Abstract
Carcinoid heart disease is a rare cardiovascular condition with high morbidity and mortality. A consequence of vasoactive peptides released by carcinoid tumors that result in plaque formation on the valve leaflets, carcinoid heart disease most often manifests with classic deformation of the tricuspid and pulmonic valves, along with symptoms of right heart failure. Treatment of carcinoid syndrome with somatostatin analogues and management of right heart failure are hallmarks of carcinoid heart disease management. Definitive treatment of carcinoid heart disease is surgical valve replacement which carries increased surgical risk but also offers better long-term survival. Percutaneous management of valve disease in carcinoid heart disease is an attractive option for patients who are too high risk for surgical repair and for those with bioprosthetic valve deterioration. In the following chapter, a comprehensive review of the current state of carcinoid heart disease is presented.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Carcinoid
- Tricuspid valve
- Pulmonic valve
- Cardiac surgery
- Tricuspid regurgitation
- Pulmonic valve regurgitation
- Valvular heart disease
- Neuroendocrine tumor
- Cancer
- Cardiac complications of cancer
Case Presentation
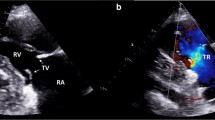
A 55-year-old male with no significant past medical history presents for evaluation of progressive shortness of breath and new onset leg swelling. He is a regular exerciser and has new windedness 20 min into a workout for the past several months. In the past few weeks, he has developed new bilateral lower extremity swelling, notes occasional palpitations, and has lightheadedness upon bending down to tie his shoes. He denies angina, orthopnea, paroxysmal nocturnal dyspnea, syncope. Review of systems notable for diarrhea. His vitals are within normal limits and his electrocardiogram is unremarkable. On exam, he is compensated, jugular venous pressure is estimated at 12 mm Hg with a prominent V-wave, he has a prominent right ventricular heave, a III/VI systolic ejection murmur at the left sternal border which increases with inspiration is heard, and he has 2+ bilateral lower extremity edema. You suspect carcinoid heart disease and N-terminal pro-B-type natriuretic peptide (NT-proBNP) of 510 ng/mL, urine 5-H1AA of 791 μmol/24 h and transthoracic echocardiogram (Fig. 8.1) confirm your diagnosis.
Transthoracic echocardiography of classic tricuspid and pulmonic carcinoid valve disease. Note the thickened and retracted leaflets with lack of coaptation causing severe tricuspid and pulmonic regurgitation. CW Doppler of tricuspid regurgitation and pulmonic regurgitation jets shows a dense, triangular waveform consistent with severe regurgitation (arrows)
Incidence and Epidemiology of Carcinoid Heart Disease
Heart disease and cancer are leading causes of morbidity and mortality in the United States. In 2019, the prevalence of cardiovascular disease in people ≥20 years old was estimated at 48.0% which in 2016 amounted to 121.5 million adults [1]. In 2018, it was estimated that 1.74 million new cases of cancer were diagnosed [2]. In contrast, neuroendocrine tumors like carcinoid are rare with an incidence of 2.5 to 5 cases per 100,000 people although incidence is increasing and a small subset of these cases will have carcinoid heart disease [3].
Neuroendocrine tumors are neoplasms that have characteristic neuroendocrine differentiation, can arise in various locations, and may cause symptoms related to vasoactive peptide release [3]. “Carcinoid” refers to neuroendocrine tumors arising from the gastrointestinal tract. Carcinoid tumors are indolent and typically do not cause symptoms until the tumor is large or metastasized. Carcinoid syndrome refers to the presence of a constellation of symptoms including secretory diarrhea, bronchospasm, and episodes of vasomotor changes (e.g., flushing and hypotension, rarely hypertension). Typically, carcinoid syndrome is thought to occur when vasoactive substances originating from the carcinoid tumor or metastases reach the systemic circulation through the hepatic vein. It is estimated that of people with neuroendocrine tumors, 30–40% will have carcinoid syndrome and 20–50% of patients with carcinoid syndrome will have carcinoid heart disease [4].
