Abstract
The rapidly growing understanding of the Omura’s whale (Balaenoptera omurai) is an example of how the drive to explore and discover has been enhanced by advances in technology, particularly in bioacoustics. This combination of inspiration and technology has allowed exploration of topics that had been out of reach for many scientists. The Omura’s whale was first described in 2003 from a handful of specimens, and for a decade remained an enigmatic “ghost” species without confirmation of live sightings, let alone any understanding of its ecology. After the chance discovery of a population off northwest Madagascar, the stage was set for the exploration of a new species. In five short years, through the application of recent advancements in bioacoustics, molecular genetics, and satellite telemetry, we may have a better understanding of the Omura’s whale (or at least one of its populations) than of many other cetaceans. In this chapter, I describe new information gathered on Omura’s whales off Madagascar during 2013–2017, with a focus on its singing behavior. Combined with growing information from other sites around the world, I make inferences about the species’ behavioral patterns and describe the potential for innovative research that could be conducted on this enigmatic species.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Omura’s whale
- Balaenoptera omurai
- Balaenopteridae
- Passive acoustic monitoring
- Spatiotemporal distribution
- Song
- Singing behavior
- Geographic variation
- Breeding ecology

(Photo credit and ©, Salvatore Cerchio)
An Omura’s whale mother with her calf near Banc du Goliath, north of Nosy Be, Madagascar, in November 2018. This female was first seen on Nosy Be without a calf in November 2015. Drone photo collected under research permit 266/18/MEEF/SG/DGF/DSAP/SCB issued by la Direction Générale de l'Environnement et des Forêts, and la Direction de la Gestion des Ressources Naturelles Renouvelables et des Écosystèmes, Madagascar.
1 Introduction
During February and March 1984, the r/v Regina Maris set anchor each day on Silver Bank, Dominican Republic, in the fading light of the late afternoon. As a young student under the tutelage of Ken Balcomb, I took that cue to hang a small hydrophone from the course yard of the foremast, and plug it into the latest advancement in commercially available acoustic recording technology, a Sony Walkman Pro. Through the night, I lay on the deck of the Regina, listening to the cacophony of a dense chorus of humpback whale singers, waiting for a single singer to come into the foreground, and then push the REC button to record his song onto a 45 m cassette audio tape. Since portable computers had yet to be invented, and digital signal processing software was not yet accessible to non-specialists, analysis of these recordings started with hand notation taught to a few students like me by Katy Payne, to scroll the sequences of song phrases onto a piece of lined engineering paper. Later at a farmhouse lab in Lincoln, Massachusetts, I followed a laborious process to create hardcopy spectrograms on long strips of 35 mm photographic paper, from which I, and other students and researchers, would qualitatively define the structure of the songs for each individual and make quantitative measurements of duration and frequency using a metric ruler. Archaic as these practices seem today, they represented remarkable innovations in the 1980s, leaps in technology from previous decades that facilitated new research outside the walls of exclusive, highly funded labs. These allowed young students like me to take part in rapidly progressing research that was laying down the foundations of what we know about cetacean bioacoustics. This was fertile ground for the city-boy that I was, who had been obsessed with nature since a child, and was mesmerized by the sounds of humpback whales when first played off a flexible little vinyl record pulled from an issue of National Geographic. My natural curiosity and penchant for discovery were ignited by these experiences on the Regina Maris and in the Paynes’ lab. And so, it was this combination of the romance of the field and the sheer exhilaration of discovery that captured my soul and set me on my career’s path. That path would ultimately lead me to a humbling discovery and the rare opportunity to explore and describe the behavior of an enigmatic species of baleen whale, the Omura’s whale, which in turn would be enhanced by a new wave of technological advancements.
2 Background
Discovery and leaps in technology progress together and feed into each other in a self-reinforcing loop, continuously advancing our understanding of the natural world. Perhaps nowhere has this been as evident in the past two decades, as with the discovery of a new species of baleen whale, an animal the size of a bus, which for centuries had been overlooked in the tropical waters of mostly remote exotic regions. The Omura's whale (Balaenoptera omurai) was only described and established as a distinct species in 2003, historically misidentified as the “small form” of the Bryde's whale (Balaenoptera edeni), when it was first encountered during Japanese scientific whaling operations in the late 1970s (Ohsumi 1980; Wada et al. 2003). In fact, it was not even part of, nor the nearest relative to the Bryde’s whale complex (which currently includes Bryde’s whale, Balaenoptera brydei; Eden’s whale, B. edeni; and most recently Rice’s whale, Balaenoptera ricei). Early research on the Omura’s whale established that it is an ancient lineage, phylogenetically distinct with a divergence time of ca. 17 million years ago, and basal to a large clade containing the three species of the Bryde’s whale complex and the sei whale (Balaenoptera borealis) (Sasaki et al. 2006). This was not the simple splitting of two closely related groups that had recently diverged, which has become quite common with advances in phylogenetic research (such as is the case with Rice’s whale, Rosel et al. 2021), but a novel discovery in the spirit of nineteenth century exploration. Until 2013, the species remained an enigma, known only from a handful of historic whaling and stranding specimens from the western Pacific and eastern Indian Oceans (Wada et al. 2003, Yamada et al. 2006a, b, 2008; reviewed in Cerchio et al. 2019). With no confirmed observations or detailed ecological studies in its natural habitat, there was a complete absence of information on species range, conservation status, ecology, acoustic repertoire, and behavior.
Enter Madagascar, an isolated island continent in the southwest Indian Ocean, off the southeastern coast of Africa. At the time of the 2003 description of the Omura’s whale in the Indo-Pacific region, little was known about the diversity of cetaceans in the coastal waters of Madagascar. More recently, field surveys and passive acoustic monitoring conducted from 2009 through 2019 off the island of Nosy Be established the northwest waters of Madagascar as a cetacean biodiversity hotspot. Research off northwest Madagascar has now documented a minimum of 22 different cetacean species with an additional seven species likely to occur (Cerchio et al. in press). Among these was the unexpected discovery in 2013 of a previously unknown population of Omura’s whale (Cerchio et al. 2015). Prior to this discovery, the nearest documentation of Omura’s whale came from a place over 5000 km away off the Coco (Keeling) Islands in the eastern Indian Ocean, where Japanese scientific whaling operations had hunted Bryde’s whales (Ohsumi 1980; Wada et al. 2003). Thus, the Omura’s whales discovered off northwest Madagascar were the first members of the species identified from the western Indian Ocean and represented a substantive expansion of the known species range. Extensive field observations during 2013 and 2014 in the Nosy Be region resulted in an initial study that in just two years established some fundamental ecological aspects of the species. This work revealed the region to be an important habitat for Omura's whales and provided the first in situ observations of this poorly known species globally (Cerchio et al. 2015). Phylogenetic analysis of tissue samples from 18 animals confirmed that the Madagascar population was definitively Omura's whale, and also suggested low genetic variation across its range. This was itself the result of remarkable leaps in molecular genetic techniques since the first recognition of the taxon in the early 1990s. Wada and Numachi (1991) first suggested a species-level classification for Omura’s whales based on allozyme analysis, a laborious time-consuming technique of identifying protein polymorphisms that predated the polymerase chain reaction (PCR) and wide use of mitochondrial DNA sequencing to assess taxonomy. Once the Nosy Be samples were imported from Madagascar (a laborious bureaucratic process that took a few years), species identification following PCR and mtDNA sequencing actually took only two weeks; so ironically, the confirmation that the medium-sized balaenopterids of Nosy Be were Omura’s whale was not delayed because of technological limitations, but rather modern bureaucracy.
