in nature, and cytotoxic chemotherapy continues to be the only treatment that offers a clear survival benefit. Most oncologists utilize a strategy of induction 5-fluorouracil-(5-FU-) or gemcitabine-based chemotherapy in patients with locally advanced disease, owing to the high likelihood of occult metastatic disease and lack of local therapies that improve survival. However, the role of radiotherapy in locally advanced pancreatic cancer remains an area of interest and investigation. Conversion to surgical resectability is uncommon in the setting of locally advanced disease, but resection should be considered by experienced surgeons for patients responding to chemotherapy, with or without radiation. In the metastatic setting, the development of combination chemotherapy regimens such as gemcitabine + nab-paclitaxel and 5-fluorouracil + folinic acid + irinotecan + oxaliplatin (FOLFIRINOX) has modestly improved patients’ life expectancy. The recent approval for the PARP inhibitor, olaparib, as a maintenance therapy for patients with germline BRCA1/2 mutations marks the first biomarker-based approved therapy in pancreatic cancer and has fueled optimism for other targeted therapies. Of note, targeted therapy in unselected patients has not shown clinically significant benefits. To date, immunotherapy has not been effective outside of the small minority with microsatellite instability, but attempts to modulate the pancreatic tumor microenvironment may open the door for future success. Finally, supportive care should not be overlooked as a vital component of managing patients with advanced pancreatic cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Locally advanced pancreatic cancer
- Metastatic pancreatic cancer
- Chemotherapy
- Chemoradiotherapy
- Targeted therapy
- Supportive care
Introduction
Up to 85% of patients with pancreatic cancer have unresectable disease at the time of diagnosis, either due to local tumor invasion or metastatic spread. As is the case for many other solid tumors, cytotoxic chemotherapy remains the mainstay of treatment for this group of patients. Goals of systemic therapy include prolongation of survival and palliation of symptoms. Occasionally, patients with locally advanced disease may become eligible for surgical resection after initial systemic therapy. Improvements in chemotherapy regimens over the past decade have led to important, albeit modest, gains in the survival of patients with advanced pancreatic cancer. Below we will review treatment approaches from the medical oncology perspective for patients with locally advanced and metastatic disease.
Locally Advanced Disease
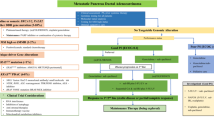
Approximately one-third of patients present with unresectable, locally advanced disease at the time of diagnosis with pancreatic cancer. While several definitions of the stages of resectability have been developed over the past decade and a half, each centers on the relationship between the tumor and surrounding vasculature [1,2,3]. The MD Anderson Criteria for resectability for pancreatic cancer define locally advanced disease as tumors that either encase the superior mesenteric artery, the celiac axis, or hepatic artery without a reconstructive option or occlude the superior mesenteric or portal veins without a reconstructive option (Fig. 9.1) [2].
Given the contraindication to surgery and the high probability of occult metastatic disease in this population, systemic chemotherapy is the recommended first-line treatment in patients with locally advanced pancreatic cancer (LAPC). The GERCOR group retrospectively analyzed patients with LAPC treated in their trials with first-line chemotherapy, which was either 5-fluorouracil (5-FU) + leucovorin + gemcitabine or gemcitabine + oxaliplatin [4]. They found that 29% of patients developed disease progression during the first 3 months of chemotherapy, a subset that had a median overall survival of 4.5 months. This data highlights the heterogeneity in aggressiveness of LAPC. Upfront systemic therapy identifies patients with particularly aggressive biology as those who would not benefit from local therapy such as radiation.
Guidelines on the optimal initial chemotherapy regimen for patients with LAPC rely primarily on data from patients with metastatic disease. Combination regimens such as FOLFIRINOX and gemcitabine + nab-paclitaxel are frequently utilized in this setting, owing to their proven efficacy in phase 3 studies of patients with metastatic pancreatic cancer [5, 6]. A 2016 systematic review evaluated the role of FOLFIRINOX in LAPC in 315 patients across 11 studies [7]. The authors reported a median overall survival of 24.2 months (95% CI 21.7–26.8) and median progression-free survival (PFS) of 15.0 months (95% CI 13.8–16.2). The pooled proportion of patients with LAPC who underwent resection was 25.9%, with 78.4% having an R0 (microscopically negative) resection.
