Abstract
Sclerosing adenosis, sclerosing lesions, microglandular adenosis, and mucocele-like lesions are benign lesions that may mimic invasive carcinoma on routine histology. Adenosis and sclerosing lesions are small glandular proliferations that may resemble well-differentiated invasive ductal or tubular carcinoma, whereas mucocele-like lesions may be confused with mucinous carcinoma. It may be especially difficult to recognize these lesions in the limited core needle biopsy sample, and therefore it is important to maintain a low threshold for diagnostic workup. Immunohistochemical studies for myoepithelial cell markers facilitate the correct diagnosis in challenging cases. However, a notable exception is that microglandular adenosis lacks myoepithelial cells and must be distinguished from well-differentiated invasive carcinoma by its S-100 protein immunoreactivity and triple-negative phenotype.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Sclerosing adenosis
- Apocrine adenosis
- Radial scar
- Complex sclerosing lesion
- Microglandular adenosis
- Mucocele-like lesion
Adenosis
Overview
Adenosis represents a group of benign proliferative lesions of the breast that feature an increased number of glands in the terminal duct lobular units. Sclerosing adenosis, the most common type, is characterized by coexistent stromal sclerosis that compresses the glands and distorts the architecture of involved lobules. In 1940, Ewing briefly described the entity of “fibrosing adenomatosis.” Foote and Stewart [1] provided one of the first detailed histopathologic studies of “sclerosing adenosis.” Subsequently, Urban and Adair [2] and Heller and Fleming [3] published early clinicopathologic studies of this lesion. A common emphasis by the authors of these early reports is the potential to misinterpret this benign small glandular proliferation as invasive carcinoma. Today, although experienced pathologists are well aware of this entity and its diagnostic pitfalls, various histopathologic variants continue to pose challenges, especially in the limited sample on core needle biopsy (CNB).
Clinical, Radiologic, and Gross Features
Sclerosing adenosis is a common lesion of the breast, most prevalent in women of reproductive or perimenopausal age, especially those in the third and fourth decades. It is present in over one quarter of all benign breast biopsies, frequently in association with other proliferative and nonproliferative fibrocystic changes [4]. Sclerosing adenosis limited to isolated lobules is often an incidental microscopic finding; however, lesions involved by microcalcifications may manifest mammographically. Confluent and florid lobules of adenosis, termed “nodular adenosis” or “adenosis tumor,” may present as a palpable or radiologically detectable mass. Clinical examination reveals a firm, mobile mass with or without associated tenderness.
Sclerosing adenosis most frequently presents as microcalcifications or masses and less frequently as atypical densities or architectural distortion [5,6,7,8,9]. On mammography, microcalcifications tend to be clustered in distribution and show amorphous, pleomorphic, or punctate morphologies [5, 6]. Sclerosing adenosis also correlates with scattered mammographic calcifications, but these radiologic lesions are seldom biopsied due to their benign appearance. Masses may be visualized on mammogram and/or ultrasound and range from an oval shape and circumscribed or indistinct margins to an irregular shape with irregular margins [5,6,7,8,9]. Sclerosing adenosis is associated with spiculated masses in few cases [5, 6]. Ultrasonography may also reveal heterogeneously echogenic areas or focal acoustic shadowing [5, 7, 10].
Gross pathology of excised adenosis varies depending on the histologic patterns of the lesion. Nodular adenosis appears as a mass with relatively circumscribed or lobulated borders, a firm consistency, and a grayish-white or pale tan cut surface. Sclerotic lesions tend to form multinodular masses with more irregular borders. Masses usually measure less than 3 cm. A gritty texture is observed if the adenosis is involved by numerous calcifications. Chalky streaks characteristic of invasive carcinoma are absent. Sclerosing adenosis involving scattered lobules is not apparent on gross examination.
Microscopic Features
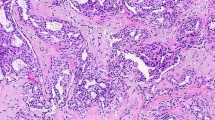
Adenosis may be a focal, diffuse, or tumor-like process (Fig. 5.1). At low magnification, it is characterized by a small glandular proliferation with a lobulocentric pattern of growth (Fig. 5.1c). Lesions range from sclerotic to florid. Myoepithelial cells are retained throughout the lesion, although it can be challenging to identify two cell layers in very florid or sclerotic lesions by hematoxylin and eosin (H&E) morphology alone.
Sclerosing adenosis displays swirling lobules of compressed and distorted glands in fibrotic stroma. Sclerosis is often accentuated in the central portion of the lobule. Areas with less sclerosis contain round glands with open lumens and elongated glands with compressed lumens (Fig. 5.1c). Eosinophilic secretions may be present within the open lumens. The luminal epithelium consists of variably attenuated cuboidal to columnar cells with bland cytomorphology. The myoepithelium is typically flattened and rather inconspicuous. With increasing sclerosis, compressed glands with obliterated lumens appear as small nests or cords of epithelioid or spindled myoepithelial cells. Atrophic epithelial cells may be barely perceptible or absent. The glands are surrounded by a thickened basement membrane and variably expanded dense fibrotic to hyalinized stroma. Scattered stromal and intraluminal microcalcifications are common (Fig. 5.2). Stromal elastosis may be seen and may be a clue that the adenosis is part of a larger sclerosing lesion. Sclerosing adenosis is often associated with other fibrocystic changes, such as columnar cell and apocrine change (Fig. 5.3), usual epithelial hyperplasia, radial sclerosing lesions, papillomas, and fibroadenomas (Fig. 5.4a, b), as well as atypical epithelial hyperplasia (especially atypical lobular hyperplasia) and carcinoma in situ (CIS).
Florid adenosis consists of enlarged or coalescent lobules of densely packed ductules and glands (Fig. 5.4c, d). The hyperplastic ductules are elongated, tortuous, and entwined. Transversely sectioned glands are round with patent lumens and tangentially or longitudinally sectioned glands are more elongated. The glands are lined by cuboidal to columnar epithelial cells. Mitoses are infrequent. “Adenosis tumor” and “nodular adenosis” refer to confluent areas of florid or sclerosing adenosis that form a mass. The mass may have circumscribed or lobulated margins. On CNB, recognition of a well-demarcated border is helpful in determining the benign nature of particularly florid cases of adenosis.
Rare cases of florid or sclerosing adenosis have an infiltrative appearance. Sclerosing adenosis may contain benign glands that extend into adjacent adipose tissue, simulating stromal invasion, or that surround nerves or lie within the epineurium of nerve fibers. Taylor and Norris [11] and Davies [12] identified the phenomenon of perineural invasion in approximately 2–4% of their cases and showed that it was not associated with the development of invasive carcinoma. In a series of 10,000 breast consult cases, Gobbi et al. [13] found 13 cases of sclerosing adenosis (4 cases) and complex sclerosing lesions (9 cases) that were involved by florid usual hyperplasia, atypical ductal hyperplasia (ADH), or ductal carcinoma in situ (DCIS) and associated with perineural invasion. In these lesions, the glands involving nerves showed cytologic and architectural features of the adjacent usual or atypical hyperplasia. Additionally, Eusebi and Azzopardi [14] reported a small series of cases with infiltration of blood vessel walls by benign glands (Fig. 5.5).
Sclerosing lesions with neural and vascular infiltration. Sclerosing adenosis involved by DCIS infiltrates the wall of a lymphatic vessel (a–c). SMM confirms the presence of myoepithelial cells in this lesion (c). A benign sclerosing lesion shows perineural invasion (d–f). A multiplex immunostain with SMM (brown) confirms the presence of myoepithelial cells in this lesion (f)
Adenosis may be partially or completely involved by other pathologic entities, including typical and atypical apocrine change (see below for separate discussion of apocrine adenosis), atypical epithelial hyperplasia, and CIS [15,16,17,18,19]. Sclerosing adenosis involved by CIS warrants special attention for its ability to mimic invasive carcinoma. Lobular carcinoma in situ (LCIS) more commonly involves sclerosing adenosis than DCIS. LCIS involving adenosis may fill and expand glands with a solid proliferation of small uniform cells with eccentric nuclei and scant pale cytoplasm, or less commonly spread through the lesion in a pagetoid fashion [16] (Fig. 5.6). In contrast, DCIS involving adenosis is characterized by a solid or cribriform proliferation of more cohesive cells with increased cytologic atypia and necrosis [15, 17,18,19] (Figs. 5.7 and 5.8). The overall impression of CIS within sclerosing adenosis is one of glands, nests, or cords of neoplastic epithelial cells in a fibrotic stroma—a diagnostic dilemma in some cases.
Two cases of DCIS involving adenosis that mimicked invasive ductal carcinoma on core biopsy. One case is characterized by an atypical small glandular proliferation with an infiltrative appearance (a–d), whereas the other represents a nodular and florid proliferation for which the circumscribed margin is only apparent on excision (e–h). A myoepithelial cell layer is confirmed in both lesions by a p63/keratin dual stain (d) and a SMM/keratin dual stain (h). SMA is not as useful in distinguishing DCIS involving adenosis from invasive tubular carcinoma (i) due to high background staining of myofibroblasts
Differential Diagnosis
Invasive carcinoma of tubular, lobular, or ductal types is the primary consideration in the differential diagnosis of sclerosing adenosis and its histopathologic variants. It must be emphasized that the key to the diagnosis of sclerosing adenosis is recognition of the lobulocentric pattern at low magnification. This approach may be limited by a small or fragmented sample on CNB. If this pattern cannot be appreciated, immunohistochemistry (IHC) should be employed to confirm the presence of myoepithelial cells in sclerosing adenosis and definitively distinguish it from invasive carcinoma.
Sclerosing adenosis with less sclerosis and retention of glandular tubules—especially those examples with extension into adipose tissue or perineural invasion—is a well-known mimic of tubular carcinoma and well-differentiated invasive ductal carcinoma. Because tubular carcinoma is composed almost exclusively of well-formed glands with bland cytology, it is in the differential diagnosis of most types of adenosis and sclerosing lesions. It is characterized by a haphazard proliferation of tubules with sharply angulated contours, tapering ends, and open lumens, which stands in contrast to the lobulocentric proliferation of rounded and compressed tubules of adenosis. The surrounding stroma is typically desmoplastic rather than fibrotic or hyalinized. At high magnification, the tubules consist solely of columnar epithelial cells with apical snouts and little epithelial attenuation. Tubular carcinoma lacks a basal lamina by ultrastructural studies and Periodic acid–Schiff (PAS) staining, and myoepithelial cells by IHC, in contrast to retention of these elements in sclerosing adenosis [20,21,22]. Sclerosing adenosis with marked sclerosis and glandular atrophy simulates invasive lobular carcinoma with classic morphology (Fig. 5.2c, d) or variant tubulolobular morphology. In these cases, most of the glands are reduced to myoepithelial cells arranged as cords and single cells and their lobulocentric distribution is distorted by the expanded stroma. Invasive lobular carcinoma, in contrast, is usually a more cellular and infiltrative lesion composed of cells with a more prominent epithelioid morphology. However, it tends to form scattered foci of more dispersed cells, and it is this pattern that most resembles sclerosing adenosis. Invasive lobular carcinoma may show focal formation of small tubules, which could further complicate this distinction.
Perhaps one of the greatest diagnostic pitfalls is confusing adenosis involved by CIS with an invasive ductal carcinoma growing in a predominantly nested pattern. We recently reported that misinterpretation of DCIS involving sclerosing adenosis as invasive ductal carcinoma was responsible for 3 of 14 major diagnostic changes (2 patients, 3 cases) in a study of revised pathology reports specific to breast pathology over a 5-year period at our institution [23]. Both cases were especially challenging. One case was a quite florid example of adenosis involved by high nuclear grade apocrine DCIS for which the lobulocentric configuration could not be fully appreciated on CNB. Another case was a CNB of a particularly infiltrative example of sclerosing adenosis and an associated sclerosing lesion partially involved by intermediate nuclear grade DCIS. Atypical glands and nests of tumor cells were present in a haphazard distribution, including freely permeating adipose tissue, which defied recognition of a lobulocentric pattern in the limited sample (Fig. 5.7). In general, it is difficult to distinguish sclerosing adenosis involved by CIS from invasive carcinoma due to the pattern of glands, nests, or cords of neoplastic epithelial cells in a fibrotic stroma. Features that favor sclerosing adenosis involved by CIS include lobular configuration, at least focally identifiable myoepithelial cells on H&E-stained sections, hyaline basement membrane surrounding tumor cell nests, uninvolved sclerosing adenosis in neighboring tissue, and separate foci of CIS [23, 24]. It is helpful to maintain a low threshold for the consideration of CIS involving sclerosing adenosis prior to making a diagnosis of invasive carcinoma on CNB.
