Abstract
Major salivary glands (MASG), and in particular parotid glands, are the main site of B-cell lymphoma in patients with primary Sjögren’s syndrome (pSS), carrying the highest relative risk of all autoimmune diseases. At present, biopsying the MASG in pSS has limited indications, since it is mostly reserved for those patients with persistent salivary glandular enlargement and strong suspicion of lymphoma.
In this chapter, the role of MASG biopsy in the evaluation and management of pSS patients is reviewed, focusing on the lymphoproliferative risk. Furthermore, technical aspects, potential complications and differences among currently available MASG biopsy techniques will be described.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Sjögren’s syndrome
- Major salivary gland biopsy
- Parotid gland
- Lymphocytic sialadenitis
- Lymphoepithelial lesion
- Histopathology
Introduction
Currently, the use of major salivary gland (MASG) biopsy is limited regarding the clinical management of primary Sjögren’s syndrome (pSS) since it is mostly reserved for those pSS patients with persistent salivary glandular enlargement and suspicion of lymphoma. The literature focuses on surgical parotid gland biopsy, while little information is reported regarding submandibular gland biopsy [1,2,3]. The open surgical parotid biopsy approach is not commonly used mainly because of the risk of surgical side effects, such as facial nerve damage, development of sialoceles, or salivary fistulae, as well as the frequently described temporary change in sensation of the skin area around the incision [3, 4]. However, when performed by experts and with specific surgical approaches (i.e., in the ‘safety zone’, as will be described), the risk of perioperative complications is usually minimal [1]. At present, only a few centres use parotid gland biopsy in clinical practice for pSS patients, and their experience seems to confirm the safety of the procedure [1, 5]. Hitherto, labial salivary gland (LSG) biopsy is preferred in obtaining histological evidence of the diagnosis of pSS. Minor salivary glands are distributed in the oral cavity (labial, buccal, and palatal mucosa) and are easily accessible; hence, LSG biopsy has been included in the diagnostic work-up of pSS since 1970 and in the international classification criteria [6, 7].
Compared with LSG biopsy, MASG biopsy may offer some advantages for the management of pSS, including improved differential diagnosis, disease progression monitoring, and treatment response assessment [8, 9]. One of the advantages is that MASG tissues can be sampled multiple times from the same gland. Moreover, MASG biopsy may identify and therefore allow the study of lesions, such as lymphoepithelial lesions, in patients suspected to be predisposed to lymphoproliferative disease [5, 10]. Overall, further studies focusing on the relevance of MASG biopsies in pSS appear worthwhile, making the issue which type of biopsy is superior (LSG or MASG) for diagnostic and follow-up purposes a subject of debate.
Major Salivary Gland Biopsy Procedures
The main indication for MASG biopsies in pSS is persistent swelling of the major salivary glands, in particular of the parotid, which appears to be a major risk factor for lymphoma development in pSS [10]. The presence of a focal lesion within an altered salivary parenchyma or a severe diffuse inhomogeneity of the glandular parenchyma within a glandular ultrasonographic appearance of MASG swelling is indicative of lymphoma [5]. The biopsy procedures in MASG require accurate knowledge of the path of the facial nerve and its possible relationship with the focal lesion.
Facial Nerve
Originating from the pons of the brainstem, the facial nerve, or the seventh cranial nerve, emerges from the skull through the stylomastoid foramen, lateral to the styloid process. The main trunk of the nerve enters the parotid gland parenchyma, where it splits into a superior temporo-facial and an inferior cervico-facial portion, subsequently dividing into five branches. The five branches, or rami, of the facial nerve are named temporal (or frontal), zygomatic, buccal, marginal mandibular, and cervical. Anastomoses usually occur between the buccal and zygomatic branches, whereas the temporal and mandibular branches are typically terminal nerve fibres and, therefore, are more vulnerable to injuries. The path of the facial nerve exhibits significant interindividual differences, as demonstrated by several anatomical studies [11, 12], with regard to both the distribution and the branching pattern of the facial nerve.
The temporal branch exits the parotid gland and travels within the superficial temporal fascia, also known as the temporoparietal fascia, which is within the superficial musculo-aponeurotic system (SMAS). It crosses over the zygomatic arch to supply the frontalis muscle from below. A ‘danger zone’ can be identified in the frontal branch using several anatomical landmarks, consisting of a triangle spanning from the ear lobe to the lateral brow and lateral extent of the highest forehead crease.
