Abstract
Neurodegenerative diseases are characterised by a progressive loss of neurons that leads to a range of cognitive and/or motor dysfunctions. During recent decades, some common pathways leading to neurodegeneration have been identified, such as protein misfolding, neuroinflammation, and the dysfunction of mitochondria and protein clearance systems. More recently, an altered gut microbiota has been identified as another potential feature seen in neurodegenerative disorders, which has been shown to play a central role in health and disease. The gut microbiota communicates with the central nervous system along the microbiota-gut-brain axis modulating host health and disease. Although the specific role of gut microbiota on the pathogenesis of these diseases is still under investigation, therapeutic approaches focusing on the modification of gut microbiota could bring novel therapeutics for neurodegenerative diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Neurodegeneration
- Alzheimer
- Parkinson
- Huntington
- Inflammation
- Protein misfolding
- Mitochondria
- Gut microbiota
1 Introduction
It’s been over a century since James Parkinson and Alois Alzheimer first published their observations of the neurodegenerative diseases that bear their names [1, 2]. Today, neurodegenerative diseases are one of the main causes of comorbidity and mortality in older adult populations, and these numbers will likely increase with the proportion (or number) of aged individuals increasing day by day. These debilitating diseases have immense emotional and financial tolls on all societies worldwide.
In broad terms, neurodegenerative diseases are conditions where neurons in the central or peripheral nervous system progressively degenerate, leading to central nervous system dysfunction. Several neurodegenerative diseases can be identified depending on the neuronal population affected, its localisation in the brain, and the clinical features observed (see Table 1). Most neurodegenerative diseases are characterised by depositions of misfolded native proteins and a widespread clinical symptomatology. The traditional method of classifying neurodegenerative disease is based on clinicopathological features from the anatomical area affected with the neuronal dysfunction supported by molecular pathology patterns of the misfolded proteins [14]. Nevertheless, classifying these diseases is a challenging subject as specific symptoms and protein aggregates can be found in multiple diseases, hampering the diagnosis of the disease [15]. Thus, the idea that neurodegenerative diseases are overlapping or even a continuum has been raised [14].
To date, there is no cure for any single neurodegenerative disease, where diagnosis usually leads to debilitating symptoms and ultimately death, due in part to the lack of complete knowledge of the aetiological factors involved and a limited understanding of their pathological progression. While degenerative mechanisms are not yet fully elucidated, some common mechanisms leading to neurodegeneration have been identified, such as protein aggregation, neuroinflammation, oxidative stress, mitochondrial dysfunction, and impairments in autophagy.
Accumulating evidence indicating that intestinal microbiota influences brain function and behaviour across the lifespan has sparked interest in the role the gut microbiome plays in neurodegenerative disease. The gut microbiota of individuals with neurodegenerative disease differs from healthy people, suggesting a connection between the brain pathology and the gut microbiota. The microbiota-gut-brain axis is a bidirectional communication pathway where gut microbes signal to the brain and the brain signals to the gut. The mechanisms of communication are not fully understood due to large interconnected complex networks but include immune, neural, endocrine, and metabolic pathways [16]. A growing body of evidence now points towards a role for the gut microbiota in age-related disorders including neurodegenerative disease; however, the level of involvement of the gut microbiota in the pathology is still under debate. Our knowledge about the implications of gut microbiota in the pathogenesis of these diseases, as well as the number of therapeutic interventions targeting the gut microbiota for these diseases, are however increasing.
2 Neurodegenerative Diseases
Neurodegeneration is the generic term that describes the loss of neurons leading to a progressive dysfunction of the central nervous system. Neurodegenerative diseases are usually of unknown origin, and the pathogenesis is considered to be driven by a combination of genetic and environmental factors (with the possible exception of Huntington’s disease—HD—which is inherited in an autosomal dominant manner).Footnote 1 The knowledge around these conditions has increased notably in recent decades thanks to improvements in neuronal imaging and molecular techniques. However, this knowledge varies greatly amongst diseases, and Alzheimer’s disease (AD) and Parkinson’s disease (PD) are by far the most studied neurodegenerative diseases to date. A brief description of the most common and studied neurodegenerative diseases is given below as they will be mentioned throughout this chapter.
AD is the most common neurodegenerative disease affecting at least 43.8 million people worldwide [17], a number that is growing year after year and expected to double by 2050 [18]. AD is the most common diagnosis of dementia. The main neuropathological hallmarks of AD are the accumulation of extracellular amyloid-beta (Aβ) plaques and intraneuronal deposits of tau protein, a microtubule-associated protein, which constitutes the primary component of neurofibrillary tangles [19]. These pathological features lead to neuronal cell death in the hippocampus and the cerebral cortex and a decline in memory, thinking, and language abilities.
PD is the second most common neurodegenerative disease, and more than six million individuals worldwide were living with PD in 2016 [20]. It is characterised by a slow and progressive degeneration of dopaminergic neurons in the substantia nigra. This neuronal decay in the nigrostriatal pathway generates motor (bradykinesia, rigidity, resting tremor, and postural instability) and non-motor symptoms (constipation, anosmia, and sleep disturbances amongst others). In addition to neuronal loss, PD is also characterised by the presence of intraneuronal protein inclusions of α-synuclein, called Lewy bodies [21]. α-Synuclein is a small protein, mostly present in the presynaptic terminals of neurons, and its function, although not fully understood, is associated with synaptic function modulation and vesicle trafficking [22].
HD is a neurodegenerative disease caused by an inherited autosomal dominant CAG trinucleotide repeat in the huntingtin gene that causes neuronal dysfunction [8]. Hence, unlike other neurodegenerative diseases, HD pathogenesis is triggered by a genetic mutation. This neurodegeneration causes a wide spectrum of movement, cognitive, and psychiatric symptoms that appear in the first decades of adult life [8].
Amyotrophic lateral sclerosis (ALS) is the most common form of motor neuron disease and affects motor neurons and causes muscular atrophy and weakness leading to difficulties in speaking and breathing and ultimately death [23]. In ALS, neurodegeneration and neuronal cell death are associated with excess synaptic glutamate and mitochondrial alterations [24]. A blockade of the neurotransmitter γ-aminobutyric acid (GABA) receptor in ALS causes muscle hyperexcitability and a moderate loss of motor neurons [25]. Thus, distortions in the fine balance between the excitatory glutamate activity and inhibitory GABA activity can severely compromise motor neuron viability. Significant particularities of ALS versus other neurodegenerative diseases include a lower age of onset, fast decay of motor neurons, and quick mortality rate [26].
Multiple sclerosis (MS) is a neurodegenerative and inflammatory autoimmune disease where T cells in the immune system react against oligodendrocytes in the CNS, resulting in neuroinflammation, demyelination, and axonal loss [27]. The consequences of demyelination depend on the area affected but include ataxia, loss of coordination, visual and sensory impairment, and fatigue. The autoimmune profile of MS makes it different from the other neurodegenerative diseases as it is characterised by an earlier onset and episodic manifestations.
The conditions mentioned above do not represent a complete list of neurodegenerative diseases characterised thus far but represent the conditions where the role of the gut microbiome has been mostly investigated. There is a plethora of proteinopathies described in the literature that include infectious acquired neurodegeneration (such as that seen in prion-like disease, transmissible spongiform encephalopathies, or Creutzfeldt-Jakob disease) and conditions with mixed clinicopathological features such as dementia with Lewy bodies.
Although each neurodegenerative disease has a clearly defined set of diagnostic symptoms, most patients present with mixed clinical symptomatology, hampering an accurate diagnosis that in most cases cannot be confirmed until post-mortem. The rising complexity of neuropathological findings demands that all aspects of the clinical picture are analysed and not limited to the cardinal symptoms. Secondary symptomatology, including gastrointestinal, cognitive, and psychiatric manifestations, are increasingly being considered as a prodromal diagnostic tool for neurodegenerative disease, especially given that they can significantly impact quality of life. For example, symptoms of gastrointestinal dysfunction such as constipation are a common symptom of the prodromal phase in PD and can arise several years before any motor function deficits.
Hence, it is becoming increasingly evident that not only do disease-specific brain-related symptoms need to be considered. Gastrointestinal microbes were initially examined for GI-related conditions such as Crohn’s disease and irritable bowel syndrome but are now under serious consideration in the field of brain neuropathies.
3 Gut Microbiota
The human microbiota is an entity that includes trillions of microorganisms (bacteria, viruses, fungi, phages, yeasts, archaea) that live in and on our bodies [28]. The gut microbiota specifically consists of a broad community of bacterial species that live in symbiosis in the human gastrointestinal tract and is essential for the digestion of non-digestible substrates in the host, such as dietary fibres [29]. The gut microbiota is also involved in many other functions including host metabolism, immune system regulation, and neuronal development.
The technological advancement of genetic sequencing techniques lately has dramatically improved our knowledge of the gut microbiota composition and abundance; it is estimated that our gastrointestinal tracts are populated with more than 500–1000 different bacterial species [30]. The human gut microbiota is mostly composed of bacterial strains belonging to the phylaFootnote 2 Firmicutes and Bacteroidetes, but other minority phyla that are commonly found include Proteobacteria, Actinobacteria, Fusobacteria, and Verrucomicrobia [31]. The gut microbiota is a dynamic entity that experiences many shifts during the host lifespan and can be modulated by many external factors such as lifestyle choices and environmental inputs [32]. However, each individual harbours radically different microbial compositions, even in conserved taxa, and our knowledge about what regulates that variability is limited. For this reason, what constitutes the best microbiome composition is unknown.
Although microbial community composition in the gut has a great inter-individual variability, the maintenance or disruption of the host gut homeostasis, per individual, is key in health and disease. The overall composition ratios of gut microbiota in any one individual are very well conserved once adulthood is reached. In fact, changes in the microbiome and the subsequent relationship with biological systems such as the immune, endocrine, and central nervous system correlate with a broad range of diseases. In particular, the relative abundance between the two major phyla, expressed as the Firmicutes/Bacteroidetes ratio, has been linked with many pathological conditions [33], and it is currently used as an estimation of gut microbiota alterations. In the context of neurodegenerative diseases, intestinal microbiota dysbiosisFootnote 3 seems to contribute to neurodegeneration [35].
The implication of the gut microbiota in disease has sparked interest in the scientific community that foresees gut microbiota modulation as a potential target for therapeutic prevention and intervention. Many therapeutic interventions focused on the enhancement of beneficial bacteria have been described, such as the oral administration of probiotics, prebiotics, or faecal microbiota transplantation (FMT). In probiotic administration, live bacteria and/or yeasts, thought to be beneficial to health, are ingested, whereas prebiotic administration consists of the ingestion of non-digestible fibres that promote the growth of beneficial bacteria. FMT is the transplantation of gut microbiota from a donor individual into the GI tract of a recipient individual. In animal models, FMT has been used to investigate the pathogenic mechanisms of neurodegenerative diseases by transplanting the gut microbiota of healthy donors into a diseased recipient, or vice versa. These approaches are under investigation for neurodegenerative diseases and will be described later in this chapter.
3.1 Gut Microbiota and Ageing
Ageing is an inevitable and progressive deterioration of physiological functions of the host that correlate with increased risk of disease and death. Ageing comes with modifications in life habits (such as diet or exercise) and physiological changes. The ageing process brings changes in gut physiology, as well as gut microbial composition and function [36]. The process of ageing has been classified as a sensitive period for gut microbiota, where it is susceptible to environmental triggers and intrinsic factors in the host [37]. However, the relationship between ageing and gut microbiota is thought to be bilateral, as the gut microbiota can also contribute to normal ageing. Ageing-associated gut microbiota changes result in increased gut permeability, modifications in the production of gut microbiota-derived metabolites, and alterations in the host immune system [38]. Age-associated shifts in the gut microbiota are linked to increased susceptibility to many diseases, including neurodegenerative diseases [39, 40].
The composition of the gut microbiota is markedly different between young and elderly populations [41,42,43] the latter being characterised by a lower microbial diversity [44, 45]. For instance, in some cohort studies, elderly populations had a reduced Firmicutes/Bacteroidetes ratio [46, 47], but not in others [48]. Also, a reduction in beneficial commensal bacteria such as Bifidobacterium and Lactobacillus and an increase in harmful bacteria such as Enterobacteriaceae are reported in aged individuals [49]. These microbial changes are linked to changes in physiology attributed to ageing but also to lifestyle changes such as modifications in diet, reduction of physical activity, and an increase in medications [46]. In ageing populations, a lower microbial diversity correlated with diseased conditions [46]. For example, loss of diversity in the core microbiotaFootnote 4 groups is associated with increased frailty and reduced cognitive performance [50]. Although the mechanisms linking gut microbiota and brain are not fully understood, it is clear that microbial dysbiosis in the gut is associated with a higher risk of brain dysfunction.
4 The Microbiota-Gut-Brain Axis
The concept of a microbiota-gut-brain axis is relatively new but is increasingly accepted due to the mounting evidence that the gut microbiota can regulate brain-specific processes such as host behaviour [37]. The microbiota-gut-brain axis is comprised of bidirectional complex communication networks between the brain and the gastrointestinal tract. This bidirectional communication between the two organs involves many signalling pathways such as the enteric nervous system (ENS), the hypothalamic-pituitary-adrenal axis, the immune and endocrine systems, as well as the gut microbiota and its metabolites [16, 51]. Despite interest in and knowledge of the microbiota-gut-brain axis increasing daily, there is still a lack of full understanding of the underlying mechanisms involved in these networks.