Gemcitabine-based combination regimens also have limited data in the locally advanced setting. A phase 2 study in Austria evaluated gemcitabine + oxaliplatin as “neoadjuvant” therapy in patients with LAPC [8]. Of the 33 patients in their study, 13 underwent resection with a 69% R0 resection rate. The patients who underwent resection had a median OS of 22 months, compared to 12 months for those who did not undergo resection. An important caveat to these results is that 15 of the 33 patients were considered to have borderline resectable disease at the time of diagnosis when evaluated by centralized imaging review. The results of the multicenter phase 2 LAPACT trial which evaluated the combination of induction gemcitabine + nab-paclitaxel in patients with LAPC were reported in 2018 [9]. One hundred seven patients were included in the study, and the median PFS was 10.2 months. Neutropenia was the most common (42%) grade 3 or 4 adverse event, but the authors found the regimen to be largely tolerable. Sixteen patients (15%) in this study underwent surgical resection with 7 having an R0 resection.
For patients who are not eligible for combination chemotherapy regimens due to comorbidities or poor performance status, single agent gemcitabine is a reasonable alternative. Evidence for the use of gemcitabine monotherapy in this setting is largely extrapolated from older clinical trials that included patients with LAPC and metastatic disease [10,11,12]. Results published from the LAP07 trial, which studied the benefit chemoradiation compared with continuation of chemotherapy in patients with LAPC, demonstrated that induction chemotherapy with single agent gemcitabine resulted in a median overall survival of 13.6 months [13]. Radiotherapy with or without concurrent chemotherapy is another first-line option for patients who cannot tolerate combination chemotherapy, an approach that will be discussed later.
Despite improvements in response rates with combination chemotherapy, most patients who are initially considered to have LAPC never become candidates for curative surgical resection. However, patients who demonstrate response to induction systemic therapy without the development of overt metastatic disease should be re-evaluated for the possibility of resection by an experienced multi-disciplinary team. This is an approach supported by both the National Comprehensive Cancer Network (NCCN) and the American Society of Clinical Oncology (ASCO) [14, 15]. A study of 415 patients with LAPC treated at Johns Hopkins from 2013 to 2017 found that 84 (20%) underwent surgical resection after a median of 5 months of pre-operative therapy. Median OS was higher in the resected cohort at 35.3 months, compared to 16.3 months in the non-resected patients. They also found that the use of FOLFIRNOX and stereotactic body radiation therapy (SBRT) was associated with an increased probability of surgical resection.
The German phase 2 NEOLAP study randomized 130 LAPC patients at 33 different institutions who had not progressed on 2 initial cycles of gemcitabine + nab-paclitaxel to either continue the doublet for 2 more cycles or switch to 4 cycles of FOLFIRINOX [16]. The study’s results, presented in 2019, included a primary endpoint of conversion rate to resectability. The conversion rate in the gemcitabine + nab-paclitaxel group was 30.6%, compared to 45.0% in the FOLFIRINOX group (p = 0.135). Median OS was not different between the two chemotherapy arms, but conversion to resectability was associated with an improved median OS (27.4 vs 14.2 months, p = 0.0035).
Determining a patient’s potential for downstaging and future resectability at the time of diagnosis with LAPC is very challenging. An attempt has been made by clinicians at the Medical College of Wisconsin to categorize LAPC patients into “type A” (potentially resectable) or “type B” (very likely not resectable) based on vascular involvement and the potential for surgical resection after neoadjuvant therapy [17]. An analysis of 108 consecutive patients with LAPC who were categorized into type A or B under this schema found that 62% of type A and 24% of type B patients underwent surgical resection [18]. The authors concluded that such a classification may help establish appropriate expectations and goals of care with patients.
Patients who continue to have stable but unresectable disease after induction chemotherapy of 4 to 6 months should be considered for either continuation of systemic therapy, a treatment break, or consolidative chemoradiotherapy. This decision should be made taking into account the patient’s goals of care, performance status, and symptoms. ASCO and NCCN guidelines recommend observation for this population in the absence of a clinical trial. For patients who are treated with chemoradiotherapy, there may be some benefit to maintenance systemic chemotherapy if their disease continues to be localized [19].
The role of radiation therapy in locally advanced disease will be discussed in greater depth later in this chapter. For LAPC patients who are treated with radiation, the addition of concurrent chemotherapy as a sensitizing agent has become commonplace. A 2009 systematic review analyzed the benefits of chemoradiotherapy in 21 published studies and concluded that chemoradiotherapy increases OS compared to radiotherapy alone, at the cost of higher toxicity [20]. The SCALOP trial was a randomized phase 2 study comparing gemcitabine-based chemoradiotherapy to capecitabine-based chemoradiotherapy for patients who had not developed disease progression during 12 weeks of induction chemotherapy with the combination of gemcitabine and capecitabine [21]. Seventy-four LAPC patients were ultimately randomized to one of the chemoradiotherapy groups in the study, and median OS was improved with capecitabine compared to gemcitabine (15.2 vs 13.4 months, hazard ratio [HR]: 0.39, p = 0.012). The adverse effect profile also favored capecitabine-based chemoradiotherapy, though quality of life scores were not different between the arms.
