Abstract
In this meta-analysis, the investigators reviewed the use of antipsychotics for treating behavioral and psychological symptoms in dementia (BPSD) and the potential side effect of decline in cognitive function from ten randomized placebo-controlled trials (RCTs).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Dementia
- Behavioral and psychological symptoms in dementia (BPSD)
- Antipsychotics
- Cognition
- Randomized placebo-controlled trials
- Meta-analysis
Alexander Wolf, Stefan Leucht, Frank-Gerald Pajonk
FormalPara Journal PublisherEuropean Archives of Psychiatry and Clinical Neuroscience
FormalPara Year of Publication2017
FormalPara Type of StudyMeta-analysis
FormalPara Funding SourcesNone
FormalPara ObjectiveTo determine the association between the use of antipsychotics for the treatment of BPSD and the side effect of further decline in cognitive function from a meta-analysis of ten randomized, double-blind, placebo-controlled trials [1].
FormalPara MethodsThe investigators of the study searched for all published and unpublished randomized controlled trials that assessed the efficacy of antipsychotics in the treatment of BPSD in the following databases: MEDLINE, Scopus, CENTRAL (last search 2014), and ClinicalStudyResults (open database for trials, last search 2008). The investigators were only looking for randomized, double-blind, placebo-controlled trials with a minimum duration of 1 week. The keywords used in the search included antipsychotic, antipsychotics, neuroleptic, neuroleptics, aripiprazole, chlorpromazine, clozapine, flupenthixol, haloperidol, melperone, olanzapine, pimozide, pipamperone, quetiapine, risperidone, sulpiride, thioridazine, ziprasidone, and zuclopenthixol, in conjunction with the keyword searches dementia, Alzheimer, Alzheimer’s, AD, vascular dementia, VD, BPSD, behavioural and psychological, behavioral and psychological, Pick, and Pick’s disease. Keywords used in the search of ClinicalStudyResults included dementia or Alzheimer dementia in conjunction with antipsychotic, neuroleptic, aripiprazole, clozapine, olanzapine, quetiapine, risperidone, or ziprasidone.
The exclusion criteria for this study were studies without a placebo arm and those studies that evaluated individuals with Lewy body dementia.
The primary outcome measure of the cognitive change in all included studies was the Mini-Mental State Examination (MMSE). However, two studies provided additional data on the Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog). A sensitivity analysis was performed using ADAS-Cog instead of MMSE where provided. A meta-regression was performed to test a correlation between baseline MMSE scores and study duration. All pooled antipsychotics were analyzed individually.
The outcome data of the studies were summarized in a meta-analysis using the random effects model to take heterogeneity among studies into account. The investigators calculated both mean differences (weighted mean difference, WMD) in MMSE raw values and standardized mean differences (SMD) as the effect size. To assess study heterogeneity, the investigators used chi-square test (P < 0.1 set a priori to assume presence of heterogeneity) and I-square statistic (values ≥50% as considerable heterogeneity). The possibility of publication bias was examined using the funnel plot method.
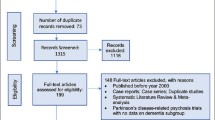
The investigators screened 27,602 records from Scopus, MEDLINE, CENTRAL, and ClinicalStudyResults, of which 27,484 records were excluded from initial screening. A total of 118 records were assessed for eligibility. Of these, 103 records were excluded for reasons as follows: combined post hoc or subgroup analysis, inappropriate diagnosis or participants, inappropriate intervention or control, no allocation concealment, length less than 1 week, and no relevant outcome.
A total of 15 records referring to 10 studies with 13 arms and 1586 participants were included in this meta-analysis. Eleven studies investigated placebo versus a second-generation antipsychotic (aripiprazole, olanzapine, quetiapine, risperidone), and two investigated placebo versus a first-generation antipsychotic haloperidol. Included study participants were more often female (mean 69%; range 55–80%), with mean age of 80 (range 75–84) years and a mean baseline MMSE score of 12.7 (range 5.2–21.5).
Antipsychotic use was associated with decline in cognitive function in the pooled analysis when compared to placebo (SMD = −0.065, WMD = −0.211). However, only two studies showed significant effects, and when those two were excluded, the SMD turned zero (P = 0.95). As mentioned above, each antipsychotic outcome data was pooled individually.
Two included studies showed cognitive worsening with aripiprazole, being significant in one trial and not significant in the other.
FormalPara OlanzapineThree out of four included studies showed no or minimal differences between olanzapine and placebo. Of note, one trial which included participants with a particularly high MMSE (21.5 points) reported significant decline in cognitive function with olanzapine as compared to placebo.
FormalPara QuetiapineThree included studies showed no significant difference between quetiapine and placebo.
FormalPara RisperidoneTwo included studies showed no significant difference between risperidone and placebo.