Once the suspected species attribution had been confirmed, detailed photographic evidence allowed the first accurate description of external appearance and scientific depiction of the species (Cerchio et al. 2015; Cerchio and Yamada 2018). The Omura’s whale had previously been confused with Bryde’s whales, primarily due to its similar size, body shape, and tropical distribution; however, its external appearance was quite distinct, with a different dorsal fin shape, and diagnostic pigmentation that includes extreme asymmetry between left and right sides (similar to a fin whale, though bolder and more striking) (Fig. 15.1). Sighting data included documentation of mothers with young calves, as indicated by their small size and bent dorsal fins, an indication that calving occurred off Madagascar. There was also extensive documentation of foraging behavior, including surface lunge feeding on swarms of euphausiids and likely other zooplankton. Acoustic recordings during these initial years, from a boat in the presence of a whale and from archival recorders, indicated a form of acoustic behavior that included stereotyped and rhythmically repetitive sounds that were at times heard in choruses of multiple individuals (Cerchio et al. 2015). These aspects of acoustic behavior are indicative of singing in other balaenopterids (e.g., fin whales, Balaenoptera physalus, Watkins et al. 1987, 2000; and blue whales, Balaenoptera musculus, MacDonald et al. 2006) and are therefore suggestive of a male breeding display. Nosy Be ecotourism operators reported sighting these “petite rorquals” while working from April through December. Taken together, these observations led to the conclusion that Nosy Be and the northwest waters of Madagascar support both breeding and feeding habitat for a potentially small, resident population of Omura’s whales.
Omura’s whale, Balaenoptera omurai, northwest Madagascar population, showing asymmetric pigmentation between right side (top) and left side (bottom) and typical external characters; illustration by Frédérique Lucas, executed with photographic and video materials from Nosy Be, Madagascar.
Since the initial publication of the Madagascar observations in Cerchio et al. (2015), multiple accounts of Omura’s whales have been reported throughout the globe, at least in part due to an elevated awareness of the species and descriptions of their diagnostic field characters and acoustic behavior. Cerchio et al. (2019) compiled accounts from all available sources, verifying records based on a descending hierarchy of genetic, morphological, photographic, acoustic, and/or detailed sighting data. It was found that the Omura’s whale is widely distributed around the globe and has currently been documented in tropical and/or warm-temperate latitudes of all ocean basins between 35°N and 35°S, although primarily in the true tropics (Fig. 15.2). There are currently no confirmed reports in the central and eastern Pacific Ocean (both north and south), even in areas that have been extensively visually surveyed such as the Eastern Tropical Pacific (ETP). This suggests that the central Pacific may represent a real barrier to dispersal as it does for other highly coastal marine mammal taxa (e.g., genera Sousa and Neophocaena). Most accounts of Omura’s whales are from neritic continental shelf areas; however, there are several deep water oceanic accounts, suggesting that preferred habitat varies among populations. Cerchio et al. (2019) suggested that some populations may have exclusive shallow water shelf habits, whereas others may utilize both shallow and nearby deep water habitats, likely related to the availability and distribution of food resources. Among the accounts reported were several cases of acoustic documentation, illustrating that Omura’s whale vocalizations are stereotyped and recognizable across a broad range of geographic locations and ocean conditions from the central Atlantic Ocean to the southeastern Indian Ocean.
Map showing global distribution of reported Omura’s whales based on a diagnostic verification method. Legend: “Genetic” refers to typing of mtDNA markers; “Morphology” refers specifically to diagnostic characters of the skull; “Photographic” refers to photographs or videos illustrating diagnostic external physical characters and pigmentation; “Acoustic” refers to recordings of vocalizations; “Visual” refers to a personal report congruent with external appearance when also substantiated by a separate account from the same general locale verified by a higher level method. When multiple methods of verification are available from a single account, the highest level is reported in order of presentation, with “Genetic” being the highest. (From Cerchio et al. (2019, Fig. 1) Global distribution of Omura’s whales (Balaenoptera omurai) and assessment of range-wide threats. Front. Mar. Sci., https://doi.org/10.3389/fmars.2019.00067 (CC BY)
3 Insights from Passive Acoustic Monitoring off Madagascar
3.1 Omura’s Whale Sightings off Nosy Be
During six field seasons of coastal boat surveys between July and November (2011–2017) within an approximately 80 km coastal range off Nosy Be, Madagascar, there was a total of 286 encounters consisting of 311 Omura’s whales, not accounting for re-sightings of individuals between groups (Fig. 15.3). Most of these encounters occurred during the 2015–2017 field seasons, with only 44 sightings documented during 2011–2014. This increase in sightings during the latter three years is believed to be a combination of both greater focus on Omura’s whales and a higher encounter rate due to a real increase in density/abundance of animals during 2015 and 2016 (also suggested by local tour operators and fishers). During boat surveys, Omura’s whales showed a strong preference for shallow continental shelf or neritic habitat, avoiding the near-coastal habitat (<20 m depth) and the deeper waters of the off-shelf pelagic habitat (>100 m depth); accordingly, the mean bottom depth among 60 encounters measured in situ was 53.8 m (range 23–81 m).
The social structure of the species, at least within this study region, may be best characterized by “loose aggregations” of individuals. When applying a traditional definition of “group” typically used for humpback whales (Megaptera novaeangliae) and other large baleen whales (i.e., multiple individuals within a few body lengths of each other), Omura’s whales were predominantly encountered as singletons (mode = 1) with a mean group size of 1.1 (s.d. 0.3, range 1–2, n = 261; Cerchio et al. unpublished). In only three cases were two adults considered ephemerally associated as a “group”; the only other “groups” encountered were mother-calf pairs (n = 22). However, despite this seemingly solitary social structure based on a restrictive definition of “group”, when one individual was encountered there were often several others within 100 s of meters to several kilometers, likely within acoustic communication range of one another. Therefore, the term “aggregation” may be a more appropriate term than “group” when considering the social structure of the species (Chap. 7). The largest aggregation of Omura’s whale documented to date was encountered in 2015, with photographic identification indicating a minimum of 12 individuals within a 2 × 5 km area that was surveyed over a 3 h period (Fig. 15.4). This aggregation was documented 10 km off the north coast of Nosy Be just south of a submerged bathymetric feature called Banc du Goliath.
Boat survey track (a) north of Nosy Be, Madagascar, on November 19, 2015, illustrating an example of an Omura’s whale aggregation (b) in an approximately 2 × 5 km area that was surveyed over 3 h, within which a minimum of 12 different individual Omura’s whales were photographically identified. Each different color circle indicates the position(s) of a different individual, and the yellow star indicates the position of the long-term recorder that was thereafter deployed at Banc du Goliath.