Metastatic Disease
The medical management of a patient with metastatic pancreatic cancer is frequently complex and often requires attention to pain control, thromboembolic disease, biliary or gastrointestinal obstruction, infection, pancreatic exocrine insufficiency, and anorexia/weight loss, in addition to side effects from chemotherapy (Fig. 9.2). However, administration of chemotherapy has been shown to have the dual benefit of prolonging survival and improving quality of life [22, 23]. The two most commonly utilized first-line chemotherapy regimens are FOLFRINOX and gemcitabine + nab-paclitaxel (Table 9.1).
FOLFIRINOX was established as a standard-of-care first-line regimen based on data from the large phase 3 study conducted by Conroy et al. comparing the combination with gemcitabine monotherapy, the previous standard-of-care [5]. A total of 342 patients with metastatic pancreatic cancer were randomized to either FOLFIRINOX or gemcitabine. Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 and adequate liver and renal function. Compared to gemcitabine, FOLFIRINOX improved median OS (11.1 vs 6.8 months, HR, 0.57, p < 0.001). The FOLFIRINOX arm also demonstrated an improved objective response rate (31.6% vs 9.4%) and median PFS (6.4 vs 3.3 months, HR, 0.47, p < 0.001). Importantly, rates of grade 3 or 4 adverse events were higher in the FOLFIRINOX group, including neutropenia (45.7% vs 21.0%) and diarrhea (12.7% vs 1.8%).
Two years after the data on FOLFIRINOX was published, the results of the phase 3 MPACT trial were published, comparing gemcitabine plus albumin-bound paclitaxel (nab-paclitaxel) to gemcitabine monotherapy in 861 patients. Similar to FOLFIRINOX, this doublet improved median OS compared to gemcitabine (8.5 vs 6.7 months, HR, 0.72, p < 0.001). Median PFS was 5.5 months with gemcitabine + nab-paclitaxel compared to 3.7 months for the single agent (HR: 0.69, p < 0.001). As expected, grade 3 or 4 adverse effects were higher in the doublet arm including neutropenia (38% vs 27%), fatigue (17% vs 7%), and neuropathy (17% vs 1%).
The selection of which first-line chemotherapy, if any, to utilize depends on patient comorbidities and performance status. For patients with an excellent performance status, either regimen is appropriate. Most clinicians consider gemcitabine + nab-paclitaxel to be a more tolerable therapy and may recommend the doublet for a patient with a borderline performances status. Single agent gemcitabine represents another option for patients whose performance status or comorbidities preclude a combination regimen [10, 24]. Comorbid conditions such as pre-existing peripheral neuropathy may necessitate treatment that omits platinum or taxanes, such as FOLFIRI or gemcitabine alone. Additionally clinicians should be cautious about prescribing irinotecan and potentially gemcitabine in patients with hepatic impairment. Despite a cutoff of 75 years in the pivotal 2011 study leading to the approval of FOLFIRINOX, age, alone, should not necessarily dictate which first-line chemotherapy is selected, as performance status is often a better predictor of tolerability [25].
Patients who maintain an ECOG performance status of ≤2 may be considered for second-line therapy. NAPOLI-1 was a phase 3 study of second-line therapy in 417 patients with metastatic pancreatic cancer whose disease had progressed on gemcitabine-based chemotherapy [26]. Patients were randomized to one of the three arms: (1) 5-FU + leucovorin, (2) nanoliposomal irinotecan, or (3) combination of 5-FU + leucovorin + nanoliposomal irinotecan. The primary endpoint, median OS, in the 5-FU + leucovorin + nanoliposomal irinotecan group was 6.1 months compared to 4.2 months in the 5-FU + leucovorin group (HR: 0.67, p = 0.012). These results led to the approval of 5-FU + leucovorin + nanoliposomal irinotecan as a second-line regimen for gemcitabine-refractory patients in 2015. For patients who were treated with first-line 5-FU-based therapy, including FOLFIRINOX, gemcitabine + nab-paclitaxel represents a reasonable option for eligible patients, despite limited prospective data [27, 28]. Many patients who progress on first-line therapy are not candidates for further systemic therapy and should be managed with best supportive and/or hospice care.