In the lower part of the face, the facial nerve divisions lay underneath the SMAS and become more superficial anteriorly to the masseter muscle. In particular, special care should be taken during invasive procedures to avoid injury of the marginal mandibular nerve, as it would result in paralysis of the lip and chin, producing a notable deformity.
Facial nerve splitting usually occurs near the superficial crossing of the retromandibular vein and the superficial temporal artery in their vertical part and downstream branches in the anterior and superficial part of the parotid gland, thereby granting the posterior and superficial portion of the parotid the label ‘safety zone’ for interventional procedures (Fig. 14.1). In this area, the facial nerve runs deeply in the glandular parenchyma.
Open Surgical Biopsy of the Parotid Gland
Parotid gland biopsy was originally described by Kraaijenhagen in 1975 [13] and modified by Pijpe et al. in 2007 [4]. The procedure is performed under local anaesthesia, but it generally requires more expertise than the LSG biopsy. The skin is infiltrated with a solution made of a local anaesthetic and a vasoconstrictor. An auriculo-temporal nerve block is performed by directing the injection with a 45° posterior inclination above the attachment of the ear lobe; additional anaesthesia can be performed by puncturing the skin and the underlying SMAS layer covering the parotid gland (Fig. 14.2).
With a scalpel blade, a subcutaneous dissection is performed following the insertion of the ear lobe along the skin crease for a length of approximately 1.5–2 cm using blunt-tipped scissors, exposing the parotid capsule.
Once haemostasis is complete and the parotid capsule is clearly visible, it is incised, and a 5 × 5 mm sample of gland parenchyma is sampled for histopathological review [1, 4, 14].
Obviously, the main risk is injury to the facial nerve. Once the facial nerve exits the skull base from the stylomastoid foramen, it runs anteriorly to innervate the mimic muscles of the face. The nerve lies underneath the superficial lobe of the parotid in the initial trait, and its main trunk is located at a depth of approximately 2 cm, depending on age and individual variations (Fig. 14.3).
The main trunk is the most important target for the surgeon to avoid since section will result in paralysis of the ipsilateral half of the face. As shown in Fig. 14.4, the main trunk is found in the same plane as the posterior belly of the digastric muscle, and its emergence is generally indicated by the ‘pointer’ tragal cartilage. In this region, the main trunk is located at a depth covered by the bulky superficial lobe and has no branches. Within the ‘safety zone’, an area located between 1 cm anterior and 1 cm below the ear lobe, the superficial lobe of the parotid protects the facial nerve for a length of approximately 2 cm, with some variability between individuals.
A superficial tissue sample taken from the ‘safety area’ is unlikely to cause any damage to the facial nerve due to the deep lie and absence of branches. Therefore, for the parotid gland biopsy, a sampling procedure might be safely performed within 1–1.5 cm from the glandular capsule. In contrast, more anteriorly, the facial nerve gives off its branches and becomes more superficial, running in close contact with the surface of the parotid gland, which makes it more vulnerable to injuries. Moreover, attention should be given not to injure the great auricular nerve, a sensitive branch that supplies the ear lobe and the skin of the periauricular region with its sensory fibres.
Once tissue sampling has been performed, a multilayer closure of the wound is performed by placing absorbable sutures beneath the skin layer and non-absorbable sutures along the skin. Sutures are removed after 1 week, and only a minimal residual scar is found along the skin crease of the ear lobe.
The aforementioned ‘safety zone’ allows the procedure to be safely performed with respect to the facial nerve, particularly when performed by expert surgeons. The procedure is not devoid of other potential complications, including sialoceles, salivary gland fistulae, accidental injury to the superficial temporal artery, infection, or numbness of the ear lobe, the latter indicating damage to the great auricular nerve [4].
Ultrasound-Guided Core Needle Biopsy Procedure of the Major Salivary Glands
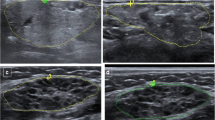
Salivary gland ultrasound (SGUS) is frequently used to assess structural abnormalities typical of pSS [15] and create a scoring system, as proposed in the past by De Vita et al. [16] and more recently by the OMERACT taskforce [17]. Recent reports suggest that focal ultrasound lesions (Fig. 14.5a) or a peculiar sonographic appearance of the gland, e.g., large, confluent hypoechoic areas spread over the gland (Fig. 14.5b), could indicate a glandular B-cell lymphoma [18, 19].
