Abstract
Activating mutations in driver oncogenes and loss-of-function mutations in tumor suppressor genes contribute to tumor progression and metastasis. Accordingly, therapies targeting key tumor cell intrinsic signaling pathways are being used in clinical trials, and some have met FDA approval. However, these treatments benefit only a small proportion of patients harboring key driver mutations and acquired resistance to these therapies presents a major impediment to effective treatment. More recently, the contribution of the tumor microenvironment (TME) has been an area of active investigation and has begun to provide critical insights into carcinogenesis. The host stromal cells in the TME co-evolve with tumors and contribute to carcinogenesis in several ways. Among the host cells, bone marrow (BM)-derived cells constitute a significant fraction and directly contribute to proliferation, invasion, intravasation, extravasation, and outgrowth at the metastatic site. While the tumor reprogrammed BM cells constitute attractive targets for anti-cancer therapy, recent studies have also begun to unravel their role as prognostic and predictive molecular markers of the disease.
In this chapter, we will focus on recent advances and emerging concepts of the contribution of BM-derived cells in various steps of primary tumor progression and the metastatic cascade (Fig. 14.1) and discuss future directions in the context of novel diagnostic and therapeutic opportunities.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Metastasis
- Tumor microenvironment
- Bone marrow
- Anti-cancer therapy
- Premetastatic niche
- Tumor progression
- Bone marrow-derived cells
- Circulating tumor cells
-
Bone marrow-derived cells contribute to primary tumor growth as well as metastatic progression. These are recruited to primary tumor sites via tumor-secreted factors or via HIF1α in hypoxic tumors. Tumor-associated macrophages, myeloid-derived suppressor cells (MDSCs), endothelial progenitor cells (EPCs), and tumor-associated neutrophils all aid in primary tumor growth via different mechanisms involving immunosuppression, angiogenesis, etc. On the other hand, dendritic cells (DCs) may have both pro-tumorigenic via ENO1 and anti-tumorigenic role via activation of CD4+ and CD8+ T-cells.
-
For intravasation, macrophages recruited at primary tumor sites downregulate E-cadherin junctions and trigger actin-rich invadopodia to promote early dissemination. Neutrophils aid in the process of intravasation by increasing MMP9 and angiogenesis.
-
Platelets are known to promote circulating tumor cells (CTCs) in blood and their metastasis. Clot formation also recruits macrophages, which in turn increase Akt-dependent signaling to promote tumor cell survival. Tregs can protect disseminated tumor cells from an immune attack as well. Neutrophils protect CTCs by forming neutrophil extracellular traps (NETs).
-
A number of tumor-secreted or BM-derived factors play a role in the formation of premetastatic niche which promotes metastatic outgrowth of tumor cells at secondary sites. Lysyl oxidase secreted from primary tumors modifies ECM at the secondary site to aid in tumor cell colonization. Primary tumor-derived CCL2 also promotes S100A8 and SAA3 secretion from lung endothelial cells that increases vascular permeability to assist in tumor cell colonization. Similarly, macrophages, MDSCs, and neutrophils are recruited at the premetastatic sites to modify the environment at these sites, aid in colonization by tumor cells, promote their survival, support their metabolic needs, and promote metastatic outgrowth.
-
Platelets aid in extravasation by promoting adhesion of tumor cells to endothelial cells. Primary tumor-derived G-CSF increases the presence of immunosuppressive G-MDSCs in lungs for metastatic outgrowth. Neutrophils promote metastasis by forming NETs and degrading thrombospondin-1. Different subsets of cancer-associated fibroblasts also enhance metastasis by NOTCH, CXCL12, and TGFβ pathways. Bone microenvironment promotes stemness and plasticity in tumor cells to promote multi-organ metastasis at different sites.
Primary Tumor Growth
The BM contributes significantly to the TME and supports tumor progression and metastasis by regulating angiogenesis, inflammation, and immune suppression. General descriptions of the contribution of the BM-derived TME to tumor growth and metastasis have been covered in several excellent reviews [1,2,3,4]. Most solid tumors harbor an immune infiltrate consisting of myeloid and lymphoid cells, whose phenotype and activation status has been shown to change with the stage of malignancy [4]. Hematopoietic stem cells (HSCs) are maintained in the BM compartment and anchored to the endosteal surface by calcium-sensing receptors present on their surface [5]. Major adhesion mechanisms that mediate HSC anchorage in the BM niche include receptor tyrosine kinase TIE2-Angiopoietin-1 (ANG1) interactions [6] and chemokine (C-X-C motif) receptor 4 (CXCR4)-stromal-derived factor-1 (SDF-1) interactions [7]. Furthermore, HSCs also adhere to osteopontin in the bone via β1 integrin [8].
Secreted tumor-specific factors systemically stimulate the quiescent BM compartment, resulting in the expansion, mobilization, and recruitment of BM progenitor cells. For instance, matrix metalloprotease 9 (MMP9) secreted by primary tumors systemically degrades osteopontin [9, 10] and mediates cleavage of SDF-1 [11], thereby releasing BM cells from the bone niche [12]. Similarly, tumor-secreted granulocyte colony-stimulating factor (G-CSF) mobilizes HSCs from the niche by promoting neutrophil elastase-mediated degradation of SDF-1 [13]. The mobilized BM-derived cells are recruited into tumor beds in response to chemoattractants. For instance, SDF-1 secreted by primary tumors recruits CXCR4+ BM-derived cells to the TME [12]. Other tumor-secreted factors such as vascular endothelial growth factor (VEGF) and placental growth factor (PlGF) bind to VEGFR1+ BM cells, while monocyte colony-stimulating factor (M-CSF) recruits monocytes and macrophages [12]. Furthermore, chemokine (C-C motif) ligand 2 (CCL2), also known as monocyte chemotactic protein (MCP-1), was identified as a tumor-derived chemokine that recruits circulating monocytes into the TME, where they undergo differentiation into tumor-associated macrophages (TAMs) [14]. Stromal-derived CCL2/MCP-1 and colony-stimulating factor (CSF1) are also involved in the recruitment of TAMs [15,16,17]. Additionally, hypoxia-inducible factor 1 alpha (HIF1α) in hypoxic tumors promotes the recruitment of BM-derived myeloid and endothelial progenitor cells (EPCs), which increase the bioavailability of VEGF via their secretion of MMP9, enhancing tumor angiogenesis [18]. Infiltrating BM cells also provide paracrine mitogenic signals to induce proliferation of tumor cells via their secretion of growth factors such as epidermal growth factor (EGF), and cytokines including interleukin-6 (IL-6) and tumor necrosis factor (TNFα) [12]. Colorectal cancer has high VEGFC expression which leads to increased activation of VEGFR3 on cancer-associated macrophages and lymph vessels, important for lymphangiogenesis in colorectal cancer [19].
Macrophages are the most abundant myeloid cells present among the recruited BM-derived cells [20]. Notably, increased macrophage infiltration has been correlated with poor prognosis, as shown in Hodgkin’s lymphoma, breast cancer, and lung cancer [21,22,23]. In ovarian cancer, TAMs mediate an immunosuppressive environment at the primary tumor site, aiding tumor growth and metastasis [24]. These are recruited via an E3 ligase, UBR5, expressed highly by ovarian cancer cells through CCL2/CSF-1 axis [24]. Classically activated macrophages, defined by an M1 phenotype, generate host responses against the growing tumors, whereas “alternatively” activated M2 macrophages, representing “educated” TAMs, are major perpetrators of tumor progression and metastasis. M1 macrophages are characterized by an elevated expression of inducible nitric oxide synthase (iNOS), producing nitric oxide (NO) for effective pathogen killing [25], and the pro-inflammatory cytokine IL-12 [26]. Tumor-derived mediators, such as IL-4, IL-10, IL-13, transforming growth factor beta (TGFβ), and prostaglandin E2 (PGE2) mediate polarization of TAMs toward the tumor-promoting, M2 phenotype [27]. M2 TAMs are characterized by elevated expression of arginase (Arg1) and decreased expression of iNOS - ArgIhigh iNOSlow [28]. M2 TAMs promote immune suppression by expressing high levels of IL-10 and downregulating IL-12 [29]. Paclitaxel has been shown to revert TAMs to an immunocompetent M1 profile in a TLR4-dependent pathway [30]. Interestingly, TAMs have also been shown to promote tumor growth via a key inflammatory mechanism activating the classical complement pathway [31]. Production of C1q by TAMs and C1r, C1s, C4, and C3 by tumor cells leads to the assembly C1 complex and activation of the complement pathway, leading to an immunosuppressive microenvironment and tumor growth in a model of clear-cell renal cell carcinoma [31]. In the same model, mice with deficiency of complement pathway components show decreased tumor growth [31].
M2 TAMs also induce extracellular matrix (ECM) degradation and angiogenesis by producing MMPs and VEGF, respectively [28]. Furthermore, IL-4 secreted by pancreatic tumor cells induces members of the cysteine protease family cathepsins [32, 33] in TAMs, where cathepsin B and S have been shown to promote tumor growth, invasion, and angiogenesis [34]. By contributing to angiogenesis and tumor invasiveness, TAMs ultimately promote metastasis. In the MMTV-PyMT model of breast cancer, genetic ablation of macrophages by CSF1 deletion impairs angiogenesis and suppresses metastasis to the lung, mainly due to the VEGFA-mediated angiogenic action of TAMs [35,36,37]. Furthermore, deleting an effector of CSF1 signaling, Ets2, in macrophages, induced the expression of antiangiogenic factors thrombospondin 1 (Tsp-1) and Tsp-2 by macrophages, resulting in decreased angiogenesis in PyMT primary tumors and suppression of lung metastasis [38]. Moreover, macrophages promote angiogenesis and metastasis in PyMT primary tumors via their expression of WNT7b [39]. TAMs also associate with newly formed blood vessels induced by EC-derived angiopoietin 2 (ANG2), enhancing angiogenesis and tumor cell dissemination [40]. Primary tumor cells, or tumor cell supernatant, has also been shown to activate TAMs via LAMP2a-PRDX1/CRTC1 leading to enhanced tumor growth [41]. In breast cancer, obesity leads to increase in tumor-infiltrating macrophages, and activation of NLRC4 inflammasome and increased interleukin IL-1β production [42]. IL-1β in turn upregulates angiopoietin-like 4 (ANGPTL4) driving angiogenesis-mediated breast tumor growth [42]. In TNBC, CD169+ macrophages promote tumor growth by inhibiting intratumoral CD8+ T cells, resulting in an upregulation of PD-L1 on macrophages via JAK2/STAT3 signaling pathway [43]. Inhibiting STAT6 downstream of IL-4 and IL-13 inhibits the M2 differentiation of macrophages, thus inhibiting tumor growth and metastasis in an orthotopic 4T1 breast cancer model [44]. Pre-treated gliomas have been found to be infiltrated by blood-derived TAMs with M2 phenotype exhibiting immunosuppressive cytokines (IL10 and TGFB2) and oxidative metabolism [45]. Higher expression of these blood-derived TAM markers but not microglial TAM markers are associated with poor overall survival in these patients [45].
M2 TAMs promote an immune-suppressive TME by producing IL-10, which promotes Th2 cell polarization [14]. In turn, Th2 cells produce IL-4, which in a feedback loop activates M2 TAM polarization [46]. M2 TAMs also secrete CCL22, which recruits regulatory T cells (TReg) [46]. TAMs also produce TGFβ and process latent TGFβ, releasing its active form [47], and hence reducing T cell cytolytic and antitumor activity [48]. TAMs express programmed death ligand 1 (PD-L1) on their cell surface, which binds the immune inhibitory receptor, programmed death 1 (PD-1), on the surface of T cells, resulting in T cell inactivation and apoptosis [49]. TAMs inhibit T cell growth by depleting arginine in the local microenvironment through expression of Arg1 [50]. A new study recently described that a diet rich in n-3 fatty acids induces significant reactive oxygen species production and macrophage death, inhibiting tumor growth [51].
TAMs also contribute to chemoresistance in the MMTV-PyMT breast cancer model [22], and inhibiting macrophage recruitment using a CSF1R signaling antagonist enhanced the antitumor effect of cytotoxic chemotherapeutics via a CD8+ T cell-dependent mechanism [24]. CCL8 provides a positive regulatory loop between breast cancer cells and TAMs via CSF1 and TNF-α, by enhancing the expression of SIGLEC1 [52]. Interestingly, TAMs that underwent CSF1R signaling blockade failed to elicit CD8+ T cell responses and exhibited reduced immunosuppressive activity [53].
A recently identified cancer-associated fibroblast (CAF) subset expressing CD10 and GPR77 promotes chemoresistance and poor survival in breast and lung cancer patients [54]. GPR77, a C5a receptor, maintains a persistent NF-κB activation in CD10+GPR77+ CAFs and promotes cancer stem cell enrichment and chemoresistance by IL-6 and IL-8 secretion [54]. In colorectal cancer patients, there was a higher proportion of somatic copy number mutations in fibroblasts present in tumors, as compared to adjacent normal tissues and five fibroblast-specific biomarkers: BGN, RCN3, TAGLN, MYL9, and TPM2, were associated with a poor prognosis [55]. Increased granulocyte recruitment to tumors by CAFs is known to limit the efficacy of CSF-1R inhibition [56]. This is because CSF1 in tumor cells reduces granulocyte-specific chemokine expression in CAF which limits their migration to tumors. These findings advocate for combining CSF1R inhibitor with a CXCR2 antagonist [56]. CAFs can also be educated by tumor cells as demonstrated in a pancreatic cancer model with a gain of function mutation in p53 [57]. Tumor cells with mutant p53 increase CAF contractility markers (pMLC, pMYPT1, and ACTA2), which can further increase the invasiveness of cancer cells independent of p53 mutation status in these cells [57]. This crosstalk between CAFs and tumor cells can be interrupted by inhibiting perlecan, thus reducing invasion and metastasis [57].