Incorporation of targeted therapies into the armamentarium of pancreatic cancer treatment has largely been ineffective. A mutation in the KRAS gene is an almost universal finding in pancreatic cancers, and its subsequent activation is a well-described driver of pancreatic tumor development [29,30,31,32]. Despite decades of effort by researchers from the bench to clinic, the discovery of an effective therapy to target this crucial oncogene has been elusive thus far [33, 34]. Downstream to KRAS are potential therapeutic targets including mTOR, RAF, MEK, though inhibition of these proteins in pancreatic cancer patients has not demonstrated clinical efficacy in multiple clinical trials [35,36,37,38,39]. The addition of monoclonal antibodies targeting VEGF and EGFR to gemcitabine chemotherapy has similarly shown no benefit in large phase 3 clinical trials [40, 41]. One notable exception is erlotinib, an oral tyrosine kinase inhibitor with activity against EGFR, which demonstrated a statistically significant improvement in OS when added to gemcitabine compared to gemcitabine alone in a randomized phase 3 study [42]. However, erlotinib is seldom utilized in clinical practice as its addition to gemcitabine improved median OS by fewer than 2 weeks, and it is associated with non-trivial toxicities.
One targeted strategy with recent therapeutic success aims to exploit aberrancies in DNA damage repair pathways. While germline mutations in BRCA1 and BRCA2 are found in only up to 7%, broadening the umbrella of DNA damage repair mutations to include genes such as PALB2, ATM, RAD51, and CHEK1/2 may increase this population to almost a quarter of patients with pancreatic cancer [30, 43,44,45,46,47,48]. Patients with aberrant DNA damage repair are more susceptible to DNA-damaging therapies, including platinum chemotherapy as well as radiation. More recently, the development of inhibitors of poly(adenosine diphosphate-ribose) polymerase (PARP), a crucial component of the homologous recombination pathway for single-strand DNA breaks, has offered a novel therapeutic agent. Phase 2 studies of the PARP inhibitors olaparib and rucaparib as single agents have shown promising activity in pre-treated pancreatic cancer patients with germline or somatic BRCA1 and BRCA2 mutations [49, 50]. The phase 3 POLO trial randomized 154 metastatic pancreatic cancer patients with germline BRCA1 or BRCA2 mutations whose disease had not progressed on at least 16 weeks of first-line platinum-based chemotherapy to either maintenance placebo or olaparib [51]. The median PFS in the olaparib group was significantly longer (7.4 vs 3.8 months, HR: 0.53, p = 0.004). At the interim analysis, there was no difference in overall survival between the groups. In December 2019, olaparib was granted FDA approval for this indication, marking the first biomarker-based targeted therapy approved for pancreatic cancer [52]. Another rare but clinically significant molecular aberration is a fusion in the NTRK gene, which occurs in up to 1% of patients with pancreatic cancer and has been demonstrated in case reports to be a clinically significant target with the inhibitor, entrectinib [53]. In 2019, the FDA approved entrectinib as a tumor-agnostic treatment for patients with advanced solid tumors harboring NTRK fusions [54]. Tumor molecular profiling that includes evaluation for NTRK gene fusions should be considered for patients with advanced pancreatic cancer.
Over the past several years, immunotherapy has completely changed the treatment landscape in a number of malignancies including melanoma, non-small cell lung cancer, and genitourinary cancer. Unfortunately, most patients with gastrointestinal cancers have not reaped these benefits. Pancreatic cancer, in particular, has repeatedly failed to demonstrate response to single agent immune checkpoint inhibition outside of the approximately 1% of patients whose tumors harbor mutations in mismatch repair proteins or have high microsatellite instability (MSI-H) [55,56,57,58,59]. It has been hypothesized that immunotherapy in pancreatic cancer fails based on the presence of an immunologically “cold,” immunosuppressive, tumor microenvironment with abundant tumor-associated macrophages, regulatory T cells, cancer-associated fibroblasts, and myeloid-derived suppressor cells [60,61,62]. Clinical studies aiming to introduce cytotoxic lymphocytes or target these immunosuppressive components of the pancreatic tumor microenvironment in order to enhance the activity of both chemotherapy and immune checkpoint inhibitors are ongoing (e.g. NCT03336216, NCT02588443).
