Abstract
Complex pelvic ring injuries are responsible for significant morbidity and mortality in trauma patients in the setting of hemodynamic compromise and pelvic hemorrhage. An option for primary hemorrhage control for pelvic fracture-related bleeding that has become more widely accepted in the past decade is preperitoneal pelvic packing (PPP). Initial evaluation and management of patients with pelvic fractures are guided by the Advanced Trauma Life Support protocol, followed by application of a pelvic binder, chest radiograph, focused assessment with sonography in trauma (FAST) examination, and resuscitative endovascular balloon occlusion of the aorta (REBOA) placement if appropriate. If the patient remains hemodynamically unstable despite initial resuscitation and 2 units of packed red blood cells (PRBCs), the patient should be taken to the operating room emergently for external fixation and PPP. Angiography and angioembolization are reserved for patients who have persistent hemodynamic instability present after external fixation and PPP; additionally, patients who require transfusion of more than four units of PRBCs in the 12 h post-packing after normalization of coagulation indices should also undergo diagnostic angiography. Ultimately, the addition of PPP with complementary angioembolization appears to result in the lowest mortality rate for hemodynamically unstable pelvic fracture patients.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Learning Objectives-
Describe the morbidity and mortality associated with pelvic ring injuries and hemodynamic compromise.
-
Outline the principles of initial management of patients with pelvic ring injuries, in particular those who present in hemorrhagic shock.
-
Define indications and operative technique for preperitoneal pelvic packing (PPP).
-
Describe scenarios in which angiography and angioembolization are indicated after external fixation and PPP.
1 Background
Pelvic ring injuries are responsible for significant morbidity and mortality in trauma patients. The majority of injuries are due to high energy blunt trauma including falls, motor vehicle collisions, and auto-pedestrian mechanisms, and can be life threatening, with mortality rates up over 30% in modern series [1,2,3,4,5]. Patients with pelvic ring injury who present with hemodynamic compromise have a significantly higher rate of mortality, nearly four-fold higher than those without hemodynamic instability [6]. In severely injured patients with pelvic fractures and hemodynamic compromise, the primary cause of early death is due to hemorrhage, whereas late mortality is driven by traumatic brain injury and multiorgan failure [7]. Factors predictive of mortality include hemodynamic instability, lactic acidosis, age >65 years, female sex, and injury severity, specifically concomitant chest and bowel injuries [8, 9]. Despite ongoing evolutions in trauma care, the mortality rate has remained high [10, 11], highlighting potential for improvement in the current approach to pelvic fracture management.
As noted in modern series, there is a significant variation in the diagnostic and therapeutic approach to patients with pelvic fractures and hemodynamic compromise. An option for primary hemorrhage control for pelvic fracture-related bleeding that has become more widely accepted in the past decade is preperitoneal pelvic packing (PPP). Rationale for PPP includes more rapid hemorrhage control compared to angioembolization by addressing the primary source of hemorrhage [12]. The objective of this book chapter is to describe the initial management of patients with complex pelvic ring injury, as well as the indications, operative technique, and outcomes of PPP.
2 Initial Evaluation and Management of the Pelvic Fracture Patient
Initial evaluation and management of patients with pelvic fractures should be approached with attention to the primary survey and ATLS protocol [13]. In any patients with blunt mechanism and hypotension (systolic blood pressure [SBP] <90 mmHg), a pelvic binder or pelvic stabilization with a sheet should be placed at the level of the greater trochanters; pelvic binding in of itself can significantly reduce pelvic volume, prevent shifting of bony elements, and improve hemorrhage control [4, 14,15,16]. As part of the initial assessment, Focused Assessment with Sonography for Trauma (FAST) exam and chest radiograph (CXR) should be performed to rule out intraperitoneal or intrathoracic sources of hemorrhage. The FAST exam reliably identifies clinically significant hemoperitoneum in life-threatening pelvic fracture-related hemorrhage, with a false-negative rate as low as 2% [17].