Immunohistochemical Workup
Immunohistochemical studies to confirm the presence of the myoepithelial cell layer are useful for cases of sclerosing adenosis that cannot be easily distinguished from invasive carcinoma on routine histology. Although numerous myoepithelial markers are available (p63, smooth muscle myosin heavy chain (SMM), calponin, smooth muscle actin (SMA), S100 protein, CD10, and basal cytokeratins, et cetera), they differ in their sensitivity, specificity, and ease of interpretation, which may be attributed in part to the variable immunoreactivity of these markers in stromal cells, vessels, luminal epithelial cells, and tumor cells. The myoepithelial markers with the best combination of sensitivity and specificity are p63 (nuclear staining) and SMM (cytoplasmic staining). An immunostain for p63 is easy to interpret due to its nuclear staining pattern and its lack of immunoreactivity in myofibroblasts and blood vessels. An immunostain for SMM is preferable to those for other contractile proteins, calponin and SMA, because it shows only limited staining of myofibroblasts (Fig. 5.7i). All three of these markers stain blood vessels [25, 26]. It is often best to use immunostains for p63 and SMM, or one of the other markers, in a complementary approach. In addition, it is useful to note that sclerosing adenosis and other sclerosing lesions may be distinguished from invasive carcinoma by the pattern of expression of estrogen receptor (ER). Sclerosing adenosis typically shows heterogeneous immunoreactivity in contrast to the strong diffuse pattern of staining in well-differentiated invasive carcinomas.
Pathogenesis
Although the exact etiology and pathogenesis remain unknown, it is hypothesized that estrogen-stimulated proliferation plays a role in the development of sclerosing adenosis and other types of proliferative fibrocystic change. Epidemiologic studies have shown that proliferative fibrocystic change, including sclerosing adenosis, is more common in reproductive age, parous, and obese women and those receiving hormone replacement therapy [4]. Shoker et al. [27] demonstrated increased ER expression and Ki-67 proliferation index in sclerosing adenosis, radial scars, papillomas, and fibroepithelial lesions, suggesting ER dysregulation in these lesions. These authors suggested that the degree of dysregulation correlates with the risk of developing breast cancer.
Clinical Management and Prognosis
A diagnosis of sclerosing adenosis on CNB is safely managed by observation if it is concordant with the radiologic findings and if it is not associated with another lesion requiring excision. Gill et al. [6] showed that sclerosing adenosis provided sufficient correlation for clustered microcalcifications and well-circumscribed or indistinctly marginated masses on CNB. Sclerosing adenosis was accurately sampled in all but one case with adequate follow-up. In retrospect, on CNB of the only spiculated mass, a radial sclerosing lesion was present in association with sclerosing adenosis, and on excision, this lesion was involved by several small foci of invasive carcinoma with tubular and lobular features. This study highlights the point that a diagnosis of sclerosing adenosis should not be accepted as concordant for spiculated masses. Although spiculated masses sometimes represent sclerosing adenosis, the radiologic differential diagnosis includes several benign and malignant entities, including post-surgical scar, radial sclerosing lesion, and invasive carcinoma. These entities cannot reliably be distinguished by radiologic features alone [28]. Moreover, radial sclerosing lesions commonly coexist with sclerosing adenosis and their recognition at CNB may be difficult if the overall architecture is not apparent. It is important to recognize these lesions as a nontrivial number of cases are associated with in situ or invasive carcinoma [29]. Surgical excision is generally recommended for spiculated masses.
Multiple studies have demonstrated that sclerosing adenosis is associated with a modestly increased risk of breast cancer. Jensen et al. [30] found that sclerosing adenosis is an independent risk factor for breast cancer, and the relative risk was approximately 1.7, in a study of 547 cases of sclerosing adenosis with long-term follow-up identified from over 10,000 benign breast biopsies from the Nashville Breast Cohort. More recently, Visscher et al. [4] investigated the risk of breast cancer in women diagnosed with sclerosing adenosis who had been followed long term as part of the Mayo Benign Breast Disease Cohort. Breast cancer risk was assessed using standardized incidence ratios (SIRs) relative to the Iowa Surveillance, Epidemiology, and End Results (SEER) registry. Sclerosing adenosis was present in 3733 women (27.8%) out of a cohort of over 13,000 women followed for a median of 15.7 years. Across the entire cohort, women with sclerosing adenosis had a higher risk of developing breast cancer (SIR 2.10, 95% confidence interval [CI] 1.91–2.30) than those without it (SIR 1.52, 95% CI 1.42–1.63). In general, sclerosing adenosis is associated with a 1.5–2-fold increased risk of breast cancer and should be classified with other types of proliferative breast disease without atypia showing similar risk [31, 32].
Apocrine Adenosis
“Apocrine adenosis ,” apocrine metaplasia of the epithelium comprising sclerosing adenosis, is present in 3% of benign breast biopsies [33]. Apocrine adenosis occurs in an older population than sclerosing adenosis, with an average age of almost 60 years at diagnosis [33,34,35]. Apocrine adenosis and atypical apocrine adenosis belong to a spectrum of apocrine lesions of the breast, ranging from benign apocrine change to apocrine CIS to invasive carcinoma with apocrine features. Apocrine cytologic features include cellular enlargement, abundant eosinophilic granular cytoplasm, round central nuclei, and prominent nucleoli (Fig. 5.3c, d). Atypical apocrine adenosis is generally diagnosed in the presence of greater than threefold variation in nuclear size (1.73-fold increase in nuclear diameter) and nucleolar enlargement [36]. Because cells with apocrine features are enlarged, nuclear enlargement must be assessed using cells with normal apocrine cytology as a reference point. Mitoses are rare and necrosis is absent in these lesions [34].
The differential diagnosis of atypical apocrine adenosis includes apocrine DCIS and invasive ductal carcinoma with apocrine features. The distinction between atypical apocrine adenosis and apocrine DCIS involving sclerosing adenosis can be extremely difficult. Although no consensus criteria exist, features suggestive of apocrine DCIS include considerable cytologic atypia, characteristic patterns of architectural atypia, necrosis, and greater extent of the lesion [37, 38]. As noted for sclerosing adenosis involved by other atypical lesions, it is also possible to confuse atypical apocrine adenosis for invasive carcinoma, especially in particularly florid or sclerotic cases without an obvious lobulocentric pattern of growth (Fig. 5.7e–h). In a study of the reproducibility of diagnoses in breast biopsies, 4 of 12 pathologists rendered a diagnosis of invasive carcinoma in a case of apocrine adenosis [39].
Immunohistochemical studies show that apocrine lesions of the breast are characterized by lack of expression of estrogen and progesterone receptors in addition to expression of androgen receptor and gross cystic disease fluid protein-15 (GCDFP-15) [40]. Some cases of apocrine metaplasia and apocrine adenosis exhibit abnormal oncoprotein and apoptosis-related protein expression and a higher proliferation rate. By IHC, apocrine adenosis shows expression of human epidermal growth factor 2 (HER2) in over 50% of cases [41,42,43]; however, it is not accompanied by gene amplification on fluorescence in situ hybridization [42]. In one study, Selim et al. [41] detected membranous HER2 expression of variable intensity in 55.6% of apocrine adenosis cases and 10.9% of apocrine metaplasia cases as well as nuclear p53 expression in 27.8% of apocrine adenosis cases and 1.6% of apocrine metaplasia cases. All cases of apocrine adenosis and apocrine metaplasia were positive for the oncoprotein c-Myc and negative for the anti-apoptotic protein BCL2. The Ki-67 proliferation index was higher in apocrine adenosis (3.6%) than in apocrine metaplasia (1.3%) and normal breast epithelium (0.9%). The authors hypothesized that cases of apocrine adenosis with these molecular changes may represent precursor lesions to in situ and invasive apocrine carcinomas. An important implication of this study is that the expression of HER2 or p53 by IHC cannot distinguish atypical apocrine adenosis from apocrine DCIS, although studies have not directly compared markers in these two entities.
Much less data is available to inform the clinical management of atypical apocrine adenosis compared to other atypical breast lesions. In the largest study to date on atypical apocrine adenosis diagnosed on CNB, Chang Sen et al. [44] found that 4 of 20 (20%) surgically excised cases were upgraded to malignancy, including three with DCIS and one with invasive carcinoma. All four malignancies were masses on ultrasound, while three of them were associated with calcifications. In contrast, in other recent studies by Calhoun and Booth [33] and Hou et al. [45], there were no upgrades to DCIS or invasive carcinoma on surgical excision of apocrine adenosis and atypical apocrine adenosis without coexisting atypical hyperplasia or carcinoma in 7 and 10 cases, respectively. The findings raise the possibility that atypical apocrine adenosis diagnosed on CNB may not require surgical excision. However, given the limited data, as well as the difficulty in morphologically and immunohistochemically distinguishing atypical apocrine adenosis from apocrine DCIS, it would be prudent to carefully consider surgical excision of most cases of atypical apocrine adenosis on CNB. A diagnosis of non-atypical apocrine adenosis on CNB does not require further intervention.
The few long-term follow-up studies regarding the risk of breast cancer associated with atypical apocrine adenosis show conflicting results. In a series of 51 cases of atypical apocrine adenosis by Carter and Rosen [46], none of the patients developed carcinoma after an average follow-up period of approximately 3 years. Likewise, Fuehrer et al. [34] showed that the rate of breast cancer diagnosis in patients with atypical apocrine adenosis (8.1%) after an average follow-up period of 14 years did not significantly differ from that of the remainder in the Mayo Benign Breast Disease cohort (7.8%). In contrast, Seidman et al. [35] found that 4 of 37 patients diagnosed with atypical apocrine adenosis at the Armed Forces Institute of Pathology developed breast cancer after a mean follow-up of 8.7 years. This study demonstrated an overall risk of 5.5 relative to the reference population from the SEER registry, a relative risk similar to that of other atypical lesions of the breast. The authors suggested that atypical apocrine adenosis is associated with an increased risk of breast cancer, either because it is a true atypical proliferation of apocrine-type epithelium, or because some cases represent apocrine DCIS involving sclerosing adenosis with insufficient architectural features to make this diagnosis.
Radial Scars and Complex Sclerosing Lesions
Overview
Radial scars are benign lesions characterized by a central fibroelastotic core surrounded by radiating ducts and lobules with cysts and proliferative changes. In 1928, Semb [47] provided the first description of this lesion, referring to it as “rosette like lesion” and “proliferative centre.” Subsequently, radial scars have been reported by several different authors using several different terms, including sclerosing papillary proliferation [48], benign sclerosing ductal proliferation [49], nonencapsulated sclerosing lesions [50], infiltrating epitheliosis [51], and indurative mastopathy [52], amongst others. In 1975, Hamperl [53] named this same entity in German as “strahlige narben,” which was translated into “radial scar.”
Some authors have recommended use of the term “radial scar” for lesions measuring up to 1 cm and “complex sclerosing lesion” for larger lesions [54]. Others reserve the term “complex sclerosing lesion ” for lesions composed of several closely contiguous fibroelastotic areas or lesions with a less organized appearance [55]. Both terms are recommended in the most recent World Health Organization Classification of Breast Tumours [56].
As with the other small glandular proliferations in this chapter, it is well known that radial scar/complex sclerosing lesions (RS/CSLs) mimic invasive carcinoma on both radiologic and pathologic examination. Several early authors even hypothesized that these lesions represented direct precursors to breast cancer, noting “transitional features” of tubular carcinoma [57], and referring to them as “incipient tubular carcinomas” [50]. Although RS/CSLs are regarded as benign, a small proportion of lesions are associated with in situ and invasive carcinoma , posing a number of diagnostic and clinical management challenges.
Clinical, Radiologic, and Gross Features
The incidence of radial scar is 3–9 per 1000 mammograms in the screened population [58, 59]. Studies of population-based large cohorts such as the Mayo Benign Breast Disease cohort, the Nurses’ Health Study, and the Nashville cohort report incidences of radial scars in benign breast biopsies in the range of 5–10% [60,61,62]. Radial scars most frequently occur in women between 40 and 60 years of age [60,61,62]. An association with menopausal status, parity, or contraceptive use has not been identified [60]. Radial scars are often incidental microscopic findings; however, larger lesions are clinically detected on screening mammography. Radial scars form palpable and freely mobile masses in a minority of cases. Multicentric and bilateral lesions are common.
Radial scars are well-known mimics of invasive carcinoma on mammography. Radial scars may appear as stellate densities, spiculated masses, or architectural distortions. Rare cases present as isolated microcalcifications. Taber and Dean [63] proposed criteria to favor an interpretation of radial scars over invasive carcinoma based on certain mammographic features. According to these authors, radial scars are characterized by a radiolucent central core, long and thin radiating spicules, radiolucent projections parallel to the spicules, varying appearances in different projections, and an absence of a palpable mass or skin changes. In contrast, invasive carcinoma typically has a dense central zone and short spicules.
Several studies have shown that these features are not specific for radial scars. In a retrospective review of 255 stellate lesions, Mitnick et al. [64] concluded that the criteria of Tabar and Dean could not reliably distinguish radial scars and invasive carcinomas. Of 73 nonpalpable carcinomas, 14 (19%) had features attributed to radial scars, including radiolucent centers and radiating elongated spicules. Conversely, 4 (44%) of 9 radial scars had a dense central region. In this study, microcalcifications constituted the only mammographic finding capable of differentiating between the lesions with overlapping features, more common in invasive carcinomas (9 of 14 cases) than radial scars (1 of 4 cases). Subsequent studies by Hassell et al. [65] and Boute et al. [66] found that radial scars only displayed all of the classic criteria in 71.6% (48 of 67) and 48% (54 of 133) cases, respectively. Contrary to the study by Mitnick et al. [67], both studies identified microcalcifications in approximately half of the lesions, arguing against microcalcifications as a discriminating feature.