The treatment of patients with advanced pancreatic care invariably requires clinicians to incorporate supportive care. Common symptoms include pain, nausea, diarrhea, depression, anxiety, anorexia, and weight loss. Early recruitment of supportive or palliative care specialists to the treatment team can reduce the complexity of management by oncologists and has been demonstrated to decrease the aggressiveness of care near the time of death [63]. Pain is a near universal symptom, and its etiology is often multifactorial owing to local invasion into the celiac plexus or effects of distant metastases. Opioid-based therapy is the recommended treatment of cancer-related pain, but interventions such as celiac plexus neurolysis are also commonly utilized [64]. Biliary obstruction is another common effect of pancreatic cancer that can lead to infection and often precludes adequate delivery of chemotherapy. When required, biliary stenting with metal rather than plastic stents tends to improve stent patency and lowers infection risk [65]. Antidepressants, appetite stimulants, antiemetics, and pancreatic enzyme replacements are all part of the armamentarium of clinicians and should be considered for appropriate patients.
Approach to LAPC at MD Anderson
While an individualized approach is necessary for patients with locally advanced, unresectable disease, the general approach at MD Anderson Cancer Center is to begin with systemic therapy. FOLFIRINOX is typically given to patients with good performance status and no contraindications to treatment with oxaliplatin, such as pre-existing peripheral neuropathy. For those patients with frailty or comorbidities, gemcitabine and nab-paclitaxel given once every 2 weeks (rather than weekly × 3, every 28 days) are more commonly utilized. Restaging evaluations consisting of routine laboratory studies and serum tumor markers in addition to contrast-enhanced computed tomography of the chest, abdomen, and pelvis are performed every 2 months. If a patient develops metastatic disease or progression of the primary tumor with first-line chemotherapy, second line chemotherapy is usually recommended with efforts to enroll such patients on active clinical trials. For the subset of patients who have not progressed after 4–6 months of systemic therapy, referral to a radiation oncologist to consider consolidating radiation therapy is advised. Whenever possible, delivery of consolidating radiation is conducted in the context of a clinical trial. Importantly, the smaller subset of patients who have a clinical and radiographic response to chemotherapy with or without radiation at the primary tumor site without interval metastatic disease should be referred to an experienced surgical oncologist to consider surgical resection with curative intent. This is particularly true of those patients who have normal or near-normal serum tumor markers after systemic therapy ± radiation.
MD Anderson Approach to Metastatic Pancreatic Cancer
Combination chemotherapy is the most common recommendation for patients who present with metastatic disease. The choice of FOLFIRINOX or gemcitabine and nab-paclitaxel is based on the individual patient’s personal wishes, performance status, comorbidities, and frailty. FOLFIRINOX is usually preferred as first-line therapy for patients with ECOG performance status 0–1. Gemcitabine and nab-paclitaxel are more commonly offered to those patients with ECOG performance status 1–2. In some cases, only gemcitabine monotherapy should be considered, particularly for patients with frailty or those likely to experience increased toxicity with combination therapy (Table 9.2). When feasible, enrollment in a front-line clinical trial is considered, especially for patients with well-preserved performance status.
Whenever possible, germline genetic testing and molecular profiling of biopsy material are recommended to identify patients who may benefit from subsequent targeted therapy, most commonly olaparib for patients with germline BRCA mutations delivered as maintenance therapy. This is usually appropriate after 4–6 months of cytotoxic therapy with a platinum agent such as oxaliplatin. Clinical trial enrollment is considered for patients with progressive disease after front-line therapy who maintain good performance status. Importantly, attention to the results of germline testing and/or molecular profiling of biopsy material for enrollment in biomarker-specific clinical trials is strongly encouraged.
Conclusion
Patients with locally advanced or metastatic disease comprise the overwhelming majority of those with pancreatic cancer. Cytotoxic chemotherapy continues to be the only treatment that offers a clear survival benefit for this group of patients. The development of combination chemotherapy regimens over the past decade, particularly gemcitabine + nab-paclitaxel and FOLFIRINOX, has extended the life expectancy of most patients with advanced disease. While a small percentage of initially locally advanced pancreatic cancers may ultimately become surgically resectable, the goal of therapy in the vast majority of patients is palliative in nature. Improvements in biomarker selection and novel targeted agents have already begun to expand therapeutic options for a minority of patients such as those with germline BRCA1/2 mutations, and there is optimism for similar future successes in other pancreatic cancer patients. While immune checkpoint inhibitors largely have no role in pancreatic cancer, manipulation of the immunosuppressive tumor microenvironment appears necessary to unlock the therapeutic potential of immunotherapy in this disease. Finally, supportive care is a crucial, if underappreciated, component of care for patients with advanced pancreatic cancer.