In patients with hypotension unresponsive to resuscitation (persistent SBP <80 mmHg), insertion of a resuscitative endovascular balloon occlusion of the aorta (REBOA) catheter should be considered for Zone III (infra-renal) inflation [18]. REBOA ultimately allows for temporary and/or partial occlusion as a bridge to further resuscitation, imaging, and transport to the operating room; preliminary data of patients with concomitant REBOA and PPP suggest that this combination provides life-saving hemorrhage control in otherwise devastating injuries [19]. Notably, while Zone III REBOA has been shown to decrease pelvic hemorrhage, it does not generate as much pelvic pressure as preperitoneal packing, an essential factor of venous hemostasis [20]. Further, when comparing isolated REBOA to isolated PPP for pelvic hemorrhage control, patients who receive REBOA spend longer time in the emergency department with greater mortality rates than with PPP [21]. However, in conjunction, REBOA and PPP result in expeditious time to hemorrhage control [19, 22].
In conjunction initial ATLS-driven care, FAST, CXR, and REBOA, particular attention should be paid to concomitant injures, specifically chest wall, extremity, spine, and genitourinary injuries [23,24,25,26,27,28]. Over two-thirds of severely injured patients with pelvic fractures have concomitant injuries which merit surgical intervention at some point during their hospitalization, and nearly one-fourth have a concomitant injury identified on initial trauma work up that merits urgent intervention altering the initial acute operative plan [29]. Lastly, in the trauma bay, labs should be drawn, including lactate and base deficit, to assess degree of physiologic insult, and when available viscoelastic hemostatic assays should be acquired to guide precision transfusion [30]. Data suggests that trending serial lactate measurements in the early window after pelvic ring injury are a rapid and reliable estimation of true severity of hemorrhage rather than routinely used hematologic measurements [31]. Ultimately, if the patient remains hemodynamically unstable despite the aforementioned resuscitation measures and 2 units of packed red blood cells (PRBCs), the patient should be taken to the operating room emergently for external fixation and PPP.
3 Indications for Preperitoneal Pelvic Packing
Packing for retroperitoneal hemorrhage from pelvic fracture was first described in 1994 in Europe in the setting of complex pelvic fractures [32]. This technique was later modified to an anterior, preperitoneal approach [33]. In conjunction with external fixation, which closes down the pelvic space, PPP is an optimal strategy to address pelvic hemorrhage. PPP rapidly and effectively addresses venous (presacral and paravesical venous plexuses) and bony sources of pelvic hemorrhage by tamponade, while external fixation reduces the available volume of the retroperitoneal space in both open and closed ring pelvic fractures [34]. Indications for PPP are the same historical indications for angioembolization and are described in our institutional protocol (Fig. 9.1). Specifically, blunt trauma patients with hemodynamic instability in the ED despite transfusion of 2 units of PRBCs with a known pelvic fracture. Alternatively, patients undergoing laparotomy for intraabdominal hemorrhage that have an associated pelvic hematoma may require PPP if they remain hemodynamically unstable despite control of intraabdominal bleeding. If a patient is being transferred to the operating room for hemorrhage in the chest or abdomen, PPP can be performed simultaneously if concomitant pelvic hemorrhage is suspected or discovered intraoperatively. While PPP has predominantly been described in adults, there are also reports of its use and effectiveness in pediatric trauma patients as well [35, 36].
Algorithm for the evaluation and management of unstable pelvic fractures
REBOA should be employed in centers with expertise and is typically deployed in Zone III for patients with persistent hypotension despite red cell transfusion with SBP <80 mmHg
SBP Systolic blood pressure, REBOA Resuscitative endovascular balloon occlusion of the aorta, FAST Focused assessment with sonography in trauma, PRBCs Packed red blood cells, PPP Preperitoneal pelvic packing, SICU Surgical intensive care unit, CT Computed tomography, HD Hemodynamically
4 Operative Approach
In anticipation of PPP, a multidisciplinary approach should be taken, with fastidious involvement of the orthopedic team for external fixation, as well as other specialty teams for relevant concomitant injury repair such as urology in the setting of a genitourinary injury or neurosurgery in the setting of intracranial hemorrhage requiring craniotomy. A benefit of PPP is that it can be performed simultaneously in conjunction with other operative procedures. According to a review of 42,122 patients with pelvic fractures from the National Trauma Data Bank, 10% of pelvic fracture patients have a common or external iliac vascular injury, 26% have a concomitant bladder injury, and 17% have an intraperitoneal bowel injury [37], and as such, it is not surprising that nearly 90% of patients with severe pelvic fractures require more than one procedure (beyond external fixation/PPP) [38].