Myeloid-derived suppressor cells (MDSCs) have been observed in cancer patients and contribute to tumor progression. MDSCs are a heterogeneous population of immature myeloid cells [58, 59], which are activated in response to S100 calcium binding protein A8 (S100A8) and S100A9 pro-inflammatory mediators [60]. MDSCs contribute to tumor immune evasion by suppressing the CD4+ and CD8+ immune response partly via arginase production, by expanding TReg cells, and by inhibiting the cytolytic activity of natural killer (NK) cells [3, 61]. MDSCs also express the interferon gamma (IFNγ)-inducible enzyme indoleamine 2,3-dioxygenase (IDO), a well-known suppressor of T cell activation [62]. IDO catalyzes tryptophan catabolism, depleting tryptophan from the local microenvironment and blocking T cell activation [63]. IDO expression by MDSCs was found to be STAT3-dependent in breast cancer [64]. Interestingly, in a model of melanoma, tumor-derived IDO was described to recruit and expand MDSCs via a TReg-dependent mechanism, leading to an immunosuppressive microenvironment [65]. Pancreatic tumor cell-mediated production of IL1β is also known to induce an immunosuppressive environment comprising of M2 macrophages, MDSCs, CD1dhiCD5+ regulatory B cells, and Th17 cells, which leads to reduced infiltration of CD8+ cytotoxic T-cells [66]. Increased emergence of PanINs and PDAC has been also attributed to a decrease in myofibroblast-derived Col1 (type 1 Collagen) [67]. Col1 deletion enhances tumor CXCL5-associated recruitment of CD206+ARG1+MDSCs to suppress CD8+ T-cells [67]. In ovarian carcinoma, a STAT5-dependent increase in AMPKα transcription induced by GM-CSF in MDSCs mediates tumor growth and inhibits antitumor CD8+ T cell immunity [68]. In gliomas, IDH mutation leads to lesser T-cell infiltration and are thus immunologically cold tumors [69]. Immune-suppressive effects are also enhanced in the TME by increased competition for glucose between cancer cells and tumor-infiltrating lymphocytes (TILs). Immune checkpoint blockade antibodies against CTLA-4, PD-1, and PD-L1 enhance glycolysis in T-cells, along with mTOR activation and IFNγ production [70, 71]. PD-L1 inhibition on tumor cells also dampens mTOR activity and glycolysis in these cells [70].
Tumor-associated neutrophils (TANs) exert a pro-tumorigenic effect at the primary site, promoting angiogenesis and suppressing immune responses [72,73,74]. In mice, CD11b+ Gr1+ neutrophils, recruited by primary tumor-derived G-CSF, contribute to refractoriness to anti-VEGF therapy and promote angiogenesis via the expression of Bombina variegata peptide 8 (Bv8) [75, 76]. CD11b+ Gr1+ immature myeloid cells recruited to colon and lung tumors promote angiogenesis and vessel maturation via their MMP9 production, increasing VEGF bioavailability, as well as by incorporating into tumor blood vessels [77]. Interestingly, the pro-tumorigenic effects of neutrophils are TGFβ-dependent, whereupon TGFβ blockade, neutrophils switch from the “N2” pro-tumorigenic phenotype to the “N1” anti-tumorigenic phenotype [78]. In the case of dormant tumor cells, stress hormone activated neutrophils release pro-inflammatory S100A8/9, which induces activation of myeloperoxidase which results in the accumulation of oxidized lipids in neutrophils. These lipids activate FGF pathway in tumor cells to allow them to escape dormancy and form new tumor lesions [79].
Dendritic cells (DCs) are a class of antigen-presenting cells that uptake, process, and present antigens, including tumor-derived antigens, to antigen-specific T cells, resulting in T cell activation and expansion. Mature DCs express CCR7 which is essential for the migration of tumor-derived DCs to tumor draining lymph nodes [80]. In melanoma, tumor-associated DCs do not present tumor antigens and fail to activate T cells [81]. Interestingly, tumor-associated DCs (TADC) in ovarian cancer were found to be immunosuppressive, promoting tumor progression. In multiple myeloma, plasmacytoid DC-expressed enolase 1 (ENO1) suppressed CD8- and NK-cell immunity against multiple myeloma cells [82]. In this context, lipid peroxidation byproducts induce endoplasmic reticulum (ER) stress, activating an ER stress response factor, Xbp1, which reduces the ability of tumor DCs to present antigens and activate T cells [83]. TADC have been recently characterized into three subsets: pre-cDC-derived cDC1s, pre-cDC-derived cDC2s, and monocyte-derived Mo-DCs [84]. Mo-DCs scavenge tumor antigen but do not upregulate CCR7 in tumors and are immunosuppressive [84]. Both cDC1 and cDC2 migrate to lymph nodes, while cDC1 activates CD8+ T-cells and supports Th1 polarization of CD4+T-cells, cDC2s induce Th17 CD4+ T-cell phenotype (cDC2) [80, 84].
In addition to the perivascular contribution of BM-derived hematopoietic cells, BM-derived VEGFR2+ EPCs, recruited to early avascular tumors in response to tumor-derived VEGF, provide an alternative source of endothelial cells, which contributes to neovessel formation of certain tumors in mice and humans [85,86,87,88]. The contribution of EPCs to tumor vessel formation has been reported to be variable [89]. However, EPC ablation was associated with angiogenesis inhibition both in primary tumors and metastatic outgrowth [87, 90, 91]. EPCs also contributed to vascular rebound following administration of vascular disrupting agents [92], and chemotherapeutics rapidly induced circulating endothelial progenitor (CEP) mobilization and subsequent tumor homing [93]. Despite these studies, confusion has prevailed due to the extensive variability in EPC contribution to vessel formation in different tumor model systems [64, 89], and some studies have even claimed lack of EPC contribution [94]. However, in addition to vessel incorporation, EPCs have been shown to secrete proangiogenic factors, including VEGF and platelet-derived growth factor (PDGF) [90, 95], suggesting that along with providing stability to nascent vessels, EPCs contribute to vessel recruitment through paracrine mechanisms at a critical early stage of tumor growth. These observations are consistent with other studies demonstrating that paracrine signaling by specific populations of perivascular cells may have significant biological effects. For example , depletion of myeloid cell-derived VEGF caused vessel normalization even when abundant sources of VEGF were present in the TME [96]. Similarly, endothelial cell-autonomous VEGF and not the abundant extracellular VEGF was shown to be critically required for the homeostasis of blood vessels [97].
Furthermore, the BM contributes to myofibroblast populations in the tumor stroma, as shown in a mouse model of pancreatic insulinoma [98, 99]. BM-derived myofibroblasts support angiogenesis in the primary tumor by secreting a host of proangiogenic factors, such as VEGF, basic fibroblast growth factor (bFGF), TGFβ, PDGF, hepatocyte growth factor (HGF) , and they remodel the ECM via several MMPs and ADAMs [100]. The BM is also a source of pericytes, cells that support vessel maturation, where BM-derived pericyte progenitor cells are mobilized to remodel the vasculature in tumors [101,102,103,104].
Primary Tumor Invasion and Intravasation
In order to metastasize, tumor cells at the primary site invade into the basement membrane, undergo transendothelial migration, and intravasate into circulation. These events are facilitated by BM-derived cells. Cancer cells express EGFR, while TAMs express CSF1R. Secretion of EGF from macrophages stimulates cancer cells to form elongated protrusions for invading into the adjacent matrices and to also produce CSF-1, which, in turn, stimulates TAMs for further production of EGF. Molecular inhibition of either EGF or CSF1 significantly impedes the migratory behavior of both cell types, which further confirms this positive feedback loop [105, 106]. Intratumoral CD4+ T cells in PyMT tumors also induce the expression of EGF in macrophages, via IL-4 [46]. Furthermore, cancer-derived CSF1 signaling through TAM CSF1R activates Wiskott–Aldrich syndrome protein (WASP), promoting macrophage migration and EGF expression [107]. Interestingly, although breast tumor cells that express ErbB3 or the CXCR4 receptor invade in response to the ligands heregulin beta1 (HRGβ1) and SDF-1, respectively, their invasion is still dependent on the EGF-CSF1 paracrine loop, such that blocking this signaling loop results in suppression of invasion in response to other ligands [108]. A causal role for macrophages has been linked to early cancer cell dissemination in HER2+ breast cancer cells. CCL2 secreted by cancer and myeloid cells in premalignant lesions recruits CD206+/Tie2+ macrophages, upregulates Wnt pathway and in turn downregulates E-cadherin junctions in these cancer cells to promote early dissemination and metastasis [109].
Moreover, Mena, the mammalian ortholog of Drosophila Enabled (Ena), contributes to cell motility by regulating actin dynamics [110]. Breast cancer cells expressing MenaINV, an invasion-specific isoform, exhibit multicellular streaming and increased intravasation dependent on the EGF-CSF1 paracrine signaling loop between tumor cells and macrophages [111]. Transendothelial migration of breast tumor cells occurs in microanatomical structures known as “Tumor MicroEnvironment of Metastasis” (TMEM) [112]. TMEMs are composed of one TIE2high/VEGFhigh perivascular macrophage in physical contact with a MenaINV-expressing cancer cell, and an underlying endothelial cell [111,112,113]. Macrophages induce RhoA GTPase activity in tumor cells, triggering actin-rich invadopodia that allow tumor cell transendothelial migration [114]. Furthermore, transient vascular permeability was observed at the TMEM, where TIE2hi TMEM macrophages secrete VEGFA, causing local loss of vascular junctions, transient permeability, and tumor cell intravasation [112].
In pancreatic cancer, tumor-derived IL-4 induces the expression of the cysteine proteases cathepsin B and cathepsin S in macrophages, which enhance tumor cell invasion and intravasation by altering the extracellular matrix (ECM) [34]. Moreover, an in vitro study suggested that pancreatic cancer cells activate toll-like receptor 4 (TLR4) signaling in macrophages, inducing IL-10 expression and M2 polarization. TAM TLR4/IL-10 signaling promotes epithelial-to-mesenchymal transition (EMT) in tumor cells, characterized by downregulation of E-cadherin, and upregulation of vimentin and Snail, as well as induction of MMP2 and MM9 proteolytic activity, suggesting a mechanism for TAM-driven tumor cell migration [115]. Recently a novel role of Myosin II was discovered. The migratory behavior of rounded-amoeboid like tumor cells is perpetuated by high ROCK-Myosin II expression which drives IL-1α secretion and NF-κB activation, and these rounded tumor cells are found at the leading invasive edge in close proximity to CD206+CD163+ TAMs and vessels to promote tumor progression [116]. High DAB2 (disabled homolog 2 mitogen-responsive phosphoprotein) expression in tumor-infiltrating TAMs is associated with a worse prognosis in patients. DAB2+ TAMs are at the invasive edge of the tumor and assist in tumor cell dissemination by ECM remodeling, integrin recycling, and mechanosensing cues via YAP-TAZ signaling [117]. In hepatocellular carcinoma, cancer-associated fibroblasts (CAFs) via the expression of endosialin on their surface recruit macrophages through CD68 interaction, regulating GAS6 expression in CAFs to mediate M2 polarization in macrophages and tumor growth [118].
Neutrophils recruited by malignant fibrosarcoma and prostate cancer cells enhance angiogenesis and intravasation in primary tumors by secreting MMP9 [119]. In intrahepatic cholangiocarcinoma, neutrophils recruited by tumor-derived CXCL5, a ligand of CXCR2, enhance metastasis [120]. CXCR2 knockout hosts exhibited smaller tumors and reduced metastasis of breast cancer cells, and a decrease in tumor cell proliferation and angiogenesis, coinciding with significantly suppressed recruitment of CD11b+ Gr1+ myeloid cells and F4/80+ macrophages, suggesting a role of the infiltrating pro-inflammatory immune cells in tumor progression and metastasis [121]. Myeloid cells recruited to mammary tumors harboring a Tgfbr2 deletion secrete MMPs and TGFβ1 that mediate tumor cell invasion and metastasis [122].
In a mouse model of colorectal cancer, collective invasion of cancer cells occurs via a paracrine loop between CD34+ immature myeloid cells (iMCs) and tumor cells. Tumor cells secrete CCL9, which recruits CCR1+ iMCs to the invasive front, where they express MMP2 and MMP9 and promote tumor collective migration [123]. Loss of SMAD4 in human colorectal cancer cells leads to an enhanced expression of CXCL1 and CXCL8 in tumor cells, which in turn leads to an increased recruitment of neutrophils via CXCR2 and higher cancer progression [124].
BM-derived mesenchymal stem cells (MSCs) injected subcutaneously with weak metastatic human breast cancer cells enhanced the migration and dissemination of the tumor cells, via the signaling of MSC-derived chemokine CCL5 to its receptor CCR5 on tumor cells [125]. Direct interaction between BM-MSC-derived periostin and CCL2 in B-ALL cells is responsible for increased leukemia burden [126]. Furthermore, myofibroblasts expressing alpha-smooth muscle actin (αSMA) and derived from BM-MSCs secrete MMP13, increasing tumor invasiveness in a model of skin cancer [127].