3.2 Acoustic Monitoring Effort
Omura’s whale vocalizations were initially described from boat-based recordings in the presence of individuals off Nosy Be, Madagascar (Cerchio et al. 2015). Thereafter, autonomous archival recorders were deployed in the waters of the Nosy Be region that had been documented as Omura’s whale habitat. Initially, a pilot study was conducted over a three week period in 2014, in which three SoundTrap-202 recorders (oceaninstruments.co.nz), sampling continuously at a sample rate (SR) of either 96 kHz or 144 kHz, were deployed at approximately 20 m depth at four different sites for periods of 7–14 days. These recordings were useful for obtaining long, uninterrupted sequences of Omura’s whale sounds, and proved the effectiveness of using remote archival recorders to document spatiotemporal occurrence of vocalizing Omura’s whales. Following on from the pilot study, four SoundTrap-300 recorders were used to monitor for Omura’s whale and other cetacean vocalizations from 1 December 2015 to 10 November 2016, recording on a 33% duty-cycle (10 min every 30 min) at a SR of 24 kHz. The recorders were deployed in water depths of approximately 40 m and distributed across an approximately 80 km range within the study area (Fig. 15.3). Two recorders were placed at the extreme north (Banc du Goliath) and south (Baramahamay) of the study area, respectively, and two were placed in the core of the study area, separated by approximately 20 km (Banc de Rosario and Ankazoberavina). The southern site and the two core sites were previously monitored during the pilot deployment in 2014, during which many Omura’s whale vocalizations were recorded. The Banc du Goliath site was chosen to monitor the area near the site of the large aggregation encountered in 2015 (Fig. 15.4). The recorders operated for an aggregate of 1161 recorder-days, yielding a total of 9287 h of acoustic data. These data were used to define the spatiotemporal occurrence of Omura’s whale throughout the study area over a one year period (Cerchio et al. 2018).
3.3 Description of Omura’s Whale Song
As initially described in Cerchio et al. (2015, 2018), vocalizations recorded in the presence of Omura’s whale were classified as songs. An Omura’s whale “song,” as recorded off northwest Madagascar, consists of a single stereotyped phrase comprised of one to two unit types; this is repeated at a regular interval of approximately two to three minutes for extended periods of time in long sequences (Figs. 15.5 and 15.6). In northwest Madagascar to date, a single “song type” has been positively attributed to Omura’s whales through simultaneous visual observations and acoustic recordings, referred to as Type1 song. Type1 song occurs in two similar forms (or two similar “phrase types”), which are referred to as Type1A and Type1B. Type1A is composed from a single, partially amplitude-modulated (AM), low-frequency sound (Unit A, Fig. 15.5A). Unit A has an average duration of 10.2 (s.d. 0.31) sec in the ~15–50 Hz frequency band, its main energy in an initial 8.2 (s.d 0.27) sec wideband section with a slightly bimodal energy distribution (20.8 and 40.0 Hz) (Fig. 15.5C), and terminates with a short multi-narrowband “tail” of 1.9 (s.d. 0.37) sec. Type1B is composed from Unit A and a distinct constant frequency sound (Unit B, Fig. 15.5B), which has a duration of approximately 4 s in the 16–17 Hz band (Cerchio et al. 2018; Moreira et al. 2020; Fig. 15.5B). Songs are repeated at regular intervals, with a singer varying between phrase types in its song sequence (Fig. 15.5D). Frequently, overlapping series with different received levels and acoustic quality were recorded, indicating choruses of several individual singers (Fig. 15.5E).
Northwest Madagascar Omura’s whale song. Sound spectrograms illustrating A phrase Type1A, and B phrase Type1B (2000 Hz SR; 2048 pt FFT, 95% overlap, Hann window); C power spectral density level of Unit A (averaged across 22 units from 6 different series; 2000 Hz SR, 32,768 pt FFT, 0% overlap, Hann window); and spectrograms of D a sequence of four songs from a single singer, and E a chorus of an estimated five or six Omura’s whale singers with 18 separate songs audible (with a subjective assessment of individual attribution indicated by colored numbers; 2000 Hz SR; 4096 pt FFT, 95% overlap, Hann window)
Uninterrupted sequences of songs can, at times, last for periods in excess of several hours, with the longest recorded uninterrupted sequence lasting for at least 11.6 h, during which time the whale was within detection range of the recorder. Figure 15.6 illustrates an 8 h example observation of singing, in which the song sequence fades in at the start and then fades out at the end, suggesting that the whale was singing prior to moving within detection range of the recorder, sung for many hours within detection range, and then continued to sing as it moved out of the detection range. An interesting aspect of this observation is that the singer had to be actively maintaining its relative position in the vicinity of the recorder to be detected for this period of time. The currents in this area range from approximately 1–4 km/hr depending on tidal regime, and given the relatively short detection range of the recorder in this habitat (likely <5 km, see below) a passively drifting whale likely would have drifted out of detection range during this period; similarly, if moving even at less than the average documented movement speed (3.9 km/hr from four satellite tagged individuals; Cerchio et al. 2018), the whale would have traversed through the detection range in less time. Recordings of long sequences in excess of several hours are not uncommon, suggesting that some singers may remain relatively stationary by actively maintaining position in a specific area while singing.
To assess song repetition rate, spectrograms (2000 Hz SR, 4096 pt FFT, 50% overlap) of 963 h of recordings from the four locations monitored in 2014 were manually scanned to define unambiguous, continuous song sequences. Each sequence was presumed to be a single individual singer based upon an uninterrupted, consistent song repetition rate throughout the sequence (Fig. 15.6), and defined either as a solo singer or as a prominent foreground singer when more than one singer was audible. A total of 161 individual sequences were isolated ranging from 10 to 244 repeated songs, typically a mix of the two phrase types. As a measure of song repetition rate, the interval from the onset of one song to the onset of the next (hereafter referred to as the song repetition interval) was measured from 111 individual series with ≥20 consecutive songs. Mean repetition interval for individual series ranged from 141.6 s (s.d. 34.5, n = 44 intervals) to 219.7 s (s.d. 45.73, n = 32 intervals) with a population mean across the 111 individual means of 177.5 s (s.e. 15.3) (Fig. 15.7).
Variation in song repetition rate, as expressed by the distribution of mean song repetition intervals, measured from 111 individual singers. Data included are for continuous series of ≥ 20 songs, presumed to be from a single individual and typically with mixed phrase types (Type1A and Type 1B) repeated uninterrupted at a relatively consistent repetition rate
Since these 111 song sessions indicated that the range of song repetition intervals is consistent and typically 150–200 s, the summation of the number of detected song phrases in a 10-min sample of the duty-cycled data (33%, 10 min every 30 min) should be a function of the number of singing whales. Therefore, total number of songs across an entire day of samples provides a reliable index of daily singing activity and the relative number of singing whales within detection range of the recorder. The actual detection ranges of the recorders were undetermined, but short deployments of pairs of recorders separated by 2, 3, and 5 km, suggested that in this shallow water environment songs can be detected at ranges > 2km, but likely <5 km (unpublished data). Under the assumption that singing is a display limited to reproductively mature males, the number of singers only reflects a portion of the population, and therefore, summation of songs, or acoustic presence, represents only a general proxy for Omura’s whale daily presence.