On ultrasound, a radial scar most often appears as an irregular hypoechoic mass with ill-defined borders and posterior acoustic shadowing. Parenchymal distortion may be seen in the absence of a mass. It has been claimed that an echogenic halo, peripheral cysts, and complex echotexture represent other features more commonly seen with radial scars than invasive carcinomas [66]. However, there is a significant overlap in the appearance of radial scar and invasive carcinoma on this imaging modality as well.
Most radial scars cannot be identified on gross examination. If the radial scar is large enough to be appreciated grossly, usually greater than 1 cm, it appears as a stellate mass, quite similar in appearance to invasive carcinoma. It is a firm lesion with central puckering and an irregular edge that infiltrates into the surrounding fatty breast tissue. Creamy white or yellow streaks of elastosis emanate from a pale gray central core. Dilated cysts may be visible at the periphery of the lesion. Some radial scars appear as vague masses without distinct features.
Microscopic Features
A radial scar features a central zone of sclerosis and elastosis with radiating ducts and lobules (Fig. 5.9), giving it a stellate appearance on low magnification. Obliterated ducts and smaller angulated glands are entrapped within the fibroelastotic stroma of the central zone. The entrapped glands are lined by one or more inner layers of benign epithelium and an outer layer of inconspicuous myoepithelium. The epithelium may appear separated from the underlying myoepithelium and stroma in some areas as a result of retraction artifact. It is important to note that myoepithelial cells may be reduced in numbers or absent at the periphery of some entrapped glands due to plane of sectioning. The entrapped glands are invested in a paucicellular stroma with a mixture of bright pink hyalinized collagenous tissue and pale pink or pale blue elastosis. Ducts and lobules radiate from the sclerotic center along with interdigitating bands of fibrous tissue and interspersed adipose tissue and appear to expand in a centrifugal manner. The ducts and lobules are involved by cysts, apocrine metaplasia, and proliferative changes, such as sclerosing adenosis, usual and atypical epithelial hyperplasia, and papillomatosis (Fig. 5.9). Calcifications are often associated with the lesion. As with sclerosing adenosis, a minority of sclerosing lesions may demonstrate perineural invasion by benign ducts (Fig. 5.5d–f) [11, 13]. Complex sclerosing lesions (CSLs) are characterized by the same elements—fibroelastotic core(s), entrapped glands, and ducts and lobules with fibrocystic changes (Figs. 5.10, 5.11, and 5.12). However, some authors would use this designation if the lesion is larger and does not have the radial configuration of a radial scar [55]. CSLs with intraductal papillomatosis show considerable overlap in histologic features with sclerosing intraductal papillomas and the classification of lesion may be best performed based on the predominant finding (Fig. 5.13).
Radial scar and complex sclerosing lesions. Radial scars may be discrete lesions with a single central sclerotic focus and radiating epithelial elements (a, b), possibly removed in large part by the biopsy. CSLs may have a more disorganized architecture with multiple sclerotic foci, often involved by various types of fibrocystic change (c, d). Myoepithelial markers aid in the distinction from invasive carcinoma, but must be interpreted with caution. For lesions that mimic invasive carcinoma, it is best to perform immunohistochemistry for multiple myoepithelial cell markers, as a sclerosing lesion with attenuated staining may be confused for invasive carcinoma (SMM, e)
Radial scars may be involved by CIS and invasive carcinoma as evidenced by the numerous studies on the incidence of upgrade to malignancy on surgical excision of these lesions. LCIS and DCIS tend to involve the peripheral ducts and lobules with characteristic patterns of growth and cytomorphologies. However, it is possible for CIS to extend into the distorted glands of the fibroelastotic core, and in these instances the lesion bears resemblance to invasive carcinoma. Low to intermediate nuclear grade DCIS is most common. Invasive carcinoma is typically ductal or tubular in histology, well to moderately differentiated, small (less than 2 cm), and limited to the periphery of the lesion [29].
Metaplastic carcinomas , including low-grade adenosquamous carcinomas (LGASC) and spindle cell carcinomas, may also arise in association with complex sclerosing lesions and sclerosing papillomas [68, 69] (Fig. 5.14). Such tumors may be related to adenosquamous proliferation (ASP) of the breast, which is recognized by some pathologists as a common finding in sclerosing breast lesions and a hallmark of these lesions. ASP consists of compact ducts with both glandular and squamous features within spindle cell stroma in the nidus of the lesion. It is similar in appearance and immunophenotype to LGASC, but limited in extent and lacking infiltration into the surrounding breast tissue [70, 71].
Sclerosing papillary lesion associated with a subtle atypical adenosquamous proliferation worrisome for LGASC (a–d). Small nests and tubules are present in the background spindled stroma. This focus lacks evidence of a surrounding myoepithelial cell layer on a SMM and keratin double stain (c), and lesional cells display dual positivity for p63 and keratin (d). LGASC was confirmed on excision (not shown)
Infiltrating epitheliosis , a controversial entity, is considered by most pathologists to represent a RS/CSL involved by florid usual ductal hyperplasia (UDH) (“epitheliosis”). Other pathologists have argued that it should be classified as a separate entity due to its distinctive histologic features, including (1) a florid epithelial proliferation comprising the bulk of the lesion, involving ducts with jagged edges, and “flowing out” into the adjacent breast tissue, (2) scleroelastotic stromal changes present throughout the lesion rather than confined to the central nidus, (3) frequent desmoplastic stroma and keloid-like fibrous bands, and (4) a discontinuous-to-absent myoepithelial cell layer. However, the epithelial proliferation is reminiscent of florid UDH with a solid growth pattern, frequent intranuclear inclusions, and heterogeneous CK5 and ER expression [72]. Moreover, reduced staining for myoepithelial cells is a recognized phenomenon in RS/CSLs [73]. As such, optimal classification of infiltrating epitheliosis remains a matter of debate.
Differential Diagnosis
RS/CSLs are well-recognized mammographic, gross, and histologic mimics of invasive carcinoma, especially tubular carcinomas. Both entities may appear as spiculated lesions on imaging and stellate lesions on gross examination. Histologically, the glands entrapped within the fibroelastotic core of the RS/CSLs are most worrisome for invasion. This is a pitfall encountered particularly in CNBs in which recognition of the overall architecture may be difficult. Again, as with other small glandular proliferations, it is important to have a high degree of suspicion for these lesions on core biopsy. Although these glands may be distorted and haphazardly arranged, they are confined to the central portion of the lesion and encircled by benign elements, typically with hyperplasia present at the periphery. Moreover, on high magnification, at least some, if not all, of the glands contain an outer layer of myoepithelial cells. In contrast, tubular carcinoma contains angulated tubules that are typically embedded in a desmoplastic stroma throughout the entire lesion; however, some tumors may have areas of fibrotic or elastotic stroma similar to that of sclerosing lesions. The tubules infiltrate into the breast parenchyma and adipose tissue at the periphery of the tumor. Myoepithelial cells are completely absent. It is particularly challenging to distinguish RS/CSLs from tubular carcinoma on CNB, especially if only the fibroelastotic core of the lesion has been sampled (Fig. 5.12).
LGASC is another carcinoma in the differential diagnosis of RS/CSLs, which may be rather subtle and difficult to recognize when it arises in the background of these lesions [68]. LGASC is characterized by well-formed glandular structures and solid nests of squamous cells within reactive cellular stroma [74]. RS/CSLs are often associated with an adenosquamous proliferation within the nidus of the lesion; however, LGASC extends beyond the sclerosing lesion into the surrounding breast parenchyma [70]. At scanning magnification, the tumor may have a spiculated appearance resembling the configuration of a RS/CSL, while adenosquamous elements of the central nidus may be mistaken for entrapped benign glands. Clues to the diagnosis of LGASC include the squamoid and syringoid features of tumor cell nests, the lamellar arrangement of the spindled stroma around glandular structures, and frequent peripheral lymphoid aggregates. LGASC is characterized by variable immunohistochemical staining at the periphery of lesional glands for myoepithelial markers (p63, SMM, SMA, CD10, and calponin), with complete circumferential staining in a minority of cases, which may further complicate the distinction from RS/CSLs. In contrast to benign RS/CSLs, in our experience, LGASC is more likely to display loss of myoepithelial markers, dual p63 and cytokeratin staining of tumor cells in squamoid nests, and variable staining of stromal cells with cytokeratins (CKAE1/AE3, CK5/6, CK7, CK 34βE12, and Cam5.2) [74]. Immunoreactivity for p63 is rare in the stromal cells of both RS/CSLs and LGASC [74, 75] and should prompt consideration for spindle cell carcinoma , which may arise in association with either of these lesions.
Immunohistochemical Workup
As with other small glandular proliferations, immunohistochemical staining for myoepithelial cells aids in the differential diagnosis of radial scars versus invasive carcinoma. A study by Hilson et al. [73] revealed that myoepithelial cells in benign sclerosing lesions demonstrate immunophenotypic differences from myoepithelial cells surrounding normal mammary ducts and lobules. Almost half of the cases of sclerosing lesions in their study showed reduced expression of at least one of seven myoepithelial cell markers. Expression of CK5/6 was reduced in approximately one third of cases. Expression of SMM, CD10, p63, and calponin was reduced in 21%, 15%, 9%, and 6%, respectively. A failure to confirm the presence of myoepithelial cells in sclerosing lesions could lead to the erroneous classification of these benign lesions as invasive cancer. This study highlights the importance of using two or more myoepithelial cell markers in combination (Fig. 5.9e). Most benign sclerosing lesions show no staining for p63 or cytokeratins in stromal cells, which helps in ruling out metaplastic carcinoma, although stromal positivity for CK MNF116 is a diagnostic pitfall [75]. Like sclerosing adenosis, sclerosing lesions show a heterogeneous pattern of estrogen receptor expression, which aids in the distinction of this lesion from strongly and diffusely staining well-differentiated invasive carcinoma and triple-negative LGASC.
Pathogenesis
The exact pathogenesis of radial scars is unknown. It has been hypothesized that these lesions result from an unknown injury that leads to fibrosis, possibly related to duct ectasia and obliteration [76], chronic inflammation [77], or vascular compromise [78], or that they result from an abnormal epithelial/stromal interaction. Radial scars are characterized by a cellular phase with abundant central myofibroblasts and a mature phase with few myofibroblasts and prominent collagen and elastin fibers [77]. A number of factors involved in vascular stroma formation, such as collagen type 1, fibronectin, thrombospondin 1, and vascular endothelial growth factor, are expressed [78]. Like other proliferative lesions of the breast, the epithelium shows evidence of estrogen receptor dysregulation [27, 79].
PIK3CA-activating point mutations have been detected in over 60% of RS/CSLs [80], over three quarters of ASPs microdissected from the nidus of early cellular RS/CSLs [71], and most cases of so-called infiltrating epitheliosis [72]. Given recurrent PIK3CA mutations in carcinomas of the breast (TCGA), including a high rate of these mutations in LGASC [81], some authors have suggested that RS/CSLs and related lesions may represent clonal proliferations and precursors to invasive carcinoma, although this has not been clearly supported by evidence of shared molecular alterations between coexistent RS/CSLs and carcinoma [71, 72]. However, PIK3CA-AKT pathway mutations have also been found in several other benign proliferative lesions, such as UDH (50%) [82], papillomas (over 60%) [83], and columnar cell lesions (over 50%) [84], some of which harbored a different mutation than coexistent carcinoma [84], and therefore it is postulated that PIK3CA mutations may play a greater role in epithelial proliferation than malignant transformation [80].
Clinical Management and Prognosis
A diagnosis of RS/CSL on CNB is routinely managed by surgical excision. Surgical excision is a consensus recommendation for RS/CSL involved by atypical hyperplasia, as a significant fraction of cases (24–37%) will be upgraded to malignancy (DCIS or invasive carcinoma) [29]. On the other hand, clinical management of RS/CSLs without associated atypical hyperplasia has been a matter of debate over the years. Bianchi et al. [29] conducted one of the largest single center studies on the surgical outcomes of radial scars without atypical hyperplasia diagnosed on CNB revealing that 8.2% (4 of 49 cases) of lesions were upgraded to malignancy. The same authors found a similar mean upgrade rate of 8.3% in a review of the literature to date, which included 21 prior series with a total of 899 cases of radial sclerosing lesions without atypia diagnosed at CNB [59, 85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104]. The upgrade rate differed widely among series, ranging from 0% to 40%, with most in the range of 4% to 16%. This most likely reflects variability in study design, inconsistent radiologic-pathologic concordance and small sample sizes. In a large multi-institutional study of radiologic lesions of uncertain malignant potential (Breast Imaging-Reporting and Data System 3), Rahka et al. [105] also found that 8.9% (25 of 278 cases) of radial sclerosing lesions without atypical hyperplasia were upgraded to malignancy on excision (14 DCIS, 6 invasive carcinoma). Although it can be argued based on this data that the risk of malignancy is sufficiently high to warrant routine surgical excision for RS/CSLs.