The relevance of the gut-brain axis in neurodegenerative diseases became evident 20 years ago when Dr. Braak and colleagues proposed an intriguing hypothesis that PD spread from the gut to the brain as a result of an infection [52]. They presented evidence of Lewy bodies outside the nigrostriatal pathway, in locations such as the olfactory bulb, the ENS, and the vagus nerve. According to Braak and colleagues, the first inclusions of α-synuclein occur in the vagus nerve and olfactory bulbs and then the pathology spreads in an ascending manner to the brainstem and forebrain [52]. Interestingly, the vagus nerve is one of the best characterised communication pathways between the brain and the gut [53]. Animal models were then developed to assess the gut-to-brain spread hypothesis. Now we know that α-synuclein can spread in a prion-like manner both in vitro and in vivo [54]. Moreover, the transport of different forms of α-synuclein from the gut to the brain through the vagus nerve has been reported in rats [55]. However in a recent animal study, although the expected brain-to-gut spread of α-synuclein could not be confirmed, alterations in the ENS and the gut microbiome were apparent [56]. Interestingly, gut-seeded α-synuclein fibrils promoted gut dysfunction and brain pathology in aged mice but not in young mice [57]. Despite the point of origin of PD in the body still being a matter of debate, these investigations have highlighted the importance of the role that the gut-brain axis plays in PD.
Most neurodegenerative diseases were initially viewed as neuronal brain-exclusive diseases, but recent findings have challenged this idea and neurodegenerative diseases are now viewed as a multisystemic disease. Although ageing, genetics, and the environmental are important risk factors for neurodegeneration, as we will see later on, the involvement of the gut microbiome through the bidirectional gut-brain axis cannot be diminished. As a result, much research now focuses on the potential implications of the microbiome in these diseases.
5 Towards Neurodegeneration
For decades, neuroscientists were focused on the specifics of the neuronal decay in each neurodegenerative disease. However, it is now more evident that there are clinical, cellular, and molecular differences that neurodegenerative diseases share, which contribute to the development of neurodegeneration. Furthermore, they share common pathogenic pathways that lead to neurodegeneration. Below, we discuss the main factors contributing to neurodegenerative disease (see Fig. 1).
Known factors that contribute to neurodegenerative disease. The aetiology of neurodegenerative diseases is mostly unknown, but several factors such as ageing, genetics, environmental, and lifestyle choices contribute to the pathogenesis of neurodegenerative diseases. SNCA, synuclein alpha; LRRK2, leucine-rich repeat kinase 2; PARK2, Parkinson disease-2; PINK1, PTEN [phosphatase and tensin homolog]-induced kinase 1; DJ-1, protein deglycase; APP, β-amyloid precursor protein; PM (particulate matter) 10 (<10 μm) 2.5 (<2.5 μm); O3 (ozone/trioxygen); NOX, nitrogen oxides; Fe, iron; Cu, copper; Zn, zinc; Mn, manganese; Al, aluminium; Cd, cadmium; Pb, lead; CMV, cytomegalovirus; ND, neurodegenerative disease
5.1 Factors that Contribute to the Development of Neurodegeneration
5.1.1 Ageing
The growth of an ageing population worldwide is increasing rapidly, and with it, the incidence of neurodegenerative diseases. From a cellular and molecular perspective, the nine hallmarks of ageing have been identified and grouped into primary, antagonistic, or integrative hallmarks (see Fig. 2) [58]. The primary hallmarks of ageing include genomic instability, epigenetic alterations, telomere attrition, and loss of proteostasis,Footnote 5 which are considered to be unequivocally negative processes. The antagonistic hallmarks—mitochondrial dysfunction, cellular senescence, and deregulated nutrient sensing—unlike the primary hallmarks, can have beneficial or deleterious effects depending on their intensity. The integrative hallmarks—stem cell exhaustion and altered intercellular communication—arise as the culprit of the accumulative damage induced by primary and antagonistic hallmarks. These central biological mechanisms of ageing and their relationship with neurodegeneration have been reviewed elsewhere [59].
The absence of disease-free brains in the oldest population suggests that brain ageing and neurodegeneration are a continuum rather than a simplistic cause-effect relationship [60]. Thus, not only ageing but congenital predisposition and environmental factors will determine the lesions that will lead to specific diseases.
5.1.2 Congenital Factors
Genetic studies have shown that genetic predisposition plays an important role in the development of neurodegenerative disorders such as AD or PD, especially in young adult onset cases where specific mutations have been identified [61, 62]. In early onset familial AD, mutations in three genes (amyloid precursor protein (APP), presenilin-1, and presenilin-2) involved in the Aβ plaque formation have been identified as inherited in an autosomal dominant pattern [61]. In the familial forms of PD, genes (SNCA, LRRK2, PARK2, PINK1, DJ-1, and ATP13A2) have been identified to be hereditable monogenic PD [63]. In contrast, HD is an exception to neurodegenerative diseases, as the genetic component is needed for the development of the disease.
However, most chronic neurodegenerative diseases are considered to have a multifactorial aetiology since most of the cases are sporadic and cannot be attributed solely to genetic factors.
5.1.3 Environmental Factors
There is mounting evidence that environmental factors play a crucial role in the development of neurodegenerative diseases, in particular in AD and PD. Pesticides, herbicides, fertilisers, and particulate matter (PM), ozone (O3), nitric oxide, and heavy metals have been demonstrated as having an increased risk of developing AD [64, 65], PD [66, 67], and ALS [68] (see Fig. 1). Pesticides and heavy metals can be neurotoxic leading ultimately to neurodegeneration [69]. Interestingly, some of these environmental factors can directly affect gut microbiota. It is believed that a complex combination of genetic and environmental interactions is key for disease pathogenesis and that the microbiome is a participant in this intricate network of factors. However, how these environmental factors interact is not fully understood, and more investigations on these interactions are needed as they could elucidate mechanisms of pathogenesis and improve prevention and personalised therapy for these diseases [70].
5.1.4 Lifestyle
Lifestyle choices and experiences have also been linked to the development of these diseases. For example, sport-related traumatic brain injury has been reported to increase the risk of developing both AD and PD [71, 72], whereas coffee consumption, smoking [73], and vigorous exercise [74] correlated with a decreased risk for PD and the Mediterranean diet for AD [75]. Lifestyle risk factors for MS include smoking, vitamin D deficiency or low sun exposure, and infections of Epstein-Bar virus or cytomegalovirus [76]. These lifestyle factors can impact gut microbiota, once more highlighting the complexity of factors and systems involved in neurodegeneration diseases.
5.2 Common Pathways to Neurodegeneration
Although each neurodegenerative disease has its own singular pathogenic mechanisms, some commonalities arise in the pathways leading to neurodegeneration.
5.2.1 Protein Misfolding
The most common neurodegenerative disorders are proteinopathies, characterised by the misfolding, aggregation, and accumulation of disease-specific proteins. These proteins change their conformation resulting in a loss of their biological function and can become toxic. AD, characterised by the formation of Aβ deposits as amyloid plaques, as well as neuronal tau inclusions, is probably the most salient proteinopathy, although many others exist such as PD, HD, frontotemporal dementia, and spinocerebellar ataxia type 1. The molecular mechanisms, causing a normal protein with a physiological function to transform into an abnormal conformation, are not well understood [77].
Protein homeostasis is a tightly regulated process essential for cellular integrity. Misfolded or aggregated proteins are quickly targeted by molecular chaperones that repair or degrade faulty proteins to maintain cellular homeostasis [78]. Molecular chaperones use the ubiquitin proteasome system and autophagy pathways to degrade these proteins [79]. However, these systems are altered in many neurodegenerative diseases, facilitating the accumulation of these aberrant proteins. Moreover, cellular ageing, proteotoxic stress, or genetic mutations can interfere with this process, resulting in proteins that escape the cell’s quality control system and aggregate into non-native structures [78]. Consequently, although misfolded proteins in neurodegenerative diseases (α-synuclein, Aβ, huntingtin) have very different biological function and location, they share a β-sheet-rich tertiary structure in their pathological form, which facilitates their aggregation into oligomeric fibrillar formations [80].
Thus, despite having differential molecular agents implicated, neurodegenerative diseases share many common altered hallmarks that explain this accumulation of aberrant proteins.
5.2.2 Glial Cells and Neuroinflammation
The brain is not only populated by neurons, in fact, it’s estimated that glial cells are at least as abundant as neurons [81]. Neurodegenerative diseases are multicellular in nature, and the implication of both neuronal and non-neuronal populations is now being investigated. This shift was based on extensive research data, showing first the presence of neuroinflammation in neurodegenerative disease, and second, the involvement of glial cells in disease progression. There is compelling evidence that neurodegenerative diseases such as AD, PD, and MS, are strongly associated with immune activation and neuroinflammation [82, 83].
Most neurodegenerative diseases display increased levels of neuroinflammation, which is the inflammatory response in the brain. This permeabilisation leads to lymphocyte infiltration. Neuroinflammation involves the activation of microglia and astrocytes, which secrete inflammatory molecules such as cytokines and chemokines. Evidence from both individuals and animal models reports that there is a recruitment of glial cells into the afflicted areas. For example, activated microglia and/or astrocytes are found in the substantia nigra of PD patients [84, 85] and AD patients, respectively [86]. Not only are innate immune cells found in neurodegenerative processes, cytotoxic T lymphocytes—major cell components of the adaptive immune system—were also reported to be higher in blood and in the affected PD brain regions, than in healthy subjects [87, 88]. The selective location of these T cells indicates an infiltration to the brain parenchyma, suggesting a disruption of the blood-brain barrier (BBB). Altered permeability in the BBB is a common pathological feature in many neurodegenerative diseases [89, 90].
At a molecular level, neuroinflammation has also been confirmed, where increased levels of pro-inflammatory molecules such as tumour necrosis factor α (TNF-α), interleukin 1β (IL-1β), and interferon γ (IFN-γ) were present in the serum and cerebrospinal fluid of PD patients and in the nigrostriatal pathway at post-mortem analysis [91]. Thus, although the identification of neuroinflammation during the progression of neurodegeneration is largely understood, these data do not confirm the involvement of neuroinflammation in the degenerative process. However, genetic and epidemiological studies have shown that polymorphisms in neuroinflammation-related genes increase the susceptibility for PD [91] and mutations in APOE and TREM2 genes, mainly expressed in glial cells, increase the risk for AD [92]. Further, genes linked to major histocompatibility complex (MHC) class II have been found to be a risk factor for MS [93].
This evidence together suggests that neuroinflammation is linked to the neurodegenerative process, playing a crucial role in disease progression. First, protein aggregates cause a direct inflammatory reaction that eventually leads to neuronal cell death. Immune responses are a double-edged sword however, with beneficial or deleterious consequences depending on the specific situation. Furthermore, these cellular and molecular changes seen in patients have been observed in animal models too.
Independent of the origin of neuroinflammation, immunotherapies targeting the neuroinflammation in neurodegenerative diseases could help halt or modify the course of disease. Many immune-based therapeutic interventions are under investigation, including targeting the clearance of protein aggregates, with the inhibition of inflammation and apoptosis amongst other mechanisms of action [94].
5.2.3 Mitochondrial Dysfunction and ROS Generation
Neurons consume high amounts of energy to perform [95]; hence, they rely on mitochondria to fulfil these high metabolic demands. Mitochondria not only produce high quantities of ATP, but they also regulate calcium concentration and generate reactive oxygen species (ROS) from the respiratory chain [96].
Misfolded proteins can negatively affect mitochondria by several mechanisms including direct damage to mitochondrial DNA, trafficking impairment, or promoting mitochondria-dependent cell death pathways [97]. This mitochondrial dysfunction is well known in AD, where Aβ and tau proteins disrupt mitochondrial DNA maintenance, protein import, electron transport chain activity, and reduction-oxidation (redox) balance [97]. Similarly, α-synuclein accumulation in mitochondria reduced mitochondrial complex I activity and increased free radical production in dopaminergic neurons [98]. Mitochondria in motor neurons of ALS patients have an altered structure and appear swollen and vacuolated under histological analysis [99]. Post-mortem analysis of ALS brains showed alterations in respiratory chain complexes within mitochondria [100, 101].
Gene mutations are another factor linked to mitochondrial dysfunction. In PD, mutations in PINK1, Parkin, and DJ-1 are closely associated with mitochondrial dysfunction [102, 103]. Parkin and PINK-1 are known regulators of mitophagy, the autophagic process responsible for clearing the cell of defective mitochondria [104, 105].
If mitochondrial dysfunction in neurodegenerative diseases is a cause or a consequence, it’s still under consideration, but it seems plausible that a reciprocal toxic cycle exists. Mitochondria are the main source of ROS production, including superoxide (O2−), hydroxyl (HO), and hydrogen peroxide (H2O2) radicals, which are a by-product of oxidative phosphorylation in cellular respiration. In neurodegeneration, the implication of oxidative damage as a pathogenic factor is well known, and as a result, has often been a target of potential therapeutic treatments; however, clinical trials assessing the benefits of antioxidants in neurodegenerative diseases have been generally negative [106].