3.4 Spatiotemporal Occurrence of Omura’s Whale Singing
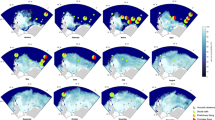
There was apparent spatiotemporal variation in the occurrence and relative prominence of Omura’s whale singing activity among the four recorder sites during the 2015/2016 recording period (Cerchio et al. 2018). Initial review of data from 1 December 2015 to 10 November 2016 indicated that singing was clearly most prominent at the northernmost site, and relatively rare at the two most southern sites after December. An automated detector for song Unit A was developed using the Band-limited Energy Detector of Raven Pro 1.5 (Bioacoustics Research Program 2014) in the 15–50 Hz frequency band in order to detect occurrences of songs composed of either phrase Type1A or Type1B. Several SNR thresholds for automatic detection were tested on one week of data from Banc du Goliath. A detection threshold of 5 dB was chosen, yielding a detection rate of 81.3% for all phrases and a false detection rate of only 1.9%, thus providing a conservative estimate of the total number of true detections. The detector was run on all data from all sites, and examination of one week from each month (ca. 25% of data) indicated widely varying rates of “false positive” detections due to particularly high levels of ambient noise at some recorders during certain months. Therefore, detector results were exhaustively browsed manually to verify all detections, removing detections of other sounds and all false positives. The resultant output provided confident and conservative counts of songs (recognizing an estimated 81% detection rate since missed detections, or “false negatives,” were not logged during the elimination of false positives). Songs were recorded at all sites during December on a daily basis, sometimes in choruses of multiple individuals; singing activity subsided in January, particularly at the three southern sites where it was thereafter sporadic and uncommon from February through April, and varied substantially among the four sites from May through November (Fig. 15.8) (Cerchio et al. 2018, unpublished data).
Daily counts of songs from December 01, 2015, to November 30, 2016, at each of the four recording sites in the Nosy Be region (see Fig. 15.3): A Banc du Goliath; B Ankazoberavina; C Banc de Rosario; and D Baramahamay. Data were recorded on a 33% duty-cycle of 10 min every 30 min (on the hour and half hour, local time), so totals represent the number of songs detected during a total of 8 h of recording each day. Gray indicates periods with no data or incomplete data
Whereas singing activity declined during January in the three southern sites, song was recorded extensively at the most northern site, Banc du Goliath, in all months with choruses of multiple individuals occurring throughout the year (Fig. 15.8A). Moreover, there was no indication of strict seasonality despite fluctuation in singing activity throughout the year. Singing was present daily from 1 December to the end of January and then was intermittently present throughout the year with several strong peaks in activity, the most prominent being late May to late June, and late October to mid-November when our recording effort ended (Fig. 15.8A). It is assumed that singing activity remained high throughout November, as this was the period of boat surveys when many animals were sighted in the vicinity of the recorder. The longest stretch of days without any song detections was eight days in late August; however, even this hiatus was shortly followed by a period of high singing activity in mid-September (Fig. 15.8A). The presence of singing year-round off Banc du Goliath indicates definitively that this population of Omura’s whales is non-migratory, in that it does not seasonally move out of northwest Madagascar waters. This was a key discovery of the acoustic monitoring effort, since residency of the population and a non-migratory life history of the species had been previously suspected, but not confirmed.
The Banc Rosario recording site, 35 km south of Goliath, had the second highest levels of singing activity, with relatively high levels of singing during December and from May to November, but very little from late December through mid-May (Fig. 15.8B). These two sites are considered hotspots for Omura’s whale as indicated by boat-based sightings (Fig. 15.3), and also indicated by the long-term experience of local ecotourism companies. Singing activity at the Ankazoberavina recorder, 20 km to the southwest of Banc Rosario, and Baramahamay recorder, another 30 km south of Ankazoberavina, was markedly less than at Goliath and Rosario. At Ankazoberavina, singing was heard consistently only in December, but even in that month at less than half the occurrence rate as during the same days at Goliath (Fig. 15.8C). After December, song was recorded at Ankazoberavina on less than one day per month overall, with no activity from late January to late March, or late May to late August (when the recorder prematurely stopped recording). At Baramahamay, singing was recorded more frequently, during December into early January, and from late May to early November when our recording effort ended, but at a far lower rate than Goliath or Rosario (Fig. 15.8D). This indicates that despite whales being resident year-round in the Nosy Be region, there was distinct micro-geographic variation in their distribution within the region throughout at least the monitored year. Moreover, the variation throughout the year at Goliath and Rosario suggests influxes of singing animals moving throughout the area, into and out of detection range of the recorders. This result underscores the importance of a broad sampling strategy throughout a region; e.g., a very different conclusion regarding local residency would have been reached if acoustic monitoring had only been conducted at Ankazoberavina and not at Banc du Goliath. Further monitoring effort in additional years will be required to determine if this pattern is consistent across years, suggesting stable micro-habitat preferences, or conversely if spatial heterogeneity changes across years, perhaps suggesting the influence of fluctuating and variable oceanographic conditions on distribution of prey resources, and consequently on the distribution of whales.
Multiple simultaneous singers were heard throughout the recording period, at times in choruses of several individuals within detection range, as indicated by the presence of multiple overlapping sequences of songs. The frequency of occurrence of multiple singers was assessed at the Banc du Goliath site, since it had the highest measured levels of singing activity (Fig. 15.8A). From 1 December 2015 to 10 November 2016, a total of 15,349 10-min samples were evaluated, of which 40.5% (n = 6,213 samples) had at least one song indicating the presence of a singing Omura’s whale. A total of 26,438 songs were counted, with the number of songs per sample ranging from 0 to 23. The daily average number of songs per sample closely tracked the total number of songs per day (Fig. 15.8A), suggesting little to no seasonal variation in tendency to chorus. Based on a mean song repetition interval of 177.5 s (Fig. 15.7), on average a single singer produces 3.4 songs per 10 m sampling period. Considering this interval ± 2 s.d. (i.e., an approximated 95% C.I.), the number of individual singing males can be estimated based on number of songs per sample. There were no singers detected in 59.5% of all samples, 1 singer estimated present in 20–27% of all samples, and ≥2 singers estimated present in 14–20% of all samples (Fig. 15.5E for a 10-min sample with 18 songs from an estimated 5–6 different singers). This indicated that when singing whales were present, there were multiple singing males within detection range of the recorder from one-third to one-half of the time, suggesting that choruses were a common phenomenon.
Assessment of hourly rates of song phrases/10-min (using Goliath site data) gave no indication of a cyclic diel trend in singing activity across the complete sampling period (Cerchio et al. 2018), unlike other baleen whales for which diel trends have been repeatedly found. For example, the tendency of blue whales to sing more frequently at twilight or night than during the day in several locales (Stafford et al. 2005; Wiggins et al. 2005; Chap. 9), humpback whales to increase singing activity during dark hours in multiple ocean basins (Cholewiak 2008; Cerchio et al. 2014; Chap. 11), North Atlantic right whales to produce “gunshot” sounds, a signal that has been associated with a male display, more often at night in spring and summer (Matthews et al. 2001; Parks et al. 2005; Chap. 13), and minke whales to sing more at night in the North Atlantic and off South Africa, but either have no distinct pattern or be more common during daylight hours in the western Antarctic Peninsula (Chap. 14).
3.5 Acoustic Detections of Other Song Types
Two additional novel forms of song were detected off Nosy Be with lower occurrence rates than the confirmed Omura’s whale song (Type1A and 1B). Both were in the 20–60 Hz frequency band, with stereotyped units repeated in a series at regular intervals, and therefore considered a form of balaenopterid singing. They were never produced within the same series as an Omura’s whale Type1 song; therefore, it is unclear if they represent a different form of Omura’s whale song or were produced by Omura’s whales at all. Each shared similarities with the Type1 Omura’s whale song, in terms of AM unit structure, unit duration and repetition rate. To our knowledge, these specific forms have not been previously reported in the literature, and since they were never recorded in the presence of a whale, species attribution can only be speculated. The first novel song was recorded sporadically at three of the sites (Banc du Goliath, Banc de Rosario and Ankazoberavina) from late February to mid-September, and at times a series of high SNR novel songs overlapped with a series of Omura’s whale Type1 songs; however, during its peak occurrence, this first novel song was never as common as Omura’s whale Type1 song. The second novel song form was very rare and documented only twice on the Banc du Goliath recorder.
