Abstract
Recurrent pregnancy loss (RPL) represents a disheartening and distressing loss for couples, as well as a complex clinical challenge for physicians. Clinically recognised pregnancy loss is common, occurring in over 15% of pregnancies, whilst recurrent pregnancy losses are a distinct disorder defined by two or more recognised pregnancy losses (ESHRE Guideline Group on RPL et al., Hum Reprod Open 2018(2):hoy004, 2018; Practice Committee of the American Society for Reproductive Medicine, Fertil Steril 98:1103–1111, 2012). It is estimated that less than 5% of women experience two consecutive miscarriages and only 1–2% experience three or more (Stirrat, Lancet 336:673–675, 1990; Rai and Regan, Lancet 368:601–611, 2006).
A comprehensive evaluation may include investigations into anatomic, genetic, endocrine, autoimmune and iatrogenic causes of recurrent pregnancy loss. The psychological impact that may result from repeated pregnancy losses should also not be underestimated, and supportive psychological care should also be offered to couples (ESHRE Guideline Group on RPL et al., Hum Reprod Open 2018(2):hoy004, 2018; Gibbins and Porter, Clin Obstet Gynecol ;59(3):456–463, 2016). The investigations and management of RPL are unsolved problems, and there is no clear aetiology found in up to 50% of cases. However, despite this, women with unexplained recurrent first trimester miscarriage have an excellent pregnancy outcome if offered supportive care and conceive a subsequent pregnancy with 50–60% success rate (Royal College of Obstetricians and Gynaecologists, Scientific Advisory Committee, Guideline No. 17. The Investigation and treatment of couples with recurrent miscarriage, 2011). Assessment of RPL should focus on screening for genetic factors, antiphospholipid syndrome and assessment for uterine malformations, endocrine disorders and optimising healthy lifestyle behaviours (ESHRE Guideline Group on RPL et al., Hum Reprod Open 2018(2):hoy004, 2018; Practice Committee of the American Society for Reproductive Medicine, Fertil Steril 98:1103–1111, 2012). Overall, evidence-based and up-to-date clinical practice guidelines are required to inform the effective management of couples with RPL, and the goal remains to optimise value and adhere to evidence-based care (Branch and Silver, Clin Obstet Gynecol 59(3):535–538, 2016).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Key Points-
Most miscarriages are sporadic, and thought to be associated with genetic causes, influenced by maternal age.
-
More than 50% of recurrent pregnancy loss will not have a clearly defined cause after thorough evaluation.
-
Most women with unexplained recurrent pregnancy loss will have successful outcomes in subsequent pregnancies.
15. Case Vignette
A 33-year-old G2P0 woman presents with her partner of 2 years for a consultation for recurrent pregnancy loss. She has been trying to conceive with her partner for 2 years and has experienced two first trimester miscarriages, which were both managed surgically with dilation and curettage (D&C). She reports she has always had her pregnancies confirmed on ultrasound on an early dating scan at 6 weeks with subsequent foetal loss before 12 weeks on repeat ultrasound. Genetic evaluation was not performed on products of conception from either of these pregnancies.
She has a known history of polycystic ovarian syndrome but is otherwise medically well with no other significant medical history. Her only surgeries were her two D&C procedures. She has no gynaecological history and reports regular menses every 28 days with no heavy menstrual bleeding or dysmenorrhoea and onset of menarche at age 13. She reports normal and up-to-date cervical screening tests and denies a history of sexually transmitted infections. She has been taking regular antenatal vitamins and has no known allergies.
Her husband is medically well with no significant medical or surgical history. They both deny the use of alcohol, tobacco or substance use.
15.1 Definition
The definition of recurrent pregnancy loss (RPL) has traditionally included only couples with three or more spontaneous, consecutive miscarriages. Professional organisations such as the American Society for Reproductive Medicine (ASRM) and European Society of Human Reproduction and Embryology (ESHRE) have now redefined RPL as the loss of two or more clinically recognised pregnancies, excluding molar and ectopic pregnancies [1, 2]. However, the previous definition of three or more consecutive pregnancy losses remains in use by other organisations such as the Royal College of Obstetricians and Gynaecologists (RCOG) in the UK, as well as the French College of Gynaecologists and Obstetricians [4, 5]. As the revised definition of recurrent miscarriage is used across more countries and regions, more women and couples will be able to access services for investigation and management. For most investigations, the decision on when to start investigations will need to be decided in a shared decision-making process between couples and their physician [6].
15.2 Evaluation and Treatment
15.2.1 Introduction
The following chapter will review a general approach to investigation and management of recurrent pregnancy loss. A detailed description of potential contributing factors to RPL will follow, as well as the appropriate diagnostic and therapeutic strategies. An initial evaluation for early pregnancy loss is included in ◘ Fig. 15.1, followed by a summary overview of standard workup for RPL in ◘ Table 15.1.
Factors generally accepted to be associated with RPL include embryonic chromosomal abnormalities, uterine malformations, endocrine dysfunction, autoimmune disorders and acquired thrombophilia such as antiphospholipid syndrome [4, 7, 8]. Other causes have been proposed but remain controversial, including chronic endometritis, infectious diseases, inherited thrombophilia, luteal phase deficiency and high sperm DNA fragmentation levels [1, 4, 7].
Investigations for RPL were traditionally only initiated after three consecutive miscarriages. However, recent data does not support this protocol. Several recent studies showed that women with two or three pregnancy losses had similar obstetric characteristics and investigation results with two RPL and three RPL had very similar obstetric characteristics and associated causative factors. Hence, couples with two pregnancy losses should be offered the same care pathway [11,12,10].
Over the years, evidence-based treatments such as surgical management of uterine anomalies and aspirin and heparin for antiphospholipid syndrome have improved outcomes for couples with RPL. However, more than 50% of cases of recurrent miscarriage will not have a clearly defined aetiology [7]. Most investigations and treatment also remain controversial, with a lack of consensus amongst international groups regarding standard investigations and treatment options for RPL [11]. Nevertheless, standard investigations for recurrent miscarriage continue to be important in evaluating potential factors responsible for pregnancy loss [12].
A standard initial evaluation of RPL should include a complete patient history including medical, obstetric and family history, as well as lifestyle factors such as smoking, alcohol consumption and exercise. Medical and family history could be used to tailor diagnostic investigations in RPL. Any previous investigations on prior miscarriages should be reviewed, as well as any evidence of acute or chronic disease.
