Abstract
This chapter addresses how the embodiment approach may represent a unifying perspective for examining the cerebellar role in emotional behavior and psychological traits. It is not intended to be exhaustive, but rather it can be a good starting point for advancing the cerebellar neural mechanism underlying embodiment. Our goal is to provide illustrative examples of embodied emotions and psychological traits in the emerging field of emotional and cognitive cerebellum. We illustrate how the cerebellum could be an important hub in the embodiment processes, associated with empathic abilities, impaired emotional identification and expression (as occurring for example in the presence of alexithymia), and specific psychological constructs (i.e., hypnotizability).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 The Embodied Foundations of Emotions
Influential theories suggest that bodily factors are constitutive of cognition in the sense that any human cognition is embodied and passes through bodily experiences.
Embodiment theory posits that cognitive and emotional processes are shaped and rooted in our biological constitution (Critchley and Garfinkel 2017). The general ideas that the mind is grounded in the whole body, rather than being a piece of “software” installed only in the “hardware” of the brain and that emotional states arise from physiological changes from within the whole body allowed to bridge the Cartesian dichotomies between mind and body, cognition and emotion, culture and nature, rationality and irrationality (Damasio and Carvalho 2013). This point of view had novel implications for understanding the content of the conceptual system for emotion and the implied structures (Damasio and Carvalho 2013; Niedenthal et al. 2005). Crucially, embodiment involves the central processing of bottom-up afferent signals from the body along with top-down regulatory directives in a bidirectional relationship. The physiological signals may be represented as subjective feelings and thus may lead to behaviors adjusting the current state.
According to embodiment theory, processing of information about concrete facts (i.e., songs, emotional faces, personality characteristics) or abstract concepts (i.e., social, emotional, or psychological constructs) is triggered, influenced, updated, associated with, and even dependent on perceptual, somatosensory, motor, neuroendocrine, and autonomic nervous system activities (Niedenthal et al., 2005). Importantly, the embodied emotion is formulated through bodily sensations and usually expressed through action. Notably, long before scientists came to demonstrate how our emotions affect our bodies, William James in the essay titled “What is an Emotion?” (1884) asserts that “the bodily changes follow directly the perception of the exciting fact, and that our feeling of the same changes as they occur is the emotion” and subsequently in the volume “The Principles of Psychology” (1890/1907) he writes “the world experienced comes at all times with our body as its center, center of vision, center of action, center of interest. Where the body is ‘here’; when the body acts is ‘now’; what the body touches is ‘this’; all other things are ‘there’ and ‘then’ and ‘that’.” Challenging common presuppositions about the ordering of an emotional episode, James argued that it is not an emotion causing the bodily changes, but the corporeal reverberations are actually the raw material of the emotion itself. Note that for more recent emotion theories and researches (such as Izard 2009), a common complaint against James’ theory is that it fails to assign a cognitive role to emotions, identifying them with feelings of bodily changes.
Embodiment involves the brain capturing modality-specific states and then re-instantiates parts of the same states to process the emotion-related information. The somatosensory ability allows feeling what is occurring inside the body, still discriminating between physiological and emotion-related bodily states (Khalsa et al. 2018). Note that interoception encompasses proprioceptive and visceral signaling, and it is related to all physiological organs that relay signals to the brain about the current physiological status of the body. Mapping onto the brain, this information allows for a nuanced representation of the body physiological state, important for maintaining homeostatic conditions and critical for emotion processing and self-awareness recognition. Distinct emotions have been associated with distinct patterns of bodily sensations and actions that have a certain universality as to how emotions are organized and represented in the body (Nummenmaa et al. 2014). For example, in all cultures most basic emotions are associated with sensations of elevated activity in the upper chest area, likely corresponding to changes in breathing and heart rate, the sensations in the upper limbs are prominent in approach-oriented emotions, anger, and happiness, whereas the sensations of decreased limb activity are a defining feature of sadness, and finally, the sensations in the digestive system and around the throat region are mainly related to disgust. Whether negative or positive, emotions are experienced in the body, facilitating our ability to identify, respond to, and interact with our internal and external environment.
2 Empathy as Embodied Emotional-Cognitive Process and Its Relation with the Cerebellum
After being a longstanding center of philosophical debate, the concept of empathy has crossed the borders of the philosophical domain and has been addressed by social, developmental, clinical, and dynamic psychology and subsequently even by neuroscience. Contributions from this rich variety of fields resulted in an overabundance of operational definitions. As Husserl suggested (1931), any intersubjective experience should be conceived as an empathic experience in which we consciously ascribe intentional acts and feelings to another subject. Such an experience is made possible because of physical, sensorial, and perceptual similarities with the “other” seen as Leib (notably, Husserl distinguishes between Leib, the component that is experientially based in our living body, and Körper, the physical structure).
The premise to understand the empathic process is that self-awareness and sensitivity of our own emotional states are prerequisites to accurately comprehend the other’s states. At a phenomenological level, the empathy construct can be conceived as a primary interaction between individuals, with one experiencing and sharing the feelings of the other. In fact, the empathic capacity allows sharing the affective states of others, exerting cognitive control, predicting, and understanding others’ feelings, motivations and actions, without losing sight of whose feelings belong to whom, and behaving accordingly (de Waal and Preston 2017). The self/other distinction is one of the basic characteristics distinguishing empathy from other forms of “feeling with the other”: empathy presupposes alterity. Empathy promotes prosocial and cooperative behaviors (de Waal and Preston 2017; Leblanc and Ramirez 2020; Preston and de Waal 2002) and enables people to navigate the social world they live in. Without the capacity to empathize, we would be lost in our complex world, requiring ever-increasing flexibility to adapt to fast-changing social relationships and mutual understanding (Luyten et al. 2020).
Empathy is regulated by both affective and cognitive components that produce emotional understanding (Shamay-Tsoory et al. 2009a). Affective empathy refers to the ability to share the state of other persons through observation or imagination of their experience, and as a consequence of other’s state usually leads to an appropriate isomorphic emotional response. Cognitive empathy refers to the abilities of Perspective Taking and Theory of Mind (ToM) that allow predicting and understanding other’s mental state by using cognitive processes. Combined, these processes enable to understand beliefs, desires, and emotions of others in real-life or imaginary situations. The idea that empathic capacities are associated with somatosensory, interoceptive, and autonomic processes that tend to simulate those of another person closely fits with the notion of embodiment. In the context of embodied empathy, it is critical to consider that we use our own bodies to simulate information originating from others’ body and face to share and understand their emotions, since empathically experiencing other’s emotional states comes from “re-creating” other’s feelings in ourselves. It has been proposed that the individuals have an understanding of the mind and emotions of others through “mirroring” or “resonance” mechanisms responsive to the other’s bodily states (Gallese and Goldman 1998).
