Abstract
In this study, the investigators reviewed the efficacy and safety data from a pooled analysis of four randomized placebo-controlled trials of duloxetine for the treatment of late-life generalized anxiety disorder (GAD).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Generalized anxiety disorder
- Duloxetine
- Randomized controlled trial (RCT)
- Double-blind
- Placebo-controlled trial
- Late-life anxiety disorders
Jonathan Davidson, Christer Allgulander, Mark H Pollack, James Hartford, Janelle S Erickson, James M Russell, David Perahia, Madalaine M Wohlreich, Janice Carlson, Joel Raskin
FormalPara Journal PublisherHuman Psychopharmacology: Clinical and Experimental
FormalPara Year of Publication2008
FormalPara Type of StudyPooled analysis of randomized placebo-controlled trials
FormalPara Funding SourcesEli Lilly and Company and Boehringer Ingelheim
FormalPara ObjectivesTo determine the efficacy and safety of duloxetine for the treatment of generalized anxiety disorder among older adults from a pooled analysis of four randomized placebo-controlled trials [1].
FormalPara MethodsThis study combined the results of four separate multicenter, randomized, double-blind, placebo-controlled, parallel-group studies of outpatients with a diagnosis of generalized anxiety disorder (GAD) in mixed age populations and reported results from their post hoc analyses of elderly individuals.
For this analysis, 73 individuals ≥65 years in age with a DSM-IV-TR diagnosis of GAD were included. Participants were required to have moderate–severe illness, reflected by Hospital Anxiety and Depression Scale (HADS) anxiety subscale score of ≥10, a Covi anxiety scale (CAS) score of ≥9, and clinical global impressions–severity of illness (CGI-S) score of ≥4.
Study 1 had a 9-week acute therapy phase in which participants were randomized to receive duloxetine 60 mg, duloxetine 120 mg, or placebo. Participants in both duloxetine treatment groups were started at 60 mg/day, which could initially and temporarily be lowered to 30 mg/day if a higher dose was not tolerated. Study 2 consisted of a 10-week acute therapy phase, followed by a 2-week discontinuation phase. Participants were randomized to duloxetine or placebo. Participants in the duloxetine treatment group were started at 60 mg/day, which could initially and temporarily be lowered to 30 mg/day if tolerability concerns arose. After titration to 60 mg/day, flexible dosing was allowed in weekly increments of 30 mg/day up to a maximum dose of 120 mg/day. Study 3 consisted of a 10-week acute therapy phase. Participants were randomized to receive duloxetine 60–120 mg/day, venlafaxine 75–225 mg/day, or placebo in a 1:1:1 ratio. Duloxetine treatment was initiated at 30 mg/day for 1 week, followed by an increase to 60 mg/day, after which flexible dosing was allowed in weekly increments of 30 mg/day up to a maximum dose of 120 mg/day. Study 4 consisted of a 10-week acute therapy phase, followed by a 2-week tapering phase. Participants were randomized in a 2:1:2:2 ratio to duloxetine (60–120 mg/day), duloxetine (20 mg/day), venlafaxine (75–225 mg/day), or placebo. Duloxetine treatment was initiated at 30 mg/day for 1 week and increased to 60 mg/day. After titration to 60 mg/day, flexible dosing was allowed in weekly increments of 30 mg/day up to a maximum dose of 120 mg/day. Data from the venlafaxine arms and duloxetine 20 mg/day arm were not included in the pooled analysis.
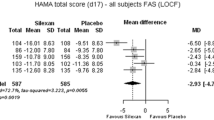
The primary outcome measure was the mean baseline-to-endpoint change in Hamilton anxiety scale (HAM-A). Secondary measures included the HADS, the CGI-I and Patient Global Impressions of Improvement scales, and the Sheehan disability scale (SDS) impairment scores. Response was defined as ≥50% reduction from baseline in HAMA total score, or a CGI-I score of ≤2 at endpoint. Sustained improvement was defined as a ≥30% reduction from baseline in HAMA total score at any visit before endpoint, sustained until the last visit; and remission was defined as a HAMA total score of ≤7 at endpoint. Two-tailed chi-square tests and linear modeling was used to compare the rates of response and side effects between the duloxetine and placebo groups.
Of the 1491 participants who were randomly assigned to treatments in the 4 studies, 73 (4.9%; duloxetine = 45; placebo = 28) were included in this pooled analysis. There were no significant differences between the treatment groups on the demographic variables or the severity of illness at baseline. Thirty participants in the duloxetine group (66.7%) and 20 participants in the placebo group (71.4%) completed treatment (P = 0.610). Significantly more participants randomized to duloxetine discontinued treatment due to an adverse event [10 (22.2%) vs. 0 (0.0%), P = 0.011]. The treatment groups did not differ significantly with respect to other reasons for discontinuation. The mean age of the participants was 70.1 years in the duloxetine group and 70.9 years in the placebo group. A total of 95.6% of participants in the duloxetine group and 96.4% of participants in the placebo group were white.
