Abstract
Diatoms play key roles in aquatic ecosystems, from being important primary producers to driving major biogeochemical cycles. Understanding what determines diatom growth and the taxonomic and functional diversity is essential for our general knowledge of aquatic ecosystems and for predicting their responses to changing environmental conditions. This chapter provides a brief overview of diatom ecology from a trait-based perspective. First, it reviews traits that determine diatom responses to the environment, including the nutrient-, light-, and temperature-related traits. It then describes traits that are important for the ecological interactions of diatoms with other members of planktonic food webs, such as competitors, predators, parasites, and mutualists. The chapter also briefly discusses trait plasticity and evolution, as well as highlights traits that determine diatom responses to global environmental change. Finally, the chapter briefly outlines some challenges and future directions in the ecology of diatoms.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Diatoms are a highly successful and abundant group of marine and freshwater phytoplankton that contributes more than 20% of global carbon fixation on Earth (Nelson et al. 1995). They are among the main primary producers in planktonic and benthic (especially in freshwaters) food webs and a major food source for primary consumers (zooplankton in planktonic food webs) (Sommer et al. 2002). Diatoms also have a disproportionately large role in carbon sequestration because of their relatively heavy silica frustules that contribute to their sinking and carbon immobilization (Smetacek 1999; Basu and Mackey 2018; Tréguer et al. 2018).
While this book is primarily about the molecular aspects of diatom life, understanding those aspects without the knowledge of ecology is impossible. Many significant advances in the field of diatom biology occurred while looking at different levels of biological organization simultaneously, from genomes to populations and communities. This chapter provides a brief overview of diatom ecology through the mechanistic lens of trait-based approaches. Focusing on traits rather than species helps understand the mechanisms of ecological responses of organisms to the environment, their biotic interactions, and how ecological communities are structured. In this chapter, I discuss key ecological dimensions of diatom life and the eco-physiological traits that determine diatom responses to the environment and their interactions with grazers, parasites, and bacterial symbionts. I also briefly discuss trait plasticity and evolution and how they may complicate our trait-based predictions by altering trait values. I finish the chapter outlining the challenges and future directions in trait-based diatom biology.
2 Ecological Success of Diatoms
What makes diatoms so successful and how will this group fare in the future, with the ongoing changes in climate and other anthropogenic influences? To understand the past, current, and future ecological success of diatoms in aquatic food webs, we can use trait-based approaches that focus on functional traits rather than on species per se. Trait-based approaches are now a major research framework in terrestrial plant ecology (McGill et al. 2006; Lavorel and Garnier 2002; Lamanna et al. 2014) and is becoming more and more popular in studies of other organisms, including phytoplankton and other microbes (Litchman and Klausmeier 2008; Green et al. 2008; Martiny et al. 2015; Westoby et al. 2021). Trait-based approaches can help increase our mechanistic understanding of how ecological communities assemble and function and how they change along different environmental gradients. They can also help reduce the complexity of a system, while incorporating diversity. A consideration of different trait combinations and correlations, including trait trade-offs, could help mechanistically define different ecological strategies, for example, a grazer-resistant strategy (through large cell size) may also be associated with poor nutrient competitive ability and vice versa, so that species highly susceptible to grazing may be good nutrient competitors (Edwards et al. 2011).
Trait-based approaches can also be useful in predicting the future of ecological communities under the ongoing anthropogenic climate change by determining what traits or suites of traits could be selected for or against under different global change scenarios, such as warming, changes in physical mixing patterns and nutrient regimes, or food web alterations.
Below I will briefly outline the main concepts relevant to trait-based ecology and discuss how they can be applied to diatoms to understand their ecology and evolution at present, in the past, and in the future. I will also outline future directions in trait-based ecology of diatoms.
3 Trait-Based Framework
To develop a trait-based ecological framework for any group of organisms, we need to identify relevant traits and investigate whether and how different traits are correlated and whether there are trade-offs among traits (Litchman and Klausmeier 2008). We also need to determine how traits are related to fitness and define how fitness would be assessed (McGill et al. 2006). We should also define the most important environmental gradients and what trait combinations could be selected for under different environmental conditions (Litchman et al. 2007). We can also use trait-based mechanistic models to explain and predict the distributions of organisms with different traits in different environments (Follows et al. 2007; Klausmeier et al. 2020).
Traits are often defined as “any morphological, physiological, or phenological heritable feature measurable at the level of the individual, from the cell to the whole organism, without reference to the environment or any other level of organization” (Garnier et al. 2016). Functional traits are the most commonly studied traits that can be defined as traits that affect fitness (Violle et al. 2007). Traits can also be classified as morphological, physiological, life history, behavioral, as well as genomic and metabolic traits (Litchman and Klausmeier 2008). In microbes, including diatoms, genomic and metabolic traits may be important for inferring ecological strategies. Traits can be grouped as related to different organismal functions, such as growth and reproduction, resource acquisition, and avoidance of consumers and parasites/pathogens (Litchman and Klausmeier 2008). Another trait classification divides traits into “response” and effect” traits (Lavorel and Garnier 2002, Litchman et al. 2015). The response traits determine an organism’s responses to the environment, and the effect traits characterize the effects of an organism on the environment. Some traits can be the response and effect traits at the same time, and they may be more influential and informative than other traits. For example, nitrogen (N) fixation can be viewed both as a response trait (N-fixation increases under N limitation) and an effect trait (N-fixation can increase N availability in an ecosystem).
Many traits are not independent from each other but positively or negatively correlated, and those correlations do not have to be linear. Many such trait relationships can represent trade-offs, meaning that having certain values for a trait may lead to a limited range of another trait and those values would not necessarily maximize the performance, if viewed in isolation. For example, a common trade-off in phytoplankton is a negative relationship between the ability to compete for scarce resources such as nutrients and vulnerability to grazing (Grover 1995; Leibold 1996). This trade-off is often related to cell size, so that a small-celled phytoplankter has a competitive advantage in acquiring a resource at low concentrations due to less diffusion limitation, but the smaller size makes it more vulnerable to grazing (Litchman and Klausmeier 2008; Edwards et al. 2013). These trade-offs likely apply to diatom algae as well. Some trade-offs are not universal but more evident in certain environments. For example, the competitive ability for phosphorus trade-offs with the competitive ability for nitrogen in freshwater phytoplankton but appears to be positively correlated (so, no trade-off) in marine phytoplankton (Edwards et al. 2013). Trade-offs may be not only pairwise but higher-dimensional as well, where more than two traits are correlated to form a trade-off surface (Edwards et al. 2013).
4 Diatom Origin and Macroevolutionary Patterns in Traits
Diatoms evolved around 150 to 200 million year ago, around the Jurassic period of the Mesozoic era (Kooistra et al. 2007; Medlin 2016). The evidence of subsequent massive diversification of diatoms is found in the Cretaceous (Medlin 2016). The three major clades are Coscinodiscophyceae (radial centrics) that appeared in the Jurassic, Mediophyceae (polar centrics + radial Thalassiosirales) that appeared in early Cretaceous, and Bacillariophyceae (pennates) that appeared in late Cretaceous (Kooistra et al. 2007, Medlin 2016). Interestingly, the major extinction of the K-T boundary was not particularly detrimental for diatoms, and they kept diversifying after that (Kooistra et al. 2007).
The evolution of silica frustule in diatoms was a major innovation that contributed to diatoms’ ecological success (Smetacek 2001; Raven and Waite 2004; Kooistra et al. 2007). The evolutionary emergence of new traits often allows different groups of organisms to colonize novel ecological niches, and increase their competitive abilities and the overall fitness. In addition, with respect to trait trade-offs, where traits are not independent but correlated, leading to constraints, the evolution of novel traits may lead to breaking the trade-offs, where novel lineages become outliers, deviating from established relationships among traits. Being an outlier may help gain competitive advantage under different environmental scenarios.
Diatom evolution of silica frustule may have increased diatom fitness in different ways. Cells became heavier, and their sinking rates increased (Smetacek 1985). Sinking is often viewed as contributing to phytoplankton’s ability in general and diatoms’ in particular to move toward nutrient-rich lower layers of the water column (Smetacek 1985). Both in the ocean and freshwater lakes, temperature and water density gradients and the resulting water column stratification lead to pronounced gradients in nutrients, where nutrient-rich water sits below the nutrient-depleted (due to phytoplankton uptake) water (Klausmeier and Litchman 2001). Rapid sinking of heavy cells with silica frustules to gain access to nutrients can afford a competitive advantage to diatoms over other phytoplankton groups (Raven and Waite 2004; Gemmell et al. 2016; Du Clos et al. 2021).