The affective and cognitive components (emotional regulation, affective representations, self-awareness, cognitive flexibility, perspective-taking, and mentalizing) of empathy are mediated by specific and interacting neuronal systems, as indicated by the possibility of distinct impairments of the affective or cognitive empathy in specific clinical disorders. For example, schizophrenia, depersonalization, and narcissistic personality disorder are characterized by deficits in affective empathy (Ritter et al. 2011; Shamay-Tsoory et al. 2007), while bipolar disorder and borderline personality traits are associated with impairment in the cognitive empathy (Harari et al. 2010; Shamay-Tsoory et al. 2009a, b). Even within non-clinical populations, the balance between the capacities of affective and cognitive empathy varies from one individual to another, uniquely defining the empathic experience for each person (Moore et al. 2015).
At the neurobiological level, most research has assessed empathy as a state rather than a trait and has mainly focused on the neocortical activation associated with empathy-eliciting situations (Lamm et al. 2007). Neuroimaging studies (Bilevicius et al. 2018; Fan et al. 2011) have reported consistent activation of brain structures specifically associated with each component of empathy. Namely, the anterior cingulate cortex (ACC) and anterior insula (AI) are mostly recruited in affective empathy, whereas the medial cingulate cortex (MCC) and adjacent dorsomedial prefrontal cortex (DMPFC) in cognitive empathy.
As in the prefrontal cortex, the two empathic processes appear topographically distinct even in the cerebellum, with the posterior vermis being activated mainly in affective processing of empathy, and the posterior lateral cerebellum, and particularly Crus 1 and Crus 2 regions, being activated in cognitive components. fMRI studies (Gu et al. 2012; Moriguchi et al. 2007; Singer et al. 2004) reported that empathy for other’s pain is associated with cerebellar activation. Bilateral lesions to the cerebellar posterior vermis and hemispheres provoke deficits of empathy and ToM (Clausi et al. 2019; Roldan Gerschcovich et al. 2011), and patients with various types of cerebellar damage are impaired in ToM tasks (Sokolov 2018). Furthermore, a distinct mentalizing network directly connected to the cerebral mentalizing network has been identified in Crus 1 and 2 (Buckner et al. 2011).
The cerebellar involvement mainly in cognitive empathy fits with the significant covariation in activity of the right lateral cerebellum with self-rated individual differences in empathy for pain described by Singer et al. (2004), with the repeatedly reported cerebellar involvement in social cognition (Van Overwalle et al. 2014, 2020) and with the activation throughout right Crus 1 and 2 associated with ToM tasks (King et al. 2019). In a pediatric brain-injured sample, the individual differences in cerebellar volumes predicted ToM outcomes, and the volumetric reductions in the Cerebro-Cerebellar Mentalizing Network predicted poor ToM performances (Ryan et al. 2017). In children affected by autism spectrum disorders, voxel-based morphometry analyses revealed reduced volumes in right Crus 1 and 2, and the degree of cerebellar volumetric reductions correlated with the severity of symptoms in social interaction, communication, and repetitive behavior (D’Mello et al. 2015). More specifically, it has been reported that the size of the empathic imbalance between cognitive and emotional components positively correlates with autism traits in a neurotypical population (Shalev and Uzefovsky 2020). Interestingly, when predominant on affective empathy, cognitive empathy is related to stronger connectivity in interoception, autonomic monitoring, mentalizing and socio-cognitive networks that include the cerebellum (Cox et al. 2012). Furthermore, the cerebellum contributes to the empathic aspects linked to cognitive flexibility that allows adopting the subjective perspective of the other and to executive and regulatory processes that modulate the subjective feelings associated with emotions.
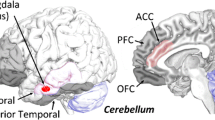
In contrast to these numerous findings, few investigations have specifically addressed the structural underpinning of empathy as a trait in healthy subjects, and the vast majority of research has so far mainly focused on the cerebrum, neglecting cerebellar regions. Recently, Picerni et al. (2021) analyzed the associations between macro- and micro-structural cerebellar measures and levels of affective and cognitive trait empathy (measured by the self-report Interpersonal Reactivity Index, IRI) in a large sample of healthy subjects of both sexes. The scores of Fantasy IRI-subscale that assesses one’s ability to imaginatively transpose themselves into feelings and actions of fictitious characters in books, movies, and plays were positively associated with the volumes in right cerebellar Crus 2 and pars triangularis of inferior frontal gyrus (Fig. 16.1, upper part). Furthermore, the increased volumes in Crus 2 were accompanied by diminished values of Mean Diffusivity in the same area, indicating an increased functional capacity. Reading a book or watching a movie are non-innate brain activities, which may occupy a very significant part in the daily life of many people, presumably because of their considerable adaptive value. During such activities, it often opens a window into characters’ thoughts and feelings, so that people respond in thought and feeling to fictive situations as if they actually occur. Ultimately, these activities help subjects to optimize decisions and actions, learn about existing or fictive worlds, and stimulate motivation and imagination, functioning thus as a sort of “emotional gym,” in which empathic capacities may be exerted. In watching a movie or reading a book, subjects may be so emotionally moved to get lost in the fictive happenings of the stories as if these were real, and imaginatively perceive themselves as transposed into the character’s thoughts and feelings, experiencing the character’s happenings from the character’s perspective, and merging with or “being” that character. Thus, empathy abilities are involved in emotional information processing not only “on-line,” when we respond to real emotional objects, but also “off-line,” when we represent emotional symbols (Niedenthal et al. 2005). Notably, the empathic responses are embodied either when we tend to mimic the behavior of others actually present and when we process bodily signals originating from information about others stored in long-term memory, even in the case the others are fictional characters.