Carcinoid heart disease (CHD) is associated with high morbidity and mortality. In 1993, Pellikka et al. reported on 74 patients with carcinoid syndrome and found that those with echocardiographic evidence of carcinoid heart disease had a median survival of 1.6 years, which was significantly worse than patients without cardiac involvement [5]. A more recent retrospective study on 200 patients with carcinoid heart disease also at the Mayo Clinic found that that the median survival for patients diagnosed between 1981 and June 1989 was 1.5 years (95% CI 1.1–1.9 years), for patients diagnosed in July 1989–May 1995 it was 3.2 years (95% CI 1.3–5.1 years), and for patients diagnosed in June 1995–2000 it was 4.4 years (95% CI 2.4–7.1 years) [6]. Survival rates have likely improved due to use of somatostatin analogues and surgical intervention.
Pathophysiology of Carcinoid Heart Disease
Carcinoid heart disease is characterized by the development of plaques along the endocardial surface of the valve leaflet, and primarily affects the right-sided valves; the left side is seldom affected, owing to inactivation of vasoactive substances by the lung vasculature. Grossly, the plaques present as thickening of the valve with focal commissural fusion and associated thickening of the subvalvular apparatus, like findings seen in rheumatic heart disease (Fig. 8.2). Microscopically, the plaques are composed of a proliferation of bland-appearing myofibroblasts; extracellular components such as collagen, myxoid matrix, and elastin; and an overlying endothelial layer. Chronic inflammation as well as neovascularization can be seen within the plaques. Damage to the underlying valve is not characteristic (Fig. 8.3). The elaboration of serotonin by the primary or metastatic tumor is implicated in the development of CHD. Activation of 5-hydroxytryptamine 2B receptors has been shown to promote myofibroblast deposition and the activation of valvular interstitial cells, which results in the fibrosis seen in CHD [7,8,9].
Histology of carcinoid heart disease. (a) Myofibroblastic proliferation (carcinoid plaque) involving the left side of the tricuspid valve (arrow); the strip of valve on the right is uninvolved (*). (b) Smooth muscle actin immunohistochemistry highlighting the myofibroblastic proliferation (arrow); the uninvolved valve is negative (*). (c) Bland proliferation of spindled myofibroblasts with associated extracellular matrix; underlying cardiac valve is present in the upper left-hand corner (*) and chronic inflammation in the lower right-hand corner (arrow). (d) Neovascularization within the carcinoid plaque
Surveillance and Diagnosis of Carcinoid Heart Disease
Several biomarkers have been correlated to carcinoid heart disease including NT-proBNP, urinary or plasma 5-HIAA, chromogranin A, and actin A. In 2017, an expert consensus statement on carcinoid heart disease recommended that NT-proBNP was the best biomarker available for screening patients with carcinoid syndrome for carcinoid heart disease. This expert panel proposed that patients with metastatic neuroendocrine tumors undergo a clinical assessment and check NT-proBNP every 6 months. If the NT-proBNP is greater than 260 ng/mL or there are clinical suggestions of carcinoid heart disease, then a transthoracic echocardiogram should be performed. It can be useful to also keep track of a patient’s urinary 5-HIAA level since values >300 μmol/24 h increase a patient’s risk of developing carcinoid heart disease [4]. Unfortunately, carcinoid heart disease can progress quickly so regular follow up of patients with carcinoid syndrome is critical. Bhattacharyya et al. prospectively followed 252 patients with carcinoid syndrome at 6-month intervals and noted progression of carcinoid heart disease if the echocardiographic score they utilized increased by ≥25% from the prior 6 months. With a median follow-up for 29 months, 44 patients had progression of existing carcinoid heart disease or new carcinoid heart disease diagnosed [10].
Echocardiographic scoring systems have been developed to characterize carcinoid heart disease and predict progression. For example, in patients with carcinoid heart disease, a score which graded the following features from 0 (normal) to 3 (severe): leaflet thickening/mobility/morphology, valvular stenosis/regurgitation and right ventricular diameter/function correlated with NT-pro-BNP levels [11]. In a study of 137 patients with metastatic neuroendocrine tumors, a 5-point increase in echocardiographic score correlated with increased risk of CHD progression (OR 2.95, 95% CI 1.71–5.09, p < 0.005) and death (OR 2.66, 95% CI 1.63–4.35, p < 0.005) [12]. A comparison of six available echocardiographic scoring systems found all had similar sensitivity and specificity for identifying CHD however more complex scores may be better for patients with CHD who may need surgery [13].