Tumor Cell Survival in Circulation and Extravasation into Metastatic Organs
Cancer cells from the primary tumor intravasate into the peripheral circulation as circulating tumor cells (CTCs) [128]. Following intravasation, CTCs induce platelet coagulation by secreting thrombin, enabling platelets to shield tumor cells from shear stress encountered in circulation [129]. Platelets also protect tumor cells from the immune activity of NK cells [130, 131]. Platelet depletion or disruption of clot-forming components inhibited metastasis in mouse models [132]. Platelets have also been shown to be a major source of TGFβ1 in the circulation, and platelet-induced EMT enhanced metastasis in vivo [133]. A conditional knockout of PDGFB in platelets has been shown to negatively impact vascular integrity in the tumor microenvironment, thus enhancing hypoxia and EMT in primary tumors, elevating the levels of CTCs and promoting metastasis [134].
Clot formation also recruits macrophages, which in turn protect circulating tumor cells. Tumor-initiated clot formation induces the expression of vascular cell adhesion molecule 1 (VCAM-1) and vascular adhesion protein 1 (VAP1) on endothelial cells, which recruit macrophages [135]. Macrophages expressing integrin α4 (CD49b) bind to VCAM-1-expressing tumor cells and propagate AKT-dependent survival signals to them [136]. In colorectal carcinoma (CRC), CD163+ TAMs at the invasive edge regulate EMT, CTC in blood, and metastasis of CRC. TAMs secrete IL-6, activating the JAK2/STAT3 pathway that inhibits miR-506-3p in cancer cells, thus promoting FoxQ2 expression which increases CCL2 secretion to promote the recruitment of macrophages [137]. In TNBC, higher expression of tumor suppressor miR-149 directly targets CSF-1 to inhibit recruitment and polarization of M2 macrophages, thus suppressing lung metastasis [138].
CD4+ CD25+ FoxP3+ TReg cells isolated from lymph nodes of patients with melanoma and from malignant ascites of ovarian cancer patients inhibit CD4+ and CD8+ T cell proliferation and cytokine production in vitro [139, 140], suggesting that TReg cells can protect disseminated cancer cells at the metastatic site from immune attack. Ovarian cancer stem cells (CSCs) have been found to express CCL5 and recruit Treg through the expression of CCR5 receptor on their surface [141]. This CSC CCL5-Treg CCR5 interaction increases IL-10 and also increases the production of MMP-9 by Treg, aiding with ovarian cancer metastasis [141]. Patients with liver metastasis are known to respond poorly to anti-PD-1 therapy. Recently, the presence of a tumor-specific antigen causing systemic suppression of the immune system and increased activation of Tregs and modulation of CD11b+ monocytes was reported [142]. Thus, combining anti-PD-1 with anti-CTLA4 or EZH2 inhibitors to deplete or destabilize Tregs is considered as a potential approach against liver metastases [142].
The next challenge for CTCs is to exit the circulation and colonize the surrounding tissue of the metastatic organ. The first step of extravasation requires that a CTC properly adheres with the endothelial wall. Tumor-derived IL-8 recruits neutrophils and increases their expression of β2 integrin, promoting the interaction between tumor cell intercellular adhesion molecule-1 (ICAM-1) and neutrophil β2 integrin [143]. ICAM-1 is also expressed on endothelial cells [129], enabling neutrophils to anchor tumor cells to the endothelium, enhancing extravasation and metastatic foci formation in lungs [143] and liver [144]. Neutrophils also form structures called neutrophil extracellular traps (NETs), composed of extruded DNA and antimicrobial proteases. Neutrophils trap circulating tumor cells in NETs that form in liver and lung capillaries to promote metastasis [145]. NETs have been found to be elevated in invasive esophageal, gastric, and lung cancer patients, and shown to regulate disease progression [146]. Using lung and colon cancer murine models, it was observed that inhibiting NETs either using Sivelestat or peptidyl arginine deiminase type IV (PAD4, involved in NET formation) reduced spontaneous lung and liver metastasis in these models [146]. In the blood, circulating tumor cells (CTCs) have been found to be present in association with neutrophils, which help in the cell cycle progression of these tumor cells, followed by an increase in their metastatic potential [147].
Platelets contribute to tumor cell extravasation by promoting the adhesion of tumor cells to ECs at the distant site. In addition to EC P-selectin, platelet-expressed P-selectin promoted lung metastasis of breast cancer and melanoma [148]. CTCs themselves express the selectin ligands sialyl Lewis-a (sLea) and sialyl Lewis-x (sLex) [149]. These ligands allow tumor cells to adhere to endothelial cell E-selectin and confer increased metastatic potential [150, 151].
Inflammatory monocytes recruited to the premetastatic lungs via the CCL2-CCR2 axis increased tumor cell extravasation from the vasculature into the lung parenchyma by increasing VEGF-induced vessel permeability, resulting in transendothelial migration [152] during breast cancer metastasis to the lungs [153] and colorectal cancer metastasis to the liver [154]. Tumor antigen, CD97, has been shown to stimulate platelet activation via a bidirectional signaling through platelet-derived lysophosphatidic acid (LPA). This increases vascular permeability and transendothelial tumor cell migration [155]. In colon and breast cancer models, platelet-specific receptor for collagen and fibrin, GPVI (glycoprotein VI), interacts with galectin-3 on tumor cells to promote extravasation of tumor cells via ITAM signaling [156]. Tumor cells undergoing EMT and circulating tumor cells express Hsp-47, which has also been shown to increase platelet recruitment and metastatic lung colonization of tumor cells [157].
Tumor Cell Colonization and Initiation of Metastasis in Distant Organs
Metastatic tumors set up a BM-derived microenvironment at the distant site of metastasis, known as the premetastatic niche [158]. This niche functions as a permissive hub for supporting colonization and outgrowth of disseminated tumor cells following extravasation. BM-derived cells at the metastatic site also influence tumor cell tropism and promote metastatic outgrowth.
Contribution of the Premetastatic Niche in Colonization and Initiation of Metastasis
In 1889, Stephen Paget proposed the “seed and soil” hypothesis, in which he suggested that cancer cells, being the “seed,” had an affinity for and only colonized organs that were conducive to their growth, or had the proper “soil” [159]. Indeed, in recent years, studies have shown that conducive microenvironments are required for disseminated tumor cells to engraft at distant sites, in agreement with the “seed and soil” hypothesis. Strikingly, metastatic primary tumors systemically generate bone marrow-derived “premetastatic niches” in distant organs that serve as hubs for supporting future metastases [160].
The first account of the premetastatic niche described that the recruitment of VEGFR1+ hematopoietic progenitor cells to the lungs is necessary for tumor metastasis. In this model, Lewis lung carcinoma and melanoma primary tumors systemically induced the expression of fibronectin in lung fibroblasts, leading to the recruitment of BM-derived VEGFR1+ and CD11b+ myeloid cells to the lungs via their fibronectin receptor, VLA-4 [161]. CD11b+ myeloid cells are also recruited to premetastatic lungs by chemoattractants S100A8 and S100A9, expressed in response to primary tumor-derived VEGFA , TGFβ, and tumor necrosis factor α (TNFα) [162]. The recruited CD11b+ cells in turn express TNFα and TGFβ, which enhance tumor cell metastasis [162]. In addition, serum amyloid A3 (SAA3), induced by S100A8 and S100A9 in lungs, activates NFκB signaling via TLR4 on myeloid cells, potentiating the inflammatory response and accelerating lung metastasis [163]. Furthermore, primary tumor-derived CCL2 signaling through CCR2 on lung endothelial cells induces the secretion of S100A8 and SAA3, which increases vascular permeability, resulting in hyperpermeable foci that attract leukocytes and tumor cells [164]. A novel way to suppress pro-metastatic effects of S100A8 has been utilized in 4T1 model by inhibiting high-fat diet-enhanced premetastatic niche formation [165]. Glycyrrhizic acid prevents Gr1+ myeloid cell migration and S100A8/9 expression by decreasing M1-macrophage population and their CCL2 and TNF-α production leading to modification of premetastatic niche formation and reduction of metastasis [165].
Primary tumor-derived VEGF induces the expression of MMP9 in CD11b+ myeloid cells and endothelial cells in the premetastatic niche [166]. MMP9 in the premetastatic niche releases VEGF from the ECM, promoting angiogenesis [167], and soluble KIT ligand, which further recruits KIT receptor-expressing BM cells [168]. In melanoma metastasis to liver, pro-oncogenic miR-155 decreased levels of its target NFE2L2, a redox regulatory factor, while increasing VEGFA levels in premetastatic liver via pro-oxidative events [169]. In 4T1 models, primary tumor-secreted VEGF was elevated, leading to hyperpermeability of vessels in lungs and reduced levels of tight junction proteins: occluding and ZO-1 [170]. Inhibition of a multifunctional glycoprotein, dipeptidyl peptidase-4, has been shown to accelerate EMT in breast cancer cells and thus their metastasis via induction of CXCL12/CXCR4 and activation of mTOR pathway [171].
Furthermore, hypoxia from primary tumors induces the accumulation of MDSCs in premetastatic lungs, suppressing the cytotoxic function of NK cells [172]. Treatment with epigenetic therapy (azacitidine and entinostat) induces the differentiation of MDSCs into a more-interstitial macrophage-like phenotype by reducing expression of CCR2 and CXCR2, thus disrupting the premetastatic niche [173]. Chronic stress is known to enhance premetastatic niche formation and metastatic colonization of breast cancer cells in lungs via β-adrenergic signaling [174]. β-adrenergic signaling causes an increase in monocytes and macrophages in the premetastatic lungs, which interact with upregulated CCL2 in pulmonary stromal cells, to enhance metastatic colonization [174]. In premetastatic niche, a myeloid-rich, immune-suppressive gene signature is present which enhances metastasis [175]. Recently, this immunosuppressive effect was mitigated using genetically engineered myeloid cells (GEMys) to deliver IL-12 to the secondary site. IL12-GEMy enhanced antigen presentation and T-cell activation at the premetastatic site to reduce metastasis [175].
Lysyl oxidase (LOX), a hypoxia-inducible secreted amine oxidase, is also critical in the generation of premetastatic niches in solid tumor metastasis [176]. Secreted LOX from hypoxic primary breast cancer cells co-localizes with fibronectin in both pulmonary and hepatic premetastatic niches, and crosslinks collagens in the local microenvironment. This modification of the ECM promotes the recruitment of CD11b+ BM cells, creating a niche permissive for the colonization of metastasizing tumor cells at these secondary sites [176]. The inhibition of LOX at primary tumors abrogates the establishment of premetastatic niches and decreases metastatic burden in secondary organs. Similarly, the targeting of CD11b+ cells restricts the establishment of tumor-supportive premetastatic niches, reducing metastatic burden [176]. In addition, LOX expression and activity during the onset and development of both chemical- and radiation-induced lung and liver fibrosis has been shown to be responsible for fibrosis-enhanced metastasis to these organs. The action of LOX generates tumor cell supportive niches high in fibrillar collagen, which increase seeding, tumor cell persistence, and survival [177]. While the involvement of BM-derived cells was not directly investigated, the changes occurring during fibrosis dramatically recapitulate those observed in premetastatic niche remodeling, suggesting common overlapping mechanisms [178]. More recently, LOX has also been shown to induce the formation of premetastatic osteolytic lesions in the bone. In this case, elevated levels of LOX secreted by hypoxic primary tumors alter the homeostatic balance between osteoclasts and osteoblasts. LOX modulates the BM stroma to drive de novo osteoclastogenesis while decreasing osteoblast proliferation, both in vitro and in vivo. The net result is unbalanced coupling, osteolysis, and premetastatic niche generation within the bone. These LOX-driven premetastatic niches, in turn, support circulating tumor cell colonization and the development of overt bone metastases [179].
In a bladder cancer model, the tumor cell-derived proteoglycan, versican, enhances metastasis to the lungs via a mechanism involving increased lung CCL2 chemokine expression and increased macrophage infiltration [180]. Consistent with these data, the recruitment of CCR2-expressing monocytes and macrophages to the lungs in response to tumor cell-derived and host-derived CCL2 enhances breast tumor metastasis to lungs [153]. In epithelial ovarian cancer, higher expression of miR-590-3p suppressed FOXA2 levels by binding to its 3″ UTR, which increased versican levels to enhance tumor proliferation and metastasis [181]. High versicanV1 expression in HCC promotes metastasis via activation of EGFR-PI3K-AKT pathway [182].
Immature myeloid cells expressing the stem and progenitor cell marker CD117 are involved in premetastatic niche formation [161, 176]. Similarly, mature myeloid cells, such as CD11b+ Ly6C+ monocytes, are recruited to premetastatic lungs by CCL2, and CD11b+ CD68+ F4/80+ macrophages are recruited by fibrin clots in the premetastatic lungs, where they enhance metastasis of B16 melanoma and breast cancer cells , respectively [183, 184]. Macrophages in the premetastatic niche are derived from circulating BM-derived monocytes, which are recruited via CCL2 [153], and this implies that primary tumor-secreted factors can systemically recruit myeloid progenitors at different stages of differentiation to premetastatic sites, where they differentiate into metastasis-promoting macrophages. Interestingly, primary prostate and breast tumors with a low metastatic potential systemically induce the expression of the antiangiogenic factor Tsp-1 in myeloid cells recruited to the premetastatic lungs [185], indicating that even non-metastatic tumors can modify the microenvironment in distant organs. Recently pro-metastatic effects of chemotherapy, involving taxanes and anthracyclines, were identified that led to primary tumors releasing extracellular vesicles enriched in annexin A6 which promoted NF-κB-dependent endothelial cell activation, CCL2 induction, and Ly6C+CCR2+ monocytes in the premetastatic lungs, followed by overt metastatic outgrowth [186].