Association between cerebellar gray matter volumes and embodiment-related psychological constructs. In the upper part, the volumes in right Crus 1, Crus 2, Lobule VI were associated with Empathy scores (specifically, with the scores of Fantasy subscale of Interpersonal Reactivity Index). In the middle part, the volumes in bilateral Crus 1 were associated with Alexithymia scores (evaluated by 20-item Toronto Alexithymia Scale). In the lower part, the volumes in left Lobules IV/V and VI were associated with Hypnotizability scores (evaluated by Stanford Hypnotic Susceptibility Scale, Form A). The reported significant differences survived to familywise error correction (FWE) for multiple comparisons. z above colorbar indicates normalized t-values. In figure left is left and coordinates are in Montreal Neurological Institute space
In line with its contributions to the motor and affective domain, one of the main mechanisms underlying cerebellar functionality involves the generation of internal models (Ito 2008). The internal models are neural representations that encode the context-specific dynamics of concrete or abstract representations to facilitate predictive control of the system. The internal models directly compute the final outputs that produce the desired affective outcomes (inverse models) or encode the transition from actual sensory states to future (predicted) sensory consequences through an efference copy of the last command (forward model) (Ito 2008; Ramnani 2006). Furthermore, inputs to the cerebellum regarding the salience and motivational value of emotional stimuli guide internal models to determine how an emotional response could benefit individuals in their current state and, thus, shape how the output from the cerebellum modifies the emotional response. Therefore, the cerebellum checks whether an individual’s state deviates from the expected state during emotion processing, and if the prediction error exceeds a given threshold defined by the context, the cerebellum refines the cortical response and recalibrates the internal model. And yet, since the predictions are based on information from the cortex to the cerebellum (efferent copies), and error signals are sent from the cerebellum to the cortex, the co-activation of the cerebellum and neocortical areas appears to be needed to successfully manage any mismatch (Ito 2008; Ramnani 2006). Similarly, to what happens in the sensorimotor system, even within the emotional/social realm the cerebellar function is characterized by the associated processes of forward modeling and error sensitivity that allow anticipating the other’s behavior or one’s own reactions (Sokolov et al. 2017; van Overwalle et al. 2020). We are now proposing that the concepts of prediction and error processing may be advanced to understand the cerebellar contribution to empathic abilities toward real people or even fictional characters. When the subject empathizes with other people, the cerebellar forward model potentially generates representations and predictions regarding other’s feelings. Internal models are developed by using past perceptual, motor, and socio-emotional experiences of the empathizer, and are framed by the intentions, beliefs, and feelings of the other. The degree of matching between the subject and the other relies on such representations, but the subject can efficiently match with the state of the other to the degree that s/he has already existing representations for that state, pointing out the experience-dependence of such a process, in analogy to what previously described for the motor domain (Calvo-Merino et al. 2006).
Interestingly, in Picerni et al. (2021), in addition to volumes of right Crus 2 the Fantasy IRI-subscale scores were associated with volumes of right pars triangularis of the inferior frontal gyrus. As known, the cerebellum has vast connections with the prefrontal cortex, whose functions are related to the ability to live sociably and communicate with others, being key nodes of the mirror neuron system (MNS) (Cattaneo and Rizzolatti 2009; Molenberghs et al. 2012). Given its observation-execution matching properties, MNS provides the appropriate mechanism for empathy and imitation (Iacoboni 2009) and allows identifying goals and intentions of others by their resemblance to stored representations for the same states (experience-dependence). MNS may facilitate thus the simulation of behavior—even emotional—of the other (Kaplan and Iacoboni 2006).
It has been postulated that the prefrontal areas are activated when two or more emotional states—such as one’s own and that of the other (in real life or imaginary situations)—are simultaneously processed and integrated to form a higher-order empathic state (Shamay-Tsoory et al. 2009a, b). It has been reported the peculiar engagement of the right inferior frontal cortex when comparing conditions in which the subject attributes a mental state to a character in a story in which the subject is featured and one in which s/he is absent (Vogeley et al. 2001). Further evidence for the critical role of the right inferior frontal cortex in the inhibition of self-perspective, comes from a case report of a subject with a lesion of this area who was impaired in ToM tasks that required the suppression of his own perspective but performed well if it did not (Samson et al. 2005). Thinking of or viewing a person who experiences a powerful emotion stimulates mirroring mechanisms and, through the implementation of the internal models provided by the cerebellum, might form embodied representations of that emotion grounded in perceptual, sensorimotor, and visceral control loops. These embodiment circuitries act as a boost for subsequent socio-emotional processes, allowing the remapping of other’s states into the corresponding subject’s perceptual, sensorimotor, and visceral brain areas making the subject experience the same emotion as the other (Preston and de Waal 2002; Niedenthal 2007; Preston 2007). The more similar the other’s state is to something the subject has already experienced, the more his/her representations will match the other’s state (Preston and de Waal 2002). Specifically, embodied models of empathy suggest that the capacity to detect own internal bodily signals should allow for a simulation of others’ states on own body, leading thus to a fine-tuning to others’ emotional and affective states (Bernhardt and Singer 2012). Consistently with these considerations, in the conceptual framework of social cognition, the concepts of empathy and embodiment are closely intertwined (Niedenthal 2007). However, embodied models struggle to explain why projecting other’s affective states on own body does not cause confusion between which responses belong to ourselves and which to someone else. It has been hypothesized that the aforementioned mechanisms of affective simulation and embodiment should be matched with compensatory processes that help to distinguish self-generated stimuli from other’s stimuli, forming and maintaining a clear distinction between self and other. These compensatory processes could be the efference copy signal provided by the cerebellum and the sensory and interoceptive signal processing at cortical and cerebellar levels. To differentiate between self and other, the brain needs to predict the sensory consequences of self-produced actions. According to the efference copy theory, the brain suppresses perception of self-produced sensory stimuli just by developing efference copies. A dysfunction of the efference copy at the forward comparator might weaken the sense of self-agency (“I am the initiator of my own emotions and actions”), such that self-induced sensory changes lose their ‘self’ tag and the individual no longer feels that he or she controls himself or herself. Furthermore, even the processing of sensory and interoceptive signals might serve to differentiate between self and other, by strengthening the representation of the subject’s real body properties, at the expense of vicarious simulations of those from others (Palmer and Tsakiris 2018). In fact, the embodied self is likely established through processing of afferent tactile, proprioceptive and interoceptive information. Recently, deactivations have been demonstrated during self-touch and activations during touch by others in areas involved in somatosensory processing, social cognition, and salience. Interestingly, among those activated areas was the cerebellum (Boehme et al. 2019).
In summary, depending on how empathy is triggered, the affective and cognitive components of empathy are differentially involved. Thus, either the automatic tendency to mimic the other’s expressions (bottom-up processing) and the capacity for imaginatively transposing ourselves into the feeling and thinking of the other (top-down processing) may be engaged. This co-recruitment of top-down and bottom-up components suggests that empathizing with the other’s states relies upon the use of affective processing, cognitive representations related to the other’s mental state, embodiment, and reactivation of information from one’s own past experiences. The bidirectional traffic in the cerebellum fits well with its processing of somatic, cognitive, and emotional signals, suggesting it as critical hub in the networks implicated in mind–brain–body interactions, highlighting the involvement of the cerebellum in socio-cognitive processes and supporting the view of a “social cerebellum,” and more specifically of an “empathic cerebellum.”
