Abstract
-
Phenylketonuria (PKU) was the first inherited metabolic disease identified by newborn screening and treated with diet to prevent the development of intellectual disability.
-
Classification of the severity of phenylketonuria is based on the type of the variants in phenylalanine hydroxylase (PAH) gene, dietary phenylalanine tolerance, pretreatment blood phenylalanine concentrations, and the necessity to introduce treatment to achieve target blood phenylalanine concentrations.
-
The etiology of brain damage in PKU has not been fully elucidated; however, high blood phenylalanine concentrations are associated with impaired transport of large neutral amino acids into the brain, decreased neurotransmitters synthesis, changes in brain morphology (gray and white matter), and impact on many enzymes involved in brain metabolism.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
-
Phenylketonuria (PKU) was the first inherited metabolic disease identified by newborn screening and treated with diet to prevent the development of intellectual disability.
-
Classification of the severity of phenylketonuria is based on the type of the variants in phenylalanine hydroxylase (PAH) gene, dietary phenylalanine tolerance, pretreatment blood phenylalanine concentrations, and the necessity to introduce treatment to achieve target blood phenylalanine concentrations.
-
The etiology of brain damage in PKU has not been fully elucidated; however, high blood phenylalanine concentrations are associated with impaired transport of large neutral amino acids into the brain, decreased neurotransmitters synthesis, changes in brain morphology (gray and white matter), and impact on many enzymes involved in brain metabolism.
1 Background
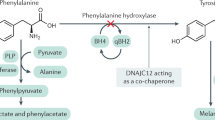
Phenylketonuria (PKU) is the most common inherited autosomal-recessive inborn error of amino acid metabolism characterized by decreased activity of the enzyme phenylalanine hydroxylase (PAH) [1, 2]. The Norwegian biochemist and physician, Asbjorn Fölling, discovered PKU in 1934 by detecting phenylketones in the urine of siblings with intellectual disabilities, with subsequent identification of altered phenylalanine metabolism as the cause of this disease [3, 4]. PAH is the enzyme that converts phenylalanine to tyrosine in the presence of the cofactor tetrahydrobiopterin (BH4), molecular oxygen, and nonheme iron [5] (Fig. 9.1). Loss of PAH activity results in elevated blood phenylalanine concentrations and is referred to as hyperphenylalaninemia (HPA) or phenylketonuria (PKU).
Phenylalanine hydroxylase (PAH) and tetrahydrobiopterin (BH4) in the presence of molecular oxygen (O2) and nonheme iron converts phenylalanine to tyrosine. The alternate pathway of phenylalanine metabolism results in the accumulation of phenylalanine as well as phenylpyruvic acid and other phenylketones that are excreted in the urine. (Adapted from Acosta [1] and Donlon J et al. [5])
PKU is the exemplar of the effectiveness of newborn screening as it was the first inherited metabolic disease in which infants were identified by newborn screening and treated with diet before the development of intellectual disability associated with untreated PKU. If left untreated or ineffectively managed, PKU can cause severe intellectual disability, as well as complex neurological and behavioral disorders. Severely affected patients are unable to live independently, often requiring specialized and continuous supervised care. Conversely, early and continuously treated patients typically have normal or nearly normal cognitive development. [6,7,8]
2 Biochemistry
Phenylalanine is an indispensable amino acid that cannot be synthesized by humans (Chap. 6). It comprises 3–7% of all dietary protein. After protein ingestion and digestion, phenylalanine is absorbed from the gastrointestinal tract to the liver via the portal vein. Phenylalanine is either hydroxylated into tyrosine via PAH in the liver or is incorporated into new proteins in tissues [5]. Hyperphenylalaninemia due to decreased activity of PAH manifests as a spectrum of disorders (severe, moderate, or mild PKU and non-PKU hyperphenylalaninemia). Deficiencies in the activity of PAH cofactor – tetrahydrobiopterin (BH4) – represent a group of inherited metabolic diseases that result not only in hyperphenylalaninemia but also in alterations in tyrosine and tryptophan metabolism. BH4 is also a cofactor for tyrosine hydroxylase and tryptophan hydroxylase, as well as three isoforms of nitric oxide synthase. Therefore, proper functioning of BH4 is essential for the synthesis of dopamine, catecholamines, serotonin, melanin, and nitric oxide [9]. Phenylalanine can also be transaminated to phenylpyruvic acid as an alternative to hydroxylation by PAH. Phenylpyruvic acid, along with other ketones, is excreted in the urine as phenylacetic acid, phenylacetylglutamine, and phenyllactic acid. This pathway of phenylalanine metabolism is much less effective than hydroxylation [1, 5] (Fig. 9.1).