References
Bockhorn M, Uzunoglu FG, Adham M, Imrie C, Milicevic M, Sandberg AA, et al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2014;155(6):977–88.
Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13(8):1035–46.
Abrams RA, Lowy AM, O’Reilly EM, Wolff RA, Picozzi VJ, Pisters PW. Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol. 2009;16(7):1751–6.
Huguet F, Andre T, Hammel P, Artru P, Balosso J, Selle F, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25(3):326–31.
Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25.
Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–703.
Suker M, Beumer BR, Sadot E, Marthey L, Faris JE, Mellon EA, et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. 2016;17(6):801–10.
Sahora K, Kuehrer I, Eisenhut A, Akan B, Koellblinger C, Goetzinger P, et al. NeoGemOx: gemcitabine and oxaliplatin as neoadjuvant treatment for locally advanced, nonmetastasized pancreatic cancer. Surgery. 2011;149(3):311–20.
Hammel P, Lacy J, Portales F, Sobrero AF, Cid RAP, Mozo JLM, et al. Phase II LAPACT trial of nab-paclitaxel (nab-P) plus gemcitabine (G) for patients with locally advanced pancreatic cancer (LAPC). J Clin Oncol. 2018;36(4_suppl):204.
Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–13.
Stathopoulos GP, Syrigos K, Aravantinos G, Polyzos A, Papakotoulas P, Fountzilas G, et al. A multicenter phase III trial comparing irinotecan-gemcitabine (IG) with gemcitabine (G) monotherapy as first-line treatment in patients with locally advanced or metastatic pancreatic cancer. Br J Cancer. 2006;95(5):587–92.
Herrmann R, Bodoky G, Ruhstaller T, Glimelius B, Bajetta E, Schuller J, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol. 2007;25(16):2212–7.
Hammel P, Huguet F, van Laethem JL, Goldstein D, Glimelius B, Artru P, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA. 2016;315(17):1844–53.
Network NCC. Pancreatic Adenocarcinoma (Version 1.2020). Available from: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic_blocks.pdf.
Balaban EP, Mangu PB, Khorana AA, Shah MA, Mukherjee S, Crane CH, et al. Locally advanced, unresectable pancreatic cancer: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2016;34(22):2654–68.
Kunzmann V, Algui H, Goekkurt E. Conversion rate in locally advanced pancreatic cancer (LAPC) after nab-Paclitaxel/Gemcitabine- or FOLFIRINOX-based induction chemotherapy (NEOLAP) - Final Results of a multicenter randomised Phase 2 AIO trial. ESMO 2019 Congress. 2019:Abstract 671O.
Evans DB, George B, Tsai S. Non-metastatic pancreatic cancer: resectable, borderline resectable, and locally advanced-definitions of increasing importance for the optimal delivery of multimodality therapy. Ann Surg Oncol. 2015;22(11):3409–13.
Chatzizacharias NA, Tsai S, Griffin M, Tolat P, Ritch P, George B, et al. Locally advanced pancreas cancer: staging and goals of therapy. Surgery. 2018;163(5):1053–62.
Mizrahi JD, Moningi S, Nogueras-Gonzalez GM, Wolff RA, Javle MM, Varadhachary GR, et al. Abstract B36: maintenance chemotherapy after chemoradiation in patients with locally advanced pancreatic cancer. Cancer Res. 2019;79(24 Suppl):B36–B.
Huguet F, Girard N, Guerche CS, Hennequin C, Mornex F, Azria D. Chemoradiotherapy in the management of locally advanced pancreatic carcinoma: a qualitative systematic review. J Clin Oncol. 2009;27(13):2269–77.
Mukherjee S, Hurt CN, Bridgewater J, Falk S, Cummins S, Wasan H, et al. Gemcitabine-based or capecitabine-based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): a multicentre, randomised, phase 2 trial. Lancet Oncol. 2013;14(4):317–26.
Yip D, Karapetis C, Strickland A, Steer CB, Goldstein D. Chemotherapy and radiotherapy for inoperable advanced pancreatic cancer. Cochrane Database Syst Rev. 2006;(3):CD002093.
Glimelius B, Hoffman K, Sjoden PO, Jacobsson G, Sellstrom H, Enander LK, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol. 1996;7(6):593–600.
Carmichael J, Fink U, Russell RC, Spittle MF, Harris AL, Spiessi G, et al. Phase II study of gemcitabine in patients with advanced pancreatic cancer. Br J Cancer. 1996;73(1):101–5.