3 Alexithymia as Embodied Emotional-Psychological Process and Its Relation with the Cerebellum
Alexithymia is a construct of personality characterized by impairment in cognitive, emotional, and affective processing. It describes people with deficiencies in identifying, processing, or describing subjective feelings or emotional aspects of social interaction, difficulty in distinguishing between feelings and bodily sensations of emotional arousal, and limited affect-related fantasy and imagery. In the mid-1960s, the psychiatrist John C. Nemiah and his colleague Peter E. Sifneos undertook systematic studies of the cognitive style of individuals that found it extremely difficult to describe their subjective feelings. This was what in 1972 led Sifneos to coin the term “alexithymia,” which is formed by the roots of several Greek words, and literally means “lack of words for emotion.” People with alexithymic traits have a tendency to focus on facts without affective involvement rather than inner experiences (Sifneos 1972; Taylor and Bagby 2004). Internal experiences are believed to be minimized, and attention is focused externally, an effect that some Authors have attributed to altered interoception (Dobrushina et al. 2020). Although alexithymia is not a psychological disorder in itself, it is associated with enhanced risk of psychological impairment and is present in a broad spectrum of psychiatric and psychosomatic disorders, as chronic pain, somatoform disorders, addictive disorders, anxiety, and depression (Taylor and Bagby 2004). The presence of high alexithymic traits results in impairment in empathy, and the inverse relationship of these two constructs characterizes the broad psychiatric and psychosomatic spectrum (Moriguchi et al. 2007).
Neuroimaging studies in subjects with high alexithymic traits have shown less activation in brain areas associated with emotional awareness, such as the ACC, fusiform gyrus, amygdala, parahippocampal gyrus and insula (Kano et al. 2003; Pouga et al. 2010; Reker et al. 2010). As for volumetric variations, negative correlations between alexithymia scores and amygdala and insula volumes were described (Laricchiuta et al. 2015), supporting the view that in alexithymia altered processing of emotional stimuli is associated with a reduction of reactivity and volume in limbic structures. Furthermore, negative correlations between alexithymia scores and ACC volumes were reported (Laricchiuta et al. 2015).
More connected with the cerebellar lens through which we are viewing the alexithymic brain, there are alterations in the cerebellar activity (Kano et al. 2003; Moriguchi et al. 2007; Kh et al. 2012) or volumes (Laricchiuta et al. 2015) reported in the presence of alexithymia. Positive associations have been found between alexithymia scores (obtained in the 20-item Toronto Alexithymia Scale), and gray matter (GM) volumes in bilateral cerebellar Crus 1 (Fig. 16.1, middle part), without significant alterations of micro-structural (density, surface, and orientation of cells) parameters (Laricchiuta et al. 2015). The enlarged volumes in Crus 1 described in subjects with high alexithymic traits corroborate the notion of cerebellar involvement in cognitive, emotional, and affective processes and fit with the consistent activation unique to emotional processing described in bilateral Crus 1 (Kh et al. 2012; Stoodley and Schmahmann 2010). How the brain structure—specifically, the volume—relates to function is a debated issue. In fact, on the assumption that larger populations of neurons can produce larger outputs, and can therefore be more influential than smaller populations of neurons, a greater-than-average volume may signify greater-than-average power to carry out specific functions. However, a greater-than-average volume may signify even a smaller-than-average power, considering that for example a deficient pruning might render the area suboptimal in terms of a less fine-tuned and functionally optimized structure. At the same time, even a smaller-than-average volume may be related to increased and more tuned efficiency. Human and experimental evidence tends to favor the “larger-is-more-powerful” position. In fact, training on particular tasks or experiencing complex environments does increase the volume of the functionally related brain structures (Boyke et al. 2008; Di Paola et al. 2012), supporting that volume tends to positively covary with function. Thus, it is possible to suggest that the increased volumes of Crus 1 could result in an enhanced inhibitory output of Purkinje cells, the only efferent fibers of the cerebellar cortex, on the deep cerebellar nuclei, modulating thus their excitatory output.
Neuroanatomical (Bostan et al. 2013) and fMRI (Habas et al. 2009) studies indicate that Crus 1 and lobule VI constitute a node in the cortico-limbic network centered on the dorsal ACC and fronto-insular cortex, and involved in detecting, integrating and filtering emotional information. Even the act of identifying emotional intonation (affective prosody) produces activation in Crus 1 and lobule VI, VII (Wildgruber et al. 2005). Furthermore, it has been reported that negative emotional faces evoke prominent activation in Crus 1 and 2 as well as in lobules VI and IX (Schraa-Tam et al. 2012). Aversive stimuli in the form of noxious heat and unpleasant images produce increased activation in Crus 1 and lobule VI negatively correlated with the activation of limbic and para-limbic areas, as para-hippocampal gyrus, ACC, and hypothalamus (Moulton et al. 2011).
The link between alexithymia levels and cerebellum (positive relation) and limbic system (negative relation) suggests a specific functional role for the cerebellar involvement in emotional processing in general and in alexithymia in particular (Fusar-Poli et al. 2009). Cerebellar nuclei project to extra-cerebellar targets, including the limbic system (Bostan et al. 2013). The inhibited nuclear activity could result in a reduced excitatory input to limbic and para-limbic structures that, in turn, could undergo a volumetric reduction because of the diminished activation level. Such a mechanism, however hypothetical, is in line with classical electrophysiological evidence indicating that cerebellar nuclear stimulations have suppressive effects on limbic sites, including ACC and amygdala (Snider and Maiti 1976). In the same vein, smaller ACC volumes and greater posterior cerebellar volumes have been described in patients with Cushing’s disease reporting depressive and anxiety symptoms as well as cognitive, affective, and personality disorders (Andela et al. 2013). Structural neuroimaging studies on patients affected by obsessive-compulsive disorder also indicate smaller ACC volumes associated with greater cerebellar GM volumes (de Wit et al. 2014), offering additional insights into the reciprocal structural relation between the cerebellum and limbic and para-limbic areas.