Recent studies suggest that a reliable diagnosis can be made on CNB if the lesion has been sufficiently sampled. In a study of 80 cases at MD Anderson Cancer Center, Resetkova et al. [106] argued that extensive sampling by 9- and 11-gauge needles in conjunction with radiologic-pathologic correlation may eliminate the need for surgical excision in a majority of patients. Similarly, in a multi-institutional study, Brenner et al. [93] showed that missed carcinomas are less likely with larger gauge (11-gauge) vacuum-assisted biopsy devices than with 14-gauge automated biopsy devices (0% and 9% upgrade rates, respectively), especially if at least 12 cores are examined. Some authors have proposed complete excision of radial scars without atypical hyperplasia by vacuum-assisted mammotomy as a way to avoid surgery and some centers have adopted this practice [104].
Other studies suggest that smaller radial scars can be managed conservatively with close follow-up rather than surgical excision. Sloane and Mayers [107] examined 126 radial scars and CSLs and noted that carcinoma was very uncommon in lesions smaller than 0.6–0.7 cm. More recently, in a series of 77 small radial scars (all measuring less than 0.5 cm), Matrai et al. [108] did not find any malignancy on surgical excision. Similarly, Lee et al. [109] found no upgrade to malignancy for incidental or “microscopic” radial scars diagnosed during the evaluation of another target lesion, while Park et al. [110] noted the same for mammographically occult asymptomatic RS/CSLs detected on ultrasound guided CNB. Consistent with this, a recent meta-analysis of 49 studies that included 3163 radial scars with surgical outcomes revealed a pooled estimate for upgrade to malignancy for RS without atypia of 5%, which dropped to 1% for the subgroup diagnosed on 8–11-gauge vacuum-assisted biopsies, with an upgrade of 0% for special case scenarios of small or incidental lesions [111].
While management of RS/CSLs remains controversial and institution-dependent, this newer data suggests that radiological-pathological concordant lesions without atypia may not require surgical excision, especially those that are small and well sampled by larger bore needles. It may be prudent to make management decisions on a case-by-case basis through careful radiologic-pathologic correlation and multidisciplinary discussion.
In the past, radial sclerosing lesions were hypothesized to be direct precursors of invasive carcinoma due to the morphologic similarities to invasive carcinoma and the coexistence with it in a fraction of cases [50, 57]. Currently, radial scars are regarded as benign proliferative lesions and risk factors for breast cancer. Like sclerosing adenosis, much of the data regarding the risk of breast cancer associated with radial scars comes from the three large cohorts of benign breast disease in the United States. In a nested case control study of 1396 women followed for a median of 12 years in the Nurses’ Health Study, Jacobs et al. [61] reported an incidence of 7.1% and a relative risk of 1.8. The risk of breast cancer was independent of the histologic category of benign breast disease and additive to the risk associated with other well-established pre-malignant lesions such as ADH. The risk increased with larger lesions and multiple lesions. There was no significant difference in the number of cases with subsequent development of ipsilateral or contralateral breast cancer. In contrast, Sanders et al. [62] showed that radial scars were associated with only a minimally increased risk of breast cancer (relative risk = 1.11) in women followed for a mean of 20.5 years in the Nashville Breast Cohort. Radial scars were almost always associated with proliferative breast disease and their risk was dependent on the category of proliferative breast disease present. Similarly, radial scars did not increase the risk of breast cancer above the presence of other proliferative breast disease in women with a mean follow-up of 14 years in the Mayo Benign Breast Disease cohort [60]. Overall, a diagnosis of radial scar places a patient into the same risk category as proliferative breast disease.
Microglandular Adenosis
Overview
Microglandular adenosis (MGA) is a small glandular proliferation with a lack of myoepithelial cells and an infiltrative growth pattern that may be confused with invasive carcinoma. It was initially illustrated by McDivitt et al. [112] in 1968 and subsequently characterized in greater detail in clinicopathologic studies by Clement and Azzopardi [113], Rosen [114], and Tavassoli and Norris [115] in 1983.
Clinical, Radiologic, and Gross Features
MGA is a rare lesion that has only been reported in women. Although it affects patients across a broad range of ages, the majority of patients are 45–55 years of age. MGA may present as an incidental microscopic finding or as a palpable mass or thickening in the breast. Some cases are associated with pain or tenderness [113,114,115,116,117,118,119]. On mammography, MGA may correlate with various findings, including a density, a mass, parenchymal distortion, or calcifications [120]. Sebate et al. described the radiologic features of MGA in a single case found at surveillance screening in a 22-year-old BRCA1 germline mutation carrier. Although mammography revealed dense breast tissue with no abnormality, sonography demonstrated a hypoechoic mass with an irregular border, and magnetic resonance imaging showed a small non-circumscribed mass with moderate early and delayed enhancement [121].
Grossly, MGA forms an ill-defined and infiltrative induration, plaque-like lesion, or mass with a firm or rubbery consistency. Masses typically measure 3–4 cm, but lesions as large as 20 cm have been reported [122]. Many cases of MGA cannot be appreciated grossly.
Microscopic Features
MGA is characterized by a haphazard proliferation of small uniform round glands in mammary stroma or fat. The glands are lined by a single layer of cuboidal epithelial cells completely invested by basement membrane. The epithelial cells contain a round nucleus and clear to slightly eosinophilic vacuolated cytoplasm. In a minority of cases, the cytoplasm contains coarse, eosinophilic granules that express alpha-1-antitrypsin and alpha-1-antichymotrypsin reminiscent of serous-type salivary glands. Rarely, chondromyxoid metaplasia can be found in association with MGA. Inspissated secretion is present as brightly eosinophilic globules within the glands, highlighted on PAS, mucicarmine, and Alcian blue special stains. Calcification of the secretions is occasionally observed. Mitotic figures are uncommon. Myoepithelial cells are absent throughout the lesion. A discrete basement membrane is present on reticulin and PAS special stains and ultrastructural studies (Fig. 5.15). Immunohistochemical stains for collagen type IV and laminin can also be utilized. The glands are embedded in a hypocellular dense collagenous stroma and freely permeate the mammary adipose tissue. There is no particular orientation of the glands relative to normal ducts and lobules. The glands may be crowded, but each gland remains distinct from its neighbor [113,114,115, 123].
Microglandular adenosis, typical (a–h). MGA consists of a haphazard proliferation of round glands with attenuated epithelium and bright pink secretions (a, b). Occasional cases feature cuboidal cells with bright eosinophilic granules (e, f). It simulates invasive carcinoma due to this growth pattern and lack of a myoepithelial cell layer (p63, c), but it is distinguished by intense staining for S100 protein (d) and retention of the basement membrane (reticulin, g; type IV collagen, h)
MGA encompasses a spectrum of lesions ranging from uncomplicated MGA, as described above, to atypical MGA and MGA associated with in situ and invasive carcinoma. Atypical MGA is composed of typical elements as well as foci with more complicated architecture and mild to moderate cytologic atypia. It consists of a pleomorphic mixture of small and large glands that are more closely packed together, sometimes in a “back-to-back” configuration. The glands are irregularly shaped and can be interconnected. Cellular proliferation within the lumen results in cribriform glands and solid nests. Intraluminal secretions are reduced or absent. The epithelial cells display enlarged hyperchromatic or vesicular nuclei with coarse chromatin and prominent nucleoli. Mitotic activity is increased (Fig. 5.16). A diagnosis of CIS involving MGA is rendered when MGA contains glands with an expansile solid proliferation obliterating the lumen, severe cytologic atypia, and frequent mitotic or apoptotic figures. It tends to retain the underlying alveolar growth pattern of MGA (Fig. 5.17) [116, 124].
Invasive carcinoma arises in a background of atypical MGA and CIS as a coalescent growth of the CIS or as infiltrating cords and single tumor cells within a desmoplastic stroma (Fig. 5.18). It may be difficult to determine the extent of invasive carcinoma due to the disorderly growth pattern and lack of myoepithelial cells throughout the entire lesion. One distinguishing feature is that the basement membrane, which is preserved in MGA and atypical MGA, is disrupted in invasive carcinoma. Several case series have reported that the invasive carcinomas associated with MGA are frequently high grade and display various growth patterns and histologic subtypes. Although a solid growth pattern (Fig. 5.18) is most common, cribriform, glandular, and trabecular growth patterns may be seen. Invasive carcinoma with acinic cell differentiation, secretory differentiation, basaloid features, or metaplastic elements (chondromyxoid metaplasia, squamous metaplasia, spindle cell carcinoma) have been reported [116,117,118,119, 124,125,126,127,128,129,130,131] (Fig. 5.19). Moreover, Acs et al. described 17 cases of adenoid cystic carcinoma transitioning from MGA.
Poorly differentiated carcinoma arising from atypical MGA and CIS (a–d). Atypical MGA features irregular glands with lumens obliterated by a solid proliferation of atypical cells (b). CIS is characterized by expanded solid nests of tumor cells that retain the alveolar growth pattern (c). Invasive carcinoma frequently manifests as a solid nodule arising from coalescent CIS (a). All components stain for S100 protein, although it may be reduced in atypical MGA and carcinoma (d)
Differential Diagnosis
MGA may be confused with other types of adenosis. Khalifeh et al. [116] retrospectively identified 65 cases diagnosed as MGA at MD Anderson Cancer Center from 1983 to 2007 and found that 54 (83%) of these cases contained myoepithelial cells and 48 (70%) would be better classified as adenosis. Most of these cases were diagnosed before 1990 and before the routine use of immunohistochemical studies to aid in this diagnosis. In general, most cases of adenosis are easily distinguished from MGA by their lobulocentric pattern of growth and variable glandular contours in contrast to the haphazard and infiltrative pattern and uniform round glands of MGA. The cases of adenosis that may be misdiagnosed as MGA exhibit clear cell change, thick eosinophilic luminal secretions, and/or an infiltrative growth pattern. These cases can be identified as adenosis by confirmation of a myoepithelial cell layer on IHC.
MGA is notorious for the potential to be mistaken for well-differentiated invasive ductal or tubular carcinoma, especially on CNB, due to the infiltrative growth pattern of its glands and their lack of myoepithelial cells (Fig. 5.20). Tubular carcinoma features variably sized angulated glands lined by columnar epithelium with apical snouts in contrast to the uniform round glands with cuboidal epithelium characteristic of MGA. The glands of both tubular carcinoma and MGA lack myoepithelial cells, but those of tubular carcinoma lack basement membrane as well. Although tubular carcinoma commonly has a desmoplastic stroma, MGA does not elicit a stromal reaction. Instead, MGA has a hypocellular and hyalinized fibrous stroma. Despite these differences, this differential diagnosis is often challenging and requires immunohistochemical studies to definitively distinguish the two entities [132].
Well-differentiated invasive ductal carcinoma is an important consideration in the diagnosis of sclerosing lesions, adenosis, and MGA (a–f). This paucicellular tubule-forming carcinoma in sclerotic stroma is difficult to distinguish from a sclerosing lesion on core biopsy (a, b). This carcinoma composed of round tubules with intraluminal eosinophilic secretions bears striking resemblance to MGA (c–f). Lack of p63-positive myoepithelial cells (e) may be seen with invasive carcinoma and MGA, but strong and diffuse ER expression (f) supports the diagnosis of well-differentiated invasive ductal carcinoma
MGA, in particular atypical forms, may demonstrate significant overlap with acinic cell carcinoma (ACCa) in clinicopathologic, immunohistochemical, and molecular features and may represent part of the same spectrum of lesions. Like MGA, ACCa may display an infiltrative proliferation of small glands lacking myoepithelial cells, strong expression of S100 protein, and a triple-negative immunophenotype. ACCa is characterized by diffuse serous differentiation with the presence of intracytoplasmic zymogen-type granules and the expression of immunohistochemical markers of acinar differentiation, such as lysozyme and amylase; however, MGA can also exhibit these features (Fig. 5.21). ACCa may show greater architectural heterogeneity than MGA, with well-differentiated microglandular and less-differentiated solid areas [133, 134], although this may also be observed in cases of MGA transitioning to carcinoma. In fact, it has been argued that primary ACCa of the breast represents an example of carcinoma arising in MGA with acinic cell differentiation [131], while others contend that MGA and ACCa are distinct entities [134]. Recently, Geyer et al. [133] provided molecular evidence that MGA and ACCa are related lesions that belong to a low-grade triple-negative breast neoplasia family, as they harbor similar genomic profiles including recurrent TP53 mutations. While this may be true, only ACCa is currently considered an indolent form of triple-negative breast cancer (TNBC). Both entities may be associated with high-grade TNBC.
