Abstract
Water is required for sustaining life. It is also used in anthropogenic activities such as agriculture, washing, and industries. Emerging technologies to decontaminate wastewater spent filter backwash water (SFBW) and waste sludge have been widely investigated in wastewater treatment plants. Most of the industries produce spent filter backwash water (SFBW). SFBW utilization is important due to the feasible heavy metals recycle, microorganisms and predecessor for disinfection outcomes. Modernization in coagulation and membrane techniques, particularly in Ultrafiltration and micro- treatment, provides an appropriate method for SFBW to assure the water quality needed for reuse. The main advantages of Ultrafiltration (UF) are less land consumption and reliable water quality. It can remove microorganisms from the water completely, improving the biological quality of water. The suspended particles, viruses and colloidal substances in water are purified using this method. As the primary purification technology of urban drinking water, Ultrafiltration is an alternative to recycling industrial wastewater and sewage drains. Compared with the conventional water treatment process, the EC and UF process has higher efficiency, better effects of treatment and low energy consumption. It is important to further investigate ultrafiltration technology to improve the quality of water, protect water resources, and balance the ecological environment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Due to urbanisation, industrialisation and household consumption, a huge amount of wastewater is being generated across the globe. Nevertheless, this unsustainable growth releases toxic chemicals into the air, water, and land and thus contaminating them to an unprecedented scale. Among the pollutants released into water bodies, several were found to be causing serious issues to human health, and analyzing the release mechanism, degradation process, implementing their removal process before discharging into natural water bodies are needed (Karri et al. 2021; Dehghani et al. 2021).
In the traditional water treatment processes, to remove pollutants, protozoa, consisting of organic/inorganic particles, viruses and bacteria, from the coagulation basin effluents, sand filters are used (Adin et al. 2002). They are regularly backwashed to restore the quantity and the SFBW resultant, including the pollutants sedimented in the sand filter (Bourgeois et al. 2004). During backwashing of filter, SFBW consisting of the organism, colloidal materials and inorganic metals are produced by dislodging impurities (Cornwell and Macphee 2001) from the filter. SFBW is reversed to the top of the Water Treatment Plant (WTP) to redeem waste streams (Cornwell and Lee 1994).
SFBW has a negative effect on the finished quality of water if directly recycled (Arora et al. 2001a, b) and endanger the safety of drinking because pathogens, Cryptosporidium and Giardia or Disinfection outcomes might be sedimented in wastewater treatment (Nasser et al. 2002). Among all the approaches for water treating, membrane filtration has the best performance. (LeGouellec et al. 2004) indicates that gradual reduction of DBP, particles and microbes SFBW elements is obtained by separating a membrane known as Ultrafiltration (UF) (Reissmann and Uhl 2006).
SFBW restoring by filtration using a membrane (Walsh et al. 2008), fouling of membrane and clogging of pores due to organic or colloidal particles reduces the membrane flux and the recovery rate next to backwashing (Guigui et al. 2002). A hybrid technique is employed to enhance the flux in the membrane (Howe et al. 2006). Pre-coagulation in combination with separation of the membrane is used to form higher and porous floc on the membrane plane (Chen et al. 2007), deducing the fouling of membrane and clogging, in turn, increases the flux in the membrane (Lin et al. 2008).
Pre-treatment coagulation constraints like dosage of Alum coagulants and pH significantly affects the properties of flocs, that affects the separation of subsequent solid–liquid (Walsh et al. 2008), comprising specific resistance and reduced water ability of coagulated element that controls the efficiency of consequent filtration (Song et al. 2001). The coagulation filtration using membrane facilitates the value of water purified, SFBW with running (Lee et al. 2000), and quality cost at certain conditions near the existing water treatment process.
Recently, the water reuse of SFBW has gained importance in many countries due to water scarcity. SFBW serves as a forever source for the working of WTP (Raj et al. 2008). During the water treatment process, SFBW generated is about 2–10% of total production in the plant. Backwashing using a filter is performed to detach all the sedimented elements using the bed at filtration. There are constraints (Walsh et al. 2008) regarding its reuse due to a higher percentage of metals, organic elements, microbes, heavy metals, and colloidal elements.
Exposure to heavy metals routes consists of absorption, ingestion and inhalation. Insertion through the raw water is significant for exposure to heavy metals. Major health issues like breathing shortness, mutagenic, neurotoxic effects with various cancers are caused by heavy metal contamination in drinking water (Chowdhury et al. 2016). SFBW restoration endangers the superiority of the water processed due to the contaminants with more concentration percentage (Ang et al. 2015). SFBW is conducted using various methods.