Intriguingly, the enlarged volumes in Crus 1 described in subjects with high alexithymic traits are nicely compatible with the functional findings by Moriguchi and Komaki (2013), who reported that people with high alexithymic traits show reduced neural response in the limbic system to external and internal emotional stimuli and conversely increased neural response in somatosensory and sensorimotor areas to stimuli closely associated with physical information. Note that subjects with high alexithymic traits exhibit hypersensitivity to physical sensations, associated with a tendency to rely on or to amplify physical symptoms. The network comprising the cerebellum and limbic system (and also the sensorimotor and prefrontal cortices) is involved in sensing and monitoring the physiological bodily conditions (Critchley and Garfinkel 2017; Moulton et al. 2011), in representing the interoception within the context of ongoing activities, and in feeling self- and externally induced emotions (Anders et al. 2004).
In the condition of efficient functioning, subjects have internal models of their internal or external environment that serve the function of representing it. Such internal models form embodied representations grounded in sensorimotor control loops, and these representations in turn are internally manipulated before or instead of acting directly on the environment, even if the final goal of this form of embodied emotion and cognition is acting on the environment (Barsalou 2008; Niedenthal et al. 2005; Pezzulo and Castelfranchi 2009).
On such a basis, alexithymia may be considered an altered embodiment process related to an altered perception of physiological correlates of the emotional activation, resulting in a deficit in emotional awareness. Karlsson et al. (2008) suggested that in highly alexithymic individuals the brain regions involved in bodily awareness may be hyperactive during emotional processing, possibly reflecting the alexithymic tendency to experience physical symptoms when emotionally aroused. In the same line, Zhang et al. (2011) interpreted the increases in GM density in relation to alexithymia as indicative of a greater reliance on bodily sensations during the subjective experience of emotion. Accordingly, a somatosensory amplification has been described in the presence of definite alexithymia (Lumley et al. 2005). Kano et al. (2007) described the aberrant manner of perceiving body signals of subjects with high alexithymic traits and reported positive associations between alexithymia scores and cerebellar regional cerebral blood flow (rCBF) following visceral stimulation. Furthermore, high cerebellar rCBF was reported in subjects with high alexithymic traits when viewing emotional facial expressions (Kano et al. 2003), recalling emotional autobiographic traces (Huber et al. 2002), or observing a classic mirror neuron task, as the observation of goal-directed hand actions (Moriguchi et al. 2009).
In the presence of alexithymia, an altered referential process can lead to emotions that are somato-sensorially perceived but not verbally expressed, due to not having words for the emotions or being without symbols for the somatic states. In line with the lack of emotional awareness, it has been described the lack of somatic awareness, the “alexisomia” (Shitsu-taikan-sho in Japanese). Such an intriguing psychological construct is characterized by difficulty in the awareness of somatic sensations, disconnection between cortical and subcortical systems, and homeostatic inadequacy by blunt interoception. Alexisomia might be an important variable in the pathology of psychosomatic disorders (Ikemi and Ikemi 1986; Moriguchi and Komaki 2013). Reduced interoceptive awareness featuring alexisomia would result by impairment of the senses necessary to maintain homeostasis (as hunger and sleepiness), the senses associated with adaptive processes to environmental changes normally felt as warning signs (such as fatigue), and the senses accompanying physical diseases (such as chill and pain). Ikemi and Ikemi (1986) added that individuals prone to Shitsu-taikan-sho show unhealthy and self-destructive lifestyles and have difficulties in awareness and expression of bodily feelings. Thus, if the awareness of bodily states, including autonomic and hormonal status, is the basis of emotional awareness, deficits of emotional awareness underlying alexithymia might be related to deficits of bodily sensation awareness underlying alexisomia. To exemplify this unhealthy condition, we can mention that patients with reduced interoceptive awareness may experience somatosensory amplification (the tendency to perceive normal somatic and visceral sensations as intense, disturbing, and noxious), accompanied by persistent pain, such as myalgia of some part of the body, or may not perceive their own somatic state properly. Considering the bottom-up component of the emotional expression, the altered awareness of bodily states featuring the alexisomia might be the rudimentary form of altered emotional awareness featuring alexithymia.
4 Hypnotizability as Embodied Psychological-Cognitive Process and Its Relation with the Cerebellum
Hypnotizability, or hypnotic susceptibility, is a term used to describe the degree to which a subject is responsive to suggestion, taking into account that not everyone is susceptible to hypnosis. In other words, hypnotizability is a personality construct that predicts the proneness to modify perception, memory, emotion, and behavior according to the content of specific imaginative suggestions after hypnotic induction as well as in the ordinary state of consciousness (Elkins et al. 2015). Hypnotizability can be assumed to be a psychological trait facilitating the embodiment of suggestions and the involuntariness in action. Indeed, differences in its main cognitive-emotional components (i.e., imagery, fantasy proneness, expectancy, attention/absorption, acquiescence, and motivation) are accompanied by differences in somatic and autonomic correlates, as sensorimotor integration, cardiovascular control, functional equivalence between imagery and perception (Santarcangelo and Scattina 2016). The degree of hypnotic susceptibility determines the differences in the ability of detachment from bodily signals, interoceptive sensitivity, tendency toward ideomotor behaviors, and in the way to represent and reconstruct sensorimotor information. In particular, the highly hypnotizable individuals exhibit higher proneness to modify memory, perception, and behavior according to specific imaginative suggestions and display greater embodiment of mental images related to both imagery or perception of a position of a body part (Ibáñez-Marcelo et al. 2019). The different embodiment of mental images supports the proneness to respond to sensorimotor suggestions and to report involuntariness in action featuring highly hypnotizable individuals. Additionally, different levels of hypnotizability are related to morpho-functional peculiarities of several brain areas. In particular, different activation and volumes not only of specific cortical regions, including the dorsolateral prefrontal cortex, inferior frontal gyrus, ACC, parietal and temporal regions, para-hippocampal gyrus, and insula), but also of defined specific cerebellar areas have been described (Hoeft et al. 2012; Jiang et al. 2017; McGeown et al. 2015; Picerni et al. 2019). fMRI studies have shown that the report of involuntariness in action is associated with the activation of the parieto-cerebellar network, which differentiates the activations during suggestion-induced movements misattributed to an external source with respect to movements experienced as self-produced and controlled (Blakemore et al. 2003).