Primary tumor-secreted G-CSF recruits Ly6G+ neutrophils to premetastatic lungs, where they contribute to the formation of lung metastasis via their expression of Bv8, which promotes tumor cell migration [187]. Interestingly, the CCL2-CCR2 axis was also shown to recruit CCR2+ neutrophils to the premetastatic lung [188]. However, instead of promoting metastasis, these neutrophils inhibited the survival of disseminated cells through CCL2-dependent activation of H2O2-mediated killing [188]. These results suggest that CCL2 both promotes and blocks metastasis initiation; hence, insights into these processes will be critical for developing anti-metastatic therapies. A novel subset of neutrophils, CD62Ldim, have been found to have stronger adhesion and longer survival [189]. These neutrophils are necessary for the formation of premetastatic niche in lungs in breast cancer models via CXCL12-CXCR4 pathway [189]. Migration of neutrophils to distant tissues to form premetastatic niche was recently identified in the presence of tumors. Bone marrow neutrophils in mice-bearing early-stage tumors have higher OXPHOS and glycolysis, higher ATP production, as well as increased autocrine ATP signaling via purinergic receptors than control neutrophils from non-tumor bearing mice [190]. In osteosarcoma metastasis, tumor-secreted ANGPTL2 stimulates lung epithelial cells to recruit neutrophils to the lungs and form premetastatic niche for osteosarcoma metastasis [191]. Regulators of lipid metabolism, oxysterols, also regulate premetastatic niche in lungs of mice-bearing 4T1 tumors by recruiting neutrophils to the lungs via oxysterol/LXR (nuclear Liver X Receptors-(LXR)α and LXRβ) signaling [192]. Chronic nicotine exposure also increases recruitment of pro-tumor N2-neutrophils to the lungs to form premetastatic niche [193]. This niche promotes STAT3-activated lipocalin 2 (LCN2) release, which has been detected in serum and urine of breast cancer patients and cancer-free women who smoke [193]. Thus, LCN2 can possibly be used as a prognostic biomarker for lung metastasis of breast cancer.
Non-myeloid cells also compose the premetastatic niche. For instance, CD4+ T cells in premetastatic bones increase osteoclastogenesis by secreting receptor activator of nuclear factor-kB ligand (RANKL), thus enhancing the metastasis of breast cancer cells to the bones [194]. Moreover, 4T1 breast cancer cells systemically induce the expression of CCL22 in lung stroma, thereby inducing the recruitment of TReg cells to premetastatic lungs [195]. A study defining premetastatic niche in tumor draining lymph node using MMTV-PyMT breast cancer model by single-cell RNA sequencing identified that CD4+ and CD8+ T cells have higher angiogenesis pathway genes, upregulated T-reg genes, downregulated IFN and inflammatory gene signatures [196]. The niche in these tumor draining lymph nodes also had upregulation of OXPHOS in fibroblastic reticular cells and genes like Prdx3, Ndufa4, and Uqcrb, demonstrating enhanced ATP consumption, TCA cycle and a metabolic switch to OXPHOS by breast cancer cells [196].
In addition to soluble tumor-derived factors generating the premetastatic niche, exosomes released from primary tumors also induce the mobilization of BM-derived cells which are then recruited to the secondary site to generate the premetastatic niche [197]. Metastatic B16 melanoma cells release exosomes that carry MET, transferring MET to BM progenitors, leading to their recruitment to premetastatic lungs and enhancing metastasis [198]. Bone metastasis of prostate cancer cells was recently found to be dependent on exosome-mediated transfer of pyruvate kinase M2 (PKM2) from prostate cancer cells into bone marrow stromal cells (BMSCs), which then increases CXCL12 production by BMSCs via HIF-1α to mediate bone tropism [199].
Organ Tropism
Correlations have been found between primary tumors and their preferred metastatic destination, and more recent studies have begun to identify mechanism of metastasis organotropism. The tropism, or preferential metastasis, of tumors to specific organs has been shown to be determined in part by cancer cell intrinsic pathways, and as a consequence, gene signatures that mediate organ-specific metastasis have been described [200,201,202]. However, tumor non-cell-autonomous mechanisms have also been shown to play a necessary role in organ tropism. For example, chemoattractants in metastatic organs are able to recognize cognate chemokine receptors expressed on cancer cells that promote homing. Breast cancer cells expressing CXCR4 and CCR7 migrate toward SDF-1 and CCL21 chemokine gradients, respectively, in metastatic sites [203]. Signaling via CXCR4 and CCR7 mediates actin polymerization and pseudopodia formation, leading to chemotactic responses and invasion [203]. Moreover, blocking CXCR4/SDF-1 signaling suppresses metastasis of breast cancer cells to the lymph nodes and lungs [203]. CXCR4 expression is also required for human epidermal growth factor receptor 2 (HER2)-mediated breast cancer metastasis [204]. In addition to CXCR4 and CCR7, CCR10 expression on melanoma cells confers tropism to the skin [203]. Furthermore, chemokine (C-X3-C motif) receptor 1 (CX3CR1) expression on pancreatic cancer cells mediates their metastasis to chemokine (C-X3-C motif) ligand 1 (CX3CL1)-expressing peripheral neurons [205].
A major step after homing is adhesion of tumor cells at the distant site. During the early steps of pulmonary metastasis, disseminated breast cancer cells arrest in lungs via contacts between tumor α3β1 integrin and laminin 5 expressed on pulmonary vasculature basement membrane [206]. Furthermore, TNFα secreted by primary tumors [162] and by myeloid cells in lungs [207] upregulates the expression of the adhesion molecules E-selectin, P-selectin, and VCAM-1, promoting tumor cell adhesion and migration [3]. TNBC primary tumors increase CD117+ hematopoietic progenitor cells in the bone marrow in the mice compared to luminal primary tumors and control mice [208]. TNBC primary tumors also increase fibronectin, tenascin-c, and periostin in lungs to increase lung metastasis, and the lung-conditioned media from these mice are rich in metastasis proteins like CCL7, FGFR4, GM-CSF, MMP3, TSP-1, and VEGF [208]. A recent study [209] established cell lines derived from circulating tumor cells in breast cancer patients and cultured in vivo. These ex vivo cultured cells recapitulated the metastasis phenotypes in mouse models and also identified semaphorin 4D as an important mediator of migration through blood-brain barrier and brain metastasis in breast cancer patients [209]. Breast tumor cells and stroma together create distinct extracellular matrix niches for different organ metastasis [210]. Proteins enriched in CD109, SERPINB1, HCFC1, and cerebellin-1 are prominent in brain metastases, COL4A4 and laminin-121 are rich in lung metastases, and bone marrow metastatic niche is rich in S100A6 and S100A11 [210]. In breast cancer, enhanced expression of POU1F1 transcription factor (or Pit-1) is associated with increased CXCR4 and CXCL12 expression, along with specific breast cancer metastasis to liver and lungs [211].
Primary tumors from multiple organs metastasize to bone. Breast cancer causes osteolytic lesions in bones, stimulating the formation and activity of osteoclasts. These osteolytic lesions cells express CSF1, which activates osteoclasts that break down bone. Breast cancer cells also express parathyroid hormone-related protein (PTHRP) and TNFα, which activate RANKL and inhibit osteoprotegerin synthesis, inducing formation and activity of osteoclasts [212, 213]. In melanoma, inhibition of RANKL reduced metastasis to the bone, but not to other organs [213].
Breast cancer cells that home to the bone express upregulated CXCR4, osteopontin, MMP1, and IL-11 [200]. The bone stroma is rich in SDF-1, the ligand for CXCR4, which may mediate the tropism of the breast cancer cells. Once in the bone, IL-11 activates osteoclasts, and MMP1 releases matrix-sequestered factors, enhancing tumor outgrowth and bone degradation [3]. When bone matrix is degraded, several sequestered factors are released, including insulin-like growth factor 1 (IGF1), TGFβ, and bone morphogenetic proteins (BMPs), which enhance metastatic survival and outgrowth and induce PTHRP synthesis, leading to further bone degradation [212]. This leads to a positive feedback loop of increased bone loss and enhanced metastatic tumor growth. In the case of neuroblastoma that metastasizes to the bone, while some tumors secrete RANKL, others induce IL-6 expression in BM-MSCs. IL-6 activates osteoclasts and is required for bone metastasis [214]. Furthermore, BM-derived IL-6 mediates survival and proliferation of IL-6R+ neuroblastoma cells [215].
Prostate cancer, on the other hand, generates osteoblastic lesions in the bone, characterized by disrupted bone deposition. Prostate metastatic lesions release endothelin 1, TGFβ2, FGF, and BMPs, all of which are osteoblastic and alter bone structure [3]. Moreover, prostate cancer cells also produce urokinase-type plasminogen activator (uPA) and prostate-specific antigen (PSA), which can release growth factors from the bone matrix, enhancing metastatic outgrowth [212].
More recently, tumor-derived exosomes were shown to be implicated in organ-specific metastasis. Exosomes carrying integrins α6β4 and α6β1 directed metastasis to the lungs, while exosomal integrin αvβ5 directed metastasis to the liver, in both cases by inducing the expression of S100 chemoattractants in the target organ [216].
Metastatic Outgrowth
After seeding in the secondary site, metastatic tumors establish vasculature in order to outgrow. This occurs via the production of angiogenic factors, such as VEGFA, and the recruitment of endothelial cells and pericytes. Metastasis-associated macrophages (MAMs) support tumor outgrowth at the metastatic site [152] via a TIE2-dependent mechanism that promotes angiogenesis [40]. On the contrary, a membrane tetraspan molecule, MS4A4A, selectively expressed by macrophage-lineage cells and associated with Dectin-1 in macrophages, is responsible for activation of macrophages and NK cells via Syk-dependent signaling and reactive oxygen species production, to reduce metastasis [217]. In the MMTV-PyMT breast cancer model, BM-derived EPCs are recruited to metastatic lesions, where they incorporate into nascent vessels and contribute to the angiogenic switch, promoting the progression of micrometastases to macrometastases [90]. EPCs express the transcription factor inhibitor of differentiation 1 (ID1), which is required for EPC mobilization and recruitment [90, 91]. Moreover, several proangiogenic genes are upregulated in recruited EPCs, suggesting an additional mechanism whereby EPCs promote angiogenesis and metastatic progression [90].
Furthermore, the contribution of neutrophils to metastatic progression was demonstrated in models of extrinsic lung inflammation, where the neutrophil-secreted serine proteases neutrophil elastase (NE) and cathepsin G (CG) degrade antiangiogenic Tsp-1, coinciding with increased lung metastasis [218]. Furthermore, neutrophils in the premetastatic lungs were shown to expand the metastasis-initiating cell population of breast cancer cells by expressing leukotrienes [219]. Neutrophils also secrete proangiogenic Bv8, which promotes metastatic progression [187].
As in earlier stages of tumor progression, myeloid cells also play a role in metastatic outgrowth. In the MMTV-PyMT breast cancer model, BM-derived CD11b+ Ly6Chigh myeloid progenitor cells in premetastatic lungs secrete the extracellular matrix protein, versican, which promotes mesenchymal-to-epithelial transition (MET) of metastatic tumor cells via the TGFβ pathway, thereby increasing proliferation and accelerating lung metastatic outgrowth [220]. Primary tumor-secreted G-CSF is known to increase immunosuppressive CD11b+GR1+ MDSCs in the spleen and lungs of a 4T1 breast cancer model . In 4T1 model of metastasis it has been found that presence of orthotopic primary tumor increases (G-) MDSCs, monocytic (M-) MDSCs, macrophages, eosinophils, and NK cells in the lungs [221]. Even upon resection of primary tumor, immunosuppressive G-MDSCs still persist in the lungs, aiding in subsequent metastatic outgrowth. Thus, reduction of G-MDSCs via gemcitabine treatment in addition to primary tumor removal significantly reduces metastasis [221].
Neutrophil accumulation in cancer leads to a worse prognosis in patients and is one of the major reasons for increased cancer metastasis [222]. Interplay between CXCR4 and CXCR2 dictates neutrophil release from the bone marrow, where inhibition of CXCR4 or secretion of CXCL1 and CXCL2 by endothelial cells and megakaryocytes to mediate CXCR2 signaling, promotes neutrophil release into circulation and downstream activity [222]. Neutrophils are known for premetastatic conditioning at secondary sites, T-cell suppression, and promotion of cancer cell survival [223, 224]. In cancer, there is evidence of increased granulopoiesis which enhances neutrophil formation. Obesity has also been shown to increase breast-to-lung metastasis in a GM-CSF- and IL5-dependent manner by causing lung neutrophilia [225]. Weight loss significantly reverses these effects and lowers serum levels of both GM-CSF and IL-5 [225]. Recent evidence also suggests that increased recruitment of neutrophils at the metastatic site via cathepsin C-mediated activation of neutrophil PR3 to increase IL-1β secretion, enhances breast-to-lung metastasis [226]. This increased recruitment also promotes extrusion of their genomic DNA to form NETs via neutrophil-induced reactive oxygen species by NADPH oxidase NOX2 [224], degrading TSP-1 and promoting metastatic outgrowth [226]. Therefore, cathepsin C inhibition via AZD7986 could prove to be an effective strategy for mitigating these effects.