Filtration using membranes such as MF and UF effectively removes colloids, particulates and pathogens. It requires a lower footprint, low consumption of energy and quality water production. Fouling is the major issue in the membrane process, particularly for SFBW processes. UF membrane processes have proven capabilities to reject turbidity and suspended solids (Zhang et al. 2008). With proper pre-treatment, such as coagulation-flocculation-sedimentation (CFS), UF membranes can also remove viruses, bacteria, and pathogens (Gao and Yue 2005).
The required treatment for SFBW prior to restoration at the plant differs between sites provided by the purpose of the treatment (Zhao et al. 2008). A conventional approach is needed to minimize the SFBW reusing effect on raw water quality (Lai et al. 2015). SFBW has to be processed previous to its reuse (Yu et al. 2013). The choice for disposal includes discharge to a receiving stream or a sewer (Chen et al. 2014). SFBW contains heavy metals and disinfectants that are harmful to the biological life of the stream, discharging directly to streams is usually restricted (Peter et al. 2011). Discharge to the drain has to be controlled depending on the ingredients and SFBW’s total amount (Wang et al. 2016). For most water processing plants, particularly in water scarce areas or arid, SFBW recycling holds to be a feasible option (Wang et al. 2014).
To control this problem, membrane elements combined with a process like coagulation pre-treatment, sedimentation and Ultrafiltration are applied (Huang et al. 2011). Coagulation is the essential technique to reduce the particulate elements, colloids, suspended solid elements, organic and dissolved elements (Yu et al. 2013) prior to the process in the membrane. The biopolymer (Uday Kumar et al. 2021) and biopolymer composites easily fit into different parts of the treatment process by acting as filtration media, adsorbents, coagulants and as flocculants. Upon increasing the aggregation of flocs (Thirugnanasambandham et al. 2021), most flocs settle down, thus improving removal efficiencies during the coagulation process.
UF is an efficient method to reduce microbes, colloids and suspended elements. Application of UF membrane (Li et al. 2012) for treatment of backwash water aids in addressing the issues of fouling and (Raj et al. 2008) the quality of treated water for drinking and other application. Many investigators have identified EC as a promising water purification method (Song et al. 2017) for potable water and wastewater. Compared to other processes, EC (Das and Nandi 2021) shows several enticing advantages like minimal expenses, ease of install, no requirement of chemicals, and lower treatment time.
2 Spent Filter Backwash Water Treatment Methods
2.1 Determination of Optimum Coagulant Dose Using Jar Test
Jar tests are the experimental way to find the optimum coagulant dosage for the SFBW treatment. The test is performed in a jar-test apparatus (Fig. 1), consisting of six beakers at room temperature with one litre volume. The test comprises three subsequent steps: rapid mixing takes place initially at 200 rpm for about 1 min, after that, mixing is done at 30 rpm for 20 min. Stirring is then stopped for the sludge to sediment. Once the sedimentation is done, the residue liquid is drawn from a zone about 2 cm beneath the liquid level of the beaker to obtain the turbidity of the SFBW treatment using a turbidimeter. pH test to observe the pH value for the water using pH electrodes, pH meters and PerpHect Ag/AgCl Gel triode. Total dissolved solids (TDS) are calculated using the Conductivity Meter equipment.
Electrical conductivity (EC) is measured to indicate changes in water composition by conductivity equipment. Chlorine test to show the amount of chlorine residue in wastewater, evaluated by Stable neutral Orth tolidine method, at a range of 625 nm with neutral Orth tolidine reagent, Buffer—stable reagent. A spectrophotometer does detection of aluminium in wastewater at 553 nm with Eriochrome cyanine R stock solution, dye solution, Sulfuric acid and Ascorbic acid. Determination of the sulphate concentration using a spectrophotometer at 420 nm with conditioning reagent, BaCl2—crystals (20–30 mash).
To measure the Fe II, Fe III content in water, a spectrophotometer at 510 nm with Hydrochloric acid, Hydroxylamine chloride, Ammonium acetate, Sodium acetate, Phenanthroline are used. The total alkalinity test indicates the amount of carbonates, bicarbonates, and hydroxides in the water. It is noticed by titration of a sample electrometric aliquot with a standard strong acid solution (H2SO4), the result is identified by pH meter.
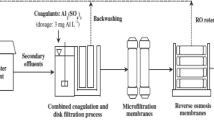
To improve filtration performance, the SFBW is to be pre-treated by two levels: sedimentation elements and coagulation. Large particles are preferably removed through pre-settling, and solid particles from supernatants become low, improving the flux in the membrane during filtration. SFBW is allowed to settle at various time durations prior to the filtration. After allowing to settle for 15 min, the supernatant liquid is used as the pre-settled SFBW for microfiltration.