It is possible that these novel forms of song are from another baleen whale species, such as Bryde’s whale (B. edeni or B. brydei); however, Bryde’s whales have only been confidently documented far to the south of Madagascar during Japanese scientific whaling operations and other visual boat surveys (Ohsumi 1980; Best 2001), and never off the west coast of Madagascar, nor off Nosy Be despite the extensive effort to study Omura’s whales (Cerchio et al. in press). Alternatively, these may be songs of Omura’s whale, representing different song types that are distinct from the common Type1 song. If these songs were from Omura’s whales, it is not obvious why they are less prevalent than Type1 song. The comingling of Omura’s Type1 song and the first novel form of song in close proximity (likely within a few kilometers) suggests that they may both be from males of the same species singing in a chorus. Little is known about the range and dispersal of Omura’s whale populations, but our data from Nosy Be, including photographic identification and satellite telemetry data (Cerchio et al. 2018), suggest that this Nosy Be population is resident and has a restricted range along a ca. 400 km stretch of the northwest coast of Madagascar. Nothing is known about population structure, long distance movements or population mixing of Omura’s whale, nor is it clear whether there exist multiple populations in the southwest Indian Ocean. It is conceivable that there are other as yet undetected populations in the region, that populations have diagnostic song types, and the presence of these two song types represents males from nearby populations temporarily moving into the range of the northwest Madagascar population. In the next section, we explore what is currently known about the geographic variation in Omura’s song across its global range.
4 Global Geographic Variation of Omura’s Whale Song
It is now recognized that Omura’s whales have a nearly pan-tropical distribution, with documented accounts in all ocean basins except the central and eastern Pacific Ocean (Cerchio et al. 2019). Among the accounts that have been reported are several acoustic documentations of Omura’s whale song occurring widely throughout the global range: in the equatorial Atlantic Ocean off the São Pedro and São Paolo Archipelago, Brazil (Moreira et al. 2020), and Ascension Island (Leroy et al. 2021); in the central Indian Ocean off the Chagos Archipelago (Sousa and Harris 2015); in the South China Sea (G. C. Gagnon, pers. comm.); and broadly along the northwestern coast of Australia (McCauley 2009, 2011; McPherson et al. 2016; Erbe et al. 2017). All of these cases of acoustic identification were based upon strong similarities in acoustic features with the published description of Omura’s whale song from Madagascar (Cerchio et al. 2015). Only some were accompanied by independent photographic verification of the presence of the species in the general region (only off Australia), but none was recorded in the presence of Omura’s whales, as was done off Madagascar. That the similarities of these songs with Madagascar Omura’s whale song were strong enough to infer the occurrence of the species in these other regions, indicates that Omura’s whale song has diagnostic, species-specific acoustic features across an extended range (Fig. 15.9).
Waveforms and spectrograms illustrating similarities for songs attributed to Omura’s whales from four disparate sites in the species’ range as presently documented. Top panel: spectrogram examples of four song types from: A northwest Madagascar (Type1B), (Cerchio et al. 2015, 2018) with confirmed attribution to Omura’s whales; B Diego Garcia, Chagos Archipelago in the central Indian Ocean originally described as a baleen whale of unknown species (Sousa and Harris 2015, sound contributed by D. Harris from Diego Garcia CTBT acoustic data); C the northwest coast of Australia (IMOS acoustic data, Kimberley site); and D São Pedro and São Paulo Archipelago on the mid-Atlantic Ridge (from Moreira et al. 2020). Lower four panels show short sequences of songs from: E northwest Madagascar, F Diego Garcia, Chagos Archipelago, G northwest coast of Australia and H São Pedro and São Paulo Archipelago, Brazil. All four of these song types have been attributed to Omura’s whales due to their common acoustic features and repetition rates compared to song features from other baleen whale species (2 kHz SR, FFT 4096 DFT, 95% overlap, frequency resolution 0.5 Hz, time resolution of 200 ms)
The first reports of Omura’s whale songs came from the northwest coast of Australia and initially were attributed to Bryde’s whales (McCauley 2009), before the description of Madagascar Omura’s whale song in 2015. The song was documented across an extensive range, from at least ten recording sites from Exmouth northeast into the Timor Sea to north of Darwin (McCauley 2009, 2011). After the description of Omura’s whale song from Madagascar, the songs in these first reports were independently attributed to Omura’s whales in three separate studies (McPherson et al. 2016; Erbe et al. 2017; Cerchio et al. 2019). Off the Chagos Archipelago, Sousa and Harris (2015) reported two vocalizations of unknown origin off Diego Garcia, attributing them to a baleen whale and speculating that they could represent undescribed blue whale vocalizations. One of these, the “Diego Garcia Croak” (DGC), was recognized as an Omura’s whale song by Cerchio et al. (2019). In the equatorial Atlantic, sounds recognized as Omura’s whale song were reported from deep water recorders near the São Pedro and São Paulo Archipelago on the mid-Atlantic Ridge (Moreira et al. 2020), and very similar songs have more recently been reported from Ascension Island (Leroy et al. 2021), using hydrophones of the Comprehensive Nuclear Test Ban Treaty Organization, CTBTO).
The songs from four disparate locations for which acoustic descriptions are available (Madagascar, the Chagos Archipelago, Australia and the Equatorial Atlantic) broadly share structural characteristics and acoustic features that make them unambiguously identifiable as Omura’s whale song and distinguish them from all other described baleen whale vocalizations (Table 15.1, Fig. 15.9). All combine wideband, noisy, AM, and narrowband components with predominant energy in the 15–50 Hz band, a bimodal energy distribution and spectral energy peaks at approximately 20 and 40 Hz. The three Indian Ocean songs all end with a low-frequency narrowband component or a separate low-frequency narrowband unit, at approximately 16–20 Hz, which appears optional in at least the Madagascar population (e.g., Type1A vs Type1B song). Song phrase duration is between approximately 10–18 s, with lower variability when considering only the first unit or sub-unit (duration between 10-11 s). Repetition intervals are consistent with averages of one song every approximately 170-200 s. Despite these broad similarities, there are distinct geographic differences in the structure and acoustic features of the songs from the different regions. Such geographic variation often develops between populations that have been at least semi-isolated and undergone vocal or dialectical divergence (Fig. 15.9; Cerchio et al. 2019; Moreira et al. 2020; Leroy et al. 2021).
