Abstract
The nervous system branches throughout the body in a way similar to the circulatory system, innervates almost all tissues, and regulates normal tissue homeostasis and function. Although it is well known that both the vascular system and the immune system display strong influences on cancer, nerves have often been seen as more silent partners in the tumor microenvironment. However, studies from different tissue types have revealed similarities in how the nervous system regulates normal and neoplastic cellular function. Therefore, neural-cancer crosstalk, both systemically and locally within the tumor microenvironment, is now emerging as a crucial hallmark of cancer initiation, growth, and metastasis.

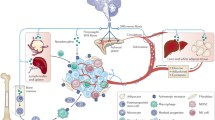
Regulation of the tumor microenvironment mediated by autonomic innervation. The autonomic nervous system (ANS) interacts directly with tumor epithelial cells, along with multiple stromal components in the tumor microenvironment (TME). Remodeling of extracellular matrix components triggered by sympathetic signaling promotes angiogenesis, tumor growth and dissemination. The immune system is another TME component regulated by nerves. Sympathetic nerves stimulate the mobilization of tumor-associated macrophages. The ANS has a dual effect on the expression of immune checkpoints such as PD-1 and PD-L1. The parasympathetic innervation can downregulate these proteins, allowing the immune system to fight malignant cells. Created with BioRender.com
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Tumor microenvironment (TME)
- Central nervous system (CNS)
- Peripheral nervous system (PNS)
- Sympathetic and parasympathetic nervous systems
- Perineural invasion (PNI)
- Axonogenesis
- Neo-neurogenesis
- Cancer progression
-
Presence of nerves in and around malignant tumors has recently emerged as an important feature of the tumor microenvironment
-
Nerve fibers influence cancer growth, spread, therapeutic resistance and prognosis
-
The function of different nerve types must be understood in a cancer-specific manner
-
Different mechanisms for tumor-nerve interactions have been described, including perineural invasion, axonogenesis, and neo-neurogenesis
Background
The nervous system is involved in physiological processes from organogenesis and growth to tissue homeostasis and repair throughout the body. Similar to these roles, neural elements can also influence the initiation, growth, and metastasis of malignant tumors. This has currently emerged as an expanding and exciting field within cancer research.
The mechanisms underlying peripheral nerve-tumor interactions are largely unexplored. It is not known whether this crosstalk reflects paracrine-signaling events leading to increased neural activity in the local tumor microenvironment (TME), whether nerve-to-cancer cell synapse-like structures are formed, and whether electrical coupling exists outside the central nervous system (CNS) that enable peripheral nerves to interact with cancer cells [1].
It is now known that nerves infiltrate the TME and actively stimulate cancer cell growth and dissemination [2, 3]. This mechanism involves the paracrine release of neurotransmitters [4] into the vicinity of cancer cells and stromal cells to activate corresponding membrane receptors. In addition, the secretion of neurotrophic growth factors by cancer cells drives the outgrowth of nerves in solid tumors. In this way, reciprocal interactions between nerves and cancer cells provide new insights into the cellular and molecular basis of tumorigenesis [4, 5].
Studies in pancreatic [6, 7] and prostate cancer [4] have shown that neurotransmitters and cytokines secreted from nerves can enhance the malignant phenotype of cancer cells, including proliferation, cell survival, and invasiveness. Further, cancer cells secrete neuromodulating signals to induce neuroplasticity, neural invasion, and neuropathic pain sensation [8]. Therefore, reciprocal interactions between nerves and cancer cells cooperate to promote cancer progression.
Tumor-nerve crosstalk may also occur indirectly, by the nervous system regulating other cell types of the TME, e.g., immune cells, endothelial cells, and fibroblasts [9]. This communication may occur locally within the TME or tumor niche, or more systemically through circulating signals that might influence distant pre-metastatic tissue niches.
To better understand the mechanisms by which nerves interact with the TME to drive cancer initiation and progress, we will first review some aspects of developmental biology and regeneration. The parallels between embryonic development and cancer were first considered by Waddington in 1935 [10], linking mechanisms of development or regeneration to uncontrolled tumor growth. Waddington hypothesized that common signaling pathways in regeneration and cancer could provide cues to control cancer progression. Nerve dependence for regeneration and tissue growth was discovered in 1823 (as discussed in Boilly et al. [11]) from studies of salamander limb amputation, demonstrating that innervation was crucial for adult regeneration. The ingrowth of nerves into the blastema (the part of the tissue converted into a zone of undifferentiated progenitors [12]) starts during the early stages of wound healing in the epithelium covering the amputation site. Nerves infiltrate the blastema and recreate the neural networks necessary for regeneration, which is similar to the findings in cancer, indicating that nerves are active participants in tumor progression [11]. This emphasizes the need to investigate links between development, regeneration, and cancer.
Division of the Nervous System
In humans, the nervous system consists of the central nervous system (CNS) and the peripheral nervous system (PNS). The brain and spinal cord constitute the CNS, while the PNS consists of external nerves which connect the rest of the body to the CNS.
Central Nervous System
The CNS consists primarily of the brain and the spinal cord. It is mainly composed of grey and white matter. Grey matter is composed of cell bodies of neurons, glial cells, and capillaries, and it constitutes the outermost layer of the brain. White matter is primarily formed by myelinated axons, with thin elongated cell projections covered in myelin and created by oligodendrocytes [13].
There are two main cell types found within the CNS : neurons and glial cells. Neurons are responsible for the information relay, sensory and motor processing via specialized connections called synapses. The neuron structure varies based on their function, but generally, dendrites on the outer rim of the cell body will receive signals from other nerves. Glial cells, on the other hand, are all non-neuronal supporting cells of the CNS , including astrocytes, oligodendrocytes, microglia, and ependymal cells [14]. These cells are responsible for tissue repair upon damage, creating myelin sheaths around nerve axons, acting as the primary immune defense, and creation and locomotion of the cerebrospinal fluid of the brain, respectively.