Another likely advantage of silica frustule is the increased range of shapes and sizes that diatom cells can evolve (Ryabov et al. 2021). Compared to other phytoplankton groups, diatoms exhibit a greater diversity of shapes, including extremely elongated, needle-like cells that would not be possible in the absence of the hard supporting silica crystal structure (Ryabov et al. 2021) (Fig. 1). This diversity of shapes may help achieve higher fitness under different environmental conditions and, thus, contribute to the overall ecological success of diatoms. Shape variation may help more efficient nutrient acquisition and escape from grazers by exceeding the dimensions of grazing windows by different grazers.
The evolution of silica cell wall in diatoms also allowed a more efficient protection from zooplankton grazers in a more direct way, as silica frustules are exceptionally strong and can withstand significant force from grazers, both due to the frustule architecture and silica as the material (Hamm et al. 2003). The evolution of better defenses against grazers in diatoms (and other phytoplankton) has led to so called “arms race,” where both the phytoplankton (prey) and the grazers (predators) evolve various means to better defend themselves and to overcome defenses, respectively (Smetacek 2001).
Over the geological time, diatom traits changed, and one such change that is relatively well documented is the change in cell size (Finkel et al. 2005). The mean frustule size decreased 2.5-fold over the Cenozoic; however, the range of sizes, as well as species diversity increased (Finkel et al. 2005). Several drivers, such as temperature and nutrient availability, are hypothesized to control diatom size on long time scales (Finkel et al. 2005). Diatom size has major effect on carbon sequestration and biogeochemical cycling; consequently, changes in cell size influenced these processes over the geological time.
5 Traits That Determine Diatom Responses to the Environment
Diatoms, as all other phytoplankton, need nutrients (macro- and micronutrients), sunlight, and CO2 to survive and grow. These are major resources for diatoms. Nutrient- and light-dependent growth and utilization are well studied, and several mathematical relationships are commonly used to describe these processes. The parameters of those equations are often determined experimentally and viewed as traits (Litchman and Klausmeier 2008). These traits can thus be called “response” traits. Comparing those traits across groups can provide novel insights into the mechanistic basis of diatom ecological strategies and how they differ from strategies of other groups. In addition to resources (consumed by diatoms), several environmental factors, such as pH and temperature, are the important dimensions of diatom ecological niches. Below I briefly outline common ways to describe the dependence of diatom growth on those factors.
5.1 Nutrients
The ability of diatoms to take up nutrients is often described as the Michaelis–Menten relationship:
where V is the uptake rate, R is the resource (nutrient) concentration, Vmax is the maximum uptake rate for a given resource, and ks is the half-saturation constant for uptake.
The dependence of growth on a nutrient is also assumed to follow a saturating curve (Monod curve):
where μ is the growth rate, μmax is the maximum growth rate, and Ks is the half-saturation constant for growth (note that it is different from the half-saturation constant for uptake, reflected here by using the capital K).
If we want to determine the overall change of biomass or cell density, we need to include mortality m, and the simplest way is to assume constant mortality. Then, the change in biomass B with time t would be:
Often, growth rate is assumed to depend not on the external but on the internal/intracellular nutrient concentration or nutrient quota, according to the Droop model (Droop 1973; Grover 1991a; Litchman and Klausmeier 2008):
where μ∞ is the growth rate at infinite quota, Q is nutrient quota (intracellular nutrient concentration), and Qmin is the minimum nutrient quota at which growth stops. There is also a separate equation to describe nutrient uptake V (Litchman and Klausmeier 2008) (Eq. 1).
The Droop model works better than the Monod model under fluctuating resource supply, as it tracks more realistically the dynamics of intracellular resource (nutrient) content (Grover 1991b). However, it has more parameters (traits) that need to be measured. A significant number of studies measured nutrient uptake in diatoms, as well as in other phytoplankton. These nutrient-related traits have been compiled for diatoms and other phytoplankton, so we can determine if diatoms tend to differ from other groups in some of those traits (Edwards et al. 2015a). Mean maximum growth rates tend to be higher in marine diatoms compared to other marine phytoplankton groups (Edwards et al. 2012). This suggests that marine diatoms should be adapted to high or fluctuating nutrient conditions (Grover 1997; Edwards et al. 2012). Interestingly, the pattern is different in freshwaters: Freshwater diatoms tend to have intermediate values of nutrient-related traits among freshwater groups. For many other nutrient-related traits, differences across taxonomic groups can be explained by cell size differences. When cell size effects are accounted for, diatoms tend to have intermediate values of nutrient-related traits (Edwards et al. 2012). Some general trends revealed by the analysis of trait compilations apply to diatoms, as well as other taxonomic groups. For example, the ability to compete for limiting nutrients declines with increasing cell size, with the scaled nutrient affinity declining with size (Edwards et al. 2012). Interestingly, marine and freshwater diatoms differ in their nutrient-related traits, such as affinities for N and P, which suggests different selective pressures (e.g., degree of limitation by different nutrients) in the two environments (Edwards et al. 2011, 2012).
Diatoms may benefit from fluctuating nutrient supply because their ability to store nutrient pulses is high, as they tend to have large vacuoles (Sicko-Goad et al. 1977). This is especially true for nitrogen, as it is stored primarily in the vacuole, while phosphorus is stored in the cytoplasm, so the advantage of having a large vacuole does not matter as much for this nutrient (Syrett 1981; Litchman et al. 2009). Similarly, for iron, another frequently limiting nutrient, vacuole storage may be of a lesser importance, at least in some diatoms, as ferritins, proteins that bind to iron, may be used for storing it (Marchetti et al. 2009; Lampe et al. 2018). The scaling of the vacuole size may be greater than that of the cell volume, so that the relative vacuole size is greater in large-celled species, implying that large cells have enhanced storage capacity. Given that larger cells tend to have slower growth rates, there is likely a trade-off between the maximum growth rate and nutrient (at least nitrogen) storage capacity. This trade-off, as trade-offs in general, defines contrasting ecological strategies that can be favored by different environmental conditions or coexist spatially and/or temporally, especially under fluctuating conditions (Sommer 1984; Litchman et al. 2009; Edwards et al. 2013).
The parameters of the equations used to describe the dependence of diatom (and other phytoplankton) growth on nutrients can be considered traits and used to predict growth under different conditions. Connecting these traits with the underlying physiological and molecular mechanisms and determining the mechanistic explanations of the observed trait differences across taxa are active areas of research that include looking at the genomes, transcriptomes, and cell molecular structure of different species. Diatoms up- or downregulate numerous genes under nutrient limitation, leading to differences in transcription and abundance of proteins, that likely lead to changes in nutrient-related traits. For example, under phosphorus limitation, the model diatom Thalassiosira pseudonana exhibited changes in the transcription and abundance of proteins associated with cellular P allocation and transport, increased utilization of dissolved organic phosphorus (DOP), change in the cell surface, and regulation of glycolysis and translation (Dyhrman et al. 2012). Under nitrogen limitation, T. pseudonana upregulated genes involved in glycolysis, the tricarboxylic acid cycle (TCA) cycle, and N metabolism and downregulated genes for the Calvin cycle, gluconeogenesis, pentose phosphate, oxidative phosphorylation, and lipid synthesis (Jian et al. 2017).
5.2 Light
For light utilization, several functions are commonly used to describe the dependence of phytoplankton, including diatom, growth on light. The growth rate can either be a saturating function of irradiance, such as an equivalent of the Monod function, or can include inhibition of growth by high light levels, called photoinhibition. Here is an example of a saturating function that is used to describe light-dependent growth (Schwaderer et al. 2011):
where μ is the growth rate, I is the irradiance, μmax is the maximum growth rate, and α is the initial slope of the growth–irradiance curve.
For the growth rate that declines under high irradiance, Eilers and Peeters (1988) proposed the following function:
where μmax is the maximum growth rate (d−1) achieved at Iopt, I is the irradiance (μmol photons m−2 s−1), and α is the initial slope of the growth–irradiance curve (Edwards et al. 2016). Key traits that characterize diatom growth responses to light are the α, μmax, and Iopt. α characterizes the ability to grow at low irradiances, and high μmax is usually selected for at high or fluctuating nutrients (Litchman et al. 2009). High α is probably achieved through the high concentration of photosynthetic pigments, and there is a positive relationship between α and cellular chl a concentration (Edwards et al. 2015b).