Imaging
Echocardiography is the gold standard diagnostic test for the screening and serial evaluation of carcinoid heart disease [4]. As the right-sided heart chambers are anteriorly located, close to the chest wall, transthoracic echocardiography (TTE) typically provides excellent imaging of the right ventricle, tricuspid valve, and pulmonic valve. Two-dimensional and three-dimensional imaging provides information on valvular structure and function, and Doppler assessment is used to quantitate the severity of valve dysfunction. Echocardiographic evaluation of right ventricular size and function is also essential. If TTE does not provide adequate valvular evaluation, transesophageal echocardiography (TEE) may also be a useful imaging modality.
As previously mentioned, carcinoid heart disease is characterized by the deposition of carcinoid plaques, primarily on the right-sided heart structures. There is a wide spectrum of imaging findings that have been described [14], ranging from mild valvular thickening to severe valvular dysfunction. On echocardiography, there is a classic appearance of the tricuspid and pulmonic valves. The leaflets and subvalvular apparatus appear thickened and retracted, and in the case of severe disease, the valves appear rigid and stuck in a semi-open position (Fig. 8.1). This leads to a combination of stenosis and regurgitation. Valvular function is assessed with the use of color flow Doppler and continuous wave (CW) doppler, and the severity of stenosis or regurgitation should be graded accorded to guidelines [15]. With severe carcinoid tricuspid valve disease, color Doppler will reveal turbulent diastolic inflow suggestive of tricuspid stenosis as well as severe tricuspid regurgitation. CW Doppler through the tricuspid valve will reveal a dense, triangular-shaped regurgitant jet consistent with severe tricuspid regurgitation, as well as elevated inflow gradients indicative of tricuspid stenosis. The combination of stenosis and regurgitation will often show “to and fro” flow across the valve.
With three-dimensional (3D) imaging, the valves can be visualized en face and, in the case of severe disease, may reveal a triangular-shaped orifice with no central coaptation, and little change in systole or diastole (Fig. 8.4). Multiplanar reconstruction (MPR) can be used to evaluate all leaflets and 3D planimetry can be performed to estimate valve area or regurgitant orifice area (Fig. 8.4) [16].
Three-dimensional transesophageal echocardiography (3D TEE) of the tricuspid and pulmonic valves. 3D en face views allow simultaneous visualization of all three leaflets and highlight minimal leaflet excursion in systole versus diastole. 3D multiplanar reconstruction (MPR) allows simultaneous visualization of long axis and short axis views. 3D planimetry of the short axis view can be performed to measure valve area or regurgitant orifice area
Although carcinoid disease most commonly involves the tricuspid and pulmonic valves, left-sided structures, such as the mitral and aortic valves, may also be affected, particularly in the presence of a patent foramen ovale [14]. Rarely, carcinoid tumors may metastasize to the heart, which is characterized by a well-circumscribed mass that may be seen in either of the ventricular walls or interventricular septum [7]. For this reason, a comprehensive echocardiographic exam is necessary, including evaluation for right to left shunt with agitated saline contrast study.
In addition to echocardiography, cardiac magnetic resonance imaging (MRI) may be helpful in the evaluation of patients with carcinoid heart disease. Cardiac MRI can provide a quantitative assessment of right ventricular size and function, evaluate for the presence of metastases, and can also provide information on valvular structure and function [7].
Management of Carcinoid Heart Disease
Successful management of carcinoid heart disease relies on a team-based approach. Key members of the team include the patient, cardio-oncologist, oncologist, cardiothoracic surgeon, and cardiothoracic anesthesiologist. From a cardiac standpoint, the focus of care is on management of right heart failure and identification of the need for cardiac surgery. Daily weights, fluid restriction, and careful diuretic administration are necessary given the side effects of decreasing preload in right heart failure. Compression stockings and salt restriction are also important. Spironolactone can be considered given the potential benefit to the right heart [17].
Somatostatin analogues are the cornerstone of carcinoid treatment and prevent the development and/or progression of carcinoid heart disease [4]. Somatostatin analogues decrease circulating levels of serotonin and bioactive metabolites. The two currently available somatostatin analogues are octreotide and lanreotide and long-acting formulations are used for treatment of symptomatic carcinoid. Use of these analogues is expanding as studies have shown benefit in asymptomatic patients with carcinoid with regards to progression-free survival [18, 19]. Telotristat ethyl is approved by the Food and Drug Administration for refractory symptoms and interferon alfa is an approved agent in Europe. Everolimus, an mTOR inhibitor, should be used with caution in carcinoid heart disease and held for 2–4 weeks prior to procedures, including heart surgery [4]. Peptide receptor radionucleotide therapy (PRRT) is a type of treatment in which radionucleotide therapy is delivered systemically to tumor cells and is contraindicated in patients with decompensated heart failure due to the administration of intravenous fluids involved with the treatment [4, 20]. Transcatheter arterial embolization or chemoembolization of hepatic metastases should be used with caution in severe carcinoid heart disease and right ventricular dysfunction. Surgical debulking of hepatic metastases may be an option after valve replacement for carcinoid heart disease [4].