15.3 Epidemiological Risk Factors
15.3.1 Age
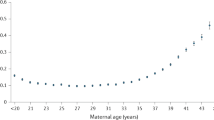
Advanced maternal age is a well-established risk factor for subfertility, foetal anomalies, stillbirth and obstetric complications. Fecundity decreases gradually beginning from 32 years and more rapidly from 37 years, reflecting a decline in the number and quality of remaining oocytes. This progressive atresia of oocytes is associated with elevated levels of follicle-stimulating hormone and anti-Mullerian hormone [13]. Furthermore, age-related decline in fertility is also accompanied by significant increases in rates of aneuploidy and spontaneous miscarriage [4, 7]. Advanced paternal age has also been identified as a risk factor for pregnancy loss, with the highest combined risk seen in couples where the woman is 35 years or older and the man is 40 years or older [14, 15].
15.3.2 Previous Reproductive History
The risk of further miscarriages increases after each successive pregnancy loss, reaching 40% after three consecutive pregnancy losses. A previous live birth does not prevent a woman from experiencing RPL, and the prognosis worsens with increasing maternal age [16, 17].
15.4 Anatomic Risk Factors
Anatomic defects are found in up to 15% of women with RPL and may be classified as congenital malformations or acquired anomalies including intrauterine adhesions, myomas and endometrial polyps. They are thought to interrupt endometrial vasculature and prompt abnormal placentation [1, 2, 4].
15.4.1 Congenital Malformations
Congenital malformations of the reproductive tract result from failure of development, fusion and degeneration of the paramesonephric ducts. Congenital uterine malformations have a well-established association with recurrent pregnancy loss, in addition to other complications such as preterm birth, foetal malpresentation and increased rates of Caesarean delivery [18, 19]. Congenital uterine abnormalities may have varying degrees of symptomatology but can be broadly classified into unification defects (unicornuate, bicornuate or didelphys uterus) and/or canalisation defects from incomplete resorption of the midline septum (subseptate or septate uterus) [20].
Imaging for the detection of uterine malformations is commonly performed via hysterosalpingography but now may be more fully characterised by sonohysterography, laparoscopy, magnetic resonance imaging or ultrasound imaging (two-dimensional or three-dimensional) [21]. Sonohysterography uses the introduction of saline or contrast fluid into the uterine cavity to enhance ultrasound imaging studies and has higher sensitivity and specificity compared to hysterosalpingography or diagnostic hysteroscopy in diagnosis of uterine malformations, with ◘ Fig. 15.2 demonstrating a hysteroscopic approach. In contrast, hysterosalpingography has a good sensitivity for diagnosing more pronounced uterine malformations, but it is limited in differentiating between the types of malformations [22, 23].
There is still insufficient evidence on the efficacy and safety of surgical interventions for improving reproductive outcomes. Some reports have shown hysteroscopic metroplasty for women with septal divisions to reduce miscarriages and improve live birth rates [27,28,26], whilst other studies show surgical management of fusion or unification defects to be of limited benefit [27]. The primary limitation to this data is the lack of large randomised controlled therapeutic trials. An international randomised controlled trial found no evidence that hysteroscopic septum resection improved live birth rates in women with a septate uterus, as compared with expectant management. However, this was limited by a small sample size of 80 women [28]. Until such a study can robustly prove an improvement in reproductive outcomes, patients should be adequately counselled regarding the potential risks of surgery, such as uterine perforation, and participate in a shared decision-making process for ongoing management.
15.4.2 Intrauterine Adhesions
Intrauterine adhesions , or synechiae, occur in sites where the endometrial basal layer has been destroyed, most frequently from curettage, uterine surgery or infection. Endometrial trauma may cause minimal or significant interruption to vasculature, resulting in menstrual abnormalities, infertility and recurrent pregnancy loss. Asherman syndrome describes the presence of intrauterine adhesions with oligo- or amenorrhoea [29].
Hysteroscopic adhesiolysis is now the treatment of choice due to its minimally invasive nature and being able to be performed under direct vision. It does carry significant risks of uterine perforation, and dense scar tissue and difficult entry into the cervix may necessitate laparoscopic or ultrasound guidance, ultrasonography or laparoscopic guidance [30]. Some studies have reported on the use of a Foley catheter introduced into the uterine cavity with an inflated balloon for several days after lysis of adhesions to prevent recurrence. However, this has the added risk of infection and patient discomfort, as well as potential disadvantages of reducing blood flow to the regenerating uterine walls. There is no current consensus regarding surgical method, instruments and any barriers to prevent recurrence and hormonal treatment for endometrial regeneration [34,35,33].
15.4.3 Intrauterine Masses
Intrauterine masses such as endometrial polyps (as seen in ◘ Fig. 15.3) and myomas (in ◘ Fig. 15.4) are postulated to have space-occupying effect that impedes embryonic implantation and potential acts as a foreign body causing subacute endometritis, hence causing pregnancy loss. However, they are also benign growth, which may be present in up to 30–40% of the normal population, and their effect on reproductive outcomes is controversial. There is no evidence for surgical management to improve fertility outcomes [37,38,36].
15.4.4 Cervical Incompetence
Cervical incompetence is a clinical diagnosis, based on a history of late miscarriage preceded by spontaneous rupture of membranes or painless cervical dilation. It is frequently cited as a cause of RPL, more commonly occurring in mid-trimester. However, there are no objective investigations that can identify non-pregnant women with underlying cervical incompetency [37]. It is thought to arise from previous surgical trauma such as cone biopsies, large loop excision of the transformational zone, repeated dilation and curettage or obstetric lacerations. A rarer cause is exposure to diethylstilbestrol (DES) whilst in utero. A Cochrane review identified no conclusive evidence that prophylactic cervical cerclage reduces the risk of recurrent mid-trimester miscarriage. Hence the benefits of serial cervical length measurements and the prophylactic cervical cerclage may be questionable [38].
15.5 Genetic Factors
Over 50% of spontaneous miscarriages are the result of chromosomal abnormalities, which may be of parental origin, or arise de novo in the embryo from parents with normal chromosomes [39]. The most common genetic errors include aneuploidy (gain or loss of a chromosome), chromosomal imbalances (from translocations, inversions, deletions or duplications) and single gene mutations.
15.5.1 Cytogenetic Abnormalities
Embryonic aneuploidies are the most common cause of early pregnancy loss, with up to 90% of chromosomally abnormal embryos spontaneously aborted, as a natural selection mechanism [2, 4, 39]. The most common abnormalities are numeric chromosome errors such as trisomy (60%), polyploidy (20%) and monosomy X (20%). The autosomal trisomies typically arise de novo owing to meiotic nondisjunction during gametogenesis and are associated with increase in age. The parental karyotypes are normal in most of these cases conferring a minimal recurrence risk [40, 41].
15.5.2 Parental Chromosomal Disorders
Most miscarriages occur in chromosomally normal parents. Abnormal parental karyotypes such as translocations, inversions and rarely ring chromosomes are found in around 2–5% of individuals referred for genetic testing after RPL [42, 43].