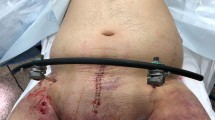
The technique for PPP has been previously described [39, 40] and in experienced hands, can be completed in less than 5–10 min [40]. The patient should be positioned supine on a table compatible with fluoroscopy and prepped in the standard fashion from neck to knees. PPP should be preceded by external fixation to stabilize the bony pelvis, create a smaller pelvic volume, and provide a stable counter-pressure for the pelvic packing. For unstable anterior-posterior compression and lateral compression injuries, anterior frames can be placed via the faster but potentially less stable iliac crest route or the more stable but fluoroscopy-dependent supra-acetabular approach; in contrast, vertical shear injuries are best stabilized with a posterior C-clamp [41]. It is important that the trauma team is present in the operating room for placement of the external fixation to ensure that the anterior fixation bar is positioned such that access for the suprapubic PPP incision is not obstructed.
After external fixation, a 6–8 cm vertical midline incision is sharply made from the pubic symphysis cephalad, sharply cutting the subcutaneous tissue and using bovie cautery to divide the fascia (Fig. 9.2a). This step requires special attention to ensure that the incision is distinct from the incision for exploratory laparotomy; the peritoneal pelvic space boundary should not be violated, preventing the tamponade effect of PPP. After dissection through the midline fascia, the pelvic space can be entered, leaving the peritoneum intact; at this time, it is often apparent that the pelvic hematoma has performed a majority of the pelvic space dissection, which extends around the bladder down to presacral plane (Fig. 9.2b). Once the paravesicular pelvic space is entered, packing can be performed by retracting the bladder to the contralateral side and inserting a laparotomy pad into the pelvic space (Fig. 9.2c). The laparotomy pads should be inserted deep towards the sacrum down to the presacral space using a ringed forceps or Cobb elevator to place them deeply into this space (Fig. 9.2d). The second laparotomy pad is placed laterally along the wall of the bladder, and the third laparotomy pad is placed anteriorly along the pubic rami bilaterally. A total of six laparotomy pads is most commonly used. While laparotomy pads are consistent with the traditional description of PPP, there are newer reports of using hemostatic gauze for packing to optimize hemorrhage control and decrease transfusion requirement [42].
Rarely, in the cases of vertical shear injuries, only one hemipelvis is affected and unilateral packing can be performed to avoid dissecting the pelvic space contralaterally. Once packing has been completed, suprapubic tubes for urethral or bladder injuries may be placed through separate stab incisions just lateral to the vertical PPP incision; it is essential at the end of the procedure that there is a mechanism in place to drain the bladder. The fascia is closed with a running 0-PDS suture, and the skin is closed with staples. Upon completion of PPP, remarkable increases in systolic blood pressure may be observed, with near doubling of the SBP after packing [43]. Once PPP and other operative procedures are performed during the index surgery, transfer to the ICU should be arranged and CT imaging performed.
After PPP, packs are left in place until the patient’s physiologic derangements, including coagulopathy, have resolved, usually within 24–36 h. When removing the pelvic packs, hemostatic interventions including suture, electrocautery, and topical agents should be used preferentially over the option of repacking the pelvis. Repacking of the pelvic space is associated with a marked increase in infections complications; with almost 50% of repacked patients developing pelvic space infections, repeat packing should be avoided [38]. While the optimal timing of definitive internal fixation of pelvic fractures remains debated, internal fixation at the time of preperitoneal pack removal has been described; in a retrospective review of patients with hemodynamically unstable pelvic fractures who underwent PPP, internal fixation at the time of pack removal resulted in shorter length of stay in the intensive care unit, faster time to definitive pelvic fixation, and less infectious complications [44].