Carcinoid Crisis
Carcinoid crisis is of concern in the peri-procedure period [21]. Carcinoid crisis refers to the constellation of flushing, hypotension, and bronchospasm, which can result from administration of catecholamines and histamine-releasing drugs or from events that trigger catecholamine release like emotional stress, hypercapnia, hypothermia, hypotension, or hypertension [22]. Octreotide administration prior to and during the procedure can provide hemodynamic stability and should be readily available during procedures for patients with carcinoid. Patients should be educated on their potential need for octreotide prior to procedures and encouraged to proactively discuss the sedation plan with the anesthesiology team at preoperative visits. Cardiac surgery in patients with carcinoid heart disease can be particularly challenging given the need to manage low cardiac output secondary to right heart failure on top of carcinoid crisis. Cardiothoracic anesthesiologists with experience managing carcinoid heart disease may be best suited to provide anesthesia support in these cases. Many institutions have octreotide protocols in place to manage carcinoid crisis in the perioperative period.
Surgery
Surgical timing depends on heart failure symptoms, right heart function, and how well-controlled carcinoid syndrome is. Cardiac surgery for carcinoid heart disease typically focuses on tricuspid valve replacement. However patients may also need pulmonic valve replacement, closure of a patent foramen ovale to avoid transmission of vasoactive substances to the left-sided valves, replacement of other valves, bypass grafting, removal of intramyocardial carcinoid metastases, and patch enlargement of the right ventricular outflow tract. Optimal timing of surgery is not clear and requires a collaborative discussion between members of the patient’s care team. Current consensus guidelines recommend referral to cardiac surgery when symptoms or ventricular dysfunction are present, anticipated survival of at least 12 months postoperatively, and rarely as a precursor to hepatic surgery [4]. The decision of mechanical versus bioprosthetic valves should be individualized with considerations given to the need for future oncologic procedures, bleeding risks associated with liver disease and structural valve deterioration [23]. The oncologist should weigh in regarding how controlled a patient’s carcinoid syndrome is in the presurgical period and recommendations for somatostatin analogue dosing in the perioperative period.
Cardiac surgery can improve heart failure functional class and lengthen survival. A study of 22 patients who underwent valve replacement between 2006 and 2010 showed that those who survived surgery had significant improvement in New York Heart Association functional class at 3 months [24]. A contemporary review of 240 patients who underwent valve replacement for carcinoid heart disease between November 1985 and January 2018 revealed survival estimates at 1 year was 69%, 3 years was 48%, and 5 years was 34%. Early mortality rate after valve surgery also decreased over time, 29% between 1985 and 1994, 7% between 1995 and 2004, and 5% since 2005 [25].
Bioprosthetic Valve Deterioration
Bioprosthetic valve deterioration has been reported after valve replacement to treat carcinoid heart disease. Castillo et al. reported the need for redo tricuspid and pulmonic valve replacement at 25 months in a 47-year-old male with carcinoid heart disease who underwent resection of the primary carcinoid tumor in the ileum and liver lobectomy after his initial cardiac surgery. These authors surmise that high levels of serotonin and 5-HIAA despite optimal medical therapy contributed to the early deterioration of the initial prosthetic valves. They performed the patient’s redo surgery with mechanical tricuspid and pulmonic valves [26]. In Bhattacharya et al.’s aforementioned study of 22 patients undergoing cardiac surgery, 2 developed bioprosthetic valve degeneration [24]. Another surgical outcomes study of 39 patients treated with surgery for CHD reported 2 had valve deterioration treated with valve-in-valve transcatheter aortic valve replacement [27].