Phenylalanine hydroxylase (PAH) and BH4 in the presence of molecular oxygen (O2) and nonheme iron convert phenylalanine to tyrosine. The alternate pathway of phenylalanine metabolism results in the accumulation of phenylalanine as well as phenylpyruvic acid and other phenylketones that are excreted in the urine (Adapted from Acosta [1] and Donlon J et al. [5]).
The enzyme PAH has a complex structure consisting of three domains: regulatory, catalytic, and C-terminal domains. The regulatory domain contains a serine residue that is involved in activation by phosphorylation. The catalytic domain is responsible for cofactor and ferric iron binding, while the C-terminal domain is associated with inter-subunit binding [10]. The liver is the primary site of PAH activity, but it is also synthesized in the kidneys, pancreas, and brain.
3 Genetics
Phenylketonuria is an autosomal-recessive disorder. The majority (98%) of genetic variants associated with PKU occur at the phenylalanine hydroxylase locus [5], on the long arm of chromosome 12, in the region of q22-q24.1 [11]. Almost 1291 variants in the PAH locus have been described thus far, with 60% being missense mutations [12]. It is estimated that globally 450,000 individuals have PKU, with global prevalence of 1:23,930 live births [2]. Globally, the incidence in screened populations is estimated at 1:12,000 with a carrier frequency of 1:55 [13] (Box 9.1).
Box 9.1: Global Incidence of PKU [2]
Country | Incidence |
|---|---|
Italy | 1:4000 |
Ireland | 1:4545 |
Iran, Jordan | 1:5000 |
Turkey | 1:6667 |
Germany | 1:5360 |
Austria | 1:5764 |
Estonia | 1:7143 |
Poland | 1:8039 |
France | 1:9091 |
United Kingdom | 1:10,000 |
Saudi Arabia | 1:14,245 |
Canada | 1:15,000 |
China | 1:15,924 |
United States of America | 1:25,000 |
Mexico | 1:27,778 |
Peru | 1:46,970 |
Japan | 1:125,000 |
Thailand | 1:227,273 |
The prevalence of PKU varies widely among ethnic groups and geographical regions. It is highest in European and some Middle European countries.
The correlation between genotypes and biochemical phenotype, pretreatment phenylalanine concentrations, and phenylalanine tolerance is well established; however, the correlation between genotype and clinical phenotype, including neurological, intellectual, and behavioral outcomes, is weak [13].
4 Diagnosis
In most developed countries, PKU is identified by newborn screening by the presence of elevated phenylalanine and/or phenylalanine to tyrosine (Phe:Tyr) ratio in the dry blood spot collected in the first days to week of life (Chap. 2). Tandem mass spectrometry (MS/MS) is the method of choice to analyze the blood spots; however, other methods such as enzymatic techniques or high-pressure liquid chromatography (HPLC) are also used in some laboratories. After a positive newborn screening result, the patient is evaluated at a metabolic center for confirmatory testing and to rule out BH4 deficiency by the analysis of pterin, as well as dihydropteridine (DHPR) activity. In many centers, a BH4 loading test is often performed to identify patients with BH4-responsive variants of PKU, as well as BH4 deficiencies caused by the disturbance in the production and/or recycling of BH4 [14]. Table 9.1 describes classifications of PAH deficiency.
5 Clinical Presentation
Untreated, late-treated, or poorly controlled patients have chronically elevated blood phenylalanine concentrations that lead to progressive and irreversible neurological, psychological, behavioral, as well as physical impairments that significantly impact quality of life. The degree of impairment depends on the blood concentration of phenylalanine with the most severe symptoms observed in untreated patients with the severe (classical) form of the disease. Although severe intellectual disability (with IQ scores often below 50) is the most typical presentation, untreated patients may demonstrate many other symptoms of persistent hyperphenylalaninemia (Box 9.2).
Box 9.2: Symptoms of Untreated Classical PKU
-
“Musty” odor (urine and body)
-
Hypopigmentation of the skin, hair, and iris
-
Eczema
-
Intellectual disability
-
Neurological (seizures, tremor)
-
Behavioral (hyperactivity, self-injury)
-
Psychological (depression, anxiety, agoraphobia)
The outcome of early detected and treated PKU is generally favorable; however, even with good metabolic control, some individuals may demonstrate a higher prevalence of neuropsychological complications, including decreased executive function, internalizing disorders, and low self-esteem. Some patients, especially adults with PKU, are at higher risk of developing mood, anxiety, and attentional disorders across the lifespan [16,17,18].
6 Nutrition Management
The cornerstone of dietary management in PKU is limiting consumption of the offending amino acid, phenylalanine. In general, the diet is restricted in all high protein foods and includes medical foods that contain little or no phenylalanine but supply other amino acids in the diet (Chap. 10).