5 Role of Angiography
Angiography and angioembolization are reserved for patients who have persistent hemodynamic instability present after external fixation and PPP [38, 45,46,47]. The trigger for diagnostic angiography after external fixation/PPP is transfusion of more than four units of red blood cells (RBC) in the 12 h post-packing after normalization of coagulation indices. Diagnostic angiography may identify arterial sources for angioembolization, but as previously noted, an arterial source of pelvic hemorrhage only occurs in approximately 15% of patients and is usually from internal iliac artery branches, gluteal artery branches, obturator artery, or pudendal artery [38, 48]. Even in patients with an arterial source, the likelihood of concomitant venous bleeding is nearly 100% [45]. In the small percentage of patients who ultimately undergo angiography after PPP, 80% have positive findings for arterial injury which can be localized and targeted [49]. Empiric embolization should not be pursued given the risk of perineal necrosis, infection, impotence, and persistent hemorrhage [50, 51]. While research has explored the predictors of angioembolization need in patients with pelvic injuries, it remains difficult to predict in the first hour of admission which patients will require angioembolization after PPP; the only predictor in external fixation/PPP series described thus far is post-packing PRBCs [2].
6 PPP Outcomes
Since its first description in Europe in the 1990s, PPP has shown promise in reducing morbidity and mortality in patients with severe pelvic fractures and pelvic hemorrhage, with both decreased transfusion rates and decreased mortality from exsanguination [12, 31, 52,53,54]. Adoption of PPP into institutional protocols for management of patients with pelvic fractures and related hemorrhage has been shown to decrease mortality by up to 30% post-PPP adoption [43, 55, 56]. In a retrospective cohort of patients, outcomes after progressive protocolized implementation of angiography and then PPP, overall 30-day mortality decreased from 63% to 42% after implementation of angiography and then even further to 30% in PPP, suggesting that PPP had a greater impact on overall survival than angiography [55]. A comparison of several modern day series finds similar findings. There was a 32% mortality rate for modern management of complex pelvic fracture patients in shock [6], 41% mortality for those managed with angioembolization alone [57], 35% mortality for an algorithm guided protocolized care using angiography [4], and 37% mortality in a study that prioritizes hemostatic resuscitation [5]. Patient managed with PPP/EF followed by complementary angioembolization demonstrated a 21% mortality [39, 48], and when REBOA was added to PPP/EF mortality which was only 14% with no deaths due to pelvic fracture bleeding [19].
While PPP has been associated with improved morbidity and mortality in treatment of pelvic hemorrhage, there are a few notable complications which occur infrequently. Surgical site infections have been described at a particularly high rate in patients who undergo repeating packing (47% versus 6% in patients with single packing) [38, 54]. Infectious complications are also more common in patients with open fractures, acetabular fractures, and associated perineal wounds (bladder injuries) [48, 58]. In addition to infectious complications, a case report of lower extremity abdominal compartment syndrome with PPP has been described [36]. Recently, a case series described a high rate of venous thromboembolism (VTE) in PPP patients, with a deep venous thrombosis incidence of 23% and pulmonary embolism incidence of 8% [59]. With such a high incidence of VTE in this patient population, patients should undergo bilateral lower extremity surveillance duplex ultrasounds following PPP.
7 Conclusion
Pelvic ring injuries continue to pose a great clinical challenge to trauma providers, as the addition of physiologic insult can drastically increase mortality risk. Despite advances in trauma care, the mortality rates of pelvic fractures patients in many modern series have failed to decrease in a corresponding manner and remain high [1,2,3,4,5, 10, 11]. Adoption of targeted protocols for patients with pelvic fractures and hemodynamic instability can drastically improve outcomes in these high-risk patients [56, 60, 61]. The addition of PPP with complementary angioembolization appears to result in the lowest mortality rate for hemodynamically unstable patients with pelvic fracture.
Key Concepts
-
The primary cause of early death in severely injured patients with pelvic fractures and hemodynamic compromise is hemorrhage.
-
In the ED, a pelvic binder or pelvic stabilization with a sheet should be placed at the level of the greater trochanters for patients with hypotension (systolic blood pressure [SBP] <90 mmHg); pelvic stabilization significantly reduces pelvic volume, prevents shifting of bony elements, and improves hemorrhage control.