5.2.4 Protein Clearance Systems: UPS and Lysosome Dysfunction
The ubiquitin-proteasome system (UPS) is a crucial protein degradation process in cells; briefly, proteins tagged with ubiquitin are targeted for proteasome degradation. Proteasomal turnover is particularly challenging for neurons due to their distinctive morphology (long axons and complex dendritic ramifications) [107]. Lysosomes—the organelle responsible for clearance of cellular debris—become dysfunctional in the pathogenesis of neurodegenerative disease. Lysosomes may be one of the key mechanisms underlying the accumulation of aberrant proteins in neuronal cells.
Interestingly, α-synuclein is degraded via UPS and autophagy-lysosome pathways [108, 109], leading to UPS regulation and lysosomal modification as potential methods of ND therapies.
5.2.5 Microbial Metabolites
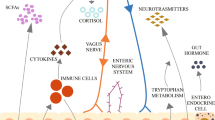
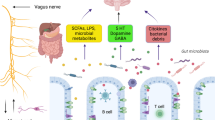
Gut microbiota alterations have been observed in neurodegenerative diseases. Short-chain fatty acids (SCFAs)—which include acetate, propionate, and butyrate—are metabolites produced by bacterial fermentation of dietary fibres in the colon and are thought to be key mediators in the gut microbiota-brain axis crosstalk. However, most of the mechanisms by which SCFAs exert these effects remain yet unknown and need to be investigated further.
Tryptophan metabolism is one of the most important signalling pathways of the gut microbiota. Tryptophan is an essential amino acid which serves as a precursor to biosynthetic compounds, such as serotonin, melatonin, and nicotinamide adenine dinucleotide (NAD+). The tryptophan-kynurenine metabolic pathway degrades tryptophan into several metabolites with inflammatory, oxidative, and neuronal modulatory properties [110]. Moreover, kynurenine enzymes further influence inflammatory processes [111]. Thus, this complex balance between neuroprotective and neurotoxic agents is crucial for the brain, and disturbances in the gut microbiota or other relevant processes such as inflammation could destabilise this equilibrium. In fact, in a systematic review, neurotoxic kynurenines were invariably increased in all major neurodegenerative diseases, while neuromodulatory kynurenines were decreased in AD, PD, and HD [110].
6 The Role of Gut Microbiota in Neurodegenerative Disorders
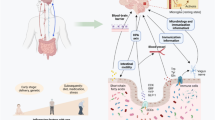
Through the microbiota-gut-brain axis, gut microbiota can modulate brain function and behaviour across the lifespan, both in health and disease [37]. However, the microbiota-gut-brain axis is bidirectional, as neurodegenerative brain dysfunction can also impact on the gut microbiota [112]. It encompasses a tentative relationship that is somewhat sensitive to neuronal cell death and generalised inflammation. Furthermore, as mentioned earlier, neurodegenerative disorders are most frequent in aged populations, and ageing directly modifies the gut microbiota. Consequently, the gut microbiota composition of patients suffering from neurodegenerative diseases differs significantly from healthy subjects. In most cases, the relationship between the gut microbiota and neurodegeneration has been reported recently, but if dysbiosis is the cause or the consequence of the pathogenesis is still being investigated.
The gut microbiota and its metabolites interact with many of the pathways leading to neurodegeneration. Thus, it comes as no surprise that many studies have linked microbial dysbiosis to the pathology of neurodegenerative diseases [113, 114]. For instance, reduced diversity of gut microbiota during ageing and neuroinflammation are two common features of gut dysbiosis and neurodegeneration. Gut microbiota are constantly regulating microglial activation [115], and this could have great implications in neurodegeneration. These findings suggest that this process could be manipulated by microbiome-targeted strategies (see Fig. 3). It has been suggested that SCFA-producing bacteria could modulate immune activation in the brain [35]. Similarly, the porousness of the BBB could also be targeted via gut microbiome, as BBB permeability depends on microbiota composition [116].
Microbiota-targeted therapeutic interventions for neurodegenerative disease. The microbiota-gut-brain axis is linked to neurodegenerative diseases and interacts with many factors involved with neurodegeneration. Thus, new therapeutic approaches targeted to the gut microbiota are emerging for neurodegenerative diseases. ALS, amyotrophic lateral sclerosis; AD, Alzheimer’s disease; PD, Parkinson’s disease; HD, Huntington’s disease; FMT: faecal microbiota transplantation
Next we will summarise the main gut microbiota alterations observed in neurodegenerative diseases (see Table 2).
6.1 PD and Microbiome
Evidence for a role of the microbiome in PD comes from both animal and human studies. Regarding mouse models, PD pathophysiology is greatly reduced in the germ-free mouse—mice lacking any gut microbes—models of induced PD, and this effect can be reversed with oral administration of bacterial metabolites or an FMT [154]. Accordingly, antibiotic treatment ameliorated, while microbial recolonisation promoted, pathophysiology in mice overexpressing α-synuclein. Recently, a preclinical study reported that a gut bacterial amyloid promoted α-synuclein aggregation and motor impairment in mice [155]. These investigations suggest that gut microbiota is required for motor deficits, microglia activation, and α-synuclein pathology, at least in mice.
Gastrointestinal symptoms such as constipation are known to appear in PD patients well before the onset of the motor symptoms [156]. These gastrointestinal comorbidities—constipation, diarrhoea, and microbial dysbiosis—are common in most neurodegenerative diseases, which implicate the gut microbiome in neurodegenerative processes. Interestingly, the full removal of the vagus nerve—a surgical procedure called vagotomy—reduced the risk of developing PD in a clinical cohort [157].
Recently, the gut microbiota of PD patients has been investigated and compared to healthy controls (HCs) in a growing number of studies. Gut microbiota in PD individuals is characterised by a decrease in taxonomic diversity and significant differences in the bacterial community. Overall, PD patients showed reduced levels of anti-inflammatory-associated butyrate-producing bacteria such as Blautia and Roseburia [136, 138], lower concentrations of SCFAs in faeces [135], and increased levels of pro-inflammatory-associated bacteria Ralstonia in the mucosa [136]. Moreover, non-significant reductions were observed for Prevotellaceae in PD patients, which may contribute to increased gut permeability in PD [136, 137].
Interestingly, gut microbiota alterations were linked with clinical characteristics [126, 128]. For instance, an increase in Enterobacteriaceae found in these patients [135, 138] positively correlated with postural instability [138] and disease severity [131]. These alterations in the microbiota of individuals with PD persisted over disease progression [125].
Together, these investigations suggest a pro-inflammatory environment in the gut of PD individuals. A bacterial metabolite, which is a marker of gut dysbiosis, was found in higher concentrations in individuals with PD [158]. On top of that, individuals suffering from PD have increased intestinal permeability that correlates with intestinal α-synuclein [159]. These findings in the gut microbiota of PD patients could be microbial biomarkers for PD used as supplemental evidence for PD diagnosis [123, 135,136,137,138].
When other facets of PD have been studied, such as the prodromal phase of PD or PD associated with mild cognitive impairment, the gut microbiota has been reported to be differently altered in comparison to individuals with PD and HCs [124], indicating that the microbiota changes alongside the progression of the disease. Thus, more studies are needed that target these phases of the disease.
6.2 AD and Microbiome
The evidence linking AD and gut dysbiosis is less pronounced than in PD. The modulation of the gut microbiome using germ-free mice, or conventional mice treated with antibiotics or probiotic administration, has shown that changes in the gut microbiota correlate with changes in host cognitive behaviours. For instance, germ-free mice exhibited impairments in spatial and working memory [160]. Temporary depletion of gut microbiota using antibiotics in rats also led to increased anxiety-like behaviours and deficits in spatial memory [161]. The administration of Lactobacillus fermentum NS9 reduced the alterations in behaviour induced by antibiotic treatment [161]. Moreover, administration of SCFAs promoted Aβ deposition in germ-free mice and exacerbated Aβ deposition in colonised mice via modulation of microglial phenotype [162]. As well, faecal transplantation of healthy microbiota reduced the formation of Aβ plaques and neurofibrillary tangles, glial reactivity, and cognitive impairment [163]. Thus, the role of gut microbiota in Aβ pathology and cognitive behaviour suggests that they could have a role in the pathogenesis of AD and therefore act as a novel avenue for therapeutic intervention.
Diet has long been considered linked with AD pathogenesis through the modulation of the immune system [164]. A Mediterranean diet is characterised by abundant plant-based foods such as fruits and vegetables, olive oil, and nuts as the main fat component, with a moderate intake of dairy products, fish, poultry, and eggs. Epidemiological investigations have shown that higher adherence to the Mediterranean diet correlated with a reduced risk of AD [165]. For example, omega-3 polyunsaturated fatty acids (ω-3 PUFAs) are essential for normal brain function [166], and abundant ω-3 PUFAs in the diet of elderly populations correlated negatively with cognitive decline [167]. Furthermore, frequent intake of fruits and vegetables, naturally high in antioxidants and vitamins, can also lower the risk of dementia and cognitive impairment [168]. Diets that are high in fats and sugars, such as the Western diet, can lead to cognitive impairment, memory decay, and increase the risk of AD [169]. High fat diets can induce changes in the gut microbiota and promote intestinal permeability, ultimately increasing inflammation, promoting disease. This suggests that gut microbiota play an important role in the increase/decrease of diet-associated AD risk. Nevertheless, the mechanisms driving the effects of gut microbiota in AD need further study.
Evidence from human studies assessing the role of the microbiome in the pathogenesis of AD is still very recent and limited. Some small case-control studies have indirectly evaluated the oral microbiome of AD individuals, as AD has long been linked to poor dental hygiene. When the gut microbiome of AD individuals was compared to healthy age- and sex-matched controls, AD individuals exhibited lower microbial diversity, decreased abundances of Firmicutes and bifidobacteria; moreover, levels of differentially abundant genera correlated with cerebrospinal fluid biomarkers of AD pathology [120].
Calprotectin is a protein biomarker used to assess intestinal inflammation. In a small study, AD individuals presented with a high calprotectin level in their faeces, indicating a disturbed intestinal barrier function in AD [170]. Another study comparing cognitively impaired individuals with and without amyloidosis to HCs found that the group with cognitive impairment and amyloidosis showed a lower abundance of Eubacterium rectale and a higher abundance of Escherichia/Shigella, which correlated with pro-inflammatory cytokines in blood, suggesting that those patients suffered from peripheral inflammation [119]. Another study found differences in abundance of Bacteroides, Actinobacteria, Ruminococcus, and Lachnospiraceae between AD patients and HCs [118].
Moreover, alterations in the GABAergic system are linked to cognitive impairment [171], and the evidence of GABA dysregulation in AD and ageing is substantial [172]. Interestingly, Lactobacillus and Bifidobacterium can produce GABA in the gut that can influence GABA in the CNS [173].
Although less direct, other evidence also supports the role of gut microbiota in the pathogenesis of AD. The hygiene hypothesis states that reduced microbial exposure due to improved sanitation and lifestyle changes in modern societies induces a malfunction of immunoregulation processes, contributing to autoimmune and inflammatory diseases. AD has many similarities with autoimmune diseases as it is an inflammatory disease with elevated Th1-mediated inflammation [23, 174]. When the relationship between the incidence of AD and environmental microbial diversity were investigated, countries with a greater degree of sanitation, and a lower extent of microbial diversity, had higher incidence of AD [175].
As summarised here, there are multiple connections that link AD and gut microbiome alterations, making microbiome-targeted interventions worth investigating further.
6.3 HD and Microbiome
Gut dysbiosis and increased intestinal permeability in HD are frequently reported together in both clinical and preclinical studies [140, 176, 177]. In a rodent model, although substantial changes in bacterial species abundance in the gut microbiota were not found in a longitudinal study of HD and wild-type mice, the HD gut microbiome was perturbed in the premotor symptom phase, suggesting the occurrence of gut dysbiosis in HD [178]. The sequencing data of another rodent study reinforces this subtle change in the gut microbiome of HD since they observed similar bacterial populations in HD and wild-type mice but differences in abundances [178]. In humans, it is still unclear whether individuals with HD present greater bacterial diversity in the gut, but alterations in bacterial abundances have been reported [139, 140]. For example, the abundance of Intestinimonas was higher in individuals with HD and correlated with HD clinical characteristics [139]; here, a correlation was established between altered gut microbiota and the occurrence of chronic inflammation [139]. Nevertheless, the alterations of the gut microbiome in HD are only recently being investigated, and a better understanding of this should become clearer in the future.
6.4 MS and Microbiome
Autoimmune diseases such as MS are characterised by a dysregulation of the immune system, and recently it was shown that the gut microbiota can modulate the immune system. For example, germ-free animals develop an attenuated experimental autoimmune encephalomyelitis (EAE) response, which is a rodent model of MS, unless they are transplanted with gut microbes from colonised mice [179], suggesting that the gut microbiota are key for disease progression. Interventional preclinical studies showed that probiotic administration can ameliorate disease severity in EAE by reducing inflammation and inhibiting Th17 cell differentiation [180, 181]. Similarly, oral administration of broad-spectrum antibiotics ameliorated EAE development in mice [182]. These data together suggest that the gut microbiome is implicated in MS pathogenic severity.
In terms of gut microbial community composition, the level of diversity between patients with MS and HCs was similar, but the relative abundances of specific bacteria differed significantly. In contrast to AD/PD, MS is not presented exclusively in an adult or elderly population, and consequently the microbiome does not necessarily have the particularities seen in an aged microbiome. Nevertheless, MS patients exhibited reduced levels of Clostridia family and Bacteroidetes, known to produce SCFAs and induce Treg cells [146], potentially facilitating autoimmune processes. Furthermore, Methanobrevibacter smithii and Akkermansia muciniphila are increased in stool samples of MS individuals, which can affect T cell differentiation [142, 145]. Moreover, excessive expansion of intestinal Th17 cells correlated with microbiota alterations and disease activity [141]. There is strong evidence surrounding the involvement of the microbiome in MS; however, is it still unclear if the microbiome is the trigger or a driver of the neuroimmune pathogenesis.