2.2 Spent Filter Backwash Water Treatment by Chemical Disinfection
Chemical disinfection is an alternative technique for SFBW treatment before recycling the water for further process. (Arora et al. 2001a, b) Determination of disinfectant for the treatment is essential to measure the demand of oxygen obtained by various elements in SFBWs. Filtration techniques in wastewater treatment discharge wash water consisting of suspended solids with minimum concentration varying in the range 30 to 400 mg/l, based on precipitations of raw water treated and the amount of backwash water recycled. About 2–3% of the flow in treatment is the filter backwash water. SFBW contains 10–20% total elements, both organic elements and microbes, as the effect of microbial growth in the filters. While recycling the SFBW, there is an increase in concentrations of Cryptosporidium and Giardia in the wastewater, resulting in unwanted growth of the microorganisms into the water supply (DiGiovanni et al. 1999). Particles in SFBW affect disinfection's efficiency due to their mechanism involved in particle-less wastewaters. In the process, Potassium permanganate and Chlorine dioxide are utilized for demand in oxidant and measurement of disinfection since it does not produce Tri Halo Methane (THM) outcomes. For each sample of SFBW, oxidant demands are obtained. Particle-less samples are measured by slow centrifugation for about 10 min along with filtration using a 0.22 µm filter (bottle top). Elements containing particles are extracted utilizing a 0.22 µm filter (syringe) by a prior filtration before oxygen demand, measurements of glass-fibre residue are done. For the deactivation of Cryptosporidium, various SFBW elements are utilized.
Cell culture–quantitative sequence detection method (CC–QSD) is considered to find the disinfection mechanism of Cryptosporidium. Treated Cryptosporidium oocysts are obtained. In every oxidant sample, dosages are implemented for oxygen demand in SFBW samples, considering with and without elements. SFBW elements mixed with Cryptosporidium oocysts are processed for 10 min by KMnO4 and ClO2. After the designated contact time, samples are evaluated by CC–QSD technique for Cryptosporidium invasion. Since the pre-settling removes large particles, the sub micrometre elements are left in the residue liquid, the initial flux is lowered by the sub micrometre elements that are lower compared to the pore of the membrane. Due to the filtration of SFBW, sludge obtained from large particles removes a particular range of submicrometric elements before passing the surface of the primary zone. The amount of solid in SFBW improves microbial removal in the pilot run. Polymer treatment for SFBW (cationic and anion) results in an efficient deduction of total solids along with microbes in the treated wastewater.
2.3 Spent Filter Backwash Water Treatment by Coagulation
This process includes primary deposit, coagulation, thickening and Ultrafiltration using membranes for SFBW treatment. Conventional poly aluminium chloride (PACl) is generally used as a coagulant. In the pilot unit for all stages, except UF, the rate of flow to be maintained is about 10 L/h. Hydraulic retention time (HRT) in this stage is to be maintained approximately at 60, 6, 48, and 192-min (Mahdavi et al. 2016). Figure 2 represents the experimental setup used in the coagulation experiment. Coagulation is performed at standard pH (8.3) and estimated PAFCl amount (15 mg/L) with FeCl3 amount (40 mg/L) being regularly added into the agitating zone (with HRT for 6 min, at 80 rpm speed). Next, the coagulated effluent passes through two tanks for flocculation, with a 40-rpm agitating intensity. The SFBW is pre-treated with PACl prior to the filtration using a membrane. A specific percentage of coagulant is mixed with SFBW sample at a maintained pH 7, then by fast agitation for 1 min, 20 min of slow mix and the end settling for about 20 min. Pre-treatment before coagulation depicts the decline in membrane flux and the effect of coagulation efficiency.
The coagulated sample is utilized as the feed in the membrane UF module. To identify the optimum dose, selection has been done for both coagulants for 4 days separately and continuously (Fig. 2). The optimum dose is determined to produce the reliable water quality depending on precipitation and percentage of colour. Elements are then evaluated after the second clarification of two HRT. Then about 1,000 L of SFBW is processed separately by coagulants. Next, 800 L volume processed water by PAFCl coagulant, and 800 L volume of processed water by FeCl3 coagulant enters through UF membrane. Various parameters, like colour, turbidity, TC, FC and pH, are observed about ten times at pilot scale measurement, while the concentration of heavy metals is observed three times for optimum dose and processed water quality.
Electrocoagulation (EC) has been considered a more efficient process for removing multiple water contaminants simultaneously from contaminated water in recent years as an alternative to other conventional techniques. The main advantage of the EC process is low investment cost, no addition of chemical requirement, easy to operate and most importantly, very low production of sludge (Thakur and Mondal 2017). The electrocoagulation process works on the principle of a standard electrochemical cell. The sacrificial metal anode dissolute in the aqueous medium and generates hydrolysis product (hydroxo-metal species) which adequately destabilizes the contaminant particles and structures coagulants. Electrolysis gases (H2 and O2) produced due to electrochemical reduction (Das and Nandi 2019) of water in the cathode initiates soft turbulence in the aqueous medium and works in combination to enhance the flocculate of the coagulant materials.