Balanced translocations are the most common chromosomal abnormalities contributing to RPL. They occur where there is an exchange of chromosome segments and can be categorised as reciprocal or Robertsonian translocations. Robertsonian translocations occur where the two acrocentric chromosomes (numbers 13, 14, 15, 21, 22) are combined near the centromere, with loss of the short arms [44]. Balanced translocations include an exchange of chromosome segments with no loss in the two non-homologous chromosomes. The risk of miscarriage is influenced by the chromosome involved, the size and type of rearrangement as well as the genetic content of the rearranged chromosomal segments. Notably, parents carrying balanced translocations are usually asymptomatic [42, 43].
Rarer chromosomal abnormalities include inversions and ring chromosomes. Inversions occur where a piece of chromosome breaks at two points and reinserts within the same chromosome. Paracentric and pericentric inversions are much rarer but are also associated with an increased risk of RPL. Ring chromosomes occur when two breaks are created in one chromosome and the resulting ends fuse to form a ring [45, 46].
15.5.3 Genetic Evaluation in RPL
If no foetal product of conception histopathology is available from previous pregnancy losses, then parental karyotyping may be considered after an individual risk assessment [1, 2, 4]. However, routine screening of parental karyotyping is not recommended. In couples with no other cause of RPL other than a structural chromosomal rearrangement, nearly two-thirds are likely to have a normal outcome in subsequent pregnancy [42, 47, 48].
Genetic counselling is crucial when a structural genetic factor is identified, as strategies to prevent recurrence will depend on the underlying cause of pregnancy loss. Reproductive options may include preimplantation genetic diagnosis and antenatal genetic diagnosis such as chorionic villus sampling and amniocentesis . This allows for foetal karyotyping to be performed followed by more specific genetic testing such as gene sequencing, polymerase chain reactions (PCR) and restriction fragment length polymorphism (RFLP). Preimplantation genetic diagnosis (PGD) with embryo transfer allows for the transfer of only unaffected foetuses or for the use of donor gametes [49,50,48].
15.6 Endocrine Risk Factors
15.6.1 Luteal Phase Deficiency
Progesterone is necessary for the maintenance of early pregnancy and is initially produced by the corpus luteum, until the developing placenta takes over production between 7 and 9 weeks of gestation. Luteal phase deficiency was first described in 1949, and its defining characteristic is a deficiency in endogenous progesterone, hence affecting normal embryo implantation and maintenance of early pregnancy [49]. Luteal phase insufficiency can be caused by endocrinopathies such as stress, PCOS and prolactin disorders [50].
Although luteal phase deficiency is known to be associated with RPL, finding consistent, accessible and reliable diagnostic criteria for luteal phase deficiency has been challenging, and there remains no consensus regarding its definition [51]. Classically, serum progesterone levels below 10 ng/ml have been associated with an increased risk of miscarriage. However, serum progesterone levels are subject to high levels of fluctuations due to the pulsatile release of luteinising hormone. Traditionally, serial endometrial biopsies would be performed for diagnosis; however, this is not recommended currently for diagnosis, as there is poor reproducibility of findings and high interobserver variation with histological diagnoses [53,54,52].
Progesterone use has been found to be of limited benefit in an unselected population with sporadic miscarriages; however, there is a potential benefit in a subpopulation with three or more consecutive miscarriages [53, 54].
15.6.2 Polycystic Ovarian Syndrome (PCOS)
PCOS is a complex disorder involving abnormalities within the hypothalamic-pituitary-ovarian axis, to cause anovulatory dysfunction, hyperandrogenism and polycystic ovarian morphology. Women may have oligo- or amenorrhoea, obesity and laboratory evidence of elevated androgens, elevated levels of luteinising hormone (LH), insulin resistance and hyperinsulinemia [50]. PCOS is associated with several complications of pregnancy including gestational diabetes, hypertensive disorders including pre-eclampsia and early pregnancy loss. Several of the abnormalities seen in PCOS patients have been independently associated with RPL, including insulin resistance, hyperinsulinemia, hyperandrogenemia and obesity. However, there is a lack of clear evidence that PCOS predisposes to recurrent pregnancy loss, as available studies have not used the Rotterdam criteria to define PCOS, but rather have only used polycystic ovarian morphology [55, 56]. Furthermore, current research indicates that metformin does not reduce risk of pregnancy loss in PCOS , and there is limited evidence to suggest that clomiphene citrate or ovarian drilling is beneficial in this population [56, 57].
There is a need for a reappraisal of available evidence to determine the true prevalence and the role of PCOS in recurrent pregnancy loss.
15.6.3 Thyroid Dysfunction
Thyroid function may vary significantly during normal pregnancy. If overall thyroid homeostasis is to be maintained, the thyroid gland is challenged to increase thyroid hormone production. A study of thyroid function and pregnancy outcome revealed a positive linear relationship between miscarriage and maternal thyroid-stimulating hormone (TSH) levels [58]. Thyroid disorders, especially hypothyroidism and increased thyroid peroxidase antibodies, are correlated with disturbed folliculogenesis, spermatogenesis, fertilisation and embryogenesis and have long been associated with RPL, preterm birth, low birth weight and detrimental effects with foetal neurocognitive development [59, 60].
15.6.3.1 Hypothyroidism
The most common cause of hypothyroidism in pregnant women, affecting nearly 0.5% of patients, is chronic autoimmune (Hashimoto) thyroiditis, followed by endemic iodine deficiency through poor supplementation, poor radioactive iodine therapy and post-thyroidectomy state [60].
Whilst overt hypothyroidism is associated with an increased rate of pregnancy loss, the association between subclinical hypothyroidism (SCH) and miscarriage is less clear. SCH refers to elevated TSH levels with preserved free thyroxine levels [60, 61]. Hypothyroidism may be easily diagnosed with a serum thyroid-stimulating hormone level; however, the threshold level for diagnosing subclinical hypothyroidism is contentious. It is controversial whether to lower the upper limit of normal TSH from 4–5mIU/L to 2.5mIU/L, which represents two standard deviations above the euthyroid population [61, 62]. However, studies which utilised the pre-pregnancy TSH threshold of 2.5mIUL/L found no association with increased pregnancy loss and no improvement with thyroxine replacement therapy [65,66,67,68,66].
There is fair evidence that subclinical hypothyroidism when defined as TSH > 4 mIU/L during pregnancy is associated with miscarriage and routine screening with TSH levels should be offered in RPL [63].
15.6.3.2 Thyroid Autoimmunity
The presence of anti-thyroid antibodies may imply abnormal T-cell function, suggesting an additional immune-mediated role in causing pregnancy loss. For women with thyroid antibodies and a serum TSH 2–4mIU/L, treatment should be considered in early pregnancy [67].
