Abstract
Tinea capitis is the most common infection in prepubertal individuals and it is most frequent in children aged 3–7 years. There is connection between the disease with low socioeconomic status and poor hygiene. Clinical presentation of T. capitis may be divided into two subtypes: Inflammatory and non-inflammatory. Inflammatory and suppurative type is known as kerion. Living in rural areas and immune system disorders may facilitate the development of kerion. Zoophilic dermatophytes are more prominent in kerion formation. The clinical presentation is painful and erythematous, inflammatory crusted nodule(s). Pustules may accompany. Regional lymphadenopathy may be present. Clinical examination and fungal culture are very essential in the diagnosis. Wood’s lamp examination and dermoscopy may help in the diagnosis process of a kerion. Wood’s lamp investigation reveals green fluorescence in M. canis-caused infection. Broken hairs, scales, follicular keratosis, black dots, bent hairs, erythema, comma hairs, crusts, forked hairs, barcode-like hair, follicular pustules, V-shaped hairs may be observed with a dermoscopy in patients having kerion celsi.
In this article, a 5-year-old girl with multiple painful alopecic plaques on the scalp will be presented.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
An otherwise healthy five-year-old girl was presented with a six-week history of a painful lesions and hair loss. The lesions started as a small area of hair loss with coexisted erythema, folliculitis and scaling. Pruritus was reported. The patient was previously treated with oral amoxicilin combined with clavulanic acid and topical fucidic acid with no improvement. With time, edema and pain occured. Moreover, the progression of hair loss was reported. The patient’s family lived in rural areas and carried out cattle and ovine breeding. Moreover, numerous contacts with stray dogs and cats were reported. Siblings, parents, and other family members did not have similar symptoms or signs.
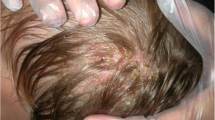
A physical examination of the patient revealed red-colored five alopecic plaques (5 × 5, 3 × 2, 0.5 × 0.3, 0.5 × 0.2, and 1 × 1 cm) on the right temporal region and a solitary plaque (1 × 1 cm) on the frontal area of her scalp. All the plaques were covered with yellow adherent crusts. The lesions were filled with pus and discharge was observed (Fig. 56.1). The presence of multiple pustules especially within the largest plaque was detected (Fig. 56.2). A dermatological examination of other body parts was normal. Enlarged and painful right cervical and occipital lymph nodes (approximately 1 × 1.5 cm in diameter) and were palpated. Hepatosplenomegaly was not detected. Dermoscopy revealed comma hairs, pustules, bent hairs, scales, crusts, and erythema. A Wood’s lamp examination showed green fluorescence. A direct mycological examination was negative.
Complete blood count showed mild anemia and leukocytosis (11.19 × 103 μL). C-reactive protein and erythrocyte sedimentation rate were increased (7.6 mg/L and 65 mm/h, respectively). There were no other noticeable abnormalities. Viral Hepatitis and HIV serology were negative. In fungal culture, the growth of Microsporum canis was observed.
Based on the case description and the photographs, what is your diagnosis?
-
1.
Scalp abscess.
-
2.
Kerion celsi.
-
3.
Alopecia areata.
-
4.
Discoid lupus erythematosus.
Kerion celsi.
Discussion
Tinea capitis is the most common infection in prepubertal individuals and it is most frequent in children aged three to seven years. An increased incidence of tinea capitis in this age group is associated with the absence of sebum secretion. The disease is not expected in infants and adults, but it can be rarely seen. There is a relation between the disease with low socioeconomic status and poor hygiene. Several pathogens may lead to tinea capitis such as Microsporum canis, Trichophyton verrucosum, Trichophyton mentagrophytes, Trichophyton tonsurans, and Trichophyton schoenleinii. Clinical presentation of T.capitis may be divided into two subtypes: inflammatory and non-inflammatory. Living in rural areas and immune system disorders may facilitate the development of kerion. Zoophilic dermatophytes are more prominent in kerion formation [1]. Kerion celsi is an inflammatory or suppurative form of tinea capitis which occurs as a result of an increased hypersensitivity to dermatophyte infection. It presents as a painful and erythematous, inflammatory crusted nodule(s). Pustules may be observed. Although the lesion is mostly solitary, multiple plaques may also be present. Purulent drainage and regional lymphadenopathy usually accompany. The hair is easily pulled in the infected area.