Peripheral Nervous System
The peripheral nervous system (PNS) consists of the nerves and ganglia that connect the CNS to the rest of the body. The PNS can be subdivided into three systems: the somatic, the enteric, and the autonomic nervous system. The somatic nervous system is the voluntary division of the PNS and consists of afferent nerves (which transmit the sensory information from the body to the CNS), and efferent nerves (which exit commands from the CNS to the muscles) [15]. The enteric nervous system consists of a mesh-like network of neurons that governs the function of the gastrointestinal tract. It is influenced by the autonomic nervous system (ANS) although it might also be capable of acting independently [16]. The ANS has two branches: the sympathetic nervous system and the parasympathetic nervous system. The ANS acts mainly unconsciously, and innervates and regulates all organs in the body, except skeletal muscle. It is organized as two neurons in series, where the first originates in the CNS and connects the second neuron, which originates in a ganglion in the periphery and innervates the target gland or organ [17].
Autonomic Innervation in Cancer Tissues
A growing body of evidence suggests a link between the ANS and cancer. Multiple studies in animal models and humans have shown that sympathetic and parasympathetic nerves innervate cancer tissues and influence their behavior to promote tumor growth and distant metastasis [18,19,20,21]. The fact that the postganglionic neuronal bodies are located relatively close to the target tissues give them the ability to respond to changes in the TME, not only through neurotransmitter secretion, but also through alterations in the transcription or translation and cytoskeletal changes [9]. In addition , the different cells that make up the TME express receptors for sympathetic and parasympathetic neurotransmitters and various neuropeptides and can thereby react to nerve stimulation in ways that affect tumor growth and progression [19, 22,23,24].
Tumor-Nerve Interactions in the Tumor Microenvironment
Recent advances in genetical engineering and imaging have shed light on the mechanisms of neuronal regulation in cancer. An initial and more straightforward view of nerves affecting tumor proliferation, survival, and migration consider the direct action of neurotransmitters on cancer cells. For example, aberrant innervation of the epithelial cells in the stomach promotes the initiation and progression of gastric cancer [25]. Besides this, there is new evidence indicating that regulation of the TME by the nervous system has a strong impact on the tumor properties and aggressiveness (See Graphic abstract figure):
Angiogenesis is necessary for the availability of nutrients and oxygen in tumors, and therefore, for their expansion and spread. Blood vessels and sympathetic nerves share common patterning cues [26]. In prostate cancer, it has been shown how adrenergic nerves indirectly affect tumor growth by stimulating angiogenesis in the TME [22].
The immune system is another component of the TME highly regulated by the nervous system. By using genetic engineering techniques, the modulation of sympathetic and parasympathetic nerve systems in breast cancer has been shown to affect the expression of immune checkpoints and regulators, including PD-1, PD-L1, and FOXP3, with subsequent impact on antitumor immune response and breast cancer progression [21]. In a different study, sympathetic nerves were shown to stimulate the mobilization of tumor-associated macrophages that activated a metastatic switch within the primary tumor [27].
Finally, cancer-associated fibroblasts (CAFs), a major component of the TME, have been shown to actively remodel the extracellular matrix (ECM) by producing type I collagen in response to sympathetic stimuli [28]. Such changes in the TME might promote both angiogenesis and neurogenesis, and hence cancer dissemination.
Taken together, nerves interact with both malignant cells and various components of the TME, and this multifaceted nerve-stroma interaction is decisive for tumor growth and dissemination.
Mechanisms of Nerve Involvement in Cancer
Nerve infiltration in TME has recently emerged as a key player in cancer pathogenesis [1, 2, 9], including regulation of cancer initiation, growth, and metastasis. The interaction between cancer cells and nerves is bidirectional and involves trophic factors released by nerves towards both cancer cells and surrounding stromal cells, and cancer cells secrete neurotrophic factors to stimulate nerve infiltration. In combination, the molecular mediators of the tumor-nerve crosstalk represent a dangerous duo that promote tumor-associated neural networks that boosts tumor growth and spread [4, 25, 29].
Perineural invasion was for long the only acknowledged cancer-nerve interaction, but cancer and nerves can also interact via other mechanisms, including axonal outgrowth (sprouting) of pre-existing nerves through a process of axonogenesis, and cancer-stimulated formation of new nerves from neural progenitor cells through the process of neo-neurogenesis [30] . The effects that nerves have on a specific cancer, can vary dependent on tissue type [1].
Perineural Invasion
Perineural invasion (PNI) is a process in which cancer cells invade the perineural space of surrounding nerves and move around them [31], providing a route for metastatic spread along nerves [32] (Fig. 16.1, left). Many cancer types have been observed to attract neural interaction and PNI, at least at advanced disease stages, including gastric cancer [33], lung [34], head and neck [35], pancreas [7], prostate [4], but also breast cancer [36], and PNI is considered an important pathological feature of many tumors that can also be observed in the absence of vascular invasion [31]. Although PNI has been found in many tumor types, the underlying molecular mechanisms by which cancer cells invade perineural spaces—including which factors are involved—have not been defined and no treatment targeting PNI is currently available.