Selenium is postulated to play a key role in thyroid homeostasis through integration into thyroid enzymes responsive for protection against immune-mediated oxidative damage. There have been several studies suggesting selenium treatment to reduce antibody levels, which may allow for lower doses or thyroxine supplementation in women with Hashimoto’s thyroiditis. Unfortunately, there are no current randomised controlled trials to support this treatment in RPL [68].
15.6.3.3 Hyperthyroidism
Hyperthyroidism, found in 0.1–0.4% of pregnancies, is not a known causative factor of RPL. Nevertheless, it is noted that women with untreated overt hyperthyroidism are at high risk of thyroid storm, congestive heart failure, pre-eclampsia, preterm birth and spontaneous miscarriage [69, 70].
15.6.4 Management of Thyroid Dysfunction in RPL
In conclusion, screening with TSH and thyroid autoantibodies and treatment of subclinical hypothyroidism are recommended in women with RPL. Thyroxine administration, commencing at a low dose such as 50 microg daily, is a safe and effective method in reducing early pregnancy loss in women with overt hypothyroidism or in euthyroid women with anti-thyroid antibodies. Current recommendations support thyroxine administration for TSH >4 mIU/L but not at TSH of 2.5–4 mIU/L in the absence of thyroid antibodies [63, 71].
15.6.5 Abnormal Glucose Metabolism
Pregestational diabetes complicates around 1% of pregnancies, and many studies have shown patients with poorly controlled diabetes are known to have an elevated risk of spontaneous miscarriage, preterm birth and hypertensive disorders. The main underlying cause of miscarriage is thought to be lethal embryonic malformations due to glucose teratogenicity if the patient has poorly controlled diabetes in the periconceptional period [72, 73]. Current evidence suggests that well-controlled diabetes is not a risk factor for RPL and that optimal metabolic control for diabetic women is crucial in the periconceptional period and first trimester [1, 4]. Metformin is known to be a safe, effective and low-risk oral hypoglycaemic agent for management of diabetes [73].
15.6.6 Hyperprolactinaemia
Prolactin is commonly measured because elevated prolactin levels are associated with ovulatory dysfunction. The underlying mechanism is unclear, but prolactin is postulated to maintain corpus luteum function and progesterone secretion, although the mechanism is still unclear [74]. Normalisation of prolactin levels in RPL population, with a dopamine agonist such as bromocriptine, was effective in preventing miscarriages but showed no significant difference in conception and live birth rates [75]. Due to the absence of consistent evidence on its association with RPL, prolactin testing is not routinely recommended in the absence of symptoms of hyperprolactinaemia such as oligo- or amenorrhoea [1].
15.6.7 Diminished Ovarian Reserve
Diminished ovarian reserve (DOR) , defined as reduced ovarian reserve markers with regular menstrual cycles, has been suggested to be a causative or prognostic factor in RPL. Ovarian reserve can be assessed with measurements of FSH, oestrogen (E2), inhibin B and anti-Mullerian hormone (AMH) or ultrasound investigation to determine antral follicle count (AFC) and ovarian volume [1, 2, 3].
DOR may be seen following pelvic surgery, chemotherapy and radiotherapy but also conversely in the general population of young women conceiving naturally and is not necessarily considered as a pathological entity. Additionally, ovarian aging may lead to increased rates in foetal aneuploidy, which makes investigation into a direct causative effect with RPL difficult [76, 77]. A recent systematic review and meta-analysis has found an apparent association between DOR and RPL as measured by low AMH levels and AFC [78]. However, more studies are required to evaluate their prognostic value in RPL, and assessment of ovarian reserve is not recommended as part of routine screening [1, 2, 4, 5].
15.7 Immunological Factors
The immune system of pregnant women is tightly controlled to defend against microbial infections and to accept an embryo or the foetus, and inflammation-like processes are crucial for tissue growth, remodelling and differentiation of the decidua during pregnancy. A failure in normal immune control mechanisms may result in an autoimmune response to a developing foetus, like those that develop after rejected grafts in organ transplantation [79]. Autoantibody formation against phospholipids, thyroid antigens and nuclear antigens has been investigated as a potential causative factor in RPL, and 20% of women with RPL will have increased serum levels of autoantibodies, most commonly antiphospholipid antibodies [80].
There is currently insufficient evidence to recommend immune testing such as human leukocyte antigen (HLA) determination, cytokine levels and natural killer cell analyses [81]. Antinuclear antibodies (ANA) will be detected in 10–15% of women, with no clear relationship with pregnancy outcomes. However, some studies have shown a weak association between ANA and RPL, and there is evidence that the presence of ANA may confer a poorer prognosis [82, 83]. Overall, there is no evidence that available immunotherapies such as intravenous immunoglobulins, paternal cell immunisation or donor leukocytes provide any benefit for improving live birth rates. Hence, no immunological tests are recommended as part of routine RPL workup [84].
15.8 Thrombophilia
15.8.1 Antiphospholipid Syndrome
Antiphospholipid syndrome (APS) is the only proven thrombophilia associated with recurrent pregnancy loss, with international consensus diagnostic criteria outlined in ◘ Table 15.2 [85]. Between 15 and 20% of women with RPL have positive antiphospholipid antibodies, with the three most clinically recognised and relevant antibodies including lupus anticoagulant (LA), anticardiolipin antibody and anti-beta-2 glycoprotein I which contribute to the laboratory diagnosis of APS. It has been suggested that the presence of anti-beta-2 glycoprotein may indicate an increased risk of thrombosis [86, 87].
The diagnosis of antiphospholipid syndrome can be complex and is based on a combination of clinical manifestations of vascular thrombosis or pregnancy morbidity, as well as the presence of autoantibodies, on two tests performed 12 or more weeks apart [85]. The hypothesis behind APS and recurrent miscarriage is the encouragement of a hypercoagulable state, inflammatory processes and defective angiogenesis. This is corroborated by the presence of microthrombi within placental vasculature and decidua in pregnancy samples of women with RPL [87, 88].
Standard treatment for APS includes a combination of low-dose aspirin and low-dose heparin, which may reduce pregnancy loss by 54% [89]. Aspirin may be commenced in the periconceptual period, whilst heparin should be commenced after the first positive pregnancy test, with both continued until delivery [90]. The use of prednisolone increases risks of hypertensive disorders, gestational diabetes and preterm birth and is not recommended for treatment of APS [91]. Postpartum thromboprophylaxis is considered for a short interval, and women with known APS should consider avoiding oestrogen containing oral contraceptives due to the persistent thrombotic risk [89, 90].