Percutaneous Interventions in Carcinoid Heart Disease
Percutaneous interventions to treat carcinoid heart disease is a burgeoning area. Percutaneous native pulmonic valve replacement with the Melody valve and the Edwards Sapien XT have been reported to treat CHD in patients who were considered high surgical risk [28,29,30]. Conradi et al. reported on two patients who previously had bioprosthetic tricuspid and pulmonic valve replacements who were treated with valve-in-valve replacement of the pulmonic valve [31]. Transcatheter, transapical tricuspid, and pulmonary valve-in-valve replacements with the Edwards Sapien XT valves has been described to treat failing bioprosthetic tricuspid and pulmonic valves in carcinoid heart disease [32]. Transcatheter treatment of native tricuspid valve disease (without CHD) is challenging due to the large tricuspid annular size, lack of fibrous skeleton for support, highly variable tricuspid anatomy, and complex subvalvular apparatus. There are several transcatheter tricuspid valve replacement devices under investigation for the treatment of native tricuspid valve regurgitation; however, to date, percutaneous valve replacement of the native tricuspid valve affected by carcinoid heart disease has not yet been reported, but this is an area of great interest for future investigation.
Abbreviations
- 3D:
-
three-dimensional
- CHD:
-
carcinoid heart disease
- CW:
-
continuous wave
- MPR:
-
multiplanar reconstruction
- MRI:
-
magnetic resonance imaging
- NT-proBNP:
-
pro-B-type natriuretic peptide
- TEE:
-
transesophageal echocardiography
- TTE:
-
transthoracic echocardiography
References
Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: a report from the American heart association. Circulation. 2019;139(10):e56–e528.
National Cancer Institute. https://www.cancer.gov/about-cancer/understanding/statistics. Accessed 24 May 2020.
Kunz PL. Carcinoid and neuroendocrine tumors: building on success. J Clin Oncol. 2015;33(16):1855–63.
Davar J, Connolly HM, Caplin ME, Pavel M, Zacks J, Bhattacharyya S, et al. Diagnosing and managing carcinoid heart disease in patients with neuroendocrine tumors: an expert statement. J Am Coll Cardiol. 2017;69(10):1288–304.
Pellikka PA, Tajik AJ, Khandheria BK, Seward JB, Callahan JA, Pitot HC, et al. Carcinoid heart disease. Clinical and echocardiographic spectrum in 74 patients. Circulation. 1993;87(4):1188–96.
Moller JE, Pellikka PA, Bernheim AM, Schaff HV, Rubin J, Connolly HM. Prognosis of carcinoid heart disease: analysis of 200 cases over two decades. Circulation. 2005;112(21):3320–7.
Hassan SA, Banchs J, Iliescu C, Dasari A, Lopez-Mattei J, Yusuf SW. Carcinoid heart disease. Heart. 2017;103(19):1488–95.
Laskaratos FM, Rombouts K, Caplin M, Toumpanakis C, Thirlwell C, Mandair D. Neuroendocrine tumors and fibrosis: an unsolved mystery? Cancer. 2017;123(24):4770–90.
Luis SA, Pellikka PA. Carcinoid heart disease: diagnosis and management. Best Pract Res Clin Endocrinol Metab. 2016;30(1):149–58.
Bhattacharyya S, Toumpanakis C, Chilkunda D, Caplin ME, Davar J. Risk factors for the development and progression of carcinoid heart disease. Am J Cardiol. 2011;107(8):1221–6.
Bhattacharyya S, Toumpanakis C, Caplin ME, Davar J. Usefulness of N-terminal pro-brain natriuretic peptide as a biomarker of the presence of carcinoid heart disease. Am J Cardiol. 2008;102(7):938–42.
Dobson R, Burgess MI, Valle JW, Pritchard DM, Vora J, Wong C, et al. Serial surveillance of carcinoid heart disease: factors associated with echocardiographic progression and mortality. Br J Cancer. 2014;111(9):1703–9.
Dobson R, Cuthbertson DJ, Jones J, Valle JW, Keevil B, Chadwick C, et al. Determination of the optimal echocardiographic scoring system to quantify carcinoid heart disease. Neuroendocrinology. 2014;99(2):85–93.
Bhattacharyya S, Toumpanakis C, Burke M, Taylor AM, Caplin ME, Davar J. Features of carcinoid heart disease identified by 2- and 3-dimensional echocardiography and cardiac MRI. Circ Cardiovasc Imaging. 2010;3(1):103–11.
Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American society of echocardiography developed in collaboration with the society for cardiovascular magnetic resonance. J Am Soc Echocardiogr. 2017;30(4):303–71.