6.5 ALS and Microbiome
ALS pathology is intricately linked to alterations in glutamate, GABA, and serotonin, and some strains of our gut microbiota can modulate the production of these neurotransmitters. Moreover, in a transgenic mouse model of ALS, the disruption of the junction structure in the intestine led to increased gut permeability, abnormal Paneth cells in the intestine [183], and reduced levels of Butyrivibrio (butyrate-producing bacteria) fibrisolvens, Escherichia coli, and Firmicutes bacteria, in comparison to wild type mice [183], suggesting microbial dysbiosis.
The gut microbiota in individuals with ALS exhibits a reduction of the ratio Firmicutes/Bacteroidetes phyla [153], which has been associated with detrimental health outcomes. In particular, butyrate-producing bacteria are reduced at early stages of the disease [183, 184]. Furthermore, ALS disease was associated with reduced levels of beneficial bacteria from the genera Oscillibacter and Anaerostipes and the family Lachnospiraceae and increased levels of harmful bacteria such as genus Dorea [153]. More evidence supports these changes in the abundance of microbial species between individuals with ALS and healthy subjects. In a randomised controlled trial, ALS patients had higher abundance of Escherichia coli and enterobacteria [185]. Furthermore, gut microbiota composition in ALS changes over the course of the disease; significant fluctuations of certain microbial strains were observed in a longitudinal study [149]. Overall, recent studies support the idea that relative abundance of beneficial microorganisms is decreased in ALS [151, 183, 185, 186]. On top of that, a higher vegetable fibre intake was shown to be associated with a slower-progression ALS disease [187].
Microbiota signatures as an element in the aetiology or pathogenesis of the disease have been broadly discussed [188, 189] and have led to investigative approaches towards modulating the gut microbiota in ALS patients as a novel therapeutic. Furthermore, these human studies have not only helped characterise the gut microbiota of ALS patients during the progression of the disease, but they are also the basis for a characterisation of these microbiota changes into an ALS biomarker.
In summary, host gut microbiota of neurodegenerative disease patients is markedly different than healthy subjects and is characterised by an overgrowth of pathogenic and reduction of commensal microbial strains, leading to altered production of beneficial metabolites such as SCFAs, which eventually increases the pathogenic milieu, setting up a vicious cycle. The development of gut microbiota-targeted interventions could help disrupt this endless loop of pro-inflammation and ameliorate at least some of the pathophysiological events, benefiting the patient.
7 Microbiota-Targeted Therapeutic Interventions for Neurodegenerative Diseases
In the search for therapeutic interventions for neurodegenerative disease, much effort has gone into trying to halt or reduce the aggregation of the aberrant protein involved, as well as directly targeting the pathways that lead to neurodegeneration, such as neuroinflammation. Microbiome modulation is an innovative approach that could address those targets indirectly and provide novel microbiome-targeted interventions for these diseases. Directly targeting neuroinflammation through the gut microbiota is one of the most common objectives of these therapeutic investigations. Evidence highlighting the role of gut microbiome in neurodegeneration has uncovered new insights in potential microbiome-based therapeutic approaches, interventions not only targeted to the direct modulation of the gut microbiota but also to their metabolites such as SCFAs.
Therapies include the use of antibiotics, probiotics, prebiotics, and FMT. Below we summarise some of the investigations carried out to date, regarding the potential of the gastrointestinal microbiota and metabolites, to ameliorate some facets of neurodegenerative diseases.
7.1 Microbial Metabolite-Based Interventions
SCFAs are neuroactive biomolecules and as such have a potential interest as a therapeutic agent for neurodegenerative disease. Although the specific signalling around neuroprotective and anti-inflammatory effects of SCFAs are not completely understood, it has thus far been attributed, at least in part, to their histone deacetylase (HDAC) inhibitory action. First, SCFAs are well known to have anti-inflammatory effects [190] and to be involved in the modulation of microglial function [115]. For example, butyrate can decrease microglial activation and pro-inflammatory cytokines in vivo [191, 192]. Second, SCFAs are able to modulate neurotransmitter synthesis and expression. For example, butyrate and propionate can control catecholamine synthesis by regulating tyrosine hydroxylase gene expression [193]. This is very interesting for PD research in particular, as tyrosine hydroxylase is an enzyme involved in dopamine synthesis. Moreover, SCFAs can modulate the concentrations of other neurotransmitters such as glutamate, glutamine, and GABA [194].
These promising neuroprotective and anti-inflammatory properties make SCFAs a good candidate for a potential therapeutic agent in neurodegenerative diseases; for example, HDAC dysregulation has been implicated in memory impairment, and levels of SCFAs are reduced in preclinical models of AD [195]. In a rodent model of AD, sodium butyrate was able to improve associative memory and increase expression of genes associated with learning even at advanced stages of pathology [196]. Butyrate has also shown promising beneficial effects in improving cognitive and motor impairments while reducing dopamine neurodegeneration in several animal models [197, 198]. If we look at MS, oral administration of SCFAs ameliorated the disease severity in an EAE model [199], and butyrate in particular was able to suppress demyelination and enhance remyelination [200].
SCFAs can also exert anti-inflammatory effects via astrocytes, as SCFAs downregulate the astrocytic production of IL-1β and TNF-α [201]. Further, SCFAs can also contribute to reduce inflammation by inhibition of NF-kB on peripheral blood mononuclear cells, which further reduces the production of pro-inflammatory cytokines [202].
These results provide strong evidence that SCFAs can regulate several CNS processes related to neurodegeneration as well as modulate cognitive and motor behaviours, especially when administered in advanced stages of neurodegeneration. However, caution has to be taken when extrapolating these results to humans as these were mainly observed in animal models.
Ferulic acid (FA) is a gut-derived compound found in fruits and vegetables that can also be synthetised by gut bacteria. FA can prevent Aβ toxicity by inhibiting Aβ aggregation both in vitro and in vivo [203]. Two long-term studies assessed the benefits of oral administration of FA in transgenic mouse models of amyloidosis and found that FA reversed spatial memory deficits, reduced Aβ aggregates in the brain, attenuated neuroinflammation, and stabilised oxidative stress [203, 204].
Dysregulation of the kynurenine pathway is associated with neurodegenerative and other neurological disorders. Targeted interventions with metabolites from the kynurenine pathway could potentially be used to modulate brain physiology and normalise imbalances in this pathway in pathological conditions. For example, indoleamine 2,3-dioxygenase (IDO) inhibitors are being investigated for their protective role against oxidative damage [205]. Since gut microbiota are a key regulator of the kynurenine pathway, probiotic products may be potentially beneficial in regulating kynurenine/tryptophan dynamics [205].
Recently, researchers manipulated disease severity in a rodent model of ALS via supplementation with gut microbial strains; where Ruminococcus torques and Parabacteroides distasonis increased severity of the disease, Akkermansia muciniphila ameliorated it [206]. Interestingly, this reduction of the pathogenesis by Akkermansia muciniphila was attributed to increasing levels of nicotinamide, together with changes in mitochondrial function and oxidative stress pathways. Moreover, nicotinamide was associated with functional improvements in ALS patients [207]. Also the therapeutic potential of hydrogen sulphide and molecular hydrogen was tested in mice; it was shown that hydrogen-rich saline administration could preserve mitochondrial function and reduce ROS production [208].
7.2 Probiotic-Based Interventions
Probiotic interventions are being screened in several contexts including neuroprotection, neurodegeneration, and inflammation. These studies inform us of the potential of these probiotic products (see Table 3). For example, a combined administration of Lactobacillus helveticus and Bifidobacterium longum in a myocardial infarction model reduced pro-apoptotic pathways (caspase-3 and Bax/Bcl-2) and increased anti-apoptotic pathways (Akt phosphorylation), suggesting a role in neuroprotection [221]. Clostridium butyricum was also reported to have neuroprotective effects in a vascular dementia model in rats by increasing brain-derived neurotrophic factor (BDNF), Bcl-2, and Akt phosphorylation [222].
Moreover, probiotic administration can modulate long-term memory. In a rodent AD model, administration of Lactobacillus and Bifidobacterium strains improved memory and learning outcomes and reduced oxidative stress in the hippocampus [223]. In a similar study, along with behavioural recovery and a reduction of Aβ plaques, a probiotic mix formulated of lactic acid bacteria and bifidobacteria was able to partially restore proteasome and autophagy functionality [224]. In a recent study using APP/PS1 transgenic mice, exercise training and probiotic administration reduced Aβ plaques in the hippocampus and improved cognitive performance in a spatial learning task [225]. Such investigations demonstrate that disease progression could potentially be ameliorated by microbiota-targeted approaches.
For instance, probiotics could reduce duration of the clinical symptoms or reduce their severity, while also reducing levels of pro-inflammatory cytokines in the EAE rodent model of MS [226]. Similarly, a combination of Lactobacillus strains produced these same effects by inhibiting pro-inflammatory activation of Th17 cells, while enhancing IL-10+ producing regulatory T cells [227].
Hence, preclinical investigations have shown the potential use of probiotics as a therapeutic strategy against neurodegeneration. However, few clinical investigations have been carried out to date investigating the potential benefits of probiotic products in neurodegenerative diseases (see Table 3). Overall, these clinical trials have shown that probiotic products can modulate gut microbiota composition, ameliorate comorbidities such as constipation, and even improve cognitive and motor deficits.
However, there are few clinical trials available to date, with low numbers of patients, and they only assessed the effects of short-term use of probiotics. Moreover, the results of these clinical trials are based on limited analysis of the microbiome and the cognitive and motor functions. Nevertheless, they are a first step into the evaluation of probiotics as a potential therapeutic avenue for neurodegenerative diseases and their comorbidities.
7.3 Antibiotic-Based Interventions
Antibiotic administration is another effective means of gut microbiome modulation. In vitro, many antibiotics can prevent or reduce protein aggregation in the context of neurodegenerative disease. Further, antibiotics such as ceftriaxone—which is a β-lactam antibiotic—have been shown to have neuroprotective and anti-inflammatory effects in many neurodegenerative diseases. For instance, in an AD transgenic mouse model, ceftriaxone reduced the increased levels of glutamate present in the vicinity of Aβ plaques and restored neuronal activity via glutamate transporter 1 [228]. There are several mechanisms by which ceftriaxone could act, including upregulation of glutamate transporter 1 expression, as well as the amelioration of oxidative stress and neuroinflammation [229]. Preventive and therapeutic treatment of ceftriaxone in an EAE mouse model indirectly hampered T cell proliferation and pro-inflammatory cytokine secretion [230]. Thus, antibiotic treatment can attenuate disease course and severity in a rodent model of MS.
Rifampicin inhibited the aggregation and fibril formation of synthetic Aβ peptides [231]. Similarly, doxycycline induced remodelling of α-synuclein oligomers into non-toxic species in vitro [232] and prevented Aβ fibrillisation both in vitro and in vivo [233]. Moreover, a combination of long-term broad-spectrum antibiotics decreased Aβ plaque deposition in a rodent model [234]. However, when doxycycline and rifampicin (alone or in combination) were tested in clinical trials, they had no beneficial effects on cognition in AD patients [235]. However, eradication of Helicobacter pylori resulted in improvement of cognition outcomes in AD patients [236] and in motor improvements in PD patients [237]. These results indicate that antibiotic administration could be an interesting therapeutic option for particular cases dealing with detrimental bacteria, instead of using of antibiotics as a universal therapy for all neurodegenerative diseases.
AD might be the best candidate to test antibacterial drugs on since it has been postulated that it could have an infectious aetiology. Thus, testing antibiotics in clinical trials could shed some light onto this issue and verify if antibacterial therapy could be beneficial for a subset of (or all) AD patients [238].
7.4 Faecal Microbiota Transplantation
FMT can reconstruct the healthy gut microenvironment and alleviate clinical symptoms of many metabolic, autoimmune, and neuropsychiatric diseases. Recently, FMT has been postulated as a potential therapeutic intervention to restore the microbiome in neurodegenerative disease. Despite limited information about its long-term benefits and risks, some case reports have confirmed the efficacy of FMT for use in the treatment of neurological disorders [239]. In MS, two case reports have shown amelioration or stabilisation of MS symptoms several years after the transplant [240, 241]. In PD, one report stated that a PD patient observed improvements in constipation until the end of the follow-up 3 months after FMT, but no long-term motor improvements [242]. In a more recent study, 15 PD patients were exposed to a colonic or nasointestinal FMT and concluded that although both procedures were safe, colonic FMT achieved significant improvement and longer maintenance of efficacy than nasointestinal FMT [243]. In this study, two patients reported self-satisfying outcomes that lasted for more than 2 years [243]. In AD, there is only one case reported of a patient with rapid reversal of AD symptoms following FMT for recurrent Clostridioides difficile infection [244].