15.8.2 Hereditary Thrombophilia
Hereditary thrombophilia predisposing patients to venous thromboembolisms include Factor V Leiden mutation, prothrombin mutation, protein C, protein S and antithrombin deficiency. The prevalence of hereditary thrombophilia in women with RPL is unclear, and there is no clear association between RPL and hereditary thrombophilia [92]. Current guidelines recommend screening only in the presence of additional risk factors , such as a positive family history of thrombophilia or a personal history of VTE [93]. Where possible, this should occur 6 weeks following a pregnancy loss or thrombotic event and whilst not on anticoagulants, with suggested testing methods outlined in ◘ Table 15.3.
15.9 Infection
Ureaplasma urealyticum, Mycoplasma hominis, chlamydia, Listeria monocytogenes, Toxoplasma gondii, rubella, cytomegalovirus, herpes virus and other less frequent pathogens have been identified more frequently in vaginal and cervical cultures and serum from women with sporadic miscarriages [94]. Similarly, chronic endometritis has been linked to RPL, as small studies showed an increased prevalence in this subpopulation [95]. However, there remains uncertainty regarding the true impact of chronic endometritis on reproductive outcomes, and there is no consensus on treatment options, coupled with the need for an endometrial biopsy to confirm resolution. Overall, there is no convincing evidence that infections cause RPL, and hence there is a limited benefit of routine screening and antibiotic prophylaxis [96].
15.10 Environmental and Psychological Factors
15.10.1 Lifestyle Factors
Smoking is strongly associated with adverse obstetric outcomes such as miscarriage, stillbirth, ectopic pregnancy, placenta praevia as well as poor neonatal outcomes including preterm birth, foetal growth restrictions and congenital abnormalities. It is thought that cigarette smoking causes adverse trophoblastic function and hence is linked to sporadic pregnancy loss [97].
Other lifestyle factors such as alcohol consumption (3–5 drinks per week) and caffeine consumption (more than three cups of coffee) have been associated with the risk of miscarriage in a dose-dependent manner. However, current evidence is insufficient to confirm this association [98].
15.10.2 Obesity
Obesity represents a major public health challenge worldwide, as it poses a high disease burden and mortality, affecting not only general health but also in the periconceptual period. It is associated with poor reproductive outcomes, including miscarriages, congenital malformations, gestational diabetes, pre-eclampsia, higher rates of Caesarean delivery, thromboembolic events and postpartum infection [99, 100]. A recent systematic review found higher rates of pregnancy loss in obese women with a history of RPL, but not in women who were overweight [101].
15.10.3 Psychological Factors
Recurrent pregnancy loss can have significant emotional impacts on couples, with feelings of loss of and grief intensified with repeated losses [102]. Several reports have tried to find a possible psychological aetiology for RPL, but such associations are inherently difficult to prove, due to the presence of various confounding factors and variables. International societies recommend offering supportive care in dedicated miscarriage clinics for couples with RPL [1, 4].
15.11 Unexplained Pregnancy Loss
Therapeutic interventions should be targeted to the cause of RPL. However, after a thorough evaluation, almost half of the patients will remain without a definite diagnosis. In this subpopulation with unexplained RPL with early pregnancy bleeding, progestogen supplementation may reduce the risk of miscarriage and increase live birth rates in subsequent pregnancies [54, 103].
15.12 Future Pregnancy Outcomes
Pregnancy loss can be an extremely traumatic event for couples, with feelings of helplessness, desperation and despair compounded with subsequent pregnancy losses. These symptoms may also be exacerbated by unnecessary tests that fail to enhance reproductive outcomes [104]. In this way, it is imperative that clinical and diagnostic tests focus on minimising the risk of future miscarriages, optimising the time required to become pregnant again and optimising the chances of live birth. Thankfully, women with unexplained recurrent first trimester miscarriage have an excellent pregnancy outcome if offered supportive care and conceive a subsequent pregnancy with 50–60% success rate [4].
15.13 ReviewQuestions
-
1.
Spontaneous pregnancy loss is most commonly due to:
-
A.
Uterine malformations
-
B.
Lifestyle factors
-
C.
Endocrine dysfunction
-
D.
Genetic abnormalities
-
A.
-
2.
What is the most common anatomic defect associated with recurrent pregnancy loss?
-
A.
Leiomyomas
-
B.
Bicornuate uterus
-
C.
Endometrial polyps
-
D.
Uterine synechiae
-
A.
-
3.
Which of the following is not a diagnostic criterion for antiphospholipid syndrome?
-
A.
Antithrombin antibody
-
B.
Anticardiolipin antibody
-
C.
Lupus anticoagulant
-
D.
Anti-beta-2 glycoprotein antibody
-
A.
15.14 Answer
-
1.
D
-
2.
B
-
3.
A
References
ESHRE Guideline Group on RPL, Bender Atik R, Christiansen OB, Elson J, Kolte AM, Lewis S, Middeldorp S, Nelen W, Peramo B, Quenby S, Vermeulen N, Goddijn M. ESHRE guideline: recurrent pregnancy loss. Hum Reprod Open. 2018;2018(2):hoy004.
Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: A committee opinion. Fertil Steril. 2012;98:1103–11.
Gibbins KJ, Porter TF. The importance of an evidence-based workup for recurrent pregnancy loss. Clin Obstet Gynecol. 2016;59(3):456–63.
Royal College of Obstetricians and Gynaecologists, Scientific Advisory Committee, Guideline No. 17. The Investigation and treatment of couples with recurrent miscarriage, 2011.
Huchon C, Deffieux X, Beucher G, Capmas P, Carcopino X, Costedoat-Chalumeau N, Delabaere A, Gallot V, Iraola E, Lavoue V, Legendre G, Lejeune-Saada V, Leveque J, Nedellec S, Nizard J, Quibel T, Subtil D, Vialard F, Lemery D. Collège National des Gynécologues Obstétriciens Français. Pregnancy loss: French clinical practice guidelines. Eur J Obstet Gynecol Reprod Biol. 2016;201:18–26.
Hennessy M, Dennehy R, Meaney S, Linehan L, Devane D, Rice R, O’Donoghue K. Clinical practice guidelines for recurrent miscarriage in high-income countries: a systematic review. Reprod Biomed Online. 2021;42(6):1146–71.
El Hachem H, Crepaux V, May-Panloup P, Descamps P, Legendre G, Bouet PE. Recurrent pregnancy loss: current perspectives. Int J Women’s Health. 2017;17(9):331–45.
van Dijk MM, Kolte AM, Limpens J, Kirk E, Quenby S, van Wely M, Goddijn M. Recurrent pregnancy loss: diagnostic workup after two or three pregnancy losses? A systematic review of the literature and meta-analysis. Hum Reprod Update. 2020;26(3):356–67.
Youssef A, Lashley L, Dieben S, Verburg H, van der Hoorn ML. Defining recurrent pregnancy loss: associated factors and prognosis in couples with two versus three or more pregnancy losses. Reprod Biomed Online. 2020;41(4):679–85.