Acinic cell carcinoma (a–d). ACCa may have a microglandular and nested growth pattern that resembles atypical MGA, while the presence of eosinophilic zymogen granules is usually more prominent than seen in MGA (a, b). ACCa stains for S100 protein like MGA (c); however, it also shows strong and diffuse expression of lysozyme (d) corresponding to diffuse serous differentiation
Immunohistochemical Workup
MGA, atypical MGA, and carcinoma arising in MGA have common immunohistochemical profiles, negative for ER, PR, and HER2 (i.e., triple negative) and positive for S100 protein and cathepsin D [116, 125, 126, 135]. Staining for S100 protein is characterized by an intense “chocolate brown” quality in MGA (Fig. 5.15d) but may be decreased in intensity in atypical and carcinomatous forms. The epithelial cells stain for CKAE1/AE3 and Cam5.2, but neither for epithelial membrane antigen nor GCDFP-15 [135]. Myoepithelial cell markers, such as p63 (Fig. 5.15c), SMM (Fig. 5.16c), and calponin, are negative throughout this spectrum of lesions. In contrast, immunohistochemical studies for collagen type IV (Fig. 5.15h) and laminin (Fig. 5.16d) demonstrate the presence of basement membrane in MGA and atypical MGA, distinguishing these components from coexistent invasive carcinoma. In a study of 11 cases of MGA with transition into in situ and invasive carcinoma, Khalifeh et al. [116] showed that the Ki-67 and p53 labeling indices varied in uncomplicated MGA (<3%), atypical MGA (5–10%), and carcinoma associated with MGA (>30%). All of the components expressed both CK8/18 and EGFR, including MGA-associated invasive carcinoma, suggesting that these lesions are characterized by a dual luminal and basal-like phenotype.
Uncomplicated MGA may be confused with well-differentiated invasive ductal carcinoma or tubular carcinoma when immunohistochemical studies reveal a lack of myoepithelial cells. However, the studies for ER, PR, and HER2 provide a clue to the correct diagnosis since a triple-negative phenotype would be highly unusual for these types of invasive carcinoma. A diagnosis of MGA over invasive carcinoma is supported by the demonstration of staining for S100 protein and lack of staining for epithelial membrane antigen [135].
Pathogenesis
As the only benign epithelial entity of the breast in which a myoepithelial cell layer is completely absent, the true nature of MGA has long been a matter of controversy. It has been argued that this lesion is a benign lesion [115], a precursor of invasive breast cancer [116,117,118,119, 124], or a form of ER-negative invasive breast cancer with indolent clinical behavior. Accumulating evidence suggests that it represents a nonobligate precursor lesion of TNBC. Rosenblum et al. [124] provided the first compelling morphologic evidence that MGA is a precancerous lesion. The authors described seven cases of invasive carcinoma arising in MGA in which there was progressive architectural and cytologic atypia in the transition from uncomplicated and atypical MGA to invasive carcinoma as well as unusual histologic features shared by these components. Since then, several case series of MGA have been published, with an incidence of invasive carcinoma ranging from 23% to 64% of cases [116,117,118,119, 125,126,127,128,129,130]. A rate of 64% may be an overestimation due to referral bias in one study [116]. As mentioned above, immunohistochemical studies of cases with morphologic transitions from MGA to atypical MGA to invasive carcinoma have shown that all components are “triple negative” and positive for S100 protein, basal markers, and CK8/18 (luminal marker) [116, 125, 126, 135].
Recent molecular genetic studies provide further support that MGA represents a precancerous lesion. Shin et al. [119] and Geyer et al. [118] identified DNA copy number alterations (CNAs) in a subset of MGA cases employing high-resolution chromosomal and microarray comparative genomic hybridization, respectively, supporting that at least some cases are clonal and neoplastic rather than hyperplastic. Recurrent alterations included gains of 1q, 2q, 7p, 7q, and 8q and losses of 1p, 8p, 14q, 16q, and 17q. Microdissected components of MGA, atypical MGA, and invasive carcinoma displayed similar genomic alterations. In few cases, there was acquisition of additional alterations in the progression from MGA to the invasive component, consistent with clonal evolution. Some cases of MGA had complex patterns of CNAs similar to those seen in high-grade TNBC, while others lacked any CNAs. Utilizing massively parallel sequencing, Guerini-Rocco et al. [136] found limited CNAs and an absence of clonal non-synonymous somatic mutations in pure MGA (2 cases), but identical TP53 mutations and similar patterns of CNAs in the microdissected components of MGA (7 cases) and atypical MGA (3 cases) associated with TNBC. Another recent effort to comprehensively profile MGA suggested epigenetic inactivation of GATA3 via aberrant hypermethylation of CpG sites within the gene may play a role in pathogenesis [137].
TNBC is a heterogeneous group with varying morphology, genomic alterations, and clinical behavior. It is associated with MGA in only very rare cases. One hypothesis is that MGA exists in a transient stage in which its progression to invasive carcinoma happens rapidly before it is ever detected. Another more likely possibility is that MGA serves as a nonobligate precursor to only a subset of TNBCs.
Clinical Management and Prognosis
Complete excision of uncomplicated and atypical MGA is recommended. Because MGA does not elicit a stromal response, and therefore forms a grossly ill-defined lesion, it may be challenging for the surgeon to achieve clear margins. If incompletely excised, local recurrence of MGA has been reported [114, 123]. Limited data are available regarding cases treated by breast conservation. Resetkova et al. [127] reported the case of a patient who developed recurrent carcinoma 10 years after breast conservation surgery with incomplete resection of MGA. The clinical and pathologic findings of this case suggested that the MGA gave rise to two metachronous primary mammary carcinomas, with the second carcinoma arising in residual MGA. Of note, this patient demonstrated a rather indolent course following a diagnosis of TNBC. If invasive carcinoma associated with MGA is managed conservatively, it is prudent to completely excise the MGA in addition to the invasive component.
It is not known whether carcinomas arising in MGA have unusual clinical behavior and outcome in comparison to other TNBCs. A study by James et al. [126] suggested that carcinomas arising in MGA have a relatively favorable prognosis. Fourteen cases of invasive carcinoma, mostly triple negative (11 cases), were identified from 60 cases of MGA. Ten patients treated by mastectomy were recurrence-free after a median follow-up period of approximately 5 years. Of the three patients treated by breast conservation, two were recurrence-free 12 and 105 months after treatment and the third was still alive at 98 months after developing bone metastases at 51 months. In another study, however, 2 of 6 patients had distant metastases at presentation and died of disease within 2 years [116].
Mucocele-Like Lesions
Overview
Mucocele-like lesions (MLLs) of the breast were first described by Rosen [138] in 1986 as benign lesions analogous to mucoceles of the salivary glands. Rosen defined these lesions as mucin-containing cysts lined by flat or low cuboidal epithelium with or without extravasated mucin. Subsequent series revealed that MLLs encompass a spectrum of pathologic changes, including completely benign lesions, ADH, DCIS, and mucinous carcinoma [139, 140]. Therefore, the terms “mucocele-like lesion” and “mucocele-like tumor” are descriptive terms for mucin-filled cysts that may contain associated extravasated mucin, but they do not provide a diagnosis of the pathologic process at hand. A major focus of the literature is whether radiologic or pathologic features can discriminate benign from malignant MLLs in order to inform the clinical management of these lesions.
Clinical, Radiologic, and Gross Features
MLLs are identified in 0.5–1% of breast CNBs [141, 142]. MLLs have been reported in patients who ranged from the third to eighth decade in age. The majority of MLLs are detected by mammography, most frequently as indeterminate calcifications, and less frequently as a mass lesion [143,144,145]. Trends in the literature suggest that screen-detected benign MLLs tend to present in postmenopausal women, whereas those that present as palpable masses tend to present in premenopausal women. Mucinous carcinoma, in contrast, presents as masses in an older age group.
In a recent study, Park and Kim [143] described the imaging findings for 27 cases diagnosed as pure MLLs on CNB. Microcalcifications were the most common mammographic feature (48%), and most of them had a round shape (50%) and grouped distribution (70%). Few (21%) cases presented as asymmetries or masses without calcifications. The ultrasound features included oval shape (95%), circumscribed margin (76%), parallel orientation (91%), complex cystic and solid echo pattern (95%), and no posterior feature (76%).
Other studies have compared the radiologic features of both benign and malignant MLLs. Kim et al. [144] reviewed 15 benign and 10 malignant (9 DCIS, 1 mucinous carcinoma) cases of MLL. At mammography, all of the MLLs showed calcifications and 5 cases (2 benign and 3 malignant) appeared as either a single round mass or multiple “rosary-like” masses. Pleomorphic calcifications were characteristic of the majority of both benign (73%) and malignant (80%) MLLs, often admixed with large coarse eggshell-shaped calcifications or fine linear or granular calcifications. Of note, in this study, the distribution of calcifications differed between benign and malignant lesions. Benign MLLs were associated with grouped calcifications (73% of cases), whereas in malignant MLLs, calcifications displayed a segmental or diffuse distribution (80% of cases) and extended over a wider area. At sonography, benign and malignant MLLs showed similar features, most commonly presenting as complex cysts associated with calcified or noncalcified mural nodules.
In a series of 47 cases of MLLs with benign features, ADH and DCIS, Carkaci et al. [145] found that many (61%) of these lesions were associated with calcifications on mammography, likewise either grouped coarse heterogeneous or fine pleomorphic calcifications in most instances. A small subset of MLLs from each pathologic category presented as masses, characterized by round/oval, lobular, or irregular shapes and circumscribed, indistinct, or irregular margins. At sonography, MLLs appeared as complex cysts and solid hypoechoic masses with varying shapes and margins as well. Neither the features of the calcifications nor the masses could reliably differentiate between benign, atypical, and malignant MLLs. Consistent with these findings, other smaller studies have demonstrated that the predominant radiologic lesions are clustered pleomorphic calcifications or well-defined nodules on mammogram and complex cysts or hypoechoic nodules on ultrasound. Each of these studies included too few cases to draw conclusions regarding the distinguishing characteristics of benign and malignant lesions [146, 147]. Although some features overlap with mucinous adenocarcinoma, it is useful to note that mucinous adenocarcinoma is much more likely to present as a mass and less likely to contain calcifications [148].
Grossly, MLLs may be well-defined multiloculated cystic nodules or ill-defined shiny areas with a gelatinous or mucoid cut surface. If prominent calcifications are present, yellow flecks may be apparent on the cut surface and a gritty texture may be appreciated near the lesion. Lesions ranging in size from 0.5 to 10 cm have been reported. One study showed that the average size of malignant lesions is greater (2.8 versus 1.8 cm) [139].
Microscopic Features
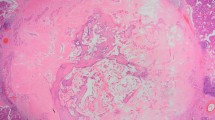
MLLs are characterized by aggregates of mucin-containing cysts that may be associated with mucin extravasation. Benign MLLs consist of cysts partially or completely lined by flat to cuboidal epithelium. Ruptured cysts show strips of benign epithelium that have lifted from the ductal wall and that are floating within the mucin. Benign MLLs may show clusters of epithelial cells in the luminal or extravasated mucin, but these clusters must be rare and must lack cytologic atypia. Extravasated mucin is present as pools of various shapes and sizes with a pushing border or with dissection through the fibrous stroma (Fig. 5.22). The mucin elicits little to no inflammatory response. Rarely, multinucleated giant cells are present within or surrounding the extravasated mucin. Mucicarmine and PAS special stains highlight the mucin. The mucin is frequently associated with characteristic coarse calcifications with distinct areas of finer granularity (Fig. 5.23). The epithelium may exhibit columnar cell metaplasia or proliferative changes ranging from UDH to ADH (Fig. 5.24).
Mucocele-like lesions without atypia (a–e). Benign mucinous cysts with focal rupture and extravasated mucin in stroma (a). Pools of extravasated mucin adjacent to a duct with epithelial hyperplasia and mucin production (b). Small MLL with associated calcifications (c). Small, incidental MLL (d). Large, mass forming MLL on excision (e)
Rare case reports have described a unique lesion with hybrid features of columnar cell change and mucocele-like lesion (Fig. 5.25). Coyne [149] noted a single enlarged lobular unit consisting of cystically dilated glands lined by columnar cells with basally oriented nuclei, abundant eosinophilic cytoplasm, and prominent apical snouts. Foci of columnar cell hyperplasia were present. Alcian blue-positive mucin was observed within the dilated glands and as small lakes within the stroma. The glands contained large round basophilic calcifications and numerous crystalloids. Areas of conventional MLL with attenuated epithelium were not seen. Fadare and Mariappan [150] reported a mucocele-lesion with large cysts lined by attenuated epithelium admixed with tubules with columnar cell change and hyperplasia. There was a gradual morphologic transition between columnar cell areas and conventional MLL areas in some cysts. Both reported cases showed diffuse positivity for estrogen and progesterone receptors. The findings suggest that the pathogenesis of MLLs may be linked to columnar cell lesions in some cases.
Malignant MLLs include those with intraductal carcinoma and those with foci of invasion (Figs. 5.26 and 5.27). Intraductal carcinoma typically displays papillary, micropapillary, or cribriform patterns of growth. Lesions with the cytological and architectural features of low-grade DCIS, but measuring less than 0.2 cm or involving only a single duct space, should be categorized as ADH. Also, if it is difficult to assess the true extent of the lesion due to significant dilatation of multiple duct spaces, a diagnosis of DCIS should be rendered only when at least two duct spaces or lobules are completely involved by the atypical proliferation. Invasive carcinoma can be diagnosed on a background of MLL when there are foci of detached clusters of cytologically malignant epithelial cells within separate pools of extravasated mucin. Adjacent intraductal carcinoma is usually present. Hamele-Bena et al. [139] noted that large, coarse calcifications within the cysts and the extravasated mucin were a striking characteristic of MLLs, more often identified in malignant cases (71%) than benign cases (46%). Moreover, ADH was present in 7 of the 11 benign cases with these calcifications. It can be argued that identification of large, coarse calcifications in a MLL should prompt careful examination of the tissue to rule out involvement by atypia or malignancy.