The amount of phenylalanine a patient can consume daily depends on the residual activity of PAH and other factors including the patient’s age and growth rate [19, 20]. The concept of limiting dietary phenylalanine was first demonstrated in the early 1950s by Bickel et al. as they showed positive effects on behavior in a young patient with PKU [6]. The development of medical foods that were low in phenylalanine but contained other amino acids made the dietary treatment of PKU possible. During the early years of PKU treatment, it was generally believed that a low phenylalanine diet could be discontinued at around 6 years of age with no adverse effects [21,22,23]; however, “treatment for life” is the optimal mode of treatment [24,25,26,27]. According to recommendations from the National Institute of Health in 2014 and the first European PKU Guidelines in 2017, treatment should be started in all patients with hyperphenylalaninemia with blood phenylalanine concentrations greater than or equal to 360 μmol/L [14, 15]. Target phenylalanine concentrations used for the long-term follow-up in many centers are age-specific. European countries/centers follow recommendations from European PKU Guidelines suggesting optimal phenylalanine levels between 120 and 360 μmol/L for children up to the age of 12 years with higher values (up to 600 μmol/L) acceptable in older patients [14, 15]. In the United States, the goal is to maintain plasma phenylalanine concentrations below 360 μmol/L [15, 24, 28] across all age groups.
7 Phenylalanine Neurotoxicity
Eighty years after the discovery of PKU, the pathogenesis of brain dysfunction and the exact mechanisms of phenylalanine neurotoxicity are yet to be elucidated. Although there is a common agreement about the relationship between blood phenylalanine concentration and cognitive outcome in PKU, the concentration of phenylalanine and a deficiency of other large neutral amino acids in the brain are believed to be the main factors causing neurotoxicity. The impact of elevated blood phenylalanine concentrations, which is especially harmful during early infancy, is complex and multidirectional [11, 18, 29,30,31,32,33,34,35,36] (Box 9.3).
Box 9.3: The Main Theories of the Pathogenesis of PKU
-
Impairment of large neutral amino acid (LNAA) transport across the blood-brain barrier (BBB) with disturbances in neurotransmitter metabolism [11, 29, 32,33,34,35, 37].
-
Impairment in cholesterol synthesis and disturbances in myelin metabolism [29, 32, 34, 38, 39].
-
Interference with the glutamatergic system directly involved in brain development [30, 33, 37, 40, 41].
-
Altered glycolysis via inhibition of pyruvate kinase and other enzymes involved in brain energy metabolism [31, 33].
-
Damage to cellular DNA, protein, and lipid, as well as decreased antioxidant defenses [33].
The typical symptoms of untreated individuals with PKU are the manifestation of the neurotoxic effect of phenylalanine on the central nervous system. A morphological change in the brain in patients with PKU affects both white and gray matters. Microcephaly, where the brain mass can be only 80% of that of a healthy individual, is a characteristic feature for many untreated PKU patients [37]. This symptom is caused by myelin structure anomalies that result in a loss of myelin volume, disturbances in cortical neuronal development, diffuse cortical atrophy, and general abnormalities in protein synthesis [33, 37,38,39,40,41,42].
Phenylalanine neurotoxicity affects the brain and related structures during critical windows of growth and development. Periods of particularly rapid growth make neuronal cells especially vulnerable to excessive amounts of toxic factors (e.g., phenylalanine) or a lack of substances needed for optimal development [43].
In PKU, similarly as in the other inherited disorders of amino acid metabolism, the fast-growing brain of the fetus is protected by the mother’s enzymatic activity. The disturbances appear after birth, and the central nervous system is at risk of damage until the brain is fully developed and matured [44]. Despite the fact that the increase in brain mass and the creation of synaptic connections occur mainly during the first year of life, the full development of some areas (e.g., prefrontal cortex or white matter myelination) is not complete until adulthood (Box 9.4).
Box 9.4: Brain Development
-
The increase in brain mass and the creation of synaptic connections occurs mainly during the first year of life.
-
Full development of some areas (e.g., prefrontal cortex and white matter myelination) is not complete until adulthood.