Mizrahi JD, Rogers JE, Hess KR, Wolff RA, Varadhachary GR, Javle MM, et al. Modified FOLFIRINOX in pancreatic cancer patients age 75 or older. Pancreatology. 2020;20(3):501–4.
Wang-Gillam A, Li CP, Bodoky G, Dean A, Shan YS, Jameson G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387(10018):545–57.
Portal A, Pernot S, Tougeron D, Arbaud C, Bidault AT, de la Fouchardiere C, et al. Nab-paclitaxel plus gemcitabine for metastatic pancreatic adenocarcinoma after Folfirinox failure: an AGEO prospective multicentre cohort. Br J Cancer. 2015;113(7):989–95.
Mita N, Iwashita T, Uemura S, Yoshida K, Iwasa Y, Ando N, et al. Second-line gemcitabine plus nab-paclitaxel for patients with unresectable advanced pancreatic cancer after first-line FOLFIRINOX failure. J Clin Med. 2019;8(6):761.
Biankin AV, Waddell N, Kassahn KS, Gingras M-C, Muthuswamy LB, Johns AL, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491(7424):399–405.
Waddell N, Pajic M, Patch A-M, Chang DK, Kassahn KS, Bailey P, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518(7540):495–501.
Zeitouni D, Pylayeva-Gupta Y, Der CJ, Bryant KL. KRAS mutant pancreatic cancer: no lone path to an effective treatment. Cancers (Basel). 2016;8(4):45.
Collins MA, Bednar F, Zhang Y, Brisset J-C, Galbán S, Galbán CJ, et al. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J Clin Invest. 2012;122(2):639–53.
Cox AD, Fesik SW, Kimmelman AC, Luo J, Der CJ. Drugging the undruggable RAS: mission possible? Nat Rev Drug Discov. 2014;13(11):828–51.
Bournet B, Buscail C, Muscari F, Cordelier P, Buscail L. Targeting KRAS for diagnosis, prognosis, and treatment of pancreatic cancer: hopes and realities. Eur J Canc (Oxford, England: 1990). 2016;54:75–83.
Javle MM, Shroff RT, Xiong H, Varadhachary GA, Fogelman D, Reddy SA, et al. Inhibition of the mammalian target of rapamycin (mTOR) in advanced pancreatic cancer: results of two phase II studies. BMC Cancer. 2010;10:368.
Chung V, McDonough S, Philip PA, Cardin D, Wang-Gillam A, Hui L, et al. Effect of selumetinib and MK-2206 vs oxaliplatin and fluorouracil in patients with metastatic pancreatic cancer after prior therapy: SWOG S1115 study randomized clinical trial. JAMA Oncol. 2017;3(4):516–22.
Ko AH, Bekaii-Saab T, Van Ziffle J, Mirzoeva OM, Joseph NM, Talasaz A, et al. A multicenter, open-label phase II clinical trial of combined MEK plus EGFR inhibition for chemotherapy-refractory advanced pancreatic adenocarcinoma. Clin Cancer Res. 2016;22(1):61–8.
Infante JR, Somer BG, Park JO, Li C-P, Scheulen ME, Kasubhai SM, et al. A randomised, double-blind, placebo-controlled trial of trametinib, an oral MEK inhibitor, in combination with gemcitabine for patients with untreated metastatic adenocarcinoma of the pancreas. Eur J Cancer. 2014;50(12):2072–81.
Gonçalves A, Gilabert M, François E, Dahan L, Perrier H, Lamy R, et al. BAYPAN study: a double-blind phase III randomized trial comparing gemcitabine plus sorafenib and gemcitabine plus placebo in patients with advanced pancreatic cancer. Ann Oncol. 2012;23(11):2799–805.
Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol Off J Am Soc Clin Oncol. 2010;28(22):3617–22.
Philip PA, Benedetti J, Corless CL, Wong R, O’Reilly EM, Flynn PJ, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol Off J Am Soc Clin Oncol. 2010;28(22):3605–10.
Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–6.
Holter S, Borgida A, Dodd A, Grant R, Semotiuk K, Hedley D, et al. Germline BRCA mutations in a large clinic-based cohort of patients with pancreatic adenocarcinoma. J Clin Oncol. 2015;33(28):3124–9.
Golan T, Kindler HL, Park JO, Reni M, Mercade TM, Hammel P, et al. Geographic and ethnic heterogeneity in the BRCA1/2 pre-screening population for the randomized phase III POLO study of olaparib maintenance in metastatic pancreatic cancer (mPC). J Clin Oncol. 2018;36(15_suppl):4115.