When diatoms are compared to other groups of phytoplankton, they tend to differ in their light-related traits and appear to have high α and high μmax (Edwards et al. 2015b). Thus, diatoms may be adapted to low light availability and high nutrients, which are the conditions associated with high mixing regimes that may favor diatoms, keeping them suspended in the water column (Lindenschmidt and Chorus 1998). Interestingly, turbulence was shown to stimulate carbon assimilation within diatom chains (Bergkvist et al. 2018), suggesting that diatoms may be adapted to turbulence in many ways. Microturbulence also changed gene expression in diatoms under nutrient-replete conditions (Amato et al. 2017), demonstrating a link between turbulent conditions and diatom physiology. There also seems to be a difference between coastal and oceanic diatoms, where coastal isolates have higher α and μmax (Edwards et al. 2015b). This could suggest adaptation to low light availability and high nutrient availability in coastal environments. There are also correlations between traits, with α and Iopt being negatively correlated, suggesting that there could be different ecological strategies with respect to light, the high light and low light-adapted species and strains (Edwards et al. 2015b).
5.3 Temperature
Similarly, for temperature, several functions have been used recently to describe the shape of dependence of growth rates or other eco-physiological processes on temperature (Thomas et al. 2012, 2017). One of them is the Norberg function (Norberg 2004; Thomas et al. 2012):
where μ(T) is the temperature-dependent growth rate (day−1), z is the midpoint of the growth curve, ω is the width of the unimodal response to temperature, and a and b determine the height, steepness, and skewness of the curve (Edwards et al. 2016). Key temperature-related traits are the optimum temperature for growth, Topt, minimum Tmin, and maximum Tmax temperatures that have nonnegative growth and bracket the thermal niche of an organism and the growth rate at Topt. These traits can be calculated from the fitted Norberg curve (Thomas et al. 2012).
Most of the growth–temperature curves are left-skewed, meaning that growth rate declines more sharply when temperatures increase above the optimum temperature, at which the growth rate is the highest (Thomas et al. 2012). There are other functions that have been used, for example, a relationship where both growth and mortality processes (births and deaths) are temperature-dependent. This dependence also produces a skewed, unimodal curve (O’Donnell et al. 2018). The skewness of the growth–temperature curve or the thermal performance curve (TPC) is an important feature that means that even slight increases of temperature above the optimum lead to significantly lower growth rates (Thomas et al. 2012). This may in part explain why most species have Topt that are higher than the average temperature of their environments (Thomas et al. 2012).
The compilations of temperature traits of different phytoplankton taxa allow us to determine if diatom temperature traits differ from other groups. Thomas et al. (2016) found that diatoms tend to have lower Topt, Tmax, and Tmin than other taxonomic groups, but this difference is significant only for the taxa from temperate regions, and in the tropics, different taxa have more similar Topt and Tmax. Interestingly, Tmin of marine diatoms is lower than Tmin of marine cyanobacteria even in the tropics, suggesting that tropical diatoms may have wider thermal niches compared to cyanobacteria (Thomas et al. 2016). It may thus be that diatoms are greater thermal generalists than other taxonomic groups. Diatoms tend to have higher maximum temperature-dependent growth rates, compared to dinoflagellates and cyanobacteria (Kremer et al. 2017). Interestingly, marine and freshwater diatoms differ in their temperature traits, and freshwater diatom taxa have on average higher temperature traits than the marine counterparts from similar latitudes, which in part could be explained by greater temperature variation in lakes compared to the ocean (Thomas et al. 2016).
We are learning more about the underlying mechanisms of thermal tolerance in diatoms and other phytoplankton. The different scaling of photosynthesis and respiration with temperature may explain some of the thermal tolerance differences (Padfield et al. 2016; Feijão et al. 2018). Taxon-specific patterns of up- or downregulation of genes involved in lipid metabolism, heat shock protein synthesis, and photosynthetic pathways likely contribute to thermal tolerances (Kinoshita et al. 2001; Rousch et al. 2004; Schaum et al. 2018). However, we still cannot explain the taxonomic differences in temperature traits at the molecular level, and this should be one of the research directions in thermal biology.
5.4 Trait Dependence on Environmental Factors
It is important to note that traits that determine the responses of diatoms to a given environmental factor may also depend on other factors. Broadly, this dependence can be viewed as phenotypic plasticity, and it is important for both ecological and evolutionary responses of organisms to environment (Stearns 1989; Hendry 2015; Hattich et al. 2017). Determining these dependencies requires experimental measurements of traits of interest while varying other factors one at a time or in combination, which is a daunting task. Meta-analyses of existing data can also help assess the interdependencies of traits. Edwards et al. (2016) showed that light-related traits depend on temperature, and vice versa, temperature traits depend on irradiance. For example, the optimum temperature for growth Topt is a unimodal function of irradiance, with the highest Topt at about 100 μmol quanta m−2 s−1 and declining at low and high irradiances. In turn, the irradiance-related traits also exhibit a unimodal distribution with respect to temperature, peaking at the optimum temperature (Edwards et al. 2016).
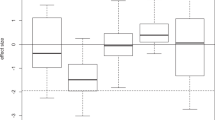
An experimental study with the model diatom Thalassiosira pseudonana showed that the optimum temperature for growth, Topt, is also a function of nutrient concentration, declining with increasing nutrient limitation (Thomas et al. 2017) (Fig. 2). This dependence may have important consequences for predicting the responses of diatoms and other phytoplankton to global environmental change. The simultaneous warming and a decreasing nutrient supply due to stronger stratification (Doney 2006) may be more detrimental than either of these factors alone (Thomas et al. 2017).
The optimum temperature for growth Topt of Thalassiosira pseudonana depends on nutrient concentrations. a) Topt vs. phosphate concentration in the medium, b) Topt vs. nitrate concentration in the medium. From Thomas et al. (2017)
Understanding how different traits depend on various environmental factors is essential for a more environment-tailored use of trait-based approaches and should be also helpful for the development of more realistic trait-based models.
6 Traits Important in Ecological Interactions
Diatoms engage in a multitude of ecological interactions, such as competition for resources, predator–prey, host–parasite, and mutualistic interactions. All these interactions involve different traits that can be used to characterize those interactions. Those traits may be correlated with each other and represent trade-offs that can define different ecological strategies (Litchman et al. 2012). Here, I briefly discuss these interactions and what traits may be particularly important in defining various biotic interactions, both within and across trophic levels.
6.1 Competition for Resources
As most microalgae, diatoms require several essential nutrients such as nitrogen, phosphorus, and iron, several other micronutrients, as well as light and carbon for growth. In addition, diatoms require silica for their cell walls. Each of these resources may at times become limiting, due to either low supply or consumption by other phytoplankton or nonphytoplankton organisms (e.g., heterotrophic bacteria can consume inorganic nutrients and, thus, compete for them with phytoplankton, including diatoms). Many traits determine competitive abilities for resources, such as the maximum growth rate, maximum rate of resource uptake, and the affinity of resource that characterizes species ability to grow at low resource concentrations (Edwards et al. 2011). There are several measures of competitive ability that are widely used to predict the outcomes of competition for resources. The most widely accepted composite trait that characterizes species competitive ability is the break-even nutrient concentration, termed R* (Tilman 1982). For the Monod equation for growth (Eq. 3 above), the R* is a function of maximum growth rate, mortality, and the half-saturation constant for growth:
For the Droop model (Eq. 4), the R* is a more complex function with more parameters (Litchman and Klausmeier 2008). The R* is thus a composite trait, so that it depends on several physiological parameters, including mortality. In different species, the same competitive ability may be achieved by modifying different parameters, such as a decrease in the half-saturation constant for growth Ks or/and mortality, or an increase in maximum growth rate μmax.
For the low nutrient concentrations, the scaled nutrient uptake affinity C is also used as a measure of competitive ability (Edwards et al. 2011, 2012):
where Vmax is the maximum rate of nutrient uptake, K is the half-saturation for nutrient uptake, and Qmin is the minimum cellular nutrient concentration allowing growth. High scaled affinity implies superior nutrient competitive ability. Again, nutrient competitive ability may be improved through the reduction of K or Qmin, or an increase in Vmax. When scaled nutrient affinity is compared across taxa, marine diatoms appear to be intermediate nutrient competitors, while small prymnesiophytes and haptophytes appear to be good nutrient competitors (Edwards et al. 2012).