Mechanisms of nerve involvement in cancer . Perineural invasion (PNI) (left) is the invasion of cancer cells to the space surrounding nerves. In this process, trophic factors are released by the nerves towards cancer cells, and cancer cells secrete neurotrophic factors to stimulate nerve infiltration. PNI can be used as a route for metastatic spread. Axonogenesis (center) is the outgrowth of axons from pre-existing nerves in the TME, stimulated by axonal guidance factors. Neo-neurogenesis (right) is the de novo formation of functional neurons from neural progenitor cells that are attracted to the TME by unknown mechanisms. Created with BioRender.com
Axonogenesis
Axonogenesis is a process that involves the outgrowth of pre-existing nerves (Fig. 16.1, center) and which expands tumor-associated nerve networks that generate neural signaling for the regulation of tumorigenesis and metastasis [4, 25]. The process of axonal sprouting shows some parallels with vascular biology [37], where growth factors that direct vessel sprouting, such as axonal guidance molecules, also regulate axonal branching. The process of axonogenesis can therefore be analogous to angiogenesis and lymphangiogenesis (i.e., the development of new lymphatic vessels in tumors) [38]. Adult neurogenesis is a dynamic process that can be stimulated under different pathological and pharmacological settings, such as injury, but is considered limited in normal physiological conditions [39].
Neo-Neurogenesis
Neo-neurogenesis involves the de novo production of functional neurons from neural progenitor cells and occurs throughout life (in rodents) in specific regions of the brain, the subgranular zone in the dentate gyrus of the hippocampus, the subventricular zone of the lateral ventricle [39], and to some extent in the striatum [40] and the cerebellum [41]. However, whether neo-neurogenesis also occurs in the adult human brain, is still up for discussion.
Recently, a process of cancer-specific neo-neurogenesis within the TME of prostate cancer was reported [5]. The process was defined as cancer-specific, as regeneration of sympathetic innervation after injury in normal prostate is driven by axonogenesis, without the presence of newly formed nerves [42]. In prostate cancer, neural progenitor cells (arising from the CNS) are abundantly present in the TME, and these progenitors direct the formation of new autonomic nerves in the tumor tissue [5] (Fig. 16.1, right).
This process is explained in a mouse model of prostate cancer, in which cancer development leads to an accumulation of Doublecortin (DCX) expressing neural progenitor cells in the subventricular zone. By cell tracing experiments, these authors were able to trace DCX+ neural progenitors, as these cells enter the circulation by disrupting the blood-brain barrier and migrate to the prostate to innervate the surrounding TME. The process was further associated with cancer-induced differentiation of the neural progenitors towards adrenergic nerves that have been shown to support early stages of prostate tumor development [4]. The mechanism by which the DCX+ neural progenitors are triggered to leave the brain is still unknown, but it is thought that the tumor may produce signals that attract neural progenitor cells, in turn leading to TME remodeling with increased number of cancer-associated autonomic nerves. Removal of neural progenitors from the TME of prostate cancer, significantly inhibited tumor progression in this mouse model.
The findings by Magnon et al. [5] were recently evaluated in a large cohort of prostate cancer patients [43], in which DCX expression did not differ by disease state, grade or outcome. These findings indicate that further work is needed to define the role of DCX expressing candidate neural progenitor cells in the TME of prostate tumors and other cancer types, and future studies are needed to describe the process of cancer-associated neo-neurogenesis and elucidate the mechanisms for cancer-stimulated targeted differentiation of CNS derived neural progenitors to adrenergic nerves within the TME.
Nerve Involvement in Different Cancer Types
Recent studies have demonstrated neural participation in malignant tumors, evident by the presence of nerve fibers innervating malignant tumors and the TME. Next, we will briefly review data on nerve innervation of gastric, pancreatic, prostate, and breast cancer.
Gastric Cancer
The incidence and mortality rates of non-cardia gastric cancer has been steadily decreasing within the last half century, with large fluctuations dependent on populations [44]. However, even after curative resections, a sizable proportion of patients experience tumor recurrence with poor prognosis. In an attempt to improve patient outcomes, new pathological indicators are being explored, such as neural participation in gastric cancer.
It has been documented that PNI plays a role in cancer progression and dissemination [31]. In a meta-analysis by Zhao et al., the authors found PNI to correlate with more aggressive tumor features, such as a diffuse tumor type, larger tumor size, and tumor metastasis. Notably, the prognostic value of PNI is influenced by the variability introduced by its evaluation process [45].
The enteric nervous system is linked to epithelial homeostasis within the gastrointestinal crypts and signaling between gastric cancer cells and nerves have been reported [46, 47]. Hayakawa et al. demonstrated that the neurotransmitter acetylcholine, from both Doublecortin-like kinase 1 positive tuft cells and nerves, induces neuronal growth factor (NGF) production and expression in gastric epithelial cells . In turn, this overexpression of NGF leads to enteric nerve system expansion and innervation that promote carcinogenesis [25].
The nervous system is known to regulate both stem and progenitor cells of the epithelium, with crosstalk between tumor cells and nerves being evident [48]. This can be seen in tumors inducing active neurogenesis, and nerves in turn stimulate the growing tumor through muscarinic acetylcholine receptor activation by the release of acetylcholine, that has been shown to promote cancer progression [49].
Pancreatic Cancer
The pancreas is innervated by sympathetic and parasympathetic nerves [50]. However, in contrast to normal pancreatic tissue , pancreatic ductal adenocarcinoma (PDAC) is characterized by high neuronal activity, marked by high neural density and hypertrophy, thought to be caused by secretion of neurotrophins such as NGF and brain neurotrophic factor (BDNF) [6, 7]. In a recent study, Renz et al., studied the effects of stress as a growth promotor in PDAC, via β-adrenergic signaling [51]. It was found that in the crosstalk between adrenergic signaling and cancer cells, neurotrophins secretion is central to the growth of PDAC. With catecholamine signaling, induced by chronic stress, increased cancer cell secretion of NGF and BDNF has been observed, in turn stimulating axonogenesis through Trk receptors, and creating a positive feedback loop.
PDAC is thought of as a neurotropic cancer, as 70–100% of PDAC patients show PNI [52,53,54]. PNI in PDAC is associated with a poor prognosis and increased cancer aggressiveness [55]. The high incidence of PNI in PDAC is not clearly understood, but it reflects the strong neurotropic effects of the tumor, and the proximity of the pancreas to multiple neural plexuses might be important [56, 57]. The pain often observed in pancreatic cancers also seems to be related to PNI, as many of the molecular mechanisms involved in this process are also implicated in pain generation, such as NGF, artemin, and granulocyte colony-stimulating factor [6, 7].