NETs have been shown to promote liver metastasis in early-stage breast cancer patients [227]. In short, NETs in liver or lungs act as a chemoattractant for cancer cells via a transmembrane protein CCDC25 on cancer cells which activates ILK-β-parvin pathway to enhance their motility and thus, metastasis to the secondary sites [227]. Tumor-secreted CXCR1 and CXCR2 ligands also promote NETosis which shields tumor cells from cytotoxic effects of NK cells and lymphocytes [228]. Inhibition of adipose triglyceride lipase activity in neutrophils also promotes breast-to-lung metastasis by increasing lipid storage by these neutrophils in premetastatic lungs [229]. These lipids via macropinocytosis–lysosome pathway are transported to metastasizing tumor cells to enhance their survival and proliferation [229]. Additionally in an obesity model, neutrophils have been shown to regulate transendothelial migration leading to higher extravasation of circulating tumor cells into secondary organs and increased breast cancer metastasis [230]. The increased metastatic potential is due to lowered vascular integrity by neutrophils via MMP9 and LCN2 secretion [230]. Genes like Ltf and Lcn2 were also upregulated in obese mice which led to increased neutrophil ROS production and enhanced NETs to promote extravasation and metastasis [230]. NETs were also shown to convert dormant cancer cells to aggressive lung metastases in vivo in the presence of lung inflammation [231]. Sustained lung inflammation caused elevated NET formation, NET DNA then cleaved laminin by NE and MMP9, causing integrin-mediated FAK/ERK/MLCK/YAP signaling to promote proliferation of dormant cancer cells [231].
A recent study demonstrated that periostin (POSTN) secreted in the lung metastatic niche by stromal αSMA+ vimentin (VIM)+ fibroblasts was required to maintain cancer stem cells (CSCs) and allow metastatic outgrowth of breast cancer cells by inducing Wnt signaling in the CSCs [232, 233]. Although this study did not determine the exact identity of the fibroblasts secreting POSTN, a previous study had shown that BM-derived MSCs are the source of POSTN [234], suggesting that BM cells in the premetastatic niche could be supporting metastasis by maintaining CSCs via POSTN secretion. In a model of pancreatic cancer, αSMA+ myofibroblasts produce majority of type I collagen (Col1). Deletion of this Col1 leads to spontaneous emergence of PanINs and PDAC, and SOX-9-mediated Cxcl5 upregulation in cancer cells. Cxcl5 causes recruitment of MDSCs and suppression of CD8+ T-cells, thus enhancing PDAC progression [67]. A recent study demonstrated the presence and role of four different cancer-associated fibroblasts in metastatic lymph nodes and how they influence cancer cell invasion. Two myofibroblastic subsets in the lymph node: CAF-S1, enhanced cell invasion by CXCL12 and TGFβ pathways; whereas CAF-S4 enhanced 3-D invasion by NOTCH signaling [235]. CAF-S4 in patient lymph node was also found to correlate with late distant metastases [235].
Recently, studies on metastatic dormancy have identified a mechanism whereby EC-derived Tsp-1 promotes breast cancer dormancy [236]. Interestingly, in another study, metastasis-incompetent primary tumors were shown to induce the expression of Tsp-1 in BM-derived myeloid cells recruited to premetastatic lungs [185], suggesting that myeloid-derived Tsp-1 could be similarly mediating metastatic dormancy.
Clinical Significance, Perspectives, and Future Directions
BM-derived cells contribute to various stages of cancer progression, and given their prevalence in patient tumors, BM-derived cells are being evaluated as prognostic tools and as therapeutic targets. Elevated levels of circulating inflammatory monocytes correlate with a poor prognosis in pancreatic cancer patients [237]. A high preoperative neutrophil to lymphocyte ratio is associated with poor prognosis after resection in NSCLC [238]. In human breast cancer, a high TMEM score, i.e., the number of tumor cell, TAM, and endothelial cell interactions, is correlated with an increased risk of metastasis [239, 240]. Of note, TMEM score predicted the risk of distant metastasis in ER(+)/HER2(–) breast cancer [240]. The increased risk of metastasis observed in patients is consistent with pre-clinical studies showing the role of macrophages in tumor cell egress from the primary site, and intravital imaging revealing the direct contact between perivascular TAMs, endothelial cells, and tumor cells, forming the TMEM [113]. Recently, a correlation between an imbalance in gut microbiota and colorectal cancer (CRC) was found. The imbalance has been shown to upregulate metastasis-related secretory protein cathepsin K (CTSK) in both human CRC samples and mouse models of CRC, leading to a higher expression of M2 TAMs in the stroma, higher CRC metastasis, and a worse clinical prognosis [241]. Thus, making CTSK a novel biomarker and target for CRC.
There is increased translational significance of CSF-1R inhibition in advanced solid tumors. Combination of CSF-1R inhibition with a CD40 agonist led to tumor rejection in multiple solid tumor models [242]. In glioblastoma (GBM), radiotherapy has shown to increase relative abundance of brain-resident microglia and monocyte-derived macrophages which promote recurrence [243]. Pre-clinical models have shown that radiotherapy combined with CSF-1R inhibition is able to effectively overcome this resistance [243]. Inhibiting CSF-1R with PLX3397 has also shown to interfere with the education of TAMs [244] in the GBM tumor microenvironment (depolarizes them from their original M2 state and upregulates M1-like state) [245]. Inhibition of CSF-1R increases the sensitivity of glioma cells to tyrosine kinase inhibitors and improved outcomes in pre-clinical trials [245].
In NSCLC patients, high tumor islet CD68+ macrophage density predicted increased survival, whereas high stromal macrophage density predicted reduced survival [246,247,248]. Further characterization of macrophage populations revealed that CD68+ M1 macrophages, defined as HLA-DR+ iNOS+ TNFα+ MRP8/14+, were significantly increased in tumor islets of NSCLC patients with extended survival compared to patients with poor survival, whereas M2 macrophages (CD163+ VEGF+) were reduced [249]. On the other hand, several reports showed a correlation of TAMs with poor prognosis in lung cancer. Adenocarcinoma patients with high CD68+ TAM density had significantly lower 5-year survival rates [250]. Furthermore, CD68+ TAM density correlated with higher tumor expression of the angiogenic factor IL-8, and higher microvessel density, but worse prognosis [251]. CD68+ CD163+ M2-like TAMs were significantly higher in patients whose disease progressed in the presence of EGFR tyrosine kinase inhibitor therapy [23]. M2 TAMs defined as CD204+ were associated with poor outcome [252], and CD206+ TAMs correlated with lymph node metastasis [253].
High levels of IL-10 expression by TAMs were significantly correlated with advanced tumor stage and predicted poor overall survival [254, 255]. Expression of MMP9 and VEGF by CD68+ IL-10hi TAMs correlated with late stage of disease [256]. On the other hand, several studies have failed to find a correlation between macrophage density and NSCLC patient prognosis [27]. In lung cancer, developmental origin of TAMs (tissue-resident vs. CCR2-dependent recruited macrophages) dictates their function, distribution, and response to cancer therapies [257]. In this regard, anti-VEGF therapy combined with chemotherapy reduced both resident- and recruited-TAMs (expressing VEGFR1) without impacting monocyte infiltration and prolonged mouse survival in pre-clinical models [257].
In colorectal cancer higher SPP1 levels and lower mTORC2 activity in TAMs are associated with a worse clinical prognosis [258]. IL-15 receptor alpha chain or hetIL-15 of the heterodimeric cytokine IL-15 treatment in colon and epithelial carcinoma models has shown increased intratumoral accumulation of XCR1+IRF8+CD103+ conventional type 1 dendritic cells (cDC1), with enhanced levels of IFNγ and XCL1, leading to an increased infiltration of CXCR3+ NK and CD8+ T cells in tumors [259]. Thus, enabling tumor immunotherapy approaches to promote effector responses over regulatory lymphocytes to delay tumor growth.
Several therapeutic combinations involving immune checkpoint blockade are being developed as effective antitumor therapeutics. In HCC, a dual anti-PD-1/VEGFR-2 therapy has shown promising effects of inhibiting tumor growth and increasing overall survival in mouse models of cancer by increasing CD8+ T cell activation and infiltration, shifting the M1/M2 ratio of TAMs, reducing T-regs, and reducing CCR2+ monocyte infiltration [241]. A bispecific antibody, A2V, targeting VEGFA and angiopoietin-2 (ANGPT2) enhances recruitment of IFNγ-expressing CD8+ T lymphocytes, with concomitant expression of PD-L1 on tumor endothelial cells in an MMTV pre-clinical model [260]. Thus, supporting the rationale for combining antiangiogenic therapy with immune checkpoint blockade to improve survival.
Immune-suppressive MDSCs, defined as Lin−HLA-DR−CD33+ and CD14−CD11b+CD33+ [261], have been found to be increased in patients with NSCLC and were associated with increased metastasis and a poor response to chemotherapy [262]. Increased levels of circulating and tumor-infiltrating MDSCs were observed in patients with colon cancer and correlated with prognosis and cancer stage [263]. Furthermore, the frequency of circulating monocytic MDSCs predicted patient response to the checkpoint inhibitor ipilimumab (anti-CTLA-4), where patients with low frequencies of MDSCs benefited more from ipilimumab treatment [264]. Glioblastoma patients showed an increase in circulating MDSCs, primarily of granulocytic lineage, that mediated immunosuppressive functions [265]. In patients with breast cancer, an immune signature consisting of CD68high/CD4high/CD8low, denoting the infiltration of the different immune cell types into tumors, significantly correlated with reduced OS [22]. Interestingly, this immune signature predicted OS independently of histopathological grade or receptor status [22].
Another important finding was that the composition of the TME landscape which is complex at the metastatic sites and can be defined by the origin of primary tumors. For instance, melanoma brain metastasis is composed of an abundance of CD4 and CD8 lymphocytes whereas breast cancer brain metastases have a prominence of neutrophils [69]. Even the TME in glioma, a CNS tumor, predominantly is comprised of higher tissue-resident microglia than tumor-infiltrating leukocytes [266]. Brain metastasis presents with the highest frequencies of Tregs, followed by IDH1-wt and IDH1-mutant gliomas [266]. Thus, dictating the differences in the efficacy of immunotherapy.
In addition to their prognostic role, a growing body of literature describing the contribution of the BM-derived microenvironment to tumor progression and metastasis reveals potential therapeutic avenues that bypass the need to target highly mutagenic tumor cells and instead focus on the more genetically stable stromal cells that support the tumor. In that respect, studies targeting BM-derived cells in the TME have shown some promise. For instance, inhibiting the recruitment of TAMs by blocking CSF1R signaling enhanced the cytotoxic effect of standard chemotherapy in a mouse model of breast cancer [22] and increased the efficacy of immunotherapy in a pancreatic cancer model [53]. Tumor refractoriness to anti-VEGF therapy was shown to be dependent on CD11b+ Gr1+ myeloid cell recruitment [73]. Blocking neutrophil recruitment to lungs by administering anti-G-CSF suppressed lung metastasis [187]. Moreover, inhibiting the myeloid cell-secreted angiogenic factor Bv8 reduced primary tumor growth, produced synergistic antitumor effects when combined with anti-VEGF treatment or chemotherapy [76], and reduced lung metastasis [187].
In a model of lung inflammation, deleting the neutrophil proteases NE and CG in the BM compartment significantly suppressed metastatic outgrowth [218], suggesting that targeting neutrophil proteases could present a way to block metastasis. Furthermore, inducing the expression of Tsp-1 by myeloid cells in the lungs by administering a peptide derived from the protein Prosaposin significantly reduced lung metastatic burden [185]. On the other hand, given the immunosuppressive function of MDSCs in cancer, strategies are being developed that promote the differentiation of MDSCs into mature, non-suppressive cells, decrease MDSC levels, or inhibit MDSC function [267]. Pre-clinical studies revealed that all trans retinoic acid (ATRA) induced the differentiation of MDSCs and enhanced T cell antigen-specific immune responses, but only induced an anti-cancer response when combined with a peptide vaccine [58, 268]. Administration of ATRA reduced MDSC levels in metastatic renal cell carcinoma (mRCC) patients that achieved a high plasma concentration of ATRA [269]. Sunitinib, an oral receptor tyrosine kinase inhibitor that targets PDGFR, VEGFR, and c-kit signaling and is FDA-approved for the treatment of advanced RCC, reduces MDSC levels in patients [270] and blocks the expansion of monocytic MDSCs while inducing apoptosis of granulocytic-MDSCs in a mouse model of breast cancer [271]. Furthermore, the immunosuppressive activity of MDSCs was abrogated by the synthetic triterpenoid, CDDO-Me, which upregulated several antioxidant genes and decreased tumor growth in mice [261]. Moreover, CDDO-Me completely blocked the inhibitory function of MDSCs isolated from RCC patients [261].
Another way MDSC function is being targeted is by inhibiting IDO, the rate-limiting enzyme in tryptophan degradation, which leads to suppression of T cell responses [48, 63]. IDO inhibition using 1-methyl-tryptophan (1MT) retarded tumor growth via a T cell-dependent mechanism in mouse models [272,273,274]. Furthermore, combining 1MT with therapies targeting immune checkpoints on T cells, such as CTLA-4, PD-1/PD-L1, synergizes the antitumor response in a mouse model of melanoma [275]. A recent clinical trial is testing the efficacy of IPI-549, a small molecule PI3Kγ inhibitor, in order to improve the efficacy of immune checkpoint blockade (NCT02637531). This is because resistance to immune checkpoint blockade has been directly correlated to increased infiltration of suppressive myeloid cells in the tumors, which have increased expression of PI3Kγ [276].
Concluding Remarks/Summary
The BM-derived TME constitutes a relatively untapped resource of novel therapeutic targets (Fig. 14.1). A major goal is to target only the “tumor-educated” BM cells and spare normal counterparts so that side effects are drastically reduced. Consistent with this notion, the analysis of enriched stromal compartments derived from human breast cancer revealed gene expression changes associated with cancer progression [277]. Similar analyses have led to the identification of activated stromal transcriptomes and tumor-stroma crosstalk pathways in human [278] and mouse [279] lung cancer. Future studies encompassing genomic, epigenetic, and proteomic analyses have the potential to provide insights into mechanisms that govern activation, expansion, mobilization, and recruitment of specific subsets of BM cells to the tumor bed leading to tumor growth and metastasis.