Currently, there are two randomised double-blind clinical trials assessing the safety and efficacy of FMT for PD patients with or without constipation (NCT04854291, NCT03808389) and other minor clinical pilot studies with the same aim (NCT03876327, NCT04837313). In parallel, other FMT clinical trials are ongoing at the moment, evaluating the safety, feasibility, and efficacy of FMT in AD patients (NCT03998423) and in ALS patients (NCT03766321). We will have to wait for their findings. However, a clinical trial of FMT for MS (NCT03183869) was finalised recently, reporting that FMT did not have any serious adverse effects, but no measures of efficacy have been reported yet.
The benefits of FMT as a therapeutic intervention in neurodegenerative disease are mostly based on animal studies and only a few case reports. Despite promising results, large-scale clinical trials are needed to evaluate the efficacy of this treatment option. Numerous trials of FMT in neurodegenerative diseases are currently ongoing, and it is expected that evidence on the efficacy of FMT will increase in the near future. Furthermore, these ongoing clinical trials will improve the logistics of FMT that still need to be refined, such as best donor selection or mode of delivery of the microbiota.
Even if microbiota-targeted interventions prove not to be successful in the goal of stopping or ameliorating the progression of neurodegenerative diseases, they could still be very beneficial in treating gastrointestinal comorbidities.
7.5 Microbiota Modulation Through Diet
Diet has a major impact on gut microbiota. Hence, it has been postulated that diet could be a beneficial avenue for treatment of neurodegenerative diseases, as they are usually characterised by a prevalent and strong microbiota dysbiosis. For example, antioxidants can directly act on gut microbiota to reduce pathogenic bacteria and increase beneficial bacteria [245]. Consequently, these beneficial bacteria can produce beneficial metabolites for brain health, conferring neuroprotection.
Many dietary compounds such as PUFAs, vitamins B and D, or resveratrol have been found to be beneficial with anti-inflammatory properties [246]. In ALS, many preclinical investigations have shown that polyphenols such as resveratrol or curcumin could improve the prognosis of the disease [247]. Some compounds such as vitamin C have largely been investigated in preclinical studies as a treatment option for neurodegenerative disease, but clinical data in humans are limited [248].
However, a growing body of evidence points to the combination of these compounds as a more efficient way to fight neurodegeneration. Clinical and preclinical data assessing dietary interventions for neurodegenerative disease is extensive and has mostly been studied in AD. For example, the ketogenic (high-fat and low-carbohydrate) diet forces the brain to use fatty acids as the main source of energy and alter energy metabolism mechanisms. These metabolic changes reduce the usage of impaired glucose metabolism in neurodegenerative pathologies and neuroinflammation, while improving mitochondrial function, thus conferring neuroprotection to ageing brain cells [249]. In addition, this diet could help to reduce the accumulation of amyloid plaques. Two clinical trials assessed the effects of triglyceride administration on AD patients, resulting in improved cognitive outcomes [250, 251].
Further, adherence to a Mediterranean-styled diet could be a potential preventive therapy as it confers a reduced risk of developing AD and cognitive impairment [252, 253]. Moreover, it has been hypothesised that the Mediterranean diet—abundant in antioxidants, vitamins, flavonoids, polyphenols, and probiotics—could attenuate neuroinflammation via the gut microbiome. The Mediterranean dietary approach to systolic hypertension (DASH) diet intervention for neurodegenerative delay (MIND) diet (that combines Mediterranean and DASH diets) is specific for dementia prevention and can slow cognitive decline [254]. Although clinical trials have shown interesting results, there is a paucity of data surrounding the long-term benefits of these interventions in patients with neurodegenerative disease.
8 Conclusions
Neurodegenerative diseases are a heterogeneous group of disorders where neurons degenerate and ultimately die. These diseases have an unknown cause and include many complex pathological processes that have frustrated the development of a remedy or cure to stop neurodegeneration. However, the scientific knowledge gathered has greatly expanded our general understanding and treatment of neurodegenerative disease. The expert view has shifted from being neuron centric to a more global disease where even entities such as the gut microbiota are now considered.
Gut microbiota have been shown to be implicated in the pathogenesis of neurodegenerative disease, although to what extent remains to be elucidated. It is quite likely that single bacterial perturbations will not be adequate, and perhaps there will not even be a disease-specific bacterial signature but rather an overall alteration of the microbial gut environment. Nevertheless, a new era of potential microbiota-targeted interventions has emerged.
Despite numerous failures in developing therapeutic interventions that can effectively modify the course of the disease, researchers have now new molecular tools to investigate the underlying pathogenic mechanisms involved and assess the efficacy of new compounds or therapeutic interventions. Currently, the evidence supporting a beneficial impact on neurodegeneration due to microbiome modification is limited.
It will be interesting to observe if psychobiotics are assessed in neurodegenerative diseases in the future. Psychobiotics are live organisms that can produce health benefits in patients suffering from psychiatric illnesses through the microbiota-gut-brain axis [255].
The evidence that gut microbiota may be involved in the onset or progression of many neurodegenerative diseases is increasing rapidly, but causality has not been proven. However, gut microbiota could be used as a clinical biomarker for the diagnosis of many neurodegenerative diseases. Furthermore, there is an opportunity to establish potential microbiome-targeted therapies to treat particular aspects of neurodegenerative disease, such as common comorbidities, resulting in improvements of host health.
Nevertheless, adequately powered longitudinal studies are needed to investigate the complex relationship between neurodegenerative disease and the microbiome and should be studied at the onset, the initial progression, and the establishment of the neurodegenerative processes. Of high importance would be the appropriate selection of patients and adequate management of confounding variables. Many factors that were overlooked in traditional neurological studies could have confounding effects in the microbiome field. This could present opportunities for interdisciplinary approaches for the treatment of neurodegenerative disease.
Notes
- 1.
Autosomal dominant inheritance pattern refers to how a mutation is inherited. In autosomal dominant inheritance, the mutation gene is located in a non-sex chromosome, and only one copy of the mutated gene is needed to be affected.
- 2.
- 3.
Dysbiosis is an ambiguous term frequently used to describe disruptions of the gut microbial populations, and it is commonly associated to disease [34].
- 4.
The core microbiota refers to the taxa that are present in the vast majority of the subjects [41].
- 5.
Regulatory processes that involve synthesis or degradation of proteins to maintain cell health.
References
Moller HJ, Graeber MB. The case described by Alois Alzheimer in 1911. Historical and conceptual perspectives based on the clinical record and neurohistological sections. Eur Arch Psychiatry Clin Neurosci. 1998;248(3):111–22.
Parkinson J. An essay on the shaking palsy. 1817. J Neuropsychiatr Clin Neurosci. 2002;14(2):223–36; discussion 2.
Piaceri I, Nacmias B, Sorbi S. Genetics of familial and sporadic Alzheimer’s disease. Front Biosci (Elite Ed). 2013;5:167–77.
Rajan KB, Weuve J, Barnes LL, Wilson RS, Evans DA. Prevalence and incidence of clinically diagnosed Alzheimer’s disease dementia from 1994 to 2012 in a population study. Alzheimers Dement. 2019;15(1):1–7.
Balestrino R, Schapira AHV. Parkinson disease. Eur J Neurol. 2020;27(1):27–42.
von Campenhausen S, Bornschein B, Wick R, Botzel K, Sampaio C, Poewe W, et al. Prevalence and incidence of Parkinson’s disease in Europe. Eur Neuropsychopharmacol. 2005;15(4):473–90.
Ramos-Arroyo MA, Moreno S, Valiente A. Incidence and mutation rates of Huntington’s disease in Spain: experience of 9 years of direct genetic testing. J Neurol Neurosurg Psychiatry. 2005;76(3):337–42.
McColgan P, Tabrizi SJ. Huntington’s disease: a clinical review. Eur J Neurol. 2018;25(1):24–34.
Cozzolino M, Carri MT. Mitochondrial dysfunction in ALS. Prog Neurobiol. 2012;97(2):54–66.
Kingwell E, Marriott JJ, Jette N, Pringsheim T, Makhani N, Morrow SA, et al. Incidence and prevalence of multiple sclerosis in Europe: a systematic review. BMC Neurol. 2013;13:128.
Westerlind H, Ramanujam R, Uvehag D, Kuja-Halkola R, Boman M, Bottai M, et al. Modest familial risks for multiple sclerosis: a registry-based study of the population of Sweden. Brain. 2014;137(Pt 3):770–8.
Schattling B, Engler JB, Volkmann C, Rothammer N, Woo MS, Petersen M, et al. Bassoon proteinopathy drives neurodegeneration in multiple sclerosis. Nat Neurosci. 2019;22(6):887–96.
Browne P, Chandraratna D, Angood C, Tremlett H, Baker C, Taylor BV, et al. Atlas of multiple sclerosis 2013: a growing global problem with widespread inequity. Neurology. 2014;83(11):1022–4.
Armstrong RA. On the ‘classification’ of neurodegenerative disorders: discrete entities, overlap or continuum? Folia Neuropathol. 2012;50(3):201–8.
Kovacs GG. Concepts and classification of neurodegenerative diseases. Handb Clin Neurol. 2017;145:301–7.
El Aidy S, Dinan TG, Cryan JF. Gut microbiota: the conductor in the orchestra of immune-neuroendocrine communication. Clin Ther. 2015;37(5):954–67.
Nichols E, Szoeke CEI, Vollset SE, Abbasi N, Abd-Allah F, Abdela J, et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(1):88–106.
2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021;17:327.
Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7(3):137–52.
Collaborators GBDPsD. Global, regional, and national burden of Parkinson’s disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):939–53.
Goedert M, Spillantini MG, Del Tredici K, Braak H. 100 years of Lewy pathology. Nat Rev Neurol. 2013;9(1):13–24.
Burre J, Sharma M, Sudhof TC. Cell biology and pathophysiology of alpha-synuclein. Cold Spring Harb Perspect Med. 2018;8(3):a024091.
Dionisio-Santos DA, Olschowka JA, O’Banion MK. Exploiting microglial and peripheral immune cell crosstalk to treat Alzheimer’s disease. J Neuroinflammation. 2019;16(1):74.
Federico A, Cardaioli E, Da Pozzo P, Formichi P, Gallus GN, Radi E. Mitochondria, oxidative stress and neurodegeneration. J Neurol Sci. 2012;322(1–2):254–62.
Lazo-Gomez R, Tapia R. Motor alterations induced by chronic 4-aminopyridine infusion in the spinal cord in vivo: role of glutamate and GABA receptors. Front Neurosci. 2016;10:200.
Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377(9769):942–55.
Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359(9313):1221–31.
Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV, Knight R. Current understanding of the human microbiome. Nat Med. 2018;24(4):392–400.
Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:k2179.
Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449(7164):804–10.
Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65.
Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90(3):859–904.
Magne F, Gotteland M, Gauthier L, Zazueta A, Pesoa S, Navarrete P, et al. The firmicutes/bacteroidetes ratio: a relevant marker of gut dysbiosis in obese patients? Nutrients. 2020;12(5):1474.
Walker WA. Dysbiosis. In: The microbiota in gastrointestinal pathophysiology. London: Academic Press; 2017. p. 227–32.
Dinan TG, Cryan JF. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol. 2017;595(2):489–503.
Konturek PC, Haziri D, Brzozowski T, Hess T, Heyman S, Kwiecien S, et al. Emerging role of fecal microbiota therapy in the treatment of gastrointestinal and extra-gastrointestinal diseases. J Physiol Pharmacol. 2015;66(4):483–91.
Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877–2013.
Kim M, Benayoun BA. The microbiome: an emerging key player in aging and longevity. Transl Med Aging. 2020;4:103–16.
Askarova S, Umbayev B, Masoud AR, Kaiyrlykyzy A, Safarova Y, Tsoy A, et al. The links between the gut microbiome, aging, modern lifestyle and Alzheimer’s disease. Front Cell Infect Microbiol. 2020;10:104.
Bosco N, Noti M. The aging gut microbiome and its impact on host immunity. Genes Immun. 2021;22:289.
Jeffery IB, Lynch DB, O’Toole PW. Composition and temporal stability of the gut microbiota in older persons. ISME J. 2016;10(1):170–82.
Ragonnaud E, Biragyn A. Gut microbiota as the key controllers of “healthy” aging of elderly people. Immun Ageing. 2021;18(1):2.
Kim S, Jazwinski SM. The gut microbiota and healthy aging: a mini-review. Gerontology. 2018;64(6):513–20.
Xu C, Zhu H, Qiu P. Aging progression of human gut microbiota. BMC Microbiol. 2019;19(1):236.
Claesson MJ, Cusack S, O’Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4586–91.
Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(7410):178–84.
Mariat D, Firmesse O, Levenez F, Guimaraes V, Sokol H, Dore J, et al. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9:123.
Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, et al. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One. 2010;5(5):e10667.
Rahayu ES, Utami T, Mariyatun M, Hasan PN, Kamil RZ, Setyawan RH, et al. Gut microbiota profile in healthy Indonesians. World J Gastroenterol. 2019;25(12):1478–91.
O’Toole PW, Jeffery IB. Gut microbiota and aging. Science. 2015;350(6265):1214–5.
Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28(2):203–9.
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211.
Fulling C, Dinan TG, Cryan JF. Gut microbe to brain signaling: what happens in vagus. Neuron. 2019;101(6):998–1002.
Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J Exp Med. 2012;209(5):975–86.
Holmqvist S, Chutna O, Bousset L, Aldrin-Kirk P, Li W, Bjorklund T, et al. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128(6):805–20.
O’Donovan SM, Crowley EK, Brown JR, O’Sullivan O, O’Leary OF, Timmons S, et al. Nigral overexpression of alpha-synuclein in a rat Parkinson’s disease model indicates alterations in the enteric nervous system and the gut microbiome. Neurogastroenterol Motil. 2020;32(1):e13726.