If the disease is not diagnosed early and treatment is not started quickly, the infection may result in fibrosis and cicatricial alopecia. Trichophyton mentagrophytes , Trichophyton verrucosum , Trichophyton megnininii , and Trichophyton tonsurans can be listed as the causing factors of kerion. Besides, Microsporum canis and Microsporum gypseum infections may also lead to the formation of kerion celsi [2]. Clinical examination and fungal culture are very essential in diagnosing tinea capitis. Skin biopsy should be avoided in pediatric population. In direct mycology test with 10–30% potassium hydroxide, fungal elements such as hyphae and spores may be observed [1]. A Wood’s lamp examination may be utilized in the diagnosis. In infections caused by Microsporum canis, a Wood’s lamp investigation reveals green fluorescence due to ectothrix infections. Trichophyton verrucosum may also lead to ectothrix infections. Endothrix type of infection caused by Trichophyton tonsurans, Trichophyton soudanense, Trichophyton rubrum, Trichophyton violaceum, or Trichophyton rubrum does not reflect any color under Wood’s light [3]. Dermoscopic evaluation is also valuable in the process of diagnosis. Broken hairs, scales, follicular keratosis, black dots, bent hairs, erythema, comma hairs, crusts, forked hairs, bar code-like hairs, follicular pustules, V-shaped hairs may be observed in patients with kerion celsi [4].
Skin abscess refers to collection of pus in the epidermis, dermis, and/or deeper layers. A more superficial form is called a furuncle. Carbuncles are deeply located skin abscesses formed by the fusion of multiple furuncles. Gram-positive bacteria such as Staphylococcus aures and Streptococci cause more than 90% of skin abscesses, while Gram-negative bacteria are isolated in <10% of cases. Skin abscesses can be induced by trauma, insect bites, animal scratches and bites, poor hygiene, atopic dermatitis, and immune disorders and may occur on any skin surface. On clinical examination, erythematous and sensitive induration can be palpated, and a central pustule usually accompanies. Besides, the lesion may fluctuate during palpation. Skin abscesses can vary in size but are usually 1–3 cm. Fever and fatigue may be accompanied in larger skin abscesses. In addition, leukocytosis and an increase in inflammatory markers can be observed in the peripheral blood. Lymphadenopathy may also be observed. Spontaneous drainage generally occurs. Diagnosis is made mainly by clinical examination. Ultrasound, tissue material culture, PCR, and hemoculture can be utilized in the diagnosis [5].
Alopecia areata is a common inflammatory hair loss. This disease can occur in people of all ages, sexes, and ethnic backgrounds. Although the exact pathophysiological mechanism of the disease is still unknown, autoimmune attack and other immune alterations are emphasized. The frequency of the disease is higher in patients with autoimmune disease and personal atopy history. In alopecia areata, small round alopecic areas (patchy alopecia areata) are usually observed. However, a complete scalp hair loss (alopecia totalis) to complete scalp and body hair loss (alopecia universalis) may be present [6]. Although alopecia areata generally shows an asymptomatic course, some patients may experience burning and stinging sensation, itch, or pain [7]. There is no obvious inflammation or scar. Nail findings such as pitting and brittleness in patients with alopecia areat may be detected [6]. In addition to dermatological examination, dermoscopy is also important. Characteristic dermoscopic findings of alopecia areata are exclamation mark hairs, yellow dots, and dystrophic hairs.
Discoid erythematosus among the pediatric population is rare. The disease causes alopecic scarring plaques on the scalp with ill-defined borders. Adherent scales cover the alopecic surfaces and follicular plugs may be observed macroscopically when the scale is removed [8]. In general, the lesions are characterized by skin atrophy, telangiectasia, changes in pigmentation, follicular plugging, and scarring [9]. Diagnosis of discoid lupus erythematosus may be difficult; the lesions can mimic bacterial and fungal infections. Characteristic dermoscopic features of discoid lupus erythematosus are loss of follicular ostia, follicular keratotic plugs, arborizing vessels, honeycomb pigmented network, dyschromia, scaling, and follicular red dot pattern [10]. A direct immunofluorescence test shows complement or immunoglobulin deposits in the dermo-epidermal junction [8].