In each of these regions, long-term monitoring has indicated that singing occurs throughout the year with little to no seasonality, as described above for Madagascar. Additional song types have also been described in multiple regions, some of which may also be attributed to Omura’s whales. Off northwest Australia, McCauley (2009, 2011) reports that song was detected in all months of the year from at least three sites (Scott’s Reef, northwest of Broome, and Joseph Bonaparte Gulf). In the eastern Timor Sea, north of Darwin, Australia, McPherson et al. (2016) report that song was detected in all months from August 2014 through July 2015 except November and December, describing apparent movement of whales in and out of the area over the course of several months. McPherson et al. (2016) also described two distinct types of Omura’s whale song in the Timor Sea with overlapping but slightly varying temporal distributions: a two-part song type (shown in Fig. 15.9c), and a single-part broadband song type that is much more similar to the Madagascar song Type1A (Fig. 15.5a). Off the Chagos Archipelago, Sousa and Harris (2015) reported peaks of occurrence in both the Austral winter and summer of 2002 and 2003, with hiatuses of no occurrence during spring and autumn, and it was concluded that the population may reside year-round at Diego Garcia. Sousa and Harris (2015) also reported two different song types, only one of which has been positively attributed to Omura’s whale based upon similarity with Madagascar song; the other song type, the “Diego Garcia Downsweep” may represent a second Omura’s whale song type for the region, although some researchers argue it to be from a blue whale (Leroy et al. 2021). In the Equatorial Atlantic, Omura’s whale song was detected off the São Pedro and São Paolo Archipelago during nine of 11 monitored months between February and December 2013, with a strong peak in singing activity during April through June, and no occurrence during September and October (Moreira et al. 2020). Currently, there is no evidence for additional song types off São Pedro and São Paulo, and the song recorded off Ascension Island appears similar, but not identical (Leroy et al. 2021). The observations of temporal occurrence for each of these regions suggest local movements within a region, without a strong seasonal signal, similar to what has been more fully described off Nosy Be, Madagascar (Fig. 15.8).
5 Synthesis and Implications for Breeding Ecology
Acoustic displays, referred to as song, have been described in many Mysticetes, particularly Balaenopteridae, and for those for which data exist, the trait is sex-limited to the males of the population. It is widely accepted that these vocal displays are therefore a product of sexual selection, represent a male breeding tactic, and are an integral part of each species’ breeding system. As in other taxa (e.g., passerine birds), the displays take the form of stereotyped songs that are repeated with a consistent rhythm, and singing sometimes occurs in aggregations of male singers (choruses, although not the synchronized choruses of some anuran amphibians or insects). Communal display in aggregations is a common characteristic of lek mating systems (Höglund and Alatalo 1995), and a form of lekking has been proposed to describe the breeding system for at least humpback whales, the best studied singers among the Balaenopteridae (Herman and Tavolga 1980; Clapham 1996). As we learn more about the acoustic behaviors of Balaenoptera species¸ it is becoming clearer that they share the general trait of having male vocal displays, and that song, communal singing (i.e., displaying in aggregations), and perhaps even lek mating systems might eventually be considered ancestral balaenopterid behavioral traits.
The Omura’s whale has only recently been added to the list of Balaenoptera species; however, because of the advancements in technology and methodology in the twenty-first century, along with the identification of a tractable population off Madagascar, we are learning about this species at a relatively rapid rate compared to previous work on other Mysticetes. The current understanding of Omura’s whale behavioral ecology and vocal display behavior fits well within the context of what is known about other balaenopterids. Omura’s whales produce stereotyped songs that are uttered in consistent, highly repetitive forms. Singing occurs uninterrupted over extended periods of time (several hours without pause) and therefore represents a time-intensive vocal display. There are currently no data on the sex of Omura’s whale singers, but given what is known about sexually selected breeding displays, it seems highly likely that singing is a male-limited vocal display important in the breeding system. Social structure appears to be characterized by loose aggregations, with groups of individuals spread over a few kilometers in a patchy and ephemeral spatial distribution. Observations in Madagascar suggest that these aggregations probably are related to both feeding and social behavior, sometimes associated with patchy food resources and other times not. Similarly, long-term acoustic monitoring indicates that chorusing by singers is a prominent part of singing behavior. Micro-geographic variation in spatial distribution is high (i.e., there is a high degree of spatial and temporal heterogeneity over an 80 km stretch of coastal habitat in Madagascar revealed by both acoustic monitoring and satellite telemetry), with apparently preferred areas for aggregation and singing. Some degree of the spatial heterogeneity is probably in response to prey distribution, but aggregating and displaying in preferred “arenas” is also characteristic of lekking behavior (Höglund and Alatalo 1995).
Whereas some characteristics of Omura’s whale behavior appear to be general to Balaenopteridae as a whole, several other emerging characteristics for the species are unusual for the family. Populations of Omura’s whales appear to be non-migratory, with likely restricted ranges and small sizes, and the species has an exclusively tropical and warm-temperate distribution, all of which are rare among balaenopterids. There is no apparent seasonality to singing behavior, which is also apparently rare among balaenopterids. Like other balaenopterids, their general song structure is diagnostic to the species across a broad global range, allowing it to be readily recognized in many cases. Currently recognized macro-geographic variation in songs types appears moderate to low on a global scale (Fig. 15.9), in comparison to some balaenopterids, for which there is markedly greater variation in song types among populations (e.g., among the song types of different populations of pygmy blue whales, Chap. 9; or among songs of non-interacting populations of humpback whales, Chap. 11).
There are indications of different song types within regions, but in most cases, the attribution of the additional song types to Omura’s whales is speculative. Thus, the perception of song similarity across their global range may be inflated by ascertainment bias, in that researchers are currently only able to identify those regional song types that are most similar to the only one that has been confidently described for the species off Madagascar (Cerchio et al. 2015). When we consider songs of unknown origin already documented, then there may be two to three possible song types off northwest Madagascar (described above), two or more off the Chagos Archipelago (Sousa and Harris 2015), and at least two off northwest Australia (McPherson et al. 2016). Therefore, it is possible that neighboring populations have distinctive song dialects, and migrants/wanderers move between populations; this would explain the presence of a “minority” song type among a much more common song type (as may be the case off Madagascar). However, there is as yet no evidence of “cultural diffusion” (Mundinger 1980; Whiten et al. 2016) of song types from one population to another, as would be indicated by the replacement of a local song by another different one (but more time for observation is needed). Alternatively, different song types may serve different roles, and each population may have a song that is similar to those currently recognized as Omura’s whale song, as well as more divergent song type(s). These observations elicit wide open questions that require expanded research on multiple populations and further interpretation of new results.
In addition to the novelty of new species-specific discoveries, Omura’s whales may present a unique opportunity for the study of balaenopterid vocal/breeding behavior. Humpback whales have been the most intensively studied baleen whale, in large part due to their tendency to densely aggregate in accessible coastal areas; however, their song and singing behavior is complex, presenting challenges to interpretation and inference (Chap. 11). Conversely, blue and fin whales have comparatively simple songs and singing behaviors (Chap. 9); however, they are challenging to study due to their dispersed pelagic distributions. The relatively simple songs and spatial distribution characteristics of Omura’s whale populations appear to combine some of the most tractable characteristics of other balaenopterids, and therefore might make them favorable for research compared to other species within the family.
Blue and fin whales are pelagic and inhabit large oceanic ranges, and their spatial distribution is likely strongly influenced by the long distance propagation of their low-frequency songs (Chap. 2). Singing males are consequently spread over large regions of deep water, and if in fact their mating systems resemble leks, their display arenas may spread over substantial portions of an ocean basin. So far, Omura’s whales appear to be neritic and coastal, with relatively small core ranges, based upon what has been learned off Madagascar. Their song propagation is limited by shallow water stripping of low frequencies and likely moderate source levels due to their small size; pairs of recorders deployed on the shelf habitat off Madagascar suggested that individual singers were simultaneously audible on hydrophones separated by 2–3 km but not on hydrophones separated by 5 km. This limited acoustic range is congruent with the visual observations of aggregations that are spatially tight in comparison to larger pelagic balaenopterids, and therefore, chorusing individuals may be separated by only 100 s of meters to several kilometers, compared to likely 10 s to 100 s of kilometers for blue and fin whales. Consequently, the lekking grounds of Omura’s whales may be of a tractable size for study.