FormalPara HaloperidolTwo included studies showed no significant difference between haloperidol and placebo.
Test of heterogeneity was significant for the trials with aripiprazole, olanzapine, and risperidone, and not significant for quetiapine and haloperidol. Two included olanzapine studies also provided ADAS-Cog scores. When using the mean change in ADAS-Cog scores instead of MMSE scores, there was an enhanced effect of cognitive worsening. Furthermore, meta-regression found a significant and strong linear correlation between study length and SMD in MMSE change: the longer the study duration, the greater the cognitive decline with antipsychotic treatment when compared to placebo. There was also a high correlation between baseline MMSE and SMD: the higher the baseline MMSE, the higher the cognitive worsening with antipsychotic treatment when compared to placebo. However, in a sensitivity analysis where the two studies with the largest impact were removed, neither a correlation between study length and cognitive worsening nor a correlation between baseline MMSE score and cognitive worsening was found.
In this meta-analysis that evaluated data from ten randomized, double-blind, placebo-controlled trials, the random effect model did not show a significant decline in cognitive function with antipsychotic use, when compared to placebo in treatment of BPSD. The meta-regression showed a significant correlation between cognitive impairment and treatment duration, and between cognitive impairment and baseline MMSE score.
FormalPara Strengths of the Study-
1.
Meta-analysis used random effects model for the purpose of taking heterogeneity among various studies into account.
-
2.
Included studies were randomized, double-blind, and placebo-controlled trials.
-
3.
There was inclusion of both first-generation and second-generation antipsychotics.
-
4.
There was comparison of each antipsychotic agent individually with placebo.
-
1.
Though a funnel plot was used, there might be a publication bias, as negative results are less likely to be published.
-
2.
MMSE is a widely used cognitive assessment tool for initial detection of cognitive impairment, but MMSE is not a very sensitive tool to detect changes in cognitive functioning, especially when short study duration is not taken into account.
-
3.
Only two studies investigated cognitive changes for more than 12 weeks, and only one more than 20 weeks.
-
4.
Dosage and the number of antipsychotics used (if more than one medication was used) for each participant are not identified.
-
5.
Confounding factors for cognitive function, such as general health, active medical illness, use of cognitive enhancers, variable progression, and prognosis in Alzheimer’s disease vs vascular dementia vs Pick’s disease, were not accounted for in this study.
Despite some limitations, this meta-analysis indicates no significant difference in the potential side effect of cognitive impairment in antipsychotic treatment for BPSD when compared to placebo. There was also no significant difference found among different generations of antipsychotic and the different agents used. This is in line with the results from the CATIE-AD study [2]: antipsychotics do not seem to cause significant cognitive changes as compared to placebo, and show improvement in symptoms such as anger, agitation, and paranoia which may help with BPSD. Of note, one out of the four olanzapine studies showed a significant decline in cognitive function as compared to placebo, which suggests similarity to one of the findings from the CATIE-AD study showing worsening of functional skills with olanzapine use as compared to placebo.
FormalPara Practical Applications of the Take-Home PointsBPSD such as delusions, hallucinations, agitations, and aggression are common in dementia and pose a significant barrier in the care of patients with dementia. However, there are only limited options for pharmacological treatment, with limited data on efficacy of these agents. Moreover, most of the agents used carry a side effect profile that is not favorable to the elderly population. Antipsychotics should be used after careful evaluation of risks vs benefits for each individual patient, targeting the minimum effective dose being used, for as brief a time as possible, and the use of these agents should be monitored closely.
References
Wolf A, Leucht S, Pajonk FG. Do antipsychotics lead to cognitive impairment in dementia? A meta-analysis of randomised placebo-controlled trials. Eur Arch Psychiatry Clin Neurosci. 2017;267(3):187–98.
Sultzer DL, Davis SM, Tariot PN, Dagerman KS, Lebowitz BD, Lyketsos CG, Rosenheck RA, Hsiao JK, Lieberman JA, Schneider LS, CATIE-AD Study Group. Clinical symptom responses to atypical antipsychotic medications in Alzheimer’s disease: phase 1 outcomes from the CATIE-AD effectiveness trial. Am J Psychiatry. 2008;165(7):844–54.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kim, S., Holsinger, T. (2022). Do Antipsychotics Lead to Cognitive Impairment in Dementia? A Meta-analysis of Randomized Placebo-Controlled Trials. In: Tampi, R.R., Tampi, D.J., Young, J.J., Balasubramaniam, M., Joshi, P. (eds) Essential Reviews in Geriatric Psychiatry. Springer, Cham. https://doi.org/10.1007/978-3-030-94960-0_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-94960-0_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-94959-4
Online ISBN: 978-3-030-94960-0
eBook Packages: MedicineMedicine (R0)