Ductal carcinoma in situ with extracellular mucin production (a–d). The DCIS is associated with several ducts with marked dilation by mucinous contents and few free-floating atypical epithelial clusters. The residual epithelial lining at the perimeter of the mucin noted on H&E (a, b) and the underlying myoepithelial layer confirmed on immunohistochemical stain for SMM (c–d) are reassuring that the free-floating clusters do not represent mucinous carcinoma
Mucocele-like lesions associated with DCIS and invasive carcinoma (a–f). One case shows DCIS lifting off of the duct wall and rare clusters of atypical cells floating in mucin (a–c), indefinite for microinvasion (c). Another case contains DCIS adjacent to multiple clusters of atypical cells in extravasated mucin (d–f), consistent with a small focus of invasive mucinous carcinoma (f)
Differential Diagnosis
The primary consideration in the differential diagnosis of MLLs is mucinous (or “colloid”) carcinoma of the breast. Mucinous carcinoma features tumor cells that grow as clusters, trabeculae, festoons, micropapillae, or cribriform structures within pools of extracellular mucin. The tumor cells usually show low to intermediate grade nuclear atypia. The cellularity of the tumor varies from case to case. Multiple sections may be necessary to detect carcinoma cells if the carcinoma is composed almost entirely of mucin or if only pools of acellular mucin are seen. Intraductal carcinoma is associated with 75% of these tumors, generally located at the periphery, and occasionally there are morphologic transitions from intraductal to invasive components. MLLs in which small fragments of epithelium have dislodged into the extracellular mucin raise the greatest suspicion for mucinous carcinoma. It is particularly problematic if DCIS is associated with the MLL and the free-floating clusters of cells are clearly neoplastic; in this setting, focal invasion is a possibility [151].
Careful evaluation of the epithelial elements of the MLL will aid in determining the true nature of the free-floating epithelium (Fig. 5.28). If a well-sampled MLL is devoid of significant atypia or CIS, it is unlikely that small epithelial fragments floating within the mucin represent a malignant process. In cases of MLL associated with DCIS, a diagnosis of displaced epithelium is favored when the floating clusters of tumor cells are few and focal; when they are associated with myoepithelial cells; or when they are contiguous with the ductal epithelium and appear to have lifted off of the duct wall (Fig. 5.29). It may be nearly impossible to determine the significance of floating epithelial clusters in some cases. In this situation, it would be appropriate to express uncertainty and to acknowledge the possibility of a small focus of invasion.
Epithelial clusters of uncertain significance in the setting of atypical (a, b) or malignant (c, d) MLLs with lesional distortion by abundant mucin and surrounding stromal response. The small clusters in sclerotic stroma cannot be definitively diagnosed as microinvasion (a, b) nor can the rare, minute clusters floating in mucin be diagnosed as mucinous carcinoma (c, d). A diagnosis of mucinous carcinoma can be more confidently made with there are several atypical epithelial clusters within a pool of extravasated mucin separate from nearby DCIS and this pool shows more complex fibrovascular septation
Immunohistochemical Workup
Immunohistochemistry for myoepithelial cell markers may aid in the distinction of epithelial displacement from focal invasion in cases of MLLs associated with DCIS. A diagnosis of epithelial displacement is suggested by the identification of preserved myoepithelial cells within the floating clusters of tumor cells [151]. A myoepithelial cell layer is confirmed beneath the epithelium of the cysts and distended ducts of MLLs on these studies.
Pathogenesis
MLLs most likely result from excess production of mucinous secretions or ductal obstruction, which lead to ductal distention, rupture, and extravasation of mucin. Minor trauma may contribute to the rupture of the cysts. Some authors have suggested that MLLs with DCIS may be a precursor of mucinous carcinoma of the breast. Kryvenko et al. [152] characterized patterns of transition from intraductal to invasive carcinoma in 130 cases of mucinous carcinoma. Interestingly, one pattern of transition, which was exclusive to cases of type A mucinous carcinoma (dominant mucin component), involved overdistention and rupture of ducts with DCIS by luminal mucin. Extravasated mucin contained floating carcinoma cells in predominantly papillary clusters. Foci of residual DCIS and residual benign ducts with mucinous contents were located at the periphery of the invasive mucinous carcinoma. This study provided evidence in support of the theory that malignant MLLs serve as a precursor of mucinous carcinoma of the breast, more specifically those with type A morphology.
Clinical Management and Prognosis
Early series of MLLs consisting of excisional biopsies and mastectomies demonstrated that these lesions encompass a morphologic spectrum from benign to atypical to malignant. With the advent of CNBs, it was assumed that all MLLs should be excised in order to avoid issues related to inadequate sampling of a heterogeneous lesion, and this became the prevailing practice. At that time, there was little data to support or refute this recommendation. Since then, many studies have been limited by small numbers, insufficient radiologic-pathologic correlation, and varying definitions of what constitutes an upgrade (ADH versus DCIS), leading to widely variable upgrade rates ranging from 0% to 43% in the literature [98, 141, 142, 145, 147, 153,154,155,156,157,158,159,160]. Reflecting this, conflicting opinions exist regarding the management of MLLs diagnosed on CNB.
Several studies have shown that mucinous lesions can be reliably diagnosed on CNB. In these studies, the upgrade rates are acceptable, if an upgrade is strictly defined as malignancy (DCIS or invasive carcinoma) on excision. Renshaw [141] reviewed 22 cases of mucinous lesions diagnosed on CNB, ranging from benign MLLs to mucinous carcinomas, and found no changes in diagnosis from benign to malignant or vice versa for the 15 cases that were excised. Similarly, Wang et al. [142] evaluated benign and malignant mucinous lesions in 32 CNBs and corresponding excisions and found that all of the benign MLLs were benign on excision as well. Some authors have shown that important differences in upgrade rates exist for benign MLLs with and without atypia. Carkaci et al. [145] identified 44 cases of MLL diagnosed on CNB or fine needle aspiration, albeit only 16 of which were surgically excised. Three (19%) of 16 cases were upgraded to malignancy on excision. None of the cases of MLL without atypia were found to be malignant. However, all three cases of MLL with atypia on biopsy were upgraded to DCIS. Similarly, Sutton et al. [161] found an overall upgrade rate of 13% in their series of 38 excised MLLs. All 22 MLLs without atypia were found to be benign on excision, while five (31%) of the MLLs with atypia were upgraded to DCIS.
Other recent studies have shown that MLLs without atypia have a small risk of upgrade to malignancy, although this risk is still much lower than observed for atypical MLLs. For example, Towne et al. [162] found that 2 of 25 (8%) MLLs without atypia diagnosed on CNB were upgraded to DCIS on excision, while Zhang et al. [163] noted the same in 1 of 19 cases (5%). Rakha et al. [164] reviewed their own data as well as the literature to date. Two (4%) of 54 cases of MLL without atypia on CNB demonstrated DCIS on the subsequent excision specimen, concordant with an upgrade rate of 4% (4 of 106) for benign MLLs in the literature. A review of previous studies revealed that the frequency of malignancy is much higher at 21% (7 of 33) if there is atypia in the CNB. Overall, an analysis of the literature reveals that surgical excision of all MLLs involved by ADH or DCIS is clearly recommended. In contrast, it may be appropriate to consider more conservative management of benign MLLs without atypia in the setting of no radiologic mass lesion and close follow-up.
Some authors advocate for the excision of all MLLs regardless of the presence of atypia. Much of this perspective results from few studies showing that ADH is found on the excision of some benign MLLs without atypia. In a small series by Carder et al. [154], 2 (33%) of 6 cases of benign MLL contained ADH in the excision. Again, 3 (75%) of 4 cases of MLL with ADH were upgraded to malignancy (two DCIS and one mucinous carcinoma). More recently, Jaffer et al. [159] evaluated 45 cases of benign MLL on CNBs and identified ADH in seven cases (16%) and DCIS is one case (2%) on excision. Both sets of authors argued that finding ADH would inform the patient and the clinician of an overall increased risk of breast cancer, providing an opportunity for more frequent screening and endocrine prophylaxis in certain patients. However, a diagnosis of ADH may or may not be treated in such a manner, and considering it as a true upgrade overestimates the risk associated with benign MLLs. It appears reasonable to recommend excision for MLLs, even those without atypia, in the setting of a radiologic mass to exclude the possibility of undersampling. However, in small lesions up to 1.0 cm that might be entirely sampled on core biopsy, clinical and radiologic follow-up without excision may be appropriate as long as there is no atypia and radiologic-pathologic correlation does not reveal discordant findings.
Recurrences of MLL are rare but have been noted in the literature. Fisher and Millis [165] reported a case of a mucocele-like tumor with atypia excised from a 31-year-old woman. One year later, three masses with histologic features of pure mucinous carcinoma with associated papillary intraductal carcinoma recurred at the same site. In a long-term follow-up study of 13 MLLs (5 with ADH, 3 with low grade DCIS) diagnosed on excisional biopsy, Ohi et al. [166] found that one MLL with ADH recurred as an MLL with DCIS at 37 months after excision. Additionally, Hamele-Bena et al. [139] identified two patients with local recurrences in their series of 53 MLLs. One patient developed an MLL with coexistent LCIS 2 years after an excisional biopsy of an MLL with UDH. Another patient, who initially presented with an MLL with focal invasion and underwent excision of the lesion, subsequently developed an ipsilateral MLL with focal invasion 5 years later. The patient also had a contralateral MLL with DCIS. In this series, one of the largest ones, the length of follow-up for the entire group ranged from less than 1 to 15.3 years, with a mean follow-up of 3.7 years. All patients were alive and well without evidence of disease at the time of the report.
In summary, adenosis, radial scars, MGA, and MLLs constitute lesions that may sometimes be confused with invasive carcinoma, especially when they are only partially represented on CNB. Familiarity with these lesions, a high degree of suspicion, and judicious use of immunohistochemical studies will help the pathologist resolve most potential diagnostic dilemmas.
References
Foote FW, Stewart FW. Comparative studies of cancerous versus noncancerous breasts. Ann Surg. 1945;121(1):6–53.
Urban JA, Adair FE. Sclerosing adenosis. Cancer. 1949;2(4):625–34.
Heller EL, Fleming JC. Fibrosing adenomatosis of the breast. Am J Clin Pathol. 1950;20(2):141–6.
Visscher DW, et al. Sclerosing adenosis and risk of breast cancer. Breast Cancer Res Treat. 2014;144(1):205–12.
Taskin F, et al. Sclerosing adenosis of the breast: radiologic appearance and efficiency of core needle biopsy. Diagn Interv Radiol. 2011;17(4):311–6.
Gill HK, Ioffe OB, Berg WA. When is a diagnosis of sclerosing adenosis acceptable at core biopsy? Radiology. 2003;228(1):50–7.
Gunhan-Bilgen I, et al. Sclerosing adenosis: mammographic and ultrasonographic findings with clinical and histopathological correlation. Eur J Radiol. 2002;44(3):232–8.
Nielsen NS, Nielsen BB. Mammographic features of sclerosing adenosis presenting as a tumour. Clin Radiol. 1986;37(4):371–3.
DiPiro PJ, et al. Mammographic and sonographic appearances of nodular adenosis. AJR Am J Roentgenol. 2000;175(1):31–4.
Chen YL, et al. Sclerosing adenosis: ultrasonographic and mammographic findings and correlation with histopathology. Mol Clin Oncol. 2017;6(2):157–62.
Taylor HB, Norris HJ. Epithelial invasion of nerves in benign diseases of the breast. Cancer. 1967;20(12):2245–9.
Davies JD. Neural invasion in benign mammary dysplasia. J Pathol. 1973;109(3):225–31.
Gobbi H, et al. Atypical ductal hyperplasia and ductal carcinoma in situ of the breast associated with perineural invasion. Hum Pathol. 2001;32(8):785–90.
Eusebi V, Azzopardi JG. Vascular infiltration in benign breast disease. J Pathol. 1976;118(1):9–16.
Chan JK, Ng WF. Sclerosing adenosis cancerized by intraductal carcinoma. Pathology. 1987;19(4):425–8.
Fechner RE. Lobular carcinoma in situ in sclerosing adenosis. A potential source of confusion with invasive carcinoma. Am J Surg Pathol. 1981;5(3):233–9.
Fechner RE. Carcinoma in situ involving sclerosing adenosis. Histopathology. 1996;28(6):570.
Oberman HA, Markey BA. Noninvasive carcinoma of the breast presenting in adenosis. Mod Pathol. 1991;4(1):31–5.
Moritani S, et al. Topographical, morphological and immunohistochemical characteristics of carcinoma in situ of the breast involving sclerosing adenosis. Two distinct topographical patterns and histological types of carcinoma in situ. Histopathology. 2011;58(6):835–46.