Miyasaka R, Mehta A, Pettersson GB, Desai MY. Carcinoid tricuspid valve disease: applications of three dimensional transesophageal echocardiography. Circ Cardiovasc Imaging. 2019;12(12):e009555.
Dos L, Pujadas S, Estruch M, Mas A, Ferreira-Gonzalez I, Pijuan A, et al. Eplerenone in systemic right ventricle: double blind randomized clinical trial. The evedes study. Int J Cardiol. 2013;168(6):5167–73.
Caplin ME, Pavel M, Ruszniewski P. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371(16):1556–7.
Rinke A, Wittenberg M, Schade-Brittinger C, Aminossadati B, Ronicke E, Gress TM, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide lar in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors (PROMID): results of long-term survival. Neuroendocrinology. 2017;104(1):26–32.
Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of (177)Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376(2):125–35.
Kaltsas G, Caplin M, Davies P, Ferone D, Garcia-Carbonero R, Grozinsky-Glasberg S, et al. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: pre- and perioperative therapy in patients with neuroendocrine tumors. Neuroendocrinology. 2017;105(3):245–54.
Castillo JG, Silvay G, Solis J. Current concepts in diagnosis and perioperative management of carcinoid heart disease. Semin Cardiothorac Vasc Anesth. 2013;17(3):212–23.
Korach A, Grozinsky-Glasberg S, Atlan J, Dabah A, Atlan K, Rudis E, et al. Valve replacement in patients with carcinoid heart disease: choosing the right valve at the right time. J Heart Valve Dis. 2016;25(3):349–55.
Bhattacharyya S, Raja SG, Toumpanakis C, Caplin ME, Dreyfus GD, Davar J. Outcomes, risks and complications of cardiac surgery for carcinoid heart disease. Eur J Cardiothorac Surg. 2011;40(1):168–72.
Nguyen A, Schaff HV, Abel MD, Luis SA, Lahr BD, Halfdanarson TR, et al. Improving outcome of valve replacement for carcinoid heart disease. J Thorac Cardiovasc Surg. 2019;158(1):99–107 e2.
Castillo JG, Filsoufi F, Rahmanian PB, Zacks JS, Warner RR, Adams DH. Early bioprosthetic valve deterioration after carcinoid plaque deposition. Ann Thorac Surg. 2009;87(1):321.
Kuntze T, Owais T, Secknus MA, Kaemmerer D, Baum R, Girdauskas E. Results of contemporary valve surgery in patients with carcinoid heart disease. J Heart Valve Dis. 2016;25(3):356–63.
Heidecker B, Moore P, Bergsland EK, Merrick SH, Rao RK. Transcatheter pulmonic valve replacement in carcinoid heart disease. Eur Heart J Cardiovasc Imaging. 2015;16(9):1046.
Kesarwani M, Ports TA, Rao RK, Mahadevan VS. First-in-human transcatheter pulmonic valve implantation through a tricuspid valve bioprosthesis to treat native pulmonary valve regurgitation caused by carcinoid syndrome. JACC Cardiovasc Interv. 2015;8(10):e161–3.
Loyalka P, Schechter M, Nascimbene A, Raman AS, Ilieascu CA, Gregoric ID, et al. Transcatheter pulmonary valve replacement in a carcinoid heart. Tex Heart Inst J. 2016;43(4):341–4.
Conradi L, Schaefer A, Mueller GC, Seiffert M, Gulbins H, Blankenberg S, et al. Carcinoid heart valve disease: transcatheter pulmonary valve-in-valve implantation in failing biological xenografts. J Heart Valve Dis. 2015;24(1):110–4.
Khan JN, Doshi SN, Rooney SJ, Bhabra MS, Steeds RP. Transcatheter pulmonary and tricuspid valve-in-valve replacement for bioprosthesis degeneration in carcinoid heart disease. Eur Heart J Cardiovasc Imaging. 2016;17(1):114.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kondapalli, L., Berning, A., Clasen, S.C., Miyasaka, R. (2022). Carcinoid Heart Disease. In: Mathelier, H., Lilly, S.M., Shreenivas, S. (eds) Tricuspid Valve Disease. Contemporary Cardiology. Springer, Cham. https://doi.org/10.1007/978-3-030-92046-3_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-92046-3_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-92045-6
Online ISBN: 978-3-030-92046-3
eBook Packages: MedicineMedicine (R0)