The vagus nerve, a major component of the parasympathetic nervous system, has been shown to stimulate the proliferation of pancreatic exocrine cells [58]. In contrast, clinical studies have indicated that vagus nerve signaling might inhibit cancer progression and metastasis [59]. Comparable to what has been shown for stomach cancer, parasympathetic nerve signaling seems to suppress tumorigenesis.
Prostate Cancer
Nerve innervation of the prostate is one of the most studied due to its anatomical distinct neural inputs. Prostate stroma is abundantly innervated by both sympathetic and parasympathetic nerves [60], and PNI has been shown to result in increased tumor growth and spread [61]. In prostate cancer, innervation from newly formed sympathetic nerves contributes to initiation [4], while parasympathetic signaling is important for cancer progression [29].
Magnon et al. were the first to demonstrate the ability of nerves to stimulate prostate cancer progression [4]. In a mouse model of prostate cancer, the authors reported that prostate tumors were infiltrated by sympathetic adrenergic nerves (expressing tyrosine hydroxylase) and parasympathetic cholinergic fibers (expressing vesicular acetylcholine transported (VAChT)). A kinetic analysis of autonomic nerve infiltration, coupled to a measurement of tumor size and metastasis occurrence, suggested that sympathetic nerves stimulated the early stages of cancer progression, while parasympathetic nerves were found to activate cancer cell dissemination at later stages. Potential clinical relevance was evaluated, and the density of sympathetic and parasympathetic nerves was higher in tumors with poor clinical outcomes [4]. In a later study, Mauffrey et al. reported increased expression of DCX+ neural progenitors derived from the CNS in the stroma of prostate cancer, where they are differentiated towards adrenergic neurons that were further associated with tumor aggressiveness, invasion, and recurrence [5].
Breast Cancer
The role of nerve innervation in breast cancer is an understudied phenomenon. Anatomical and histological assessments show that the normal breast is innervated by sympathetic and sensory nerves [62], in which sensory nerves supply the skin and nipple, while sympathetic nerves innervate blood vessels and ducts. The breast is not considered a highly innervated tissue, and from immunohistochemical staining for selected nerve markers in normal breast, nerve fibers are rarely detected, with the exception of nerve bundles [36, 63].
The presence of nerves in the TME of breast tumors have been reported in several studies [36, 63,64,65], and in breast cancer, axonogenesis has been assumed to be associated with tumor aggressiveness that is driven by NGF production [36, 66]. In a study of over 350 breast cancer tissue specimens [36], tumor-associated nerve fibers (marked by protein gene product 9.5, III beta-tubulin, and neurofilament) correlated with poor differentiation, lymph node metastasis, high clinical staging, and a triple-negative subtype.
As mentioned before, an increasing number of studies are hinting towards a link between autonomic innervation and cancer [9, 18]. Experimental rodent studies suggest that chronic stress accelerates cancer growth and progression via β-adrenergic stimulation, potentially via sympathetic neural mechanisms [27]. Findings from the study suggest activation of the sympathetic nervous system as a regulator of breast cancer metastasis and further proposed antimetastatic treatment targeting the β-adrenergic induction of pro-metastatic gene expression in primary breast cancer. Clinical studies have shown that blocking of β-adrenergic receptors reduces recurrence rates and morbidity in breast cancer patients [67, 68]. In an experimental study of rodents, Kamiya et al. showed the antagonistic innervation of sympathetic and parasympathetic nerves in the breast TME, demonstrating that sympathetic innervation stimulated tumor growth and progression, while parasympathetic innervation had the opposite effect. These findings were further supported by assessment of sympathetic and parasympathetic nerve density in a relatively small sample size of breast cancer patients (n = 29), in which a higher density of tumor-associated sympathetic nerves and a lower density of parasympathetic nerve fibers was associated with poor clinical outcome [21].
With regards to the recent findings in prostate cancer, demonstrating that sympathetic and parasympathetic nerves contribute to cancer initiation and progression, and that neural progenitors from the CNS contributes to neo-neurogenesis [4, 5], it will be interesting to study more closely the molecular mechanisms of nerve dependence of breast cancer and the role of different nerve types on tumor progression, and also to search for signals that drive the differentiation of peripheral nerve fibers from CNS derived neural progenitor cells.
The function of a given nerve type must be understood in a context-specific manner, as parasympathetic nerves inhibit growth and progression in PDAC [51], while in the stomach, parasympathetic nerves appear to promote gastric tumorigenesis [25]. Prostate cancer is highly innervated by both sympathetic and parasympathetic nerves, in which both nerve types promote cancer progression [4]. In breast cancer, the recent finding by Kamiya et al. indicates the opposite effect of sympathetic and parasympathetic nerves [21]. Importantly, these studies suggest that nerves may drive opposite effects on different tumor types, by promoting growth in one tissue while inhibiting cancer growth in other organs.
Overview of Nerve Markers
Most nerves in the TME are small fibers or individual axons that require specific neuronal biomarkers to be detected by immunohistochemical staining. In this chapter, we will highlight a few of these neural markers often used in translational cancer studies.
Neurofilament
Neurofilaments are a unique group of cytoskeletal intermediary filaments. The neurofilament triplet proteins—light, medium, and heavy (NF-L, NF-M, and NF-H, respectively)—are neuron-specific (NF-L in breast cancer, Fig. 16.2). In the CNS, the neurofilament triplet proteins have been shown to co-express Internexin-alpha (INA) [69]. Neurofilaments are primarily expressed in axons, where they stabilize axonic protrusions [70], which begin with the polymerization of NF-L and INA. The two largest subunits NF-M and NF-H are expressed in more mature and protruded axons. NFs are therefore the main component of the cytoskeleton in mature neurons, with NF-L and INA being expressed earlier than NF-M and NF-H [71].