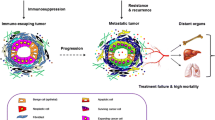
Key for Fig. 14.1

In the primary tumor , TAMs secrete VEGF and WNT7b, to promote angiogenesis, and MMPs and cathepsins, to mediate ECM degradation and tumor cell invasion. TANs secrete Bv8 and MMP9, promoting angiogenesis and ECM degradation. Myofibroblasts can also secrete MMPs and VEGF, contributing to angiogenesis. EPCs secrete angiogenic factors like VEGF and PDGF, generating a paracrine angiogenic signal, in addition to incorporating into nascent vessels. Furthermore, MDSCs suppress the activity of T cells and NK cells by secreting Arg1 and IDO, while TReg accomplishes immunosuppression by secreting IL-10 and TGFβ. There is a competition for glucose between tumor cells and T-cells. Immune checkpoint blockade is able to increase glycolysis in T-cells and elevate interferon-gamma production. Cancer stem cells (CSCs) recruit Tregs to the primary tumor via CCL5. A paracrine loop exists, whereby tumor cells secrete CSF1 to recruit macrophages, which in turn secrete EGF to promote tumor cell migration. Tumor cells, TAMs, and ECs establish the TMEM, where perivascular TAMs secrete VEGF, increasing local permeability and allowing tumor cell intravasation.
In the circulation , tumor cells activate platelet aggregation. Platelets protect tumor cells from shear stress and NK cell attack. Furthermore, platelets promote epithelial-to-mesenchymal transition (EMT) by secreting TGFβ. Platelets promote tumor cell adhesion to blood vessels at the secondary site via P-selectin. Moreover, tumors in circulation recruit neutrophils via IL-8. Neutrophils bridge tumor cells to blood vessels via neutrophil-expressed β2 integrin and tumor cell- and EC-expressed ICAM-1. Neutrophils also trap tumor cells in NETs.
In the metastatic organ , tumor cell-derived CCL2 recruits inflammatory monocytes, which differentiate into MAMs and in turn secrete VEGF to allow tumor cell extravasation. Macrophages also promote tumor cell survival via α4 integrin signaling to VCAM-1 on tumors cells. Tumor-derived factors generate a premetastatic niche, characterized by the recruitment and activation of myeloid cells in response to S100A8 and S100A9 chemokines and SAA3 inflammatory mediator. Myeloid cells are trapped in areas of crosslinked collagen. Increased presence of NETs at the secondary site promotes extravasation and cancer metastasis and can also lead to re-awakening of dormant cells to aggressive metastatic outgrowth. Furthermore, tumor-derived factors induce the secretion of fibronectin in fibroblasts, promoting the recruitment of HPCs via VLA-4. Recruited myeloid progenitor cells induce mesenchymal-to-epithelial transition (MET) via their secretion of versican, promoting metastatic outgrowth. Moreover, macrophages and neutrophils secrete angiogenic factors, and neutrophil serine proteases NE and CG degrade the antiangiogenic factor Tsp-1, enhancing metastatic outgrowth. Finally, recruited EPCs incorporate into the nascent tumor vasculature, and secrete angiogenic factors, inducing the angiogenic switch and contributing to macrometastasis formation.
Abbreviations: Arg1, arginase 1; BM, bone marrow; Bv8, Bombina variegata peptide 8; CCL2, chemokine (C-C motif) ligand 2; CCL5, chemokine (C-C motif) ligand 5; CG, cathepsin G; CSC, cancer stem cell; CSF1, colony-stimulating factor 1; ECM, extracellular matrix; EGF, epidermal growth factor; EPC, endothelial progenitor cell; HPC, hematopoietic progenitor cell; ICAM-1, intercellular adhesion molecule-1; IDO, indoleamine 2,3-dioxygenase; IL-8, interleukin-8; IL-10, interleukin-10; MAM, metastasis-associated macrophage; MDSC, myeloid-derived suppressor cell; MMP, matrix metalloprotease; NE, neutrophil elastase; NET, neutrophil extracellular trap; NK, natural killer; PDGF, platelet-derived growth factor; S100A8, S100 calcium binding protein A8; S100A9, S100 calcium binding protein A9; SAA3, serum amyloid A3; TAM, tumor-associated macrophage; TAN, tumor-associated neutrophil; TGFβ, transforming growth factor beta; TMEM, tumor microenvironment of metastasis; TReg, regulatory T cell; Tsp-1, thrombospondin-1; VCAM-1, vascular cell adhesion molecule-1; VEGF, vascular endothelial growth factor; VLA-4, very late antigen-4; WNT7b, wingless-type MMTV integration site family member 7b
References
Murdoch C, et al. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8(8):618–31.
Gao D, Mittal V. The role of bone-marrow-derived cells in tumor growth, metastasis initiation and progression. Trends Mol Med. 2009;15(8):333–43.
Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9(4):239–52.
Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–22.
Adams GB, et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439(7076):599–603.
Arai F, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118(2):149–61.
Hattori K, Heissig B, Rafii S. The regulation of hematopoietic stem cell and progenitor mobilization by chemokine SDF-1. Leuk Lymphoma. 2003;44(4):575–82.
Nilsson SK, et al. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005;106(4):1232–9.
Kollet O, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12(6):657–64.
Takafuji V, et al. An osteopontin fragment is essential for tumor cell invasion in hepatocellular carcinoma. Oncogene. 2007;26(44):6361–71.
McQuibban GA, et al. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem. 2001;276(47):43503–8.
Chantrain CF, et al. Bone marrow microenvironment and tumor progression. Cancer Microenviron. 2008;1(1):23–35.
Petit I, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3(7):687–94.
Allavena P, et al. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66(1):1–9.
Fujimoto H, et al. Stromal MCP-1 in mammary tumors induces tumor-associated macrophage infiltration and contributes to tumor progression. Int J Cancer. 2009;125(6):1276–84.
Priceman SJ, et al. Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of antiangiogenic therapy. Blood. 2010;115(7):1461–71.
Gao F, et al. Role of inflammation-associated microenvironment in tumorigenesis and metastasis. Curr Cancer Drug Targets. 2014;14(1):30–45.
Du R, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13(3):206–20.
Tacconi C, et al. Activation of the VEGFC/VEGFR3 pathway induces tumor immune escape in colorectal cancer. Cancer Res. 2019;79(16):4196–210.
Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61.
Steidl C, et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med. 2010;362(10):875–85.
DeNardo DG, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1(1):54–67.
Chung FT, et al. Tumor-associated macrophages correlate with response to epidermal growth factor receptor-tyrosine kinase inhibitors in advanced non-small cell lung cancer. Int J Cancer. 2012;131(3):E227–35.
Song M, et al. Tumor derived UBR5 promotes ovarian cancer growth and metastasis through inducing immunosuppressive macrophages. Nat Commun. 2020;11(1):6298.
Ganster RW, et al. Complex regulation of human inducible nitric oxide synthase gene transcription by Stat 1 and NF-kappa B. Proc Natl Acad Sci U S A. 2001;98(15):8638–43.
Krausgruber T, et al. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12(3):231–8.
Quatromoni JG, Eruslanov E. Tumor-associated macrophages: function, phenotype, and link to prognosis in human lung cancer. Am J Transl Res. 2012;4(4):376–89.
Redente EF, et al. Tumor progression stage and anatomical site regulate tumor-associated macrophage and bone marrow-derived monocyte polarization. Am J Pathol. 2010;176(6):2972–85.
Biswas SK, et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation). Blood. 2006;107(5):2112–22.
Wanderley CW, et al. Paclitaxel reduces tumor growth by reprogramming tumor-associated macrophages to an M1 profile in a TLR4-dependent manner. Cancer Res. 2018;78(20):5891–900.
Roumenina LT, et al. Tumor cells hijack macrophage-produced complement C1q to promote tumor growth. Cancer Immunol Res. 2019;7(7):1091–105.
Joyce JA, et al. Cathepsin cysteine proteases are effectors of invasive growth and angiogenesis during multistage tumorigenesis. Cancer Cell. 2004;5(5):443–53.
Gocheva V, et al. Distinct roles for cysteine cathepsin genes in multistage tumorigenesis. Genes Dev. 2006;20(5):543–56.
Gocheva V, et al. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev. 2010;24(3):241–55.
Lin EY, et al. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193(6):727–40.
Lin EY, et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66(23):11238–46.
Lin EY, Pollard JW. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 2007;67(11):5064–6.
Zabuawala T, et al. An ets2-driven transcriptional program in tumor-associated macrophages promotes tumor metastasis. Cancer Res. 2010;70(4):1323–33.
Yeo EJ, et al. Myeloid WNT7b mediates the angiogenic switch and metastasis in breast cancer. Cancer Res. 2014;74(11):2962–73.
Mazzieri R, et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell. 2011;19(4):512–26.
Wang R, et al. Tumor cells induce LAMP2a expression in tumor-associated macrophage for cancer progression. EBioMedicine. 2019;40:118–34.
Kolb R, et al. Obesity-associated inflammation promotes angiogenesis and breast cancer via angiopoietin-like 4. Oncogene. 2019;38(13):2351–63.
Jing W, et al. Breast cancer cells promote CD169(+) macrophage-associated immunosuppression through JAK2-mediated PD-L1 upregulation on macrophages. Int Immunopharmacol. 2020;78:106012.
Binnemars-Postma K, et al. Targeting the Stat6 pathway in tumor-associated macrophages reduces tumor growth and metastatic niche formation in breast cancer. FASEB J. 2018;32(2):969–78.
Muller S, et al. Single-cell profiling of human gliomas reveals macrophage ontogeny as a basis for regional differences in macrophage activation in the tumor microenvironment. Genome Biol. 2017;18(1):234.
DeNardo DG, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16(2):91–102.
Chong H, et al. Immunocytochemical localization of latent transforming growth factor-beta1 activation by stimulated macrophages. J Cell Physiol. 1999;178(3):275–83.
Thomas DA, Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8(5):369–80.
Kuang DM, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206(6):1327–37.
Rodriguez PC, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64(16):5839–49.
Liu L, et al. Consumption of the fish oil high-fat diet uncouples obesity and mammary tumor growth through induction of reactive oxygen species in protumor macrophages. Cancer Res. 2020;80(12):2564–74.
Cassetta L, et al. Human tumor-associated macrophage and monocyte transcriptional landscapes reveal cancer-specific reprogramming, biomarkers, and therapeutic targets. Cancer Cell. 2019;35(4):588–602 e10.
Zhu Y, et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74(18):5057–69.
Su S, et al. CD10(+)GPR77(+) cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell. 2018;172(4):841–856 e16.
Zhou Y, et al. Single-cell multiomics sequencing reveals prevalent genomic alterations in tumor stromal cells of human colorectal cancer. Cancer Cell. 2020;38(6):818–828 e5.
Kumar V, et al. Cancer-associated fibroblasts neutralize the anti-tumor effect of CSF1 receptor blockade by inducing PMN-MDSC infiltration of tumors. Cancer Cell. 2017;32(5):654–668 e5.
Vennin C, et al. CAF hierarchy driven by pancreatic cancer cell p53-status creates a pro-metastatic and chemoresistant environment via perlecan. Nat Commun. 2019;10(1):3637.
Ostrand-Rosenberg S. Immune surveillance: a balance between protumor and antitumor immunity. Curr Opin Genet Dev. 2008;18(1):11–8.
Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–74.
Sinha P, et al. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181(7):4666–75.
Marigo I, et al. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–79.
Yu J, et al. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol. 2013;190(7):3783–97.
Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol. 2003;24(5):242–8.
Yu J, et al. Noncanonical NF-kappaB activation mediates STAT3-stimulated IDO upregulation in myeloid-derived suppressor cells in breast cancer. J Immunol. 2014;193(5):2574–86.
Holmgaard RB, et al. Tumor-expressed IDO recruits and activates MDSCs in a Treg-dependent manner. Cell Rep. 2015;13(2):412–24.
Das S, et al. Tumor cell-derived IL1beta promotes desmoplasia and immune suppression in pancreatic cancer. Cancer Res. 2020;80(5):1088–101.
Chen Y, et al. Type I collagen deletion in alphaSMA(+) myofibroblasts augments immune suppression and accelerates progression of pancreatic cancer. Cancer Cell. 2021;39(4):548–565 e6.
Trillo-Tinoco J, et al. AMPK alpha-1 intrinsically regulates the function and differentiation of tumor myeloid-derived suppressor cells. Cancer Res. 2019;79(19):5034–47.
Klemm F, et al. Interrogation of the microenvironmental landscape in brain tumors reveals disease-specific alterations of immune cells. Cell. 2020;181(7):1643–1660 e17.
Chang CH, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162(6):1229–41.
Kaymak I, et al. Immunometabolic interplay in the tumor microenvironment. Cancer Cell. 2021;39(1):28–37.
Pekarek LA, et al. Inhibition of tumor growth by elimination of granulocytes. J Exp Med. 1995;181(1):435–40.
Shojaei F, et al. Role of Bv8 in neutrophil-dependent angiogenesis in a transgenic model of cancer progression. Proc Natl Acad Sci U S A. 2008;105(7):2640–5.
Youn JI, et al. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181(8):5791–802.
Shojaei F, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;25(8):911–20.
Shojaei F, et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature. 2007;450(7171):825–31.
Yang L, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6(4):409–21.
Fridlender ZG, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16(3):183–94.