The diagnosis of B-cell lymphoma must be pathologically proven, and differential diagnoses must be excluded. In non-pSS individuals who show SGUS-detected focal lesions of MASG, fine-needle aspiration cytology (FNAC) is performed to differentiate between benign and malignant lesions. FNAC is a safe technique, but it frequently fails to provide enough material for diagnosis [20]. In pSS patients at high risk for lymphoma development, i.e., those who develop persistent MASG enlargement or with suspected SGUS appearance, FNAC is inadequate as a diagnostic tool; therefore, histological sampling is usually needed by open surgical biopsy or core needle biopsy guided by ultrasound [19].
Ultrasound-guided core needle biopsy (CNB) has the potential to overcome both FNAC and surgical limitations. For decades, ultrasound (US)-guided CNB has been an established method for accurate diagnosis of a breast lump [21] or masses in other body parts. Recent evidence suggests that in pSS patients with MASG enlargement, a major risk factor for B-cell lymphoma [10], US-guided CNB can provide a sufficient sample for pathological examination, allowing immunohistochemical staining and flow cytometry [21]. In the clinical setting of suspected B-cell lymphoma in pSS patients, obtaining a pathological diagnosis with US-guided CNB requires a different strategy for the parotid and submandibular glands, respectively.
US-Guided CNB of a Suspected B-Cell Lymphoma in the Parotid Gland
In the setting of a diffusely inhomogeneous parotid parenchyma, US-guided CNB can be performed safely by targeting the postero-caudal part of the gland, and inserting the needle in the caudocranial direction anteriorly to the ear lobe (in the ‘safety zone’), keeping it as superficial as possible within 1–1.5 cm from the glandular surface.
In the presence of a focal parenchymal lesion, it is mandatory to determine the relationship between the sonograph-detected focal lesion and the facial nerve. For superficial masses, the procedure is generally feasible and safe, targeting the lesions from posteriorly through the ‘safety zone,’ while maintaining the shortest approach and a superficial depth of the biopsy needle (within 1–1.5 cm from the surface of the gland). For deeper lesions, the risks and benefits of the procedure have to be weighted. When safety is not guaranteed, the traditional surgical approach should be chosen.
US-Guided Core Needle Biopsy Technique
Currently, this technique is mainly applied in relation to epithelial tumours [22, 23]. The patient is positioned supine, with the shoulders slightly lifted (usually with a pillow below the upper back). The neck is hyperextended, turned towards the contralateral side of the target gland (Fig. 14.6).
After complete disinfection of the skin and probe, a local anaesthetic (5 mL of mepivacaine chlorhydrate) is injected under ultrasound guidance with a fine needle (23 G) in the subcutaneous tissue and in the posterior, superficial part of the parotid gland, while moving the needle in the caudocranial direction (Fig. 14.7).
This targeted access area, anterior to the ear lobe, corresponds to the caudal part of the parotid gland, i.e., the ‘safety zone’. Shortly after the local anaesthetic injection, a small skin incision is made with a scalpel (Fig. 14.8).
Then, a 14 G semi-automatic needle is inserted through the incision and moved into the parotid gland under ultrasound guidance, following the same path used for local anaesthesia (Fig. 14.9a, b).
Once the 14 G semi-automatic needle is stable inside the focal lesion of the parotid gland, sampling can be performed. Commonly, two to four samples will suffice for histologic examination. Biopsy samples should be placed in a fixative (i.e., formalin) or sent fresh to the pathology department (Fig. 14.10), depending on the requested analysis.
US-Guided CNB of a Suspected B-Cell Lymphoma in the Submandibular Gland
In the case of suspected B-cell lymphoma of the submandibular glands, nerve injuries are of no concern. The patient can be positioned as described above for the US-guided biopsy of the parotid gland, with the head completely turned to the opposite side. The gland can be accessed from anteriorly or posteriorly, depending on patient cooperation, operator preferences, and the eventual presence of a US-detected focal lesion. Disinfection, skin incision, and tissue sampling must be performed in the same way described above, with the significant difference that there is no concern for nerve damage.
Histopathology of Major Salivary Gland
Lymphoid Proliferation in pSS
Lymphoid proliferation in the setting of pSS can pose formidable issues in differential diagnosis since it ranges from a seemingly reactive process to a neoplastic lesion with overlapping morphological features. ‘Lymphoepithelial lesions’ (LELs) were described as lymphoid proliferations consisting of lymphoid hyperplasia and epithelial alterations, while ‘epi-myoepithelial islands’ were reported as a compact cellular island surrounded by a stroma of lymphoid tissue. These islands contribute to the definition of a specific histopathological lesion called myoepithelial sialadenitis (MESA), and later named lymphoepithelial sialadenitis (LESA) (Fig. 14.11a) [24, 25]. Over the years, studies have focused on the presence of lymphoepithelial lesions (LELs) and germinal centres (GCs) as premalignant pathologic features correlated with subsequent lymphoma development. LELs are observed in both labial and major salivary gland biopsy, although more pronounced in parotid biopsy (93 vs 33%) [4, 26]. Haacke et al. [26] hypothesised that infiltrating lymphocytes may cause hyperplasia of the epithelium, generating LELs.