Challis C, Hori A, Sampson TR, Yoo BB, Challis RC, Hamilton AM, et al. Gut-seeded alpha-synuclein fibrils promote gut dysfunction and brain pathology specifically in aged mice. Nat Neurosci. 2020;23(3):327–36.
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–217.
Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, et al. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15(10):565–81.
Wyss-Coray T. Ageing, neurodegeneration and brain rejuvenation. Nature. 2016;539(7628):180–6.
Wu L, Rosa-Neto P, Hsiung GY, Sadovnick AD, Masellis M, Black SE, et al. Early-onset familial Alzheimer’s disease (EOFAD). Can J Neurol Sci. 2012;39(4):436–45.
Camargos ST, Dornas LO, Momeni P, Lees A, Hardy J, Singleton A, et al. Familial Parkinsonism and early onset Parkinson’s disease in a Brazilian movement disorders clinic: phenotypic characterization and frequency of SNCA, PRKN, PINK1, and LRRK2 mutations. Mov Disord. 2009;24(5):662–6.
Day JO, Mullin S. The genetics of Parkinson’s disease and implications for clinical practice. Genes (Basel). 2021;12(7):1006.
Santibanez M, Bolumar F, Garcia AM. Occupational risk factors in Alzheimer’s disease: a review assessing the quality of published epidemiological studies. Occup Environ Med. 2007;64(11):723–32.
Mutter J, Naumann J, Sadaghiani C, Schneider R, Walach H. Alzheimer disease: mercury as pathogenetic factor and apolipoprotein E as a moderator. Neuro Endocrinol Lett. 2004;25(5):331–9.
Dick FD, De Palma G, Ahmadi A, Scott NW, Prescott GJ, Bennett J, et al. Environmental risk factors for Parkinson’s disease and parkinsonism: the Geoparkinson study. Occup Environ Med. 2007;64(10):666–72.
Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, et al. Rotenone, paraquat, and Parkinson’s disease. Environ Health Perspect. 2011;119(6):866–72.
Bozzoni V, Pansarasa O, Diamanti L, Nosari G, Cereda C, Ceroni M. Amyotrophic lateral sclerosis and environmental factors. Funct Neurol. 2016;31(1):7–19.
Chen P, Miah MR, Aschner M. Metals and neurodegeneration. F1000Res. 2016;5:F1000 Faculty Rev-366.
Killin LO, Starr JM, Shiue IJ, Russ TC. Environmental risk factors for dementia: a systematic review. BMC Geriatr. 2016;16(1):175.
Van Den Heuvel C, Thornton E, Vink R. Traumatic brain injury and Alzheimer’s disease: a review. Prog Brain Res. 2007;161:303–16.
Fang F, Chen H, Feldman AL, Kamel F, Ye W, Wirdefeldt K. Head injury and Parkinson’s disease: a population-based study. Mov Disord. 2012;27(13):1632–5.
Liu R, Guo X, Park Y, Huang X, Sinha R, Freedman ND, et al. Caffeine intake, smoking, and risk of Parkinson disease in men and women. Am J Epidemiol. 2012;175(11):1200–7.
Xu Q, Park Y, Huang X, Hollenbeck A, Blair A, Schatzkin A, et al. Physical activities and future risk of Parkinson disease. Neurology. 2010;75(4):341–8.
Hill E, Goodwill AM, Gorelik A, Szoeke C. Diet and biomarkers of Alzheimer’s disease: a systematic review and meta-analysis. Neurobiol Aging. 2019;76:45–52.
Olsson T, Barcellos LF, Alfredsson L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat Rev Neurol. 2017;13(1):25–36.
Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci. 2003;4(1):49–60.
Sweeney P, Park H, Baumann M, Dunlop J, Frydman J, Kopito R, et al. Protein misfolding in neurodegenerative diseases: implications and strategies. Transl Neurodegener. 2017;6:6.
Hartl FU. Protein misfolding diseases. Annu Rev Biochem. 2017;86:21–6.
Spires-Jones TL, Attems J, Thal DR. Interactions of pathological proteins in neurodegenerative diseases. Acta Neuropathol. 2017;134(2):187–205.
von Bartheld CS, Bahney J, Herculano-Houzel S. The search for true numbers of neurons and glial cells in the human brain: a review of 150 years of cell counting. J Comp Neurol. 2016;524(18):3865–95.
Chen WW, Zhang X, Huang WJ. Role of neuroinflammation in neurodegenerative diseases (Review). Mol Med Rep. 2016;13(4):3391–6.
Schain M, Kreisl WC. Neuroinflammation in neurodegenerative disorders-a review. Curr Neurol Neurosci Rep. 2017;17(3):25.
McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38(8):1285–91.
Damier P, Hirsch EC, Zhang P, Agid Y, Javoy-Agid F. Glutathione peroxidase, glial cells and Parkinson’s disease. Neuroscience. 1993;52(1):1–6.
Vanzani MC, Iacono RF, Caccuri RL, Berria MI. Immunochemical and morphometric features of astrocyte reactivity vs. plaque location in Alzheimer’s disease. Medicina (B Aires). 2005;65(3):213–8.
Bas J, Calopa M, Mestre M, Mollevi DG, Cutillas B, Ambrosio S, et al. Lymphocyte populations in Parkinson’s disease and in rat models of parkinsonism. J Neuroimmunol. 2001;113(1):146–52.
Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119(1):182–92.
Garbuzova-Davis S, Hernandez-Ontiveros DG, Rodrigues MC, Haller E, Frisina-Deyo A, Mirtyl S, et al. Impaired blood-brain/spinal cord barrier in ALS patients. Brain Res. 2012;1469:114–28.
Desai BS, Monahan AJ, Carvey PM, Hendey B. Blood-brain barrier pathology in Alzheimer’s and Parkinson’s disease: implications for drug therapy. Cell Transplant. 2007;16(3):285–99.
Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 2009;8(4):382–97.
Wolfe CM, Fitz NF, Nam KN, Lefterov I, Koldamova R. The role of APOE and TREM2 in Alzheimer’s disease-current understanding and perspectives. Int J Mol Sci. 2018;20(1):81.
Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247–69.
Stephenson J, Nutma E, van der Valk P, Amor S. Inflammation in CNS neurodegenerative diseases. Immunology. 2018;154(2):204–19.
Silver I, Erecinska M. Oxygen and ion concentrations in normoxic and hypoxic brain cells. Adv Exp Med Biol. 1998;454:7–16.
Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12(5):913–22.
Briston T, Hicks AR. Mitochondrial dysfunction and neurodegenerative proteinopathies: mechanisms and prospects for therapeutic intervention. Biochem Soc Trans. 2018;46(4):829–42.
Chinta SJ, Mallajosyula JK, Rane A, Andersen JK. Mitochondrial alpha-synuclein accumulation impairs complex I function in dopaminergic neurons and results in increased mitophagy in vivo. Neurosci Lett. 2010;486(3):235–9.
Afifi AK, Aleu FP, Goodgold J, MacKay B. Ultrastructure of atrophic muscle in amyotrophic lateral sclerosis. Neurology. 1966;16(5):475–81.
Bowling AC, Schulz JB, Brown RH Jr, Beal MF. Superoxide dismutase activity, oxidative damage, and mitochondrial energy metabolism in familial and sporadic amyotrophic lateral sclerosis. J Neurochem. 1993;61(6):2322–5.
Borthwick GM, Johnson MA, Ince PG, Shaw PJ, Turnbull DM. Mitochondrial enzyme activity in amyotrophic lateral sclerosis: implications for the role of mitochondria in neuronal cell death. Ann Neurol. 1999;46(5):787–90.
Moore DJ, Zhang L, Troncoso J, Lee MK, Hattori N, Mizuno Y, et al. Association of DJ-1 and parkin mediated by pathogenic DJ-1 mutations and oxidative stress. Hum Mol Genet. 2005;14(1):71–84.
Jones N. PINK1 targets dysfunctional mitochondria for autophagy in Parkinson disease. Nat Rev Neurol. 2010;6(4):181.
Lazarou M, Jin SM, Kane LA, Youle RJ. Role of PINK1 binding to the TOM complex and alternate intracellular membranes in recruitment and activation of the E3 ligase Parkin. Dev Cell. 2012;22(2):320–33.
Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183(5):795–803.
Kamat CD, Gadal S, Mhatre M, Williamson KS, Pye QN, Hensley K. Antioxidants in central nervous system diseases: preclinical promise and translational challenges. J Alzheimers Dis. 2008;15(3):473–93.
Tai HC, Schuman EM. Ubiquitin, the proteasome and protein degradation in neuronal function and dysfunction. Nat Rev Neurosci. 2008;9(11):826–38.
Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305(5688):1292–5.
Bennett MC, Bishop JF, Leng Y, Chock PB, Chase TN, Mouradian MM. Degradation of alpha-synuclein by proteasome. J Biol Chem. 1999;274(48):33855–8.
Tanaka M, Toldi J, Vecsei L. Exploring the etiological links behind neurodegenerative diseases: inflammatory cytokines and bioactive kynurenines. Int J Mol Sci. 2020;21(7):2431.
Mandi Y, Vecsei L. The kynurenine system and immunoregulation. J Neural Transm (Vienna). 2012;119(2):197–209.
Tilocca B, Pieroni L, Soggiu A, Britti D, Bonizzi L, Roncada P, et al. Gut-brain axis and neurodegeneration: state-of-the-art of meta-omics sciences for microbiota characterization. Int J Mol Sci. 2020;21(11):4045.
Friedland RP. Mechanisms of molecular mimicry involving the microbiota in neurodegeneration. J Alzheimers Dis. 2015;45(2):349–62.
Mulak A, Bonaz B. Brain-gut-microbiota axis in Parkinson’s disease. World J Gastroenterol. 2015;21(37):10609–20.
Erny D, Hrabe de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18(7):965–77.
Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Toth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6(263):263ra158.
Haran JP, Bhattarai SK, Foley SE, Dutta P, Ward DV, Bucci V, et al. Alzheimer’s disease microbiome is associated with dysregulation of the anti-inflammatory P-glycoprotein pathway. MBio. 2019;10(3):e00632.
Zhuang ZQ, Shen LL, Li WW, Fu X, Zeng F, Gui L, et al. Gut microbiota is altered in patients with Alzheimer’s disease. J Alzheimers Dis. 2018;63(4):1337–46.
Cattaneo A, Cattane N, Galluzzi S, Provasi S, Lopizzo N, Festari C, et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging. 2017;49:60–8.
Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC, et al. Gut microbiome alterations in Alzheimer’s disease. Sci Rep. 2017;7(1):13537.
Cirstea MS, Sundvick K, Golz E, Yu AC, Boutin RCT, Kliger D, et al. The gut mycobiome in Parkinson’s disease. J Parkinsons Dis. 2021;11(1):153–8.
Cirstea MS, Yu AC, Golz E, Sundvick K, Kliger D, Radisavljevic N, et al. Microbiota composition and metabolism are associated with gut function in Parkinson’s disease. Mov Disord. 2020;35(7):1208–17.
Vidal-Martinez G, Chin B, Camarillo C, Herrera GV, Yang B, Sarosiek I, et al. A pilot microbiota study in Parkinson’s disease patients versus control subjects, and effects of FTY720 and FTY720-mitoxy therapies in Parkinsonian and multiple system atrophy mouse models. J Parkinsons Dis. 2020;10(1):185–92.
Ren T, Gao Y, Qiu Y, Jiang S, Zhang Q, Zhang J, et al. Gut microbiota altered in mild cognitive impairment compared with normal cognition in sporadic Parkinson’s disease. Front Neurol. 2020;11:137.
Aho VTE, Pereira PAB, Voutilainen S, Paulin L, Pekkonen E, Auvinen P, et al. Gut microbiota in Parkinson’s disease: temporal stability and relations to disease progression. EBioMedicine. 2019;44:691–707.
Lin A, Zheng W, He Y, Tang W, Wei X, He R, et al. Gut microbiota in patients with Parkinson’s disease in southern China. Parkinsonism Relat Disord. 2018;53:82–8.
Li F, Wang P, Chen Z, Sui X, Xie X, Zhang J. Alteration of the fecal microbiota in North-Eastern Han Chinese population with sporadic Parkinson’s disease. Neurosci Lett. 2019;707:134297.
Qian Y, Yang X, Xu S, Wu C, Song Y, Qin N, et al. Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav Immun. 2018;70:194–202.
Heintz-Buschart A, Pandey U, Wicke T, Sixel-Doring F, Janzen A, Sittig-Wiegand E, et al. The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov Disord. 2018;33(1):88–98.
Hopfner F, Kunstner A, Muller SH, Kunzel S, Zeuner KE, Margraf NG, et al. Gut microbiota in Parkinson disease in a northern German cohort. Brain Res. 2017;1667:41–5.
Li W, Wu X, Hu X, Wang T, Liang S, Duan Y, et al. Structural changes of gut microbiota in Parkinson’s disease and its correlation with clinical features. Sci China Life Sci. 2017;60(11):1223–33.
Petrov VA, Saltykova IV, Zhukova IA, Alifirova VM, Zhukova NG, Dorofeeva YB, et al. Analysis of gut microbiota in patients with Parkinson’s disease. Bull Exp Biol Med. 2017;162(6):734–7.