Population characteristics of Omura’s whales might also be favorable for long-term latitudinal studies of individuals within a population. Considering humpback whales in comparison, most modern populations are large, on the order of >10,000 individuals, requiring prohibitively large samples of individuals (i.e., genetic and behavioral sampling) to study the breeding system. Additionally, long-range movements of individuals result in mixing and substantive immigration/emigration between populations. There is still much to be learned about Omura’s whale population characteristics, but based on the initial information from northwest Madagascar, the species may be characterized by small resident populations. This presents the potential for long-term latitudinal study of individuals, including for example, assessments of genetic relatedness relative to individual associations and male reproductive success.
Lastly, song characteristics of Omura’s whales are relatively conducive to study. In comparison, humpback whales (the most widely studied species in terms of song and mating behavior) have complex singing behaviors, representing the most highly derived balaenopterid in terms of evolutionary exaggerated traits. Humpback whale song is organized into a relatively complex hierarchal structure, with substantive variation on several interacting levels, including intra-individual, inter-individual, temporal, and inter-population (Chap. 11). Such complexity makes it difficult to identify unambiguous differences among individual males. This presents great challenges in attributing variation to consequential behavioral metrics, such as reproductive success, thus making it difficult to correlate song features with selection. In contrast, Omura’s whale song is comparatively simple, with a single phrase and non-hierarchal structure, uttered in a consistent repetitive rhythm. Inter-individual variation is not readily apparent, nor has it yet been properly assessed, but under the assumption that it is a male sexually selected trait, such variation must exist between individuals and should be measurable. Possible acoustic features that may contain measurable variation include the pattern of pulses in the AM units, quantitative details of phrase structure, or the repetition rates of songs. The salient feature is that the simplicity of the song increases the likelihood that consistent variation between individuals can be reliably measured and regressed against important physical and behavioral variables, such as size, age, and ultimately male mating success. It may therefore be possible to correlate song acoustic features with selection and make some inference on the evolutionary process of this species’ singing behavior and mating system.
There are several challenges that also need to be acknowledged. Perhaps most complicating is the difficulty in identifying singing individuals in the field, due to the low-frequency nature of the song, the long intervals between songs, and the difficulty in sighting and tracking individuals at the surface due to small size and an inconspicuous blow. Therefore, obtaining focal animal song samples paired with genetic samples is not straight forward. This may be surmountable using combinations of emergent technology, such as acoustic tags deployed on known (recognizable) males in an area that is being monitored by a synchronized array of hydrophones with localizing capability. Also, there are challenges inherent to working with a species that feeds throughout the year, unlike breeding humpback whales that are fasting and therefore not linked to food resources. Dependence on patchy and ephemeral prey resources results in somewhat unpredictable temporal variability in Omura’s whale distribution within their range, limiting the reliability of finding aggregations within and across years. Observations off Madagascar suggest that such variability complicates field work, but some areas exist where aggregations are more consistently found. These challenges aside, Omura’s whales may embody the simplicity and tractability of singing systems that are apparent in species such as fin and blue whales, in a species that has an accessible coastal distribution like the humpback whale, and furthermore may have small tractable populations. Therefore, the species may represent a potential model for assessing questions about general balaenopterid vocal and mating behavior, aside from species-specific questions.
6 Afterward: Consequences of Leaps for Improving Our Understanding of Poorly Known Species and Habitats
Over the past four to five decades, scientific technology has steadily developed on all fronts, and passive acoustic monitoring devices have become increasingly more powerful, portable, and affordable. These technological advancements have enabled us to expand our study of the marine environment, in regions around the world where resources and technology have been limiting. The technological advancements of the 1970s made it possible for small-scale research groups to acquire the means to conduct cutting-edge research, and make available learning experiences to undergraduate students, like myself, at local universities that would in turn motivate careers in bioacoustics. Remarkable as this was, these opportunities were still limited to developed countries and the privilege of relatively wealthy societies when considered in a global context. Advancements continue, and during the past 5–10 years in particular, there has been another major technological leap that is again revolutionizing the field of cetacean bioacoustics. There are now commercially available archival recorders the size of a soda can, at a modest price, and these recorders can record continuously for months at sample rates covering the frequency range of all mysticetes and many odontocetes. Analysis methods have shifted from analog to digital, and modestly priced high-powered computers and open-source software have pushed the limits beyond what was conceivable in the 1980s and 1990s. Today’s advancements allow researchers in remote locations, on limited budgets, access to technology that will revolutionize our understanding of previously poorly studied regions of the world. This ability opens the door to conduct studies that only ten years ago would have had orders of magnitude higher cost, if they were logistically possible at all. In addition, the discoveries that this technology facilitates will in turn synergistically inspire the wonder and passion of a new generation of explorers, as individuals discover in themselves the drive to push the boundaries of discovery itself. The case study of the Omura’s whale of Madagascar, and related work applying passive acoustic technologies to study marine mammals in Madagascar, the Mozambique Channel, and other parts of coastal Africa, are examples of what could be considered a new age of discovery in the twenty-first century. In the coming decades, as technology continues to advance, costs become more affordable, and capacity around the globe is increased through access to education and global networking, we should expect to see the inspiration of new explorers and continued discovery in regions that previously have been poorly studied.