While the R* and C are measures of nutrient competitive abilities at equilibrium, under fluctuating nutrient supplies, these composite traits may not characterize the best nutrient competitors. Fluctuations may favor species that are capable of fast nutrient uptake and/or high storage capacity (Sommer 1985; Grover 1991b; Litchman et al. 2009). Some fluctuation regimes may allow coexistence of contrasting strategies, such as small, fast growing cells and large, slower growing cells with high nutrient storage capacity (Sommer 1985; Litchman et al. 2009; Edwards et al. 2013).
6.2 Consumption by Grazers
Diatoms are consumed by zooplankton and, as other phytoplankton, have evolved a number of adaptations to minimize consumption. Silica frustule can potentially be a deterrent for some grazers, and it takes significant force to break it (Smetacek 2001; Hamm et al. 2003; Pančić et al. 2019). In the presence of grazers, more thick-shelled diatoms may persist, while thin-shelled diatoms could be grazed preferentially (Assmy et al. 2013). Silica in the cell wall may also have helped diatoms to evolve diverse cell shapes and sizes that may inhibit consumption by some grazers (Ryabov et al. 2021). Diatoms exhibit an astounding diversity of shapes, greater than any other group of phytoplankton. Shapes include extremely elongated needle-like forms that may be inedible to many zooplankton grazers (Ryabov et al. 2021). In general, cell size is a good predictor of susceptibility of diatoms to grazing. The window of diatom sizes susceptible to grazing depends on the grazer, with larger grazers consuming larger diatoms. As many diatoms form chains of cells, thus modifying their overall dimensions, chain length in is an adaptive trait to decrease susceptibility to grazing. Several studies showed that some chain-forming diatoms decrease chain length under grazing pressure by copepods but not microzooplankton (O’Connors et al. 1976; Bergkvist et al. 2012; Amato et al. 2018). Interestingly, copepod clearance rates were higher when fed long chain diatoms (Bergkvist et al. 2012).
Toxicity is an important trait that often mediates palatability of phytoplankton to grazers. Among diatoms, several taxa can produce toxins that may deter consumption by zooplankton (Ianora and Miralto 2010). Presence of copepod grazers was shown to induce toxin production in toxic diatom Pseudonitzschia sp. (Lundholm et al. 2018). The toxin concentration was positively correlated with a type of lipids produced by herbivorous but not carnivorous copepods (Lundholm et al. 2018). Cellular stoichiometry of macronutrients (C:N or C:P) is another trait that may affect diatom palatability to grazers and grazers’ growth (Sterner and Elser 2002; Polimene et al. 2015). Lower palatability due to changes in elemental stoichiometry may decrease carbon and nutrient transfer from diatoms to higher trophic levels and, thus, affect carbon sequestration (Polimene et al. 2015).
Understanding how diatom–grazer interaction depends on the above-mentioned traits and, in turn, shapes them is an active area of research that should help us understand biotic interactions in aquatic environments.
6.3 Mortality from Parasites
Diatoms are known to get infected by several types of parasites, from viruses, to fungi and parasitic protists (Tomaru and Nagasaki 2011; Scholz et al. 2016). The traits that determine the interactions of diatoms with parasites are not as well-known as the traits that describe the predator–prey interactions (mostly grazing by zooplankton). Viruses of phytoplankton, including diatoms, can be more specialized, compared to grazers (Nagasaki 2008), so the traits that may determine the susceptibility to viral attacks are more difficult to identify. Viruses that infect diatoms have been documented recently, with twenty or so viruses described; they include lytic single-strand RNA or the single-strand DNA viruses with high specificity (Arsenieff et al. 2019). Diatom viruses may be among the smallest viruses (Kranzler et al. 2019), possibly because the pores of silica frustules can be as small as 40 nm, and viruses need to enter the cell through those pores (Losic et al. 2006). An interesting question is whether the structure of the frustule could in part be shaped by the selective pressure from viruses and whether there could be a coevolutionary arms race between diatoms and their viruses. Viral mortality in diatoms may be exacerbated under nutrient limitation: A recent study showed that Si limitation was associated with higher viral abundance and diatom mortality (Kranzler et al. 2019). Chapter “Diatom Viruses” of this book describes the role of viruses in diatom biology in more detail.
Fungal (chytrid) infections in freshwater diatoms are well documented and may contribute to the termination of the spring diatom blooms (Reynolds 1984). These infections may increase in prevalence with warming, thus exerting more control on the bloom’s end (Frenken et al. 2016). Interestingly, chytrid infections may increase diatom genetic diversity through negative frequency-dependent selection (Gsell et al. 2013). A recent study (Chambouvet et al. 2019) found that fungal infection may be common in marine diatoms as well, where fungal symbionts are located inside diatom cells. What traits describe the likelihood and severity of such parasitic interaction is currently unknown. It may even be possible that the interaction is not completely parasitic.
Understanding how diatoms interact with viruses and other pathogens is a new frontier in diatom ecology, as well as in phytoplankton ecology in general. Compared to the interactions of diatoms with zooplankton, the diatom–parasite, especially, virus, interactions appear less generalizable, as the ability of viruses to infect diatoms and other phytoplankton may be species- or even strain-specific. In contrast, zooplankton’s ability to consume diatoms mostly depends on size matching and can, thus, be predicted better (Litchman et al. 2021).
6.4 Mutualistic Interactions
Diatoms engage in mutualistic interactions with many bacteria, including cyanobacteria, and these interactions include both extracellular and intracellular cross-feeding (exchange of metabolites). Recent research has revealed close mutualistic interactions between diatom cells and bacteria that live attached to diatom frustules or in the immediate vicinity of it (Seymour et al. 2017). The bacteria may provide a wide range of compounds to phytoplankton, from vitamins and growth factors, to micronutrients and lipids (Amin et al. 2015; Cooper et al. 2019; Kim et al. 2019). In turn, phytoplankton provide bacteria with organic carbon (Fu et al. 2020). The close associations of species are termed “symbiotic” and may not necessarily be mutualistic, where both (or all) partners receive benefit. Symbiotic associations may also, although probably less often, be competitive or parasitic (Baker et al. 2018; Drew et al. 2021). What kind of symbiotic relationship it is depends on many factors, including environmental drivers, such as resources (e.g., nutrients). In the associations of diatoms with attached bacteria, there may be competition for nutrients, and the overall relationship could depend on the degree of nutrient limitation. It is possible that changing environmental conditions may alter the nature of symbiotic relationships, as was found for other symbioses (Johnson et al. 1997; Grman et al. 2012). A more in-depth treatment of the diatom–bacteria interactions is presented in Chapter “Diatom Viruses” of this book.
A fascinating symbiotic relationship has been observed between some oceanic diatoms and nitrogen-fixing cyanobacteria living inside diatom cells, a so called diatom diazotroph association, DDA (Villareal 1991; Caputo et al. 2019). There are some DDAs in freshwaters, but their ecological and biogeochemical importance is unknown. Time-calibrated phylogenies suggest that these associations arose ca. 100–50 MYA, in late Cretaceous, when the oceans were highly stratified, warm, and low in nutrients (Caputo et al. 2019). Diatoms that harbor cyanobacterial diazotrophs (N-fixers) occur in different oceanic regions, but mostly in low latitudes, with blooms occurring in the North Pacific Gyre and close to large river deltas, such as the Amazon (Villareal et al. 2012). Several genera of diatoms, such as Hemiaulus, Rhizosolenia, Chaetoceros, and Climacodium, are known to have cyanobacterial symbionts (heterocystous Richelia intracellularis and Calothrix rhizosoleniae) (Caputo et al. 2019). In this intracellular symbiosis, diatom and cyanobacterium exchange metabolites, with the diatom providing carbon and cyanobacterium providing fixed nitrogen (Follett et al. 2018). The association of diazotrophs with diatoms may also help diazotrophs acquire phosphorus due to efficient sinking to deeper, P-rich water (Follett et al. 2018). The efficient acquisition of P through sinking alleviates P limitation and, thus, may stimulate nitrogen fixation.
The DDAs differ in how integrated the symbioses are: The oldest symbioses have partners that coevolved and adapted to relying on each other for key functions, such as uptake of nutrients and carbon fixation. Consequently, intracellular cyanobacterial symbionts have lost some genes that encode for nitrogen transporters and reductases, while more recent symbioses have diazotrophs outside diatom cells and those cyanobacteria have larger genomes (Caputo et al. 2018, 2019). Less is known about the changes in diatom genomes as a consequence of symbiotic association. Some of the traits found in diatoms with symbiotic diazotrophs are strong silicification and coloniality (Caputo et al. 2019). Future studies of diatom genomes should shed light on the role of symbioses in shaping genomes.