2.4 Water Treatment Using Ultrafiltration Membranes
The ultrafiltration membrane extracts effluents of SFBW is a purified flow of water called permeate and a concentrated flow of water known as concentrate or retentate (Mulder et al. 1997). Membranes generally comprise a supportive porous layer (100 µm) and a thin upper layer of about 0.1 to 1.0 µm (Aptel et al. 1996). Most of the fibre utilized in the SFBW process are made of aromatic polyamides, PPL, thin-film composite (TFC) or cellulose acetate. Inorganic fibres contain extra zirconium oxide (ZrO2) and aluminium oxide (Al2O3). The membrane module describes a unit including membranes, feed inlet, pressure support structure, effluent outlet and overall structure. Various membrane modules utilized in wastewater treatment are as follows:
-
Tubular type has a diameter of more than 3 mm fibre inside a bundled module;
-
Capillary or spiral type fiber membranes have internal diameter lower than 3 mm, inside a bundled module with 100’s–1000’s of particles of fibre;
-
Round wound type membranes are generally flat membranes that are captured inside a spacer;
-
Plate type and frame type membranes consist of flat sheets connected in series as membrane and its support sheets.
The course of flow in tube type and swirl fibre type are either interior-out or exterior-in. For the interior-out mode, the flow of influent is from the interior side of the swirl tube to the external side of the membrane, later purified water (known as permeate) is obtained. The exterior-in mode depicts flow in the opposite course.
UF membrane comprises polypropylene fibre, porous, with a range of about 0.01–0.2 μm. The area of the total UF membrane is around 0.1 m2/module. In this module, the membrane is run in end mode using filtration at a constant rate of about 8 L/ m2 h1 at the pressure of 300 Pa inside the membrane. It is processed in a duration of 60 min for filtration and SFBW for 1 min in a combination of permeate in the opposite direction.
As indicated in Table1 (Mahdavi et al. 2018), turbidity of the residue and the color obtained after coagulation and ultrafiltration process shows major reduction (Berthon et al. 2002). The filtration's measured parameter level is comparatively very low and complies with the EPA drinking water standard. Variables in SFBW restored samples are the concentration of heavy metals that has major health effects on the environment and humankind. It is observed that most of the heavy metals and metals are reduced by coagulation in treated SFBW. It is inferred that a major percentage of metal content is connected by elements related to clay, biological content, silt or particles while at the process of effluent treatment. Along with reducing suspended elements of solids during coagulation technique, colloids and particles, a major percentage of the heavy metals are removed efficiently.
3 Recent Developments and Research
3.1 Limiting Microbial Quality
The outcome of different doses of about 5 to 60 mg/L of FeCl3 and PAFCl is to identify the optimum dose. It is inferred that the optimum dosage level for FeCl3 and PAFCl is about 15 and 40 mg/L. In this dose level, the removal percentage of turbidity is 99.6 and 99.4%. In each level of dose, precipitation level is determined after HRT. While the PAFCl removal efficiency of precipitation level is enhanced to 15 mg/L. Beyond this limit, balancing of coagulant and a subsequent enhancement in precipitation occurs.
UF membrane process, the input water quality is considered important due to fouling problems. Utilizing optimum doses of FeCl3 and PAFCl, turbidity of water treated levels up to 2.4 and 3.9 Nephelometric Turbidity Units (NTU). Next, SFBW is passed through the UF process, and the value of processed wastewater microbes are evaluated. Due to coagulation and flocculation, most microorganisms like particles refer to bacteria and protozoans, and virus refers to organic elements. Turbidity reduction is indirectly related to the reduction of microbial content in SFBW. In this process, both the coagulants depicted an influence on microbe reduction (Sutherland et al. 2003).
3.2 Minimizing Metals and Heavy Metal
It is observed that the coagulants had better efficiency for removing heavy metals (Appel et al. 2002). However, heavy metal removal by PAFCl is comparatively better than FeCl3. Figure 3 depicts the Concentration of heavy metals in SFBW processed with PAFCl have been resulting in the sequence: Aluminium > Iron > Cadmium > Arsenic and Lead. After coagulation by FeCl3, the sequence is Iron > Aluminium > Cadmium > Arsenic and Lead. Next, the PAFCl-UF process sequence of heavy metals is Aluminium > Cadmium > Iron, Arsenic and Lead. consequently, sequence for FeCl3 with UF are Iron > Aluminium > Cadmium > Arsenic and Lead.
It is noticed, the coagulation process has deduced the majority of the percentage of the heavy metal in SFBW treatment. Clay and soil possess great properties in heavy metal adsorption like Lead are obtained from fractionation column. Heavy metals can be captured using the Fe-oxide fraction (Orrono and Lavado 2009).