-
Intraperitoneal or intrathoracic sources of hemorrhage should be excluded; the FAST exam reliably identifies clinically significant hemoperitoneum in life-threatening pelvic fracture-related hemorrhage, with a false-negative rate as low as 2%.
-
External fixation and PPP are performed if a patient remains hemodynamically unstable despite initial resuscitation with 2 units of packed red blood cells.
-
REBOA catheter should be considered for Zone III inflation in patients with hypotension unresponsive to resuscitation (persistent SBP <80 mmHg).
-
Patients undergoing laparotomy for intraabdominal hemorrhage that have an associated pelvic hematoma may require PPP if they remain hemodynamically unstable despite control of intraabdominal bleeding.
-
PPP should be preceded by external fixation to stabilize the bony pelvis, create a smaller pelvic volume, and provide a stable counter-pressure for the pelvic packing.
-
PPP can be completed in less than 10 min.
-
PPP is effective in pediatric trauma patients.
-
Patients who require transfusion of more than four units of PRBCs in the 12 hours post-packing after normalization of coagulation indices should also undergo diagnostic angiography.
-
Repacking of the pelvic space should be avoided, with almost 50% of repacked patients developing pelvic space infections.
Take Home Messages
-
Patient managed with PPP/EF followed by complementary angioembolization demonstrated a 21% mortality, and when REBOA was added to PPP/EF mortality was only 14% with no deaths due to pelvic fracture bleeding.
-
The addition of PPP with complementary angioembolization appears to result in the lowest mortality rate for hemodynamically unstable pelvic fracture patients.
References
Sathy AK, Starr AJ, Smith WR, et al. The effect of pelvic fracture on mortality after trauma: an analysis of 63,000 trauma patients. J Bone Joint Surg Am. 2009;91(12):2803–10.
Costantini TW, Coimbra R, Holcomb JB, et al. Pelvic fracture pattern predicts the need for hemorrhage control intervention-results of an AAST multi-institutional study. J Trauma Acute Care Surg. 2017;82(6):1030–8.
Frassini S, Gupta S, Granieri S, et al. Extraperitoneal packing in unstable blunt pelvic trauma: a single-center study. J Trauma Acute Care Surg. 2020;88(5):597–606.
Lewis RH Jr, Sharpe JP, Berning B, Fabian TC, Croce MA, Magnotti LJ. Impact of a simplified management algorithm on outcome following exsanguinating pelvic fractures: a 10-year experience. J Trauma Acute Care Surg. 2019;86(4):658–63.
Gaski IA, Barckman J, Naess PA, et al. Reduced need for extraperitoneal pelvic packing for severe pelvic fractures is associated with improved resuscitation strategies. J Trauma Acute Care Surg. 2016;81(4):644–51.
Costantini TW, Coimbra R, Holcomb JB, et al. Current management of hemorrhage from severe pelvic fractures: results of an American Association for the Surgery of Trauma multi-institutional trial. J Trauma Acute Care Surg. 2016;80(5):717–23.
Smith W, Williams A, Agudelo J, et al. Early predictors of mortality in hemodynamically unstable pelvis fractures. J Orthop Trauma. 2007;21(1):31–7.
Gabbe BJ, de Steiger R, Esser M, Bucknill A, Russ MK, Cameron PA. Predictors of mortality following severe pelvic ring fracture: results of a population-based study. Injury. 2011;42(10):985–91.
Martínez F, Alegret N, Carol F, et al. Pelvic fracture in the patient with multiple injuries: factors and lesions associated with mortality. Emergencias. 2018;30(2):91–7.
Mi M, Kanakaris NK, Wu X, Giannoudis PV. Management and outcomes of open pelvic fractures: an update. Injury. 2020;S0020-1383(20):30,170–4.
Tosounidis TI, Giannoudis PV. Pelvic fractures presenting with haemodynamic instability: treatment options and outcomes. Surgeon. 2013;11(6):344–51.