The last region to mature in the prefrontal cortex is the dorso-lateral area responsible for cognitive functions [45]. During the first few years of life, patients with PKU, which are inappropriately treated and have poorly controlled blood phenylalanine concentrations, suffer from inhibited growth of the cortex and a disrupted myelination process. For example, visuospatial speed deficits that result from structural myelin damage, which occurs early in life, followed by poor metabolic control in subsequent years, are difficult to improve despite tight phenylalanine control in adulthood [46]. Therefore, the risk of progressive neuropsychiatric manifestations of PKU in adulthood is higher in patients with poor metabolic control in infancy and early childhood. Of note, the neurotoxic influence of phenylalanine is present throughout life; this is why all patients with PKU require life-long, multidisciplinary care and maintenance of blood phenylalanine concentrations within the treatment range. For example, for complex executive functions, current and adult phenylalanine concentrations are stronger predictors of performance than metabolic control in childhood, demonstrating the importance of strict treatment and follow-up even in adulthood. Additionally, maintaining stable phenylalanine concentrations and minimizing blood phenylalanine fluctuations is of significant importance [46, 47].
High concentrations of blood phenylalanine result in increased uptake of phenylalanine into the brain and concomitant decrease in the uptake of other large neutral amino acids (LNAAs). Phenylalanine is transported into the brain by one of the LNAA carriers, the L-amino acid transporter 1 (LAT-1) [18, 32, 48,49,50,51]. This transporter also selectively transports the amino acids valine, isoleucine , methionine, threonine, tryptophan, tyrosine, and histidine. The binding of the LNAA to the LAT-1 transporter is a competitive process; the rate of transport is proportionate to the blood concentration of all the transported amino acids [52]. This system has the highest affinity for phenylalanine. With high blood phenylalanine, there is a significant decrease in the transport of other LNAAs as more phenylalanine is transported into the brain. Elevated brain phenylalanine concentrations also negatively impact the synthesis of catecholamines and serotonin in the brain due to the altered uptake of tyrosine and tryptophan and metabolism of tyrosine and tryptophan hydroxylases [5, 18, 54].
The distribution of dopamine synthesis significantly alters activity of dopaminergic neurons of the prefrontal cortex, especially the dorsolateral area, which receives a large dopamine projection and is characterized by very high dopamine turnover [49]. It has been established that dopamine deficiency and disturbances in neurotransmitter balance may be responsible for cognitive and executive function deficits, as well as emotional problems, even in early-treated patients. The intensity of these dysfunctions is related to the degree of hyperphenylalaninemia. These observations form basic assumptions for the tyrosine-dopamine theory, which explains the complex abnormalities of neuropsychological function resulting from the intra-cerebral decrease of dopamine, secondary to tyrosine deficiency [18, 43, 53, 55].
Despite the significance for the clinical presentation of dopamine depletion, Pilotto et al. showed that serotonin depletion is also of great importance and may occur at much lower cerebral phenylalanine concentrations than the levels influencing dopamine synthesis. Based on the study of 10 adults with early-treated PKU, the authors postulated that the serotonergic axis is more vulnerable to high phenylalanine concentrations and that both serotonin and dopamine deficits are common in adult PKU patients. Additionally, the brain structural 3 T MRI study of these patients showed that decreased syntheses of dopamine and serotonin were correlated with specific gray matter atrophy patterns. These findings support the need for stricter metabolic control in adults to prevent neurotransmitter depletion and accelerated brain damage due to aging [32].
8 White Matter Pathology
PKU is associated with a diffuse brain pathology, including white matter changes that can be observed in both early and continuously treated patients [44]. As an integral part of the neuronal network, white matter has a crucial role in brain functioning. It is fundamental for proper motor and sensory functions, as well as sensory organ activity. White matter damage causes complex neurobehavioral syndromes, even if cortical and subcortical regions of gray matter remain intact [56]. The brains of individuals exposed to high blood phenylalanine from early childhood present with hypomyelination and astrocytic gliosis [44]. In addition, foci of segmental demyelination and areas of status spongiosis may occur [37, 39, 40, 49]. In histopathological research in mice, Malamud et al. described the above-mentioned phenomenon as diffuse vacuole formations occurring alongside the nerve fibers or in proximity of oligodendrocytes and stratifying myelin layers [57]. Complex disturbances of myelin metabolism in patients with PKU were coined with the term dysmyelination [58, 59]. The main function of the myelin sheath surrounding an axon is to facilitate the rapid conduction of action potentials along the axons for signal transmission and neurotransmitter synthesis [60]. The myelin takes part in axon maturation and, therefore, its damage causes disturbances in nervous system function. This means that altered myelin synthesis itself is the primary cause of secondary neuronal dysfunction and neurotransmitter synthesis abnormalities, including disturbances in the synthesis of dopamine (myelin-dopamine theory). Myelin-induced maturation of axons is also necessary for proper branching of dendrites during brain development, which is essential for the formation of the brain network [18, 34, 40, 60, 61].