Coagulation combined with FeCl3, experimented under high coagulant concentration of about 40 mg/L, from the flocculation process, by entrapment of organic matter and particles in the flocs that are formed, all the connected heavy metals and metals are removed consequently (Ebrahimi et al. 2015). A hydrolysed coagulant PAFCl has more positive ions than traditional coagulants. It contains Fe and Al ions. The characteristics of Al(OH)3 and Fe(OH)3 elements are utilized. Positive ions of PAFCl adsorb negatively charged colloids, particles, and organic matter attracts heavy metals. Coagulation combined with PAFCl happens under low coagulant concentration comprises adsorption kinetics. It is observed that the adsorption kinetics for heavy metals removal is better compared to PAFCl.
4 Future Research Perspective
Major operational challenges such as control of membrane fouling and the optimization of system recovery still exist for many surface water treatment plants (Gao et al. 2011; Huang et al. 2009). As a result, public water systems are motivated to mitigate membrane fouling while simultaneously reducing their residual streams in a cost-efficient manner. However, fouling causes loss of membrane permeability, considered a drawback to the universal application of membrane processes in the wastewater treatment industry. Fouling control is a major focus of water treatment research (Gao et al. 2011). Membrane fouling occurs during filtration as constituents accumulate on or adsorb to the surface of the membrane. Fouling causes loss of membrane permeability and continuous permeability decline (Jacangelo et al. 1989) that is often considered the largest barrier to membrane adoption in the water treatment industry. These goals must be met while continuing to meet existing and emerging regulations. As a result, research needs to explore issues related to membrane fouling and operational efficiency (Jermann et al. 2007). Recent studies have examined the long-term fouling behaviour of membrane systems used in water treatment, addressing strategies to reduce fouling through operational changes and new pre-treatment applications. Specifically, four concepts are explored in this method:
-
(1)
Long-term fouling behaviour of ultrafiltration membrane studied at the pilot-scale, revealing that commonly used surrogate water quality measures did not correlate well with chemically irreversible fouling.
-
(2)
Pre-oxidation with ozone as pre-treatment to decrease fouling rate at the pilot scale.
Membrane fouling from Organic Matter (OM) occurs as organic content sedimented as a deposit and forms a sludge or adsorbs to the surface of a membrane directly (Susanto et al. 2007; Zularisam et al. 2006). Fouling is often modelled as resistance-in series (Boyd et al. 2013; Huang et al. 2008; Nguyen et al. 2011) are characterized based on fouling through cleaning. Organic fouling leads to decreased permeability and is oftentimes difficult to reverse through cleaning (Lehman et al. 2009). Therefore, considerable research has been published on pre-treatment strategies that remove or destroy organic foulants to reduce the irreversible fraction of fouling. Previous bench-scale work has identified pre-oxidation with ozone (preozonation) as a treatment strategy to reduce the organic fouling of membranes.
Ozone is a powerful oxidant that can break down or destroy complex organic compounds known to cause fouling of polymeric membranes (Gao et al. 2011; Van Geluwe et al. 2011). Previous research has generally focused on applications of pre-ozonation with ceramic ozone-resistant membranes (Lee et al. 2013; Sartor et al. 2008). Furthermore, most studies have investigated the use of ozone in direct-filtration applications, while other studies, independently, have considered the use of preozonation as a coagulant aid (Bose et al. 2007; Schneider and Tobiason 2000; Singer et al. 2003). However, very few studies have evaluated the integration of ozone, coagulation, and membrane filtration. Specifically, there is a lack of knowledge regarding the downstream impact of ozone-coagulation treatment on membrane fouling.
-
(3)
The effect of ozone on organic matter in water investigated at the bench scale.
-
(4)
The impact of recycling of membrane backwash water on system performance.
The most commonly recycled waste for conventional treatment plants with traditional media filters is spent filter backwash water (Arora et al. 2001a, b). Spent filter backwash water contains concentrated levels of the constituents found in raw water, including Cryptosporidium. In lieu of conventional filters, water plants utilize ultrafiltration membranes instead produce membrane backwash water (MBWW). Most plants choose to recycle a portion of their MBWW in order to improve their system recovery. MBWW is concentrated with constituents retained by an ultrafiltration membrane and may contain membrane cleaning chemicals.
As demonstrated by (Boyd et al. 2012), if recycled within a treatment plant, these constituents may impact UF process performance. Given that fouling of UF membranes remains a major challenge for public water systems, there is a need to better understand the impacts of MBWW recycling, the fouling characteristics of these waste streams, and the necessary treatment to mitigate fouling.