Perego M, et al. Reactivation of dormant tumor cells by modified lipids derived from stress-activated neutrophils. Sci Transl Med. 2020;12(572)
Wculek SK, et al. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020;20(1):7–24.
Ma Y, et al. Dendritic cells in the cancer microenvironment. J Cancer. 2013;4(1):36–44.
Ray A, et al. Preclinical validation of Alpha-Enolase (ENO1) as a novel immunometabolic target in multiple myeloma. Oncogene. 2020;39(13):2786–96.
Cubillos-Ruiz JR, et al. ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell. 2015;161(7):1527–38.
Laoui D, et al. The tumour microenvironment harbours ontogenically distinct dendritic cell populations with opposing effects on tumour immunity. Nat Commun. 2016;7:13720.
Lyden D, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7(11):1194–201.
Peters BA, et al. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nat Med. 2005;11(3):261–2.
Nolan DJ, et al. Bone marrow-derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization. Genes Dev. 2007;21(12):1546–58.
Gao D, et al. Bone marrow-derived endothelial progenitor cells contribute to the angiogenic switch in tumor growth and metastatic progression. Biochim Biophys Acta. 2009;1796(1):33–40.
Kerbel RS, et al. Endothelial progenitor cells are cellular hubs essential for neoangiogenesis of certain aggressive adenocarcinomas and metastatic transition but not adenomas. Proc Natl Acad Sci U S A. 2008;105(34):E54. author reply E55
Gao D, et al. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319(5860):195–8.
Mellick AS, et al. Using the transcription factor inhibitor of DNA binding 1 to selectively target endothelial progenitor cells offers novel strategies to inhibit tumor angiogenesis and growth. Cancer Res. 2010;70(18):7273–82.
Shaked Y, et al. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science. 2006;313(5794):1785–7.
Shaked Y, et al. Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell. 2008;14(3):263–73.
Purhonen S, et al. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci U S A. 2008;105(18):6620–5.
Burchfield JS, Dimmeler S. Role of paracrine factors in stem and progenitor cell mediated cardiac repair and tissue fibrosis. Fibrogenesis Tissue Repair. 2008;1(1):4.
Stockmann C, et al. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature. 2008;456(7223):814–8.
Lee S, et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130(4):691–703.
Direkze NC, et al. Multiple organ engraftment by bone-marrow-derived myofibroblasts and fibroblasts in bone-marrow-transplanted mice. Stem Cells. 2003;21(5):514–20.
Direkze NC, et al. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res. 2004;64(23):8492–5.
Vong S, Kalluri R. The role of stromal myofibroblast and extracellular matrix in tumor angiogenesis. Genes Cancer. 2011;2(12):1139–45.
Rajantie I, et al. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood. 2004;104(7):2084–6.
Song S, et al. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7(9):870–9.
Jodele S, et al. The contribution of bone marrow-derived cells to the tumor vasculature in neuroblastoma is matrix metalloproteinase-9 dependent. Cancer Res. 2005;65(8):3200–8.
Lamagna C, Bergers G. The bone marrow constitutes a reservoir of pericyte progenitors. J Leukoc Biol. 2006;80(4):677–81.
Wyckoff J, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64(19):7022–9.
Goswami S, et al. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005;65(12):5278–83.
Ishihara D, et al. Wiskott-Aldrich syndrome protein regulates leukocyte-dependent breast cancer metastasis. Cell Rep. 2013;4(3):429–36.
Hernandez L, et al. The EGF/CSF-1 paracrine invasion loop can be triggered by heregulin beta1 and CXCL12. Cancer Res. 2009;69(7):3221–7.
Linde N, et al. Macrophages orchestrate breast cancer early dissemination and metastasis. Nat Commun. 2018;9(1):21.
Gertler F, Condeelis J. Metastasis: tumor cells becoming MENAcing. Trends Cell Biol. 2011;21(2):81–90.
Roussos ET, et al. Mena invasive (MenaINV) promotes multicellular streaming motility and transendothelial migration in a mouse model of breast cancer. J Cell Sci. 2011;124(Pt 13):2120–31.
Harney AS, et al. Real-time imaging reveals local, transient vascular permeability, and tumor cell intravasation stimulated by TIE2hi macrophage-derived VEGFA. Cancer Discov. 2015;5(9):932–43.
Wyckoff JB, et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67(6):2649–56.
Roh-Johnson M, et al. Macrophage contact induces RhoA GTPase signaling to trigger tumor cell intravasation. Oncogene. 2014;33(33):4203–12.
Liu CY, et al. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab Invest. 2013;93(7):844–54.
Georgouli M, et al. Regional activation of myosin II in cancer cells drives tumor progression via a secretory cross-talk with the immune microenvironment. Cell. 2019;176(4):757–774 e23.
Marigo I, et al. Disabled homolog 2 controls prometastatic activity of tumor-associated macrophages. Cancer Discov. 2020;10(11):1758–73.
Yang F, et al. Interaction with CD68 and regulation of GAS6 expression by endosialin in fibroblasts drives recruitment and polarization of macrophages in hepatocellular carcinoma. Cancer Res. 2020;80(18):3892–905.
Bekes EM, et al. Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am J Pathol. 2011;179(3):1455–70.
Zhou SL, et al. CXCL5 contributes to tumor metastasis and recurrence of intrahepatic cholangiocarcinoma by recruiting infiltrative intratumoral neutrophils. Carcinogenesis. 2014;35(3):597–605.
Sharma B, et al. Host Cxcr2-dependent regulation of mammary tumor growth and metastasis. Clin Exp Metastasis. 2015;32(1):65–72.
Yang L, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13(1):23–35.
Kitamura T, et al. SMAD4-deficient intestinal tumors recruit CCR1+ myeloid cells that promote invasion. Nat Genet. 2007;39(4):467–75.
Ogawa R, et al. Loss of SMAD4 promotes colorectal cancer progression by recruiting tumor-associated neutrophils via the CXCL1/8-CXCR2 axis. Clin Cancer Res. 2019;25(9):2887–99.
Karnoub AE, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557–63.
Ma Z, et al. Bone Marrow mesenchymal stromal cell-derived periostin promotes B-ALL progression by modulating CCL2 in leukemia cells. Cell Rep. 2019;26(6):1533–1543 e4.
Lecomte J, et al. Bone marrow-derived myofibroblasts are the providers of pro-invasive matrix metalloproteinase 13 in primary tumor. Neoplasia. 2012;14(10):943–51.
Maheswaran S, Haber DA. Circulating tumor cells: a window into cancer biology and metastasis. Curr Opin Genet Dev. 2010;20(1):96–9.
Reymond N, d’Agua BB, Ridley AJ. Crossing the endothelial barrier during metastasis. Nat Rev Cancer. 2013;13(12):858–70.
Palumbo JS, et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105(1):178–85.
Palumbo JS, et al. Tumor cell-associated tissue factor and circulating hemostatic factors cooperate to increase metastatic potential through natural killer cell-dependent and-independent mechanisms. Blood. 2007;110(1):133–41.
Camerer E, et al. Platelets, protease-activated receptors, and fibrinogen in hematogenous metastasis. Blood. 2004;104(2):397–401.
Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576–90.
Zhang Y, et al. Platelet-specific PDGFB ablation impairs tumor vessel integrity and promotes metastasis. Cancer Res. 2020;80(16):3345–58.
Ferjancic S, et al. VCAM-1 and VAP-1 recruit myeloid cells that promote pulmonary metastasis in mice. Blood. 2013;121(16):3289–97.
Chen Q, Zhang XH, Massague J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell. 2011;20(4):538–49.
Wei C, et al. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol Cancer. 2019;18(1):64.
Sanchez-Gonzalez I, et al. miR-149 suppresses breast cancer metastasis by blocking paracrine interactions with macrophages. Cancer Res. 2020;80(6):1330–41.
Viguier M, et al. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173(2):1444–53.
Curiel TJ, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–9.
You Y, et al. Ovarian cancer stem cells promote tumour immune privilege and invasion via CCL5 and regulatory T cells. Clin Exp Immunol. 2018;191(1):60–73.
Lee JC, et al. Regulatory T cell control of systemic immunity and immunotherapy response in liver metastasis. Sci Immunol. 2020;5(52)
Huh SJ, et al. Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res. 2010;70(14):6071–82.
Spicer JD, et al. Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Res. 2012;72(16):3919–27.
Cools-Lartigue J, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013;123(8):3446–58.
Rayes RF, et al. Primary tumors induce neutrophil extracellular traps with targetable metastasis promoting effects. JCI Insight. 2019;5
Szczerba BM, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 2019;566(7745):553–7.
Coupland LA, Chong BH, Parish CR. Platelets and P-selectin control tumor cell metastasis in an organ-specific manner and independently of NK cells. Cancer Res. 2012;72(18):4662–71.
Irimura T, et al. Colorectal cancer metastasis determined by carbohydrate-mediated cell adhesion: role of sialyl-LeX antigens. Semin Cancer Biol. 1993;4(5):319–24.
Insug O, et al. Role of SA-Le(a) and E-selectin in metastasis assessed with peptide antagonist. Peptides. 2002;23(5):999–1010.
Zipin A, et al. Tumor-microenvironment interactions: the fucose-generating FX enzyme controls adhesive properties of colorectal cancer cells. Cancer Res. 2004;64(18):6571–8.
Qian B, et al. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One. 2009;4(8):e6562.
Qian BZ, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–5.
Zhao L, et al. Recruitment of a myeloid cell subset (CD11b/Gr1 mid) via CCL2/CCR2 promotes the development of colorectal cancer liver metastasis. Hepatology. 2013;57(2):829–39.
Ward Y, et al. Platelets promote metastasis via binding tumor CD97 leading to bidirectional signaling that coordinates transendothelial migration. Cell Rep. 2018;23(3):808–22.
Mammadova-Bach E, et al. Platelet glycoprotein VI promotes metastasis through interaction with cancer cell-derived galectin-3. Blood. 2020;135(14):1146–60.
Xiong G, et al. Hsp47 promotes cancer metastasis by enhancing collagen-dependent cancer cell-platelet interaction. Proc Natl Acad Sci U S A. 2020;117(7):3748–58.
Peinado H, et al. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer. 2017;17(5):302–17.
Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3(6):453–8.
Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9(4):285–93.
Kaplan RN, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820–7.
Hiratsuka S, et al. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8(12):1369–75.
Hiratsuka S, et al. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat Cell Biol. 2008;10(11):1349–55.
Hiratsuka S, et al. Primary tumours modulate innate immune signalling to create pre-metastatic vascular hyperpermeability foci. Nat Commun. 2013;4:1853.
Qiu M, et al. Modulation of intestinal microbiota by glycyrrhizic acid prevents high-fat diet-enhanced pre-metastatic niche formation and metastasis. Mucosal Immunol. 2019;12(4):945–57.
Hiratsuka S, et al. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell. 2002;2(4):289–300.
Bergers G, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2(10):737–44.
Heissig B, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109(5):625–37.
Aksenenko MB, et al. miR-155 overexpression is followed by downregulation of its target gene, NFE2L2, and altered pattern of VEGFA expression in the liver of melanoma B16-bearing mice at the premetastatic stage. Int J Exp Pathol. 2019;100(5-6):311–9.
Li R, et al. Primary tumor-secreted VEGF induces vascular hyperpermeability in premetastatic lung via the occludin phosphorylation/ubiquitination pathway. Mol Carcinog. 2019;58(12):2316–26.
Yang F, et al. Inhibition of dipeptidyl peptidase-4 accelerates epithelial-mesenchymal transition and breast cancer metastasis via the CXCL12/CXCR4/mTOR axis. Cancer Res. 2019;79(4):735–46.
Sceneay J, et al. Primary tumor hypoxia recruits CD11b+/Ly6Cmed/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res. 2012;72(16):3906–11.
Lu Z, et al. Epigenetic therapy inhibits metastases by disrupting premetastatic niches. Nature. 2020;579(7798):284–90.
Chen H, et al. Chronic psychological stress promotes lung metastatic colonization of circulating breast cancer cells by decorating a pre-metastatic niche through activating beta-adrenergic signaling. J Pathol. 2018;244(1):49–60.
Kaczanowska S, et al. Genetically engineered myeloid cells rebalance the core immune suppression program in metastasis. Cell. 2021;184(8):2033–2052 e21.
Erler JT, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15(1):35–44.
Cox TR, et al. LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res. 2013;73(6):1721–32.
Cox TR, Erler JT. Molecular pathways: connecting fibrosis and solid tumor metastasis. Clin Cancer Res. 2014;20(14):3637–43.
Cox TR, et al. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature. 2015;522(7554):106–10.
Said N, et al. RhoGDI2 suppresses lung metastasis in mice by reducing tumor versican expression and macrophage infiltration. J Clin Invest. 2012;122(4):1503–18.
Salem M, et al. miR-590-3p promotes ovarian cancer growth and metastasis via a novel FOXA2-versican pathway. Cancer Res. 2018;78(15):4175–90.
Zhangyuan G, et al. VersicanV1 promotes proliferation and metastasis of hepatocellular carcinoma through the activation of EGFR-PI3K-AKT pathway. Oncogene. 2020;39(6):1213–30.
van Deventer HW, et al. Circulating fibrocytes prepare the lung for cancer metastasis by recruiting Ly-6C+ monocytes via CCL2. J Immunol. 2013;190(9):4861–7.
Gil-Bernabe AM, et al. Recruitment of monocytes/macrophages by tissue factor-mediated coagulation is essential for metastatic cell survival and premetastatic niche establishment in mice. Blood. 2012;119(13):3164–75.