Cothren CC, Osborn PM, Moore EE, Morgan SJ, Johnson JL, Smith WR. Preperitonal pelvic packing for hemodynamically unstable pelvic fractures: a paradigm shift. J Trauma. 2007;62(4):834–9.
Trauma ACoSCo. Advance trauma life support: student manual. 10th ed. Chicago: American College of Surgeons; 2018.
Croce MA, Magnotti LJ, Savage SA, Wood GW 2nd, Fabian TC. Emergent pelvic fixation in patients with exsanguinating pelvic fractures. J Am Coll Surg. 2007;204(5):935–9.
Tinubu J, Scalea TM. Management of fractures in a geriatric surgical patient. Surg Clin North Am. 2015;95(1):115–28.
Krieg JC, Mohr M, Ellis TJ, Simpson TS, Madey SM, Bottlang M. Emergent stabilization of pelvic ring injuries by controlled circumferential compression: a clinical trial. J Trauma. 2005;59(3):659–64.
Christian NT, Burlew CC, Moore EE, et al. The focused abdominal sonography for trauma examination can reliably identify patients with significant intra-abdominal hemorrhage in life-threatening pelvic fractures. J Trauma Acute Care Surg. 2018;84(6):924–8.
Magee GA, Fox CJ, Moore EE. Resuscitative endovascular balloon occlusion of the aorta in pelvic ring fractures: the Denver health protocol. Injury. 2020;S0020-1383(20):30,072–3.
Burlew CC CJ, Freedburg M, Heelan Gladden A, Moore EE, Mauffrey C, Hoehn M, Platnick KB, Cohen MJ, Coleman JJ, Campion E, Lawless R, Werner N, Cralley A, Pieracci F. Inflate and pack! Pelvic packing combined with REBOA deployment prevents hemorrhage related deaths in unstable pelvic fractures. American Association for the Surgery of Trauma; September 8th; 2020 (virtual).
Do WS, Forte DM, Sheldon RR, et al. Preperitoneal balloon tamponade and resuscitative endovascular balloon occlusion of the aorta: alternatives to open packing for pelvic fracture-associated hemorrhage. J Trauma Acute Care Surg. 2019;87(1):18–26.
Mikdad S, van Erp IAM, Moheb ME, et al. Pre-peritoneal pelvic packing for early hemorrhage control reduces mortality compared to resuscitative endovascular balloon occlusion of the aorta in severe blunt pelvic trauma patients: a nationwide analysis. Injury. 2020;51(8):1834–9.
Duchesne J, Costantini TW, Khan M, et al. The effect of hemorrhage control adjuncts on outcome in severe pelvic fracture: a multi-institutional study. J Trauma Acute Care Surg. 2019;87(1):117–24.
Dalal SA, Burgess AR, Siegel JH, et al. Pelvic fracture in multiple trauma: classification by mechanism is key to pattern of organ injury, resuscitative requirements, and outcome. J Trauma. 1989;29(7):981–1000.
Siegel JH, Dalal SA, Burgess AR, Young JW. Pattern of organ injuries in pelvic fracture: impact force implications for survival and death in motor vehicle injuries. Accid Anal Prev. 1990;22(5):457–66.
Blum L, Hake ME, Charles R, et al. Vertical shear pelvic injury: evaluation, management, and fixation strategies. Int Orthop. 2018;42(11):2663–74.
Beckmann N, Cai C. CT characteristics of traumatic sacral fractures in association with pelvic ring injuries: correlation using the Young-Burgess classification system. Emerg Radiol. 2017;24(3):255–62.
Lin EA, Min W, Christoforou D, Tejwani NC. Young and burgess type I lateral compression pelvis fractures: a comparison of anterior and posterior pelvic ring injuries. Orthopedics. 2010;33(6):389.
Lee MJ, Wright A, Cline M, Mazza MB, Alves T, Chong S. Pelvic fractures and associated genitourinary and vascular injuries: a multisystem review of pelvic trauma. Am J Roentgenol. 2019;213(6):1297–306.
Coleman JRME, Parry J, Vintimilla DR, Nelson JT, Samuels JM, Sauaia A, Cohen MJ, Burlew CC, Mauffrey C. Association between Young-Burgess pelvic ring injury classification and concomitant injuries requiring urgent intervention. J Clin Orthop Trauma. 2020;11:1099–103.