5 Conclusion
For recycling of SFBW, presetting prior to filtration using membrane worsens separation efficiency using a membrane. The pre-treatment by coagulation improves the characteristics of UF membrane operation by enhancing flux by a reduction in the pore blockage. It is observed that pre-treatment by coagulation along with charge neutralization at the optimum dosage, the better performance of consequent filtration using membrane for restoring SFBW is obtained. Coagulation, before membrane ultrafiltration, improves permeate quality and reduces the UF membrane fouling while increasing the effectiveness and efficiency of the ultrafiltration process, i.e., elimination of the hydraulic efficiency and the organic substances. The obtained water is disinfected and particle-free, possess sufficient quality for domestic use or after treatment.
Continuous processing of pilot-plant investigation is expected for evaluation of the efficiency and to optimize the process control. Traditional treatment depicts that the polymeric treatment (anionic or cationic) efficiently calculates microorganisms and solids. Treatment with cationic polymer and chemicals like FeCl3 infers an effective deduction of DOC (70%). In treated SFBW, all heavy metals and metals concentrations comply with the potable water guidelines published by EPA with both processes. Removal percentage is better with PAFCl than FeCl3 in the coagulation process. Minimizing raw water demand as well as preventing the natural stream from high sedimentation by decreasing the level of pollution. Spent filter backwash water recycling accounts for water resources conservation.
References
Adin A, Dean L, Bonner F, Nasser A, Huberman Z (2002) Characterization and destabilization of spent filter backwash water particles. Water Supply 2(2):115–122
Ang WL, Mohammad AW, Hilal N, Leo CP (2015) A review on the applicability of integrated/hybrid membrane processes in water treatment and desalination plants. Desalination 363:2–18
Appel C, Ma L (2002) Concentration, pH, and surface charge effects on cadmium and lead sorption in three tropical soils. J Environ Qual 31:581–589
Aptel P, Buckley CA (1996) Categories of membrane operations (Ch. 2). In: Mallevialle J, Odendaal PE, Wiesner MR (eds) Water treatment membrane processes. McGraw-Hill, New York
Arora H, Di Giovanni G, Lechevallier M (2001a) Spent filter backwash water contaminants and treatment strategies. J Am Water Works Assoc 93(5):100–112
Arora H, Di-Giovanni G, LeChevallier M (2001b) Spent filter backwash water contaminants and treatment strategies. J. AWWA 93:100–112
Berthon G (2002) Aluminium speciation in relation to aluminium bioavailability, metabolism and toxicity. Coord Chem Rev 228:319–341
Bose P, Reckhow DA (2007) The effect of ozonation on natural organic matter removal by alum coagulation. Water Res 41(7):1516–1524
Bourgeois JC, Walsh ME, Gagnon GA (2004) Comparison of process options for treatment of water treatment residual streams. J Environ Eng Sci 3(6):477–484
Boyd CC, Duranceau SJ (2013) Evaluation of ultrafiltration process fouling using a novel transmembrane pressure (TMP) balance approach. J Membr Sci 446:456–464
Boyd CC, Duranceau SJ, Tharamapalan J (2012) Impact of carboxylic acid ultrafiltration recycle streams on coagulation. J Water Supply Res Technol AQUA 61(5):306–318
Chen F, Peldszus S, Peiris RH, Ruhl AS, Mehrez R, Jekel M, Legge RL, Huck PM (2014) Pilot-scale investigation of drinking water ultrafiltration membrane fouling rates using advanced data analysis techniques. Water Res 48:508–518
Chen Y, Dong BZ, Gao NY, Fan JC (2007) Effect of coagulation pretreatment on fouling of an ultrafiltration membrane. Desalination 204(1–3):181–188
Chowdhury S, Mazumder MAJ, Al-Attas O, Husain T (2016) Heavy metals in drinking water: occurrences, implications, and future needs in developing countries. Sci Total Environ 569:476–488
Cornwell DA, Lee RG (1994) Waste stream recycling-its effect on water-quality. J AWWA 86:50–63
Cornwell DA, Macphee MJ (2001) Effects of spent filter backwash recycle on Cryptosporidium removal. J AWWA 93(4):153–162
Das D, Nandi BK (2019) Defluoridization of drinking water by electrocoagulation (EC): process optimization and kinetic study. J Dispersion Sci Technol 40(8):1136–1146
Das D, Nandi BK (2021) Arsenic removal from tap water by electrocoagulation: investigation of process parameters, kinetic analysis, and operating cost. J Dispersion Sci Technol 42(3):328–337