Histological pictures of different lesions diagnosed on needle core biopsies of the parotid gland: (a) A MESA/LESA showing dense lymphoid infiltrate with focal germinal centre formation (bottom left) and benign LELs in a patient with pSS; molecular studies on this case showed a polyclonal B-cell population (H&E stain; original magnification ×20). (b) A lymphoid focus in periductal location in a patient with pSS (H&E stain; original magnification ×10). (c) A typical MALT lymphoma arising in a parotid gland showing disruptive proliferation of centrocyte-like and monocytoid B-cells with features of coalescence and LELs. (H&E stain; original magnification ×10)
For a better understanding of the histopathological lesions, the following classification of LELs has been proposed [27]:
-
Stage 1: partial LEL, involving <50% of the epithelium.
-
Stage 2: developed LEL, involving 50–100% of the epithelium.
-
Stage 3: occluded LEL, fully circumferentially affecting epithelium without lumen.
With increasing severity of LEL, the relative number of B cells is also expanding, suggesting a crucial role of B cells for epithelial proliferation [27].
Germinal centres (GSs) are usually located in the secondary lymphoid organs and play a role in antigen-driven B cell selection . In the salivary glands of pSS patients, however, ectopic GCs may contribute to the maturation of B cells (i.e., B cells with somatic Ig gene hypermutation) [28] and may play a role in the pathogenesis of lymphoma and lymphoproliferation in pSS.
According to De Vita et al. [29] and Carbone et al. [30], three histological patterns can be distinguished regarding the salivary gland lymphoproliferation in pSS. The first one comprises a stage wherein LESA/MESA presents a ‘fully benign lymphoid infiltrate’, characterised by a preserved glandular lobular architecture, conspicuous reactive lymphoid follicles without the expansion of the mantle or marginal zone, and prominent LELs acquiring a monocytoid and/or centrocyte-like appearance (restricted to the LELs). The B cell population is polyclonal. The second stage considers LESA/MESA as a ‘lymphoproliferative lesion’, characterised by a diffusively or multifocally lymphoid involvement of the gland (with preservation only of the normal acini island), a more aggressive LEL appearance, aggregates of centrocyte-like cells within the diffuse lymphoid infiltrate, and nonconfluent centrocyte-like cell ‘halos’ surrounding the LELs. Areas of immunoglobulin light chain restriction may be present, and molecular analyses may show either oligo-polyclonal or monoclonal B-cell expansion [10, 29]. The last phase represents a clear-cut lymphoproliferation in pSS, particularly B-cell clonal expansion, resulting in uncontrolled progress towards B-cell lymphoma.
The most common types of lymphomas arising in the aforementioned background are non-Hodgkin malignancies of the mucosa-associated lymphoid tissue (MALT) (Fig. 14.11c). They demonstrate a dense lymphoid infiltrate with the tendency to occupy the large majority of the gland, disrupting glandular anatomy and its function; otherwise, they may generate a localised mass [31].
The earliest manifestation of lymphoma is an expansion of centrocyte-like cells that form broad halos around LELs. These neoplastic cells show cytologic variation; they may resemble small lymphocytes of the mantle zone, show a close resemblance to centrocytes, or be characterised by an abundant pale-stained cytoplasm similar to monocytoid B cells. Characteristically scattered transformed blast cells are present, and in some cases, plasma cell differentiation may be a prominent feature. As the lesion progresses, the centrocyte-like cells coalesce into broad sheets, displacing the follicles that are such a prominent feature of the earlier stages. The proliferating centrocyte-like cells also displace and destroy the epithelium in the epi-myoepithelial islands and colonise the follicles. In some cases, formation of enlarged follicles that contain transformed tumour cells is extremely prominent, that it can be confused with follicular lymphoma [31]. Addition of immunohistochemistry may demonstrate the expression on tumour cells of CD20, IgM, and the antiapoptotic factor bcl-2, while they are generally negative for IgD, CD5, and CD10. Interestingly, a key B-cell growth factor in pSS, BAFF, which can mediate resistance to anti-CD20 therapy and target biologic drugs, is also present in the local microenvironment [32]. In some cases, low-grade malignant B-cell clones can further undergo monoclonal transformation into diffuse large B-cell lymphoma (DLBC) [33].