Elevated brain phenylalanine concentrations influence the functioning of oligodendrocytes (glial cells responsible for myelin production) and thus proper axon functioning. Two types of oligodendrocytes are present in the central nervous system. The first type, phenylalanine-sensitive oligodendrocytes, is found in close proximity to neuronal networks that are myelinated after birth. With the exception of the cerebellum, these pathways are localized in frontal brain structures (optic tract, corpus callosum, subcortical white matter, and periventricular white matter). This group of oligodendrocytes is sensitive to phenylalanine concentrations, even in early-treated patients, and therefore, when the brain is exposed to high phenylalanine concentrations, myelin synthesis is disrupted. This leads to axons lacking proper myelin sheathing, further reducing the number of dendritic connections, decreasing nervous conductivity and neurotransmitter production in presynaptic areas. The second type of oligodendrocyte cells are phenylalanine non-sensitive oligodendrocytes. These cells myelinate the axon before birth and are situated primarily in hindbrain structures (internal capsule and brainstem) and in spinal cord [18, 53, 60].
Phenylalanine and related metabolites inhibit activity of 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductase (Fig. 9.2). This enzyme is critical for proper synthesis of cholesterol in phenylalanine-sensitive oligodendrocytes located in the frontal brain, especially in the prefrontal cortex. Locally synthesized cholesterol makes up approximately 30% of all myelin lipids of the brain tissue. The function of cholesterol is not only structural but is also required for proper neuronal signal transmission [18, 60]. Inhibition of HMG-CoA reductase by phenylalanine is partially reversible in some individuals. This explains the improvement in myelination observed in MRI scans of poorly controlled patients who have returned to diet and have reduced their blood phenylalanine concentrations. The reduction in phenylalanine allows for proper myelin production in the phenylalanine sensitive oligodendrocyte population [18, 53, 60, 62] (Fig. 9.3).
Phenylalanine neurotoxicity also includes the impact of elevated phenylalanine concentrations on the oligodendroglial enzyme, phenylalanine-sensitive ATP-sulfurylase. This enzyme is involved in the synthesis of cerebrosulphatides that protect the myelin base protein responsible for preventing myelin degradation. A lack of cerebrosulphatides results in an increase in the process of myelin degradation and, if not compensated for by proper synthesis, leads to complex dysmyelination changes [42, 49] (Fig. 9.4).
According to Dyer et al., white matter pathology in untreated PKU is a developmental process in which elevated phenylalanine concentrations arrest the myelination process, causing reduced myelin formation and hypomyelination. In early treated patients, myelin lesions reflect demyelination or dysmyelination and represent loss or impairment of previously assembled myelin [60] (Box 9.5).
Box 9.5: Dysmyelination Changes in PKU [40, 44, 58]
White matter abnormalities are a result of the following features:
-
Demyelination (loss of formed myelin) in treated individuals
-
Hypomyelination (lack of myelin formation) in untreated individuals
The diffuse character of white matter pathology in PKU may compromise multiple pathways, resulting in different deficits in motor skills, coordination, visual functioning, processing speed, language, memory, and learning as well as attention and executive functioning [44].
White matter abnormalities (WMAs) of the brain were first reported in patients with PKU at the end of the 1990s [66, 67]. Dysmyelination in white matter is revealed with MRI as intense lesions and cortico-subcortical atrophy on T2-weighted images with specifically high-signal intensity in periventricular white matter. WMA may be explained by cytotoxic edema and dysmyelination changes with an increase in free water trapped in myelin sheaths [47]. The size and distribution of WMA vary between patients with localization in the white matter primarily in the temporal and occipital lobes [39, 44] (Figs. 9.5 and 9.6).
Magnetic resonance imaging (MRI) of the brain: T2-weighted images using FLAIR (fluid attenuated inversion recovery) and FSE (fast spin echo) reveal enhanced signal intensity representing white matter abnormalities (WMA) in all lobes (arrows) of a female (MM) aged 27 years on low-phenylalanine diet from 3 to 8 years of age with a DQ of 32. Blood phenylalanine concentration at MRI was 1571 μmol/L
MRI of the head (T2-weighted images, FLAIR and FSE) in the same patient (Fig. 9.5) shows regression of hyperintense lesions in white matter (WMA) of all lobes (arrows) after 7 months of treatment with a low-phenylalanine diet. Mean blood phenylalanine concentration was 724 μmol/L, blood phenylalanine concentration at MRI was 690 μmol/L
9 Gray Matter Pathology
Phenylalanine also influences the gray matter, with the greatest effect in the neocortex. A state of chronic hyperphenylalaninemia, especially in the neonate, profoundly affects the neocortex on multiple levels (Box 9.6).