7 Traits Determining Diatom Responses to Global Environmental Change
Global environmental change has many aspects that are relevant to diatoms and other microalgae. The ongoing increase in CO2 concentration leads to increasing temperatures and ocean acidification (Pörtner 2008; Doney et al. 2012; Kroeker et al. 2013). Warming of aquatic ecosystems changes mixing and stratification patterns that affect nutrient supply and light regimes (Dutkiewicz et al. 2013; Voosen 2019; Li et al. 2020). Diatoms have many relevant traits that determine their responses to various aspects of global environmental change. Growth rate of diatoms depends on CO2 concentration. Higher CO2 concentrations were shown to enhance growth of diatoms under nutrient-replete conditions in a natural community (Bach et al. 2019). However, a long-term exposure of T. pseudonana to elevated CO2 did not show a significant change in physiological parameters or adaptation (Crawfurd et al. 2011). Interactions of global change stressors may be important for determining the growth rates of diatoms. Under supraoptimal temperatures (above Topt), high pCO2 decreased growth of T. pseudonana but not under optimal temperatures (Laws et al. 2020). Stoichiometric traits may also change in response to global change stressors, thus affecting biogeochemical cycling. Higher pCO2 increased C:N and N:P ratios in T. pseudonana, but this increase was not universal across taxa (King et al. 2015). Interestingly, high pCO2 was shown to increase diatom (T. pseudonana) resilience to other stressors, such as UV and fluctuating nitrogen limitation (Valenzuela 2018). In another study, high pCO2 under nitrogen limitation decreased growth rate, cell size, pigment content, photochemical quantum yield of PSII, and photosynthetic carbon fixation in T. pseudonana (Li et al. 2018).
Temperature-related traits help us determine how different taxa, including diatoms, would respond to global warming. As discussed above, diatoms tend to have lower Topt than cyanobacteria (Thomas et al. 2016), and this trait difference may potentially lead to diatoms decreasing their abundance in the warming ocean, especially relative to cyanobacteria. Higher temperature exacerbated the negative effects of high pCO2 and nitrogen limitation in T. pseudonana (Li et al. 2018), highlighting the possibility for different stressors to interact and enhance their negative effects (Thomas et al. 2017).
Because of their silica frustules, diatoms may have higher sinking rates on average compared to other taxa; consequently, stronger stratification in the future ocean may lead to decreased diatom abundances (Raven and Waite 2004; Agusti et al. 2015; Spilling et al. 2018). Ocean acidification may lead to less silicified diatom frustules, thus potentially affecting their sinking rates, decomposition, and carbon sequestration (Petrou et al. 2019). This may also make diatoms more susceptible to grazers, thus potentially altering the dynamics of food webs.
8 Trait Evolution
Organisms’ traits are not static but undergo evolutionary change. The time scales of evolutionary changes range from a single generation (a day or so) to geological time (millions of years) (Thompson 1998; Hendry 2020). The rates of evolutionary change were shown to depend on the time scales over which the rates are calculated. The rates are generally faster over shorter time scales than over longer (geological) time scales, in part because of the periods of stasis and reversal over the course of evolution (Hendry and Kinnison 1999; Stockwell et al. 2003).
Many diatom traits experience numerous selective pressures and, as a result, evolve over different time scales. Selective forces include abiotic environmental factors, such as nutrient concentrations, temperature, as well as biotic interactions, such as competition, predation, parasitism, and mutualism. Both observational and experimental studies have demonstrated trait evolution in diatoms. The evolution of diatom traits over longer time scales and across environments has been documented (Sims et al. 2006; Connolly et al. 2008; Nakov et al. 2014). Evolutionary change may also occur relatively fast, for example, recent experimental evolution studies showed that diatoms can adapt to higher temperatures in less than a year (ca. 300 generations). The optimum temperature for growth, Topt, and, to a lesser degree, the maximum temperature allowing growth, Tmax, shifted to higher values (O’Donnell et al. 2018) (Fig. 3). High-temperature adaptation also led to changes in other traits, such as fatty acid composition, cell size, and shape and elemental stoichiometry (O’Donnell et al. 2019; 2021). Interestingly, different temperature regimes can result in different evolutionary trajectories and rates of adaptation. Schaum et al. (2018) showed that adaptation to high temperature was faster under fluctuating temperature or less extreme high temperatures (26 vs. 32 °C). The high-temperature-adapted diatom lines differed from the ancestral lines in elemental composition and metabolic traits. The genes involved in adaptation were related to transcriptional regulation, cellular responses to oxidative stress, and redox homeostasis (Schaum et al. 2018).
How traits evolve depends on environmental conditions, so the differences not only in the drivers of selection (e.g., constant vs. fluctuating temperature) but in other factors as well may alter trait evolution. Aranguren-Gassis et al. (2019) found that adaptation to high temperature was impeded by nitrogen limitation. A possible mechanism is that high-temperature-adapted lines require more nitrogen and get competitively excluded under low N conditions, thus precluding adaptation.
Documenting rapid evolution of other ecologically relevant traits, such as nutrient acquisition traits, would allow us to better predict the effects of diatoms on biogeochemical cycles and competitive interactions of diatoms with other phytoplankton.
9 Conclusions and Future Directions
Diatoms are a globally important group of phytoplankton, abundant in both marine and freshwater environments. Looking at morphological, physiological, and ecological traits, and their distribution and evolution, can help us understand what controls the abundance and diversity of diatoms in different environments and how it may change in the future. When comparing eco-physiological and morphological traits of diatoms to other phytoplankton groups, we can identify both differences and similarities. Many traits that define how diatoms interact with the environment, and other taxa, within or across trophic levels, differ from other groups. These trait differences may help explain the ecological success of diatoms over different time scales, from geological to fast ecological scales. A major challenge in trait-based research is to learn about the mechanistic underpinnings of various traits that can be used in biogeochemical models to predict phytoplankton dynamics and composition. A possible research direction to better understand the molecular basis of phenotypic traits could be a hypothesis-driven investigation of molecular differences in species with different values of relevant traits. Such comparisons could help identify the genomic, transcriptomic, and proteomic characteristics responsible for the observed trait differences.
Another future direction is a better characterization of trait variation both across and within species. While for some traits there are measurements for multiple species (Edwards et al. 2015a), some traits are mostly measured in just a handful of diatoms frequently grown in the lab, especially on the model diatom T. pseudonana. Our knowledge of traits for many diatom taxa, especially open ocean oligotrophic species, is very limited. Moreover, we have even less information about the amount of trait variation within species for many taxa. For some traits, we are beginning to get more data on intraspecific variation, such as temperature-related traits, nutrient utilization traits, or cell size. Nevertheless, the intraspecific trait variation is still assessed just for several well-studied species. Characterizing the amount of intraspecific diversity for many relevant diatom traits across diverse taxa is needed to assess the evolutionary potential of different species, as selection would likely happen on standing genotypic/phenotypic variation.
As diatom traits may change in the future in response to changing environmental conditions, understanding trait evolution in diatoms and other phytoplankton is an important frontier in trait-based diatom ecology. Experimental evolution studies should tackle measuring phenotypic trait change together with genomic, transcriptomic, and metabolomic changes to establish the link across different levels of biological organization.