Catena R, et al. Bone marrow-derived Gr1+ cells can generate a metastasis-resistant microenvironment via induced secretion of thrombospondin-1. Cancer Discov. 2013;3(5):578–89.
Keklikoglou I, et al. Chemotherapy elicits pro-metastatic extracellular vesicles in breast cancer models. Nat Cell Biol. 2019;21(2):190–202.
Kowanetz M, et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci U S A. 2010;107(50):21248–55.
Granot Z, et al. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell. 2011;20(3):300–14.
Wang Z, et al. CD62L(dim) neutrophils specifically migrate to the lung and participate in the formation of the pre-metastatic niche of breast cancer. Front Oncol. 2020;10:540484.
Patel S, et al. Unique pattern of neutrophil migration and function during tumor progression. Nat Immunol. 2018;19(11):1236–47.
Charan M, et al. Tumor secreted ANGPTL2 facilitates recruitment of neutrophils to the lung to promote lung pre-metastatic niche formation and targeting ANGPTL2 signaling affects metastatic disease. Oncotarget. 2020;11(5):510–22.
Moresco MA, et al. Enzymatic inactivation of oxysterols in breast tumor cells constraints metastasis formation by reprogramming the metastatic lung microenvironment. Front Immunol. 2018;9:2251.
Tyagi A, et al. Nicotine promotes breast cancer metastasis by stimulating N2 neutrophils and generating pre-metastatic niche in lung. Nat Commun. 2021;12(1):474.
Monteiro AC, et al. T cells induce pre-metastatic osteolytic disease and help bone metastases establishment in a mouse model of metastatic breast cancer. PLoS One. 2013;8(7):e68171.
Olkhanud PB, et al. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res. 2009;69(14):5996–6004.
Li YL, et al. Single-cell analysis reveals immune modulation and metabolic switch in tumor-draining lymph nodes. Oncoimmunology. 2020;9(1):1830513.
McAllister SS, Weinberg RA. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat Cell Biol. 2014;16(8):717–27.
Peinado H, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883–91.
Dai J, et al. Primary prostate cancer educates bone stroma through exosomal pyruvate kinase M2 to promote bone metastasis. J Exp Med. 2019;216(12):2883–99.
Kang Y, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3(6):537–49.
Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9(4):274–84.
Obenauf AC, Massague J. Surviving at a distance: organ-specific metastasis. Trends Cancer. 2015;1(1):76–91.
Muller A, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–6.
Li YM, et al. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell. 2004;6(5):459–69.
Marchesi F, et al. The chemokine receptor CX3CR1 is involved in the neural tropism and malignant behavior of pancreatic ductal adenocarcinoma. Cancer Res. 2008;68(21):9060–9.
Wang H, et al. Tumor cell alpha3beta1 integrin and vascular laminin-5 mediate pulmonary arrest and metastasis. J Cell Biol. 2004;164(6):935–41.
Kim S, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457(7225):102–6.
Medeiros B, et al. Triple-negative primary breast tumors induce supportive premetastatic changes in the extracellular matrix and soluble components of the lung microenvironment. Cancers (Basel). 2020;12(1)
Klotz R, et al. Circulating tumor cells exhibit metastatic tropism and reveal brain metastasis drivers. Cancer Discov. 2020;10(1):86–103.
Hebert JD, et al. Proteomic profiling of the ECM of xenograft breast cancer metastases in different organs reveals distinct metastatic niches. Cancer Res. 2020;80(7):1475–85.
Martinez-Ordonez A, et al. Breast cancer metastasis to liver and lung is facilitated by Pit-1-CXCL12-CXCR4 axis. Oncogene. 2018;37(11):1430–44.
Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2(8):584–93.
Jones DH, et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. 2006;440(7084):692–6.
Sohara Y, Shimada H, DeClerck YA. Mechanisms of bone invasion and metastasis in human neuroblastoma. Cancer Lett. 2005;228(1-2):203–9.
Ara T, et al. Interleukin-6 in the bone marrow microenvironment promotes the growth and survival of neuroblastoma cells. Cancer Res. 2009;69(1):329–37.
Hoshino A, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–35.
Mattiola I, et al. The macrophage tetraspan MS4A4A enhances dectin-1-dependent NK cell-mediated resistance to metastasis. Nat Immunol. 2019;20(8):1012–22.
El Rayes T, et al. Lung inflammation promotes metastasis through neutrophil protease-mediated degradation of Tsp-1. Proc Natl Acad Sci U S A. 2015;112(52):16000–5.
Wculek SK, Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature. 2015;528(7582):413–7.
Gao D, et al. Myeloid progenitor cells in the premetastatic lung promote metastases by inducing mesenchymal to epithelial transition. Cancer Res. 2012;72(6):1384–94.
Bosiljcic M, et al. Targeting myeloid-derived suppressor cells in combination with primary mammary tumor resection reduces metastatic growth in the lungs. Breast Cancer Res. 2019;21(1):103.
Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16(7):431–46.
Kos K, de Visser KE. Neutrophils create a fertile soil for metastasis. Cancer Cell. 2021;39(3):301–3.
Nemeth T, Sperandio M, Mocsai A. Neutrophils as emerging therapeutic targets. Nat Rev Drug Discov. 2020;19(4):253–75.
Quail DF, et al. Obesity alters the lung myeloid cell landscape to enhance breast cancer metastasis through IL5 and GM-CSF. Nat Cell Biol. 2017;19(8):974–87.
Xiao Y, et al. Cathepsin C promotes breast cancer lung metastasis by modulating neutrophil infiltration and neutrophil extracellular trap formation. Cancer Cell. 2021;39(3):423–437 e7.
Yang L, et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature. 2020;583(7814):133–8.
Teijeira A, et al. CXCR1 and CXCR2 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune cytotoxicity. Immunity. 2020;52(5):856–871 e8.
Li P, et al. Lung mesenchymal cells elicit lipid storage in neutrophils that fuel breast cancer lung metastasis. Nat Immunol. 2020;21(11):1444–55.
McDowell SAC, et al. Neutrophil oxidative stress mediates obesity-associated vascular dysfunction and metastatic transmigration. Nat Cancer. 2021;2:545–62.
Albrengues J, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361(6409)
Malanchi I, et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2011;481(7379):85–9.
Wang Z, Ouyang G. Periostin: a bridge between cancer stem cells and their metastatic niche. Cell Stem Cell. 2012;10(2):111–2.
Coutu DL, et al. Periostin, a member of a novel family of vitamin K-dependent proteins, is expressed by mesenchymal stromal cells. J Biol Chem. 2008;283(26):17991–8001.
Pelon F, et al. Cancer-associated fibroblast heterogeneity in axillary lymph nodes drives metastases in breast cancer through complementary mechanisms. Nat Commun. 2020;11(1):404.
Ghajar CM, et al. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol. 2013;15(7):807–17.
Sanford DE, et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res. 2013;19(13):3404–15.
Tomita M, et al. Preoperative neutrophil to lymphocyte ratio as a prognostic predictor after curative resection for non-small cell lung cancer. Anticancer Res. 2011;31(9):2995–8.
Robinson BD, et al. Tumor microenvironment of metastasis in human breast carcinoma: a potential prognostic marker linked to hematogenous dissemination. Clin Cancer Res. 2009;15(7):2433–41.
Rohan TE, et al. Tumor microenvironment of metastasis and risk of distant metastasis of breast cancer. J Natl Cancer Inst. 2014;106(8)
Li R, et al. Gut microbiota-stimulated cathepsin K secretion mediates TLR4-dependent M2 macrophage polarization and promotes tumor metastasis in colorectal cancer. Cell Death Differ. 2019;26(11):2447–63.
Hoves S, et al. Rapid activation of tumor-associated macrophages boosts preexisting tumor immunity. J Exp Med. 2018;215(3):859–76.
Akkari L, et al. Dynamic changes in glioma macrophage populations after radiotherapy reveal CSF-1R inhibition as a strategy to overcome resistance. Sci Transl Med. 2020;12(552)
Kowal J, Kornete M, Joyce JA. Re-education of macrophages as a therapeutic strategy in cancer. Immunotherapy. 2019;11(8):677–89.
Yan D, et al. Inhibition of colony stimulating factor-1 receptor abrogates microenvironment-mediated therapeutic resistance in gliomas. Oncogene. 2017;36(43):6049–58.
Welsh TJ, et al. Macrophage and mast-cell invasion of tumor cell islets confers a marked survival advantage in non-small-cell lung cancer. J Clin Oncol. 2005;23(35):8959–67.
Kim DW, et al. High tumour islet macrophage infiltration correlates with improved patient survival but not with EGFR mutations, gene copy number or protein expression in resected non-small cell lung cancer. Br J Cancer. 2008;98(6):1118–24.
Dai F, et al. The number and microlocalization of tumor-associated immune cells are associated with patient’s survival time in non-small cell lung cancer. BMC Cancer. 2010;10:220.
Ohri CM, et al. Macrophages within NSCLC tumour islets are predominantly of a cytotoxic M1 phenotype associated with extended survival. Eur Respir J. 2009;33(1):118–26.
Takanami I, Takeuchi K, Kodaira S. Tumor-associated macrophage infiltration in pulmonary adenocarcinoma: association with angiogenesis and poor prognosis. Oncology. 1999;57(2):138–42.
Chen JJ, et al. Up-regulation of tumor interleukin-8 expression by infiltrating macrophages: its correlation with tumor angiogenesis and patient survival in non-small cell lung cancer. Clin Cancer Res. 2003;9(2):729–37.
Ohtaki Y, et al. Stromal macrophage expressing CD204 is associated with tumor aggressiveness in lung adenocarcinoma. J Thorac Oncol. 2010;5(10):1507–15.
Zhang B, et al. M2-polarized tumor-associated macrophages are associated with poor prognoses resulting from accelerated lymphangiogenesis in lung adenocarcinoma. Clinics (Sao Paulo). 2011;66(11):1879–86.
Wang R, et al. Increased IL-10 mRNA expression in tumor-associated macrophage correlated with late stage of lung cancer. J Exp Clin Cancer Res. 2011;30:62.
Zeni E, et al. Macrophage expression of interleukin-10 is a prognostic factor in nonsmall cell lung cancer. Eur Respir J. 2007;30(4):627–32.
Wang R, et al. Tumor-associated macrophages provide a suitable microenvironment for non-small lung cancer invasion and progression. Lung Cancer. 2011;74(2):188–96.
Loyher PL, et al. Macrophages of distinct origins contribute to tumor development in the lung. J Exp Med. 2018;215(10):2536–53.
Katholnig K, et al. Inactivation of mTORC2 in macrophages is a signature of colorectal cancer that promotes tumorigenesis. JCI Insight. 2019;4(20)
Bergamaschi C, et al. Heterodimeric IL-15 delays tumor growth and promotes intratumoral CTL and dendritic cell accumulation by a cytokine network involving XCL1, IFN-gamma, CXCL9 and CXCL10. J Immunother Cancer. 2020;8(1)
Schmittnaegel M, et al. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci Transl Med. 2017;9(385)
Nagaraj S, et al. Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin Cancer Res. 2010;16(6):1812–23.
Huang A, et al. Increased CD14(+)HLA-DR (-/low) myeloid-derived suppressor cells correlate with extrathoracic metastasis and poor response to chemotherapy in non-small cell lung cancer patients. Cancer Immunol Immunother. 2013;62(9):1439–51.
Zhang B, et al. Circulating and tumor-infiltrating myeloid-derived suppressor cells in patients with colorectal carcinoma. PLoS One. 2013;8(2):e57114.
Meyer C, et al. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol Immunother. 2014;63(3):247–57.
Raychaudhuri B, et al. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro Oncol. 2011;13(6):591–9.
Friebel E, et al. Single-cell mapping of human brain cancer reveals tumor-specific instruction of tissue-invading leukocytes. Cell. 2020;181(7):1626–1642 e20.
Najjar YG, Finke JH. Clinical perspectives on targeting of myeloid derived suppressor cells in the treatment of cancer. Front Oncol. 2013;3:49.
Gabrilovich DI, et al. Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. J Immunol. 2001;166(9):5398–406.
Mirza N, et al. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66(18):9299–307.
Ko JS, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15(6):2148–57.
Ko JS, et al. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res. 2010;70(9):3526–36.
Friberg M, et al. Indoleamine 2,3-dioxygenase contributes to tumor cell evasion of T cell-mediated rejection. Int J Cancer. 2002;101(2):151–5.
Muller AJ, et al. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11(3):312–9.
Gu T, et al. Central role of IFNgamma-indoleamine 2,3-dioxygenase axis in regulation of interleukin-12-mediated antitumor immunity. Cancer Res. 2010;70(1):129–38.
Holmgaard RB, et al. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med. 2013;210(7):1389–402.
De Henau O, et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kgamma in myeloid cells. Nature. 2016;539(7629):443–7.
Allinen M, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6(1):17–32.
Durrans A, et al. Identification of reprogrammed myeloid cell transcriptomes in NSCLC. PLoS One. 2015;10(6):e0129123.
Choi H, et al. Transcriptome analysis of individual stromal cell populations identifies stroma-tumor crosstalk in mouse lung cancer model. Cell Rep. 2015;10(7):1187–201.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ramchandani, D. et al. (2022). Regulation of Tumor Progression and Metastasis by Bone Marrow-Derived Microenvironments. In: Akslen, L.A., Watnick, R.S. (eds) Biomarkers of the Tumor Microenvironment. Springer, Cham. https://doi.org/10.1007/978-3-030-98950-7_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-98950-7_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-98949-1
Online ISBN: 978-3-030-98950-7
eBook Packages: MedicineMedicine (R0)