Hill-Burns EM, Debelius JW, Morton JT, Wissemann WT, Lewis MR, Wallen ZD, et al. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord. 2017;32(5):739–49.
Bedarf JR, Hildebrand F, Coelho LP, Sunagawa S, Bahram M, Goeser F, et al. Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naive Parkinson’s disease patients. Genome Med. 2017;9(1):39.
Unger MM, Spiegel J, Dillmann KU, Grundmann D, Philippeit H, Burmann J, et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord. 2016;32:66–72.
Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB, et al. Colonic bacterial composition in Parkinson’s disease. Mov Disord. 2015;30(10):1351–60.
Hasegawa S, Goto S, Tsuji H, Okuno T, Asahara T, Nomoto K, et al. Intestinal dysbiosis and lowered serum lipopolysaccharide-binding protein in Parkinson’s disease. PLoS One. 2015;10(11):e0142164.
Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E, et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord. 2015;30(3):350–8.
Du G, Dong W, Yang Q, Yu X, Ma J, Gu W, et al. Altered gut microbiota related to inflammatory responses in patients with Huntington’s disease. Front Immunol. 2020;11:603594.
Wasser CI, Mercieca EC, Kong G, Hannan AJ, McKeown SJ, Glikmann-Johnston Y, et al. Gut dysbiosis in Huntington’s disease: associations among gut microbiota, cognitive performance and clinical outcomes. Brain Commun. 2020;2(2):fcaa110.
Cosorich I, Dalla-Costa G, Sorini C, Ferrarese R, Messina MJ, Dolpady J, et al. High frequency of intestinal TH17 cells correlates with microbiota alterations and disease activity in multiple sclerosis. Sci Adv. 2017;3(7):e1700492.
Cekanaviciute E, Yoo BB, Runia TF, Debelius JW, Singh S, Nelson CA, et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci U S A. 2017;114(40):10713–8.
Chen J, Chia N, Kalari KR, Yao JZ, Novotna M, Paz Soldan MM, et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep. 2016;6:28484.
Tremlett H, Fadrosh DW, Faruqi AA, Zhu F, Hart J, Roalstad S, et al. Gut microbiota in early pediatric multiple sclerosis: a case-control study. Eur J Neurol. 2016;23(8):1308–21.
Jangi S, Gandhi R, Cox LM, Li N, von Glehn F, Yan R, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. 2016;7:12015.
Miyake S, Kim S, Suda W, Oshima K, Nakamura M, Matsuoka T, et al. Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to clostridia XIVa and IV clusters. PLoS One. 2015;10(9):e0137429.
Cantarel BL, Waubant E, Chehoud C, Kuczynski J, DeSantis TZ, Warrington J, et al. Gut microbiota in multiple sclerosis: possible influence of immunomodulators. J Investig Med. 2015;63(5):729–34.
Nicholson K, Bjornevik K, Abu-Ali G, Chan J, Cortese M, Dedi B, et al. The human gut microbiota in people with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2021;22(3–4):186–94.
Di Gioia D, Bozzi Cionci N, Baffoni L, Amoruso A, Pane M, Mogna L, et al. A prospective longitudinal study on the microbiota composition in amyotrophic lateral sclerosis. BMC Med. 2020;18(1):153.
Zeng Q, Shen J, Chen K, Zhou J, Liao Q, Lu K, et al. The alteration of gut microbiome and metabolism in amyotrophic lateral sclerosis patients. Sci Rep. 2020;10(1):12998.
Zhai CD, Zheng JJ, An BC, Huang HF, Tan ZC. Intestinal microbiota composition in patients with amyotrophic lateral sclerosis: establishment of bacterial and archaeal communities analyses. Chin Med J. 2019;132(15):1815–22.
Brenner D, Hiergeist A, Adis C, Mayer B, Gessner A, Ludolph AC, et al. The fecal microbiome of ALS patients. Neurobiol Aging. 2018;61:132–7.
Fang X, Wang X, Yang S, Meng F, Wang X, Wei H, et al. Evaluation of the microbial diversity in amyotrophic lateral sclerosis using high-throughput sequencing. Front Microbiol. 2016;7:1479.
Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167(6):1469–80.e12.
Sampson TR, Challis C, Jain N, Moiseyenko A, Ladinsky MS, Shastri GG, et al. A gut bacterial amyloid promotes alpha-synuclein aggregation and motor impairment in mice. elife. 2020;9:e53111.
Savica R, Carlin JM, Grossardt BR, Bower JH, Ahlskog JE, Maraganore DM, et al. Medical records documentation of constipation preceding Parkinson disease: a case-control study. Neurology. 2009;73(21):1752–8.
Svensson E, Horvath-Puho E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P, et al. Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol. 2015;78(4):522–9.
Cassani E, Barichella M, Cancello R, Cavanna F, Iorio L, Cereda E, et al. Increased urinary indoxyl sulfate (indican): new insights into gut dysbiosis in Parkinson’s disease. Parkinsonism Relat Disord. 2015;21(4):389–93.
Forsyth CB, Shannon KM, Kordower JH, Voigt RM, Shaikh M, Jaglin JA, et al. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS One. 2011;6(12):e28032.
Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307–17.
Wang T, Hu X, Liang S, Li W, Wu X, Wang L, et al. Lactobacillus fermentum NS9 restores the antibiotic induced physiological and psychological abnormalities in rats. Benefic Microbes. 2015;6(5):707–17.
Colombo AV, Sadler RK, Llovera G, Singh V, Roth S, Heindl S, et al. Microbiota-derived short chain fatty acids modulate microglia and promote Abeta plaque deposition. elife. 2021;10:e59826.
Kim MS, Kim Y, Choi H, Kim W, Park S, Lee D, et al. Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer’s disease animal model. Gut. 2020;69(2):283–94.
McGrattan AM, McGuinness B, McKinley MC, Kee F, Passmore P, Woodside JV, et al. Diet and inflammation in cognitive ageing and Alzheimer’s disease. Curr Nutr Rep. 2019;8(2):53–65.
Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol. 2006;59(6):912–21.
Yehuda S, Rabinovitz S, Mostofsky DI. Essential fatty acids and the brain: from infancy to aging. Neurobiol Aging. 2005;26(Suppl 1):98–102.
Barberger-Gateau P. Association between Mediterranean Diet and late-life cognition. JAMA. 2009;302(22):2433; author reply.
Jiang X, Huang J, Song D, Deng R, Wei J, Zhang Z. Increased consumption of fruit and vegetables is related to a reduced risk of cognitive impairment and dementia: meta-analysis. Front Aging Neurosci. 2017;9:18.
Eskelinen MH, Ngandu T, Helkala EL, Tuomilehto J, Nissinen A, Soininen H, et al. Fat intake at midlife and cognitive impairment later in life: a population-based CAIDE study. Int J Geriatr Psychiatry. 2008;23(7):741–7.
Leblhuber F, Geisler S, Steiner K, Fuchs D, Schutz B. Elevated fecal calprotectin in patients with Alzheimer’s dementia indicates leaky gut. J Neural Transm (Vienna). 2015;122(9):1319–22.
Lanctot KL, Herrmann N, Mazzotta P, Khan LR, Ingber N. GABAergic function in Alzheimer’s disease: evidence for dysfunction and potential as a therapeutic target for the treatment of behavioural and psychological symptoms of dementia. Can J Psychiatr. 2004;49(7):439–53.
Jimenez-Balado J, Eich TS. GABAergic dysfunction, neural network hyperactivity and memory impairments in human aging and Alzheimer’s disease. Semin Cell Dev Biol. 2021;116:146.
Hu X, Wang T, Jin F. Alzheimer’s disease and gut microbiota. Sci China Life Sci. 2016;59(10):1006–23.
Rubio-Perez JM, Morillas-Ruiz JM. A review: inflammatory process in Alzheimer’s disease, role of cytokines. ScientificWorldJournal. 2012;2012:756357.
Fox M, Knapp LA, Andrews PW, Fincher CL. Hygiene and the world distribution of Alzheimer’s disease: epidemiological evidence for a relationship between microbial environment and age-adjusted disease burden. Evol Med Public Health. 2013;2013(1):173–86.
Kong G, Cao KL, Judd LM, Li S, Renoir T, Hannan AJ. Microbiome profiling reveals gut dysbiosis in a transgenic mouse model of Huntington’s disease. Neurobiol Dis. 2020;135:104268.
Stan TL, Soylu-Kucharz R, Burleigh S, Prykhodko O, Cao L, Franke N, et al. Increased intestinal permeability and gut dysbiosis in the R6/2 mouse model of Huntington’s disease. Sci Rep. 2020;10(1):18270.
Kong G, Ellul S, Narayana VK, Kanojia K, Ha HTT, Li S, et al. An integrated metagenomics and metabolomics approach implicates the microbiota-gut-brain axis in the pathogenesis of Huntington’s disease. Neurobiol Dis. 2021;148:105199.
Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4615–22.
Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature. 2017;551(7682):585–9.
Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 2016;22(6):586–97.
Ochoa-Reparaz J, Mielcarz DW, Ditrio LE, Burroughs AR, Foureau DM, Haque-Begum S, et al. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J Immunol. 2009;183(10):6041–50.
Wu S, Yi J, Zhang YG, Zhou J, Sun J. Leaky intestine and impaired microbiome in an amyotrophic lateral sclerosis mouse model. Physiol Rep. 2015;3(4):e12356.
Nicholson K, Bjornevik K, Abu-Ali G, Chan J, Cortese M, Dedi B, et al. The human gut microbiota in people with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2020;22:1–9.
Mazzini L, Mogna L, De Marchi F, Amoruso A, Pane M, Aloisio I, et al. Potential role of gut microbiota in ALS pathogenesis and possible novel therapeutic strategies. J Clin Gastroenterol. 2018;52(Suppl 1). Proceedings from the 9th Probiotics, Prebiotics and New Foods, Nutraceuticals and Botanicals for Nutrition & Human and Microbiota Health Meeting, held in Rome, Italy from September 10 to 12, 2017:S68-S70.
Hertzberg VS, Singh H, Fournier CN, Moustafa A, Polak M, Kuelbs CA, et al. Gut microbiome differences between amyotrophic lateral sclerosis patients and spouse controls. Amyotroph Lateral Scler Frontotemporal Degener. 2021:1–9.
Yu H, Kim SH, Noh MY, Lee S, Park Y. Relationship between dietary fiber intake and the prognosis of amytrophic lateral sclerosis in Korea. Nutrients. 2020;12(11):3420.
Erber AC, Cetin H, Berry D, Schernhammer ES. The role of gut microbiota, butyrate and proton pump inhibitors in amyotrophic lateral sclerosis: a systematic review. Int J Neurosci. 2020;130(7):727–35.
Gotkine M, Kviatcovsky D, Elinav E. Amyotrophic lateral sclerosis and intestinal microbiota-toward establishing cause and effect. Gut Microbes. 2020;11(6):1833–41.
Soldavini J, Kaunitz JD. Pathobiology and potential therapeutic value of intestinal short-chain fatty acids in gut inflammation and obesity. Dig Dis Sci. 2013;58(10):2756–66.
Yamawaki Y, Yoshioka N, Nozaki K, Ito H, Oda K, Harada K, et al. Sodium butyrate abolishes lipopolysaccharide-induced depression-like behaviors and hippocampal microglial activation in mice. Brain Res. 2018;1680:13–38.
Patnala R, Arumugam TV, Gupta N, Dheen ST. HDAC inhibitor sodium butyrate-mediated epigenetic regulation enhances neuroprotective function of microglia during ischemic stroke. Mol Neurobiol. 2017;54(8):6391–411.
DeCastro M, Nankova BB, Shah P, Patel P, Mally PV, Mishra R, et al. Short chain fatty acids regulate tyrosine hydroxylase gene expression through a cAMP-dependent signaling pathway. Brain Res Mol Brain Res. 2005;142(1):28–38.
Frost G, Sleeth ML, Sahuri-Arisoylu M, Lizarbe B, Cerdan S, Brody L, et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5:3611.
Zhang L, Wang Y, Xiayu X, Shi C, Chen W, Song N, et al. Altered gut microbiota in a mouse model of Alzheimer’s disease. J Alzheimers Dis. 2017;60(4):1241–57.
Govindarajan N, Agis-Balboa RC, Walter J, Sananbenesi F, Fischer A. Sodium butyrate improves memory function in an Alzheimer’s disease mouse model when administered at an advanced stage of disease progression. J Alzheimers Dis. 2011;26(1):187–97.
St Laurent R, O’Brien LM, Ahmad ST. Sodium butyrate improves locomotor impairment and early mortality in a rotenone-induced Drosophila model of Parkinson’s disease. Neuroscience. 2013;246:382–90.
Liu J, Wang F, Liu S, Du J, Hu X, Xiong J, et al. Sodium butyrate exerts protective effect against Parkinson’s disease in mice via stimulation of glucagon like peptide-1. J Neurol Sci. 2017;381:176–81.
Mizuno M, Noto D, Kaga N, Chiba A, Miyake S. The dual role of short fatty acid chains in the pathogenesis of autoimmune disease models. PLoS One. 2017;12(2):e0173032.
Chen T, Noto D, Hoshino Y, Mizuno M, Miyake S. Butyrate suppresses demyelination and enhances remyelination. J Neuroinflammation. 2019;16(1):165.