References
Agusti S, González-Gordillo JI, Vaqué D, Estrada M, Cerezo MI, Salazar G, Gasol JM, Duarte CM (2015) Ubiquitous healthy diatoms in the deep sea confirm deep carbon injection by the biological pump. Nat Commun 6:7608
Amato A, Dell’Aquila G, Musacchia F, Annunziata R, Ugarte A, Maillet N, Carbone A, Ribera d’Alcalà M, Sanges R, Iudicone D, Ferrante MI (2017) Marine diatoms change their gene expression profile when exposed to microscale turbulence under nutrient replete conditions. Sci Rep 7:3826
Amato A, Sabatino V, Nylund GM, Bergkvist J, Basu S, Andersson MX, Sanges R, Godhe A, Kiørboe T, Selander E, Ferrante MI (2018) Grazer-induced transcriptomic and metabolomic response of the chain-forming diatom Skeletonema marinoi. ISME J 12:1594–1604
Amin SA, Hmelo LR, van Tol HM, Durham BP, Carlson LT, Heal KR, Morales RL, Berthiaume CT, Parker MS, Djunaedi B, Ingalls AE, Parsek MR, Moran MA, Armbrust EV (2015) Interaction and signalling between a cosmopolitan phytoplankton and associated bacteria. Nature 522:98
Aranguren-Gassis M, Kremer CT, Klausmeier CA, Litchman E (2019) Nitrogen limitation inhibits marine diatom adaptation to high temperatures. Ecol Lett 22:1860–1869
Arsenieff L, Simon N, Rigaut-Jalabert F, Le Gall F, Chaffron S, Corre E, Com E, Bigeard E, Baudoux A-C (2019) First viruses infecting the marine diatom Guinardia delicatula. Front Microbiol 9
Assmy P, Smetacek V, Montresor M, Klaas C, Henjes J, Strass VH, Arrieta JM, Bathmann U, Berg GM, Breitbarth E, Cisewski B, Friedrichs L, Fuchs N, Herndl GJ, Jansen S, Krägefsky S, Latasa M, Peeken I, Röttgers R, Scharek R, Schüller SE, Steigenberger S, Webb A, Wolf-Gladrow D (2013) Thick-shelled, grazer-protected diatoms decouple ocean carbon and silicon cycles in the iron-limited Antarctic circumpolar current. Proc Natl Acad Sci 110:20633–20638
Bach LT, Hernández-Hernández N, Taucher J, Spisla C, Sforna C, Riebesell U, Arístegui J (2019) Effects of elevated CO2 on a natural diatom community in the subtropical NE Atlantic. Front Mar Sci 6
Baker DM, Freeman CJ, Wong JCY, Fogel ML, Knowlton N (2018) Climate change promotes parasitism in a coral symbiosis. ISME J 12:921–930
Basu S, Mackey KRM (2018) Phytoplankton as key mediators of the biological carbon pump: their responses to a changing climate. Sustainability 10
Bergkvist J, Klawonn I, Whitehouse MJ, Lavik G, Brüchert V, Ploug H (2018) Turbulence simultaneously stimulates small- and large-scale CO2 sequestration by chain-forming diatoms in the sea. Nat Commun 9:3046
Bergkvist J, Thor P, Jakobsen HH, Wängberg S-Å, Selander E (2012) Grazer-induced chain length plasticity reduces grazing risk in a marine diatom. Limnol Oceanogr 57:318–324
Caputo A, Nylander JAA, Foster RA (2019) The genetic diversity and evolution of diatom-diazotroph associations highlights traits favoring symbiont integration. FEMS Microbiol Lett 366
Caputo A, Stenegren M, Pernice MC, Foster RA (2018) A short comparison of two marine planktonic diazotrophic symbioses highlights an un-quantified disparity. Front Mar Sci 5
Chambouvet A, Monier A, Maguire F, Itoïz S, del Campo J, Elies P, Edvardsen B, Eikreim W, Richards TA (2019) Intracellular infection of diverse diatoms by an evolutionary distinct relative of the fungi. Curr Biol 29:4093–4101.e4094
Connolly JA, Oliver MJ, Beaulieu JM, Knight CA, Tomanek L, Moline MA (2008) Correlated evolution of genome size and cell volume in diatoms (Bacillariophyceae). J Phycol 44:124–131
Cooper MB, Kazamia E, Helliwell KE, Kudahl UJ, Sayer A, Wheeler GL, Smith AG (2019) Cross-exchange of B-vitamins underpins a mutualistic interaction between Ostreococcus tauri and Dinoroseobacter shibae. ISME J 13:334–345
Crawfurd KJ, Raven JA, Wheeler GL, Baxter EJ, Joint I (2011) The response of Thalassiosira pseudonana to long-term exposure to increased CO2 and decreased pH. PLoS One 6:e26695
Doney SC (2006) Oceanography - plankton in a warmer world. Nature 444:695–696
Doney SC, Ruckelshaus M, Emmett Duffy J, Barry JP, Chan F, English CA, Galindo HM, Grebmeier JM, Hollowed AB, Knowlton N, Polovina J, Rabalais NN, Sydeman WJ, Talley LD (2012) Climate change impacts on marine ecosystems. Annu Rev Mar Sci 4:11–37
Drew GC, Stevens EJ, King KC (2021) Microbial evolution and transitions along the parasite–mutualist continuum. Nat Rev Microbiol 19:623–638
Droop MR (1973) Some thoughts on nutrient limitation in algae. J Phycol 9:264–272
Du Clos KT, Karp-Boss L, Gemmell BJ (2021) Diatoms rapidly alter sinking behavior in response to changing nutrient concentrations. Limnol Oceanogr 66:892–900
Dutkiewicz S, Scott JR, Follows MJ (2013) Winners and losers: ecological and biogeochemical changes in a warming ocean. Glob Biogeochem Cycles 27:463–477
Dyhrman ST, Jenkins BD, Rynearson TA, Saito MA, Mercier ML, Alexander H, Whitney LP, Drzewianowski A, Bulygin VV, Bertrand EM, Wu Z, Benitez-Nelson C, Heithoff A (2012) The transcriptome and proteome of the diatom Thalassiosira pseudonana reveal a diverse phosphorus stress response. PLoS One 7:e33768
Edwards KF, Klausmeier CA, Litchman E (2011) Evidence for a three-way tradeoff between nitrogen and phosphorus competitive abilities and cell size in phytoplankton. Ecology 92:2085–2095
Edwards KF, Klausmeier CA, Litchman E (2013) A three-way trade-off maintains functional diversity under variable resource supply. Am Nat 182:786–800
Edwards KF, Klausmeier CA, Litchman E (2015a) Nutrient utilization traits in phytoplankton. Ecology 96:2311
Edwards KF, Thomas MK, Klausmeier CA, Litchman E (2012) Allometric scaling and taxonomic variation in nutrient utilization traits and growth rates of marine and freshwater phytoplankton. Limnol Oceanogr 57:554–566
Edwards KF, Thomas MK, Klausmeier CA, Litchman E (2015b) Light and phytoplankton growth: allometry, taxonomic variation, and biogeography. Limnol Oceanogr 60:540–552
Edwards KF, Thomas MK, Klausmeier CA, Litchman E (2016) Phytoplankton growth and the interaction of light and temperature: A synthesis at the species and community level. Limnol Oceanogr 61:1232–1244
Eilers PHC, Peeters JCH (1988) A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecol Model 42:199–215
Feijão E, Gameiro C, Franzitta M, Duarte B, Caçador I, Cabrita MT, Matos AR (2018) Heat wave impacts on the model diatom Phaeodactylum tricornutum: searching for photochemical and fatty acid biomarkers of thermal stress. Ecol Indic 95:1026–1037
Finkel ZV, Katz ME, Wright JD, Schofield OM, Falkowski PG (2005) Climatically driven macroevolutionary patterns in the size of marine diatoms over the Cenozoic. Proc Natl Acad Sci U S A 102:8927–8932
Follett CL, Dutkiewicz S, Karl DM, Inomura K, Follows MJ (2018) Seasonal resource conditions favor a summertime increase in North Pacific diatom–diazotroph associations. ISME J 12:1543–1557
Follows MJ, Dutkiewicz S, Grant S, Chisholm SW (2007) Emergent biogeography of microbial communities in a model ocean. Science 315:1843–1846
Frenken T, Velthuis M, de Senerpont Domis LN, Stephan S, Aben R, Kosten S, van Donk E, Van de Waal DB (2016) Warming accelerates termination of a phytoplankton spring bloom by fungal parasites. Glob Chang Biol 22:299–309
Fu H, Uchimiya M, Gore J, Moran MA (2020) Ecological drivers of bacterial community assembly in synthetic phycospheres. Proc Natl Acad Sci U S A 117:3656–3662
Garnier E, Navas M-L, Grigulis K (2016) Plant functional diversity: organism traits, community structure, and ecosystem properties. Oxford University Press, Oxford, UK
Gemmell BJ, Oh G, Buskey EJ, Villareal TA (2016) Dynamic sinking behaviour in marine phytoplankton: rapid changes in buoyancy may aid in nutrient uptake. Proc R Soc B Biol Sci 283:20161126
Green JL, Bohannan BJM, Whitaker RJ (2008) Microbial biogeography: from taxonomy to traits. Science 320:1039–1043