Flotte TJ, Bell DA, Greco MA. Tubular carcinoma and sclerosing adenosis: the use of basal lamina as a differential feature. Am J Surg Pathol. 1980;4(1):75–7.
Ekblom P, et al. Basement membrane and apocrine epithelial antigens in differential diagnosis between tubular carcinoma and sclerosing adenosis of the breast. J Clin Pathol. 1984;37(4):357–63.
Jao W, Recant W, Swerdlow MA. Comparative ultrastructure of tubular carcinoma and sclerosing adenosis of the breast. Cancer. 1976;38(1):180–6.
Harrison BT, et al. Quality assurance in breast pathology: lessons learned from a review of amended reports. Arch Pathol Lab Med. 2017;141(2):260–6.
Cui X, Wei S. Carcinoma in situ involving sclerosing adenosis: seeking the salient histological characteristics to prevent overdiagnosis. Ann Clin Lab Sci. 2017;47(5):529–34.
Dewar R, et al. Best practices in diagnostic immunohistochemistry: myoepithelial markers in breast pathology. Arch Pathol Lab Med. 2011;135(4):422–9.
Werling RW, et al. Immunohistochemical distinction of invasive from noninvasive breast lesions: a comparative study of p63 versus calponin and smooth muscle myosin heavy chain. Am J Surg Pathol. 2003;27(1):82–90.
Shoker BS, et al. Abnormal regulation of the oestrogen receptor in benign breast lesions. J Clin Pathol. 2000;53(10):778–83.
Franquet T, et al. Spiculated lesions of the breast: mammographic-pathologic correlation. Radiographics. 1993;13(4):841–52.
Bianchi S, et al. Radial scar without associated atypical epithelial proliferation on image-guided 14-gauge needle core biopsy: analysis of 49 cases from a single-centre and review of the literature. Breast. 2012;21(2):159–64.
Jensen RA, et al. Invasive breast cancer risk in women with sclerosing adenosis. Cancer. 1989;64(10):1977–83.
London SJ, et al. A prospective study of benign breast disease and the risk of breast cancer. JAMA. 1992;267(7):941–4.
Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312(3):146–51.
Calhoun BC, Booth CN. Atypical apocrine adenosis diagnosed on breast core biopsy: implications for management. Hum Pathol. 2014;45(10):2130–5.
Fuehrer N, et al. Atypical apocrine adenosis of the breast: long-term follow-up in 37 patients. Arch Pathol Lab Med. 2012;136(2):179–82.
Seidman JD, Ashton M, Lefkowitz M. Atypical apocrine adenosis of the breast: a clinicopathologic study of 37 patients with 8.7-year follow-up. Cancer. 1996;77(12):2529–37.
Tavassoli FA, Norris HJ. Intraductal apocrine carcinoma: a clinicopathologic study of 37 cases. Mod Pathol. 1994;7(8):813–8.
O’Malley FP, Bane A. An update on apocrine lesions of the breast. Histopathology. 2008;52(1):3–10.
O’Malley FP, Bane AL. The spectrum of apocrine lesions of the breast. Adv Anat Pathol. 2004;11(1):1–9.
Bianchi S, et al. Reproducibility of histological diagnoses and diagnostic accuracy of non palpable breast lesions. Pathol Res Pract. 1994;190(1):69–76.
Selim AG, Wells CA. Immunohistochemical localisation of androgen receptor in apocrine metaplasia and apocrine adenosis of the breast: relation to oestrogen and progesterone receptors. J Clin Pathol. 1999;52(11):838–41.
Selim AG, El-Ayat G, Wells CA. Expression of c-erbB2, p53, Bcl-2, Bax, c-myc and Ki-67 in apocrine metaplasia and apocrine change within sclerosing adenosis of the breast. Virchows Arch. 2002;441(5):449–55.
Selim AG, El-Ayat G, Wells CA. c-erbB2 oncoprotein expression, gene amplification, and chromosome 17 aneusomy in apocrine adenosis of the breast. J Pathol. 2000;191(2):138–42.
Wells CA, et al. Apocrine adenosis: a precursor of aggressive breast cancer? J Clin Pathol. 1995;48(8):737–42.
Chang Sen LQ, Berg WA, Carter GJ. Upgrade rate and imaging features of atypical apocrine lesions. Breast J. 2017;23(5):569–78.
Hou Y, et al. Surgical follow-up results for apocrine adenosis and atypical apocrine adenosis diagnosed on breast core biopsy. Ann Diagn Pathol. 2016;24:4–6.
Carter DJ, Rosen PP. Atypical apocrine metaplasia in sclerosing lesions of the breast: a study of 51 patients. Mod Pathol. 1991;4(1):1–5.
Semb CB. Pathologico-anatomical and clinical investigations of fibroadenomatosis cystica mammae: and its relation to other pathological conditions in the mamma, especially cancer. Acta Chirurgica Scandinavica. 1928;64:1–484.
Fenoglio C, Lattes R. Sclerosing papillary proliferations in the female breast. A benign lesion often mistaken for carcinoma. Cancer. 1974;33(3):691–700.
Tremblay G, Buell RH, Seemayer TA. Elastosis in benign sclerosing ductal proliferation of the female breast. Am J Surg Pathol. 1977;1(2):155–66.
Fisher ER, et al. A nonencapsulated sclerosing lesion of the breast. Am J Clin Pathol. 1979;71(3):240–6.
Azzopardi JG, Ahmed A, Millis RR. Problems in breast pathology. In: Major problems in pathology. Philadelphia: W.B. Saunders Company; 1979. p. 466.
Rickert RR, Kalisher L, Hutter RV. Indurative mastopathy: a benign sclerosing lesion of breast with elastosis which may simulate carcinoma. Cancer. 1981;47(3):561–71.
Hamperl H. [Radial scars (scarring) and obliterating mastopathy (author’s transl)]. Virchows Arch A Pathol Anat Histol. 1975;369(1):55–68.
Page DL, Anderson TJ. Diagnostic histopathology of the breast. Edinburgh: Churchill Livingstone; 1987.
Schnitt SJ, Collins LC. Biopsy interpretation of the breast, Biopsy interpretation series. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009.
Sahin A, Collins LC. Radial scar/complex sclerosing lesion. In: WHO Classification of Tumours Editorial Board, editor. Breast tumours. Lyon: International Agency for Research on Cancer; 2019. p. 30–31.
Linell F, Ljungberg O. Breast carcinoma. Progression of tubular carcinoma and a new classification. Acta Pathol Microbiol Scand A. 1980;88(1):59–60.
Burnett SJ, et al. Benign biopsies in the prevalent round of breast screening: a review of 137 cases. Clin Radiol. 1995;50(4):254–8.
Cawson JN, et al. Fourteen-gauge needle core biopsy of mammographically evident radial scars: is excision necessary? Cancer. 2003;97(2):345–51.
Berg JC, et al. Breast cancer risk in women with radial scars in benign breast biopsies. Breast Cancer Res Treat. 2008;108(2):167–74.
Jacobs TW, et al. Radial scars in benign breast-biopsy specimens and the risk of breast cancer. N Engl J Med. 1999;340(6):430–6.
Sanders ME, et al. Interdependence of radial scar and proliferative disease with respect to invasive breast carcinoma risk in patients with benign breast biopsies. Cancer. 2006;106(7):1453–61.
Tabar L, Dean PB. Teaching atlas of mammography. Stuttgart: Georg Thieme Verlag; 1985.
Mitnick JS, et al. Differentiation of radial scar from scirrhous carcinoma of the breast: mammographic-pathologic correlation. Radiology. 1989;173(3):697–700.
Hassell P, et al. Radial sclerosing lesions of the breast: mammographic and pathologic correlation. Can Assoc Radiol J. 1999;50(6):370–5.
Boute V, et al. Are the criteria of Tabar and Dean still relevant to radial scar? Eur J Radiol. 2006;60(2):243–9.
Mitnick JS, Harris MN, Roses DF. Mammographic detection of carcinoma of the breast in patients with augmentation prostheses. Surg Gynecol Obstet. 1989;168(1):30–2.
Denley H, et al. Metaplastic carcinoma of the breast arising within complex sclerosing lesion: a report of five cases. Histopathology. 2000;36(3):203–9.
Gobbi H, et al. Metaplastic spindle cell breast tumors arising within papillomas, complex sclerosing lesions, and nipple adenomas. Mod Pathol. 2003;16(9):893–901.
Wilsher MJ. Adenosquamous proliferation of the breast and low grade adenosquamous carcinoma: a common precursor of an uncommon cancer? Pathology. 2014;46(5):402–10.
Wilsher MJ, Owens TW, Allcock RJ. Next generation sequencing of the nidus of early (adenosquamous proliferation rich) radial sclerosing lesions of the breast reveals evidence for a neoplastic precursor lesion. J Pathol Clin Res. 2017;3(2):115–22.
Eberle CA, et al. Infiltrating epitheliosis of the breast: characterization of histological features, immunophenotype and genomic profile. Histopathology. 2016;68(7):1030–9.
Hilson JB, Schnitt SJ, Collins LC. Phenotypic alterations in myoepithelial cells associated with benign sclerosing lesions of the breast. Am J Surg Pathol. 2010;34(6):896–900.
Kawaguchi K, Shin SJ. Immunohistochemical staining characteristics of low-grade adenosquamous carcinoma of the breast. Am J Surg Pathol. 2012;36(7):1009–20.
Oh EY, Collins LC. Keratin expression patterns in stromal cells of benign sclerosing lesions of the breast: a potential diagnostic pitfall. Arch Pathol Lab Med. 2015;139(9):1143–8.
Elston CW, Ellis IO. The breast. Edinburgh: Churchill Livingstone; 1998.
Anderson TJ, Battersby S. Radial scars of benign and malignant breasts: comparative features and significance. J Pathol. 1985;147(1):23–32.
Jacobs TW, et al. Radial scars of the breast and breast carcinomas have similar alterations in expression of factors involved in vascular stroma formation. Hum Pathol. 2002;33(1):29–38.
Iqbal M, et al. Molecular and genetic abnormalities in radial scar. Hum Pathol. 2002;33(7):715–22.
Wolters KL, et al. Frequent PIK3CA mutations in radial scars. Diagn Mol Pathol. 2013;22(4):210–4.
Bataillon G, et al. High rate of PIK3CA mutations but no TP53 mutations in low-grade adenosquamous carcinoma of the breast. Histopathology. 2018;73(2):273–83.
Ang D, et al. Novel method for PIK3CA mutation analysis: locked nucleic acid—PCR sequencing. J Mol Diagn. 2013;15(3):312–8.
Troxell ML, et al. High prevalence of PIK3CA/AKT pathway mutations in papillary neoplasms of the breast. Mod Pathol. 2010;23(1):27–37.
Troxell ML, et al. Phosphatidylinositol-3-kinase pathway mutations are common in breast columnar cell lesions. Mod Pathol. 2012;25(7):930–7.
Dahlstrom JE, et al. Diagnostic accuracy of stereotactic core biopsy in a mammographic breast cancer screening programme. Histopathology. 1996;28(5):421–7.
Dershaw DD, et al. Nondiagnostic stereotaxic core breast biopsy: results of rebiopsy. Radiology. 1996;198(2):323–5.
Lee AH, et al. Excision biopsy findings of patients with breast needle core biopsies reported as suspicious of malignancy (B4) or lesion of uncertain malignant potential (B3). Histopathology. 2003;42(4):331–6.
Lee CH, et al. Cost-effectiveness of stereotactic core needle biopsy: analysis by means of mammographic findings. Radiology. 1997;202(3):849–54.
Meyer JE, et al. Large-needle core biopsy: nonmalignant breast abnormalities evaluated with surgical excision or repeat core biopsy. Radiology. 1998;206(3):717–20.
Jackman RJ, et al. Stereotactic, automated, large-core needle biopsy of nonpalpable breast lesions: false-negative and histologic underestimation rates after long-term follow-up. Radiology. 1999;210(3):799–805.
Kirwan SE, et al. Multiple 14G stereotactic core biopsies in the diagnosis of mammographically detected stellate lesions of the breast. Clin Radiol. 2000;55(10):763–6.
Philpotts LE, et al. Uncommon high-risk lesions of the breast diagnosed at stereotactic core-needle biopsy: clinical importance. Radiology. 2000;216(3):831–7.
Brenner RJ, et al. Percutaneous core needle biopsy of radial scars of the breast: when is excision necessary? AJR Am J Roentgenol. 2002;179(5):1179–84.
Brodie C, O’Doherty A, Quinn C. Fourteen-gauge needle core biopsy of mammographically evident radial scars: is excision necessary? Cancer. 2004;100(3):652–3; author reply 653–4.
Becker L, et al. Management of radial scars found at percutaneous breast biopsy. Can Assoc Radiol J. 2006;57(2):72–8.
Lopez-Medina A, et al. Radial scars diagnosed at stereotactic core-needle biopsy: surgical biopsy findings. Eur Radiol. 2006;16(8):1803–10.
Dillon MF, et al. Predictive value of breast lesions of “uncertain malignant potential” and “suspicious for malignancy” determined by needle core biopsy. Ann Surg Oncol. 2007;14(2):704–11.