Class III Beta Tubulin
Microtubules are built by α/β heterodimers, with a linear expression where the α-tubulin is directed towards the rear end of the microtubule and the β-tubulin faces the front [72]. The expression of Class III β-tubulin (βIII-tubulin) is primarily centered around neural crest-derived cells; however, its expression can also be induced in both normal and neoplastic tissues [73, 74]. Classically, βIII-tubulin is an early neuronal marker [75, 76], and recent discoveries suggest βIII-tubulin as a marker for stemness within these cells [77].
Doublecortin
Doublecortin (DCX) , is a microtubule-associated protein that aids microtubule stabilization [78]. DCX is a marker associated with neural progenitors and axonal growth cone of migrating central and peripheral neurons [79, 80]. Although DCX is a microtubule-associated protein, it can be demonstrated immunocytochemically in pre-migratory neuroblasts [81].
Autonomic Nerve Markers
In addition to the above-mentioned nerve markers , sympathetic nerves are marked by the expression of tyrosine hydroxylase (TH), and parasympathetic nerves express vesicular acetylcholine transported (VAChT) [4, 21]. TH is present in the CNS and in sympathetic neurons of the PNS, where it functions as the rate-limiting enzyme in the biosynthesis of noradrenaline, in which it catalyzes the conversion of L-tyrosine to L-DOPA [82]. L-DOPA is the precursor for dopamine which is the precursor for neurotransmitters noradrenaline and adrenaline. VAChT is also found in neurons of both the CNS and PNS, where it functions as a neurotransmitter transporter that loads acetylcholine into secretory vesicles [83].
Surgical and Pharmacological Denervation
Retrospective clinical studies have suggested that patients with breast cancer [27, 67, 68], melanoma [84], and prostate [85, 86] cancer that already are taking beta-blockers to treat a pre-existing condition, have lower recurrence rates and morbidity. These results support the idea that reducing stress or suppressing sympathetic activity could be a beneficial adjuvant treatment option for cancer patients [87]; however, the reported impact is relatively small [88]. Administration of beta-blockers in patients would result in a systemic effect, which makes it hard to isolate the true effects on local innervation of tumors.
Similar to regeneration, in vivo denervation experiments were important for the discovery of nerve dependence in cancer. Denervation by surgical cutting of afferent nerves, or the local injection of neurotoxic drugs using botulinum toxin or 6-hydroxydopamine, have shown promising results [4, 33]. As demonstrated in mouse models, vagal denervation in gastric cancer involves inhibition of cholinergic signaling and muscarinic receptors [25, 33]. Peripheral nerves consist of groups with different types of nerve fibers such that surgical resection of a peripheral nerve leads to disruption of all the nerve fibers within that nerve. Therefore, the function of a specific nerve type, cannot be studied separately. Potential limitations of experimental denervation therefore include unspecific or incomplete denervation but does not take into account potential cancer-induced nerve regeneration. The effect of different types of nerves on cancer progression remains to be characterized in depth and highlights the need for future studies in different cancer types to study the true importance of cancer-associated neural elements.
Future Perspectives
Nerve fibers were for a long time seen as silent spectators of the TME, but recent studies have demonstrated neural participation in and around malignant tumors, and a new field of research focusing on cancer neuroscience is emerging.
Nerves are difficult to study by regular histology, but big nerve trunks can be seen and constitute the basis for assessing perineural invasion by pathological examination [31]. Although identifying nerves within the TME of different tumors can be similar to looking for needles in a haystack, several studies mentioned earlier have suggested that neural marker density correlates with aggressive tumor features.
The nervous system is among the most complex “organs” and is correspondingly difficult to study in vivo and by tissue culture models. Targeting the direct interaction between nervous components and cancer cells requires sophisticated in vitro co-culture systems. These experiments can provide useful information about the molecular crosstalk involved. Moreover, a focus on the influence of the nervous system on local stomal cells and remodeling of the TME is needed. Single-cell analysis, lineage tracing, molecular characterization, and neural differentiation profiling will be required to define the mechanisms by which different nerves, and different types of cancer cells, are connected. Precise targeting of tumor-nerve interactions will provide new opportunities for improving outcomes for many tumors.
Within the emerging field of cancer neuroscience , many questions still remain to be answered; where do the nerves in the TME of different cancer types come from? through which mechanisms can tumors recruit new nerves? does the density of nerves correlate with more aggressive behavior? are there cancer-specific differences in the mechanisms for nerve dependence? So far, researchers have only investigated the presence and role of nerves in a few cancer types, and the cellular and molecular landscape and significance of cancer-associated nerves will need to be mapped in full detail.
Concluding Remarks/Summary
The presence of nerves in and around malignant tumors has recently emerged as an important feature of the tumor microenvironment. Nerve fibers influence cancer growth, spread, therapeutic resistance, and prognosis. The function of different nerve types must be understood and investigated in a cancer-specific manner. Much remains to be discovered with respect to how the central and peripheral nervous systems influence different solid tumor types and eventually how these interactions can be blocked.
References
Monje M, et al. Roadmap for the emerging field of cancer neuroscience. Cell. 2020;181:219–22. https://doi.org/10.1016/j.cell.2020.03.034.
Ondicova K, Mravec B. Role of nervous system in cancer aetiopathogenesis. Lancet Oncol. 2010;11:596–601. https://doi.org/10.1016/S1470-2045(09)70337-7.
Jobling P, et al. Nerve-cancer cell cross-talk: a novel promoter of tumor progression. Cancer Res. 2015;75:1777–81. https://doi.org/10.1158/0008-5472.CAN-14-3180.