Gonzalez E, Moore EE, Moore HB, et al. Goal-directed hemostatic resuscitation of trauma-induced coagulopathy: a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg. 2016;263(6):1051–9.
Ertel W, Keel M, Eid K, Platz A, Trentz O. Control of severe hemorrhage using C-clamp and pelvic packing in multiply injured patients with pelvic ring disruption. J Orthop Trauma. 2001;15(7):468–74.
Pohlemann TGT, Bosch A, Tscherne U. The technique of packing for control of hemorrhage in complex pelvic fractures. Tech Orthop. 1994;9:267–70.
Smith WR, Moore EE, Osborn P, et al. Retroperitoneal packing as a resuscitation technique for hemodynamically unstable patients with pelvic fractures: report of two representative cases and a description of technique. J Trauma. 2005;59(6):1510–4.
Moskowitz EE, Burlew CC, Moore EE, et al. Preperitoneal pelvic packing is effective for hemorrhage control in open pelvic fractures. Am J Surg. 2018;215(4):675–7.
Cothren CC, Moore EE, Smith WR, Morgan SJ. Preperitoneal pelvic packing in the child with an unstable pelvis: a novel approach. J Pediatr Surg. 2006;41(4):e17–9.
Cox SG, Westgarth-Taylor CJ, Dix-Peek SI, Millar AJ. Pre-peritoneal pelvic packing in a paediatric unstable pelvic fracture: an undescribed complication of lower limb compartment syndrome. Injury. 2013;44(2):258–60.
Cho J, Benjamin E, Inaba K, Lam L, Demetriades D. Severe bleeding in pelvic fractures: considerations in planning damage control. Am Surg. 2018;84(2):267–72.
Burlew CC, Moore EE, Smith WR, et al. Preperitoneal pelvic packing/external fixation with secondary angioembolization: optimal care for life-threatening hemorrhage from unstable pelvic fractures. J Am Coll Surg 2011;212(4):628–635; discussion 635–627.
Burlew CC. Preperitoneal pelvic packing for exsanguinating pelvic fractures. Int Orthop. 2017;41(9):1825–9.
Burlew CC. Preperitoneal pelvic packing: a 2018 EAST master class video presentation. J Trauma Acute Care Surg. 2018;85(1):224–8.
Coccolini F, Stahel PF, Montori G, et al. Pelvic trauma: WSES classification and guidelines. World J Emerg Surg. 2017;12:5.
Kim K, Shim H, Jung PY, et al. Effectiveness of kaolin-impregnated hemostatic gauze use in preperitoneal pelvic packing for patients with pelvic fractures and hemodynamic instability: a propensity score matching analysis. PLoS One. 2020;15(7):e0236645.
Jang JY, Shim H, Jung PY, Kim S, Bae KS. Preperitoneal pelvic packing in patients with hemodynamic instability due to severe pelvic fracture: early experience in a Korean trauma center. Scand J Trauma Resusc Emerg Med. 2016;24:3.
Kim TH, Yoon YC, Chung JY, Song HK. Strategies for the management of hemodynamically unstable pelvic fractures: from preperitoneal pelvic packing to definitive internal fixation. Asian J Surg. 2019;42(11):941–6.
Lustenberger T, Wutzler S, Störmann P, Laurer H, Marzi I. The role of angio-embolization in the acute treatment concept of severe pelvic ring injuries. Injury. 2015;46(Suppl 4):S33–8.
Magnone S, Coccolini F, Manfredi R, et al. Management of hemodynamically unstable pelvic trauma: results of the first Italian consensus conference (cooperative guidelines of the Italian Society of Surgery, the Italian Association of Hospital Surgeons, the multi-specialist Italian Society of Young Surgeons, the Italian Society of Emergency Surgery and Trauma, the Italian Society of Anesthesia, analgesia, resuscitation and intensive care, the Italian Society of Orthopaedics and Traumatology, the Italian Society of Emergency Medicine, the Italian Society of Medical Radiology -section of vascular and interventional radiology- and the world Society of Emergency Surgery). World J Emerg Surg. 2014;9(1):18.