References
Bioacoustics Research Program (2014) Raven pro: Interactive sound analysis software (Version 1.5) [Computer software]. The Cornell Lab of Ornithology, Ithaca, NY. Available from http://ravensoundsoftware.com/
Best PB (2001) Distribution and population separation of Bryde’s whale Balaenoptera edeni off southern Africa. Mar Ecol Prog Ser 220:277–289
Cerchio S, Andrianantenaina B, Lindsay A, Rekdahl M, Andrianarivelo N, Rasoloarijao T (2015) Omura’s whales (Balaenoptera omurai) in northwest Madagascar: ecology, behavior and conservation needs. Roy Soc Open Sci 2:150301. https://doi.org/10.1098/rsos.150301
Cerchio S, Andrianantenaina B, Zerbini A, Pendleton D, Rasoloarijao T, Cholewiak D (2018) Residency, feeding ecology, local movements and potential isolation of the Madagascar Omura’s Whale (Balaenoptera omurai) population. Paper SC/67B/NH/09 presented to the International Whaling Commission Scientific Committee. Available from: https://archive.iwc.int
Cerchio S, Laran S, Andrianarivelo N, Saloma A, Andrianantenaina B, Van Canneyt O, Rasoloarijao T (In press) Cetacean species diversity in Malagasy waters. In: Goodman SM (ed) The new natural history of Madagascar. Princeton University Press, Princeton
Cerchio S, Strindberg S, Collins T, Bennett C, Rosenbaum H (2014) Seismic surveys negatively affect humpback whale singing activity off northern Angola. PLoS ONE 9(3):e86464. https://doi.org/10.1371/journal.pone.0086464
Cerchio S, Yamada TK (2018) Omura’s whale: Balaenopteridae omurai. In: Würsig B, Thewissen JGM, Kovacs K (eds) Encyclopedia of Marine Mammals, 3rd ed, Academic Press, San Diego, CA, USA, pp 656–659
Cerchio S, Yamada TK, Brownell Jr RL (2019) Global distribution of Omura’s whales (Balaenoptera omurai) and assessment of range-wide threats. Front Mar Ecol 6:67
Cholewiak DM (2008) Evaluating the role of song in the humpback whale (Megaptera novaeangliae) breeding system with respect to intra-sexual interactions. Doctoral thesis. Cornell University
Clapham PJ (1996) The social and reproductive biology of humpback whales: an ecological perspective. Mammal Rev 26:27–49
Erbe C, Dunlop R, Jenner KCS, Jenner MN, McCauley RD, Parnum I, Parsons M, Rogers T, Salgado-Kent C (2017) Review of underwater and in-air sounds emitted by Australian and antarctic marine mammals. Acoust Aust 45:179–241
Herman LM, Tavolga WN (1980) The communication systems of cetaceans. In: Herman LM (ed) Cetacean behavior: mechanisms and functions. Wiley, New York, pp 149–209
Höglund J, Alatalo RV (1995) Leks. Princeton University Press
Leroy EC, Royer JY, Alling A, Maslen B, Rogers TL (2021) Multiple pygmy blue whale acoustic populations in the Indian Ocean: whale song identifies a possible new population. Sci Rep 11:1–21
Matthews JN, Brown S, Gillespie D, Johnson M, McLanaghan R, Moscrop A, Nowacek D, Leaper R, Lewis T, Tyack P (2001) Vocalization rates of the North Atlantic right whale (Eubalaena glacialis). J Cetac Res Manage 3:271–281
McCauley RD (2009) Sea noise logger deployment Scott Reef, 2006–2008—whales, fish and seismic surveys. Report prepared for Woodside Energy, CMST R2009–15, p 88, 61 Fig
McCauley RD (2011) Woodside kimberley sea noise logger program, September 2006 to June 2009: Whales, fish and man made noise. Report R2010–50_3 prepared for Woodside Energy, Ltd., p 80
McDonald MA, Hildebrand JA, Mesnick SL (2006) Biogeographic characterization of blue whale song worldwide: using song to identify populations. J Cetac Res Manage 8:55–65
McPherson C, Kowarski K, Delarue J, Whitt C, MacDonnell, J, Martin B (2016) Passive acoustic monitoring of ambient noise and marine mammals—barossa field: JASCO document 00997, Version 1.0. Technical report by JASCO Appl Sci Jacobs. pp. 99
Moreira S, Weksler M, Sousa-Lima RS, Maia M, Sukhovich A, Royer JY, Macondes MCC, Cerchio S (2020) Occurrence of Omura’s whale, Balaenoptera omurai (Cetacea: Balaenopteridae), in the equatorial Atlantic Ocean based on passive acoustic monitoring. J Mammal 101:1727–1735
Mundinger PC (1980) Animal cultures and a general theory of cultural evolution. Ethol Sociobiol 1(3):183–223
Ohsumi S (1980) Population study of the Bryde’s whale in the southern hemisphere under scientific permit in the three seasons 1976/77–1978/79. Rep Int Whal Comm 30:319–331
Parks SE, Hamilton PK, Kraus SD, Tyack PL (2005) The gunshot sound produced by male North Atlantic right whales (Eubalaena glacialis) and its potential function in reproductive advertisement. Mar Mamm Sci 21:458–475
Rosel PE, Wilcox LA, Yamada TK, Mullin KD (2021) A new species of baleen whale (Balaenoptera) from the Gulf of Mexico, with a review of its geographic distribution. Mar Mam Sci 2021:1–34. https://doi.org/10.1111/mms.12776
Sasaki T, Nikaido M, Wada S, Yamada TK, Cao Y, Hasegawa M, Okada N (2006) Balaenoptera omurai is a newly discovered baleen whale that represents an ancient evolutionary lineage. Mol Phylogenet Evol 41:40–52. https://doi.org/10.1016/j.ympev.2006.03.032
Sousa AG, Harris D (2015) Description and seasonal detection of two potential whale calls recorded in the Indian Ocean. J Acoust Soc Am 138:1379–1388. https://doi.org/10.1121/1.4928719
Stafford KM, Moore SE, Fox CG (2005) Diel variation in blue whale calls recorded in the eastern tropical pacific. Anim Behav 69:951–958
Wada S, Numachi K (1991) Allozyme analyses of genetic differentiation among the populations and species of the Balaenoptera. Rep Int Whaling Comm Spec Issue 13:125–154
Wada S, Oishi M, Yamada TK (2003) A newly discovered species of living baleen whale. Nature 426:278–281
Watkins WA, Daher MA, Reppucci GM, George JE, Martin DL, DiMarzio NA, Gannon DP (2000) Seasonality and distribution of whale calls in the North Pacific. Oceanography 13:62–67
Watkins WA, Tyack PL, Moore KE, Bird JE (1987) The 20-Hz signals of finback whales (Balaenoptera physalus). J Acoust Soc Am 82:1901–1912
Wiggins SM, Oleson EM, McDonald MA, Hildebrand JA (2005) Blue whale (Balaenoptera musculus) diel call patterns offshore of southern California. Aquat Mamm 31:161–168
Whiten A, Caldwell CA, Mesoudi A (2016) Cultural diffusion in humans and other animals. Curr Opin Psychol 8:15–21
Yamada TK, Chou LS, Chantrapornsyl S, Adulyanukosol K, Chakravarti SK et al (2006a) Middle-sized balaenopterid whale specimens (Cetacea: Balaenopteridae) preserved at several institutions in Taiwan, Thailand, and India. Mem Natl Sci Mus Tokyo 44:1–10
Yamada TK, Kemper C, Tajima Y, Umetani A, Janetzki H, Pemberton D (2006b) Marine mammal collections in Australia. National Sci Museum Monogr 34:117–126
Yamada TK, Kakuda T, Tajima Y (2008) Middle sized balaenopterid whale specimens in the Philippines and Indonesia. Mem National Museum Natl Sci Tokyo 45:75–83
Acknowledgements
This chapter is a synthesis of work from Madagascar presented elsewhere in peer-reviewed papers and scientific reports, with other studies conducted around the globe, in an effort to cast our current understanding of Omura’s whales as a species in the context of baleen whale behavior and biology. The long-term study off Madagascar would not be possible without the dedication of my Malagasy colleagues, staff, and students, including most prominently Norbert Andrianarivelo, Boris Andrianantenaina, and Tahina Rasoloarijao, and partnership with the Malagasy institutions, Institut Halieutique et des Sciences Marines, and Center National de Recherches Océanographiques, in particular Directors Thierry Lavitra, Jamal Angelot Mahafina, and Mamy Nirina Rajaonarivelo. Danielle Cholewiak, Liam Mueller-Brennan and Sandra Dorning assisted with acoustic analysis, and Tanguy Guillemain d’Echon and Mada Megafauna contributed to field work. Funding was provided by generous grants from the US Marine Mammal Commission, the National Geographic Society Committee for Research and Exploration, the New England Aquarium Marine Conservation Action Fund, and the Woods Hole Oceanographic Institution (through the Dalio Foundation). Lastly, the work presented here has greatly benefited by the support—intellectual, motivational and inspirational—and the friendships of Robert L. Brownell, Christopher W. Clark, and Tadasu K. Yamada.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Cerchio, S. (2022). The Omura’s Whale: Exploring the Enigma. In: Clark, C.W., Garland, E.C. (eds) Ethology and Behavioral Ecology of Mysticetes . Ethology and Behavioral Ecology of Marine Mammals. Springer, Cham. https://doi.org/10.1007/978-3-030-98449-6_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-98449-6_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-98448-9
Online ISBN: 978-3-030-98449-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)