Bashiri A, Ratzon R, Amar S, Serjienko R, Mazor M, Shoham-Vardi I. Two vs. three or more primary recurrent pregnancy losses--are there any differences in epidemiologic characteristics and index pregnancy outcome? J Perinat Med. 2012;40(4):365–71.
Youssef A, Vermeulen N, Lashley EELO, Goddijn M, van der Hoorn MLP. Comparison and appraisal of (inter)national recurrent pregnancy loss guidelines. Reprod Biomed Online. 2019;39(3):497–503.
Clifford K, Rai R, Watson H, Regan L. An informative protocol for the investigation of recurrent miscarriage: preliminary experience of 500 consecutive cases. Hum Reprod. 1994;9(7):1328–32.
American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril. 2014;101(3):633–4.
de la Rochebrochard E, Thonneau P. Paternal age and maternal age are risk factors for miscarriage; results of a multicentre European study. Hum Reprod. 2002;17(6):1649–56.
Sharma R, Agarwal A, Rohra VK, Assidi M, Abu-Elmagd M, Turki RF. Effects of increased paternal age on sperm quality, reproductive outcome and associated epigenetic risks to offspring. Reprod Biol Endocrinol. 2015;13:35.
Clifford K, Rai R, Regan L. Future pregnancy outcome in unexplained recurrent first trimester miscarriage. Hum Reprod. 1997;12(2):387–9.
Nybo Andersen AM, Wohlfahrt J, Christens P, Olsen J, Melbye M. Maternal age and fetal loss: population based register linkage study. BMJ. 2000;320(7251):1708–12.
Saravelos SH, Cocksedge KA, Li TC. Prevalence and diagnosis of congenital uterine anomalies in women with reproductive failure: a critical appraisal. Hum Reprod Update. 2008;14(5):415–29.
Oppelt P, von Have M, Paulsen M, Strissel PL, Strick R, Brucker S, Wallwiener D, Beckmann MW. Female genital malformations and their associated abnormalities. Fertil Steril. 2007;87:335–42.
Chandler TM, Machan LS, Cooperberg PL, Harris AC, Chang SD. Mullerian duct anomalies: from diagnosis to intervention. Br J Radiol. 2009;82:1034–42. https://doi.org/10.1259/bjr/99354802.
Ludwin A, Ludwin I, Banas T, Knafel A, Miedzyblocki M, Basta A. Diagnostic accuracy of sonohysterography, hysterosalpingography and diagnostic hysteroscopy in diagnosis of arcuate, septate and bicornuate uterus. J Obstet Gynaecol Res. 2011;37(3):178–86.
Acholonu UC, Silberzweig J, Stein DE, Keltz M. Hysterosalpingography versus sonohysterography for intrauterine abnormalities. JSLS. 2011;15(4):471–4.
Grimbizis GF, Camus M, Tarlatzis BC, Bontis JN, Devroey P. Clinical implications of uterine malformations and hysteroscopic treatment results. Hum Reprod Update. 2001;7(2):161–74.
Carrera M, Pérez Millan F, Alcázar JL, Alonso L, Caballero M, Carugno J, Dominguez JA, Moratalla E. Effect of hysteroscopic metroplasty on reproductive outcomes in women with septate uterus: systematic review and meta-analysis. J Minim Invasive Gynecol. 2021;S1553-4650(21):01210–3.
Kim MA, Kim HS, Kim YH. Reproductive, obstetric and neonatal outcomes in women with congenital uterine anomalies: a systematic review and meta-analysis. J Clin Med. 2021;10(21):4797.
Rikken JF, Kowalik CR, Emanuel MH, Mol BW, Van der Veen F, van Wely M, Goddijn M. Septum resection for women of reproductive age with a septate uterus. Cochrane Database Syst Rev. 2017;1(1):CD008576.
Akhtar MA, Saravelos SH, Li TC, Jayaprakasan K, Royal College of Obstetricians and Gynaecologists. Reproductive implications and management of congenital uterine anomalies: scientific impact paper No. 62 November 2019. BJOG. 2020;127(5):e1–e13.
Rikken JFW, Kowalik CR, Emanuel MH, Bongers MY, Spinder T, Jansen FW, Mulders AGMGJ, Padmehr R, Clark TJ, van Vliet HA, Stephenson MD, van der Veen F, Mol BWJ, van Wely M, Goddijn M. Septum resection versus expectant management in women with a septate uterus: an international multicentre open-label randomized controlled trial. Hum Reprod. 2021;36(5):1260–7.
Hooker AB, Lemmers M, Thurkow AL, Heymans MW, Opmeer BC, Brolmann HA, Mol BW, Huirne JA. Systematic review and meta-analysis of intrauterine adhesions after miscarriage: prevalence, risk factors and long-term reproductive outcome. Hum Reprod Update. 2014;20:262–78.
Berman JM. Intrauterine adhesions. Semin Reprod Med. 2008;26(4):349–55.
Deans R, Abbott J. Review of intrauterine adhesions. J Minim Invasive Gynecol. 2010;17(5):555–69.
Polishuk WZ, Sadovsky E. A syndrome of recurrent intrauterine adhesions. Am J Obstet Gynecol. 1975;123(2):151–8.
Pabuccu R, Onalan G, Kaya C, Selam B, Ceyhan T, Ornek T, Kuzudisli E. Efficiency and pregnancy outcome of serial intrauterine device-guided hysteroscopic adhesiolysis of intrauterine synechiae. Fertil Steril. 2008;90(5):1973–7.
Bosteels J, van Wessel S, Weyers S, Broekmans FJ, D’Hooghe TM, Bongers MY, Mol BWJ. Hysteroscopy for treating subfertility associated with suspected major uterine cavity abnormalities. Cochrane Database Syst Rev. 2018;12(12):CD009461.
Donnez J, Jadoul P. What are the implications of myomas on fertility? A need for a debate? Hum Reprod. 2002;17(6):1424–30.
Metwally M, Raybould G, Cheong YC, Horne AW. Surgical treatment of fibroids for subfertility. Cochrane Database Syst Rev. 2020;1(1):CD003857.
Drakeley AJ, Quenby S, Farquharson RG. Mid-trimester loss--appraisal of a screening protocol. Hum Reprod. 1998;13(7):1975–80. https://doi.org/10.1093/humrep/13.7.1975.
Drakeley AJ, Roberts D, Alfirevic Z. Cervical stitch (cerclage) for preventing pregnancy loss in women. Cochrane Database Syst Rev. 2003;2003(1):CD003253.