Magnon C, et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341:1236361. https://doi.org/10.1126/science.1236361.
Mauffrey P, et al. Progenitors from the central nervous system drive neurogenesis in cancer. Nature. 2019;569:672–8. https://doi.org/10.1038/s41586-019-1219-y.
Ceyhan GO, et al. Pancreatic neuropathy results in “neural remodeling” and altered pancreatic innervation in chronic pancreatitis and pancreatic cancer. Am J Gastroenterol. 2009;104:2555–65. https://doi.org/10.1038/ajg.2009.380.
Bapat AA, Hostetter G, Von Hoff DD, Han H. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer. 2011;11:695–707. https://doi.org/10.1038/nrc3131.
Stopczynski RE, et al. Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Cancer Res. 2014;74:1718–27. https://doi.org/10.1158/0008-5472.CAN-13-2050.
Zahalka AH, Frenette PS. Nerves in cancer. Nat Rev Cancer. 2020;20:143–57. https://doi.org/10.1038/s41568-019-0237-2.
Waddington CH. Cancer and the theory of organisers. Nature. 1935;135:606–8. https://doi.org/10.1038/135606a0.
Boilly B, Faulkner S, Jobling P, Hondermarck H. Nerve dependence: from regeneration to cancer. Cancer Cell. 2017;31:342–54. https://doi.org/10.1016/j.ccell.2017.02.005.
Kragl M, et al. Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature. 2009;460:60–5. https://doi.org/10.1038/nature08152.
Nieuwenhuys R, Voogd J, Huijzen C, v. The human central nervous system. 4th ed. Springer; 2008.
Jessen KR, Mirsky R. Glial cells in the enteric nervous system contain glial fibrillary acidic protein. Nature. 1980;286:736–7. https://doi.org/10.1038/286736a0.
Cuevas J. Reference module in biomedical sciences. Elsevier; 2015.
Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286–94. https://doi.org/10.1038/nrgastro.2012.32.
Catala M, Kubis N. Gross anatomy and development of the peripheral nervous system. Handb Clin Neurol. 2013;115:29–41. https://doi.org/10.1016/B978-0-444-52902-2.00003-5.
Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer. 2015;15:563–72. https://doi.org/10.1038/nrc3978.
Faulkner S, Jobling P, March B, Jiang CC, Hondermarck H. Tumor neurobiology and the war of nerves in cancer. Cancer Discov. 2019;9:702–10. https://doi.org/10.1158/2159-8290.CD-18-1398.
Hanoun M, Maryanovich M, Arnal-Estape A, Frenette PS. Neural regulation of hematopoiesis, inflammation, and cancer. Neuron. 2015;86:360–73. https://doi.org/10.1016/j.neuron.2015.01.026.
Kamiya A, et al. Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nat Neurosci. 2019;22:1289–305. https://doi.org/10.1038/s41593-019-0430-3.
Zahalka AH, et al. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science. 2017;358:321–6. https://doi.org/10.1126/science.aah5072.
Li J, Tian Y, Wu A. Neuropeptide Y receptors: a promising target for cancer imaging and therapy. Regen Biomater. 2015;2:215–9. https://doi.org/10.1093/rb/rbv013.
Hondermarck H, Jobling P. The sympathetic nervous system drives tumor angiogenesis. Trends Cancer. 2018;4:93–4. https://doi.org/10.1016/j.trecan.2017.11.008.
Hayakawa Y, et al. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic Signaling. Cancer Cell. 2017;31:21–34. https://doi.org/10.1016/j.ccell.2016.11.005.
Eichmann A, Brunet I. Arterial innervation in development and disease. Sci Transl Med. 2014;6:252ps259. https://doi.org/10.1126/scitranslmed.3008910.
Sloan EK, et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70:7042–52. https://doi.org/10.1158/0008-5472.CAN-10-0522.
Oben JA, Yang S, Lin H, Ono M, Diehl AM. Norepinephrine and neuropeptide Y promote proliferation and collagen gene expression of hepatic myofibroblastic stellate cells. Biochem Biophys Res Commun. 2003;302:685–90. https://doi.org/10.1016/s0006-291x(03)00232-8.
Dobrenis K, Gauthier LR, Barroca V, Magnon C. Granulocyte colony-stimulating factor off-target effect on nerve outgrowth promotes prostate cancer development. Int J Cancer. 2015;136:982–8. https://doi.org/10.1002/ijc.29046.
Ayala GE, et al. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin Cancer Res. 2008;14:7593–603. https://doi.org/10.1158/1078-0432.CCR-08-1164.
Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115:3379–91. https://doi.org/10.1002/cncr.24396.
Amit M, Na'ara S, Gil Z. Mechanisms of cancer dissemination along nerves. Nat Rev Cancer. 2016;16:399–408. https://doi.org/10.1038/nrc.2016.38.
Zhao CM, et al. Denervation suppresses gastric tumorigenesis. Sci Transl Med. 2014;6:250ra115. https://doi.org/10.1126/scitranslmed.3009569.
Yilmaz A, et al. Clinical impact of visceral pleural, lymphovascular and perineural invasion in completely resected non-small cell lung cancer. Eur J Cardiothorac Surg. 2011;40:664–70. https://doi.org/10.1016/j.ejcts.2010.12.059.
Scanlon CS, et al. Galanin modulates the neural niche to favour perineural invasion in head and neck cancer. Nat Commun. 2015;6:6885. https://doi.org/10.1038/ncomms7885.
Huang D, et al. Nerve fibers in breast cancer tissues indicate aggressive tumor progression. Medicine (Baltimore). 2014;93:e172. https://doi.org/10.1097/MD.0000000000000172.
Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. https://doi.org/10.1038/nature03875.