Box 9.6: Effects of Hyperphenylalaninemia on Gray Matter [37, 41]
-
Inhibition of growth process of the pyramidal pathways
-
Disrupted dendritic growth resulting in formation of fewer connections
-
Increased cell density of prefrontal cortex
-
Inadequate synaptogenesis resulting in decreased synaptic density
The primary location of this effect is the posterior brain (parietal and occipital cortex), and it is strongly correlated with blood phenylalanine concentrations [38].
10 Summary
If untreated or poorly controlled, especially in early childhood, PKU can result in severe intellectual disability, neurological deficits, and/or psychological/psychiatric manifestations. Early diagnosis with early and continuous treatment allows patients with PKU to achieve normal intellectual development; however, they still may exhibit a variety of neuropsychological difficulties. The pathogenesis of phenylalanine neurotoxicity in PKU is very complex and still far from being fully understood. It consists of both white and gray matter pathologies related to high brain phenylalanine concentrations. One of the main mechanisms of neurotoxicity is the impairment of brain neurotransmitter metabolism, especially in the prefrontal cortex. It is difficult to predict the outcome of discontinuing treatment in adults with early treated PKU; however, given the multidirectional effects of high blood phenylalanine on brain function, it is recommended that patients with PKU continue to be monitored and remain in metabolic control for life.
References
Acosta PB. Nutrition management of patients with inherited metabolic disorders. Sudbury: Jones and Bartlett Publishers, LLC; 2010.
Hillert A, Anikster Y, Belanger-Quintana A, Burlina A, Burton BK, Carducci C, et al. The genetic landscape and epidemiology of phenylketonuria. Am J Hum Genet. 2020;107(2):234–50.
Christ SE. Asbjorn Folling and the discovery of phenylketonuria. J Hist Neurosci. 2003;12(1):44–54.
Scriver CR. The PAH gene, phenylketonuria, and a paradigm shift. Hum Mutat. 2007;28(9):831–45.
Donlon J, Sarkissian C, Levy H, Scriver C. Hyperphenylalaninemia: phenylalanine hydroxylase deficiency. The online metabolic & molecular bases of inherited disease. McGraw Hill; 2021.
Bickel H, Gerrard AJ, Hickman EM. Influence of phenylalanine intake on phenylketonuria. Lancet. 1953;2:812–9.
Guthrie R, Susi A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics. 1963;32:338–43.
Chace DH, Millington D, Terada N, Kahler SG, Roe CR, Lindsay FH. Rapid diagnosis of phenylketonuria by quantitative analysis for phenylalanine and tyrosine in neonatal blood spots by tandem mass spec trometry. Clin Chem. 1993;39(1):66–71.
Gibson M, Duran M. Simple tests. In: Blau N, editor. Physician’s guide to the diagnosis, treatment, and follow-up of inherited metabolic diseases. New York: Springer; 2014.
Williams RA, Mamotte CD, Burnett JR. Phenylketonuria: an inborn error of phenylalanine metabolism. Clin Biochem Rev. 2008;29(1):31–41.
Blau N, van Spronsen FJ, Levy HL. Phenylketonuria. Lancet. 2010;376(9750):1417–27.
http://www.biopku.org/home/home.asp as of March 21, 2021.
Burgard P, Lachmann RH, Walter J. Hyperphenylalaninemia: 251–263, In: Saudubray JM, Baumgartner MR, Walter J, editors. Inborn metabolic diseases. Diagnosis and treatment. 6th edn. New York: Springer Medizin; 2016.
van Wegberg AMJ, MacDonald A, Ahring K, Belanger-Quintana A, Blau N, Bosch AM, et al. The complete European guidelines on phenylketonuria: diagnosis and treatment. Orphanet J Rare Dis. 2017;12(1):162.
Camp KM, Parisi MA, Acosta PB, Berry GT, Bilder DA, Blau N, et al. Phenylketonuria Scientific Review Conference: state of the science and future research needs. Mol Genet Metab. 2014;112(2):87–122.
Janos AL, Grange DK, Steiner RD, White DA. Processing speed and executive abilities in children with phenylketonuria. Neuropsychology. 2012;26(6):735–43.
Brumm VL, Bilder D, Waisbren SE. Psychiatric symptoms and disorders in phenylketonuria. Mol Genet Metab. 2010;99(Suppl 1):S59–63.
Ashe K, Kelso W, Farrand S, Panetta J, Fazio T, De Jong G, et al. Psychiatric and cognitive aspects of phenylketonuria: the limitations of diet and promise of new treatments. Front Psych. 2019;10:561.
MacDonald A, van Wegberg AMJ, Ahring K, Beblo S, Belanger-Quintana A, Burlina A, et al. PKU dietary handbook to accompany PKU guidelines. Orphanet J Rare Dis. 2020;15(1):171.