Sugiura-Ogasawara M, Ozaki Y, Katano K, Suzumori N, Kitaori T, Mizutani E. Abnormal embryonic karyotype is the most frequent cause of recurrent miscarriage. Hum Reprod. 2012;27(8):2297–303.
Mikwar M, MacFarlane AJ, Marchetti F. Mechanisms of oocyte aneuploidy associated with advanced maternal age. Mutat Res Rev Mutat Res. 2020;785:108320.
Warren JE, Silver RM. Genetics of pregnancy loss. Clin Obstet Gynecol. 2008;51(1):84–95.
Barber JC, Cockwell AE, Grant E, Williams S, Dunn R, Ogilvie CM. Is karyotyping couples experiencing recurrent miscarriage worth the cost? BJOG. 2010;117(7):885–8.
Flynn H, Yan J, Saravelos SH, Li TC. Comparison of reproductive outcome, including the pattern of loss, between couples with chromosomal abnormalities and those with unexplained repeated miscarriages. J Obstet Gynaecol Res. 2014;40(1):109–16.
Crolla JA, Youings SA, Ennis S, Jacobs PA. Supernumerary marker chromosomes in man: parental origin, mosaicism and maternal age revisited. Eur J Hum Genet. 2005;13(2):154–60.
De Braekeleer M, Dao TN. Cytogenetic studies in couples experiencing repeated pregnancy losses. Hum Reprod. 1990;5(5):519–28.
Laurino MY, Bennett RL, Saraiya DS, Baumeister L, Doyle DL, Leppig K, Pettersen B, Resta R, Shields L, Uhrich S, Varga EA, Raskind WH. Genetic evaluation and counseling of couples with recurrent miscarriage: recommendations of the National Society of Genetic Counselors. J Genet Couns. 2005;14(3):165–81.
Franssen MTM, Musters AM, van der Veen F, Repping S, Leschot NJ, et al. Reproductive outcome after PGD in couples with recurrent miscarriage carrying a structural chromosome abnormality: a systematic review. Hum Reprod Update. 2011;17:467–75.
Kochhar PK, Ghosh P. Reproductive outcome of couples with recurrent miscarriage and balanced chromosomal abnormalities. J Obstet Gynaecol Res. 2013;39(1):113–20.
Jones GS. The luteal phase defect. Fertil Steril. 1976;27(4):351–6. https://doi.org/10.1016/s0015-0282(16)41769-3.
Ke RW. Endocrine basis for recurrent pregnancy loss. Obstet Gynecol Clin N Am. 2014;41(1):103–12.
Smith ML, Schust DJ. Endocrinology and recurrent early pregnancy loss. Semin Reprod Med. 2011;29(6):482–90.
Duggan MA, Brashert P, Ostor A, Scurry J, Billson V, Kneafsey P, Difrancesco L. The accuracy and interobserver reproducibility of endometrial dating. Pathology. 2001;33(3):292–7.
Haas DM, Ramsey PS. Progestogen for preventing miscarriage. Cochrane Database Syst Rev. 2013;(10):CD003511.
Haas DM, Hathaway TJ, Ramsey PS. Progestogen for preventing miscarriage in women with recurrent miscarriage of unclear etiology. Cochrane Database Syst Rev. 2019;2019(11):CD003511.
Homburg R. Pregnancy complications in PCOS. Best Pract Res Clin Endocrinol Metab. 2006;20(2):281–92.
Cocksedge KA, Li TC, Saravelos SH, Metwally M. A reappraisal of the role of polycystic ovary syndrome in recurrent miscarriage. Reprod Biomed Online. 2008;17(1):151–60.
Palomba S, Falbo A, Orio F Jr, Zullo F. Effect of preconceptional metformin on abortion risk in polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2009;92(5):1646–58.
Benhadi N, Wiersinga WM, Reitsma JB, Vrijkotte TG, Bonsel GJ. Higher maternal TSH levels in pregnancy are associated with increased risk for miscarriage, fetal or neonatal death. Eur J Endocrinol. 2009;160(6):985–91.
Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O’Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, Faix JD, Klein RZ. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341(8):549–55.
Abalovich M, Gutierrez S, Alcaraz G, Maccallini G, Garcia A, Levalle O. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2002;12(1):63–8.
Baloch Z, Carayon P, Conte-Devolx B, Demers LM, Feldt-Rasmussen U, Henry JF, LiVosli VA, Niccoli-Sire P, John R, Ruf J, Smyth PP, Spencer CA, Stockigt JR, Guidelines Committee, National Academy of Clinical Biochemistry. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13(1):3–126.
Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, Pessah-Pollack R, Singer PA, Woeber KA. American Association Of Clinical Endocrinologists And American Thyroid Association Taskforce On Hypothyroidism In Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22(12):1200–35.
Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104(3):545–53.
Wang S, Teng WP, Li JX, Wang WW, Shan ZY. Effects of maternal subclinical hypothyroidism on obstetrical outcomes during early pregnancy. J Endocrinol Investig. 2012;35(3):322–5.
Uchida S, Maruyama T, Kagami M, Miki F, Hihara H, Katakura S, Yoshimasa Y, Masuda H, Uchida H, Tanaka M. Impact of borderline-subclinical hypothyroidism on subsequent pregnancy outcome in women with unexplained recurrent pregnancy loss. J Obstet Gynaecol Res. 2017;43(6):1014–20.
Maraka S, Mwangi R, McCoy RG, Yao X, Sangaralingham LR, Singh Ospina NM, O’Keeffe DT, De Ycaza AE, Rodriguez-Gutierrez R, Coddington CC 3rd, Stan MN, Brito JP, Montori VM. Thyroid hormone treatment among pregnant women with subclinical hypothyroidism: US national assessment. BMJ. 2017;25(356):i6865.
Negro R, Formoso G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. J Clin Endocrinol Metab. 2006;91(7):2587–91.
van Zuuren EJ, Albusta AY, Fedorowicz Z, Carter B, Pijl H. Selenium supplementation for Hashimoto’s thyroiditis: summary of a Cochrane systematic review. Eur Thyroid J. 2014;3(1):25–31.
Marx H, Amin P, Lazarus JH. Hyperthyroidism and pregnancy. BMJ. 2008;336(7645):663–7.
Glinoer D. Thyroid hyperfunction during pregnancy. Thyroid. 1998;8(9):859–64. https://doi.org/10.1089/thy.1998.8.859.
Reid SM, Middleton P, Cossich MC, Crowther CA, Bain E. Interventions for clinical and subclinical hypothyroidism pre-pregnancy and during pregnancy. Cochrane Database Syst Rev. 2013;(5):CD007752.
Ray JG, O’Brien TE, Chan WS. Preconception care and the risk of congenital anomalies in the offspring of women with diabetes mellitus: a meta-analysis. QJM. 2001;94(8):435–44.