El-Sayed ME, et al. Predictive value of needle core biopsy diagnoses of lesions of uncertain malignant potential (B3) in abnormalities detected by mammographic screening. Histopathology. 2008;53(6):650–7.
Lieske B, et al. Screen-detected breast lesions with an indeterminate (B3) core needle biopsy should be excised. Eur J Surg Oncol. 2008;34(12):1293–8.
Hayes BD, O’Doherty A, Quinn CM. Correlation of needle core biopsy with excision histology in screen-detected B3 lesions: the Merrion breast screening unit experience. J Clin Pathol. 2009;62(12):1136–40.
Linda A, et al. Radial scars without atypia diagnosed at imaging-guided needle biopsy: how often is associated malignancy found at subsequent surgical excision, and do mammography and sonography predict which lesions are malignant? AJR Am J Roentgenol. 2010;194(4):1146–51.
Rakha EA, et al. Outcome of breast lesions diagnosed as lesion of uncertain malignant potential (B3) or suspicious of malignancy (B4) on needle core biopsy, including detailed review of epithelial atypia. Histopathology. 2011;58(4):626–32.
Rakha EA, et al. Screen-detected malignant breast lesions diagnosed following benign (B2) or normal (B1) needle core biopsy diagnoses. Eur J Cancer. 2010;46(10):1835–40.
Rajan S, Wason AM, Carder PJ. Conservative management of screen-detected radial scars: role of mammotome excision. J Clin Pathol. 2011;64(1):65–8.
Rakha EA, et al. Characterization and outcome of breast needle core biopsy diagnoses of lesions of uncertain malignant potential (B3) in abnormalities detected by mammographic screening. Int J Cancer. 2011;129(6):1417–24.
Resetkova E, et al. Management of radial sclerosing lesions of the breast diagnosed using percutaneous vacuum-assisted core needle biopsy: recommendations for excision based on seven years’ of experience at a single institution. Breast Cancer Res Treat. 2011;127(2):335–43.
Sloane JP, Mayers MM. Carcinoma and atypical hyperplasia in radial scars and complex sclerosing lesions: importance of lesion size and patient age. Histopathology. 1993;23(3):225–31.
Matrai C, et al. Advocating nonsurgical management of patients with small, incidental radial scars at the time of needle core biopsy: a study of 77 cases. Arch Pathol Lab Med. 2015;139:1137–42.
Lee KA, et al. Risk of malignancy when microscopic radial scars and microscopic papillomas are found at percutaneous biopsy. AJR Am J Roentgenol. 2012;198(2):W141–5.
Park VY, et al. Mammographically occult asymptomatic radial scars/complex sclerosing lesions at ultrasonography-guided core needle biopsy: follow-up can be recommended. Ultrasound Med Biol. 2016;42(10):2367–71.
Farshid G, Buckley E. Meta-analysis of upgrade rates in 3163 radial scars excised after needle core biopsy diagnosis. Breast Cancer Res Treat. 2019;174(1):165–77.
McDivitt RW, Stewart FW, Berg JW, Universities Associated for Research and Education in Pathology. Tumors of the breast. In: Armed Forces Institute of Pathology (US), editor. Atlas of tumor pathology, second series, fascicle 2. Washington, DC: Armed Forces Institute of Pathology; 1968.
Clement PB, Azzopardi JG. Microglandular adenosis of the breast—a lesion simulating tubular carcinoma. Histopathology. 1983;7(2):169–80.
Rosen PP. Microglandular adenosis. A benign lesion simulating invasive mammary carcinoma. Am J Surg Pathol. 1983;7(2):137–44.
Tavassoli FA, Norris HJ. Microglandular adenosis of the breast. A clinicopathologic study of 11 cases with ultrastructural observations. Am J Surg Pathol. 1983;7(8):731–7.
Khalifeh IM, et al. Clinical, histopathologic, and immunohistochemical features of microglandular adenosis and transition into in situ and invasive carcinoma. Am J Surg Pathol. 2008;32(4):544–52.
Geyer FC, et al. Microglandular adenosis or microglandular adenoma? A molecular genetic analysis of a case associated with atypia and invasive carcinoma. Histopathology. 2009;55(6):732–43.
Geyer FC, et al. Molecular evidence in support of the neoplastic and precursor nature of microglandular adenosis. Histopathology. 2012;60(6B):E115–30.
Shin SJ, et al. Molecular evidence for progression of microglandular adenosis (MGA) to invasive carcinoma. Am J Surg Pathol. 2009;33(4):496–504.
Rosen PP. Rosen’s breast pathology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. p. 1136.
Sabate JM, et al. Microglandular adenosis of the breast in a BRCA1 mutation carrier: radiological features. Eur Radiol. 2002;12(6):1479–82.
Brogi E. Adenosis and microglandular adenosis. In: Brogi E, Hoda SA, Koerner FC, Rosen PP, editors. Rosen’s breast pathology. Philadelphia, PA: Lippincott Williams & Wilkins; 2014.
Tavassoli FA, Bratthauer GL. Immunohistochemical profile and differential diagnosis of microglandular adenosis. Mod Pathol. 1993;6(3):318–22.
Rosenblum MK, Purrazzella R, Rosen PP. Is microglandular adenosis a precancerous disease? A study of carcinoma arising therein. Am J Surg Pathol. 1986;10(4):237–45.
Koenig C, et al. Carcinoma arising in microglandular adenosis: an immunohistochemical analysis of 20 intraepithelial and invasive neoplasms. Int J Surg Pathol. 2000;8(4):303–15.
James BA, Cranor ML, Rosen PP. Carcinoma of the breast arising in microglandular adenosis. Am J Clin Pathol. 1993;100(5):507–13.
Resetkova E, Flanders DJ, Rosen PP. Ten-year follow-up of mammary carcinoma arising in microglandular adenosis treated with breast conservation. Arch Pathol Lab Med. 2003;127(1):77–80.
Shui R, Yang W. Invasive breast carcinoma arising in microglandular adenosis: a case report and review of the literature. Breast J. 2009;15(6):653–6.
Lee YH, et al. Young-aged woman with invasive ductal carcinoma arising in atypical microglandular adenosis: a case report. Pathol Int. 2010;60(10):685–9.
Acs G, et al. Microglandular adenosis with transition into adenoid cystic carcinoma of the breast. Am J Surg Pathol. 2003;27(8):1052–60.
Rosen PP. So-called acinic cell carcinoma of the breast arises from microgladular adenosis and is not a distinct entity. Mod Pathol. 2017;30(10):1504.
Wen YH, Weigelt B, Reis-Filho JS. Microglandular adenosis: a non-obligate precursor of triple-negative breast cancer? Histol Histopathol. 2013;28(9):1099–108.
Geyer FC, et al. Genetic analysis of microglandular adenosis and acinic cell carcinomas of the breast provides evidence for the existence of a low-grade triple-negative breast neoplasia family. Mod Pathol. 2017;30(1):69–84.
Foschini MP, Eusebi V. Microglandular adenosis of the breast: a deceptive and still mysterious benign lesion. Hum Pathol. 2018;82:1–9.
Eusebi V, et al. Microglandular adenosis, apocrine adenosis, and tubular carcinoma of the breast. An immunohistochemical comparison. Am J Surg Pathol. 1993;17(2):99–109.
Guerini-Rocco E, et al. Microglandular adenosis associated with triple-negative breast cancer is a neoplastic lesion of triple-negative phenotype harbouring TP53 somatic mutations. J Pathol. 2016;238(5):677–88.
Radner M, et al. Chromosome 2q gain and epigenetic silencing of GATA3 in microglandular adenosis of the breast. J Pathol Clin Res. 2021;7(3):220–32.
Rosen PP. Mucocele-like tumors of the breast. Am J Surg Pathol. 1986;10(7):464–9.
Hamele-Bena D, Cranor ML, Rosen PP. Mammary mucocele-like lesions. Benign and malignant. Am J Surg Pathol. 1996;20(9):1081–5.
Weaver MG, Abdul-Karim FW, Al-Kaisi N. Mucinous lesions of the breast. A pathological continuum. Pathol Res Pract. 1993;189(8):873–6.
Renshaw AA. Can mucinous lesions of the breast be reliably diagnosed by core needle biopsy? Am J Clin Pathol. 2002;118(1):82–4.
Wang J, et al. Can core biopsy reliably diagnose mucinous lesions of the breast? Am J Clin Pathol. 2007;127(1):124–7.
Park YJ, Kim EK. A pure mucocele-like lesion diagnosed on ultrasonography-guided core-needle biopsy: is imaging follow-up sufficient? Ultrasonography. 2014; https://doi.org/10.14366/usg.14036.
Kim JY, et al. Benign and malignant mucocele-like tumors of the breast: mammographic and sonographic appearances. AJR Am J Roentgenol. 2005;185(5):1310–6.
Carkaci S, et al. Do all mucocele-like lesions of the breast require surgery? Clin Imaging. 2011;35(2):94–101.
Cheng L, Lee WY, Chang TW. Benign mucocele-like lesion of the breast: how to differentiate from mucinous carcinoma before surgery. Cytopathology. 2004;15(2):104–8.
Glazebrook K, Reynolds C. Original report. Mucocele-like tumors of the breast: mammographic and sonographic appearances. AJR Am J Roentgenol. 2003;180(4):949–54.
Chopra S, et al. Pure mucinous breast cancer-mammographic and ultrasound findings. Clin Radiol. 1996;51(6):421–4.
Coyne JD. Columnar cell hyperplasia with intraluminal crystalloids and features of a mucocoele-like lesion. Histopathology. 2004;44(4):401–3.
Fadare O, Mariappan MR. Mucocele-like tumor and columnar cell hyperplasia of the breast occurring in a morphologic continuum. J Med Case Rep. 2008;2:138.
Tan PH, Tse GM, Bay BH. Mucinous breast lesions: diagnostic challenges. J Clin Pathol. 2008;61(1):11–9.
Kryvenko ON, et al. Precursor lesions of mucinous carcinoma of the breast: analysis of 130 cases. Am J Surg Pathol. 2013;37(7):1076–84.
Deschryver K, Radford DM, Schuh ME. Pathology of large-caliber stereotactic biopsies in nonpalpable breast lesions. Semin Diagn Pathol. 1999;16(3):224–34.
Carder PJ, Murphy CE, Liston JC. Surgical excision is warranted following a core biopsy diagnosis of mucocoele-like lesion of the breast. Histopathology. 2004;45(2):148–54.
Ramsaroop R, et al. Mucocele-like lesions of the breast: an audit of 2 years at BreastScreen Auckland (New Zealand). Breast J. 2005;11(5):321–5.
Begum SM, et al. Mucin extravasation in breast core biopsies–clinical significance and outcome correlation. Histopathology. 2009;55(5):609–17.
Ouldamer L, et al. [Mucocele-like lesions of the breast: management after diagnosis on ultrasound guided core biopsy or stereotactic vacuum-assisted biopsy]. Gynecol Obstet Fertil. 2010;38(7–8):455–9.
Flegg KM, et al. Surgical outcomes of borderline breast lesions detected by needle biopsy in a breast screening program. World J Surg Oncol. 2010;8:78.
Jaffer S, Bleiweiss IJ, Nagi CS. Benign mucocele-like lesions of the breast: revisited. Mod Pathol. 2011;24(5):683–7.
Weigel S, et al. Minimal invasive biopsy results of “uncertain malignant potential” in digital mammography screening: high prevalence but also high predictive value for malignancy. Rofo. 2011;183(8):743–8.
Sutton B, et al. Mucocele-like lesions diagnosed on breast core biopsy: assessment of upgrade rate and need for surgical excision. Am J Clin Pathol. 2012;138(6):783–8.
Towne WS, Michaels AY, Ginter PS. Mucocele-like lesion of the breast diagnosed on core biopsy: histologic and clinical analysis of 78 cases with focus on features associated with upgrade. Arch Pathol Lab Med. 2021; https://doi.org/10.5858/arpa.2020-0497-OA.
Zhang G, et al. Mucocele-like lesions diagnosed on breast core biopsy: low risk of upgrade and subsequent carcinoma. Breast J. 2018;24(3):314–8.
Rakha EA, et al. Outcome of pure mucocele-like lesions diagnosed on breast core biopsy. Histopathology. 2013;62(6):894–8.
Fisher CJ, Millis RR. A mucocoele-like tumour of the breast associated with both atypical ductal hyperplasia and mucoid carcinoma. Histopathology. 1992;21(1):69–71.
Ohi Y, et al. Mucocele-like lesions of the breast: a long-term follow-up study. Diagn Pathol. 2011;6:29.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Harrison, B.T., Dillon, D.A. (2022). Adenosis, Sclerosing Lesions, Microglandular Adenosis, and Mucocele-Like Lesions. In: Shin, S.J., Chen, YY., Ginter, P.S. (eds) A Comprehensive Guide to Core Needle Biopsies of the Breast . Springer, Cham. https://doi.org/10.1007/978-3-031-05532-4_5
Download citation
DOI: https://doi.org/10.1007/978-3-031-05532-4_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-05531-7
Online ISBN: 978-3-031-05532-4
eBook Packages: MedicineMedicine (R0)