Tang J, Shi Z, Hu J, et al. Optimal sequence of surgical procedures for hemodynamically unstable patients with pelvic fracture: a network meta-analysis. Am J Emerg Med. 2019;37(4):571–8.
Burlew CC, Moore EE, Stahel PF, et al. Preperitoneal pelvic packing reduces mortality in patients with life-threatening hemorrhage due to unstable pelvic fractures. J Trauma Acute Care Surg. 2017;82(2):233–42.
Tötterman A, Madsen JE, Skaga NO, Røise O. Extraperitoneal pelvic packing: a salvage procedure to control massive traumatic pelvic hemorrhage. J Trauma. 2007;62(4):843–52.
Ishida K, Noborio M, Shimahara Y, et al. Temporal intrailiac balloon occlusion for the treatment of intractable pelvic fracture hemorrhage. Acute Med Surg. 2016;3(2):195–8.
Lai CY, Tseng IC, Su CY, et al. High incidence of surgical site infection may be related to suboptimal case selection for non-selective arterial embolization during resuscitation of patients with pelvic fractures: a retrospective study. BMC Musculoskelet Disord. 2020;21(1):335.
Magnone S, Allievi N, Ceresoli M, Coccolini F, Pisano M, Ansaloni L. Prospective validation of a new protocol with preperitoneal pelvic packing as the mainstay for the treatment of hemodynamically unstable pelvic trauma: a 5-year experience. Eur J Trauma Emerg Surg. 2019;
Petrone P, Rodríguez-Perdomo M, Pérez-Jiménez A, Ali F, Brathwaite CEM, Joseph DK. Pre-peritoneal pelvic packing for the management of life-threatening pelvic fractures. Eur J Trauma Emerg Surg. 2019;45(3):417–21.
Shim H, Jang JY, Kim JW, et al. Effectiveness and postoperative wound infection of preperitoneal pelvic packing in patients with hemodynamic instability caused by pelvic fracture. PLoS One. 2018;13(11):e0206991.
Cheng M, Cheung MT, Lee KY, et al. Improvement in institutional protocols leads to decreased mortality in patients with haemodynamically unstable pelvic fractures. Emerg Med J. 2015;32(3):214–20.
Parry JA, Smith WR, Moore EE, Burlew CCC, Mauffrey C. The past, present, and future management of hemodynamic instability in patients with unstable pelvic ring injuries. Injury. 2020;S0020-1383(20):30176–5.
Tesoriero RB, Bruns BR, Narayan M, et al. Angiographic embolization for hemorrhage following pelvic fracture: is it "time" for a paradigm shift? J Trauma Acute Care Surg. 2017;82(1):18–26.
Stahel PF, Moore EE, Burlew CC, et al. Preperitoneal pelvic packing is not associated with an increased risk of surgical site infections after internal anterior pelvic ring fixation. J Orthop Trauma. 2019;33(12):601–7.
Heelan AA, Freedberg M, Moore EE, et al. Worth looking! Venous thromboembolism in patients who undergo preperitoneal pelvic packing warrants screening duplex. Am J Surg. 2020;S0002-9610(20):30535–3.
Jang JY, Shim H, Kwon HY, et al. Improvement of outcomes in patients with pelvic fractures and hemodynamic instability after the establishment of a Korean regional trauma center. Eur J Trauma Emerg Surg. 2019;45(1):107–13.
Kam C, Lai C, Lam S, So F, Lau C, Cheung K. What are the ten new commandments in severe polytrauma management? World J Emerg Med. 2010;1(2):85–92.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Coleman, J.R., Moore, E.E., Burlew, C.C. (2022). Preperitoneal Pelvic Packing. In: Pape, HC., Borrelli Jr., J., Moore, E.E., Pfeifer, R., Stahel, P.F. (eds) Textbook of Polytrauma Management . Springer, Cham. https://doi.org/10.1007/978-3-030-95906-7_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-95906-7_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-95905-0
Online ISBN: 978-3-030-95906-7
eBook Packages: MedicineMedicine (R0)