Subjects with different hypnotizability levels differ in sensorimotor integration, indicating the possible involvement of cerebellar networks in the sensorimotor, cognitive, and emotional aspects of hypnotizability (Santarcangelo and Scattina 2016). In particular, subjects with high hypnotic susceptibility exhibit a less strict postural and locomotor control, lower accuracy and higher variability in visuomotor tasks, higher blink rate, increased pain intensity, pre-eminent parasympathetic control of heart rate. Interestingly, they display smaller volumes in left cerebellar lobules IV/V and lobule VI (Picerni et al. 2019) (Fig. 16.1, lower part). Such volumetric differences closely fit with the functional topographic organization of cerebellar regions according to their anatomical connectivity with neocortical regions. In fact, the lobule VI represents the anterior boundary between overtly sensorimotor zones (i.e., lobules IV-V) and supra-modal cognitive zones (i.e., lobule VII), so that sensorimotor tasks that involve complex, fast and sequenced movements activate specifically the lobule VI. Thus, reduced volumes of lobules IV–V and VI of subjects with high hypnotic susceptibility may sustain their altered sensorimotor processing.
Notably, lobules IV–VI are functionally linked to the insular cortex (Sultan et al. 2012), the integrative center for own-body representation and awareness that receives large quantities of interoceptive, autonomic, and emotional information from somatosensory and limbic areas, and links them with external elements in order to organize adaptive behaviors (Craig 2011). The insular cortex is involved in self-reflection, self-monitoring, and self-regulation, as well as in empathy, all processes that can be altered in hypnosis (Terhune and Hedman 2017).
By investigating the insular responses to sensory stimuli with affective valence in relation to the individual differences in emotional susceptibility, it has been demonstrated that weaker functional connections of the left anterior insula with left lobule IV are linked to higher emotional susceptibility (Ebisch et al. 2015). Such findings suggest that these changes could represent the correlate of the altered emotional processing reported in the presence of high levels of hypnotizability, when higher emotional intensity during imagery, sensitivity and empathy, tendency to somatic complaints, and vividness of pain imagery are present (Kirenskaya et al. 2011). Furthermore, an fMRI study on a sample of subjects with high levels of hypnotizability revealed parallel activations of the left insula and left cerebellum, besides prefrontal and parietal cortices, during both hypnotically and physically induced pain (Derbyshire et al. 2004).
Once more, it is important to emphasize the role of the cerebellum in forming internal models (forward or inverse) to adapt sensorimotor, cognitive, and emotional activities to information of internal and external environment (Ito 2008; Ramnani 2006). Even in the case of hypnotizability, the long-range signals from associative cortices may exert a relevant top-down control over the cerebellum broadly involved in the operational processing of information linked to movement, thought, and emotion. Once again, it has to be considered that the top-down processes work in concert with bottom-up mechanisms. The directionality of processing (bottom-up or top-down) depends on the hierarchical position of the cortical area from which the cerebellum receives its inputs relative to the cortical area to which the cerebellum directs its outputs, placing the cerebellum as a “sub-cortical hub” between hierarchically different cortical regions (Kellermann et al. 2012). Even if only speculatively, it is possible to propose that the individual differences in hypnotic susceptibility could be mediated by cortico-cerebello-cortical loops. The sensitivity of some neocortical regions might top-down modulate the activity of cerebellar lobules IV–VI, which in turn might bottom-up control other cortical regions.
5 Conclusion
Current psychological discourse debates emotions and psychological traits as ‘embodied’ phenomena, suggesting that the body helps the mind in shaping an emotional and cognitive response. The main models of embodiment describe the self as an integration of a social or conceptual self along with our physical self, suggesting that affective and psychological functions are not independent of sensorimotor functions. We use our own body and experiences to simulate information from other people’s bodies to understand their emotions, thoughts, and behaviors. This idea of a strong mutual interaction between the embodiment processing and the cerebellum (Guell et al. 2018) underscores the role of the cerebellum in emotion and psychological traits.
References
Andela CD, van der Werff SJ, Pannekoek JN et al (2013) Smaller grey matter volumes in the anterior cingulate cortex and greater cerebellar volumes in patients with long-term remission of Cushing’s disease: a case-control study. Eur J Endocrinol 169:811–819
Anders S, Lotze M, Erb M et al (2004) Brain activity underlying emotional valence and arousal: a response-related fMRI study. Hum Brain Mapp 23:200–209
Barsalou LW (2008) Grounded cognition. Annu Rev Psychol 59:617–645
Bernhardt BC, Singer T (2012) The neural basis of empathy. Annu Rev Neurosci 35:1–23
Bilevicius E, Kolesar T, Smith S et al (2018) Trait emotional empathy and resting state functional connectivity in default mode, salience, and central executive networks. Brain Sci 8:128
Blakemore SJ, Oakley DA, Frith CD (2003) Delusions of alien control in the normal brain. Neuropsychologia 41:1058–1067
Boehme R, Hauser S, Gerling GJ (2019) Distinction of self-produced touch and social touch at cortical and spinal cord levels. Proc Natl Acad Sci U S A 116:2290–2299
Bostan AC, Dum RP, Strick PL (2013) Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci 17:241–254
Boyke J, Driemeyer J, Gaser C et al (2008) Training-induced brain structure changes in the elderly. J Neurosci 28:7031–7035
Buckner RL, Krienen FM, Castellanos A et al (2011) The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol 106:2322–2345
Calvo-Merino B, Grèzes J, Glaser DE et al (2006) Seeing or doing? Influence of visual and motor familiarity in action observation. Curr Biol 16:1905–1910
Cattaneo L, Rizzolatti G (2009) The mirror neuron system. Arch Neurol 66:557–560
Clausi S, Olivito G, Lupo M et al (2019) The cerebellar predictions for social interactions: theory of mind abilities in patients with degenerative cerebellar atrophy. Front Cell Neurosci 12:510