Cleary M, et al. Fluctuations in phenylalanine concentrations in phenylketonuria: a review of possible relationships with outcomes. Mol Genet Metab. 2013;110(4):418–23.
Horner FA, Streamer CW, Alejandrino LL, Reed LH, Ibbott F. Termination of dietary treatment of phenylketonuria. N Engl J Med. 1962;266:79–81.
Vandeman P. Termination of dietary treatment for phenylketonuria. Arch J Dis Child. 1963;106:492–5.
Hudson FP. Termination of dietary treatment of phenylketonuria. Arch J Dis Child. 1967;42:198–200.
Singh RH, et al. Recommendations for the nutrition management of phenylalanine hydroxylase deficiency. Genet Med. 2014;16(2):121–31.
Cerone R, et al. Phenylketonuria: diet for life or not? Acta Paediatr. 1999;88(6):664–6.
Smith I, et al. Effect of stopping low-phenylalanine diet on intellectual progress of children with phenyl-ketonuria. Br Med J. 1978;2(6139):723–6.
Seashore MR, et al. Loss of intellectual function in children with phenylketonuria after relaxation of dietary phenylalanine restriction. Pediatrics. 1985;75(2):226–32.
Vockley J, Andersson HC, Antshel KM, Braverman NE, Burton BK, Frazier DM, et al. Phenylalanine hydroxylase deficiency: diagnosis and management guideline. Genet Med. 2014;16(2):188–200.
van Spronsen FJ, Hoeksma M, Reijngoud DJ. Brain dysfunction in phenylketonuria: is phenylalanine toxicity the only possible cause? J Inherit Metab Dis. 2009;32(1):46–51.
Martynyuk AE, et al. Impaired glutamatergic synaptic transmission in PKU brain. Mol Genet Metab. 2005;86(Suppl 1):434–42.
Feksa LR, et al. Characterization of the inhibition of pyruvate kinase caused by phenylalanine and phenylpyruvate in rat brain cortex. Brain Res. 2003;968(2):199–205.
Pilotto A, Blau N, Leks E, Schulte C, Deuschl C, Zipser C, et al. Cerebrospinal fluid biogenic amines depletion and brain atrophy in adult patients with phenylketonuria. J Inherit Metab Dis. 2019;42(3):398–406.
Schuck PF, Malgarin F, Cararo JH, Cardoso F, Streck EL, Ferreira GC. Phenylketonuria pathophysiology: on the role of metabolic alterations. Aging Dis. 2015;6(5):390–9.
Schlegel G, Scholz R, Ullrich K, Santer R, Rune GM. Phenylketonuria: direct and indirect effects of phenylalanine. Exp Neurol. 2016;281:28–36.
Pilotto A, Zipser CM, Leks E, Haas D, Gramer G, Freisinger P, et al. Phenylalanine effects on brain function in adult phenylketonuria. Neurology. 2021;96(3):e399–411.
van Spronsen FJ, van Wegberg AM, Ahring K, Belanger-Quintana A, Blau N, Bosch AM, et al. Key European guidelines for the diagnosis and management of patients with phenylketonuria. Lancet Diabetes Endocrinol. 2017;5(9):743–56.
Huttenlocher PR. The neuropathology of phenylketonuria: human and animal studies. Eur J Pediatr. 2000;159(Suppl 2):S102–6.
Christ SE, Price MH, Bodner KE, Saville C, Moffitt AJ, Peck D. Morphometric analysis of gray matter integrity in individuals with early-treated phenylketonuria. Mol Genet Metab. 2016;118(1):3–8.
Clocksin HE, Hawks ZW, White DA, Christ SE. Inter- and intra-tract analysis of white matter abnormalities in individuals with early-treated phenylketonuria (PKU). Mol Genet Metab. 2021;132(1):11–8.
Joseph B, Dyer CA. Relationship between myelin production and dopamine synthesis in the PKU mouse brain. J Neurochem. 2003;86(3):615–26.
Hartwig C, Gal A, Santer R, Ullrich K, Finckh U, Kreienkamp HJ. Elevated phenylalanine levels interfere with neurite outgrowth stimulated by the neuronal cell adhesion molecule L1 in vitro. FEBS Lett. 2006;580(14):3489–92.
Brenton DP, Pietz J. Adult care in phenylketonuria and hyperphenylalaninaemia: the relevance of neurological abnormalities. Eur J Pediatr. 2000;159(Suppl 2):S114–20.
Antshel KM, Waisbren SE. Timing is everything: executive functions in children exposed to elevated levels of phenylalanine. Neuropsychology. 2003;17(3):458–68.
Anderson PJ, Leuzzi V. White matter pathology in phenylketonuria. Mol Genet Metab. 2010;99(Suppl 1):S3–9.