Entschladen F, Palm D, Lang K, Drell TL, t. & Zaenker, K. S. Neoneurogenesis: tumors may initiate their own innervation by the release of neurotrophic factors in analogy to lymphangiogenesis and neoangiogenesis. Med Hypotheses. 2006;67:33–5. https://doi.org/10.1016/j.mehy.2006.01.015.
Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. https://doi.org/10.1016/j.neuron.2011.05.001.
Ernst A, et al. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156:1072–83. https://doi.org/10.1016/j.cell.2014.01.044.
Ponti G, Peretto P, Bonfanti L. Genesis of neuronal and glial progenitors in the cerebellar cortex of peripuberal and adult rabbits. PLoS One. 2008;3:e2366. https://doi.org/10.1371/journal.pone.0002366.
Kobayashi T, Kihara K, Hyochi N, Masuda H, Sato K. Spontaneous regeneration of the seriously injured sympathetic pathway projecting to the prostate over a long period in the dog. BJU Int. 2003;91:868–72. https://doi.org/10.1046/j.1464-410x.2003.04222.x.
Tabrizi S, et al. Doublecortin expression in prostate adenocarcinoma and neuroendocrine Tumors. Int J Radiat Oncol Biol Phys. 2020;108:936–40. https://doi.org/10.1016/j.ijrobp.2020.06.024.
Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; https://doi.org/10.3322/caac.21660.
Zhao B, et al. Perineural invasion as a predictive factor for survival outcome in gastric cancer patients: a systematic review and meta-analysis. J Clin Pathol. 2020;73:544–51. https://doi.org/10.1136/jclinpath-2019-206372.
Gross ER, et al. Neuronal serotonin regulates growth of the intestinal mucosa in mice. Gastroenterology. 2012;143:408–417 e402. https://doi.org/10.1053/j.gastro.2012.05.007.
Neal KB, Bornstein JC. Mapping 5-HT inputs to enteric neurons of the Guinea-pig small intestine. Neuroscience. 2007;145:556–67. https://doi.org/10.1016/j.neuroscience.2006.12.017.
Lundgren O, Jodal M, Jansson M, Ryberg AT, Svensson L. Intestinal epithelial stem/progenitor cells are controlled by mucosal afferent nerves. PLoS One. 2011;6:e16295. https://doi.org/10.1371/journal.pone.0016295.
Mattingly RR, Sorisky A, Brann MR, Macara IG. Muscarinic receptors transform NIH 3T3 cells through a Ras-dependent signalling pathway inhibited by the Ras-GTPase-activating protein SH3 domain. Mol Cell Biol. 1994;14:7943–52. https://doi.org/10.1128/mcb.14.12.7943.
Borden MA, Streeter JE, Sirsi SR, Dayton PA. In vivo demonstration of cancer molecular imaging with ultrasound radiation force and buried-ligand microbubbles. Mol Imaging. 2013;12:357–63.
Renz BW, et al. Cholinergic signaling via muscarinic receptors directly and indirectly suppresses pancreatic tumorigenesis and cancer stemness. Cancer Discov. 2018;8:1458–73. https://doi.org/10.1158/2159-8290.CD-18-0046.
Chen SH, et al. Perineural invasion of cancer: a complex crosstalk between cells and molecules in the perineural niche. Am J Cancer Res. 2019;9:1–21.
Ren K, et al. Clinical anatomy of the anterior and posterior hepatic plexuses, including relations with the pancreatic plexus: a cadaver study. Clin Anat. 2020;33:630–6. https://doi.org/10.1002/ca.23470.
Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg. 2014;149:565–74. https://doi.org/10.1001/jamasurg.2013.5137.
Jurcak N, Zheng L. Signaling in the microenvironment of pancreatic cancer: transmitting along the nerve. Pharmacol Ther. 2019;200:126–34. https://doi.org/10.1016/j.pharmthera.2019.04.010.
Stolinski C. Structure and composition of the outer connective tissue sheaths of peripheral nerve. J Anat. 1995;186(Pt 1):123–30.
Pour PM, Bell RH, Batra SK. Neural invasion in the staging of pancreatic cancer. Pancreas. 2003;26:322–5. https://doi.org/10.1097/00006676-200305000-00002.
Kiba T, et al. Ventromedial hypothalamic lesion-induced vagal hyperactivity stimulates rat pancreatic cell proliferation. Gastroenterology. 1996;110:885–93. https://doi.org/10.1053/gast.1996.v110.pm8608899.
De Couck M, Marechal R, Moorthamers S, Van Laethem JL, Gidron Y. Vagal nerve activity predicts overall survival in metastatic pancreatic cancer, mediated by inflammation. Cancer Epidemiol. 2016;40:47–51. https://doi.org/10.1016/j.canep.2015.11.007.
McVary KT, et al. Growth of the rat prostate gland is facilitated by the autonomic nervous system. Biol Reprod. 1994;51:99–107. https://doi.org/10.1095/biolreprod51.1.99.
Olar A, et al. Biological correlates of prostate cancer perineural invasion diameter. Hum Pathol. 2014;45:1365–9. https://doi.org/10.1016/j.humpath.2014.02.011.
Sarhadi NS, Shaw Dunn J, Lee FD, Soutar DS. An anatomical study of the nerve supply of the breast, including the nipple and areola. Br J Plast Surg. 1996;49:156–64. https://doi.org/10.1016/s0007-1226(96)90218-0.
Zhao Q, et al. The clinicopathological significance of neurogenesis in breast cancer. BMC Cancer. 2014;14:484. https://doi.org/10.1186/1471-2407-14-484.
Tsang WY, Chan JK. Neural invasion in intraductal carcinoma of the breast. Hum Pathol. 1992;23:202–4. https://doi.org/10.1016/0046-8177(92)90247-z.