With the progression from fully benign lymphoid infiltrates and non-malignant lymphoproliferation to overt salivary gland lymphoma of MALT, a progressive polyclonal expansion of rheumatoid-positive B-cells can be observed [34, 35]. Monoclonality does not imply B-cell malignancy and may be further distinguished between monoclonal localised and monoclonal disseminated [34].
Current Role of Histopathology of Parotid Gland Biopsy in the Diagnosis and Prognosis of pSS
Although labial gland biopsy is the standard of diagnosing pSS [36], parotid gland biopsy is considered a possible alternative, especially in experienced hands [1, 37]. Unlike the labial salivary gland biopsy, the parotid gland biopsy might not only provide superior diagnostic accuracy but also improved prognostic value [34], monitoring disease activity and tissue damage [38, 39], response to treatment [40, 41], early detection, and follow-up of suspect salivary lymphoma in pSS [29, 34].
In 2006, assessing the diagnostic value of the parotid biopsy compared with labial biopsy in pSS patients, Pijpe et al. [4] developed a set of validated histopathologic criteria for the diagnosis of pSS in accordance with the AECG classification criteria. According to these criteria, a parotid biopsy is considered diagnostic for pSS when a focus score (FS) ≥ 1 is observed, irrespective of the presence of lympho-epithelial lesions (LELs), or if small lymphocytic infiltrates, not fulfilling the criterion of a FS ≥ 1, in combination with the presence of benign LELs are found in the histological specimen. The application of these histological criteria showed a sensitivity of 93%, a specificity of 95%, a PPV of 93%, and NPV of 95%, using AECG criteria as gold standard [42]. Sensitivity and specificity indices were re-assessed by other investigators [43]. The authors concluded that the sensitivity of the ACR-EULAR criteria increased by using labial salivary gland biopsies, while the specificity was higher when using parotid biopsy [43]. Using parotid biopsies, the ACR-EULAR criteria were found to have excellent accuracy to discriminate pSS from non-pSS cases, as well as an absolute agreement of 92% (k = 0.82), sensitivity of 91%, and specificity of 92% [43]. To date, more research is needed to define the diagnostic role and possible exchangeability of labial and parotid biopsies in the diagnostic work-up of pSS [1, 44].
Role of MASG Histopathology in the Differential Diagnosis of pSS
Salivary glands may become involved in inflammatory processes other than pSS [45]. Noteworthy, focal lymphocytic sialadenitis may occur not only in conjunction with other autoimmune diseases or infectious diseases including hepatitis C virus (HCV) or human immunodeficiency virus (HIV) infections but also in healthy individuals. Therefore, the presence of focal sialoadenitis alone is not a diagnostic hallmark of pSS [46]. There is a specific tropism of HCV for exocrine gland epithelial cells [29, 47], and chronic focal pSS-like sialadenitis can be observed in up to 50% of HCV-infected patients [36, 47]. In the early stage of HIV infection, features similar to pSS can be identified, such as follicular lymphoid hyperplasia and atrophic glands. HIV infections can also lead to cystic LELs within the glandular tissue. In advanced stages, lymphoepithelial cystic lesions, which develop as a result of striated duct compression by hyperplastic lymphoid tissue, may occur; while such lesions are only rarely found in pSS patients [45].
Sarcoidosis is another mimicker of pSS; sarcoidosis-associated sialadenitis is characterised by the presence of noncaseating granulomas. However, granulomas may not always be present, and scattered lymphoplasmacytic infiltrates and multinucleated cells make it difficult to differentiate between the two disorders [48] (Fig. 14.12).
IgG4-related disease is another entity that enters the differential diagnosis. The three histopathological hallmarks of IgG4-related disease are dense lymphoplasmacytic infiltration, storiform fibrosis, and obliterative phlebitis. The inflammatory infiltrate tends to surround ductal structures and includes both T and B lymphocytes, with the latter organised in germinal centre structures and the former scattered within the lesion. LELs, if present, are not as prominent as they are in pSS [46]. Before making the final diagnosis, correlation should be made with the levels of circulating IgG4, which are usually markedly elevated in this condition.
Conclusions
MASG biopsy remains the standard for the diagnosis of suspected lymphoma in pSS. Open surgical biopsy certainly is a good option, yet US-guided CNB of MAGS seems convenient and may also be safer. Additional studies are required to determine the exact positioning of each procedure.