Sijens PE, Oudkerk M, Reijngoud DJ, Leenders KL, de Valk HW, van Spronsen FJ. 1H MR chemical shift imaging detection of phenylalanine in patients suffering from phenylketonuria (PKU). Eur Radiol. 2004;14(10):1895–900.
Romani C, Palermo L, MacDonald A, Limback E, Hall SK, Geberhiwot T. The impact of phenylalanine levels on cognitive outcomes in adults with phenylketonuria: effects across tasks and developmental stages. Neuropsychology. 2017;31(3):242–54.
Daelman L, Sedel F, Tourbah A. Progressive neuropsychiatric manifestations of phenylketonuria in adulthood. Rev Neurol (Paris). 2014;170(4):280–7.63.
de Groot MJ, Hoeksma M, Blau N, Reijngoud DJ, van Spronsen FJ. Pathogenesis of cognitive dysfunction in phenylketonuria: review of hypotheses. Mol Genet Metab. 2010;99(Suppl 1):S86–9.
Surtees R, Blau N. The neurochemistry of phenylketonuria. Eur J Pediatr. 2000;159(S2):109–13.
van Spronsen FJ, et al. Large neutral amino acids in the treatment of PKU. From theory to practice. J Inherit Metab Dis. 2010;33(6):671–6.
Pardridge WM. Blood-brain barrier carrier-mediated transport and brain metabolism of amino acids. Neurochem Res. 1998;23(5):635–44.
Smith QR. Glutamate and Glutamine in the Brain. J Nutr. 2000;130:1016S–22S.
Dyer CA. Comments on the neuropathology of phenylketonuria. Eur J Pediatr. 2000;159(Suppl 2):S107–8.
Christ SE, Huijbregts SC, de Sonneville LM, White DA. Executive function in early-treated phenylketonuria: profile and underlying mechanisms. Mol Genet Metab. 2010;99(Suppl 1):S22–32.
Diamond A, Prevor MB, Callender G, Druin DP. Prefrontal cortex cognitive deficits in children treated early and continuously for PKU. Monogr Soc Res Child Dev. 1997;62(4):i–v, 1–208.
Filley CM. The behavioral neurology of cerebral white matter. Neurology. 1998;50(6):1535–40.
Malamud N. Neuropathology of phenylketonuria. J Neuropathol Exp Neurol. 1966;25(2):254–68.
Pietz J. Neurological aspects of adult phenylketonuria. Curr Opin Neurol. 1998;11(6):679–88.
Pearsen KD, Gean-Marton AD, Levy HL, Davis KR. Phenylketonuria: MR imaging of the brain with clinical correlation. Radiology. 1990;177(2):437–40.
Dyer CA. Pathophysiology of phenylketonuria. Ment Retard Dev Disabil Res Rev. 1999;5:104.
Kirkpatrick LL, Brady ST. Modulation in the axonal microtubule cytoskeleton by myelinating Schwann cells. J Neurosci. 1994;14(12):7440–50.
Cleary MA, Walter JH, Wraith JE, White F, Tyler K, Jenkins JP. Magnetic resonance imaging in phenylketonuria: reversal of cerebral white matter change. J Pediatr. 1995;127(2):251–5.
Shah SN, Peterson NA, McKean CM. Cerebral lipid metabolism in experimental hyperphenylalaninaemia: incorporation of 14C-labelled glucose into total lipids. J Neurochem. 1970;17(2):279–84.
Dyer CA, Kendler A, Philibotte T, Gardiner P, Cruz J, Levy HL. Evidence for central nervous system glial cell plasticity in phenylketonuria. J Neuropathol Exp Neurol. 1996;55(7):795–814.
Hommes FA. Amino acidaemias and brain maturation: interference with sulphate activation and myelin metabolism. J Inherit Metab Dis. 1985;8(Suppl 2):121–2.
Villasana D, Butler IJ, Williams JC, Roongta SM. Neurological deterioration in adult phenylketonuria. J Inherit Metab Dis. 1989;12(4):451–7.
Shaw DW, Weinberger E, Maravilla KR. Cranial MR in phenylketonuria. J Comput Assist Tomogr. 1990;14(3):458–60.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Giżewska, M. (2022). Phenylketonuria: Phenylalanine Neurotoxicity. In: Bernstein, L.E., Rohr, F., van Calcar, S. (eds) Nutrition Management of Inherited Metabolic Diseases. Springer, Cham. https://doi.org/10.1007/978-3-030-94510-7_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-94510-7_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-94509-1
Online ISBN: 978-3-030-94510-7
eBook Packages: MedicineMedicine (R0)