Grman E, Robinson TMP, Klausmeier CA (2012) Ecological specialization and trade affect the outcome of negotiations in mutualism. Am Nat 179:567–581
Grover JP (1991a) Non-steady state dynamics of algal population growth: experiments with two chlorophytes. J Phycol 27:70–79
Grover JP (1991b) Resource competition in a variable environment: phytoplankton growing according to the variable-internal-stores model. Am Nat 138:811–835
Grover JP (1995) Competition, herbivory, and enrichment - nutrient-based models for edible and inedible plants. Am Nat 145:746–774
Grover JP (1997) Resource competition. Chapman and Hall, London
Gsell AS, de Senerpont Domis LN, Verhoeven KJF, van Donk E, Ibelings BW (2013) Chytrid epidemics may increase genetic diversity of a diatom spring-bloom. ISME J 7:2057–2059
Hamm CE, Merkel R, Springer O, Jurkojc P, Maier C, Prechtel K, Smetacek V (2003) Architecture and material properties of diatom shells provide effective mechanical protection. Nature 421:841–843
Hattich GSI, Listmann L, Raab J, Ozod-Seradj D, Reusch TBH, Matthiessen B (2017) Inter- and intraspecific phenotypic plasticity of three phytoplankton species in response to ocean acidification. Biol Lett 13:20160774
Hendry AP (2015) Key questions on the role of phenotypic plasticity in eco-evolutionary dynamics. J Hered 107:25–41
Hendry AP (2020) Eco-evolutionary dynamics. Princeton university Press
Hendry AP, Kinnison MT (1999) Perspective: the pace of modern life: measuring rates of contemporary microevolution. Evolution 53:1637–1653
Ianora A, Miralto A (2010) Toxigenic effects of diatoms on grazers, phytoplankton and other microbes: a review. Ecotoxicology 19:493–511
Jian J, Zeng D, Wei W, Lin H, Li P, Liu W (2017) The combination of RNA and protein profiling reveals the response to nitrogen depletion in Thalassiosira pseudonana. Sci Rep 7:8989
Johnson NC, Graham JH, Smith FA (1997) Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol 135:575–586
Kim M, Shin B, Lee J, Park HY, Park W (2019) Culture-independent and culture-dependent analyses of the bacterial community in the phycosphere of cyanobloom-forming Microcystis aeruginosa. Sci Rep 9:20416
King AL, Jenkins BD, Wallace JR, Liu Y, Wikfors GH, Milke LM, Meseck SL (2015) Effects of CO2 on growth rate, C:N:P, and fatty acid composition of seven marine phytoplankton species. Mar Ecol Prog Ser 537:59–69
Kinoshita S, Kaneko G, Lee JH, Kikuchi K, Yamada H, Hara T, Itoh Y, Watabe S (2001) A novel heat stress-responsive gene in the marine diatom Chaetoceros compressum encoding two types of transcripts, a trypsin-like protease and its related protein, by alternative RNA splicing. Eur J Biochem 268:4599–4609
Klausmeier CA, Kremer CT, Koffel T (2020) Trait-based ecological and eco-evolutionary theory. In: McCann K, Gellner G (eds) Theoretical ecology: concepts and applications. Oxford University Press, Oxford, pp 161–194
Klausmeier CA, Litchman E (2001) Algal games: the vertical distribution of phytoplankton in poorly mixed water columns. Limnol Oceanogr 46:1998–2007
Kooistra WHCF, Gersonde R, Medlin LK, Mann DG (2007) CHAPTER 11 - the origin and evolution of the diatoms: their adaptation to a planktonic existence. In: Falkowski PG, Knoll AH (eds) Evolution of primary producers in the sea. Academic Press, Burlington, pp 207–249
Kranzler CF, Krause JW, Brzezinski MA, Edwards BR, Biggs WP, Maniscalco M, McCrow JP, Van Mooy BAS, Bidle KD, Allen AE, Thamatrakoln K (2019) Silicon limitation facilitates virus infection and mortality of marine diatoms. Nat Microbiol 4:1790–1797
Kremer CT, Thomas MK, Litchman E (2017) Temperature- and size-scaling of phytoplankton population growth rates: reconciling the Eppley curve and the metabolic theory of ecology. Limnol Oceanogr 62:1658–1670
Kroeker KJ, Kordas RL, Crim R, Hendriks IE, Ramajo L, Singh GS, Duarte CM, Gattuso J-P (2013) Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob Chang Biol 19:1884–1896
Lamanna C, Blonder B, Violle C, Kraft NJB, Sandel B, Simova I, Donoghue JC, Svenning JC, McGill BJ, Boyle B, Buzzard V, Dolins S, Jorgensen PM, Marcuse-Kubitza A, Morueta-Holme N, Peet RK, Piel WH, Regetz J, Schildhauer M, Spencer N, Thiers B, Wiser SK, Enquist BJ (2014) Functional trait space and the latitudinal diversity gradient. Proc Natl Acad Sci U S A 111:13745–13750
Lampe RH, Mann EL, Cohen NR, Till CP, Thamatrakoln K, Brzezinski MA, Bruland KW, Twining BS, Marchetti A (2018) Different iron storage strategies among bloom-forming diatoms. Proc Natl Acad Sci 115:E12275
Lavorel S, Garnier E (2002) Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the holy grail. Funct Ecol 16:545–556
Laws EA, McClellan SA, Passow U (2020) Interactive effects of CO2, temperature, irradiance, and nutrient limitation on the growth and physiology of the marine diatom Thalassiosira pseudonana (Coscinodiscophyceae). J Phycol 56:1614–1624
Leibold MA (1996) A graphical model of keystone predators in food webs: trophic regulation of abundance, incidence, and diversity patterns in communities. Am Nat 147:784–812
Li F, Beardall J, Gao K (2018) Diatom performance in a future ocean: interactions between nitrogen limitation, temperature, and CO2-induced seawater acidification. ICES J Mar Sci 75:1451–1464
Li G, Cheng L, Zhu J, Trenberth KE, Mann ME, Abraham JP (2020) Increasing ocean stratification over the past half-century. Nat Clim Chang 10:1116–1123
Lindenschmidt K-E, Chorus I (1998) The effect of water column mixing on phytoplankton succession, diversity and similarity. J Plankton Res 20:1927–1951
Litchman E, Edwards KF, Boyd PW (2021) Towards trait-based food webs: universal traits and trait matching in planktonic predator-prey and host-parasite relationships. Limnol Oceanogr 6:3857–3872
Litchman E, Edwards KF, Klausmeier CA, Thomas MK (2012) Phytoplankton niches, traits and eco-evolutionary responses to global environmental change. Marine Ecology Progres Series 470:235–248
Litchman E, Klausmeier CA (2008) Trait-based community ecology of phytoplankton. Annu Rev Ecol Evol Syst 39:615–639
Litchman E, Klausmeier CA, Schofield OM, Falkowski PG (2007) The role of functional traits and trade-offs in structuring phytoplankton communities: scaling from cellular to ecosystem level. Ecol Lett 10:1170–1181
Litchman E, Klausmeier CA, Yoshiyama K (2009) Contrasting size evolution in marine and freshwater diatoms. Proc Natl Acad Sci U S A 106:2665–2670
Litchman E, de Tezanos Pinto P, Edwards KF, Kremer CT, Klausmeier CA, Thomas MK (2015) Global biogeochemical impacts of phytoplankton: a trait-based perspective. J Ecol 103:1384–1396
Losic D, Rosengarten G, Mitchell JG, Voelcker NH (2006) Pore architecture of diatom frustules: potential nanostructured membranes for molecular and particle separations. J Nanosci Nanotechnol 6:982–989
Lundholm N, Krock B, John U, Skov J, Cheng J, Pančić M, Wohlrab S, Rigby K, Nielsen TG, Selander E, Harðardóttir S (2018) Induction of domoic acid production in diatoms-types of grazers and diatoms are important. Harmful Algae 79:64–73
Marchetti A, Parker MS, Moccia LP, Lin EO, Arrieta AL, Ribalet F, Murphy MEP, Maldonado MT, Armbrust EV (2009) Ferritin is used for iron storage in bloom-forming marine pennate diatoms. Nature 457:467–470
Martiny JBH, Jones SE, Lennon JT, Martiny AC (2015) Microbiomes in light of traits: A phylogenetic perspective. Science 350
McGill BJ, Enquist BJ, Weiher E, Westoby M (2006) Rebuilding community ecology from functional traits. Trends Ecol Evol 21:178–185
Medlin LK (2016) Evolution of the diatoms: major steps in their evolution and a review of the supporting molecular and morphological evidence. Phycologia 55:79–103
Nagasaki K (2008) Dinoflagellates, diatoms, and their viruses. J Microbiol 46:235–243