By contrast, FNAC appears to be of limited use in pSS, since it fails to collect adequate tissue for the pathologic diagnosis of indolent marginal zone lymphoma of MALT, the usual histotype in pSS.
Finally, MASG biopsy does not trump labial salivary gland biopsy for the diagnosis and classification of pSS at present. However, the prediction of lymphoma evolution based on the presence of LELs and GCs, which are more prevalent in MASG than in labial salivary glands, remains to be defined in the MASG microenvironment. Lymphoma often develops in the parotids and, in contrast, is extremely rare in labial salivary glands in pSS. SGUS can be used very easily for the detection and follow-up of US parenchymal abnormalities in MAGS, as a possible surrogate of salivary gland biopsy in select cases of pSS, and to guide MAGS itself in core needle biopsy.
Overall, parotid biopsy in pSS deserves attention as a relevant future tool in pSS for possible major clinical and research advances. The recent use of SGUS-guided CNB on MAGS, as a safe and effective procedure, appears of particular value to this end.
References
Spijkervet FKL, Haacke E, Kroese FGM, Bootsma H, Vissink A. Parotid gland biopsy, the alternative way to diagnose Sjögren syndrome. Rheum Dis Clin N Am. 2016;42(3):485–99.
Colella G, Cannavale R, Vicidomini A, Itro A. Salivary gland biopsy: a comprehensive review of techniques and related complications. Rheumatology. 2010;49(11):2117–21.
Delli K, Vissink A, Spijkervet FKL. Salivary gland biopsy for Sjögren’s syndrome. Oral Maxillofac Surg Clin N Am. 2014;26(1):23–33.
Pijpe J, Kalk WWI, van der Wal JE, Vissink A, Kluin PM, Roodenburg JLN, et al. Parotid gland biopsy compared with labial biopsy in the diagnosis of patients with primary Sjogren’s syndrome. Rheumatol Oxf Engl. 2007;46(2):335–41.
Zabotti A, Zandonella Callegher S, Lorenzon M, Pegolo E, Scott CA, Tel A, et al. Ultrasound-guided core needle biopsy compared with open biopsy: a new diagnostic approach to salivary gland enlargement in Sjögren’s syndrome? Rheumatol Oxf Engl. 2020;60:1282.
Chisholm DM, Mason DK. Labial salivary gland biopsy in Sjogren’s disease. J Clin Pathol. 1968;21(5):656–60.
Chisholm DM, Waterhouse JP, Mason DK. Lymphocytic sialadenitis in the major and minor glands: a correlation in postmortem subjects. J Clin Pathol. 1970;23(8):690–4.
Fisher BA, Brown RM, Bowman SJ, Barone F. A review of salivary gland histopathology in primary Sjögren’s syndrome with a focus on its potential as a clinical trials biomarker. Ann Rheum Dis. 2015;74(9):1645–50.
Pijpe J, Meijer JM, Bootsma H, Van Der Wal JE, Spijkervet FKL, Kallenberg CGM, et al. Clinical and histologic evidence of salivary gland restoration supports the efficacy of rituximab treatment in Sjögren’s syndrome. Arthritis Rheum. 2009;60(11):3251–6.
De Vita S, Gandolfo S. Predicting lymphoma development in patients with Sjögren’s syndrome. Expert Rev Clin Immunol. 2019;15(9):929–38.
Davis RA, Anson BJ, Budinger JM, Kurth LR. Surgical anatomy of the facial nerve and parotid gland based upon a study of 350 cervicofacial halves. Surg Gynecol Obstet. 1956;102(4):385–412.
Baker DC, Conley J. Avoiding facial nerve injuries in rhytidectomy. Anatomical variations and pitfalls. Plast Reconstr Surg. 1979;64(6):781–95.
Kraaijenhagen HA. Letter: technique for parotid biopsy. J Oral Surg Am Dent Assoc 1965. 1975;33(5):328.
McGuirt, Jr WF, Whang C, Moreland W. The role of parotid biopsy in the diagnosis of pediatric Sjögren syndrome. Arch Otolaryngol Neck Surg [Internet]. 2002 Nov 1 [cited 2021 Mar 23];128(11):1279. Available from: http://archotol.jamanetwork.com/article.aspx?doi=10.1001/archotol.128.11.1279.
van Ginkel MS, Glaudemans AWJM, van der Vegt B, Mossel E, Kroese FGM, Bootsma H, et al. Imaging in primary Sjögren’s syndrome. J Clin Med. 2020;9(8):2492.