Mitchell BS, Schumacher U, Stauber VV, Kaiserling E. Are breast tumours innervated? Immunohistological investigations using antibodies against the neuronal marker protein gene product 9.5 (PGP 9.5) in benign and malignant breast lesions. Eur J Cancer. 1994;30A:1100–3. https://doi.org/10.1016/0959-8049(94)90465-0.
Pundavela J, et al. Nerve fibers infiltrate the tumor microenvironment and are associated with nerve growth factor production and lymph node invasion in breast cancer. Mol Oncol. 2015;9:1626–35. https://doi.org/10.1016/j.molonc.2015.05.001.
Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population- based study. J Clin Oncol. 2011;29:2635–44. https://doi.org/10.1200/JCO.2010.33.5422.
Melhem-Bertrandt A, et al. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol. 2011;29:2645–52. https://doi.org/10.1200/JCO.2010.33.4441.
Yuan A, et al. Alpha-internexin is structurally and functionally associated with the neurofilament triplet proteins in the mature CNS. J Neurosci. 2006;26:10006–19. https://doi.org/10.1523/JNEUROSCI.2580-06.2006.
Thyagarajan A, Strong MJ, Szaro BG. Post-transcriptional control of neurofilaments in development and disease. Exp Cell Res. 2007;313:2088–97. https://doi.org/10.1016/j.yexcr.2007.02.014.
Wang H, et al. Neurofilament proteins in axonal regeneration and neurodegenerative diseases. Neural Regen Res. 2012;7:620–6. https://doi.org/10.3969/j.issn.1673-5374.2012.08.010.
Mariani M, et al. Class III beta-tubulin in normal and cancer tissues. Gene. 2015;563:109–14. https://doi.org/10.1016/j.gene.2015.03.061.
Raspaglio G, et al. Hypoxia induces class III beta-tubulin gene expression by HIF-1alpha binding to its 3′ flanking region. Gene. 2008;409:100–8. https://doi.org/10.1016/j.gene.2007.11.015.
Raspaglio G, et al. Sox9 and Hif-2alpha regulate TUBB3 gene expression and affect ovarian cancer aggressiveness. Gene. 2014;542:173–81. https://doi.org/10.1016/j.gene.2014.03.037.
Blondheim NR, et al. Human mesenchymal stem cells express neural genes, suggesting a neural predisposition. Stem Cells Dev. 2006;15:141–64. https://doi.org/10.1089/scd.2006.15.141.
Foudah D, et al. Human mesenchymal stem cells express neuronal markers after osteogenic and adipogenic differentiation. Cell Mol Biol Lett. 2013;18:163–86. https://doi.org/10.2478/s11658-013-0083-2.
Draberova E, et al. Class III beta-tubulin is constitutively coexpressed with glial fibrillary acidic protein and nestin in midgestational human fetal astrocytes: implications for phenotypic identity. J Neuropathol Exp Neurol. 2008;67:341–54. https://doi.org/10.1097/NEN.0b013e31816a686d.
Ayanlaja AA, et al. Distinct features of doublecortin as a marker of neuronal migration and its implications in cancer cell mobility. Front Mol Neurosci. 2017;10:199. https://doi.org/10.3389/fnmol.2017.00199.
Francis F, et al. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron. 1999;23:247–56. https://doi.org/10.1016/s0896-6273(00)80777-1.
Schaar BT, Kinoshita K, McConnell SK. Doublecortin microtubule affinity is regulated by a balance of kinase and phosphatase activity at the leading edge of migrating neurons. Neuron. 2004;41:203–13. https://doi.org/10.1016/s0896-6273(03)00843-2.
Sarnat HB. Clinical neuropathology practice guide 5-2013: markers of neuronal maturation. Clin Neuropathol. 2013;32:340–69. https://doi.org/10.5414/NP300638.
Kaufman S. Tyrosine hydroxylase. Adv Enzymol Relat Areas Mol Biol. 1995;70:103–220. https://doi.org/10.1002/9780470123164.ch3.
Arvidsson U, Riedl M, Elde R, Meister B. Vesicular acetylcholine transporter (VAChT) protein: a novel and unique marker for cholinergic neurons in the central and peripheral nervous systems. J Comp Neurol. 1997;378:454–67.
Lemeshow S, et al. Beta-blockers and survival among Danish patients with malignant melanoma: a population-based cohort study. Cancer Epidemiol Biomark Prev. 2011;20:2273–9. https://doi.org/10.1158/1055-9965.EPI-11-0249.
Grytli HH, Fagerland MW, Fossa SD, Tasken KA. Association between use of beta-blockers and prostate cancer-specific survival: a cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. Eur Urol. 2014;65:635–41. https://doi.org/10.1016/j.eururo.2013.01.007.
Grytli HH, Fagerland MW, Fossa SD, Tasken KA, Haheim LL. Use of beta-blockers is associated with prostate cancer-specific survival in prostate cancer patients on androgen deprivation therapy. Prostate. 2013;73:250–60. https://doi.org/10.1002/pros.22564.
Saloman JL, Albers KM, Rhim AD, Davis BM. Can stopping nerves, stop cancer? Trends Neurosci. 2016;39:880–9. https://doi.org/10.1016/j.tins.2016.10.002.
Sorensen GV, et al. Use of beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and risk of breast cancer recurrence: a Danish nationwide prospective cohort study. J Clin Oncol. 2013;31:2265–72. https://doi.org/10.1200/JCO.2012.43.9190.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Vethe, H., Bjørnstad, O.V., Carrasco, M., Akslen, L.A. (2022). Neurogenesis in the Tumor Microenvironment. In: Akslen, L.A., Watnick, R.S. (eds) Biomarkers of the Tumor Microenvironment. Springer, Cham. https://doi.org/10.1007/978-3-030-98950-7_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-98950-7_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-98949-1
Online ISBN: 978-3-030-98950-7
eBook Packages: MedicineMedicine (R0)