The treatment groups did not significantly differ in the incidence of HAMA response (20/42 (48%) vs. 8/28 (29%), P = 0.149), remission (10/42 (24%) vs. 2/28 (7%), P = 0.053), or sustained improvement (26/42 (62%) vs. 10/28 (36%), P = 0.05). Compared with placebo, participants treated with duloxetine experienced significantly greater improvements on the HAMA total (P = 0.029), the HAMA psychic anxiety factor (P = 0.034), HADS anxiety (P = 0.049) and depression scales (P = 0.026), but not the HAM-A somatic anxiety factor (P = 0.074). The treatment groups did not significantly differ in CGI-I (P = 0.143) or PGI-I scores at endpoint (P = 0.064).
A total of 35 participants (70%) in the duloxetine group complained of at least 1 side effect when compared to 15 individuals (53.6%) in the placebo group. The most common side effects in the duloxetine group were nausea, dizziness, and hyperhidrosis (15, 7, and 6, respectively), and those in the placebo group were dizziness, constipation, and hyperhidrosis (5, 4, and 3, respectively). Participants treated with duloxetine reported significantly more nausea (30.0% vs. 7.1%, P = 0.023) and weight loss (P = 0.018). More participants treated with duloxetine discontinued treatment due to an adverse event (22.2% vs. 0%; P = 0.011).
Evidence from this pooled analysis of four randomized placebo-controlled trials of duloxetine for the treatment of GAD in adults ≥65 years indicates that duloxetine is more effective than placebo in reducing overall symptoms of anxiety, but the side effects may be difficult to tolerate for some older adults.
FormalPara Strengths of the Study-
1.
This was a pooled analysis of randomized, double-blind, and placebo-controlled trials.
-
2.
The study assessed on the basis of Jadad score indicates that this was a high-quality study with a score of 5 out of 5 [2].
Questions Yes (1) No (0) | Was the study described as random? | Was the randomization scheme described and appropriate? | Was the study described as double-blind? | Was the method of double blinding appropriate? (Were both the patient and the assessor appropriately blinded?) | Was there a description of dropouts and withdrawals? | Total score Range of score quality 0–2, low 3–5, high |
Score | 1 | 1 | 1 | 1 | 1 | 5 |
-
3.
The study sample included only older adults (≥65 years).
-
4.
All participants had moderate to severe anxiety symptoms based on the included anxiety scale scores.
-
5.
There was a 68% completion rate for the study.
-
1.
The study had a small sample size of 73 participants.
-
2.
It had a short duration of study period of 10 weeks in the longest trial.
-
3.
A total of 95.6% of participants in the duloxetine group and 96.4% of participants in the placebo group were white.
-
4.
There was no significant difference in the rates of response, remission, or sustained improvement in the two groups.
-
5.
Participants treated with duloxetine had more nausea (30.0% vs. 7.1%, p = 0.023) and weight loss (P = 0.018).
-
6.
More participants treated with duloxetine discontinued treatment due to an adverse event (22.2% vs. 0%; P = 0.011).
Evidence from this pooled analysis of four randomized placebo-controlled trials of duloxetine for the treatment of GAD in adults ≥65 years indicates that duloxetine is more effective than placebo in reducing overall symptoms of anxiety, psychic anxiety, and depression. Its tolerability in older adults needs to be explored further.
FormalPara Practical Applications of the Take-Home PointsAmong older adults who present with a diagnosable anxiety disorder with moderate to severe symptoms, duloxetine is an efficacious treatment option.
References
Davidson J, Allgulander C, Pollack MH, Hartford J, Erickson JS, Russell JM, Perahia D, Wohlreich MM, Carlson J, Raskin J. Efficacy and tolerability of duloxetine in elderly patients with generalized anxiety disorder: a pooled analysis of four randomized, double-blind, placebo-controlled studies. Hum Psychopharmacol. 2008;23(6):519–26.
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Joshi, P., Tampi, R.R. (2022). Efficacy and Tolerability of Duloxetine in Elderly Patients with Generalized Anxiety Disorder: A Pooled Analysis of Four Randomized, Double-Blind, Placebo-Controlled Studies. In: Tampi, R.R., Tampi, D.J., Young, J.J., Balasubramaniam, M., Joshi, P. (eds) Essential Reviews in Geriatric Psychiatry. Springer, Cham. https://doi.org/10.1007/978-3-030-94960-0_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-94960-0_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-94959-4
Online ISBN: 978-3-030-94960-0
eBook Packages: MedicineMedicine (R0)