Nakov T, Theriot EC, Alverson AJ (2014) Using phylogeny to model cell size evolution in marine and freshwater diatoms. Limnol Oceanogr 59:79–86
Nelson DM, Treguer P, Brzezinski MA, Leynaert A, Queguiner B (1995) Production and dissolution of biogeonic silica in the ocean–revised global estimates, comparison with regional data and relationship to biogenic sedimentation. Glob Biogeochem Cycles 9:359–372
Norberg J (2004) Biodiversity and ecosystem functioning: A complex adaptive systems approach. Limnol Oceanogr 49:1269–1277
O'Connors HB, Small LF, Donaghay PL (1976) Particle-size modification by two size classes of the estuarine copepod Acartia clausi. Limnol Oceanogr 21:300–308
O'Donnell DR, Du ZY, Litchman E (2019) Experimental evolution of phytoplankton fatty acid thermal reaction norms. Evol Appl 12:1201–1211
O'Donnell DR, Hamman CR, Johnson EC, Kremer CT, Klausmeier CA, Litchman E (2018) Rapid thermal adaptation in a marine diatom reveals constraints and trade-offs. Glob Chang Biol 24:4554–4565
O’Donnell DR, Beery SM, Litchman E (2021) Temperature-dependent evolution of cell morphology and carbon and nutrient content in a marine diatom. Limnol Oceanogr 66:4334–4346
Padfield D, Yvon-Durocher G, Buckling A, Jennings S, Yvon-Durocher G (2016) Rapid evolution of metabolic traits explains thermal adaptation in phytoplankton. Ecol Lett 19:133–142
Pančić M, Torres RR, Almeda R, Kiørboe T (2019) Silicified cell walls as a defensive trait in diatoms. Proc R Soc B Biol Sci 286:20190184
Petrou K, Baker KG, Nielsen DA, Hancock AM, Schulz KG, Davidson AT (2019) Acidification diminishes diatom silica production in the Southern Ocean. Nat Clim Chang 9:781–786
Polimene L, Mitra A, Sailley SF, Ciavatta S, Widdicombe CE, Atkinson A, Allen JI (2015) Decrease in diatom palatability contributes to bloom formation in the Western English Channel. Prog Oceanogr 137:484–497
Pörtner H (2008) Ecosystem effects of ocean acidification in times of ocean warming: a physiologist’s view. Mar Ecol Prog Ser 373:203–217
Raven JA, Waite AM (2004) The evolution of silicification in diatoms: inescapable sinking and sinking as escape? New Phytol 162:45–61
Reynolds CS (1984) The ecology of freshwater phytoplankton. Cambridge University Press, Cambridge
Rousch JM, Bingham SE, Sommerfeld MR (2004) Protein expression during heat stress in thermo-intolerant and thermo-tolerant diatoms. J Exp Mar Biol Ecol 306:231–243
Ryabov A, Kerimoglu O, Litchman E, Olenina I, Roselli L, Basset A, Stanca E, Blasius B (2021) Shape matters: the relationship between cell geometry and diversity in phytoplankton. Ecol Lett 24:847–861
Schaum CE, Buckling A, Smirnoff N, Studholme DJ, Yvon-Durocher G (2018) Environmental fluctuations accelerate molecular evolution of thermal tolerance in a marine diatom. Nat Commun 9:1719
Scholz B, Guillou L, Marano AV, Neuhauser S, Sullivan BK, Karsten U, Küpper FC, Gleason FH (2016) Zoosporic parasites infecting marine diatoms – A black box that needs to be opened. Fungal Ecol 19:59–76
Schwaderer AS, Yoshiyama K, de Tezanos Pinto P, Swenson NG, Klausmeier CA, Litchman E (2011) Eco-evolutionary patterns in light utilization traits and distributions of freshwater phytoplankton. Limnol Oceanogr 56:589–598
Seymour JR, Amin SA, Raina J-B, Stocker R (2017) Zooming in on the phycosphere: the ecological interface for phytoplankton–bacteria relationships. Nat Microbiol 2:17065
Sicko-Goad L, Stoermer EF, Ladewski BG (1977) A morphometric method for correcting phytoplankton cell volume estimates. Protoplasma 93:147–163
Sims PA, Mann DG, Medlin LK (2006) Evolution of the diatoms: insights from fossil, biological and molecular data. Phycologia 45:361–402
Smetacek V (1999) Diatoms and the ocean carbon cycle. Protist 150:25–32
Smetacek V (2001) A watery arms race. Nature 411:745
Smetacek VS (1985) Role of sinking in diatom life-history cycles: ecological, evolutionary and geological significance. Mar Biol 84:239–251
Sommer U (1984) The paradox of the plankton: fluctuations of phosphorus availability maintain diversity of phytoplankton in flow-through cultures. Limnol Oceanogr 29:633–636
Sommer U (1985) Comparison between steady state and non-steady state competition: experiments with natural phytoplankton. Limnol Oceanogr 30:335–346
Sommer U, Stibor H, Katechakis A, Sommer F, Hansen T (2002) Pelagic food web configurations at different levels of nutrient richness and their implications for the ratio fish production: primary production. Hydrobiologia 484:11–20
Spilling K, Olli K, Lehtoranta J, Kremp A, Tedesco L, Tamelander T, Klais R, Peltonen H, Tamminen T (2018) Shifting diatom–dinoflagellate dominance during spring bloom in the Baltic Sea and its potential effects on biogeochemical cycling. Frontiers in Marine Science 5
Stearns SC (1989) The evolutionary significance of phenotypic plasticity. Bioscience 39:436–445
Sterner RW, Elser JJ (2002) Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton University Press, Princeton
Stockwell CA, Hendry AP, Kinnison MT (2003) Contemporary evolution meets conservation biology. Trends Ecol Evol 18:94–101
Syrett PJ (1981) Nitrogen metabolism of microalgae. In: Platt T (ed) Physiological bases of phytoplankton ecology, pp 182–210
Thomas MK, Aranguren-Gassis M, Kremer CT, Gould MR, Anderson K, Klausmeier CA, Litchman E (2017) Temperature–nutrient interactions exacerbate sensitivity to warming in phytoplankton. Glob Chang Biol 23:3269–3280
Thomas MK, Kremer CT, Klausmeier CA, Litchman E (2012) A global pattern of thermal adaptaiton in marine phytoplankton. Science 338:1085–1088
Thomas MK, Kremer CT, Litchman E (2016) Environment and evolutionary history determine the global biogeography of phytoplankton temperature traits. Glob Ecol Biogeogr 25:75–86
Thompson JN (1998) Rapid evolution as an ecological process. Trends Ecol Evol 13:329–332
Tilman D (1982) Resource competition and community structure. Princeton University Press, Princeton, NJ
Tomaru Y, Nagasaki K (2011) Diatom viruses. In: Seckbach J, Kociolek P (eds) The diatom world. Cellular origin, life in extreme habitats and astrobiology. Springer, Dordrecht
Tréguer P, Bowler C, Moriceau B, Dutkiewicz S, Gehlen M, Aumont O, Bittner L, Dugdale R, Finkel Z, Iudicone D, Jahn O, Guidi L, Lasbleiz M, Leblanc K, Levy M, Pondaven P (2018) Influence of diatom diversity on the ocean biological carbon pump. Nat Geosci 11:27–37
Valenzuela JJ, de Lomana ALG, Lee A, Armbrust EV, Orellana MV, Baliga NS (2018) Ocean acidification conditions increase resilience of marine diatoms. Nat Commun 9:2328
Villareal TA (1991) Nitrogen fixation by the cyanobacterial symbiont of the diatom genus Hemiaulus. Mar Ecol Prog Ser 76:201–204
Villareal TA, Brown CG, Brzezinski MA, Krause JW, Wilson C (2012) Summer diatom blooms in the North Pacific subtropical gyre: 2008-2009. PLoS One 7:e33109
Violle C, Navas M-L, Vile D, Kazakou E, Fortunel C, Hummel I, Garnier E (2007) Let the concept of trait be functional! Oikos 116:882–892
Voosen P (2019) Warming transforms the oceans and poles. Science 365:1359–1360
Westoby M, Nielsen DA, Gillings MR, Litchman E, Madin JS, Paulsen IT, Tetu SG (2021) Cell size, genome size, and maximum growth rate are near-independent dimensions of ecological variation across bacteria and archaea. Ecol Evol 11:3956–3976
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Litchman, E. (2022). Trait-Based Diatom Ecology. In: Falciatore, A., Mock, T. (eds) The Molecular Life of Diatoms. Springer, Cham. https://doi.org/10.1007/978-3-030-92499-7_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-92499-7_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-92498-0
Online ISBN: 978-3-030-92499-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)