De Vita S, Lorenzon G, Rossi G, Sabella M, Fossaluzza V. Salivary gland echography in primary and secondary Sjögren’s syndrome. Clin Exp Rheumatol. 1992;10(4):351–6.
Jousse-Joulin S, D’Agostino MA, Nicolas C, Naredo E, Ohrndorf S, Backhaus M, et al. Video clip assessment of a salivary gland ultrasound scoring system in Sjögren’s syndrome using consensual definitions: an OMERACT ultrasound working group reliability exercise. Ann Rheum Dis. 2019;78(7):967–73.
Baer AN, Grader-Beck T, Antiochos B, Birnbaum J, Fradin JM. Ultrasound-guided biopsy of suspected salivary gland lymphoma in Sjögren’s syndrome. Arthritis Care Res [Internet] 2020 Apr 5 [cited 2021 Jan 26]; Available from: http://doi.wiley.com/10.1002/acr.24203.
Baldini C, Zabotti A, Filipovic N, Vukicevic A, Luciano N, Ferro F, et al. Imaging in primary Sjögren’s syndrome: the “obsolete and the new”. Clin Exp Rheumatol. 2018;36 Suppl 112(3):215.
Fernandes H, D’souza CRS, Khosla C, George L, Katte NH. Role of FNAC in the preoperative diagnosis of salivary gland lesions. J Clin Diagn Res JCDR. 2014;8(9):FC01–3.
Parker SH, Jobe WE, Dennis MA, Stavros AT, Johnson KK, Yakes WF, et al. US-guided automated large-core breast biopsy. Radiology. 1993;187(2):507–11.
Kim HJ, Kim JS. Ultrasound-guided core needle biopsy in salivary glands: a meta-analysis: core needle biopsy in salivary glands. Laryngoscope. 2018;128(1):118–25.
Witt BL, Schmidt RL. Ultrasound-guided core needle biopsy of salivary gland lesions: a systematic review and meta-analysis. Laryngoscope. 2014;124(3):695–700.
Morgan WS, Castleman B. A clinicopathologic study of Mikulicz’s disease. Am J Pathol. 1953;29(3):471–503.
Schmid U, Helbron D, Lennert K. Development of malignant lymphoma in myoepithelial sialadenitis (Sjögren’s syndrome). Virchows Arch A. 1982;395(1):11–43.
Haacke EA, Bootsma H, Spijkervet FKL, Visser A, Vissink A, Kluin PM, et al. FcRL4+ B-cells in salivary glands of primary Sjögren’s syndrome patients. J Autoimmun. 2017;81:90–8.
Kroese FGM, Haacke EA, Bombardieri M. The role of salivary gland histopathology in primary Sjögren’s syndrome: promises and pitfalls. Clin Exp Rheumatol. 2018;36 Suppl 112(3):222–33.
Stott DI, Hiepe F, Hummel M, Steinhauser G, Berek C. Antigen-driven clonal proliferation of B cells within the target tissue of an autoimmune disease. The salivary glands of patients with Sjögren’s syndrome. J Clin Invest. 1998;102(5):938–46.
De Vita S, De Marchi G, Sacco S, Gremese E, Fabris M, Ferraccioli G. Preliminary classification of nonmalignant B cell proliferation in Sjögren’s syndrome: perspectives on pathobiology and treatment based on an integrated clinico-pathologic and molecular study approach. Blood Cells Mol Dis. 2001;27(4):757–66.
Carbone A, Gloghini A, Ferlito A. Pathological features of lymphoid proliferations of the salivary glands: lymphoepithelial sialadenitis versus low-grade B-cell lymphoma of the malt type. Ann Otol Rhinol Laryngol. 2000;109(12 Pt 1):1170–5.
Isaacson PG. Extranodal lymphomas: the MALT concept. Verh Dtsch Ges Pathol. 1992;76:14–23.
Gandolfo S. Double anti-B cell and anti-BAFF targeting for the treatment of primary Sjögren’s syndrome. Clin Exp Rheumatol. 2019;37 Suppl 118(3):199.
Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–90.
de Vita S, Boiocchi M, Sorrentino D, Carbone A, Avellini C, Dolcetti R, et al. Characterization of prelymphomatous stages of B cell lymphoproliferation in Sjögren’s syndrome. Arthritis Rheum. 1997;40(2):318–31.