Cox CL, Uddin LQ, Di Martino A et al (2012) The balance between feeling and knowing: affective and cognitive empathy are reflected in the brain’s intrinsic functional dynamics. Soc Cogn Affect Neurosci 7:727–737
Craig ADB (2011) Significance of the insula for the evolution of human awareness of feelings from the body. Ann NY Acad Sci 1225:72–82
Critchley HD, Garfinkel SN (2017) Interoception and emotion. Curr Opin Psychol 17:7–14
Damasio A, Carvalho GB (2013) The nature of feelings: evolutionary and neurobiological origins. Nat Rev Neurosci 14:143–152
Derbyshire SWG, Whalley MG, Stenger VA et al (2004) Cerebral activation during hypnotically induced and imagined pain. Neuroimage 23:392–401
de Waal FBM, Preston SD (2017) Mammalian empathy: behavioural manifestations and neural basis. Nat Rev Neurosci 18:498–509
de Wit SJ, Alonso P, Schweren L et al (2014) Multicenter voxel-based morphometry mega-analysis of structural brain scans in obsessive–compulsive disorder. Am J Psychiatry 171:340–349
Di Paola M, Caltagirone C, Petrosini L (2012) Prolonged rock-climbing activity induces structural changes in cerebellum and parietal lobe. Hum Brain Mapp 4:2707–2714
D’Mello AM, Crocetti D, Mostofsky SH et al (2015) Cerebellar gray matter and lobular volumes correlate with core autism symptoms. Neuroimage Clin 7:631–639
Dobrushina OR, Arina GA, Dobrynina LA et al (2020) The ability to understand emotions is associated with interoception-related insular activation and white matter integrity during aging. Psychophysiology 57:e13537
Ebisch SJH, Bello A, Spitoni GF et al (2015) Emotional susceptibility trait modulates insula responses and functional connectivity in flavor processing. Front Behav Neurosci 9:1–14
Elkins GR, Barabasz AF, Council JR et al (2015) Advancing research and practice: the revised APA division 30 definition of hypnosis. Int J Clin Exp Hypn 63:1–9
Fan Y, Duncan NW, de Greck M et al (2011) Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neurosci Biobehav Rev 35:903–911
Fusar-Poli P, Placentino A, Carletti F et al (2009) Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci 34:418–432
Gallese V, Goldman A (1998) Mirror neurons and the simulation theory of mindreading. Trends Cogn Sci 12:493–501
Gu X, Gao Z, Wang X et al (2012) Anterior insular cortex is necessary for empathetic pain perception. Brain 135:2726–2735
Guell X, Gabrieli JDE, Schmahmann JD (2018) Embodied cognition and the cerebellum: perspectives from the dysmetria of thought and the universal cerebellar transform theories. Cortex 100:140–148
Habas C, Kamdar N, Nguyen D et al (2009) Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci 29:8586–8594
Harari H, Shamay-Tsoory SG, Ravid M et al (2010) Double dissociation between cognitive and affective empathy in borderline personality disorder. Psychiatry Res 175:277–279
Hoeft F, Gabrieli JDE, Whitfield-Gabrieli S et al (2012) Functional brain basis of hypnotizability. Arch Gen Psychiatry 69:1064–1072
Huber M, Herholz K, Habedank B et al (2002) Different patterns of regional brain activation during emotional stimulation in alexithymics in comparison with normal controls. Psychother Psychosom Med Psychol 52:469–478
Husserl E (1931) Ideas: general introduction to pure phenomenology. Allen and Unwin, London
Iacoboni M (2009) Imitation, empathy, and mirror neurons. Annu Rev Psychol 60:653–670
Ibáñez-Marcelo E, Campioni L, Phinyomark A et al (2019) Topology highlights mesoscopic functional equivalence between imagery and perception: the case of hypnotizability. Neuroimage 200:437–449
Ikemi Y, Ikemi A (1986) An oriental point of view in psychosomatic medicine. Psychother Psychosom 45:118–126
Ito M (2008) Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci 9:304–313
Izard CE (2009) Emotion theory and research: highlights, unanswered questions, and emerging issues. Annu Rev Psychol 60:1–25
James W (1884) What is an emotion? Mind 9:188–205
James W (1890/1907) The principles of psychology in two volumes. Macmillan, London
Jiang H, White MP, Greicius MD et al (2017) Brain activity and functional connectivity associated with hypnosis. Cereb Cortex 27:4083–4093
Kano M, Fukudo S, Gyoba J et al (2003) Specific brain processing of facial expressions in people with alexithymia: an H2 15O-PET study. Brain 126:1474–1484
Kano M, Hamaguchi T, Itoh M et al (2007) Correlation between alexithymia and hypersensitivity to visceral stimulation in human. Pain 132:252–263
Kaplan JT, Iacoboni M (2006) Getting a grip on other minds: mirror neurons, intention understanding, and cognitive empathy. Soc Neurosci 1:175–183
Karlsson H, Näätänen P, Stenman H (2008) Cortical activation in alexithymia as a response to emotional stimuli. Br J Psychiatry 192:32–38
Kellermann T, Regenbogen C, De Vos M et al (2012) Effective connectivity of the human cerebellum during visual attention. J Neurosci 32:11453–11460
Kh E, Chen SH, Ho MH et al (2012) A meta-analysis of cerebellar contributions to higher cognition from PET and fMRI studies. Hum Brain Mapp 35:593–615
Khalsa SS, Adolphs R, Cameron OG et al (2018) Interoception and mental health: a roadmap. Biol Psychiatry Cogn Neurosci Neuroimaging 3:501–513
King M, Hernandez-Castillo CR, Poldrack RA et al (2019) Functional boundaries in the human cerebellum revealed by a multi-domain task battery. Nat Neurosci 22:1371–1378
Kirenskaya AV, Novototsky-Vlasov VY, Chistyakov AN et al (2011) The relationship between hypnotizability, internal imagery, and efficiency of neurolinguistic programming. Int J Clin Exp Hypn 59:225–241
Lamm C, Batson CD, Decety J (2007) The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. J Cogn Neurosci 19:42–58
Laricchiuta D, Petrosini L, Picerni E (2015) The embodied emotion in cerebellum: a neuroimaging study of alexithymia. Brain Struct Funct 220:2275–2287
Leblanc H, Ramirez S (2020) Linking social cognition to learning and memory. J Neurosci 40:8782–8798
Lumley MA, Gustavson BJ, Partridge RT et al (2005) Assessing alexithymia and related emotional ability constructs using multiple methods: interrelationships among measures. Emotion 5:329–342
Luyten P, Campbell C, Fonagy P (2020) Borderline personality disorder, complex trauma, and problems with self and identity: a social-communicative approach. J Pers 88:88–105
McGeown WJ, Mazzoni G, Vannucci M et al (2015) Structural and functional correlates of hypnotic depth and suggestibility. Psychiatry Res 231:151–159