Melamed N, Hod M. Perinatal mortality in pregestational diabetes. Int J Gynaecol Obstet. 2009;104(Suppl 1):S20–4.
Li W, Ma N, Laird SM, Ledger WL, Li TC. The relationship between serum prolactin concentration and pregnancy outcome in women with unexplained recurrent miscarriage. J Obstet Gynaecol. 2013;33(3):285–8.
Chen H, Fu J, Huang W. Dopamine agonists for preventing future miscarriage in women with idiopathic hyperprolactinemia and recurrent miscarriage history. Cochrane Database Syst Rev. 2016;(7):CD008883.
Trout SW, Seifer DB. Do women with unexplained recurrent pregnancy loss have higher day 3 serum FSH and estradiol values? Fertil Steril. 2000;74(2):335–7.
Massé V, Ferrari P, Boucoiran I, Delotte J, Isnard V, Bongain A. Normal serum concentrations of anti-Mullerian hormone in a population of fertile women in their first trimester of pregnancy. Hum Reprod. 2011;26(12):3431–6.
Bunnewell SJ, Honess ER, Karia AM, Keay SD, Al Wattar BH, Quenby S. Diminished ovarian reserve in recurrent pregnancy loss: a systematic review and meta-analysis. Fertil Steril. 2020;113(4):818–827.e3.
Labarrere CA. Allogeneic recognition and rejection reactions in the placenta. Am J Reprod Immunol. 1989;21(3–4):94–9.
McNamee K, Dawood F, Farquharson RG. Thrombophilia and early pregnancy loss. Best Pract Res Clin Obstet Gynaecol. 2012;26(1):91–102.
Jeve YB, Davies W. Evidence-based management of recurrent miscarriages. J Hum Reprod Sci. 2014;7(3):159–69.
Cavalcante MB, Costa Fda S, Araujo Júnior E, Barini R. Risk factors associated with a new pregnancy loss and perinatal outcomes in cases of recurrent miscarriage treated with lymphocyte immunotherapy. J Matern Fetal Neonatal Med. 2015;28(9):1082–6.
Ogasawara M, Aoki K, Kajiura S, Yagami Y. Are antinuclear antibodies predictive of recurrent miscarriage? Lancet. 1996;347:1183–4.
Wong LF, Porter TF, Scott JR. Immunotherapy for recurrent miscarriage. Cochrane Database Syst Rev. 2014;2014(10):CD000112.
Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RH, de Groot PG, Koike T, Meroni PL, Reber G, Shoenfeld Y, Tincani A, Vlachoyiannopoulos PG, Krilis SA. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4(2):295–306.
Lassere M, Empson M. Treatment of antiphospholipid syndrome in pregnancy--a systematic review of randomized therapeutic trials. Thromb Res. 2004;114(5–6):419–26.
Opatrny L, David M, Kahn SR, Shrier I, Rey E. Association between antiphospholipid antibodies and recurrent fetal loss in women without autoimmune disease: a metaanalysis. J Rheumatol. 2006;33(11):2214–21.
Kutteh WH. Antiphospholipid antibody syndrome and reproduction. Curr Opin Obstet Gynecol. 2014;26(4):260–5.
Empson M, Lassere M, Craig J, Scott J. Prevention of recurrent miscarriage for women with antiphospholipid antibody or lupus anticoagulant. Cochrane Database Syst Rev. 2005;2:CD002859.
Hamulyák EN, Scheres LJ, Marijnen MC, Goddijn M, Middeldorp S. Aspirin or heparin or both for improving pregnancy outcomes in women with persistent antiphospholipid antibodies and recurrent pregnancy loss. Cochrane Database Syst Rev. 2020;5(5):CD012852.
Laskin CA, Bombardier C, Hannah ME, et al. Prednisone and aspirin in women with autoantibodies and unexplained recurrent fetal loss. N Engl J Med. 1997;337:148–53.
Arachchillage DRJ, Makris M. Inherited thrombophilia and pregnancy complications: should we test? Semin Thromb Hemost. 2019;45(1):50–60.
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. ACOG Practice Bulletin No. 197: inherited thrombophilias in pregnancy. Obstet Gynecol. 2018;132(1):e18–34.
Penta M, Lukic A, Conte MP, Chiarini F, Fioriti D, Longhi C, Pietropaolo V, Vetrano G, Villaccio B, Degener AM, Seganti L. Infectious agents in tissues from spontaneous abortions in the first trimester of pregnancy. New Microbiol. 2003;26(4):329–37.
Kitaya K. Prevalence of chronic endometritis in recurrent miscarriages. Fertil Steril. 2011;95(3):1156–8.
Giakoumelou S, Wheelhouse N, Cuschieri K, Entrican G, Howie SE, Horne AW. The role of infection in miscarriage. Hum Reprod Update. 2016;22(1):116–33.
Lindbohm ML, Sallmén M, Taskinen H. Effects of exposure to environmental tobacco smoke on reproductive health. Scand J Work Environ Health. 2002;28(Suppl 2):84–96.
Ness RB, Grisso JA, Hirschinger N, Markovic N, Shaw LM, Day NL, Kline J. Cocaine and tobacco use and the risk of spontaneous abortion. N Engl J Med. 1999;340(5):333–9.
Stang J, Huffman LG. Position of the academy of nutrition and dietetics: obesity, reproduction, and pregnancy outcomes. J Acad Nutr Diet. 2016;116:677–91.
Boots C, Stephenson MD. Does obesity increase the risk of miscarriage in spontaneous conception: a systematic review. S1emin Reprod Med. 2011;29(6):507–13.
Cavalcante MB, Sarno M, Peixoto AB, Araujo Júnior E, Barini R. Obesity and recurrent miscarriage: A systematic review and meta-analysis. J Obstet Gynaecol Res. 2019;45(1):30–8.
Bardos J, Hercz D, Friedenthal J, Missmer SA, Williams Z. A national survey on public perceptions of miscarriage. Obstet Gynecol. 2015;125(6):1313–20.
Devall AJ, Papadopoulou A, Podesek M, Haas DM, Price MJ, Coomarasamy A, Gallos ID. Progestogens for preventing miscarriage: a network meta-analysis. Cochrane Database Syst Rev. 2021;4(4):CD013792.
Speraw SR. The experience of miscarriage: how couples define quality in health care delivery. J Perinatol. 1994;14(3):208–15.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Chong, K.Y., Mol, B.W. (2022). Recurrent Early Pregnancy Loss. In: Falcone, T., Hurd, W.W. (eds) Clinical Reproductive Medicine and Surgery. Springer, Cham. https://doi.org/10.1007/978-3-030-99596-6_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-99596-6_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-99595-9
Online ISBN: 978-3-030-99596-6
eBook Packages: MedicineMedicine (R0)