Soliman ML, Combs CK, Rosenberger TA. Modulation of inflammatory cytokines and mitogen-activated protein kinases by acetate in primary astrocytes. J NeuroImmune Pharmacol. 2013;8(1):287–300.
Usami M, Kishimoto K, Ohata A, Miyoshi M, Aoyama M, Fueda Y, et al. Butyrate and trichostatin A attenuate nuclear factor kappaB activation and tumor necrosis factor alpha secretion and increase prostaglandin E2 secretion in human peripheral blood mononuclear cells. Nutr Res. 2008;28(5):321–8.
Mori T, Koyama N, Guillot-Sestier MV, Tan J, Town T. Ferulic acid is a nutraceutical beta-secretase modulator that improves behavioral impairment and alzheimer-like pathology in transgenic mice. PLoS One. 2013;8(2):e55774.
Yan JJ, Jung JS, Kim TK, Hasan A, Hong CW, Nam JS, et al. Protective effects of ferulic acid in amyloid precursor protein plus presenilin-1 transgenic mouse model of Alzheimer disease. Biol Pharm Bull. 2013;36(1):140–3.
Westfall S, Lomis N, Kahouli I, Dia SY, Singh SP, Prakash S. Microbiome, probiotics and neurodegenerative diseases: deciphering the gut brain axis. Cell Mol Life Sci. 2017;74(20):3769–87.
Blacher E, Bashiardes S, Shapiro H, Rothschild D, Mor U, Dori-Bachash M, et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature. 2019;572(7770):474–80.
de la Rubia JE, Drehmer E, Platero JL, Benlloch M, Caplliure-Llopis J, Villaron-Casales C, et al. Efficacy and tolerability of EH301 for amyotrophic lateral sclerosis: a randomized, double-blind, placebo-controlled human pilot study. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20(1–2):115–22.
Zhang Y, Li H, Yang C, Fan DF, Guo DZ, Hu HJ, et al. Treatment with hydrogen-rich saline delays disease progression in a mouse model of amyotrophic lateral sclerosis. Neurochem Res. 2016;41(4):770–8.
Akbari E, Asemi Z, Daneshvar Kakhaki R, Bahmani F, Kouchaki E, Tamtaji OR, et al. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: a randomized, double-blind and controlled trial. Front Aging Neurosci. 2016;8:256.
Tamtaji OR, Heidari-Soureshjani R, Mirhosseini N, Kouchaki E, Bahmani F, Aghadavod E, et al. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: a randomized, double-blind, controlled trial. Clin Nutr. 2019;38(6):2569–75.
Agahi A, Hamidi GA, Daneshvar R, Hamdieh M, Soheili M, Alinaghipour A, et al. Does severity of Alzheimer’s disease contribute to its responsiveness to modifying gut microbiota? A double blind clinical trial. Front Neurol. 2018;9:662.
Barichella M, Pacchetti C, Bolliri C, Cassani E, Iorio L, Pusani C, et al. Probiotics and prebiotic fiber for constipation associated with Parkinson disease: an RCT. Neurology. 2016;87(12):1274–80.
Tan AH, Lim SY, Chong KK, Maa AM, Hor JW, Lim JL, et al. Probiotics for constipation in Parkinson disease: a randomized placebo-controlled study. Neurology. 2021;96(5):e772–e82.
Tamtaji OR, Taghizadeh M, Daneshvar Kakhaki R, Kouchaki E, Bahmani F, Borzabadi S, et al. Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2019;38(3):1031–5.
Borzabadi S, Oryan S, Eidi A, Aghadavod E, Daneshvar Kakhaki R, Tamtaji OR, et al. The effects of probiotic supplementation on gene expression related to inflammation, insulin and lipid in patients with Parkinson’s disease: a randomized, double-blind, placebo controlled trial. Arch Iran Med. 2018;21(7):289–95.
Ibrahim A, Ali RAR, Manaf MRA, Ahmad N, Tajurruddin FW, Qin WZ, et al. Multi-strain probiotics (Hexbio) containing MCP BCMC strains improved constipation and gut motility in Parkinson’s disease: a randomised controlled trial. PLoS One. 2020;15(12):e0244680.
Kouchaki E, Tamtaji OR, Salami M, Bahmani F, Daneshvar Kakhaki R, Akbari E, et al. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2017;36(5):1245–9.
Tamtaji OR, Kouchaki E, Salami M, Aghadavod E, Akbari E, Tajabadi-Ebrahimi M, et al. The effects of probiotic supplementation on gene expression related to inflammation, insulin, and lipids in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. J Am Coll Nutr. 2017;36(8):660–5.
Tankou SK, Regev K, Healy BC, Tjon E, Laghi L, Cox LM, et al. A probiotic modulates the microbiome and immunity in multiple sclerosis. Ann Neurol. 2018;83(6):1147–61.
Salami M, Kouchaki E, Asemi Z, Tamtajia OR. How probiotic bacteria influence the motor and mental behaviors as well as immunological and oxidative biomarkers in multiple sclerosis? A double blind clinical trial. J Funct Foods. 2019;52:8–13.
Girard SA, Bah TM, Kaloustian S, Lada-Moldovan L, Rondeau I, Tompkins TA, et al. Lactobacillus helveticus and Bifidobacterium longum taken in combination reduce the apoptosis propensity in the limbic system after myocardial infarction in a rat model. Br J Nutr. 2009;102(10):1420–5.
Liu J, Sun J, Wang F, Yu X, Ling Z, Li H, et al. Neuroprotective effects of Clostridium butyricum against vascular dementia in mice via metabolic butyrate. Biomed Res Int. 2015;2015:412946.
Athari Nik Azm S, Djazayeri A, Safa M, Azami K, Ahmadvand B, Sabbaghziarani F, et al. Lactobacilli and bifidobacteria ameliorate memory and learning deficits and oxidative stress in beta-amyloid (1-42) injected rats. Appl Physiol Nutr Metab. 2018;43(7):718–26.
Bonfili L, Cecarini V, Berardi S, Scarpona S, Suchodolski JS, Nasuti C, et al. Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci Rep. 2017;7(1):2426.
Abraham D, Feher J, Scuderi GL, Szabo D, Dobolyi A, Cservenak M, et al. Exercise and probiotics attenuate the development of Alzheimer’s disease in transgenic mice: role of microbiome. Exp Gerontol. 2019;115:122–31.
Ochoa-Reparaz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, et al. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010;3(5):487–95.
Kwon HK, Kim GC, Kim Y, Hwang W, Jash A, Sahoo A, et al. Amelioration of experimental autoimmune encephalomyelitis by probiotic mixture is mediated by a shift in T helper cell immune response. Clin Immunol. 2013;146(3):217–27.
Hefendehl JK, LeDue J, Ko RW, Mahler J, Murphy TH, MacVicar BA. Mapping synaptic glutamate transporter dysfunction in vivo to regions surrounding Abeta plaques by iGluSnFR two-photon imaging. Nat Commun. 2016;7:13441.
Obrenovich M, Jaworski H, Tadimalla T, Mistry A, Sykes L, Perry G, et al. The role of the microbiota-gut-brain axis and antibiotics in ALS and neurodegenerative diseases. Microorganisms. 2020;8(5):784.
Melzer N, Meuth SG, Torres-Salazar D, Bittner S, Zozulya AL, Weidenfeller C, et al. A beta-lactam antibiotic dampens excitotoxic inflammatory CNS damage in a mouse model of multiple sclerosis. PLoS One. 2008;3(9):e3149.
Tomiyama T, Asano S, Suwa Y, Morita T, Kataoka K, Mori H, et al. Rifampicin prevents the aggregation and neurotoxicity of amyloid beta protein in vitro. Biochem Biophys Res Commun. 1994;204(1):76–83.
Gonzalez-Lizarraga F, Socias SB, Avila CL, Torres-Bugeau CM, Barbosa LR, Binolfi A, et al. Repurposing doxycycline for synucleinopathies: remodelling of alpha-synuclein oligomers towards non-toxic parallel beta-sheet structured species. Sci Rep. 2017;7:41755.
Costa R, Speretta E, Crowther DC, Cardoso I. Testing the therapeutic potential of doxycycline in a Drosophila melanogaster model of Alzheimer disease. J Biol Chem. 2011;286(48):41647–55.
Minter MR, Zhang C, Leone V, Ringus DL, Zhang X, Oyler-Castrillo P, et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci Rep. 2016;6:30028.
Molloy DW, Standish TI, Zhou Q, Guyatt G, Group DS. A multicenter, blinded, randomized, factorial controlled trial of doxycycline and rifampin for treatment of Alzheimer’s disease: the DARAD trial. Int J Geriatr Psychiatry. 2013;28(5):463–70.
Kountouras J, Boziki M, Gavalas E, Zavos C, Grigoriadis N, Deretzi G, et al. Eradication of Helicobacter pylori may be beneficial in the management of Alzheimer’s disease. J Neurol. 2009;256(5):758–67.
Hashim H, Azmin S, Razlan H, Yahya NW, Tan HJ, Manaf MR, et al. Eradication of Helicobacter pylori infection improves levodopa action, clinical symptoms and quality of life in patients with Parkinson’s disease. PLoS One. 2014;9(11):e112330.
Panza F, Lozupone M, Solfrizzi V, Watling M, Imbimbo BP. Time to test antibacterial therapy in Alzheimer’s disease. Brain. 2019;142(10):2905–29.
Vendrik KEW, Ooijevaar RE, de Jong PRC, Laman JD, van Oosten BW, van Hilten JJ, et al. Fecal microbiota transplantation in neurological disorders. Front Cell Infect Microbiol. 2020;10:98.
Borody T, Leis S, Campbell J, Torres M, Nowak A. Fecal microbiota transplantation (FMT) in multiple sclerosis (MS): 942. Am Coll Gastroenterol. 2011;106:S352.
Makkawi S, Camara-Lemarroy C, Metz L. Fecal microbiota transplantation associated with 10 years of stability in a patient with SPMS. Neurol Neuroimmunol Neuroinflamm. 2018;5(4):e459.
Huang H, Xu H, Luo Q, He J, Li M, Chen H, et al. Fecal microbiota transplantation to treat Parkinson’s disease with constipation: a case report. Medicine (Baltimore). 2019;98(26):e16163.
Xue LJ, Yang XZ, Tong Q, Shen P, Ma SJ, Wu SN, et al. Fecal microbiota transplantation therapy for Parkinson’s disease: a preliminary study. Medicine (Baltimore). 2020;99(35):e22035.
Hazan S. Rapid improvement in Alzheimer’s disease symptoms following fecal microbiota transplantation: a case report. J Int Med Res. 2020;48(6):300060520925930.
Ray SK, Mukherjee S. Evolving interplay between dietary polyphenols and gut microbiota-an emerging importance in healthcare. Front Nutr. 2021;8:634944.
Szczechowiak K, Diniz BS, Leszek J. Diet and Alzheimer’s dementia - nutritional approach to modulate inflammation. Pharmacol Biochem Behav. 2019;184:172743.
Casani-Cubel J, Benlloch M, Sanchis-Sanchis CE, Marin R, Lajara-Romance JM, de la Rubia Orti JE. The impact of microbiota on the pathogenesis of amyotrophic lateral sclerosis and the possible benefits of polyphenols. An overview. Metabolites. 2021;11(2):120.
Kocot J, Luchowska-Kocot D, Kielczykowska M, Musik I, Kurzepa J. Does vitamin C influence neurodegenerative diseases and psychiatric disorders? Nutrients. 2017;9(7):659.
Rusek M, Pluta R, Ulamek-Koziol M, Czuczwar SJ. Ketogenic diet in Alzheimer’s disease. Int J Mol Sci. 2019;20(16):3892.
Reger MA, Henderson ST, Hale C, Cholerton B, Baker LD, Watson GS, et al. Effects of beta-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol Aging. 2004;25(3):311–4.
Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metab (Lond). 2009;6:31.
Hersi M, Irvine B, Gupta P, Gomes J, Birkett N, Krewski D. Risk factors associated with the onset and progression of Alzheimer’s disease: a systematic review of the evidence. NeuroToxicology. 2017;61:143–87.
Singh B, Parsaik AK, Mielke MM, Erwin PJ, Knopman DS, Petersen RC, et al. Association of Mediterranean diet with mild cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;39(2):271–82.
Morris MC, Tangney CC, Wang Y, Sacks FM, Barnes LL, Bennett DA, et al. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015;11(9):1015–22.
Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74(10):720–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
Funding
All authors are funded by Science Foundation Ireland SFI/12/RC/2273_P2; J. F. Cryan is also funded by the Saks Kavanaugh Foundation, EU H2020 project DLV-848228 DIS-COvERIE, and Swiss National Science Foundation project CRSII5_186346/NMS2068.
Conflict of Interest
J. F. Cryan has received research funding from 4D Pharma, Cremo, Dupont, Mead Johnson, Nutricia, and Pharmavite and has been an invited speaker at meetings organised by Alimentary Health, Alkermes, Ordesa, and Yakult and has served as a consultant for Alkermes and Nestle.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Cabré, S., O’Riordan, K.J., Cryan, J.F. (2022). Neurodegenerative Diseases and the Gut Microbiota. In: Rook, G.A.W., Lowry, C.A. (eds) Evolution, Biodiversity and a Reassessment of the Hygiene Hypothesis. Progress in Inflammation Research, vol 89. Springer, Cham. https://doi.org/10.1007/978-3-030-91051-8_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-91051-8_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-91050-1
Online ISBN: 978-3-030-91051-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)