Dehghani MH, Omrani GA, Karri RR (2021) Solid waste—sources, toxicity, and their consequences to human health. In: Soft computing techniques in solid waste and wastewater management (Elsevier)
Di Giovanni G et al (1999) Quantitation of intact and infectious cryptosporidium parvum oocysts using quantitative sequence detection. Proc 1996 AWWA WQTC, Tampa, Fla
Ebrahimi A, Taheri E, Pashaee A, Mahdavi M (2015) The effectiveness of polyaluminum ferric chloride (PAFCl) for turbidity and color removal from Isfahan raw water. Desalin Water Treat 55:1966–1972
Gao B, Yue Q (2005) Effect of ratio and OH−/Al3+ value on the characterization of coagulant poly-aluminum-chloride-sulfate (PACS) and its coagulation performance in water treatment. Chemosphere 61:579–584
Gao W, Liang H, Ma J, Han M, Chen Z-L, Han Z-S, Li G-B (2011) Membrane fouling control in ultrafiltration technology for drinking water production: a review. Desalination 272(1–3):1–8
Guigui C, Rouch JC, Durand-Bourlier L, Bonnelye V, Aptel P (2002) Impact of coagulation conditions on the in-line coagulation/UF process for drinking water production. Desalination 147(1–3):95–100
Howe KJ, Marwah A, Chiu KP, Adham SS (2006) Effect of coagulation on the size of MF and UF membrane foulants. Environ Sci Technol 40(24):7908–7913
Huang C, Lin J-L, Lee W-S, Pan JR, Zhao B (2011) Effect of coagulation mechanism on membrane permeability in coagulation-assisted microfiltration for spent filter backwash water recycling. Colloids Surf, A 378:72–78
Huang H, Schwab K, Jacangelo JG (2009) Pretreatment for low pressure membranes in water treatment: a review. Environ Sci Technol 43(9):3011–3019
Huang H, Young TA, Jacangelo JG (2008) Unified membrane fouling index for low pressure membrane filtration of natural waters: principles and methodology. Environ Sci Technol 42(3):714–720
Jacangelo JG, Aieta EM, Carns KE, Cummings EW, Mallevialle J (1989) Assessing Hollow-Fiber Ultrafiltration for Participate Removal. Journal (Amer Water Works Assoc) 81(11):68–75
Jermann D, Pronk W, Meylan S, Boller M (2007) Interplay of different NOM fouling mechanisms during Ultrafiltration for drinking water production. Water Res 41(8):1713–1722
Karri RR, Ravindran G, Dehghani MH (2021) Wastewater—sources, toxicity, and their consequences to human health. In: Soft computing techniques in solid waste and wastewater management. Elsevier, pp. 3–33
Lai C-H, Chou Y-C, Yeh H-H (2015) Assessing the interaction effects of coagulation pretreatment and membrane material on UF fouling control using HPSEC combined with peak-fitting. J Membr Sci 474:207–214
Lee JD, Lee SH, Jo MH, Park PK, Lee CH, Kwak JW (2000) Effect of coagulation conditions on membrane filtration characteristics in coagulation-microfiltration process for water treatment. Environ Sci Technol 34(17):3780–3788
Lee W, Lee H-W, Choi J-S, Oh HJ (2013) Effects of transmembrane pressure and ozonation on the reduction of ceramic membrane fouling during water reclamation. Desalin Water Treat 52(4–6):612–617
LeGouellec YA, Cornwell DA, MacPhee MJ (2004) Treating Microfiltration Backwash. J AWWA 96(1):72–83
Lehman SG, Liu L (2009) Application of ceramic membranes with pre-ozonation for treatment of secondary wastewater effluent. Water Res 43(7):2020–2028
Li S, Heijman SGJ, Verberk J, Verliefde ARD, Amy GL, Van Dijk JC (2012) Removal of different fractions of NOM foulants during demineralized water backwashing. Sep Purif Technol 98:186–192
Lin JL, Huang CP, Pan JR, Wang DS (2008) Effect of Al (III) speciation on coagulation of highly turbid water. Chemosphere 72:189–196
Mahdavi M, Amin MM, Hajizadeh Y, Farrokhzadeh H, Ebrahimi A (2016) Removal of different NOM fractions from spent filter backwash water by polyaluminum ferric chloride and ferric chloride. Arabian J Sci Eng, 1–8
Mahdavi M, Amin MM, Mahvi AH, Pourzamani H, Ebrahimi A (2018) Metals, heavy metals and microorganism removal from spent filter backwash water by hybrid coagulation-UF processes. J Water Reuse Desalination 8(2):225–233
Mulder M (1997) Basic principles of membrane technology (2nd edition, corrected). Kluwer Academic Publishers, Dordrecht
Nasser A, Huberman Z, Dean L, Bonner F, Adin A (2002) Coagulation as a pretreatment of SFBW for membrane filtration. Water Supply 2(5–6):301–306
Nguyen AH, Tobiason JE, Howe KJ (2011) Fouling indices for low pressure hollow fiber membrane performance assessment. Water Res 45(8):2627–2637