Key Points
-
Kerion celsi is an inflammatory or suppurative form of tinea capitis which occurs as a result of increased hypersensitivity to dermatophyte infection.
-
Kerion celsi presents as a painful and inflammatory crusted nodule. Although the lesion is mostly solitary, multiple plaques may also be present.
-
If the diagnosis is not made early and treatment is not started quickly, the infection may result in fibrosis and cicatricial alopecia.
-
A clinical examination, dermoscopy, direct mycology test with 10−30% potassium hydroxide, Wood’s lamp examination, and fungal culture are valuable in the diagnosis of kerion celsi.
References
John AM, Schwartz RA, Janniger CK. The kerion: an angry tinea capitis. Int J Dermatol. 2016;57(1):3–9. https://doi.org/10.1111/ijd.13423.
Arenas R, Toussaint S, Isa-Isa R. Kerion and dermatophytic granuloma. Mycological and histopathological findings in 19 children with inflammatory tinea capitis of the scalp. Int J Dermatol. 2006;45(3):215–9. https://doi.org/10.1111/j.1365-4632.2004.02449.x.
Hay RJ. Tinea capitis: current status. Mycopathologia. 2016;182(1–2):87–93. https://doi.org/10.1007/s11046-016-0058-8.
Aqil N, BayBay H, Moustaide K, Douhi Z, Elloudi S, Mernissi FZ. A prospective study of tinea capitis in children: making the diagnosis easier with a dermoscope. J Med Case Rep. 2018;12(1):383. https://doi.org/10.1186/s13256-018-1914-6. PMID: 30591075; PMCID: PMC6309099.
Galli L, Venturini E, Bassi A, Gattinara GC, Chiappini E, Defilippi C, et al. Common community-acquired bacterial skin and soft-tissue infections in children: an intersociety consensus on impetigo, abscess, and cellulitis treatment. Clin Ther. 2019; https://doi.org/10.1016/j.clinthera.2019.01.010.
Strazzulla LC, Wang EHC, Avila L, Lo Sicco K, Brinster N, Christiano AM, Shapiro J. Alopecia areata. J Am Acad Dermatol. 2018;78(1):1–12. https://doi.org/10.1016/j.jaad.2017.04.1141.
Alkhalifah A. Alopecia areata update. Dermatol Clin. 2013;31(1):93–108. https://doi.org/10.1016/j.det.2012.08.010.
Miettunen PM, Bruecks A, Remington T. Dramatic response of scarring scalp discoid lupus erythematosus (DLE) to intravenous methylprednisolone, oral corticosteroids, and hydroxychloroquine in a 5-year-old child. Pediatr Dermatol. 2009;26(3):338–41. https://doi.org/10.1111/j.1525-1470.2009.00916.x.
Arkin LM, Ansell L, Rademaker A, Curran ML, Miller ML, Wagner A, Paller AS. The natural history of pediatric-onset discoid lupus erythematosus. J Am Acad Dermatol. 2015;72(4):628–33. https://doi.org/10.1016/j.jaad.2014.12.028.
Tosti A, Torres F, Misciali C, Vincenzi C, Starace M, Miteva M, Romanelli P. Follicular red dots. Arch Dermatol. 2009;45(12) https://doi.org/10.1001/archdermatol.2009.277.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Atsü, A.N., Caf, N., Türkoğlu, Z., Kutlubay, Z. (2022). Nodular Alopecic Lesions. In: Waśkiel-Burnat, A., Sadoughifar, R., Lotti, T.M., Rudnicka, L. (eds) Clinical Cases in Scalp Disorders. Clinical Cases in Dermatology. Springer, Cham. https://doi.org/10.1007/978-3-030-93426-2_56
Download citation
DOI: https://doi.org/10.1007/978-3-030-93426-2_56
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-93425-5
Online ISBN: 978-3-030-93426-2
eBook Packages: MedicineMedicine (R0)