Molenberghs P, Cunnington R, Mattingley JB (2012) Brain regions with mirror properties: a meta-analysis of 125 human fMRI studies. Neurosci Biobehav Rev 36:341–349
Moore RC, Dev SI, Jeste DV et al (2015) Distinct neural correlates of emotional and cognitive empathy in older adults. Psychiatry Res 232:42–50
Moriguchi Y, Komaki G (2013) Neuroimaging studies of alexithymia: physical, affective, and social perspectives. Biopsychosoc Med 7:8
Moriguchi Y, Decety J, Ohnishi T et al (2007) Empathy and judging other’s pain: an fMRI study of alexithymia. Cereb Cortex 17:2223–2234
Moriguchi Y, Ohnishi T, Decety J et al (2009) The human mirror neuron system in a population with deficient self-awareness: an fMRI study in alexithymia. Hum Brain Mapp 30:2063–2076
Moulton EA, Elman I, Pendse G (2011) Aversion-related circuitry in the cerebellum: responses to noxious heat and unpleasant images. J Neurosci 31:3795–3804
Niedenthal PM (2007) Embodying emotion. Science 316:1002–1005
Niedenthal PM, Barsalou LW, Winkielman P et al (2005) Embodiment in attitudes, social perception, and emotion. Pers Soc Psychol Rev 9:184–211
Nummenmaa L, Glerean E, Hari R, Hietanen JK (2014) Bodily maps of emotions. PNAS 111:646–651
Palmer CE, Tsakiris M (2018) Going at the heart of social cognition: is there a role for interoception in self-other distinction? Curr Opin Psychol 24:21–26
Pezzulo G, Castelfranchi C (2009) Intentional action: from anticipation to goal-directed behavior. Psychol Res 73:437–440
Picerni E, Santarcangelo EL, Laricchiuta D et al (2019) Cerebellar structural variations in subjects with different hypnotizability. Cerebellum 18:109–118
Picerni E, Laricchiuta D, Piras F et al (2021) Macro- and micro-structural cerebellar and cortical characteristics of cognitive empathy towards fictional characters in healthy individuals. Sci Rep 11:8804
Pouga L, Berthoz S, de Gelder B et al (2010) Individual differences in socioaffective skills influence the neural bases of fear processing: the case of alexithymia. Hum Brain Mapp 31:1469–1481
Preston SDA (2007) perception-action model for empathy. In: Farrow TFD, Woodruff PWR (eds) Empathy in mental illness. Cambridge University Press, Cambridge, pp 428–447
Preston SD, de Waal FBM (2002) Empathy: its ultimate and proximate bases. Behav Brain Sci 25:1–20
Ramnani N (2006) The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci 7:511–522
Reker M, Ohrmann P, Rauch AV (2010) Individual differences in alexithymia and brain response to masked emotion faces. Cortex 46:658–667
Ritter K, Dziobek I, Preissler S (2011) Lack of empathy in patients with narcissistic personality disorder. Psychiatry Res 187:241–247
Roldan Gerschcovich E, Cerquetti D, Tenca E et al (2011) The impact of bilateral cerebellar damage on theory of mind, empathy and decision making. Neurocase 17:270–275
Ryan NP, Catroppa C, Beare R et al (2017) Uncovering the neuroanatomical correlates of cognitive, affective and conative theory of mind in pediatric traumatic brain injury: a neural systems perspective. Soc Cogn Affect Neurosci 12:1414–1427
Samson D, Apperly IA, Kathirgamanathan U et al (2005) Seeing it my way: a case of a selective deficit in inhibiting self-perspective. Brain 128:1102–1111
Santarcangelo EL, Scattina E (2016) Complementing the latest APA definition of hypnosis: sensory-motor and vascular peculiarities involved in hypnotizability. Int J Clin Exp Hypn 64:318–330
Schraa-Tam CK, Rietdijk WJ, Verbeke WJ (2012) fMRI activities in the emotional cerebellum: a preference for negative stimuli and goal-directed behavior. Cerebellum 11:233–245
Shalev I, Uzefovsky F (2020) Empathic disequilibrium in two different measures of empathy predicts autism traits in neurotypical population. Mol Autism 11:59
Shamay-Tsoory SG, Shur S, Barcai-Goodman L et al (2007) Dissociation of cognitive from affective components of theory of mind in schizophrenia. Psychiatry Res 149:11–23
Shamay-Tsoory SG, Aharon-Peretz J, Perry D (2009a) Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain 132:617–627
Shamay-Tsoory SG, Harari H, Szepsenwol O et al (2009b) Neuropsychological evidence of impaired cognitive empathy in euthymic bipolar disorder. J Neuropsychiatry Clin Neurosci 21:59–67
Sifneos PE (1972) Short-term psychotherapy and emotional crisis. Harvard University Press, Cambridge
Singer T, Seymour B, O’Doherty J et al (2004) Empathy for pain involves the affective but not sensory components of pain. Science 303:1157–1162
Snider RS, Maiti A (1976) Cerebellar contributions to the Papez circuit. J Neurosci Res 2:133–146
Sokolov AA (2018) The cerebellum in social cognition. Front Cell Neurosci 12:145
Sokolov AA, Miall RC, Ivry RB (2017) The cerebellum: adaptive prediction for movement and cognition. Trends Cogn Sci 21:313–332
Stoodley CJ, Schmahmann JD (2010) Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 46:831–844
Sultan F, Augath M, Hamodeh S et al (2012) Unravelling cerebellar pathways with high temporal precision targeting motor and extensive sensory and parietal networks. Nat Commun 3:924
Taylor GJ, Bagby RM (2004) New trends in alexithymia research. Psychother Psychosom 73:68–77
Terhune DB, Hedman LRA (2017) Metacognition of agency is reduced in high hypnotic suggestibility. Cognition 168:176–181
Van Overwalle F, Baetens K, Mariën P et al (2014) Social cognition and the cerebellum: a meta-analysis of over 350 fMRI studies. Neuroimage 86:554–572
Van Overwalle F, Ma Q, Heleven E (2020) The posterior crus II cerebellum is specialized for social mentalizing and emotional self-experiences: a meta-analysis. Soc Cogn Affect Neurosci 15:905–928
Vogeley K, Bussfeld P, Newen A et al (2001) Mind reading: neural mechanisms of theory of mind and self-perspective. Neuroimage 14:170–181
Wildgruber D, Riecker A, Hertrich I et al (2005) Identification of emotional intonation evaluated by fMRI. Neuroimage 24:1233–1241
Zhang L, Zhu C, Ye R et al (2011) Impairment of conflict processing in alexithymic individuals. Neurosci Lett 504:261–264
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Laricchiuta, D., Picerni, E., Cutuli, D., Petrosini, L. (2022). Cerebellum, Embodied Emotions, and Psychological Traits. In: Adamaszek, M., Manto, M., Schutter, D.J.L.G. (eds) The Emotional Cerebellum . Advances in Experimental Medicine and Biology, vol 1378. Springer, Cham. https://doi.org/10.1007/978-3-030-99550-8_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-99550-8_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-99549-2
Online ISBN: 978-3-030-99550-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)