Bende RJ, Janssen J, Beentjes A, Wormhoudt TAM, Wagner K, Haacke EA, et al. Salivary gland mucosa-associated lymphoid tissue–type lymphoma from Sjögren’s syndrome patients in the majority express rheumatoid factors affinity-selected for IgG. Arthritis Rheumatol Hoboken Nj. 2020;72(8):1330–40.
Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, et al. 2016 American College of Rheumatology/European league against rheumatism classification criteria for primary Sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol Hoboken NJ. 2017;69(1):35–45.
Marx RE, Hartman KS, Rethman KV. A prospective study comparing incisional labial to incisional parotid biopsies in the detection and confirmation of sarcoidosis, Sjögren’s disease, sialosis and lymphoma. J Rheumatol. 1988;15(4):621–9.
Baldini C., Luciano N., Seghieri C., Ferro F., Elefante E., Mosca M. Early damage assessment and prediction of damage accrual in primary Sjögren’s syndrome using salivary gland ultrasonography during 2 years of follow-up. Ann Rheum Dis. 2017;76((Baldini C.; Luciano N.; Ferro F.; Elefante E.; Mosca M.) Clinical and Experimental Medicine, Rheumatology Unit, University of Pisa, Pisa, Italy):589.
Zabotti A, Zandonella Callegher S, Gandolfo S, Valent F, Giovannini I, Cavallaro E, et al. Hyperechoic bands detected by salivary gland ultrasonography are related to salivary impairment in established Sjögren’s syndrome. Clin Exp Rheumatol. 2019;37 Suppl 118(3):146–52.
Delli K, Haacke EA, Kroese FGM, Pollard RP, Ihrler S, Van Der Vegt B, et al. Towards personalised treatment in primary Sjögren’s syndrome: baseline parotid histopathology predicts responsiveness to rituximab treatment. Ann Rheum Dis. 2016;75(11):1933–8.
Jousse-Joulin S., Devauchelle-Pensec V., Cornec D., Gestin S., Bressollette L., Marhadour T., et al. Ultrasonographic salivary glands response to rituximab in primary sjögren syndrome patients in the tolerance and efficacy of rituximab in primary sjogren syndrome study is not associated with the anatomopathology changes. Arthritis Rheum. 2013;65((Jousse-Joulin S.) Brest University Medical School, EA 2216, UBO and CHU de la Cavale Blanche, Brest, France):S1236.
Vitali C. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American-European consensus group. Ann Rheum Dis. 2002;61(6):554–8.
van Nimwegen JF, van Ginkel MS, Arends S, Haacke EA, van der Vegt B, Sillevis Smitt-Kamminga N, et al. Validation of the ACR-EULAR criteria for primary Sjögren’s syndrome in a Dutch prospective diagnostic cohort. Rheumatology. 2018;57(5):818–25.
Carvajal Alegria G, Costa S, Jousse-Joulin S, Marcorelles P, Pers J-O, Saraux A, et al. What is the agreement between pathological features of parotid gland and labial salivary gland biopsies? Ann Rheum Dis. 2018;77(7):e37.
Barone F, Campos J, Bowman S, Fisher B. The value of histopathological examination of salivary gland biopsies in diagnosis, prognosis and treatment of Sjögren’s syndrome. Swiss Med Wkly [Internet] 2015 Sep 16 [cited 2021 Feb 25]; Available from: http://doi.emh.ch/smw.2015.14168
Carubbi F, Alunno A, Gerli R, Giacomelli R. Histopathology of salivary glands. Reumatismo. 2018;3:146–54.
De Vita S, Sansonno D, Dolcetti R, Ferraccioli G, Carbone A, Cornacchiulo V, et al. Hepatitis C virus within a malignant lymphoma lesion in the course of type II mixed cryoglobulinemia. Blood. 1995;86(5):1887–92.
Giotaki H, Constantopoulos SH, Papadimitriou CS, Moutsopoulos HM. Labial minor salivary gland biopsy: a highly discriminatory diagnostic method between sarcoidosis and Sjögren’s syndrome. Respir Int Rev Thorac Dis. 1986;50(2):102–7.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Zabotti, A. et al. (2022). Major Salivary Gland Biopsy in Sjögren’s Syndrome, with Special Reference to the Histopathology of B-Cell Proliferation. In: Bruyn, G.A.W. (eds) Sjögren’s Syndrome and the Salivary Glands. Contemporary Rheumatology. Springer, Cham. https://doi.org/10.1007/978-3-030-90977-2_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-90977-2_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-90976-5
Online ISBN: 978-3-030-90977-2
eBook Packages: MedicineMedicine (R0)