Jones S, Hruban RH, Kamiyama M, Borges M, Zhang X, Parsons DW, et al. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science. 2009;324(5924):217.
Perkhofer L, Schmitt A, Romero Carrasco MC, Ihle M, Hampp S, Ruess DA, et al. ATM deficiency generating genomic instability sensitizes pancreatic ductal adenocarcinoma cells to therapy-induced DNA damage. Cancer Res. 2017;77(20):5576–90.
Kobashigawa S, Morikawa K, Mori H, Kashino G. Gemcitabine induces radiosensitization through inhibition of RAD51-dependent repair for DNA double-strand breaks. Anticancer Res. 2015;35(5):2731–8.
Smith J, Mun Tho L, Xu N, A. Gillespie D. The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Adv Cancer Res. 2010;108:73–112.
Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmaña J, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33(3):244–50.
Shroff RT, Hendifar A, McWilliams RR, Geva R, Epelbaum R, Rolfe L, et al. Rucaparib monotherapy in patients with pancreatic cancer and a known deleterious BRCA mutation. JCO Precis Oncol. 2018;2:1–15.
Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med. 2019;381(4):317–27.
FDA approves olaparib for gBRCAm metastatic pancreatic adenocarcinoma [press release]. 30 Dec 2019.
Pishvaian MJ, Garrido-Laguna I, Liu SV, Multani PS, Chow-Maneval E, Rolfo C. Entrectinib in TRK and ROS1 fusion-positive metastatic pancreatic cancer. JCO Precis Oncol. 2018;2:1–7.
FDA approves entrectinib for NTRK solid tumors and ROS-1 NSCLC [press release]. 2019.
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–65.
Patnaik A, Kang SP, Rasco D, Papadopoulos KP, Elassaiss-Schaap J, Beeram M, et al. Phase I study of pembrolizumab (MK-3475; Anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res. 2015;21(19):4286–93.
Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, et al. Phase 2 trial of single agent ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33(8):828–33.
Hu ZI, Shia J, Stadler ZK, Varghese AM, Capanu M, Salo-Mullen E, et al. Evaluating mismatch repair deficiency in pancreatic adenocarcinoma: challenges and recommendations. Clin Cancer Res. 2018;24(6):1326–36.
Kim ST, Klempner SJ, Park SH, Park JO, Park YS, Lim HY, et al. Correlating programmed death ligand 1 (PD-L1) expression, mismatch repair deficiency, and outcomes across tumor types: implications for immunotherapy. Oncotarget. 2017;8(44):77415–23.
Upadhrasta S, Zheng L. Strategies in developing immunotherapy for pancreatic cancer: recognizing and correcting multiple immune “defects” in the tumor microenvironment. J Clin Med. 2019;8(9):1472.
Johnson BA 3rd, Yarchoan M, Lee V, Laheru DA, Jaffee EM. Strategies for increasing pancreatic tumor immunogenicity. Clin Cancer Res. 2017;23(7):1656–69.
Liu Q, Liao Q, Zhao Y. Chemotherapy and tumor microenvironment of pancreatic cancer. Cancer Cell Int. 2017;17:68.
Jang RW, Krzyzanowska MK, Zimmermann C, Taback N, Alibhai SMH. Palliative care and the aggressiveness of end-of-life care in patients with advanced pancreatic cancer. J Natl Cancer Inst. 2015;107(3):dju424.
Arcidiacono PG, Calori G, Carrara S, McNicol ED, Testoni PA. Celiac plexus block for pancreatic cancer pain in adults. Cochrane Database Syst Rev. 2011;(3):CD007519.
Moss AC, Morris E, Leyden J, MacMathuna P. Malignant distal biliary obstruction: a systematic review and meta-analysis of endoscopic and surgical bypass results. Cancer Treat Rev. 2007;33(2):213–21.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mizrahi, J.D., Wolff, R.A. (2022). Management of Locally Advanced/Metastatic Disease: Medical Oncology. In: Bhutani, M.S., Katz, M.H., Maitra, A., Herman, J.M., Wolff, R.A. (eds) Pancreatic Cancer: A Multidisciplinary Approach. Springer, Cham. https://doi.org/10.1007/978-3-031-05724-3_9
Download citation
DOI: https://doi.org/10.1007/978-3-031-05724-3_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-05723-6
Online ISBN: 978-3-031-05724-3
eBook Packages: MedicineMedicine (R0)