Orrono DI, Lavado RS (2009) Distribution of extractable heavy metals in different soil fractions. Chem Speciat Bioavailab 21:193–198
Peter-Varbanets M, Margot J, Traber J, Pronk W (2011) Mechanisms of membrane fouling during ultra-low pressure ultrafiltration. J Membr Sci 377:42–53
Raj CBC, Kwong TE, Cheng WW, Fong LM, Tiong SH, Klose PS (2008) Wash water in waterworks: contaminants and process options for reclamation. J Environ Sci 20:1300–1305
Reissmann FG, Uhl W (2006) Ultrafiltration for the reuse of spent filter backwash water from drinking water treatment. Desalination 198(1–3):225–235
Sartor M, Schlichter B, Gatjal H, Mavrov V (2008) Demonstration of a new hybrid process for the decentralized drinking and service water production from surface water in Thailand. Desalination 222(1–3):528–540
Schneider OD, Tobiason JE (2000) Preozonation effects on coagulation. Journal (Amer Water Works Assoc) 92(10):74–87
Singer PC, Arlotta C, Snider-Sajdak N, Miltner R (2003) Effectiveness of preand intermediate ozonation on the enhanced coagulation of disinfection byproduct precursors in drinking water. Ozone Sci Eng 25(6):453–471
Song H, Fan X, Zhang Y, Wang T, Feng Y (2001) Application of microfiltration for reuse of backwash water in a conventional water treatment plant-a case study. Water Supply 1(5–6):199–206
Song P, Yang Z, Zeng G, Yang X, Xu H, Wang L, Xu R, Xiong W, Ahmad K (2017) Electrocoagulation treatment of arsenic in wastewater: a comprehensive review. Chem Eng J 317:707–725
Susanto H (2007) Fouling study in ultrafiltration-mechanism and control via membrane surface modification, Universität Duisburg-Essen, Fakultät für Chemie» Technische Chemie
Sutherland RA (2003) Lead in grain size fractions of road-deposited sediment. Environ Pollut 121:229–237
Thakur LS, Mondal P (2017) Simultaneous Arsenic and fluoride removal from synthetic and real groundwater by electrocoagulation process: parametric and cost evaluation. J Environ Manag 190:102–112
Thirugnanasambandham K, Karri RR (2021) Preparation and characterization of Azadirachta indica A. Juss. plant based natural coagulant for the application of urban sewage treatment: modelling and cost assessment. Environ Technol Innov 23:101733
Udayakumar GP, Muthusamy S, Selvaganesh B, Sivarajasekar N, Rambabu K, Sivamani S, Hosseini-Bandegharaei A (2021) Ecofriendly biopolymers and composites: preparation and their applications in water-treatment. Biotechnol Adv, 107815
Van Geluwe S, Braeken L, Van der Bruggen B (2011) Ozone oxidation for the alleviation of membrane fouling by natural organic matter: a review. Water Res 45(12):3551–3570
Walsh ME, Gagnon GA, Alam Z, Andrews RC (2008) Biostability and disinfectant by-product formation in drinking water blended with UF-treated filter backwash water. Water Res 42(8–9):2135–2145
Wang H, Qu F, Ding A, Liang H, Jia R, Li K, Bai L, Chang H, Li G (2016) Combined effects of PAC adsorption and in situ chlorination on membrane fouling in a pilot-scale coagulation and ultrafiltration process. Chem Eng J 283:1374–1383
Wang Z, Teychene BT, Chalew TEA, Ajmani GS, Zhou T, Huang H, Wu X (2014) Aluminum-humic colloid formation during pre-coagulation for membrane water treatment: mechanisms and impacts. Water Res 61:171–180
Yu W-Z, Graham N, Liu H-J, Qu J-H (2013) Comparison of FeCl3 and alum pre-treatment on UF membrane fouling. Chem Eng J 234:158–165
Zhang L-L, Yang D, Zhong Z-J, Gu P (2008) Application of hybrid coagulation-microfiltration process for treatment of membrane backwash water from waterworks. Sep Purif Technol 62:415–422
Zhao H, Hu C, Liu H, Zhao X, Qu J (2008) Role of aluminum speciation in the removal of disinfection byproduct precursors by a coagulation process. Environ Sci Technol 42:5752–5758
Zularisam AW, Ismail AF, Salim R (2006) Behaviours of natural organic matter in membrane filtration for surface water treatment—a review. Desalination 194(1–3):211–231
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sukanya, K., Sivarajasekar, N., Saranya, K. (2022). Spent Filter Backwash Water Treatment by Coagulation Followed by Ultrafiltration. In: Karchiyappan, T., Karri, R.R., Dehghani, M.H. (eds) Industrial Wastewater Treatment . Water Science and Technology Library, vol 106. Springer, Cham. https://doi.org/10.1007/978-3-030-98202-7_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-98202-7_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-98201-0
Online ISBN: 978-3-030-98202-7
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)